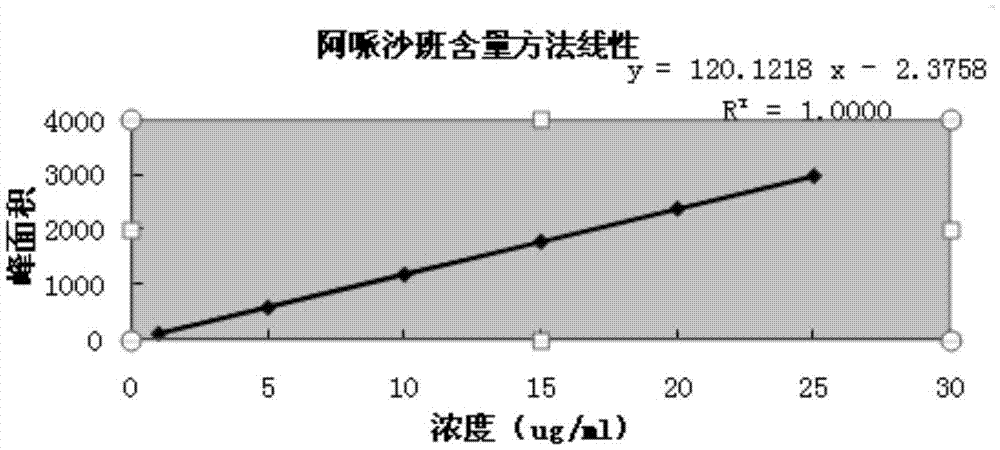
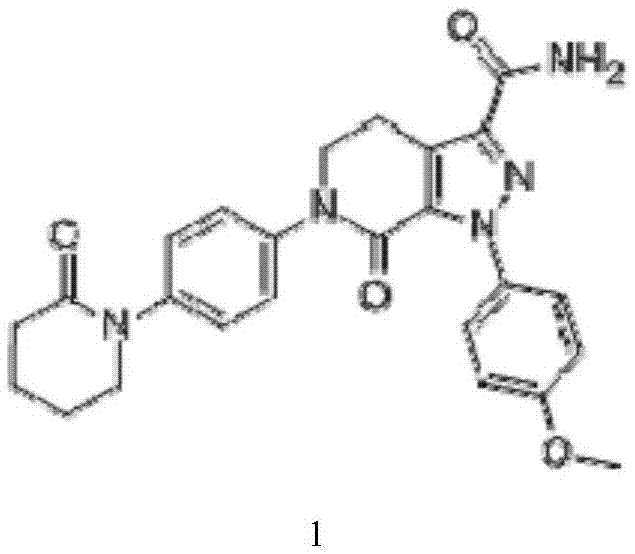
Apixaban pellet and preparation method thereof
A technology of apixaban and pellets, which is applied in the field of apixaban pellets and its preparation, can solve problems such as difficulty in mass production, adverse reactions, and cumbersome steps, and achieve good stability, stable product quality, and particle size uniform effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Example 1 Preparation of Apixaban Micropills and Micropill Capsules by Fluidized Bed Liquid Layer Method
[0076]
[0077] Grinding of the raw material drug: the apixaban raw material is pulverized with a GTM-50 jet mill until its D90=6 μm; drug-loaded layer suspension preparation: measure 2250ml of water, dissolve povidone in it, and at this time the binder The concentration is about 2.0% by mass, then add the crushed apixaban raw material, microcrystalline cellulose and croscarmellose sodium in the prescribed amount, stir until uniformly dispersed, and pass through a 60-mesh sieve to obtain a suspension of the drug-loaded layer ; Use WBF-2G multifunctional fluidized bed for drug loading, the process parameters are as follows: air volume 100m 3 / h, liquid supply speed 6g / min, atomization pressure 1.7bar, air inlet temperature 50°C, material temperature 40°C, spacer height 10cm; fluidized bed drying for 30min after drug application, the parameters are as follows: air...
Embodiment 2
[0078] Example 2 Preparation of Apixaban Micropills and Micropill Capsules by Fluidized Bed Liquid Layer Method
[0079]
[0080] Grinding of the raw material drug: GTM-50 jet pulverizer is used to pulverize the apixaban raw material until its D90=6 μm; drug-loaded layer suspension preparation: measure 4500ml of water, dissolve povidone in it, and at this time the binder The concentration is about 1.0% by mass, then add the crushed apixaban raw material, microcrystalline cellulose and croscarmellose sodium in the prescribed amount, stir until uniformly dispersed, and pass through a 60-mesh sieve to obtain a drug-loaded layer suspension ; Use WBF-2G multifunctional fluidized bed for drug loading, the process parameters are as follows: air volume 100m 3 / h, liquid supply speed 6g / min, atomization pressure 1.7bar, air inlet temperature 50°C, material temperature 40°C, spacer height 10cm; fluidized bed drying for 30min after drug application, the parameters are as follows: air vo...
Embodiment 3
[0081] Example 3 Preparation of Apixaban Pellets and Pellet Capsules by Fluidized Bed Liquid Layer Method
[0082]
[0083] Grinding of the raw material drug: GTM-50 jet pulverizer is used to pulverize the apixaban raw material until its D90=6 μm; drug-loaded layer suspension preparation: measure 900ml of water, dissolve povidone in it, and at this time the binder The concentration is about 5.0% by mass, then add the crushed apixaban raw material, microcrystalline cellulose and croscarmellose sodium in the prescribed amount, stir until uniformly dispersed, and pass through a 60-mesh sieve to obtain a suspension of the drug-loaded layer ; Use WBF-2G multifunctional fluidized bed for drug loading, the process parameters are as follows: air volume 100m 3 / h, liquid supply speed 6g / min, atomization pressure 1.7bar, air inlet temperature 50°C, material temperature 40°C, spacer height 10cm; fluidized bed drying for 30min after drug application, the parameters are as follows: air ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com