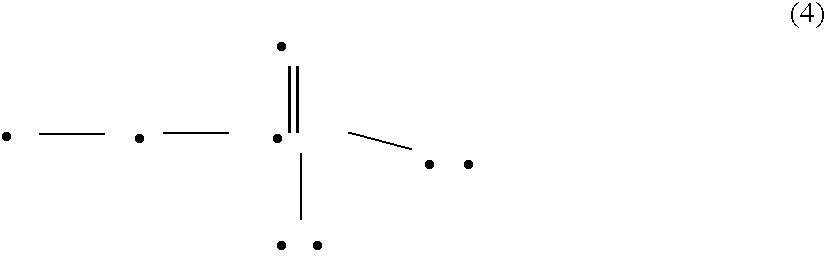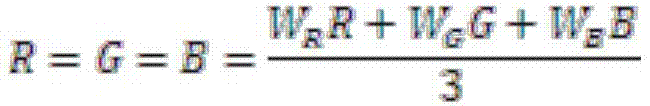Patents
Literature
577 results about "Drug application" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An Abbreviated New Drug Application (ANDA) is a written request to the U.S. Food and Drug Administration to manufacture and market a generic drug in the United States. An Investigational New Drug (IND) application is the first step in the drug review process by the U.S. Food and Drug Administration (FDA).
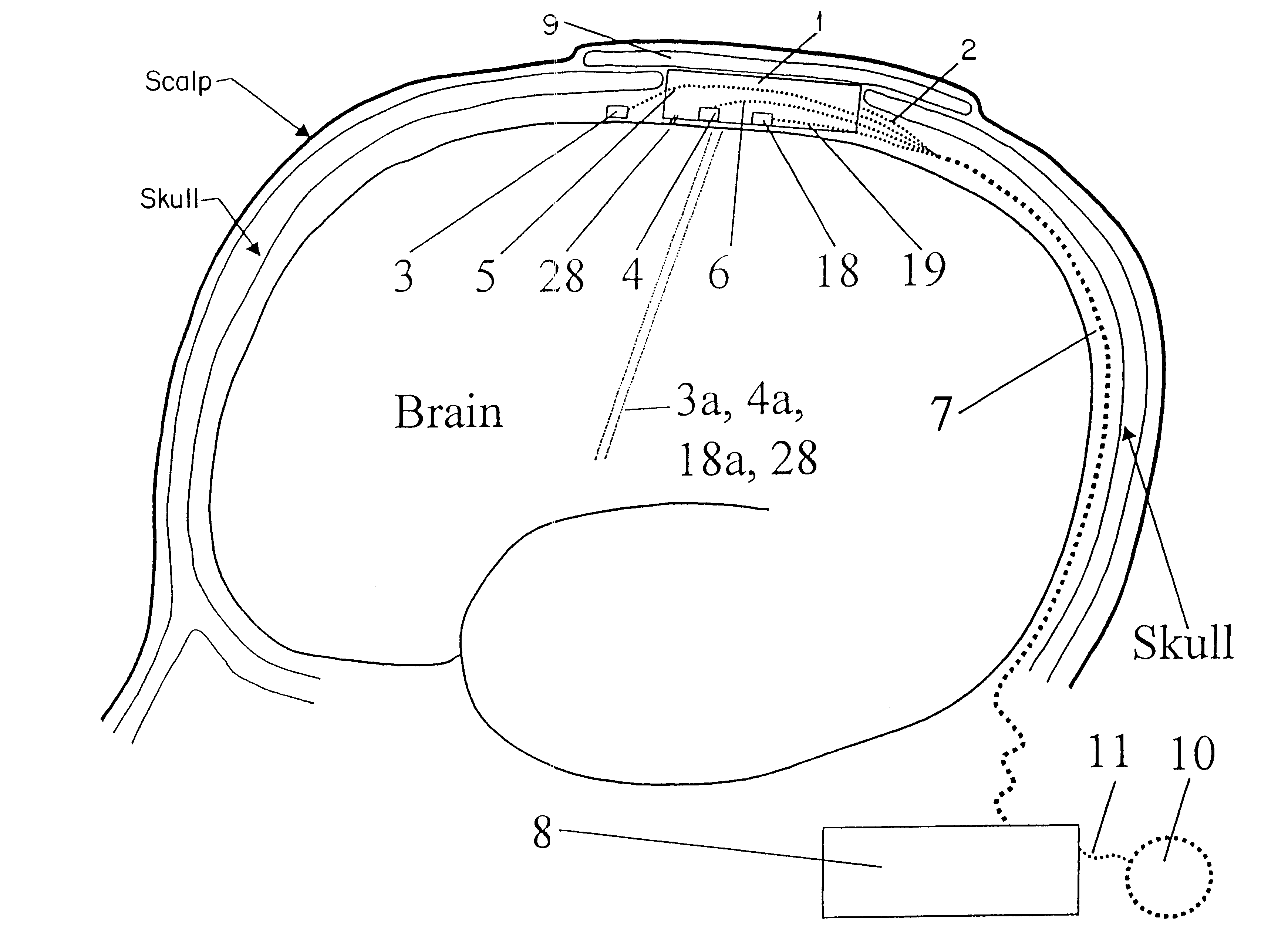
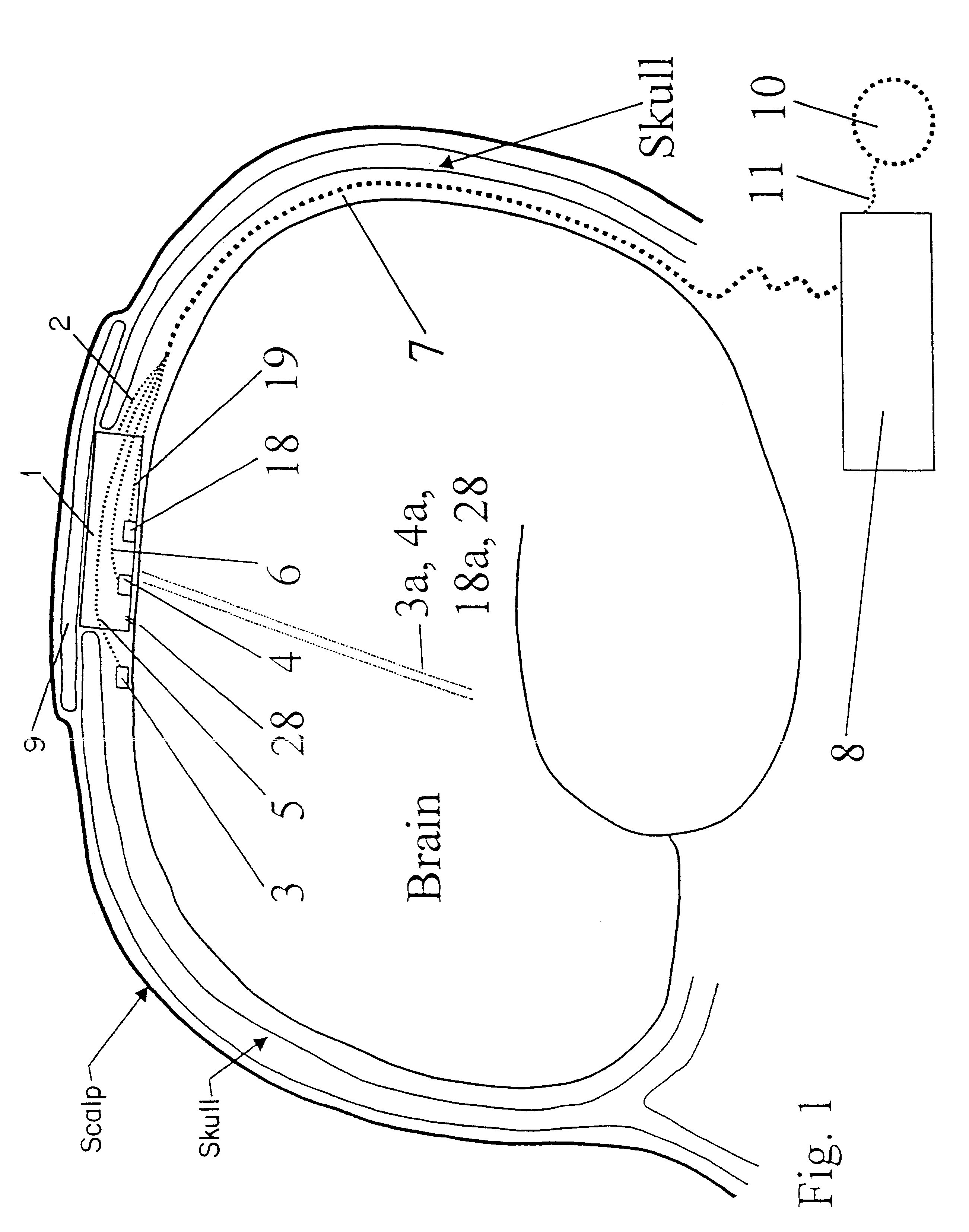
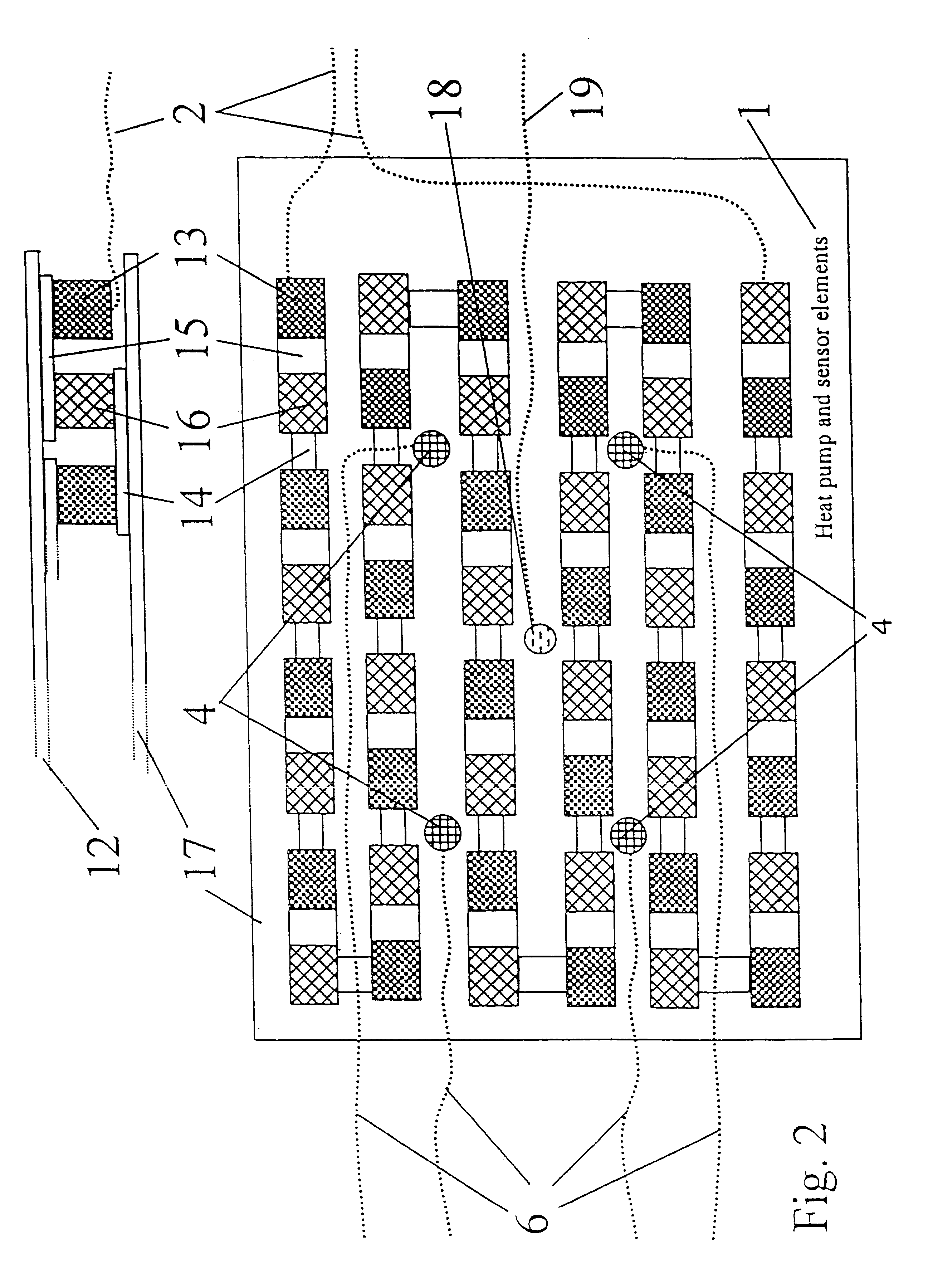
Technique for using heat flow management to treat brain disorders
InactiveUS6248126B1Prevent and reduce occurrenceModulates seizureImplantable neurostimulatorsSurgical instruments for heatingDiseaseBrain section
A method of treating a brain disorder by heat transfer from brain tissue comprising the steps of surgically cutting a heat transfer aperture into a patient's skull, thereby exposing a predetermined portion of patient's brain; surgically implanting into said heat transfer aperture a heat pump having one or more electrical sensor elements and one or more temperature sensor elements; surgically implanting a heat transfer management unit in a body cavity of said patient such that a micro controller of the heat transfer management unit is connected to one or more activity sensor elements and one or more temperature sensor elements contacting brain tissue and connecting the heat transfer management unit to said heat pump via a lead bundle. Optionally, the heat transfer unit may be located external to the patient's body. Responsive to signals from one or more activity or temperature sensor elements, mathematical algorithms of the heat transfer management unit determine abnormal brain activity, causing the heat pump to remove heat from the brain tissue into a heat sink, thereby cooling the predetermined portion of the patient's brain. This technique utilizes acute hypothermia by means of a Peltier cooler or similar device to cool the brain temperature to reduce or prevent seizure initiation and / or propagation. The method may be used in association with brain stimulation and / or drug application to acutely avoid the occurrence of a seizure episode.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
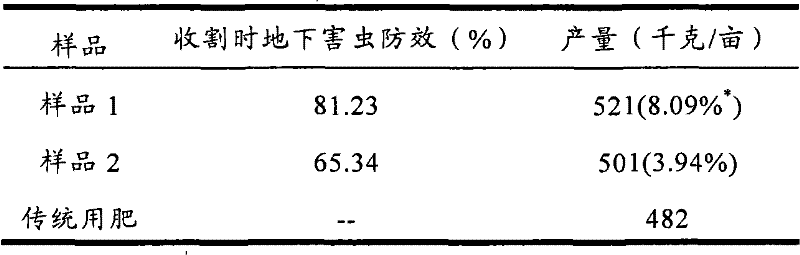
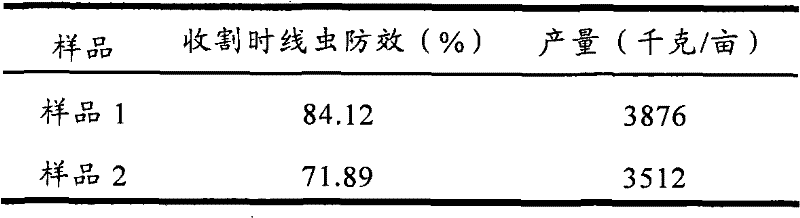
Controlled release particle fertilizer used for controlling crop insect disease
The invention relates to a controlled release particle fertilizer used for controlling crop insect diseases. The fertilizer has a double layer core shell structure and is in a granular shape and comprised of a core, an interface layer cladding the core and a controlled release layer as a casing cladding the interface layer. The core contains fertilizers required by a crop, and the interface layer cladding the core contains pesticide; or according to production requirement, the core contains particles with pesticide, and the interface layer cladding the core contains fertilizers; and an outermost layer is a controlled release layer controlling dissolving of pesticide and fertilizers. According to the invention, pesticide and fertilizer can coexist stably and release slowly, so as to reduce drug application and fertilizing times during a crop fertility stage and save field labor.
Owner:GAUNGXI TIANYUAN BIOCHEM
Aggregates with increased deformability, comprising at least three amphipats, for improved transport through semi-permeable barriers and for the non-invasive drug application in vivo, especially through the skin
InactiveUS20040105881A1Increase drug concentrationImprove distributionOrganic active ingredientsAntipyreticBiological bodyDrug carrier
Owner:IDEA AG
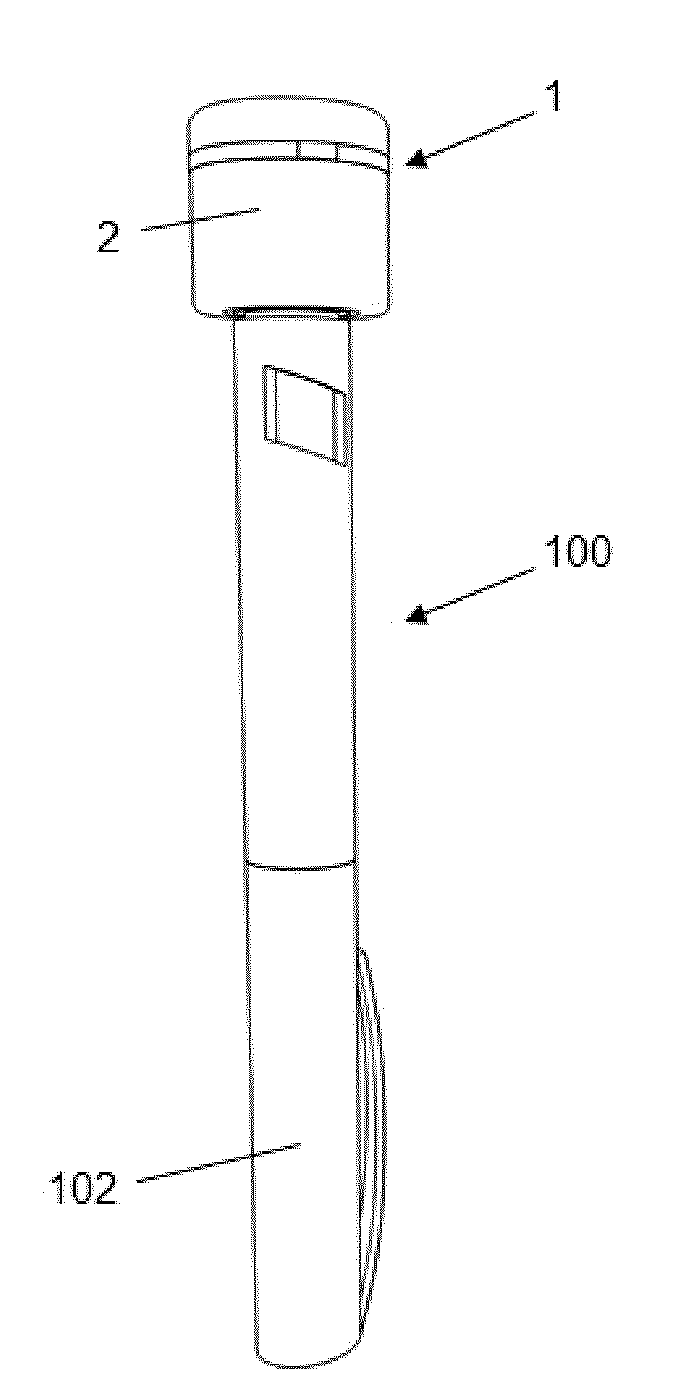
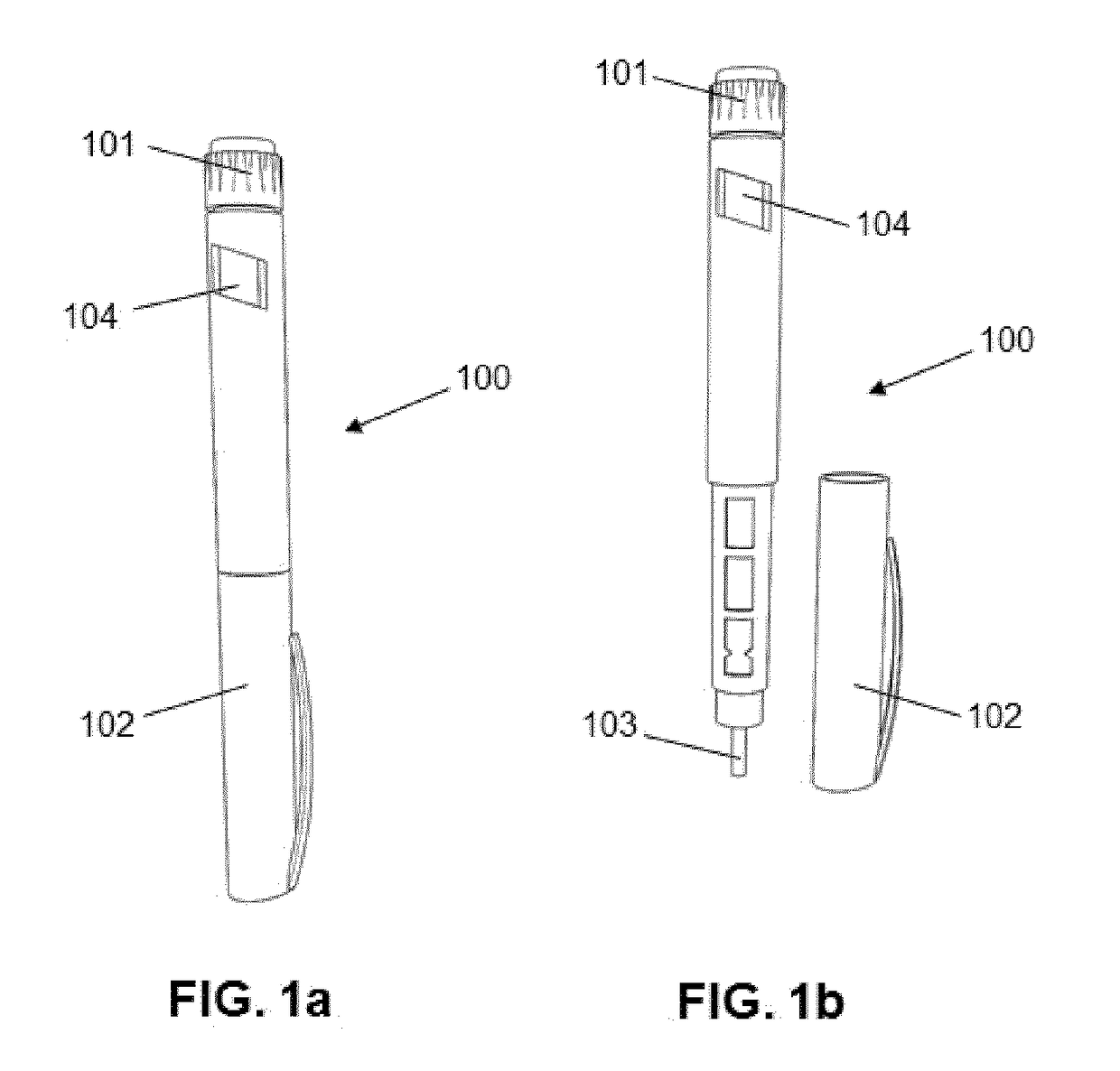
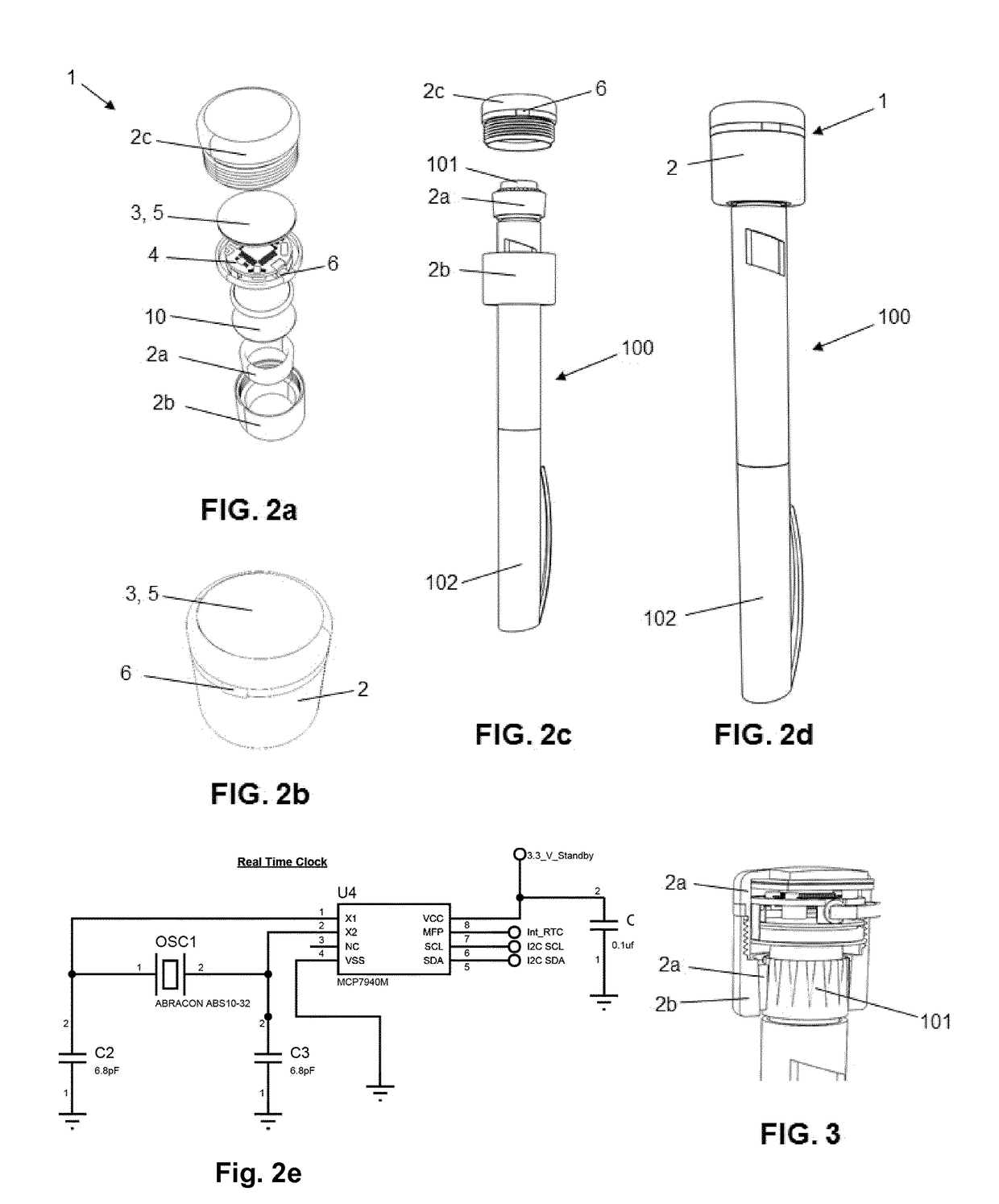
Monitoring device for drug application with a drug pen, with logging, communication and alarms
ActiveUS20180147362A1Improve adhesionImprove the quality of lifeAmpoule syringesDrug and medicationsDrug injectionLogging
The present invention provides a device (1) for monitoring the application of a drug to a patient by means of a drug pen (100), whereby the drug pen comprises a front end provided with an injection needle and a rear end provided with an actuation pushbutton, the device comprising a body (2) which can be dismountably coupled to the pen; an injection detection means determining when a drug injection is carried out; and a processing means configured for storing the date and time of the injection when the injection detection means detects that a drug injection is carried out, whereby the body is configured to be coupled to the pushbutton of the pen such that the pushbutton is actuated by pushing directly on the body, whereby the injection detection means is implemented as actuation detector configured for detecting said pushing action.
Owner:INSULCLOUD
Green long-acting zoonosis vector prevention and control medicament
InactiveCN102450282AShort durationNot easy to develop resistanceAntibacterial agentsBiocideDiseaseEcological environment
A green long-acting zoonosis vector control medicament provided by the invention is prepared from an insect growth regulator (IGR), a plant essential oil and / or a low-toxicity high-efficiency pesticide, a bactericide, an insect attractant and / or a repellant, a sustained release agent and the like. According to different use conditions and environments, by the adoption of microemulsification and sol technologies, a spraying agent, a sol coating, a film-forming inhibitor and a sol-forming inhibitor are prepared to form different release mechanisms so as to satisfy control demands in different places. The medicament provided by the invention has low toxicity and high efficiency, has effects of killing ovum, inhibiting insect growth and resisting bacteria and mildew, has a long persistent period, is not easy to generate resistance, has low residual, can be used to minimize the spread of diseases and eliminate the pollution caused by unbalance of drug application, is beneficial to the ecological environment, and is used to control zoonosis vectors (such as flea, louse, mite, tick, mosquito, fly, ant, cockroach and the like) in the fields of public place, garden, urban landscaping, means of transportation, neighborhood, hotel, school, restaurant, household, pet, animal husbandry, culturing farm and the like.
Owner:周端午
Dinotefuran insecticide having insect inducing effect
ActiveCN104472527AStrong chain killing effectGood moisturizing effectBiocidePest attractantsExcipientBalance water
The invention relates to an insecticide, in particular, relates to a dinotefuran insecticide having an insect inducing effect, and belongs to the technical field of sanitation and epidemic prevention. The dinotefuran insecticide comprises the following components by the weight percentage: 0.01-2% of dinotefuran, 0-2% of muscalure, 5-30% of sugar, 5-50% of a suggestive food ingredient, 5-30% of a moisturizing agent, 0.005-2.0% of a preservative agent, 0.01%-5.0% of an excipient, 0.5-1% of an acidifying or alkalizing agent, and the balance water. The preparation has the advantages that the preparation has a strong chain killing effect on cockroaches, has small drug application amount, has less pollution to the environment, and can penetrate into gaps for drug application. Moreover, after drug application, the cockroaches are not immediately dead after feeding but carry the attached drug back to a nest, with utilization of attributes that the cockroaches lick other cockroaches and like to eat other cockroach dead bodies, the drug is transferred to the other cockroaches, while the cockroaches are dead, other cockroach death is caused, and the whole nest of cockroaches are killed, so as to achieve the sustainable control effect.
Owner:江苏功成生物科技有限公司
Accurately targeted drug applying device and method for fruit tree rootstock
InactiveCN102907406ARapid positioningAvoid slow recognitionCharacter and pattern recognitionInsect catchers and killersPortal frameGlobal Positioning System
The invention relates to an agricultural drug applying device, in particular to an accurately targeted drug applying device for fruit tree rootstock. The device comprises a system carrying platform, a locating and identifying subsystem, a targeted drug applying subsystem and a distribution map generating subsystem. According to growing characteristics of the fruit tree rootstock, a portal frame type moving platform is adopted; a scanning type laser range finder, machine vision and GPS (global positioning system) are combined together to quickly and effectively locate and identify the fruit tree rootstock, therefore a multi-nozzle drug applying system is controlled to finish the targeted drug applying operation, and meanwhile the distribution map of the fruit, the rootstock and the drug applying amount is generated. The accurately targeted drug application to the fruit tree rootstock can be implemented; the pesticide usage amount is effectively reduced; the drug liquid utilization ratio is improved; the environment pollution is reduced; and scientific skills can be provided for the orchard digital management in the future.
Owner:BEIJING FORESTRY UNIVERSITY
Circulating water aquaculture device for aquatic animal aquaculture and drug application evaluation
The invention relates to a circulating water aquaculture device for aquatic animal aquaculture and drug application evaluation. The circulating water aquaculture device integrates aquatic animal aquaculture, water treatment and water quality monitoring, can achieve circulating water or still water aquaculture of fresh water or seawater aquatic animals, treatment and drainage of aquaculture water and aquaculture water physical and chemical parameter detection and can guarantee that the aquaculture environment is excellent, and the experiment condition is controllable in the drug application evaluation (for example, vaccine efficacy evaluation) process.
Owner:EAST CHINA UNIV OF SCI & TECH +1
C-aryl glucoside derivative, preparation method and applications thereof
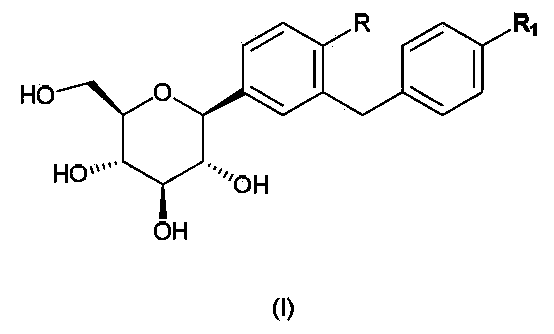
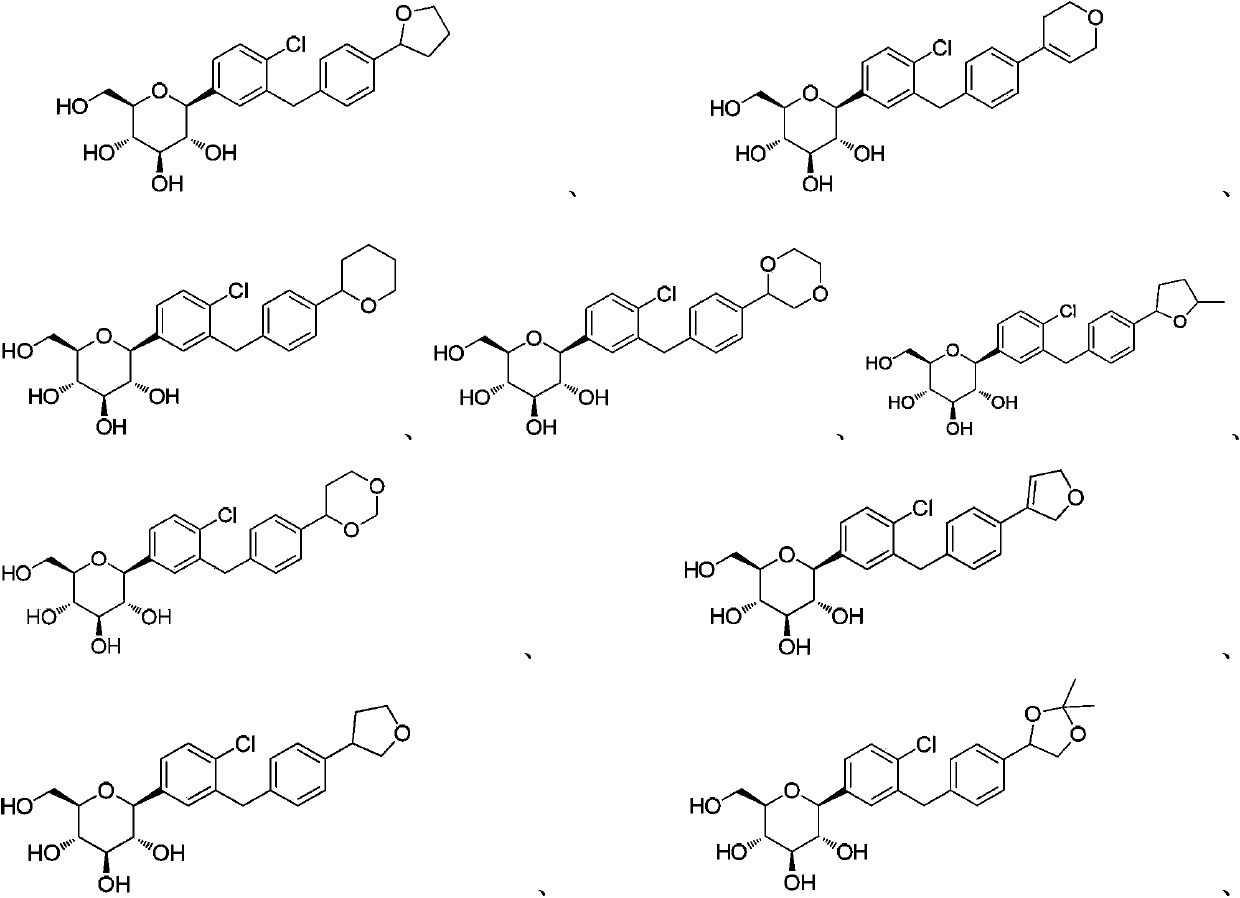
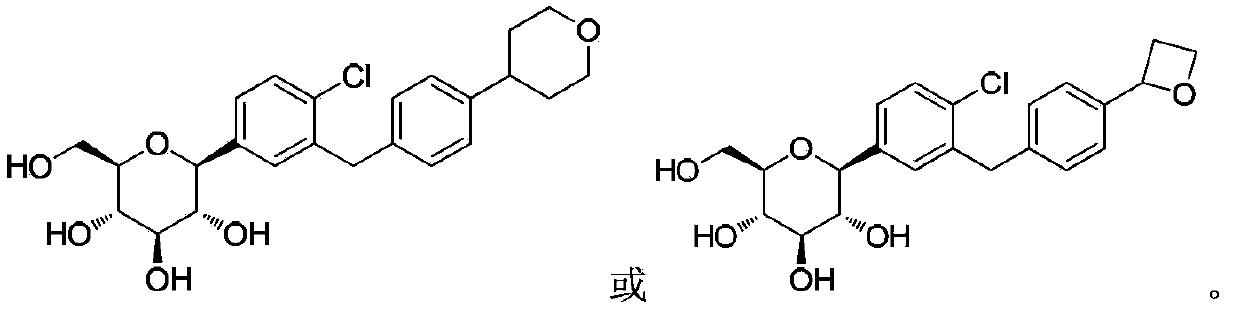
InactiveCN104109154ANovel structureStrong inhibitory activityOrganic active ingredientsOrganic chemistryDiseaseAryl
The invention discloses a C-aryl glucoside derivative, a preparation method and applications thereof. The derivative can be a compound represented by the formula (I), an optical isomer or a pharmaceutically acceptable salt of the compound, wherein the R is selected from H, chlorine, or methyl; and the R1 is selected from components whose structural formula is represented in the description. The C-aryl glucoside derivative has a novel biological structure, and has a higher SGLT-2 activity and selectivity inhibiting effect than that of the conventional SGLT-2 inhibitors. The drugs, which are prepared from the derivative, for treating SGLT-2 related diseases have a better in-vivo sugar reducing effect and higher drug application safety.
Owner:SHANGHAI SUN SAIL PHARMA SCI & TECH CO LTD
Bacteriostatic mildew-resistant insecticidal sustained release agent prepared from sol/gel also used as insect attractant
The invention relates to a bacteriostatic mildew-resistant insecticidal sustained release agent prepared from a sol / gel also used as an insect attractant. The agent is prepared into a sol plastic and a sol forming preparation with a microemulsion technology, a sol technology and the like according to different use conditions. The agent which treats an insect growth regulator (IGR) as a main component and essential oil and / or a low-toxicity high-efficiency insecticide, a bactericide, a penetrant, a water-soluble solvent, a stabilizing agent, an insect attractant, a film forming sustained release agent and the like as assistant components to satisfy prevention and control demands of different places. The agent of the invention, which has the advantages of wide insecticidal spectrum, low toxicity and high efficiency, ovum killing, insect growth inhibition, long persistent period, difficult resistance generation, bacteriostasis and mildew resistance, and low residual, can eliminate the pollution caused by unbalanced drug application, and is in favor of the ecological environment. The agent which is used for killing, preventing and controlling pests, mites and mildews on storage objects of stored grains, tobaccos, medicines, leathers, clothes, books, archives, history relics and the like and plants can reduce the loss caused by the pests and the mildews of the stored objects, the grains and the plants.
Owner:周端午
Comprehensive burning treatment couch
InactiveCN105496707ASimple structurePowerfulOperating tablesMedical applicatorsBurn treatmentDisinfectant
The invention discloses a comprehensive burning treatment couch. The comprehensive burning treatment couch comprises a couch rack, a first supporting plate, a second supporting plate, a transparent cover, a disinfectant tank, an assembly plate, a rotating ball, an atomizing nozzle, a blower, an air inlet pipe, a latch, a seal plate, a temperature sensor, a humidity sensor, a humidifier, a second rotating ball, a drug delivery pipe, an extrusion hole, a drug application cloth cover, a seal cap and an air bag. The comprehensive burning treatment couch is simple in structure and powerful in functions, can avoid a wound when a patient is treated in the treatment couch, the wound can be prevented from being pressed, secondary hurt of the wound can be prevented, dust can be effectively prevented from falling on the wound of the patient, wound infection can be avoided, and isolated disinfectant spray and ointment application can be directly provided for patients inside the transparent cover.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Preparation method for extracting flavone from purslane and uses thereof
InactiveCN101401830AWide variety of sourcesLow priceUrinary disorderPlant ingredientsCreatinine riseSide effect
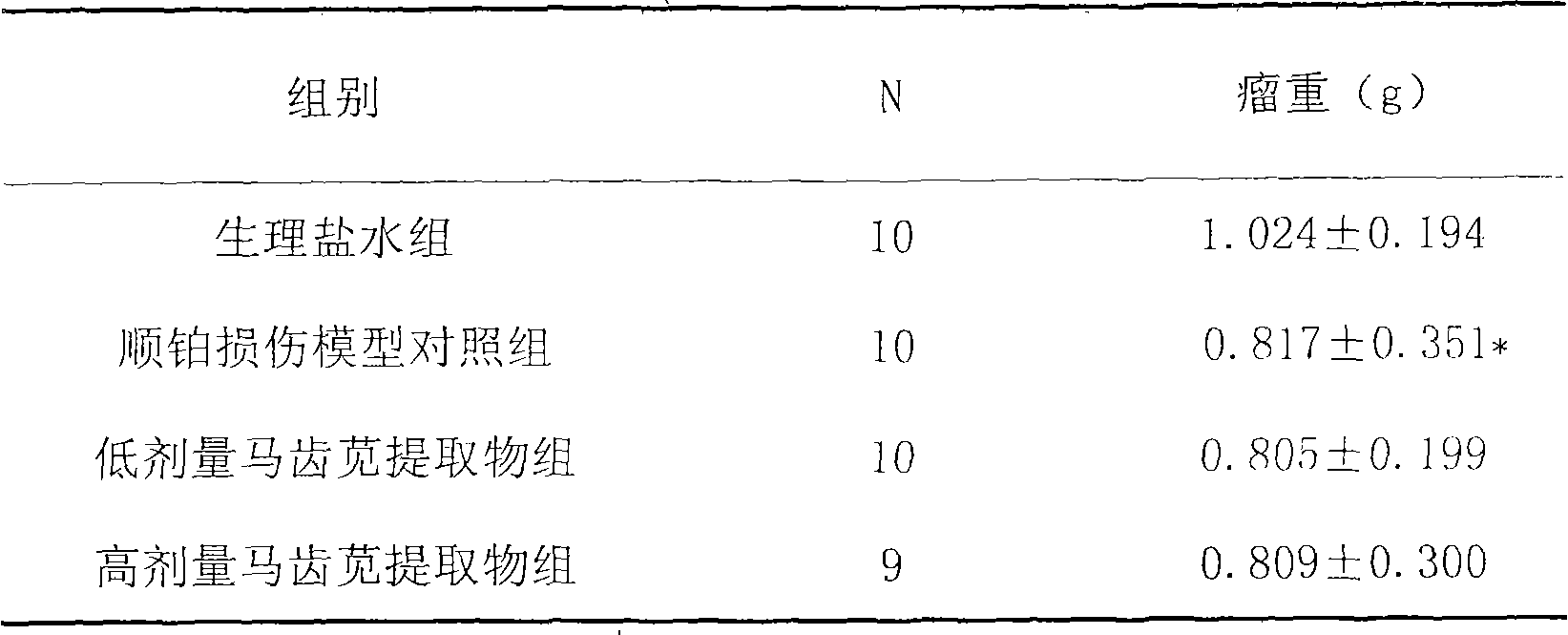
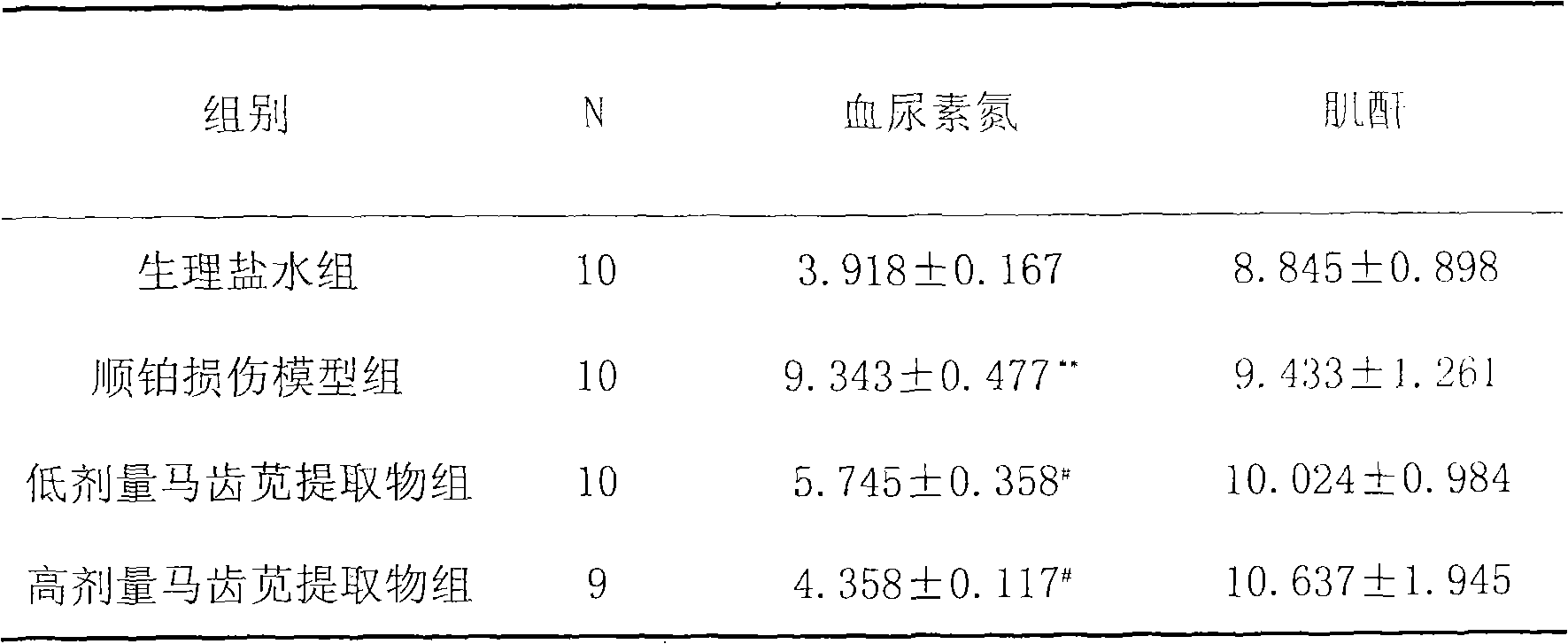
The invention discloses a preparation method for extracting flavone from purslane and application thereof. The medicine is characterized by a purslane alcohol extract. The preparation method comprises the following steps: 1. cleaning and drying the purslane; 2. cutting the purslane into short sticks; 3. extracting the flavone in the purslane by the reflux of alcohol; 4. performing the pumping filtration on the extracting solution; and 5. concentrating the liquid obtained after pumping filtration. The application method of the extract comprises the following steps: 1. inoculating tumor cells; 2. randomly dividing mice into four groups; 3. performing the group treatment; 4. sampling the blood, and weighing tumor; 5. obtaining blood serum; 6. measuring the content of blood urea nitrogen; and 7. measuring the content of serum creatinine. Animal tests prove that the extract can effectively resist kidney damage caused by the anticancer drug cisplatin; moreover, the extract has the advantages of convenient drug application, good effect and no toxic and side effects; and the purslane is wide in source, and low in price, so that the extract also has the advantages of low price and good quality.
Owner:WUHAN UNIV
Antituberculosis pharmaceutical composition
InactiveCN1602872AAvoid selective resistanceAvoid irregular medicationAntibacterial agentsOrganic active ingredientsPatient complianceHydrazine compound
The invention relates to an antituberculosis drug combination, prepared of rifampicin, isonicotinyl hydrazine, pyrazinamide, ethambutol hydrochloride and other auxiliaries, able to effectively overcome problems of drug resistance, improving compliance of patient, etc. It is applied in a compound form, which can avoid curing by single antituberculosis drug, thus avoiding selective drug resistance caused by single drug; the patients are willing to accept this, avoiding irregular drug application caused by simultaneously taking several drugs and different numbers of drug tablets, or taking more or less drugs.
Owner:浙江南洋药业有限公司
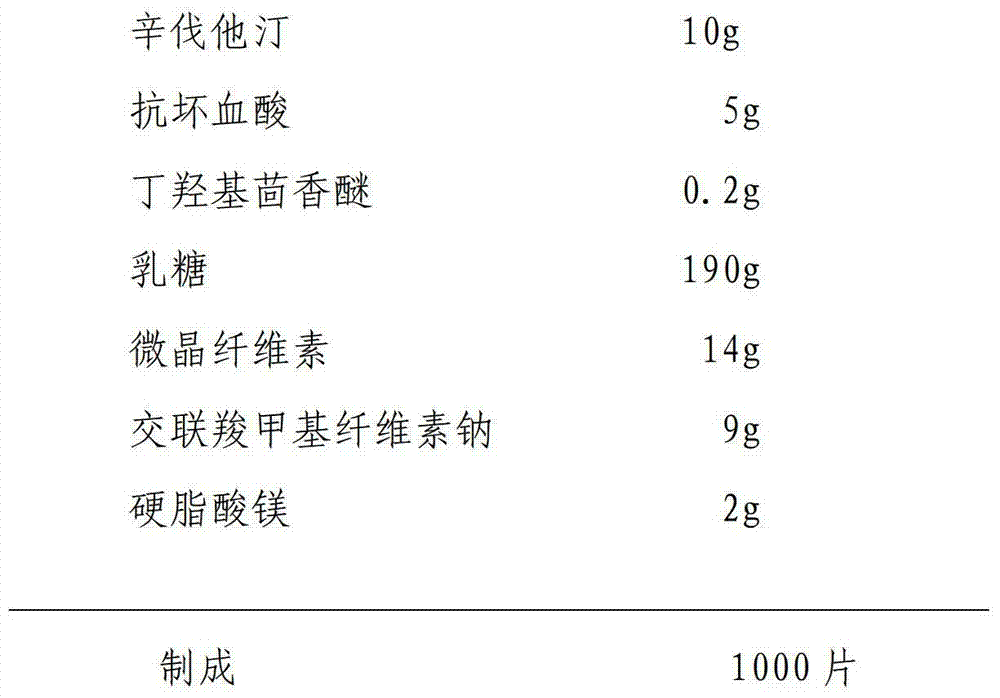
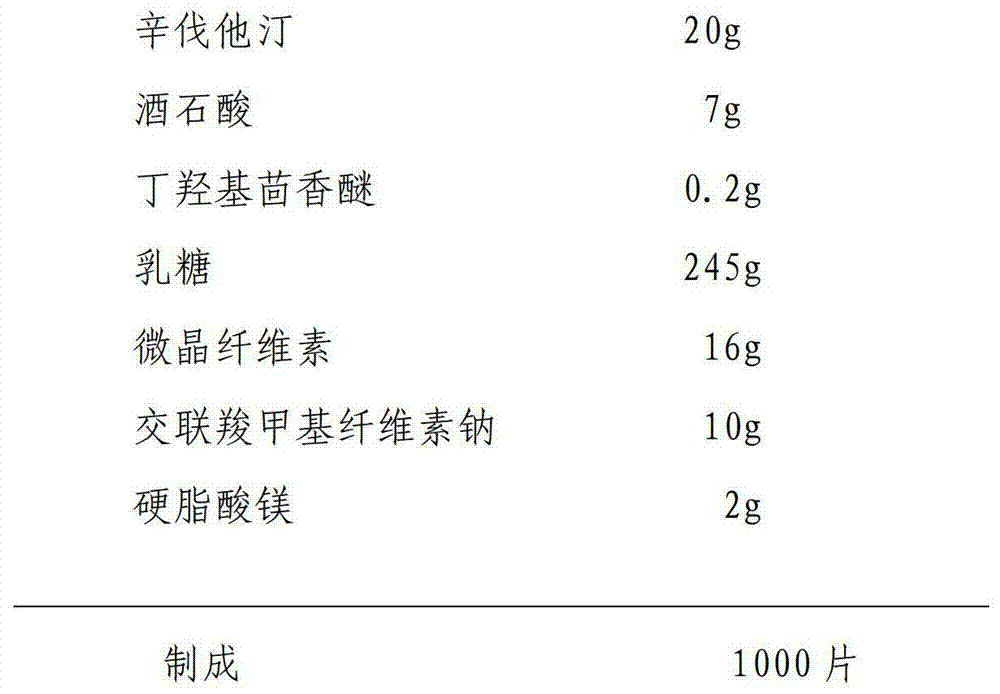
Preparation method of simvastatin tablet
InactiveCN104224736ASmall particle sizeLarge specific surface areaOrganic active ingredientsMetabolism disorderPrillDissolution
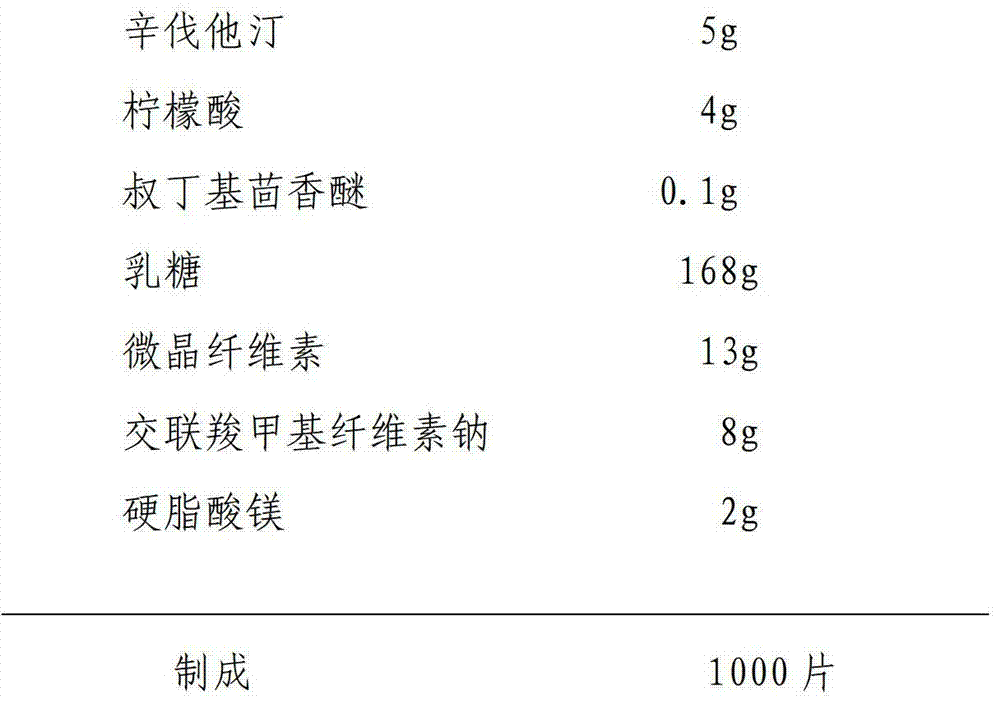
The invention relates to a preparation method of a simvastatin tablet. The simvastatin tablet is composed of simvastatin, an acidic protecting agent, an antioxidant agent, a filling agent, a disintegrating agent and a lubricant. The preparation method disclosed by the invention comprises the following steps: carrying out low-temperature superfine grinding on a simvastatin raw material and lactose in a weight ratio of 1 to (1-10), so that the particle size is reduced and the specific surface area is increased, and then the raw material is changed from a lipophilic material into a hydrophilic material; uniformly mixing the obtained mixed material with the acidic protecting agent and the antioxidant agent, the rest of lactose, microcrystalline celluloses and crosslinked carboxymethyl cellulose sodium, granulating by using a wet method, and carrying out fluidized drying while controlling the water content of a finished product grains at 1-5%, so that the simvastatin tablet is prepared. The prescription process is simple and easy to control, and prepared preparation is good in content uniformity, high in dissolution rate, good in absorption, and high in bioavailability in comparison with other preparations. The preparation method of the simvastatin tablet disclosed by the invention is good in good in reproducibility, large in productivity, and stable and controllable in quality, and can effectively guarantee the effectiveness of drugs and the safety of drug application of patients.
Owner:哈药集团人民同泰医药股份有限公司
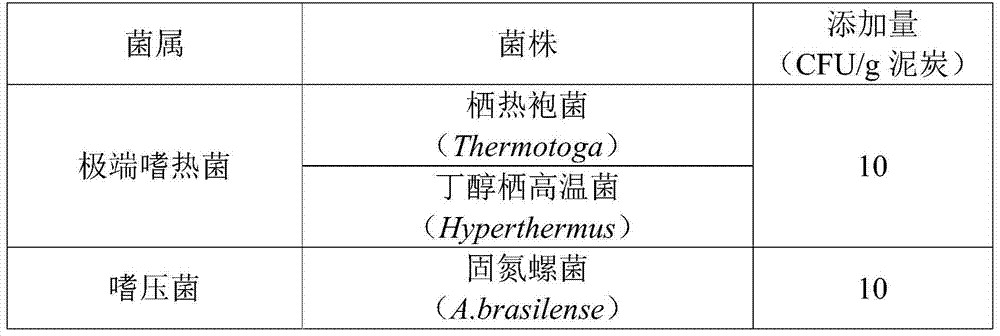
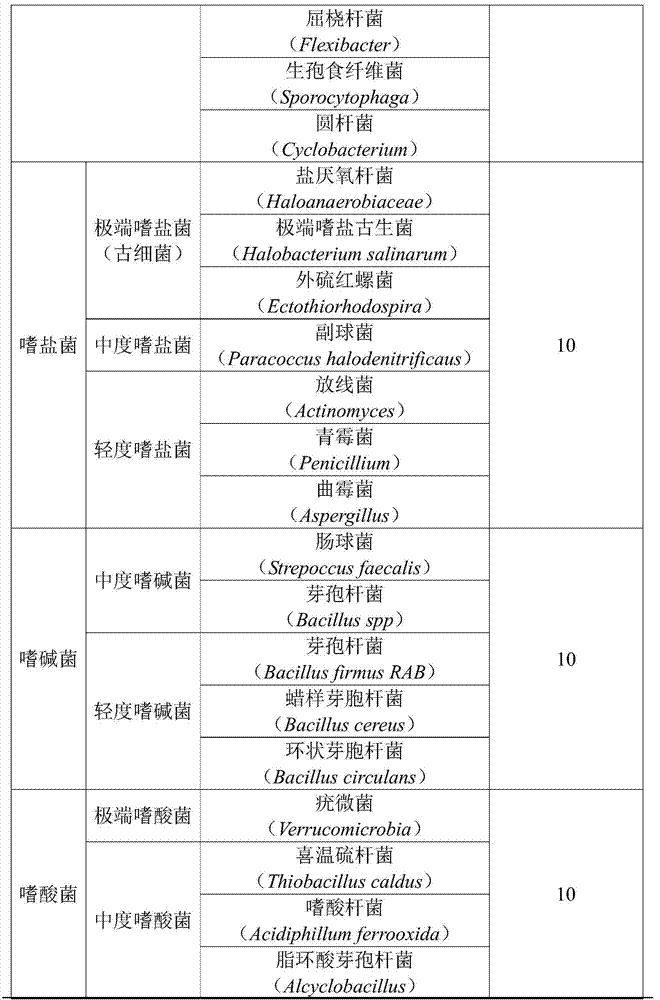
Method for extracting and preparing peat fulvic acid and drug application
InactiveCN103588977AAvoid high temperatureAvoid high pressureFungiOrganic chemistryPeatExtreme thermophile
The invention discloses an extraction method of peat fulvic acid and drug application. A method A is that mixing peat, hyperthermophilic baceria (3-13 CFU), barophilic baceria (5-11 CFU), halophilic baceria (6-12 CFU), basophilic baceria (7 to 13 CFU) and acidophilic bacteria (3-10 CFU) under three conditions including the condition I (10-130 DEG C and 0.05-0.18 MPa), the condition II (5-75 DEG C and 0.05 to 0.12 MPa) and the condition III (5-65 DEG C and 0.05 to 0.12 MPa) and treating for 1-2, 1-3 and 1-2 days respectively; a method B is that mixing the peat and part of the baceria under the conditions I and II for 1-2 and 1-3 days respectively, and mixing with the acidophilic bacteria under the condition III for 1-2 days; a method C is that mixing the peat, the hyperthermophilic baceria and the barophilic baceria under the condition I for 1-3 days, mixing with the halophilic baceria and the basophilic baceria under the condition II for 1-3 days, and mixing with the acidophilic bacteria under the condition III for 1-2 days. Fulvic acid prepared by the method is high in purity.
Owner:云南联合药业有限责任公司 +1
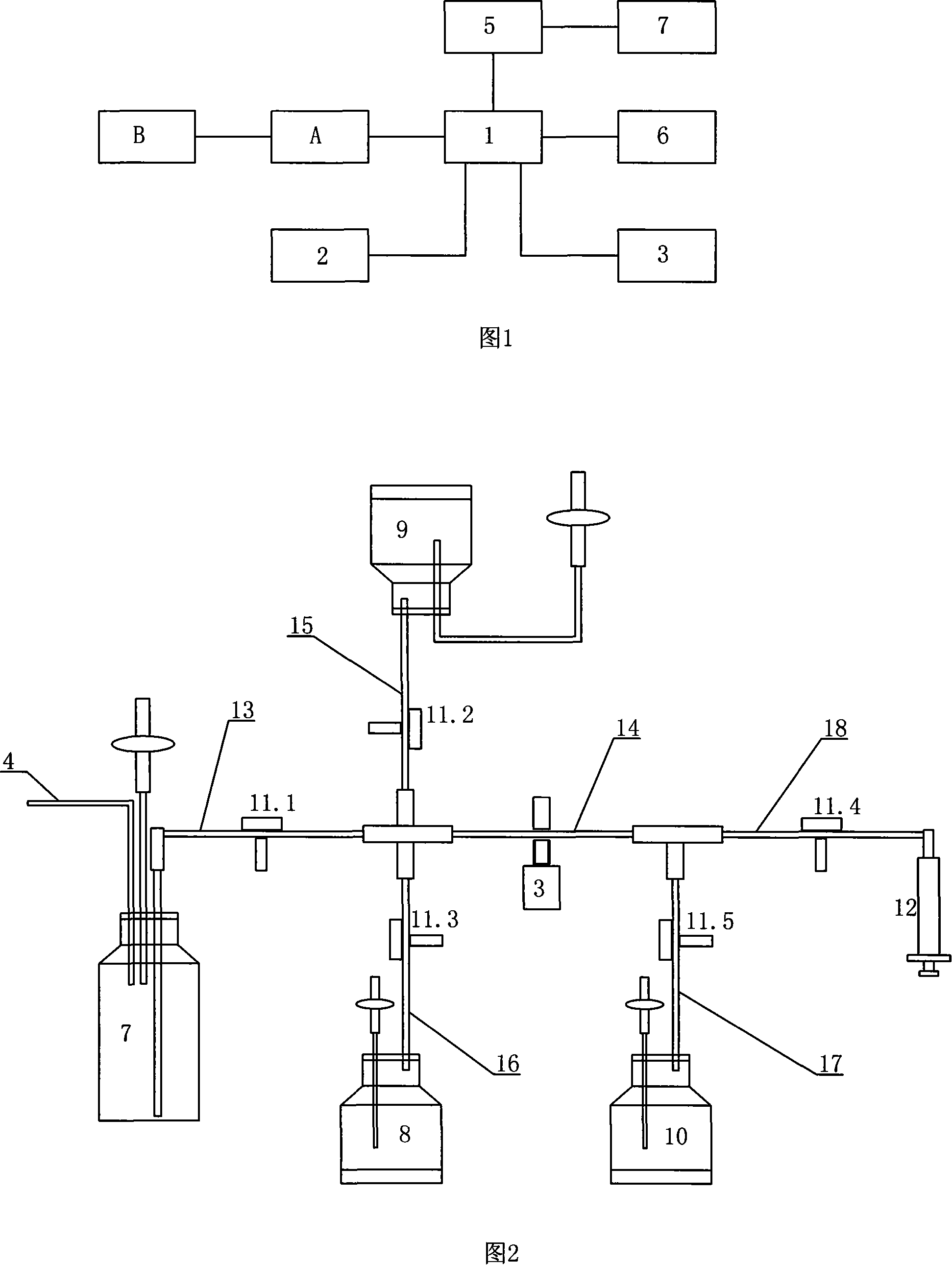
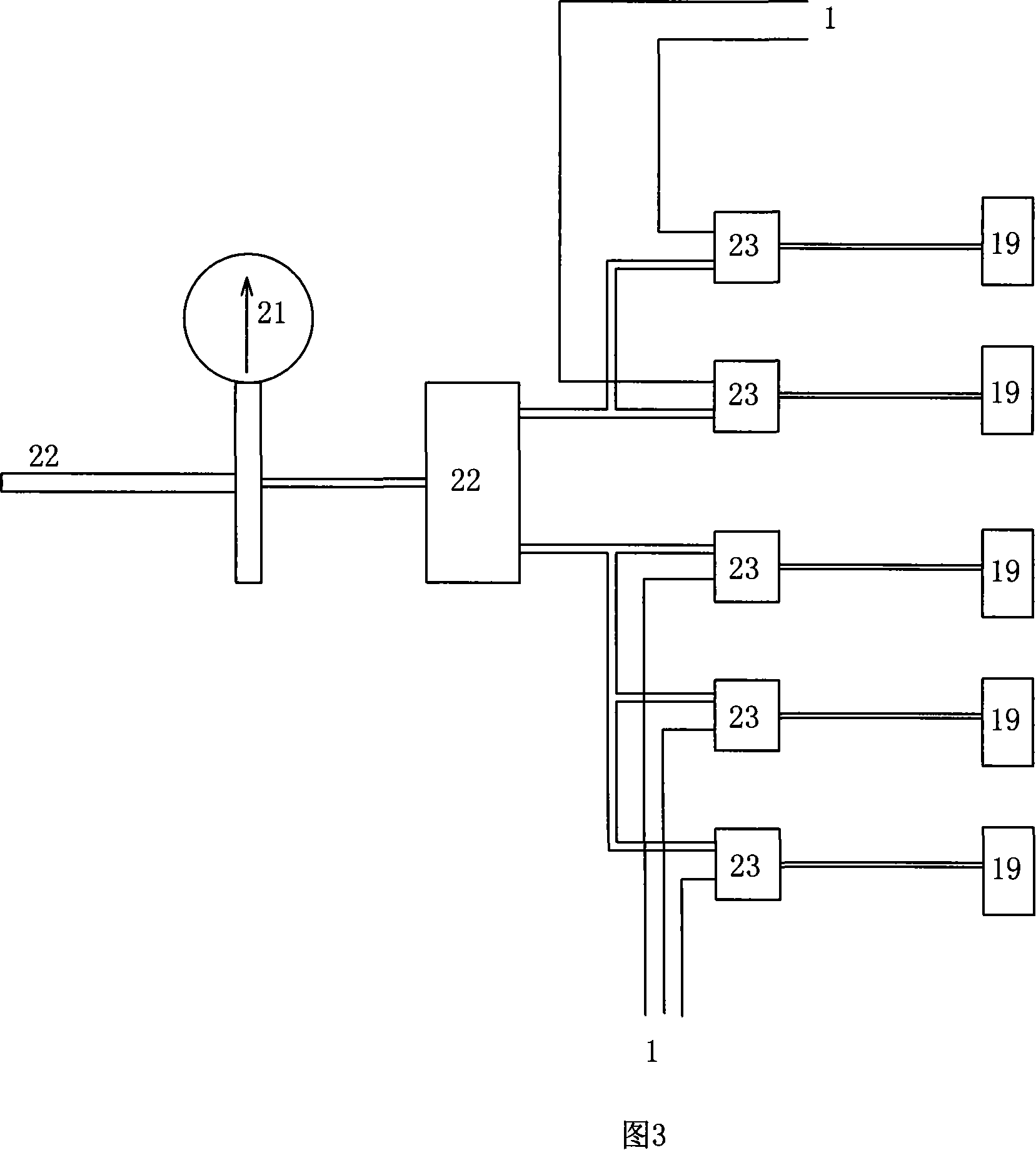
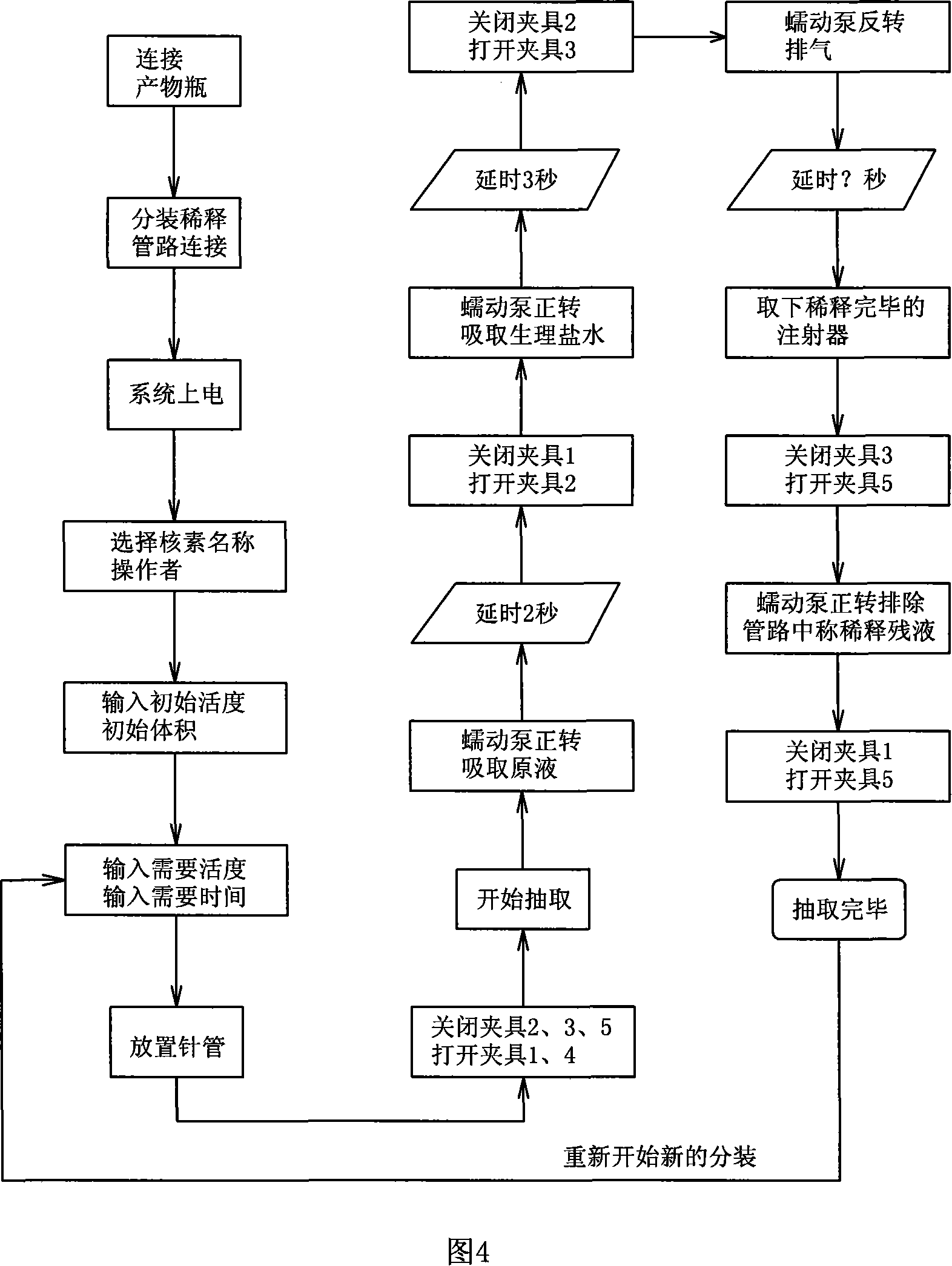
Automatic dilution split charging instrument of radionuclide liquid medicine and dilution split charging method thereof
The invention discloses radioactive nuclide liquid medicine automatic dilution diluting and dividing system and the diluting and dividing method, which belongs to the radiopharmaceutical drug application field. The structure comprises a PLC controller, a peristaltic pump, a pneumatic control device, a product bottle, an activity meter measurement barrel and diluting and dividing pipelines. The peristaltic pump, the pneumatic control device and the activity meter measurement barrel are separately connected with the PLC controller by electricity; the product bottle is arranged in the activity meter measurement barrel and the diluting and dividing pipelines are connected with the pneumatic control device. The diluting and dividing system works under the control of the procedures, which can not only ensure the correctness and the diluting and dividing accuracy but also have good protection. The invention is characterized by reasonable design, easy manufacture, flexible and convenient operation, good protective performance, high diluting and dividing accuracy and good repetitiveness.
Owner:SHANDONG PROVINCIAL HOSPITAL
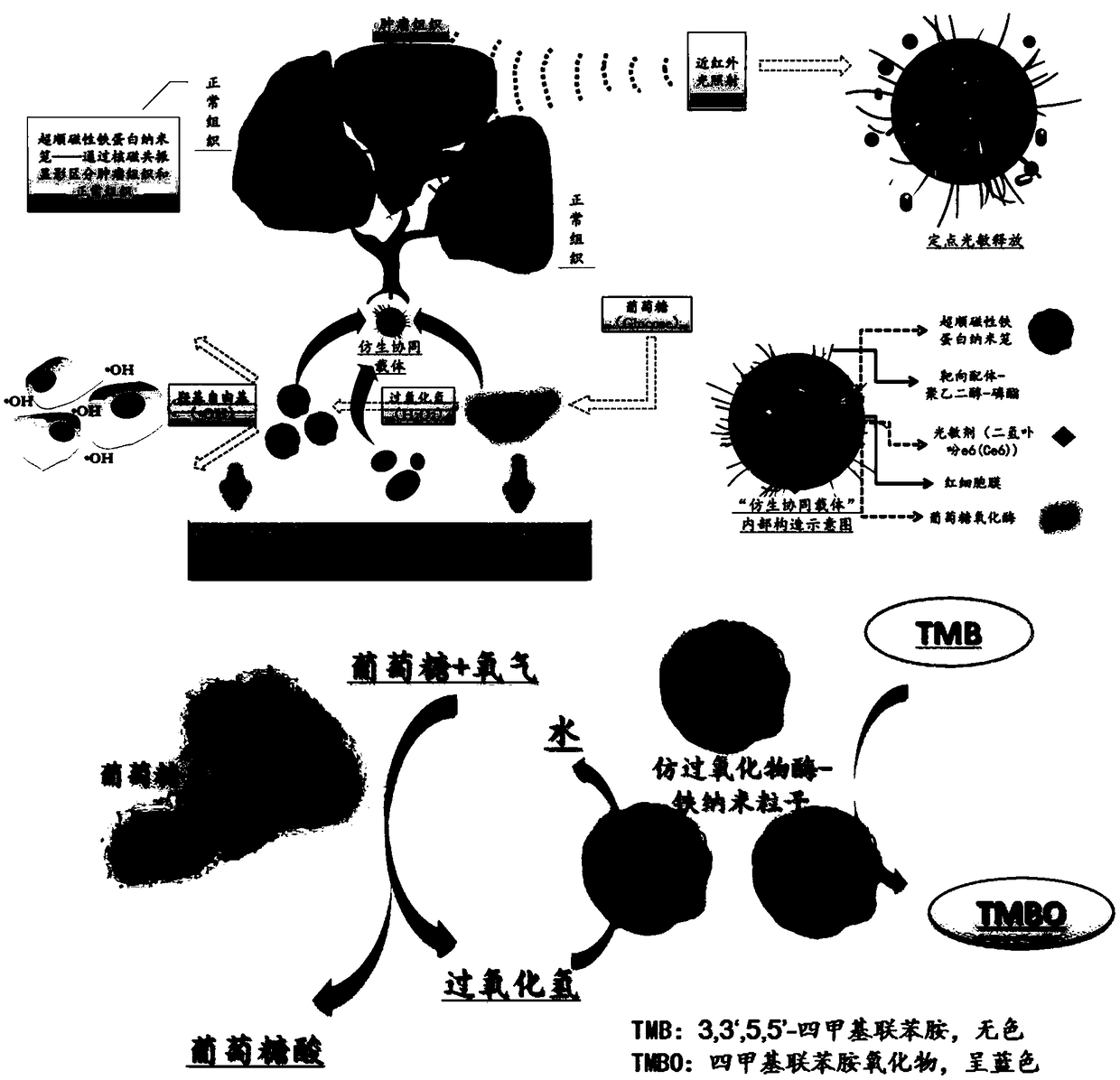
Bionic binary cooperative nano-carrier as well as preparation method and application thereof
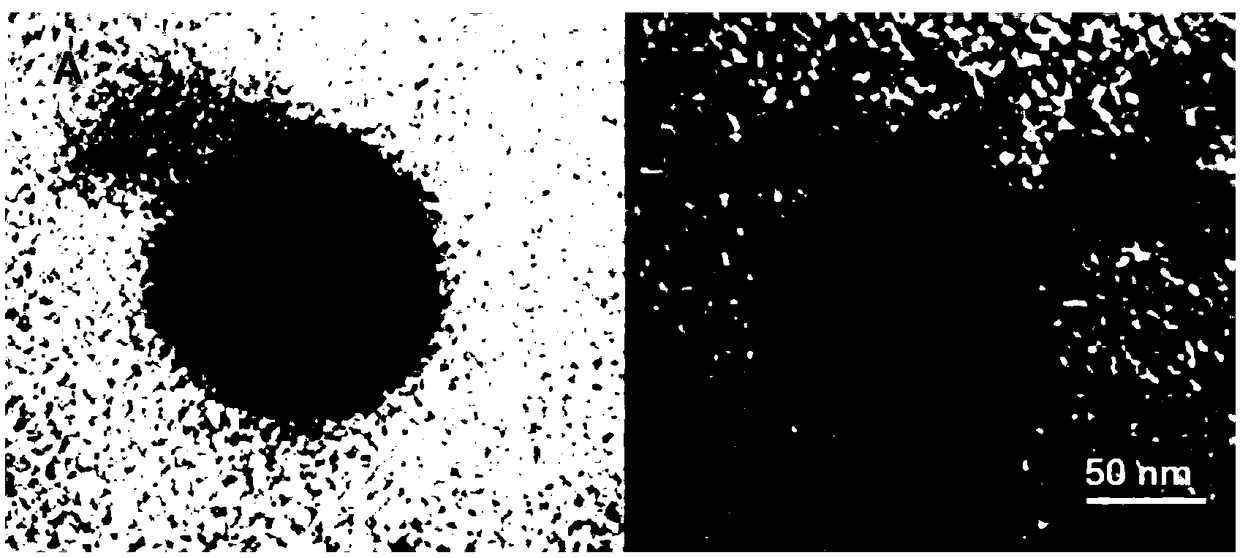
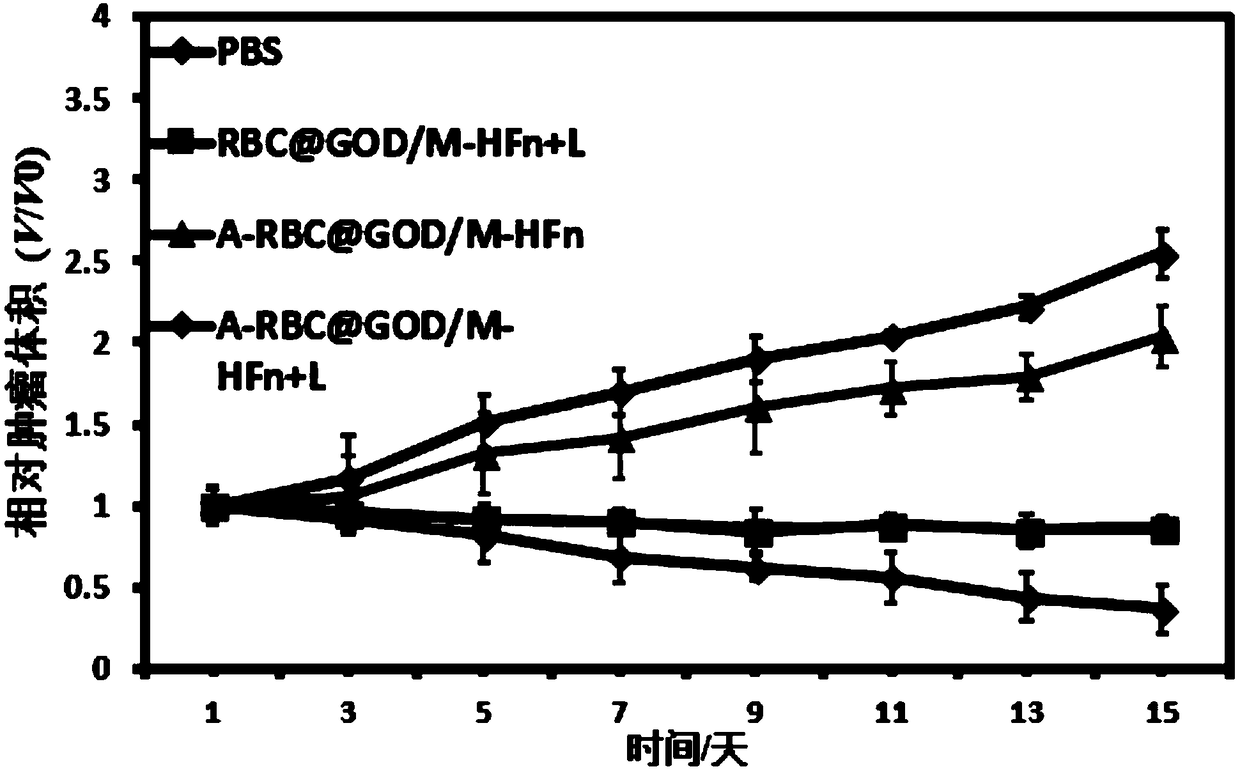
ActiveCN108815520AAccurate doseImprove bioavailabilityPeptide/protein ingredientsPhotodynamic therapyErythrocyte membraneTumor targeting
The invention provides a bionic binary cooperative nano-carrier as well as a preparation method and an application thereof. The bionic binary cooperative nano-carrier comprises an erythrocyte membrane, glucose oxidase, iron-supporting ferritin nano-particles and a photosensitizer, wherein the glucose oxidase and the iron-supporting ferritin nano-particles are coated with the erythrocyte membrane,and the photosensitizer is embedded into the surface of the erythrocyte membrane or entrapped by the erythrocyte membrane. Chain stimulative responsibility coordination of tumor hunger therapy and chemical kinetic therapy is realized, two enzymes are conveyed to a target site of an organism with the carrier on the basis of biocompatibility of the erythrocyte membrane and tumor targeting of targeting molecules, accurate administration is realized by membrane rupture based on 808 nm near-infrared light illumination in the tumors, the problem of drug resistance is solved effectively, furthermore,systemic toxicity caused by drug application is remarkably reduced, and damage to other normal tissue in an in-vivo circulation process is prevented effectively. The invention further provides the preparation method of the bionic binary cooperative nano-carrier. The bionic binary cooperative nano-carrier and the preparation method have good application prospect.
Owner:JINAN UNIVERSITY
Isaria fumosorosea oil suspension
InactiveCN104255809AImprove efficiencyReduce exposureBiocideAnimal repellantsVegetable oilIsaria fumosorosea
The invention discloses isaria fumosorosea oil suspension which mainly comprises 1%-10% of isaria fumosorosea conidial powder, 50%-80% of vegetable oil or inert mineral oil, 5%-30% of an emulsifying agent and 1%-10% of a stabilizer. The isaria fumosorosea oil suspension has the advantages as follows: the storage period of isaria fumosorosea is long, and the survival rate of the isaria fumosorosea is higher than 85% after two years of storage; after the oil suspension is diluted by water, the mixture is slightly stirred to form transparent or semitransparent emulsion, the emulsification is thorough, and the stability is good; the oil suspension can be sprayed after mixed with water, and the use is convenient in the farming area; after drug application, the vegetable oil can effectively absorb ultraviolet rays, damage of the ultraviolet rays to the isaria fumosorosea is reduced, and the protection effect of the isaria fumosorosea is improved; the processing technology is simple, and the preparation method is simple and convenient; and long-term infection biological study and indoor biological assay prove that the isaria fumosorosea oil suspension has higher infection and insecticidal efficacy on pests such as bemisia tabaci, aphids and the like.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Test equipment for research of fog drop drift, and processing method for test equipment
The invention discloses test equipment for the research of fog drop drift, and a processing method for the test equipment. The equipment comprises a dragging guide rail, a sliding block arranged on the dragging guide rail, a trailing vortex generation device hooked at the sliding block, a fog drop generation device hooked at the sliding block, a motor connected with the sliding block, and a PIV system. The sliding block slides along the dragging guide rails at a preset speed under the action of the pulling force generated by the motor, drives the trailing vortex generation device to generate trailing vortex comprising tracer particles and drives the fog drop generation device to generate fog drops. The equipment also comprises the PIV system which is used for capturing the motion images of the trailing vortex and fog drops in a fixed extending plane, carries out the processing of the motion images, and obtains the change laws of the fog drop drift and trailing vortex movement with time. The equipment can analyze and obtain the correlation of the fog drop drift and trailing vortex movement through simulating the trailing vortex movement and the fog drop drift under the real conditions, and provides theoretical guide for the research of the fog drop drift in a process of aviation drug application.
Owner:BEIJING RES CENT OF INTELLIGENT EQUIP FOR AGRI
Drug identification system based on deep learning and identification method thereof
InactiveCN107545150AAvoid instabilityCharacter and pattern recognitionSpecial data processing applicationsTest sampleDrug identification
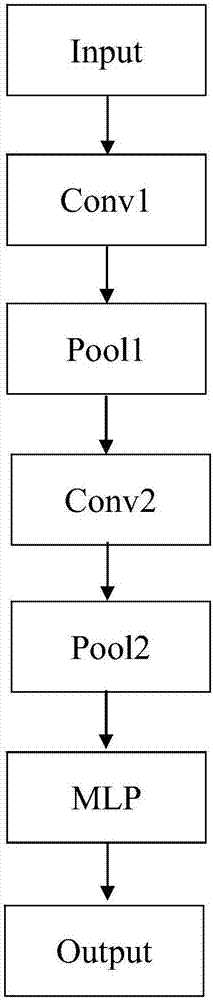
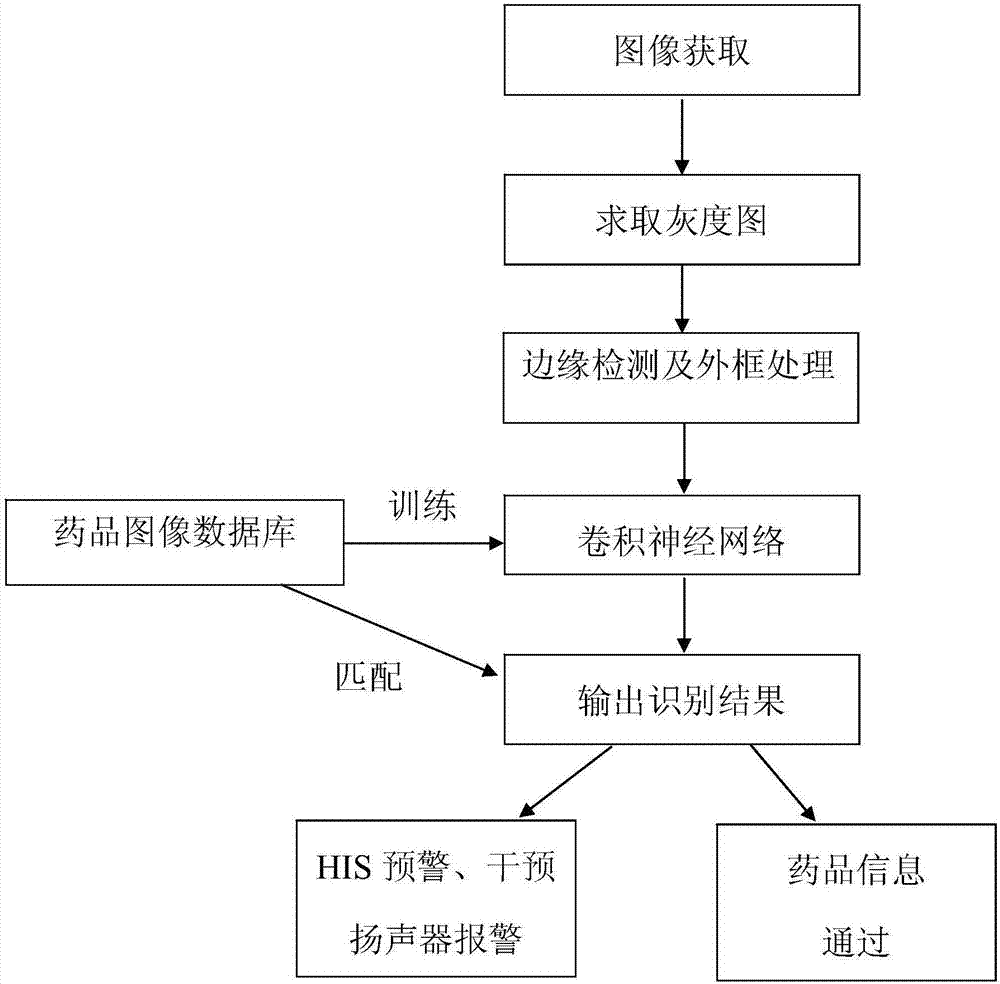
The present invention is a drug recognition system and its recognition method based on deep learning, including: 1. Preprocessing of drug image training samples, test samples, and target images. Including obtaining grayscale image, edge detection and frame processing. 2. Construct a model unit for establishing a drug recognition model based on deep learning. Including setting the initial parameters of each network layer and classifier of CNN, and inputting the above preprocessed image into CNN to obtain the features we need. 3. The identification unit is used to input the drug with identification, and then the above-mentioned established drug identification model based on deep learning obtains the convolution features of the image in the deep learning model, and cascades the obtained convolution features into the input The classifier finally outputs the recognition result. 4. Identify the hardware part of the system. It has a more accurate recognition rate and speed in various complex backgrounds and drug information, which can better reduce the error rate in drug application and the subsequent individual drug safety application platform.
Owner:张晨
Preparation method of Maxingshigan submicron powder
InactiveCN101810699AImprove broken rateImprove bioavailabilityPowder deliveryAntiviralsLong segmentGLYCYRRHIZA EXTRACT
The invention discloses a normal-temperature preparation method of veterinary drug Maxingshigan submicron powder, comprising the following steps: drug selection: selecting the following components in parts by weight: 10 parts of ephedra, 10 parts of semen armeniacae amarae, 50 parts of gypsum and 10 parts of liquorice, cleaning, drying and preparing into the long segment with the length of 3-5 cm; cribble: respectively smashing traditional Chinese medicine pieces which are cut into segments, and sieving by the sieve of 65 meshes to obtain the powder lot with grain diameter of 180-250 mu m; mixing: evenly mixing the obtained powder lot; regrinding powder: smashing the mixed powder lot into the finest powder, sieving by the sieve of 100 meshes to obtain the powder lot with grain diameter of 100-150 mu m; obtaining submicron powder: smashing the finest submicron powder into submicron powder of which the powdery lot grain diameter is 20-40 mu m, and then obtaining the finished product. The preparation method of the invention is carried out at normal temperature without low temperature or special conditions. After bulk pharmaceutical chemicals are smashed, cell wall-broken rate and bioavailability can be improved, and pharmacological action can be enhanced; drug application amount is reduced so as to save medicinal materials; the veterinary drug Maxingshigan submicron powder can be directly drunk as water, thus improving the phenomenon that traditional Chinese medicine powder only can be stirred but can not be drunk for taking.
Owner:HENAN KANGXING PHARMA
Application of NADH and NMN in preparation of drug or health caring product for Parkinson's disease
InactiveCN104758307AImprove securityObvious effect and easy to stabilizeOrganic active ingredientsNervous disorderDiseaseActive component
The invention discloses an application of NADH and NMN in preparation of a drug or a health caring product for Parkinson's disease, namely, [beta]-NADH and [beta]-NMN are applied for preparing the drug or the health caring product for the Parkinson's disease, wherein the [beta]-NADH and [beta]-NMN are prepared through enzyme catalysis. The invention provides the new drug or health caring product, which is prepared from the [beta]-NADH and [beta]-NMN which is prepared in an in-vitro enzyme catalysis manner, has cost advantage and is high in purity. The drug or health caring product is used for treating the Parkinson's disease. The [beta]-NADH and the [beta]-NMN, as active components, are used for preparing the drug or the health caring product for the Parkinson's disease, wherein the particular dosage can be decided according to the severity degree of the diseases, drug application approach and relative factors. The [beta]-NADH and the [beta]-NMN themselves exist in body cells, so that the [beta]-NADH and the [beta]-NMN are high in safety as the drug or the health caring product. In addition, the NMN is a monomer molecule so that the drug or the health is significant in function and is easy to stabilize.
Owner:HOBOOMLIFE BIO TECH SHENZHEN CO LTD
Pharmaceutical uses of black-seed grass-seed oil of tuberculate fruit
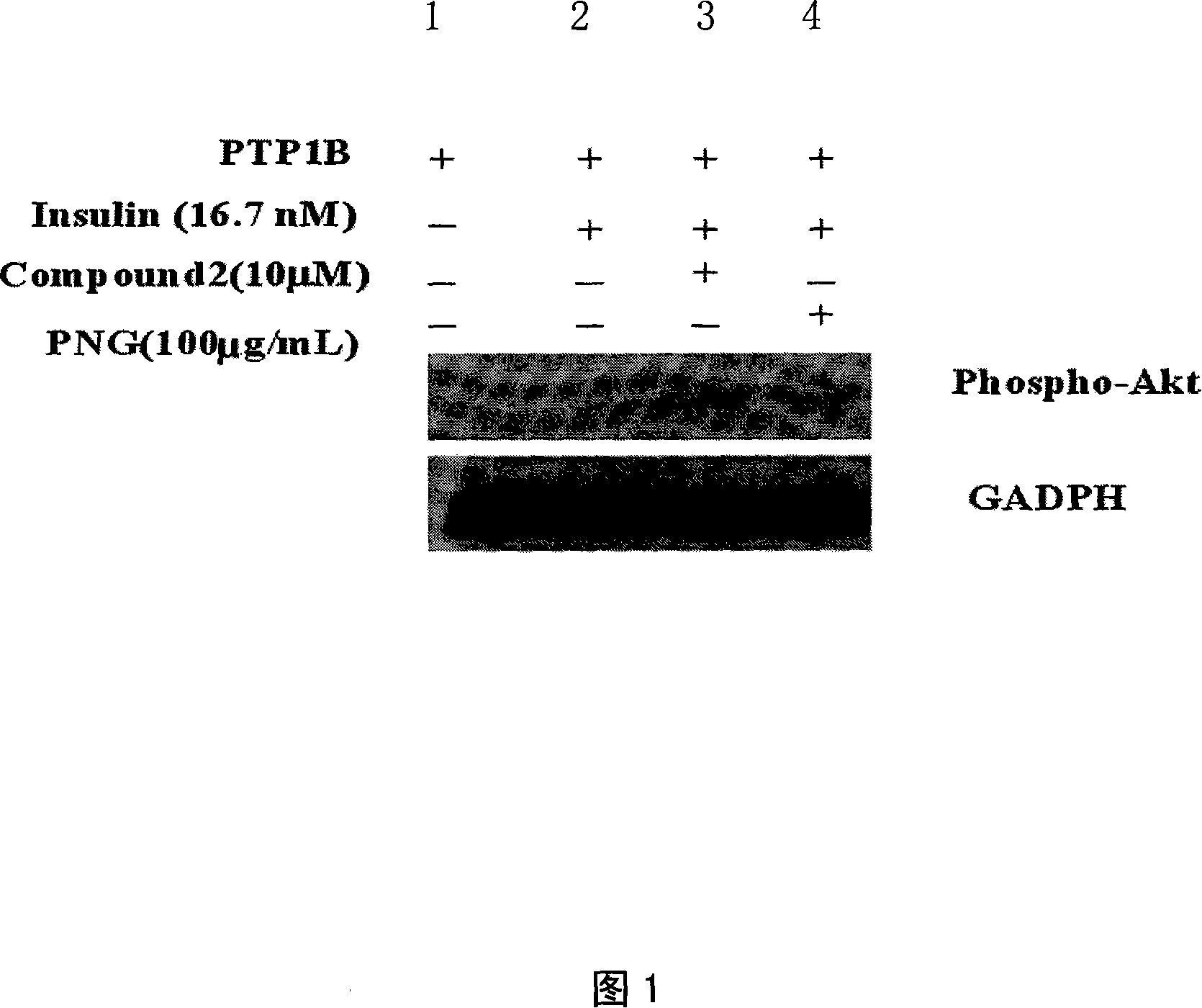
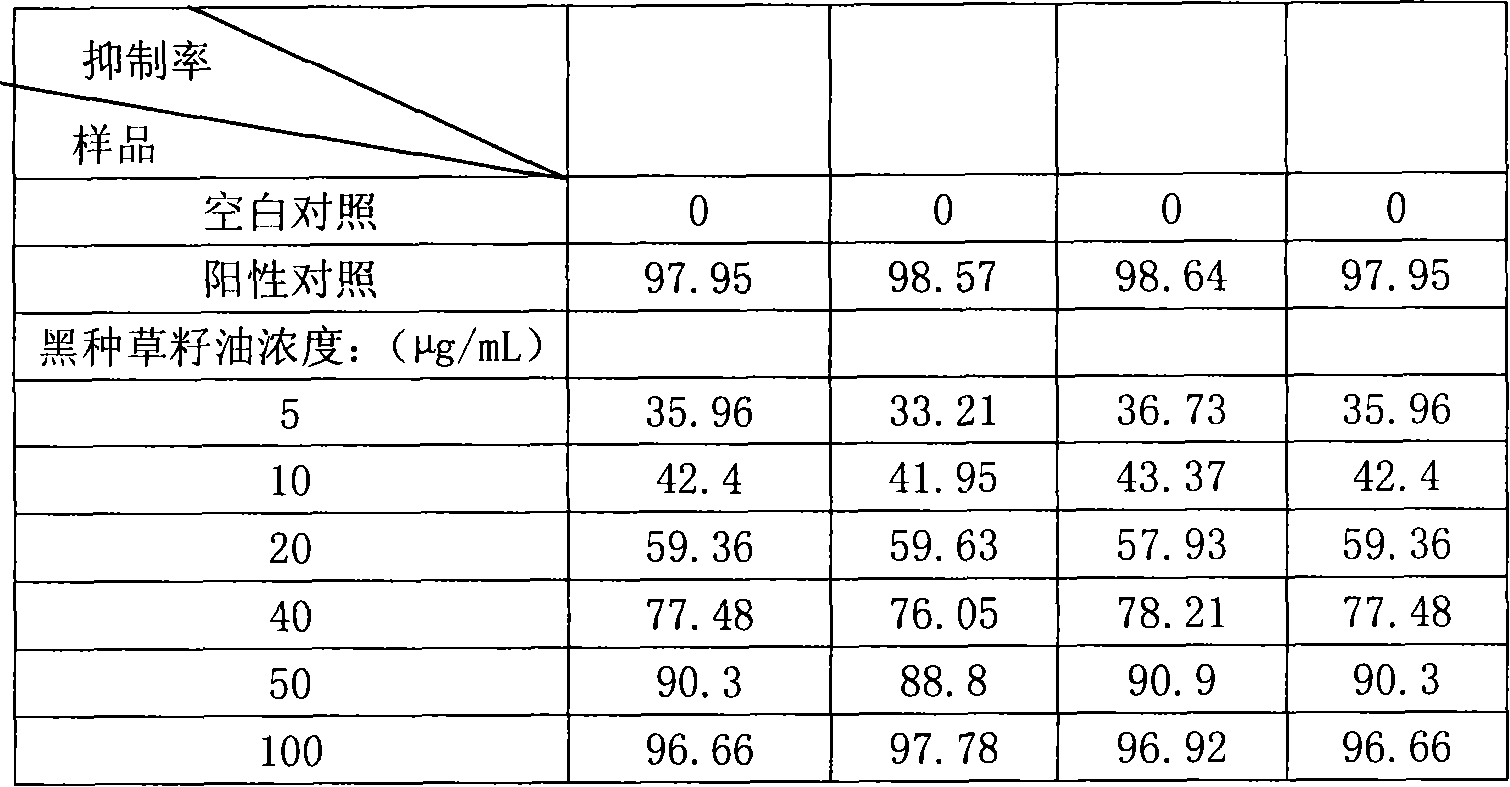
The invention discloses a drug application of tumour-fruit black-seed grass-seed oil, which is characterized by the following: squeezing from tumour-fruit black-seed through normal method; extracting in the solvent; decompressing to remove solvent; testing the effect of protein tyrosine phosphatase 1B (PTP1B) inhibitor to resist lipid peroxidization and remove free radical of oxygen. The invention also provides an application in the antidiabetic drug and anti-oxidizing drug and health products.
Owner:新疆宝康药业有限公司
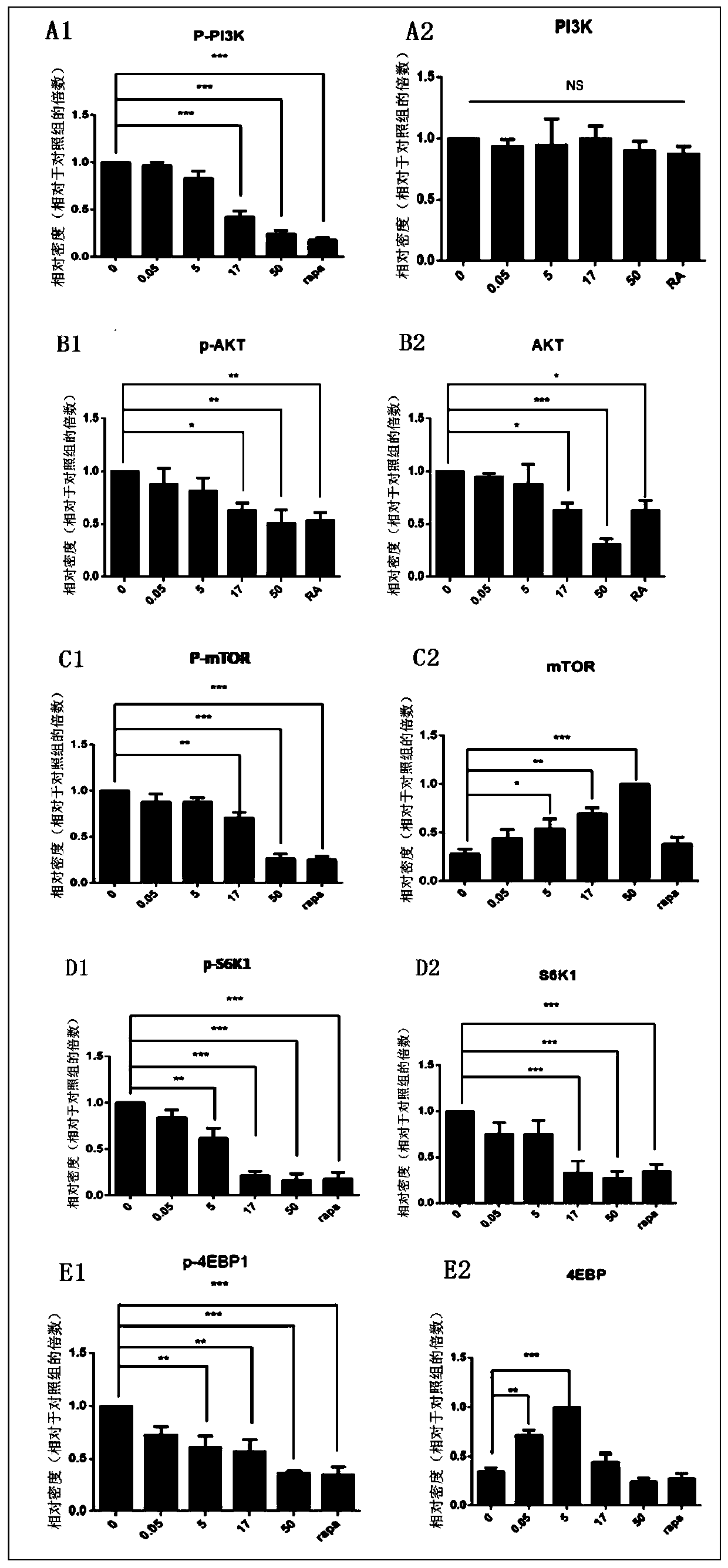
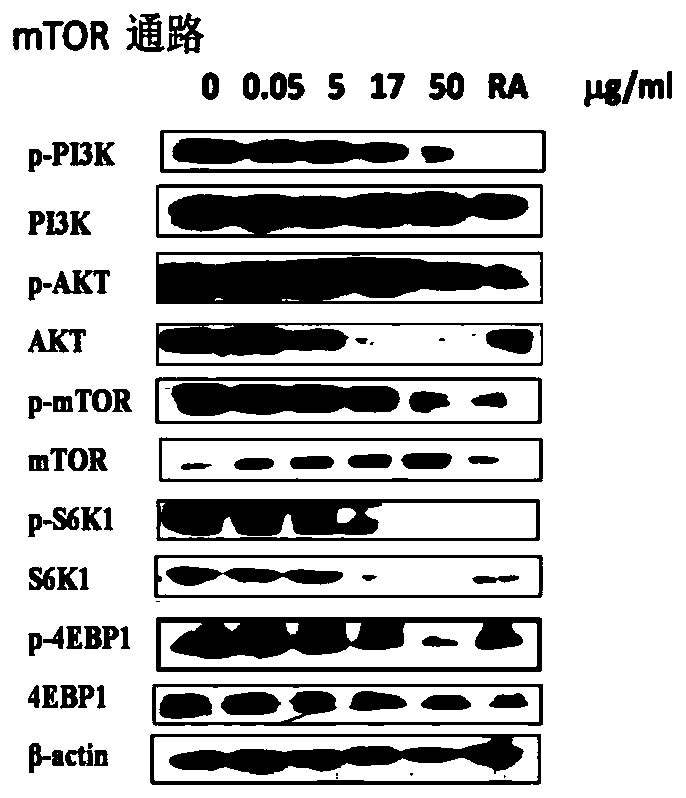
MTOR inhibitor and pharmaceutical composition, and application thereof
InactiveCN110051845APlay an inhibitory roleEnhanced inhibitory effectOrganic active ingredientsSenses disorderSocial benefitsSpiramycin II
The invention belongs to the field of drug application. Specifically, the invention discloses an mTOR inhibitor and a pharmaceutical composition, and an application thereof. The mTOR inhibitor includes one of carrimycin, isovaleryl spiramycin I, isovaleryl spiramycin II and isovaleryl spiramycin III, or a combination of two or three of isovaleryl spiramycin I, isovaleryl spiramycin II and isovaleryl spiramycin III. The mTOR inhibitor has obvious inhibitory effect on cells of mTOR pathway-related diseases, a theoretical basis is provided for application and clinical popularization of the mTOR inhibitor in preparation of drugs for treating and / or preventing mTOR pathway-related diseases, and the mTOR inhibitor has important economic and social benefits.
Owner:SHENYANG FUYANG PHARM TECH CO LTD
Powered stimulation device
ActiveUS7634314B2Reduce conductivityImproving impedanceElectrotherapyChiropractic devicesAdditive ingredientSkin surface
A device and related method of use are disclosed which employ electrically-conductive wheels to massage and contact the skin to deliver electrical current. The present invention has both cosmetic and drug applications. In a cosmetic application, the rolling action of the wheels provides a light pressure skin massage that is important to reduce the appearance of skin surface irregularities. In a drug application, the device may be used to deliver ingredients of a conductive gel to or into the skin.
Owner:AK BEAUTY ENTERPRISES LLC
Treatment apparatus with massage and drug application functions for patients with mammary gland galactostasis with inflammation
The invention discloses a treatment apparatus with massage and drug application functions for patients with mammary gland galactostasis with inflammation. The treatment apparatus comprises a fixed cover, a cavity, a sliding sleeve, an air cushion, a drug discharge pipe, a connecting cover, a drug discharge hole, a first connecting pipe, a suction nozzle, a connecting sleeve, a vacuum pump, a second connecting pipe, a multiple pipeline joint, a third connecting pipe, a getter pump, a storage box, an air pressure sensor, a first electromagnet, a track, a pressure spring, a second electromagnet,an ejecting pin, a timer, an exhaust vent, a limiting plate, an accommodating box and an air vent. The treatment apparatus with massage and drug application functions for patients with mammary gland galactostasis with inflammation is ingenious in structure, powerful in functions and simple in operation. By adoption of the apparatus, the mammary gland of the patient can be pressed and dredged without hand-operated operation of medical workers, ointment can also be uniformly applied to the surface of the mammary gland of the patient without directly contacting the ointment by hands of the medical workers, working strength of the medical workers can be reduced, and the treatment progress of the patient can also be effectively improved.
Owner:王虎霞
Medical tool capsule
The invention discloses a medical tool capsule, which comprises a tool device and an in vitro wireless remote controller. The tool device comprises a tool power source, a tool switch circuit, a tool power, a tool, a signal receiver and a microprocessor, wherein, the tool switch circuit is connected with the microprocessor, the tool switch circuit is connected between the tool power and the tool power in series, the tool power drives the tool to carry out the drug application and the acquisition operations; the in vitro remote controller sends command to the microprocessor through a receiver-transmitter, to control the tool device to carry out drug application, collection, staining, and labeling. According to the in vitro remote operation, the medical tool capsule can carry out the collection of the liquid specimen, the staining for the dubious region, the labeling of the focus and the direct drug application and treatment to the focus, thereby the diagnostic accuracy rate of the disease and the therapeutic effective rate can be greatly enhanced, the treatment cycle is shortened, the structure is simple, and the manufacture cost is low.
Owner:重庆灵方三帆生物制药有限公司
Needling device and drug applicator
ActiveUS20180280675A1Increase speedReduce in quantityPhysical therapyDiagnosticsTattoo removalPharmaceutical drug
A needling device may be used for needling of a subject's skin, and a drug applicator device may be used for applying a drug to a subject's skin. For example, a needling device may be applied to a subject's skin for hair growth applications, or may be used for wrinkle, scar, or tattoo removal. A drug applicator device may be used for multiple drug application purposes such as applying a hair growth compound to the skin of a subject.
Owner:FOLLICA
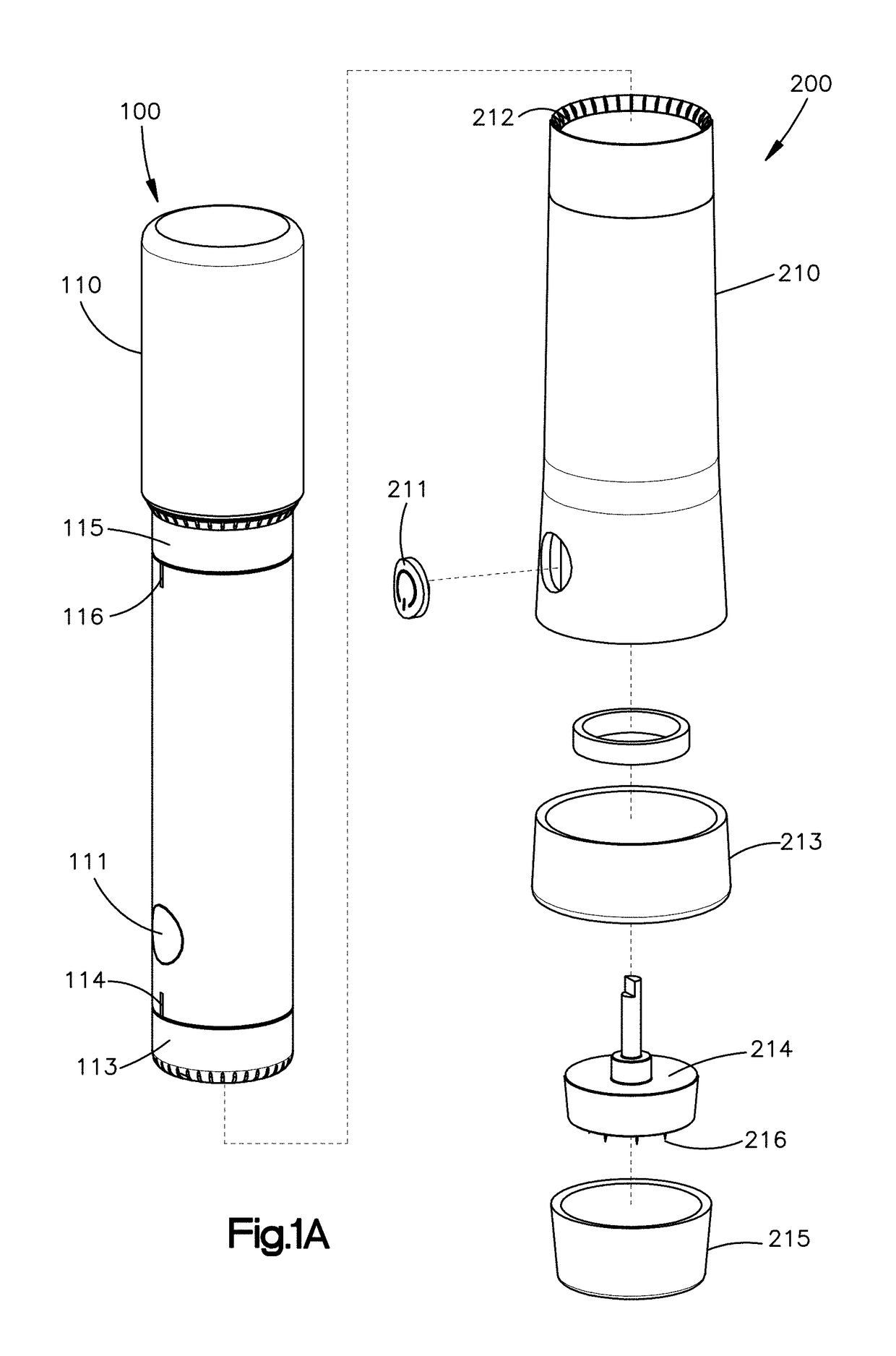
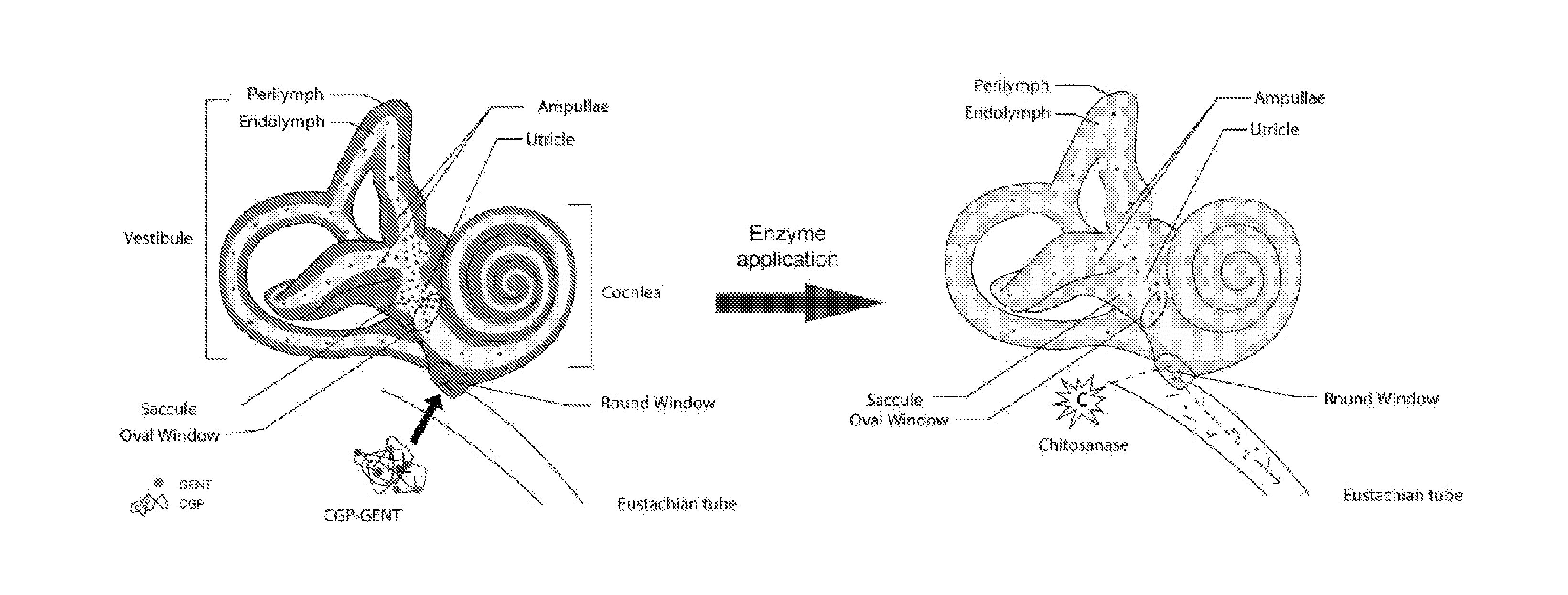
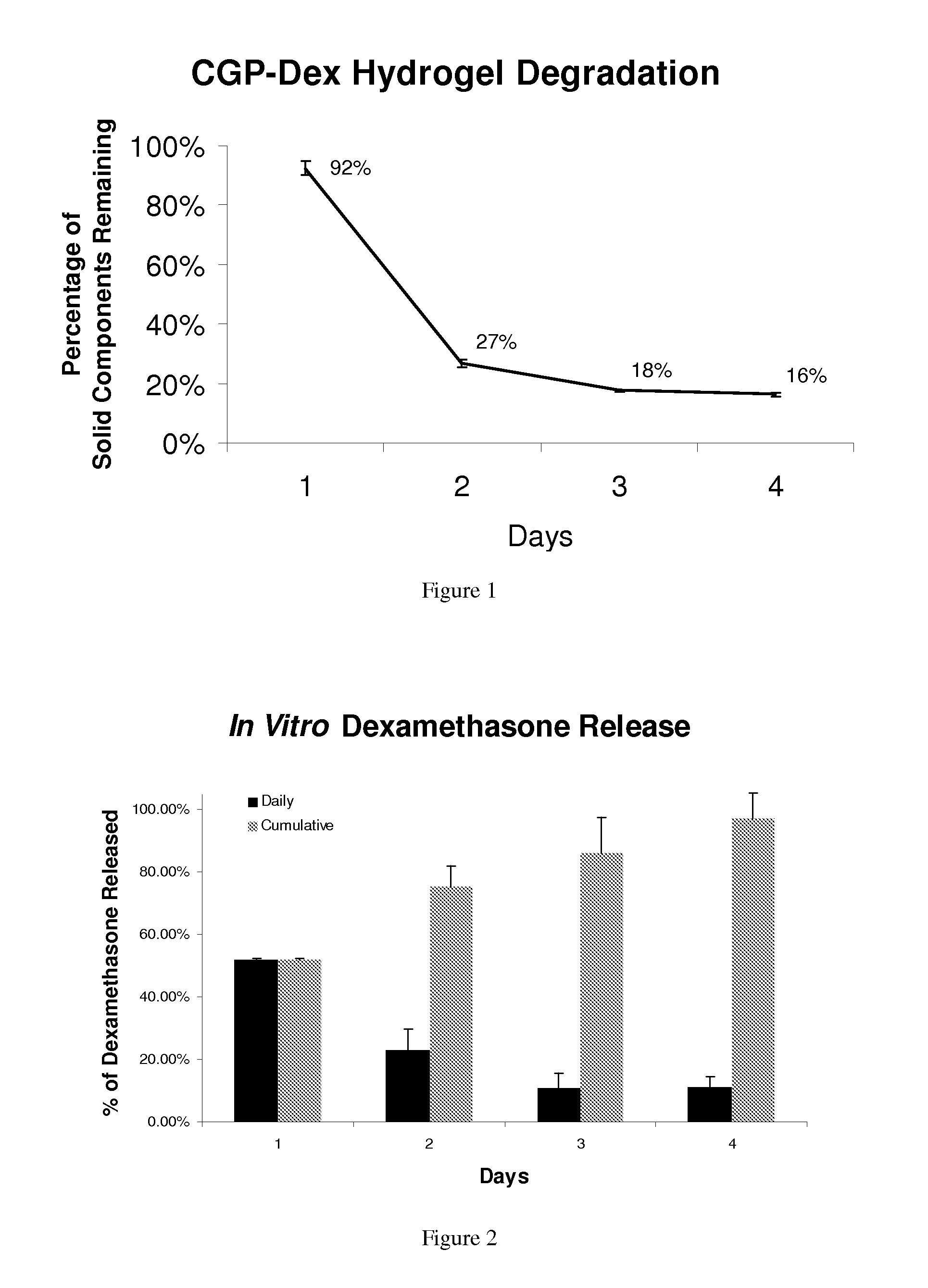
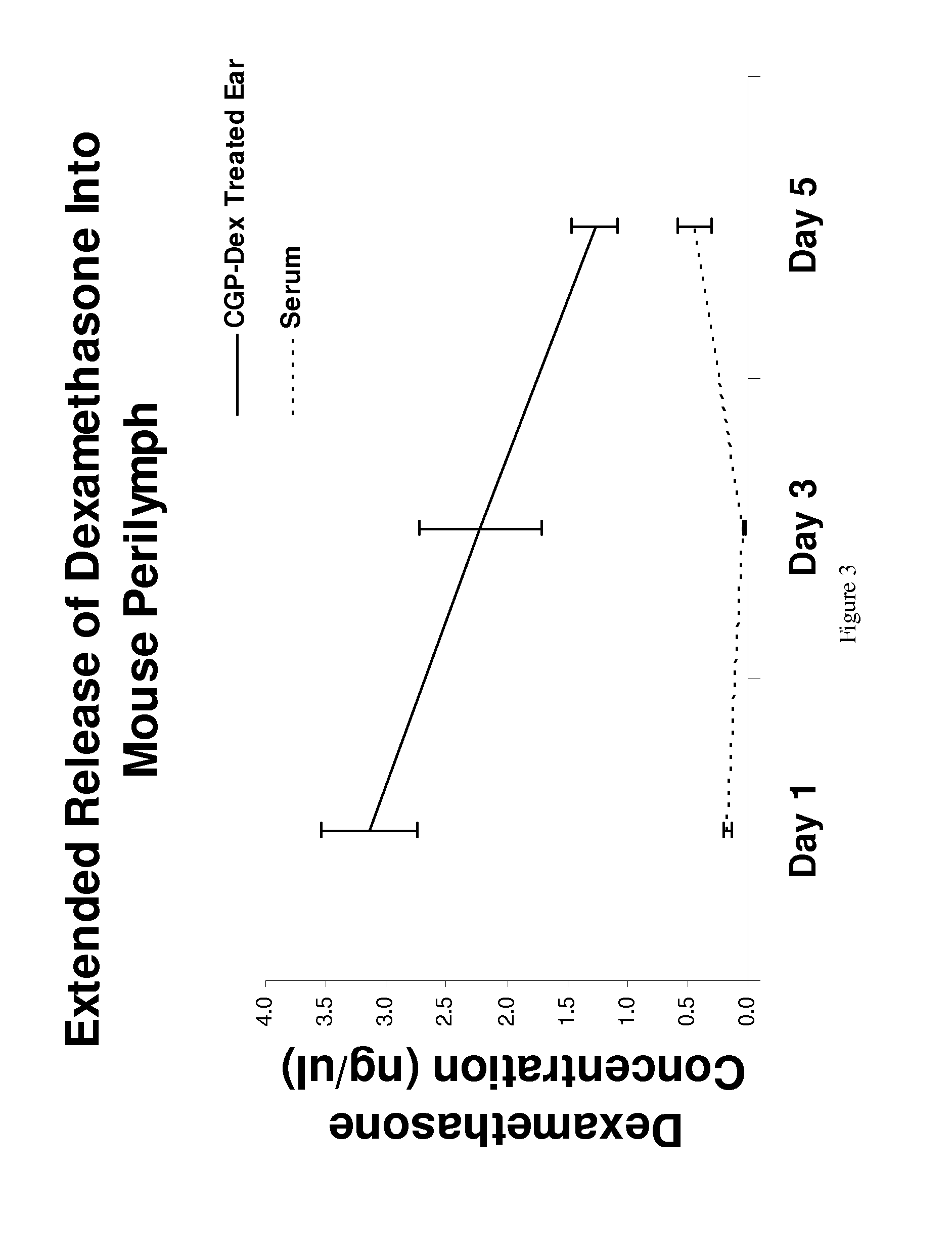
Regulated delivery systems for inner ear drug application and uses thereof
The invention relates to a controlled release delivery compositions and methods of using them for pathologies associated with Otorhinolaryngology and Head and Neck. Specifically, the invention relates to regulating drug delivery by the use of chitosan based matrices together with chitosanases.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
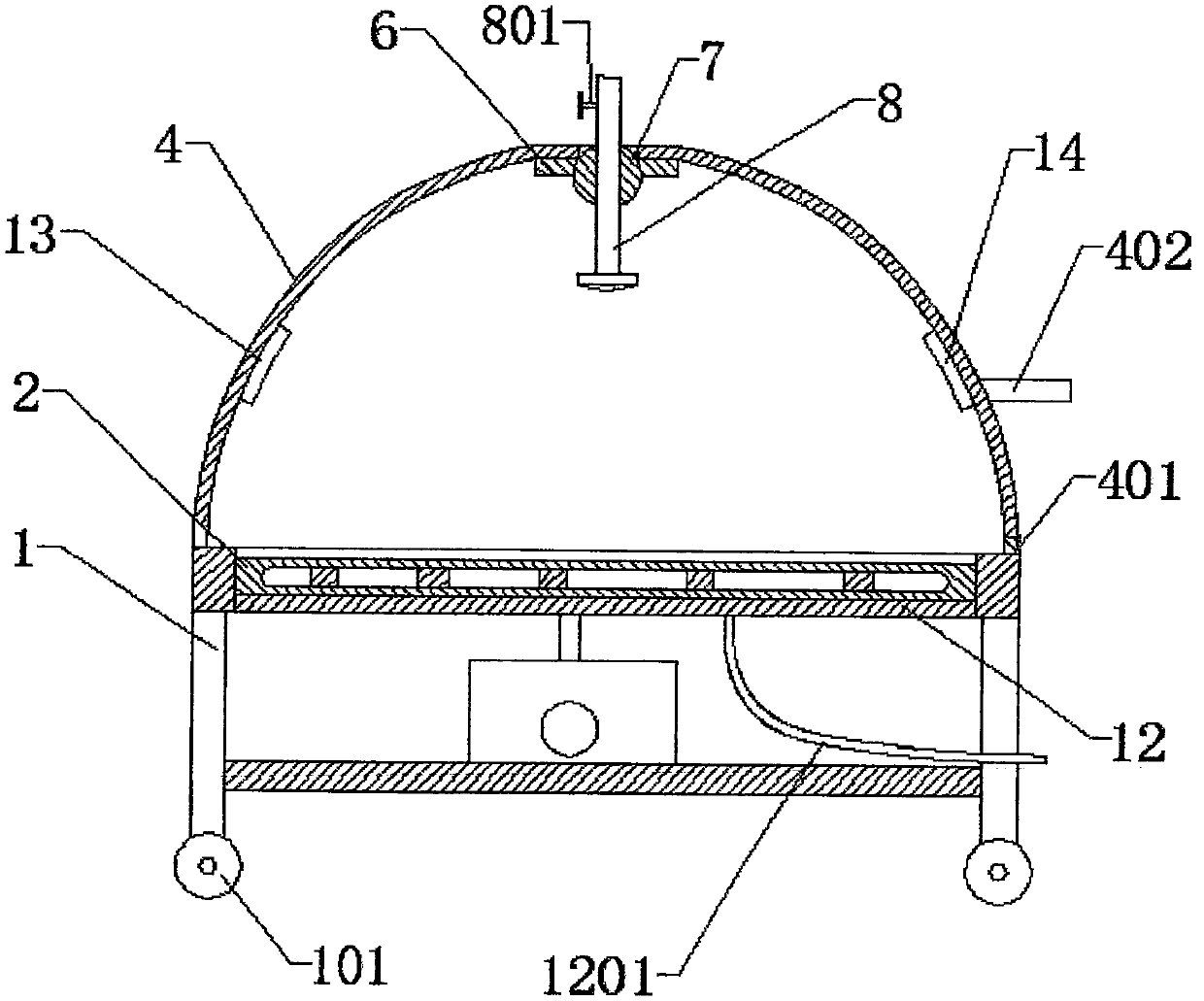
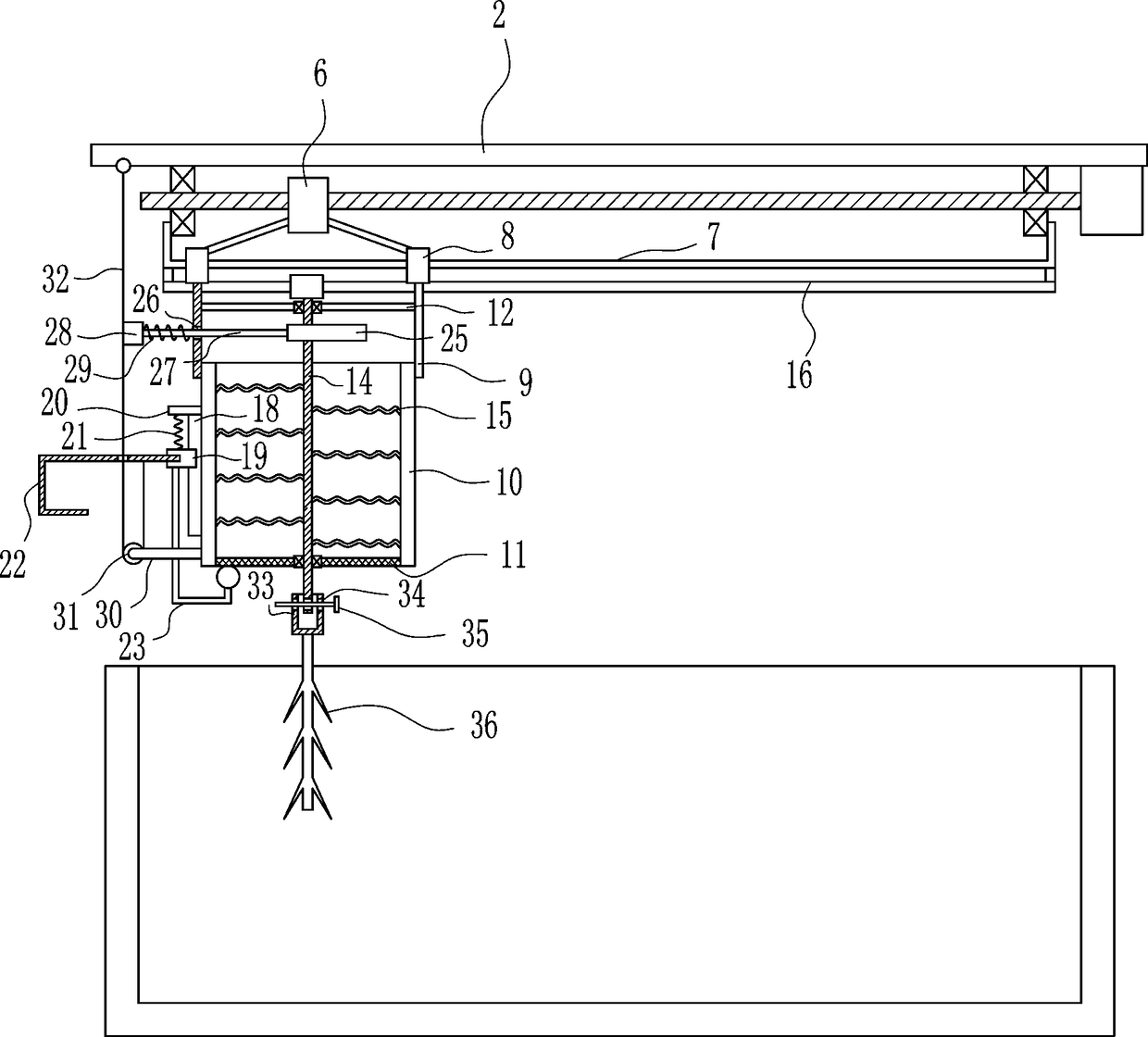
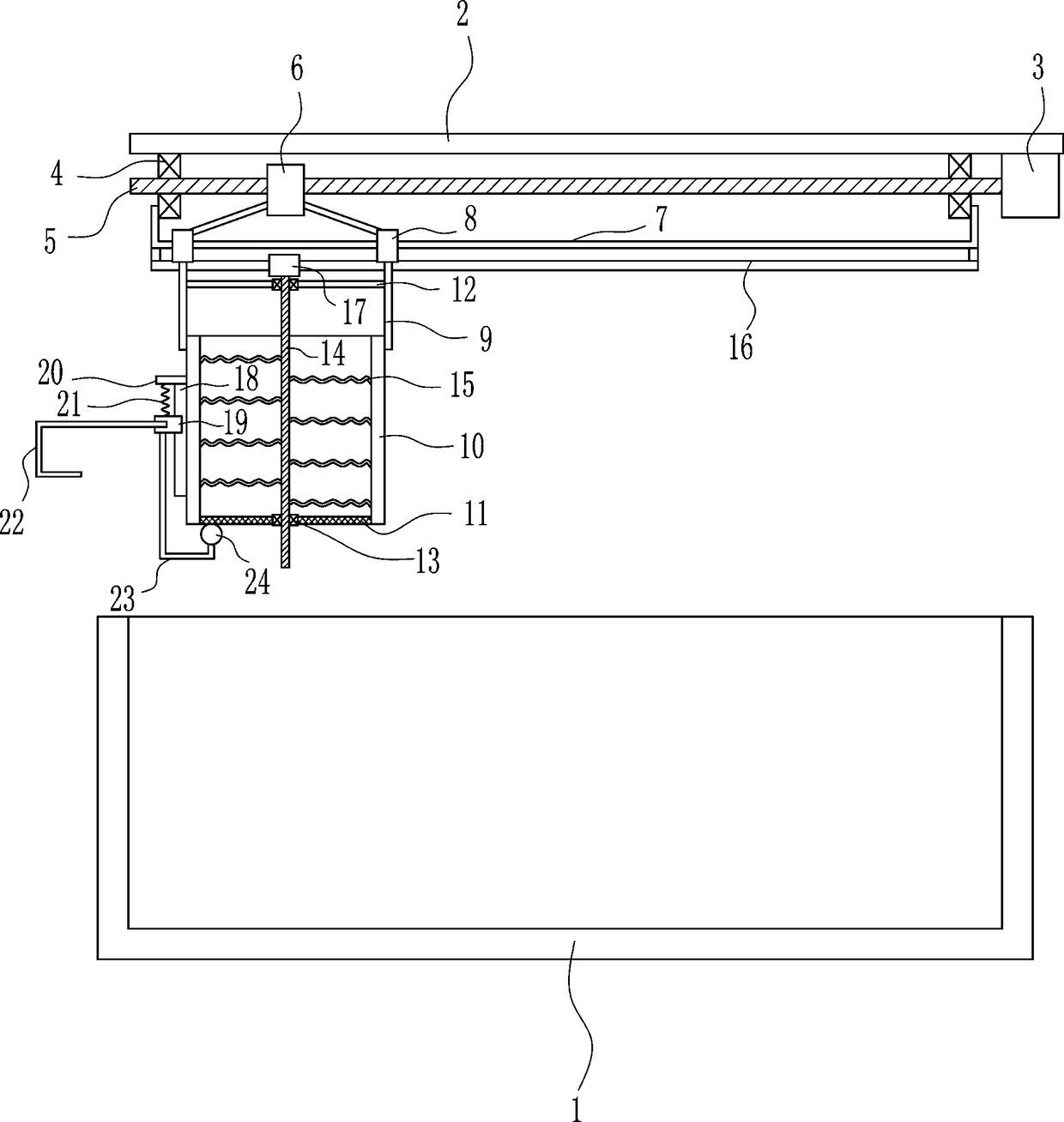
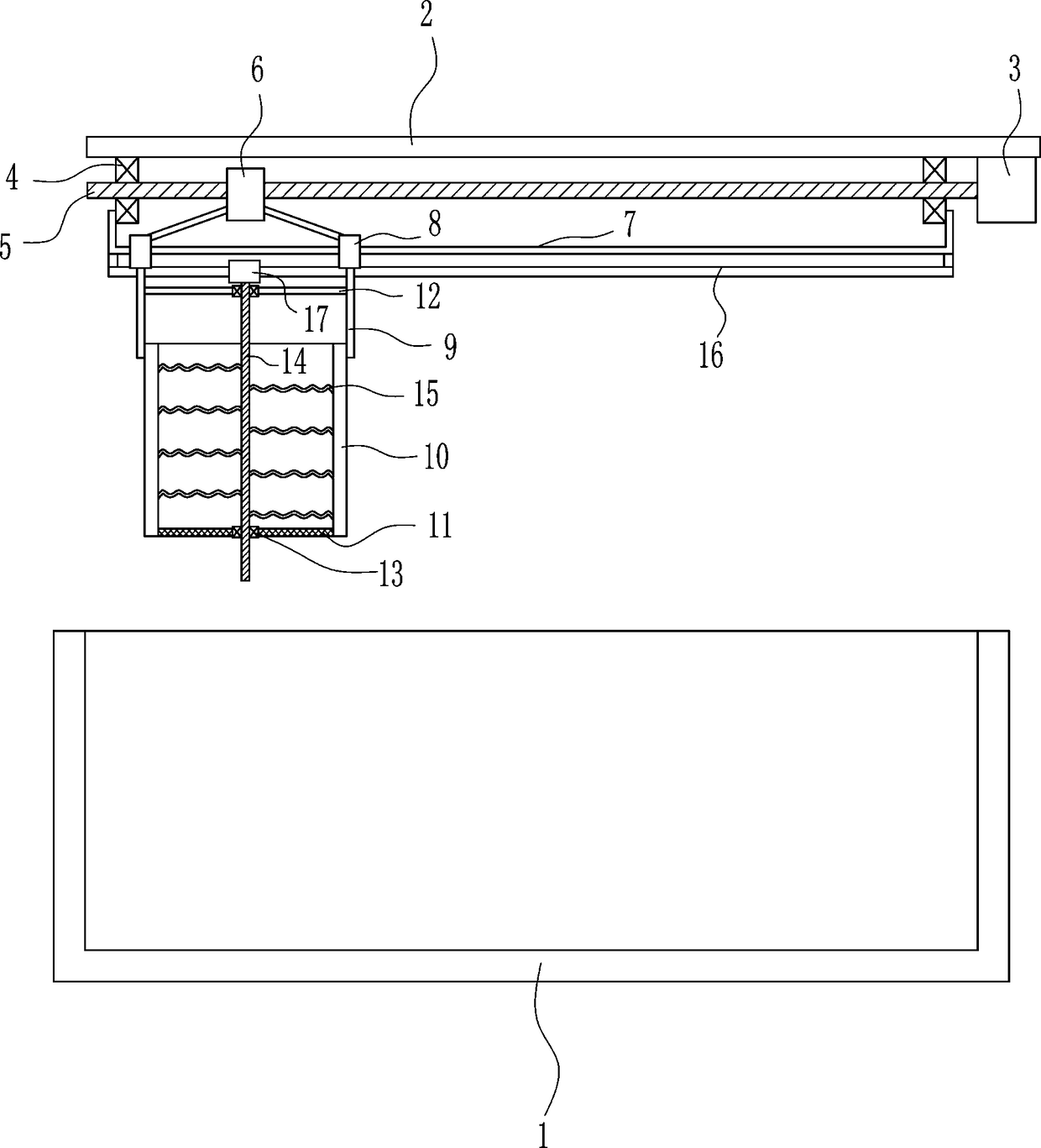
Sedimentation tank mobile drug application equipment for domestic sewage treatment
InactiveCN108609707AEasy to useDissolve fastSettling tanks feed/dischargeTreatment involving filtrationEngineeringSewage
The invention relates to sedimentation tank drug application equipment for domestic sewage treatment, in particular to sedimentation tank mobile drug application equipment for domestic sewage treatment. Technically, the invention aims to provide sedimentation tank mobile drug application equipment for domestic sewage treatment that can add drug powder movably and enable uniform mixing of drug powder with domestic sewage. According to a technical scheme, the sedimentation tank mobile drug application equipment for domestic sewage treatment comprises: a sedimentation tank, a top plate, a servo motor, a first bearing seat, a screw rod, a nut, a fixed rod, sliding sleeves, mounting plates, a drug application box, a sieving net, a connecting plate and the like. The top plate is disposed above the sedimentation tank, and the right side of the top plate bottom is connected to the servo motor. The equipment provided by the invention achieves the effects of mobile adding of drug powder and uniform mixing of drug powder with domestic sewage, the left and right movement of the drug application box enables even adding of drug powder into the sedimentation tank on the left and right, and firststirring rods can rotate continuously in the moving process to stir drug powder.
Owner:赣州汇桔科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com