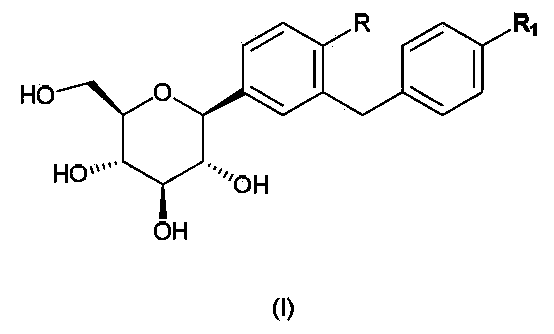
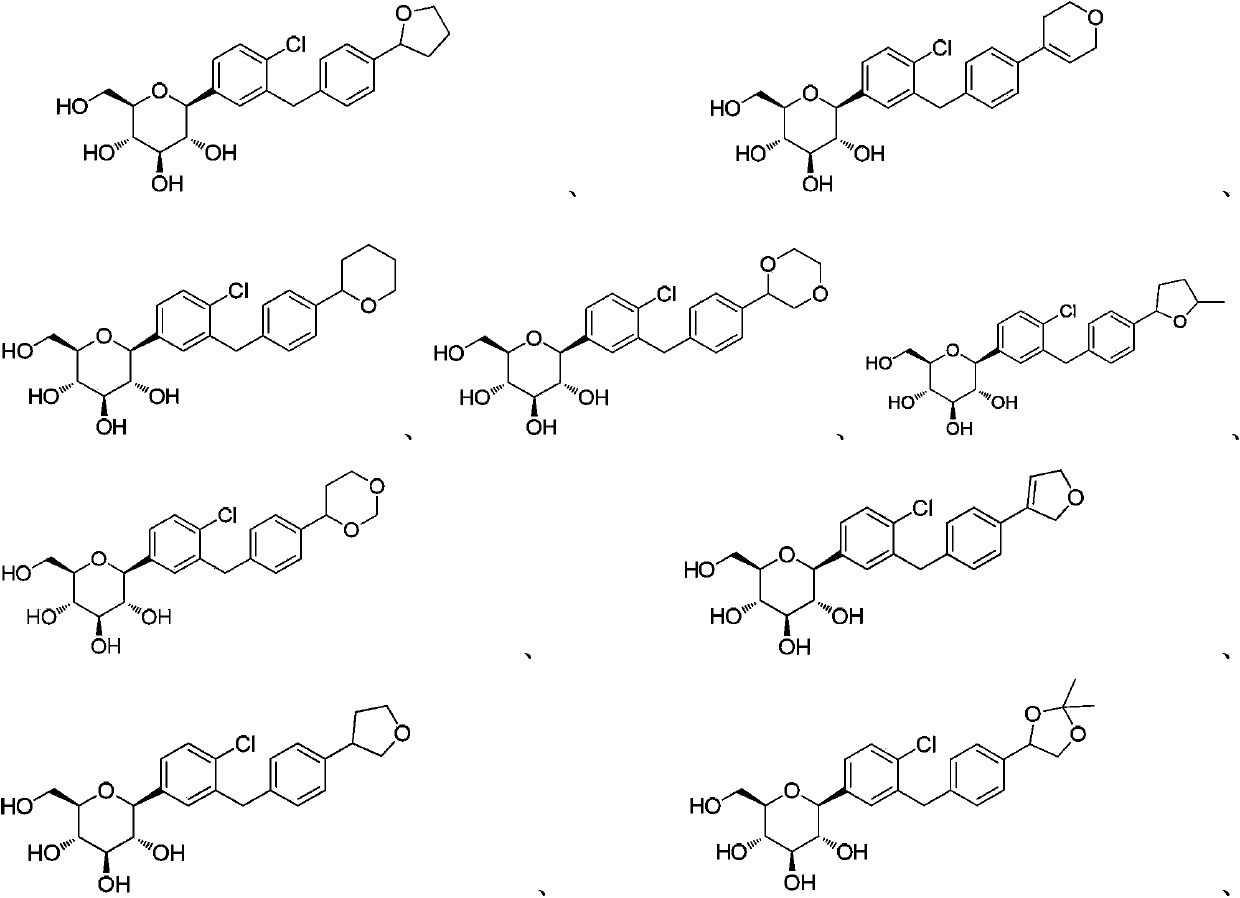
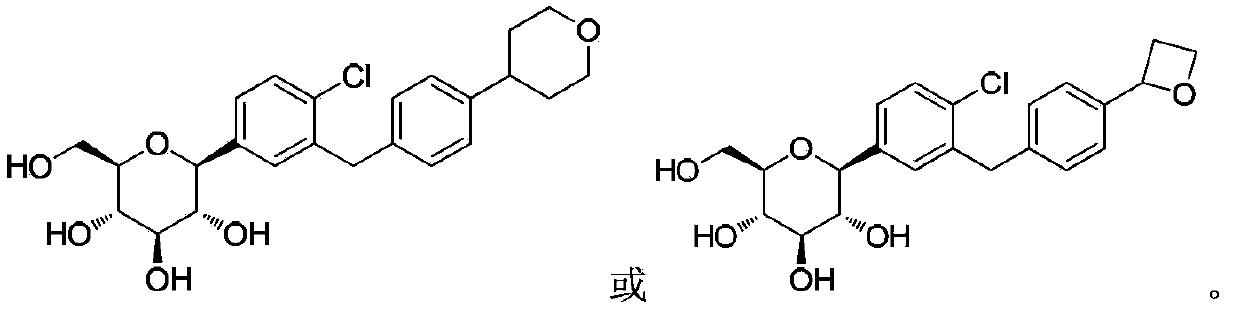
C-aryl glucoside derivative, preparation method and applications thereof
A technology of glycoside derivatives and glucose, applied in the field of derivatives in the field of chemical medicine, can solve problems such as strong inhibitory activity, and achieve the effects of high inhibitory activity and selectivity, good in vivo hypoglycemic effect, and high drug safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1 (2S, 3R, 4R, 5S, 6R)-2-(4-chloro-3-(4-(tetrahydrofuran-2-yl)benzyl)phenyl)-6-(hydroxymethyl)tetrahydro Preparation of -2H-pyran-3,4,5-triol (compound 1)
[0052] Step 1. Synthesis of 5-bromo-2-chlorobenzyl alcohol (Formula 1a)
[0053]
[0054] Dissolve 2-chloro-5-bromobenzoic acid (4.0g, 0.0170mol) in 24ml of anhydrous tetrahydrofuran, under argon protection, add dimethyl sulfide borane (8.7ml, 0.0849mol) dropwise at 0°C, and react at room temperature After 5 hours, TLC monitored until the reaction was complete, and ice-water bath, slowly added water dropwise, extracted with ethyl acetate, and spin-dried to obtain 3.6 g of white solid, the crude product was directly put into the next reaction.
[0055] Step 2. Synthesis of (5-bromo-2-chlorobenzyloxy) tert-butyldiphenylsilane (Formula 1b)
[0056]
[0057] 5-bromo-2-chlorobenzyl alcohol (formula 1a) (3.6g, 0.0163mol) was dissolved in 30ml of methylene chloride, imidazole (imidazole, 5.6g, 0.0815mol) an...
Embodiment 2
[0094] Example 2 (2S, 3R, 4R, 5S, 6R)-2-(4-chloro-3-(4-(3,6-dihydro-2H-pyran-4-yl)benzyl)phenyl) Preparation of -6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol (compound 2)
[0095]
[0096] Compound 2 was prepared in a similar manner to Example 1. Wherein, steps 1-8 are identical with embodiment 1.
[0097] Step 9. Synthesis of 4-(4-bromophenyl)-pyran-4-ol (Formula 2e)
[0098]
[0099] Under argon atmosphere, dissolve p-bromoiodobenzene (5.66g, 0.02mol) in 30ml tetrahydrofuran (THF), cool to -40°C, add isopropylmagnesium chloride (26ml, 0.026mol) dropwise, keep the reaction for 1h, add dropwise THF (10ml) solution of tetrahydropyrone (1.66g, 0.0166mol) was slowly raised to room temperature within 3h, detected by TLC, quenched with saturated ammonium chloride, extracted with ethyl acetate, and the organic phase was successively watered, saturated Washed with brine, dried, filtered, spin-dried, washed with petroleum ether to obtain a white solid (2.73g, 53%), which ...
Embodiment 3
[0112] Example 3 (2S, 3R, 4R, 5S, 6R)-2-(4-chloro-3-(4-(tetrahydro-2H-pyran-2-yl)benzyl)phenyl)-6-( Preparation of hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol (compound 3).
[0113]
[0114] Compound 3 was prepared in a similar manner to Example 1. Wherein, steps 1-8 are the same as embodiment 1.
[0115] Step 9. Synthesis of 2-(4-tributyltinphenyl)furan (Formula 3-2)
[0116]
[0117] The operation was the same as step 12 in Example 1, but compound 2c was changed to compound 3-1 (preparation reference WO20060125526) (989mg, 4.10mmol), Pd(PPh 3 ) 4 The dosage was changed to 237mg (0.205mmol), Bu 3 Sn-SnBu 3 The dosage was changed to 4.76g (8.20mmol).
[0118] Step 10. Synthesis of (2R,3R,4R,5S,6S)-2-(acetoxymethyl)-6-(4-chloro-3-(4-furan-2-)phenyl)pyran-3, 4,5-Triacetate (Formula 3-3)
[0119]
[0120] The operation was the same as step 13 in Example 1, but compound 2d was changed to compound 3-2 (284 mg, 0.627 mmol). LC-MS of the obtained product: 639.2 [M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com