Eutectic crystal formed by apixaban and carboxylic acid and preparation method thereof
A technology of apixaban and carboxylic acid, applied in the field of apixaban/carboxylic acid co-crystal and preparation thereof, can solve the problems of slow dissolution rate and low dissolution rate of apixaban
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
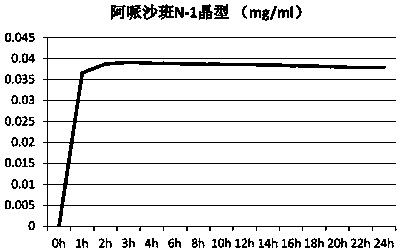
[0092] Example 1: Preparation of Apixaban / malonic acid co-crystal form I
[0093]At room temperature, add 10.2g of malonic acid, 60g of apixaban, 350ml of trifluoroethanol, and 170mL of methanol into the reaction bottle. After stirring for 1 hour to dissolve, keep the resulting solution at 30-35°C and continue stirring for 18 hours. Control the cooling rate at 10-15°C / hour, cool the crystallization solution to 0-5°C to crystallize, crystallize for 5 hours, a large number of crystals precipitate, filter the obtained crystals and wash with a small amount of methanol, and then vacuum dry at 55°C for 24 hours to obtain 53.5 g Apixaban / malonic acid co-crystal. After detection, the obtained co-crystal is Apixaban / malonic acid co-crystal form I. The X-ray powder diffraction data of the samples are as figure 1 And shown in table 1; TGA collection of illustrative plates such as figure 2 Shown; DSC spectrum such as image 3 shown.
[0094] Table 1
[0095] 2θ(°) Relat...
Embodiment 2
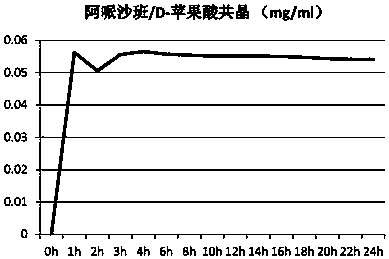
[0096] Example 2: Preparation of Apixaban / D-malic acid cocrystal form II
[0097] At room temperature, add 13.2g of D-malic acid, 60g of apixaban, 350ml of trifluoroethanol, and 170mL of methanol into the reaction flask. After stirring for 1 hour to dissolve, keep the resulting solution at 30-35°C and continue stirring for 18 hours. Then control the cooling rate of 10-15°C / hour, cool the crystallization solution to 0-5°C to crystallize, crystallize for 6 hours, a large number of crystals precipitate, filter the obtained crystals and wash with a small amount of methanol, and then vacuum dry at 55°C for 24 hours 48.6 g of apixaban / D-malic acid co-crystals were obtained. After testing, the obtained co-crystal was Apixaban / D-malic acid co-crystal form II. The X-ray powder diffraction data of the samples are as Figure 4 And shown in table 2; TGA collection of illustrative plates such as Figure 5 Shown; DSC spectrum such as Figure 6 shown.
[0098] Table 2
[0099]
Embodiment 3
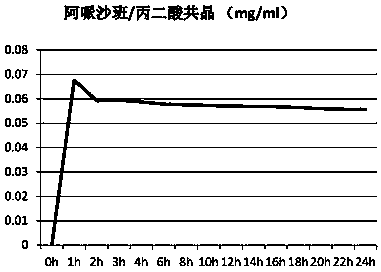
[0100] Example 3: Preparation of Apixaban / Maleic Acid Cocrystal Form III
[0101] At room temperature, add 11.3g of maleic acid, 60g of apixaban, 350ml of trifluoroethanol, and 170mL of methanol into the reaction flask. After stirring for 1 hour to dissolve, keep the resulting solution at 30-35°C and continue stirring for 18 hours. Control the cooling rate at 10-15°C / hour, cool the crystallization solution to 0-5°C to crystallize, crystallize for 5 hours, a large number of crystals precipitate, filter the obtained crystals and wash with a small amount of methanol, and then vacuum dry at 55°C for 24 hours to obtain 45.3 g Apixaban / maleic acid co-crystal. After detection, the obtained co-crystal is apixaban / maleic acid co-crystal form III. The X-ray powder diffraction data of the samples are as Figure 7 And shown in table 3; TGA collection of illustrative plates such as Figure 8 Shown; DSC spectrum such as Figure 9 shown.
[0102] table 3
[0103] 2θ(°) Rela...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com