Preparation method of simvastatin tablet
A technology of simvastatin and simvastatin tablets, which is applied in the field of preparation of simvastatin tablets, can solve the problems of dissolution rate and content uniformity, less bone tissue absorption, and poor oral absorption, etc., so as to improve bioavailability , The effect of increasing the relative absorption capacity and increasing the specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
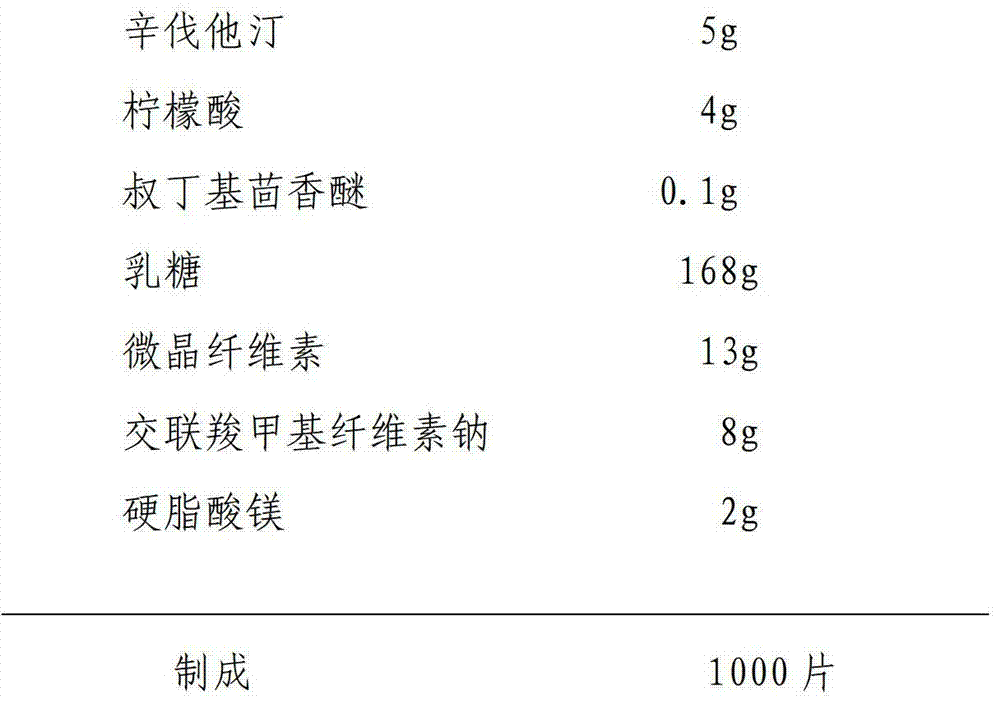
[0020] Raw material ratio:
[0021]
[0022] Preparation:
[0023] 1. The simvastatin raw material and lactose are ultrafinely pulverized at low temperature at a weight ratio of 1:5 into micropowders with a diameter less than 10 μm, and set aside;
[0024] 2. Combine the above-mentioned micronized mixed material with acid protective agent (citric acid), antioxidant (tert-butylanisole), lactose, microcrystalline cellulose, and croscarmellose sodium remaining in the prescription amount, Mix evenly, put it in a wet mixing granulator, add appropriate amount of water, wet mix and granulate, boil and dry below 60°C, and control the water content of the granules to 1-5%;
[0025] 3. Whole the simvastatin granules, add the prescribed amount of magnesium stearate, mix well, and press into 1000 tablets.
Embodiment approach 2
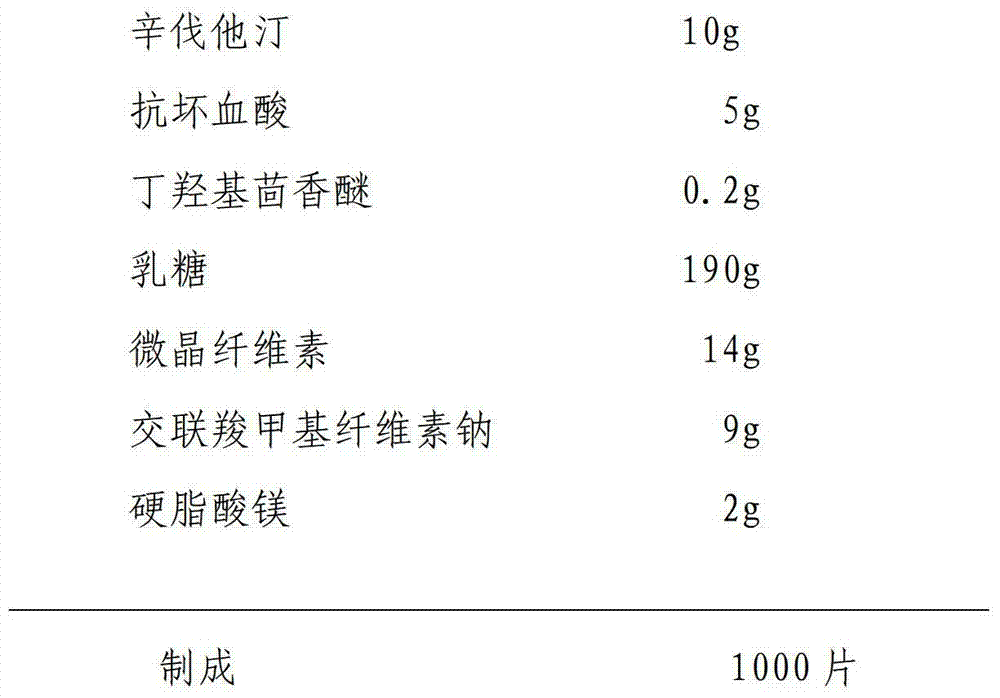
[0027] Raw material ratio:
[0028]
[0029] The preparation method is the same as the specific embodiment one.
Embodiment approach 3
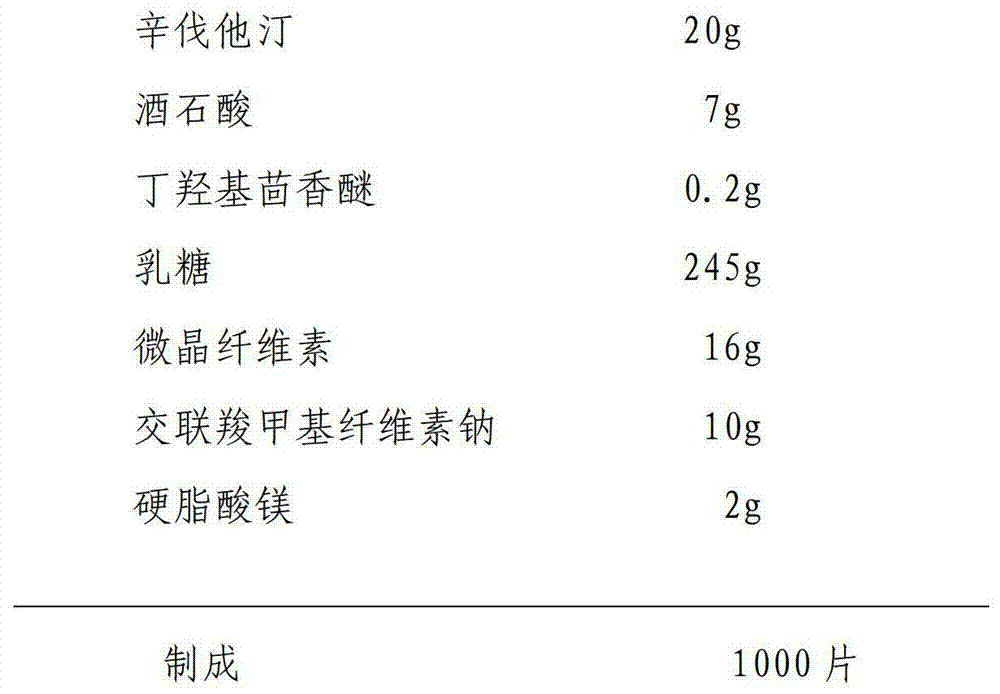
[0031] Raw material ratio:
[0032]
[0033] The preparation method is the same as the specific embodiment one.
[0034] The simvastatin sheet prepared by three embodiments and the commercially available simvastatin sheet, in vitro test characteristics are:
[0035]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com