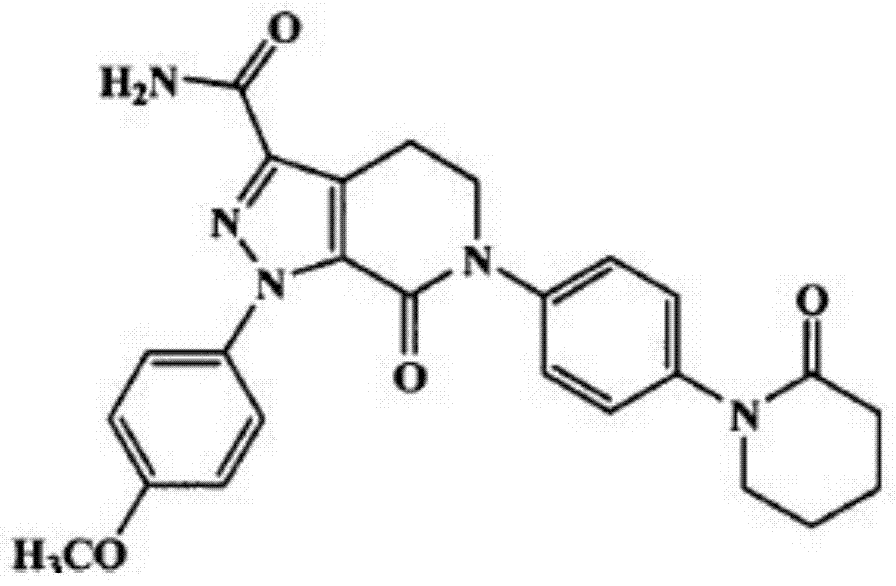
Method for preparing apixaban tablets
A technology for apixaban tablets and lubricants, which is applied in the field of preparation of apixaban tablets, can solve the problems of low in vitro dissolution rate, low bioavailability, and slow dissolution rate, and achieve high bioavailability and preparation The effect of simple method and low packaging cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
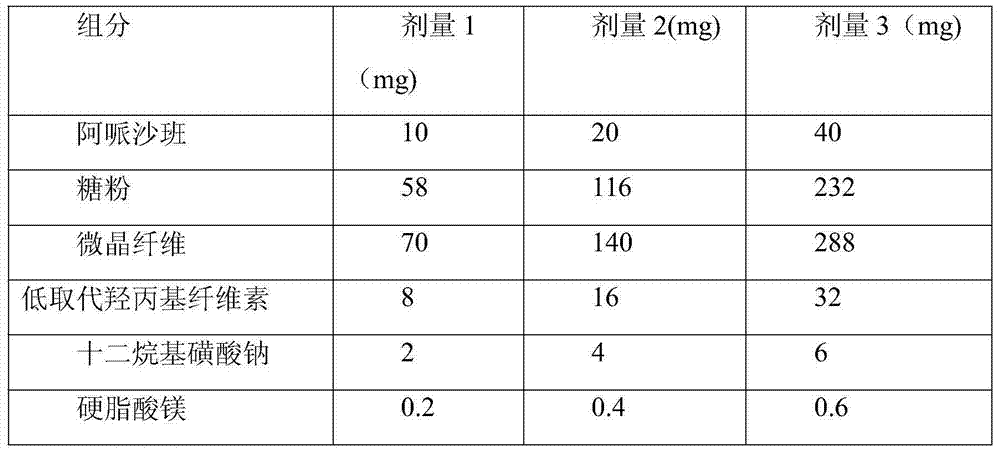
[0028] Components of apixaban tablets
[0029] Component
Dose 1 (mg)
Dose 2 (mg)
Dose 3 (mg)
10
20
30
38
76
114
70
140
210
2
4
6
2
4
6
0.2
0.4
0.6
[0030] Preparation:
[0031] (1) Weigh the raw and auxiliary materials in the above-mentioned prescription amount, except for magnesium stearate, mix all the raw and auxiliary materials and perform wet granulation. The soft material obtained is granulated with a 20-mesh sieve. The obtained granules are dried and sized. Granules, and then collect dry granules between 20 mesh and 80 mesh.
[0032] (2) After mixing the prepared granules with magnesium stearate for 30 minutes, press the tablet, and the tablet hardness is 50-60N.
Embodiment 2
[0034] Apixaban tablet components
[0035] Component
Dose 1 (mg)
Dose 2 (mg)
Dose 3 (mg)
Apixaban
10
20
40
Powdered sugar
60
120
250
22
40
88
Crospovidone
3
6
16
2
4
6
Magnesium stearate
0.2
0.4
0.6
[0036] Preparation:
[0037] (1) Weigh the raw and auxiliary materials in the above-mentioned prescription amount, except for magnesium stearate, mix all the raw and auxiliary materials and perform wet granulation. The soft material obtained is granulated with a 20-mesh sieve. The obtained granules are dried and sized. Granules, and then collect dry granules between 20 mesh and 80 mesh.
[0038] (2) After mixing the prepared granules with magnesium stearate for 30 minutes, press the tablet, and the tablet hardness is 50-60N.
Embodiment 3
[0040] Components of apixaban tablets
[0041]
[0042] Preparation:
[0043] (1) Weigh the raw and auxiliary materials in the above-mentioned prescription amount, except for magnesium stearate, mix all the raw and auxiliary materials and perform wet granulation. The soft material obtained is granulated with a 20-mesh sieve. The obtained granules are dried and sized. Granules, and then collect dry granules between 20 mesh and 80 mesh.
[0044] (2) After mixing the prepared granules with magnesium stearate for 30 minutes, press the tablet, and the tablet hardness is 50-60N.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com