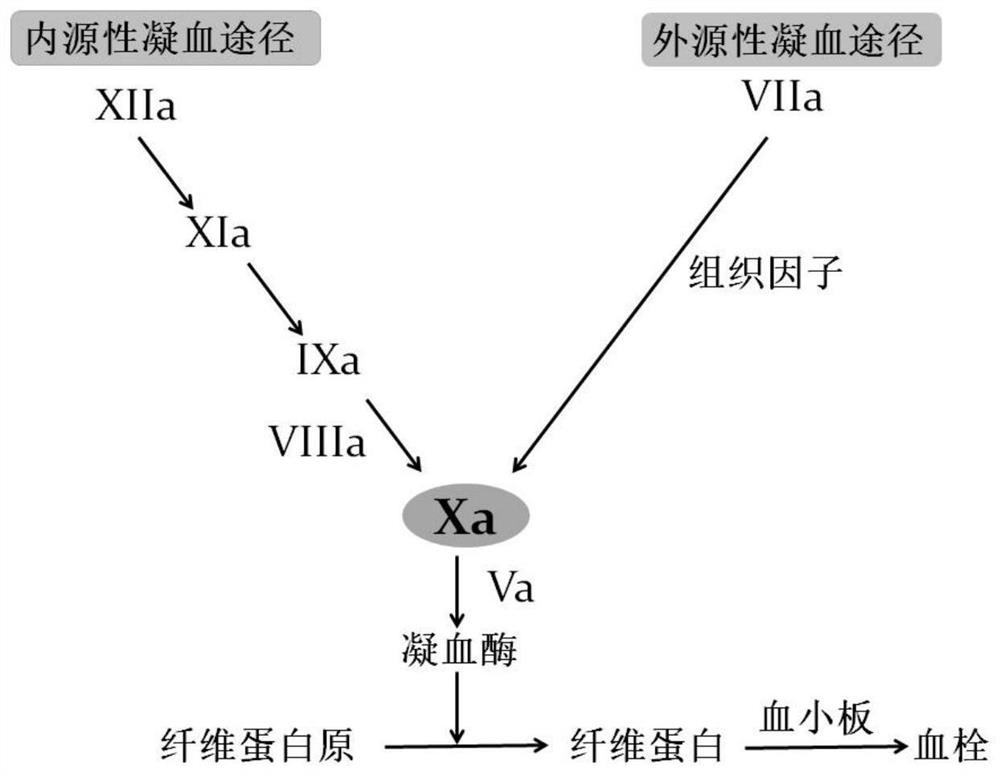
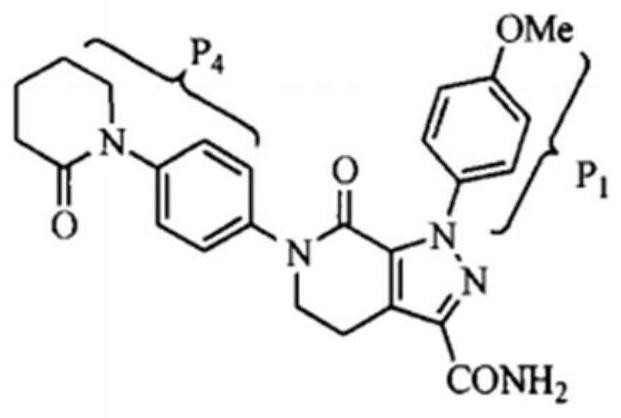
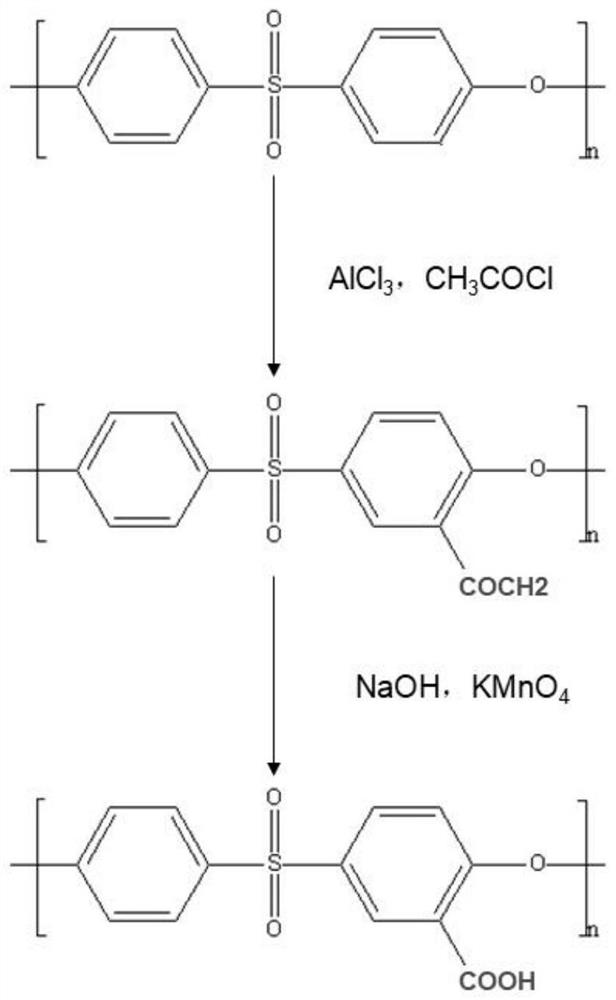
Preparation method of extracorporeal circulation anticoagulant modified membrane
An extracorporeal circulation and anticoagulant technology, applied in the field of medicine, can solve the problems of not being able to directly inhibit the core-Xa factor, aggravating the risk of bleeding in critically ill patients, and the large molecular weight of heparin substances, so as to avoid steric hindrance effects and powerful anticoagulation. coagulation and antithrombotic properties, and the effect of reducing thrombocytopenia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Step 1, synthesis of carboxylated polysulfone / polyethersulfone
[0033] Dissolve 10g of polysulfone / polyethersulfone in 40ml of NMP and stir to form a homogeneous solution, slowly blow in nitrogen, and then add 4.5g of anhydrous AlCl in sequence 3 , 6ml C 2 h 3 ClO and 100ml NMP, adjust the temperature of the reaction system to 70°C, and react for 2h. The reacted solution was washed 3 times with absolute ethanol and dried to obtain acetylated polysulfone / polyethersulfone (PSf / PES-COCH 3 ).
[0034] 10g of acetylated polysulfone / polyethersulfone (PSf / PES-COCH 3 ) was dissolved in 40ml NMP and stirred to form a homogeneous solution, and then 1g KMnO 4 , 3.7g NaOH, 9g double distilled water, 40ml NMP, adjust the temperature of the reaction system to 70°C, and react for 4h. The reacted solution was washed three times with dilute hydrochloric acid at pH=1 to obtain carboxylated polysulfone / polyethersulfone (PSf / PES-COOH).
[0035] Step 2, synthesis of apixaban modified ...
Embodiment 2
[0041] Step 1, synthesis of carboxylated polysulfone / polyethersulfone:
[0042] Dissolve 10g of polysulfone / polyethersulfone in 40ml of NMP and stir to form a homogeneous solution, slowly blow in nitrogen, and then add 5.5g of anhydrous AlCl in sequence 3 , 8ml C 2 h 3 ClO and 100ml NMP, adjust the temperature of the reaction system to 90°C, and react for 2h. The reacted solution was washed 3 times with absolute ethanol and dried to obtain acetylated polysulfone / polyethersulfone (PSf / PES-COCH 3 ).
[0043] Dissolve 10g acetylated polysulfone / polyethersulfone in 40ml NMP and stir to form a homogeneous solution, then add 1.2g KMnO4, 3.8g NaOH, 9g double distilled water, 40ml NMP in sequence, adjust the temperature of the reaction system to 80°C, and react for 6h . The reacted solution was washed three times with dilute hydrochloric acid at pH=1 to obtain carboxylated polysulfone / polyethersulfone (PSf / PES-COOH).
[0044] Step 2, synthesis of apixaban modified polysulfone / po...
Embodiment 3
[0049] Step 1, synthesis of carboxylated polysulfone / polyethersulfone:
[0050] Dissolve 10g of polysulfone / polyethersulfone in 40ml of NMP and stir to form a homogeneous solution, slowly blow in nitrogen, and then add 5g of anhydrous AlCl in sequence 3 , 7ml C 2 h 3 ClO and 100ml NMP, adjust the temperature of the reaction system to 80°C, and react for 2h. The reacted solution was washed 3 times with absolute ethanol and dried to obtain acetylated polysulfone / polyethersulfone (PSf / PES-COCH 3 ).
[0051] Dissolve 10g acetylated polysulfone / polyethersulfone in 40ml NMP and stir to form a homogeneous solution, then add 1.1g KMnO4, 3.75g NaOH, 9g double distilled water, 40ml NMP in sequence, adjust the temperature of the reaction system to 75°C, and react for 5h . The reacted solution was washed three times with dilute hydrochloric acid at pH=1 to obtain carboxylated polysulfone / polyethersulfone (PSf / PES-COOH).
[0052] Step 2, synthesis of apixaban modified polysulfone / pol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com