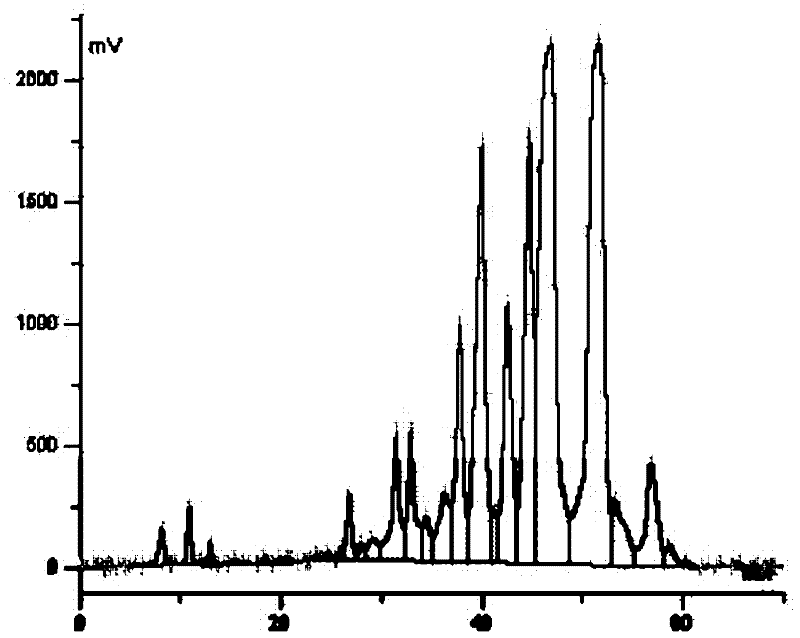
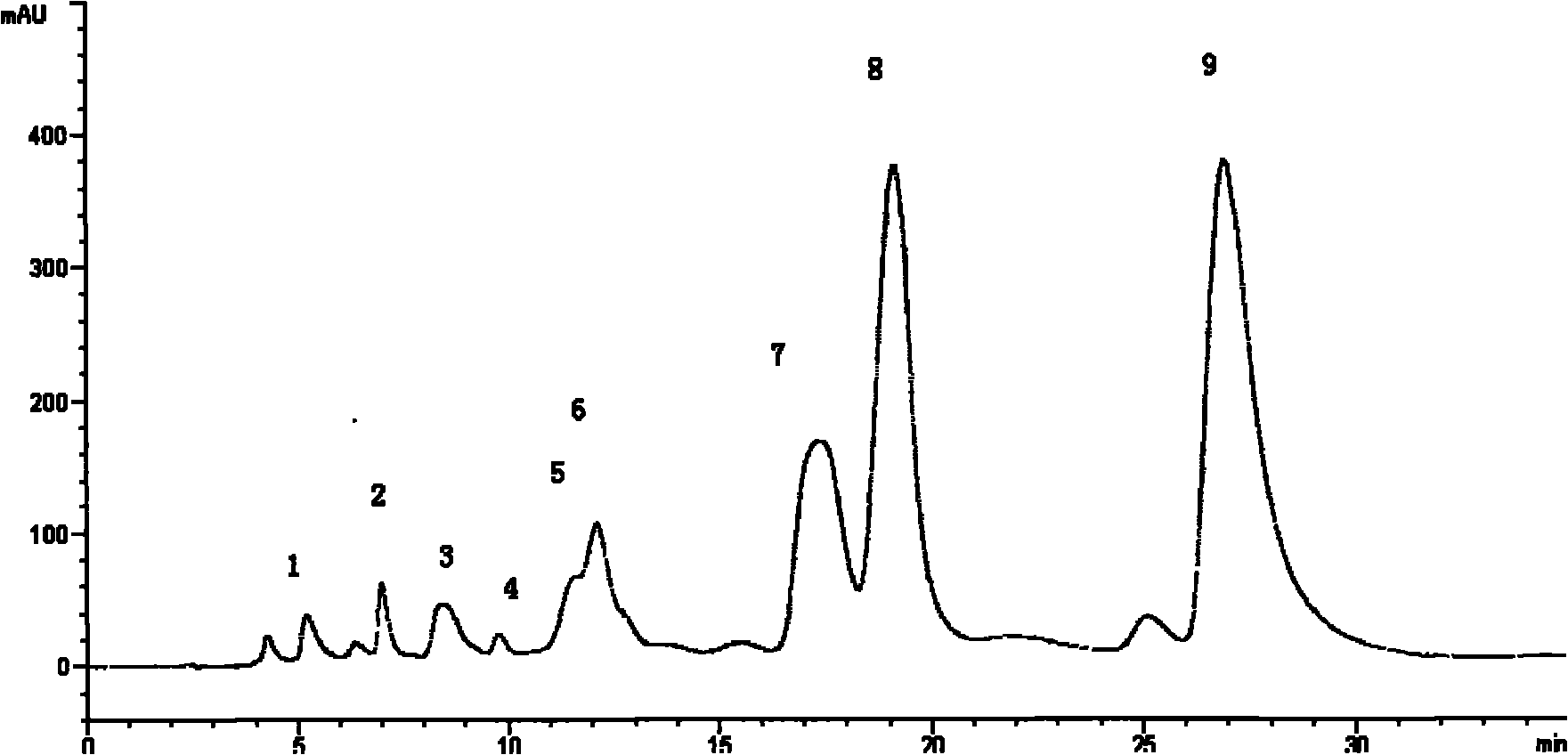
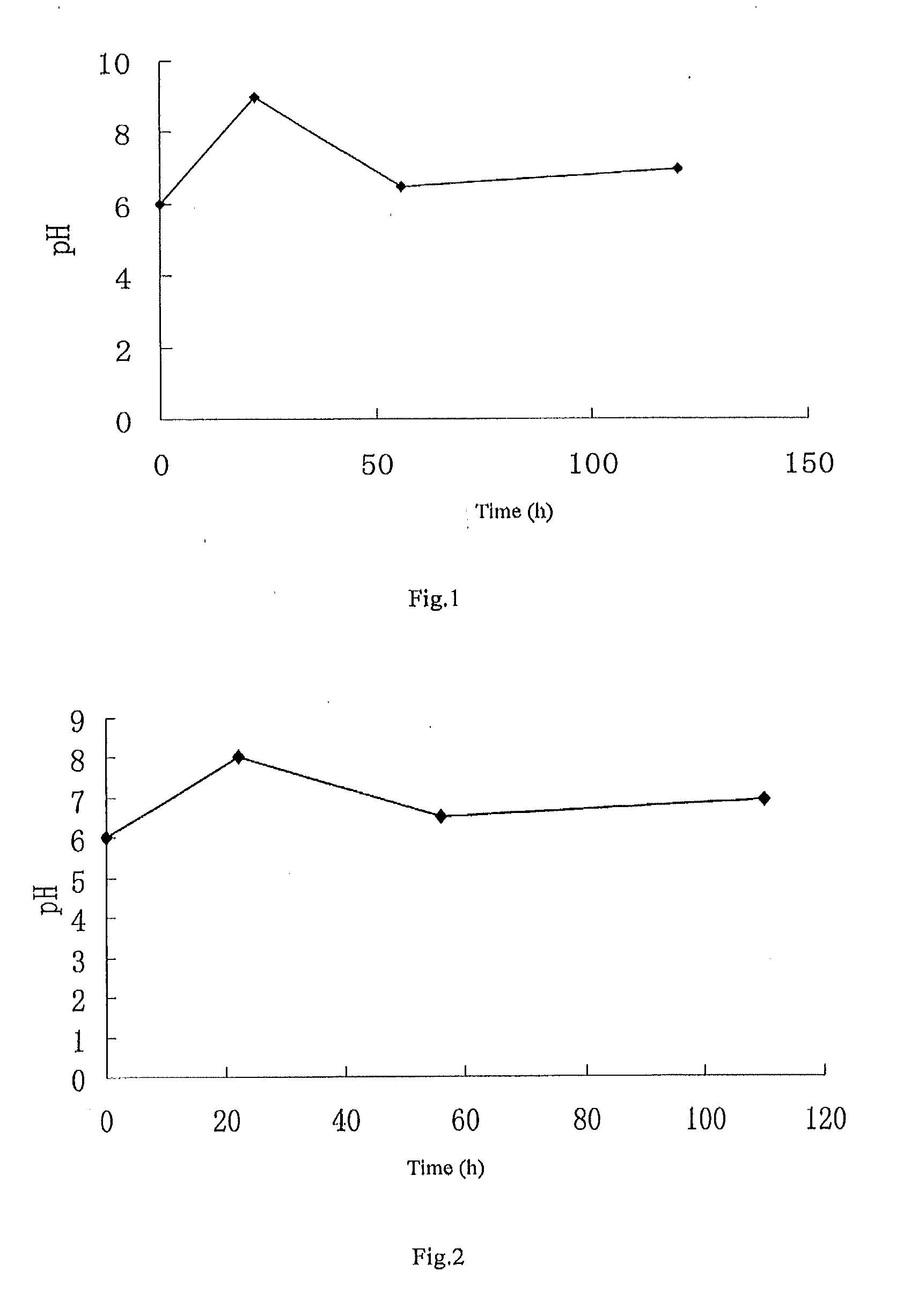
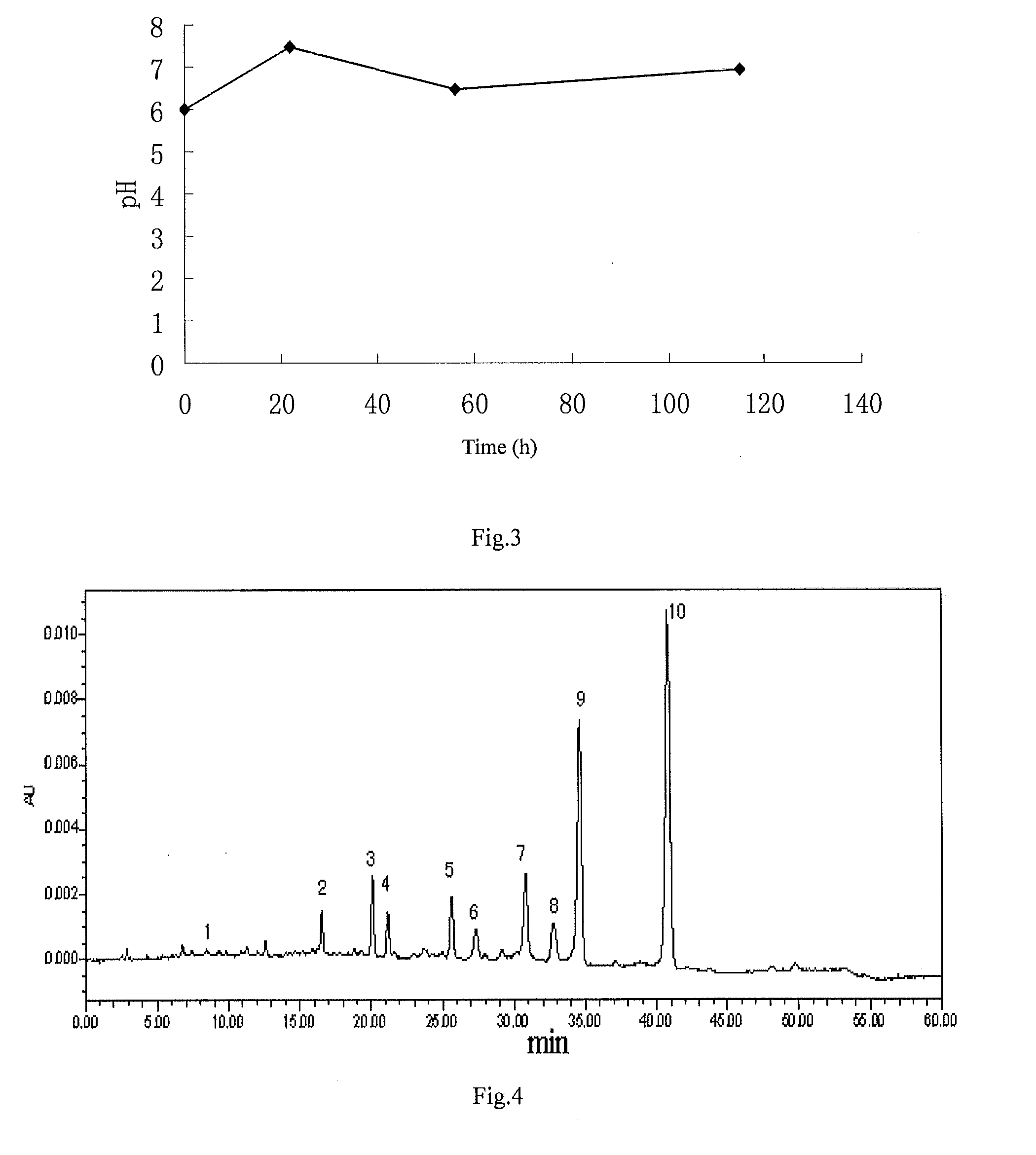
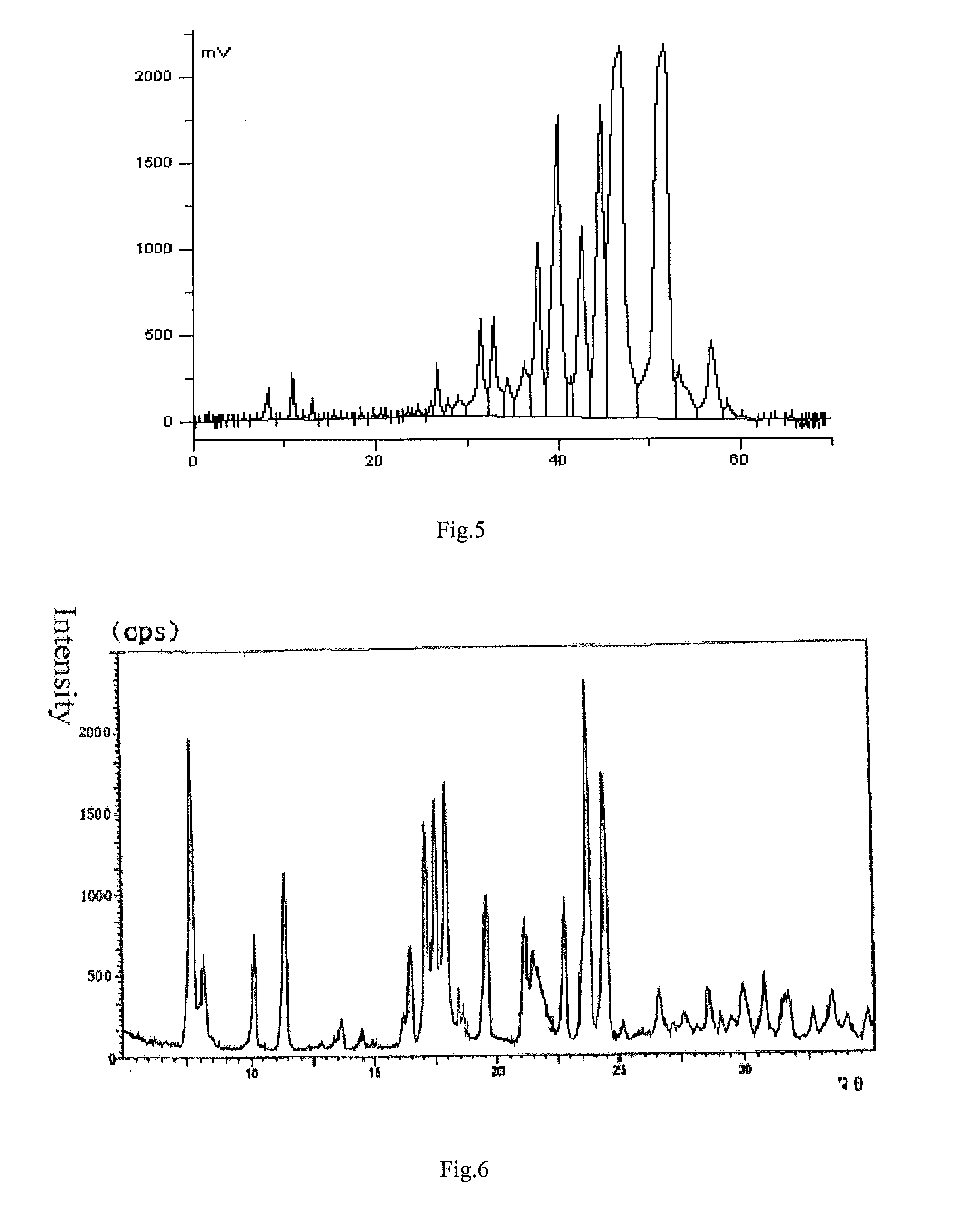
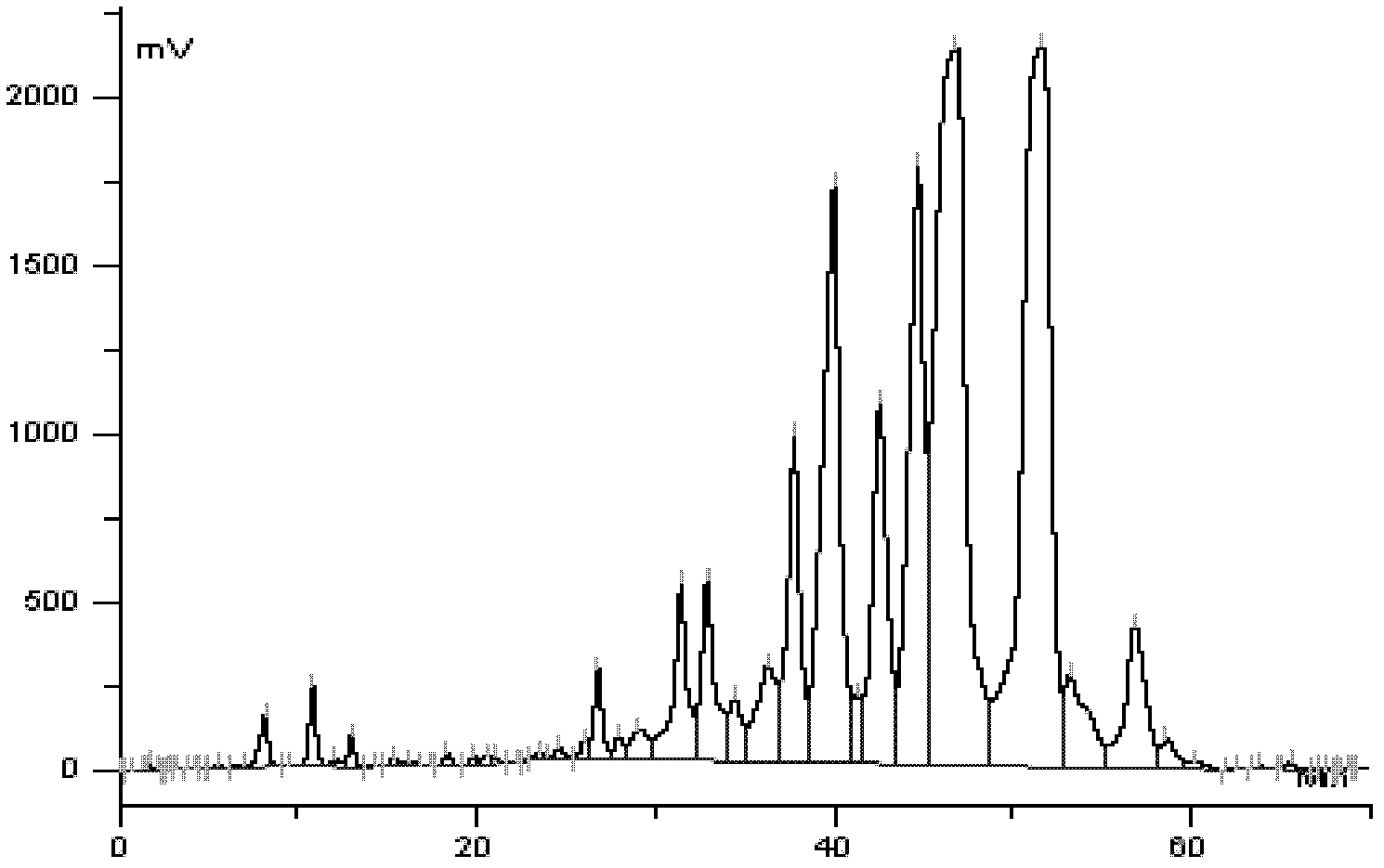
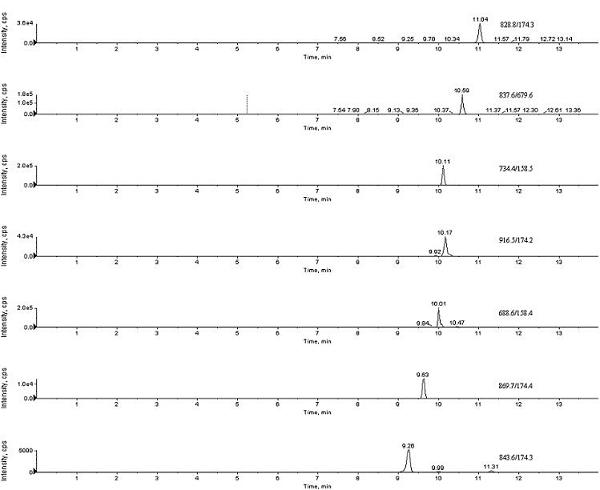
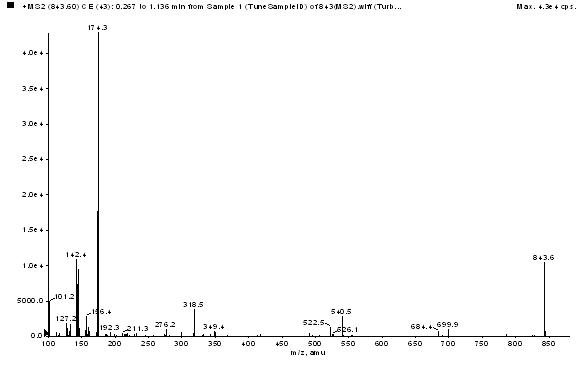
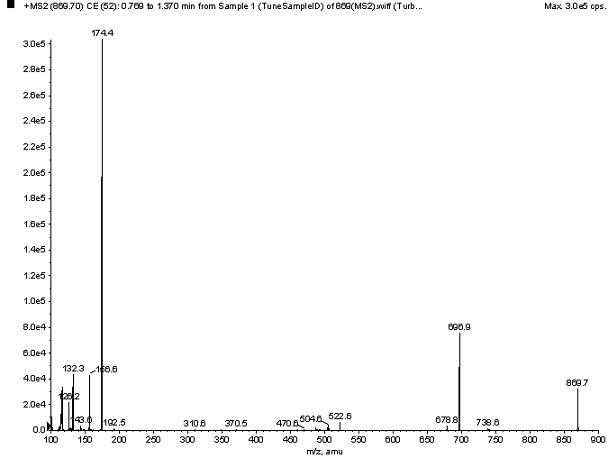
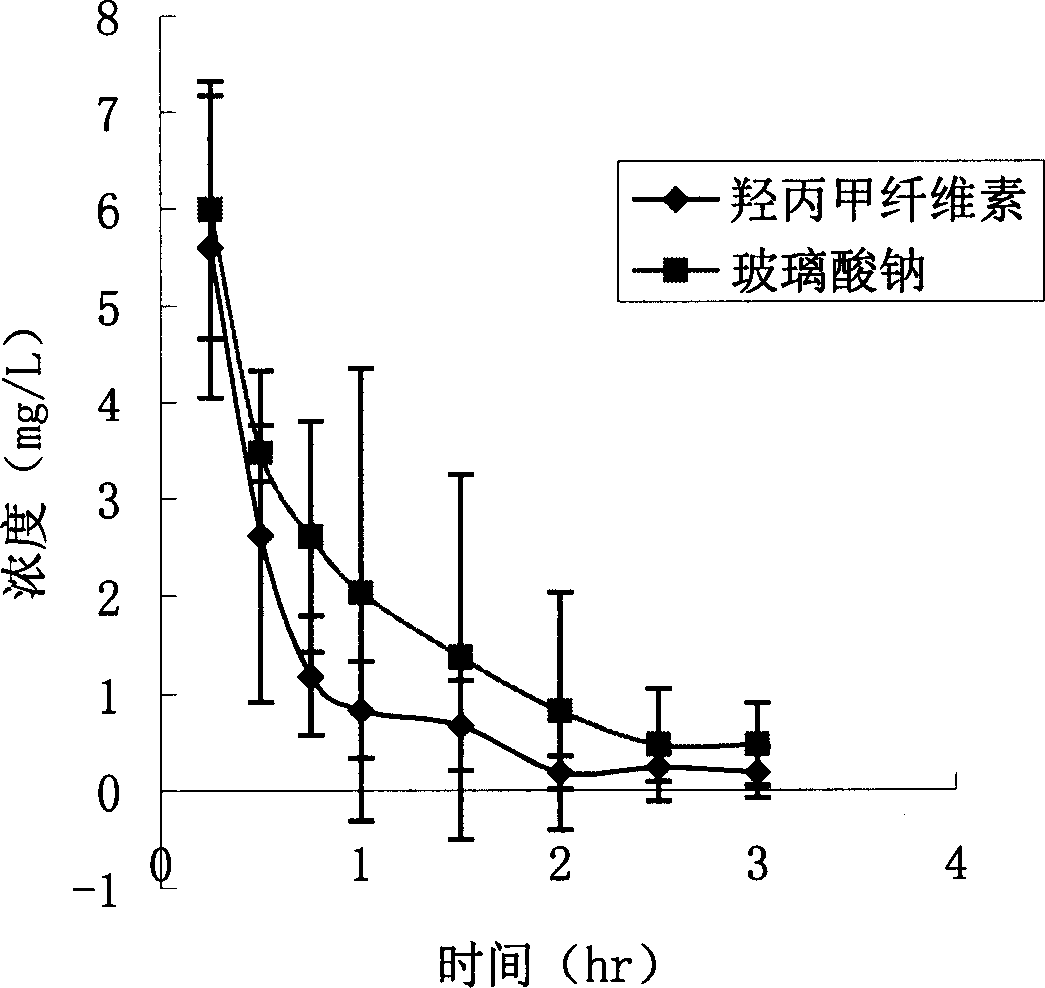
Patents
Literature
98 results about "Spiramycin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
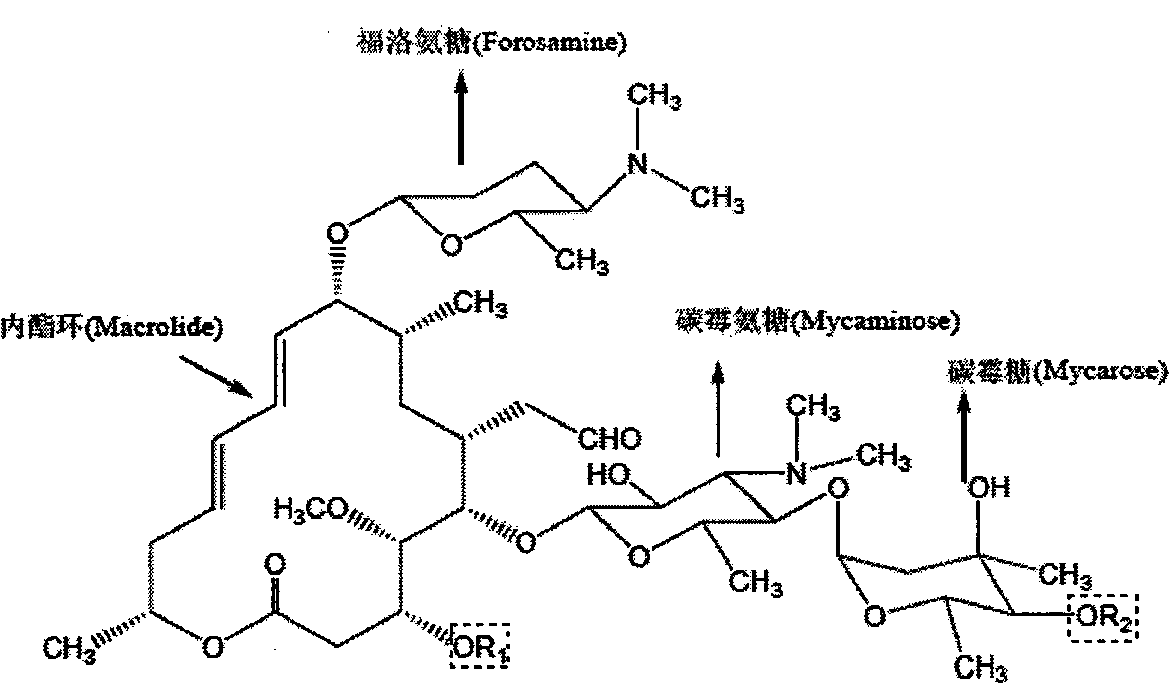
Spiramycin is a macrolide antibiotic and antiparasitic. It is used to treat toxoplasmosis and various other infections of soft tissues. Although used in Europe, Canada and Mexico, spiramycin is still considered an experimental drug in the United States, but can sometimes be obtained by special permission from the FDA for toxoplasmosis in the first trimester of pregnancy. Another treatment option (typically used after 16w gestation) are a combination of pyrimethamine and sulfadiazine (given with leucovorin).
Macrolide antibiotic bacterium dregs innocent treatment method
InactiveCN101380509ASolve pollutionRealize resource utilizationClimate change adaptationBioloigcal waste fertilisersEconomic benefitsMacrolide resistance
The invention discloses a harmless treatment method of macrolide antibiotic residues, which belongs to the technical field of solid waste treatment. The method comprises the following steps: firstly, inversely and mechanically stirring effectively broken sclertium, and then harmlessly treating the macrolide antibiotic residues with selectomycin harmless treatment microbial inoculum. The treated antibiotic residues can be used as vegetable fertilizer additive to promote the vegetable growth and solve the problem of environmental pollution caused by macrolide antibiotic residues, thus the method realizes the comprehensive utilization of resources and has better economic benefits.
Owner:HENAN UNIVERSITY OF TECHNOLOGY
Biological method for processing spiramycin bacteria residue
InactiveCN101624605ASafe and reliable performanceImprove fermentation titerMicroorganismsAnimal feeding stuffBiotechnologyMicroorganism
The invention provides a biological method for processing spiramycin bacteria residue. Through the use of a composite microbe fermenting agent and a composite enzyme preparation, the spiramycin bacteria residue is used as a main raw material and added with other auxiliary materials together to form a fermenting substrate for solid fermentation; a fermenting product is subjected to high-temperature drying and inactivation to obtain a product; and the obtained product can be used as a microbe culture medium nitrogen source or a feed protein source. The method has the characteristics of simple process, easy operation, short processing time and high processing efficiency; and the processed product has safety, reliability and excellent performance, thereby solving the problem of pollution of the spiramycin bacteria residue and realizing resource reutilization.
Owner:武汉烁森生态科技有限公司
L-isovalerylspiramycin iii, its preparation, preparation method and application
ActiveCN102260308AImprove antibacterial propertiesImprove pharmacological activityAntibacterial agentsOrganic active ingredientsFreeze-dryingAntibacterial activity
The invention relates to levorotatory isovaleryl spiramycin III, and also relates to a preparation, a preparation method and application thereof. The preparation consists of the levorotatory isovaleryl spiramycin III and a pharmaceutically acceptable carrier and / or auxiliary materials, and the purity of the levorotatory isovaleryl spiramycin III is over 90 weight percent, preferably over 95 weight percent and further preferably over 98 weight percent. The levorotatory isovaleryl spiramycin III has good antibacterial activity; the preparation of the levorotatory isovaleryl spiramycin III comprises water injection for injection, powder injection for injection and freeze-dried powder injection, fills up a blank of a single-component preparation of the isovaleryl spiramycin III in the current market and provides a new quick-response way for treating infectious diseases. The single-component preparation of the isovaleryl spiramycin III has the advantages of stable production process, easily controlled quality standard, and suitability for large-scale industrial production.
Owner:SHENYANG TONGLIAN GRP CO LTD
Methods for treating cancer
InactiveCN101568343ASuppression of premature termination mutationsOrganic active ingredientsAntineoplastic agentsMacrocyclic lactonePharmaceutical medicine
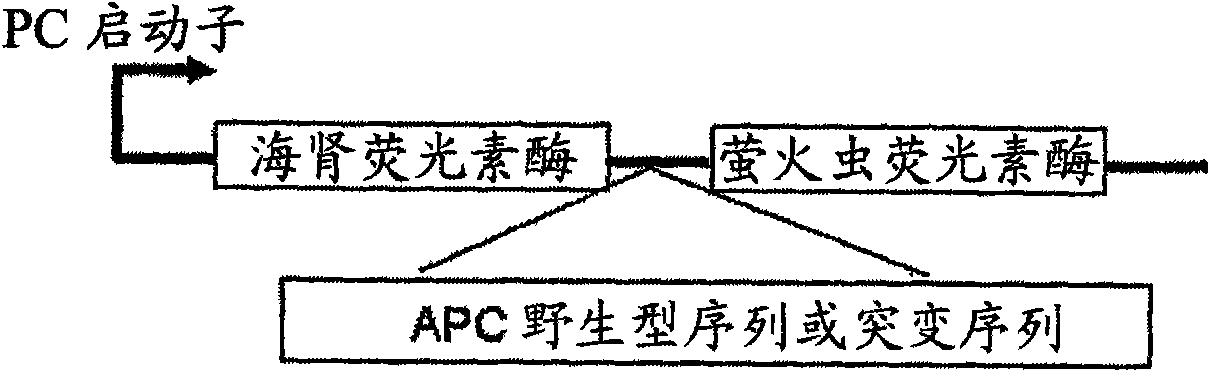
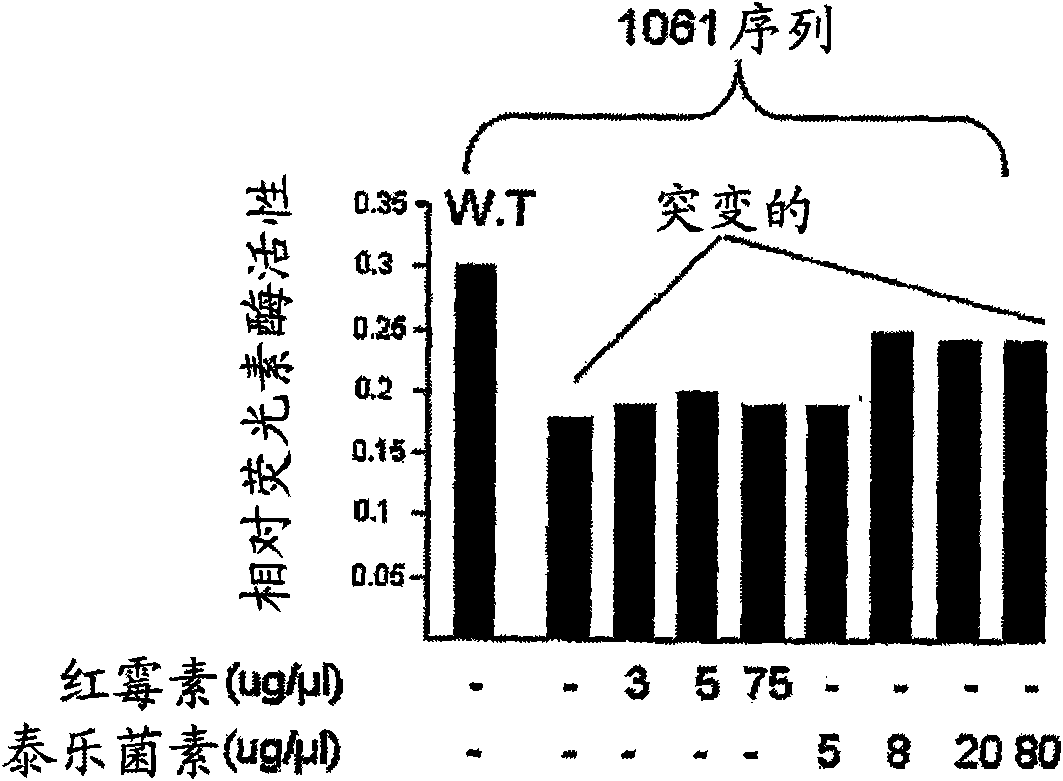
Use of a macrolide antibiotic for the manufacture of a medicament for the treatment or prevention of a cancer selected from colorectal cancer, Desmoid tumor, bladder cancer, gastric cancer, and breast cancer, the macrolide antibiotic being one or more of : tylosin, pharmaceutically acceptable salts thereof, and derivatives thereof; erythromycin, pharmaceutically acceptable salts thereof, and derivatives thereof; oleandomycin, pharmaceutically acceptable salts thereof, and derivatives thereof; and spiramycin, pharmaceutically acceptable salts thereof, and derivatives thereof. Also provided are pharmaceutical compositions and methods for the treatment or prevention of the above mentioned cancers and methods for treating or preventing, in a mammal, a cancer that expresses a mutated APC gene.
Owner:RAMOT AT TEL AVIV UNIV LTD
Purification technology of rokitamycin
InactiveCN101921302AEasy to operateQuality improvementSugar derivativesSugar derivatives preparationChemistrySpiramycin
The invention discloses a purification technology for extracting rokitamycin from fermentation liquor, comprising the following steps: (1) adding a flocculating agent in the fermentation liquor to precipitate protein, and filtering to obtain a filtrate; (2) extracting the rokitamycin from the fermented filtrate obtained from step (1) by a solvent extraction method, and obtaining an ester phase; (3) alternatively washing the ester phase obtained from step (2) with a salt solution and pure water; (4) reextracting the rokitamycin from the ester phase to a water phase by a reextraction method; and (5) crystallizing and drying the water phase rokitamycin obtained from step (4) to obtain a product. Through extraction, especially through using a buffer salt solution to wash the extraction phase, the purification method removes residual spiramycin therein and optimizes the component proportion of the rokitamycin, the obtained product meets the quality index requirements, and the spiramycin in a washing liquid can be separated and recycled, thereby greatly improving the quality and the yield of the product.
Owner:EAST CHINA UNIV OF SCI & TECH +1
Levocarrimycin, pharmaceutical compositions, preparation methods and uses thereof
The present invention relates to levocarrimycin, its pharmaceutical compositions, preparation methods and application. Levocarrimycin is a mixture of isovalerylspiramycin III, II and I as main components and contains some isobutyrylspiramycin III and II, butyrylspiramycin III and II, propionylspiramycin III and II, as well as acetylspiramycin III and II, among which, the content of isovalerylspiramycin III is no less than 30 wt %, the total content of isovalerylspiramycin III, II and I is no less than 60 wt %, and the content of acylspiramycin is 80-98 wt %. Specific optical rotation of said levocarrimycin is [α]D=−52°˜−57° in the solution of 0.02 g / ml chloroform at temperature of 25° C. The present invention also relates to the crystalline compound of isovalerylspiramycin III, II or I in levocarrimycin, and pharmaceutical compositions containing the said levocarrimycin. In present invention, the active components in levocarrimycin or its pharmaceutical compositions have optical activity and excellent anti-infective effect.
Owner:SHENYANG FUYANG PHARM TECH CO LTD
Levorotary isovaleryl spiramycin II as well as preparation, preparation method and application thereof
ActiveCN102311471AImprove antibacterial propertiesImprove pharmacological activityAntibacterial agentsOrganic active ingredientsFreeze-dryingAntibacterial activity
The invention relates to a levorotary sovaleryl spiramycin II, and also relates to a preparation, a preparation method and an application thereof. The preparation is composed of the levorotary sovaleryl spiramycin II and a pharmaceutically acceptable carrier and / or auxiliary material, and the purity of the levorotary sovaleryl spiramycin II is greater than 90wt%, preferably 95wt%, and further preferably 98wt%. The levorotary sovaleryl spiramycin II has good antibacterial activity; the preparation comprises a water injection, a powder injection, and a freeze-dried powder injection; the preparation provided by the invention fills up the blank of an isovaleryl spiramycin II individual component preparation in the markets at present, thereby providing a rapid-acting way for treating anti-infection diseases; and the isovaleryl spiramycin II individual component preparation provided by the invention has the advantages that the production process is stable, the quality standard is easy to control, and the preparation is suitable for large-scale industrial production.
Owner:SHENYANG TONGLIAN GRP CO LTD
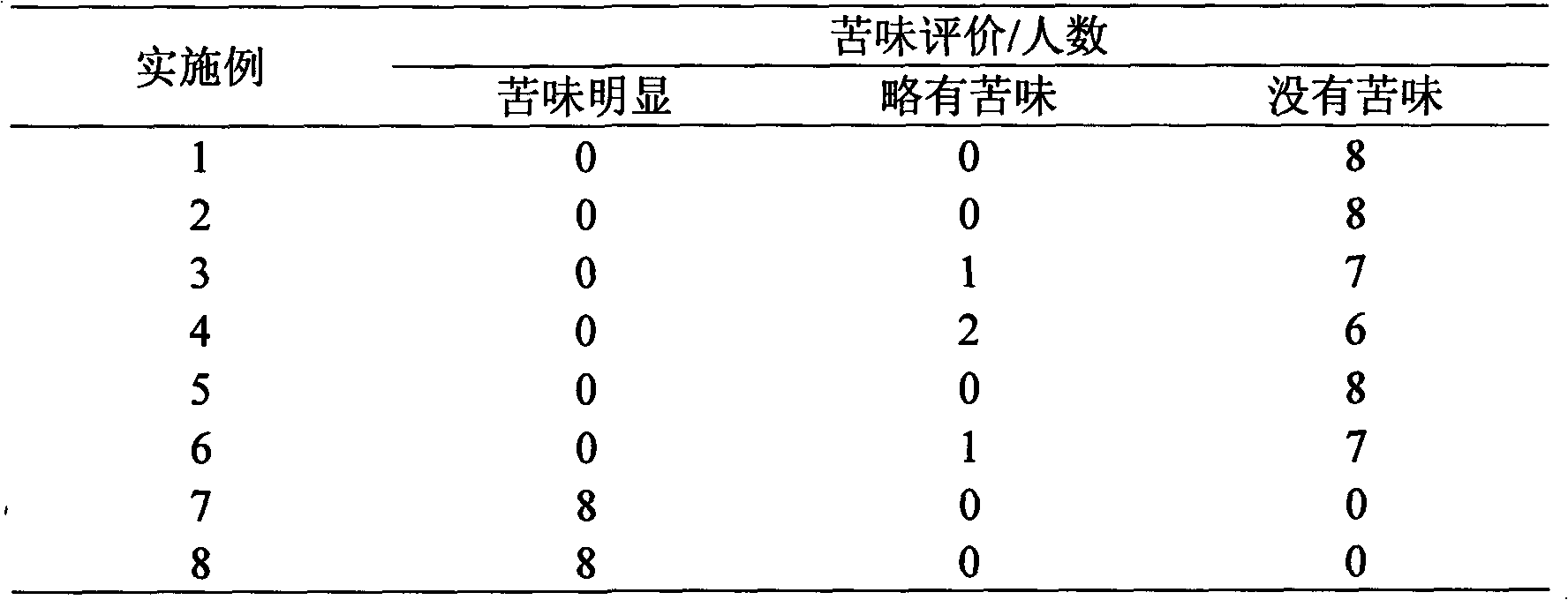
Oral composition containing spiramycin capabile of masking bitter taster
An orally taken composition containing foromacidin with masked bitter taste contains foromacidin, high-sweetness sugar, suspending aid, and excipient proportionally.
Owner:WUXI FORTUNE PHARMA
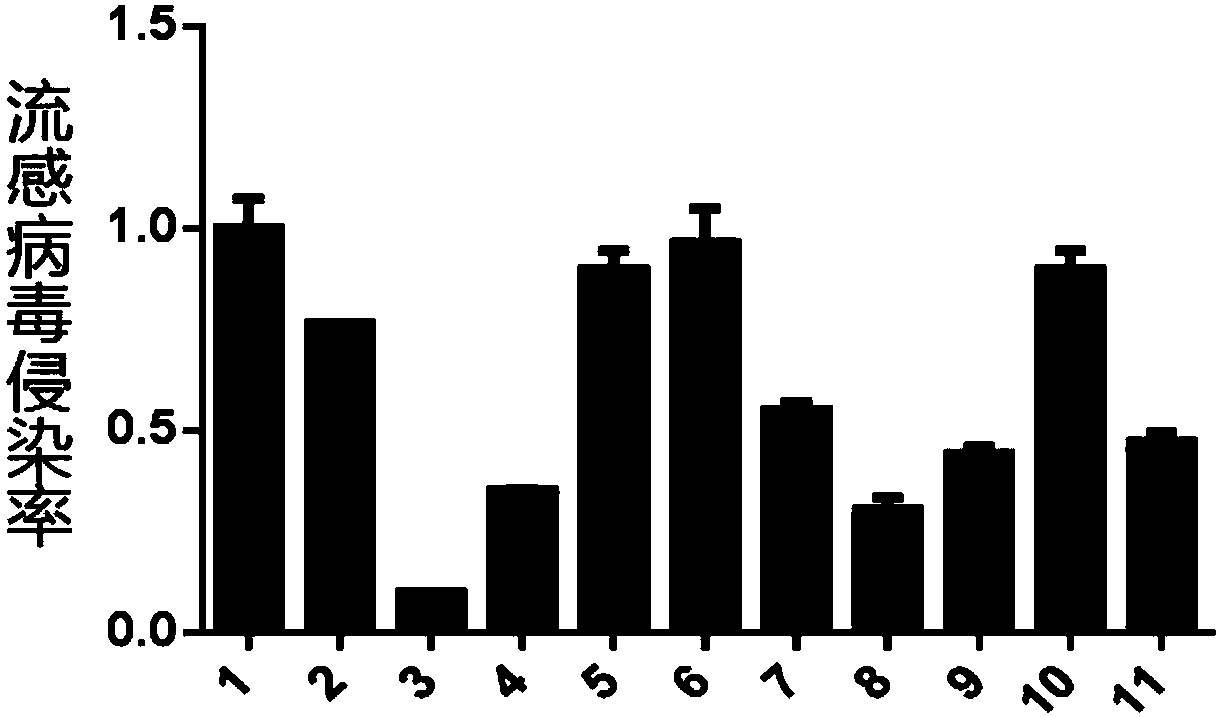
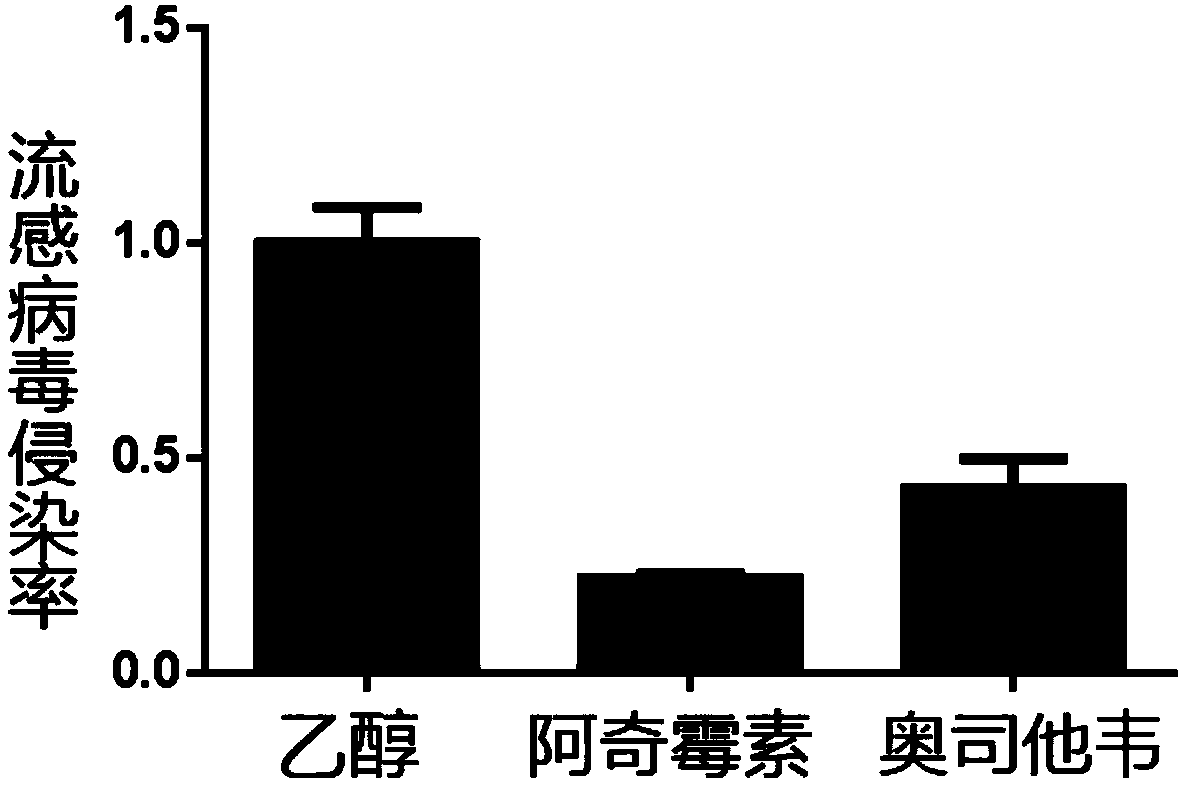
Application of macrolide antibiotics in blocking influenza virus infection
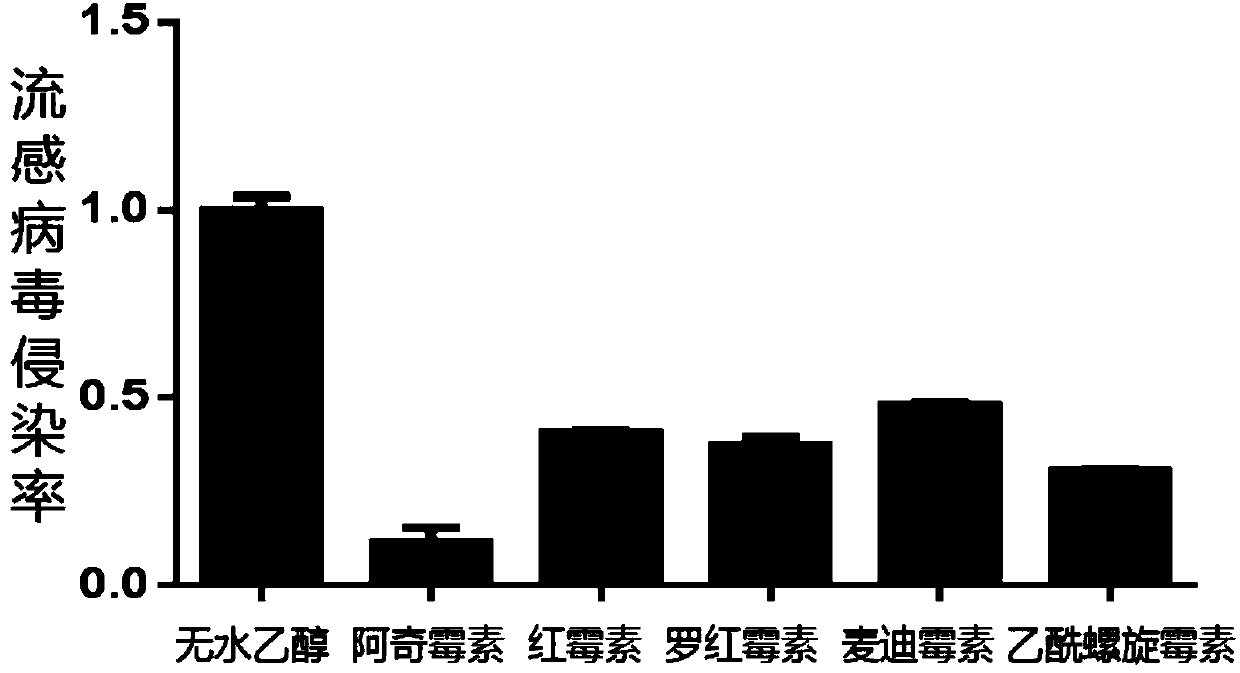
InactiveCN109833326AGood ability to inhibit influenza virusAvoid infectionAntiviralsAmine active ingredientsRoxithromycinAzithromycin
The invention relates to an application of macrolide antibiotics in blocking influenza virus infection, specifically, the invention discloses the application of macrolide antibiotics including azithromycin, erythromycin, roxithromycin, medimycin or acetylspiramycin or pharmaceutically acceptable salt thereof for blocking influenza virus infection, and the application of the macrolide antibiotics in preparation of a medicinefor treating or preventing influenza virus infection. The experiments confirm that the aforementioned macrolide antibiotics have the ability to resist influenza virus infection and are capable of performing prophylactic treatment of influenza virus infection. At the same time, the invention also validates the action mechanism of azithromycin for treatment of influenza. Therefore, the aforementioned macrolide antibiotics or the pharmaceutically acceptable salt thereof and a composition comprising the aforementioned macrolide antibiotics can be prepared as the medicinefor treating or preventing the influenza virus infection.
Owner:SUZHOU INST OF SYST MEDICINE
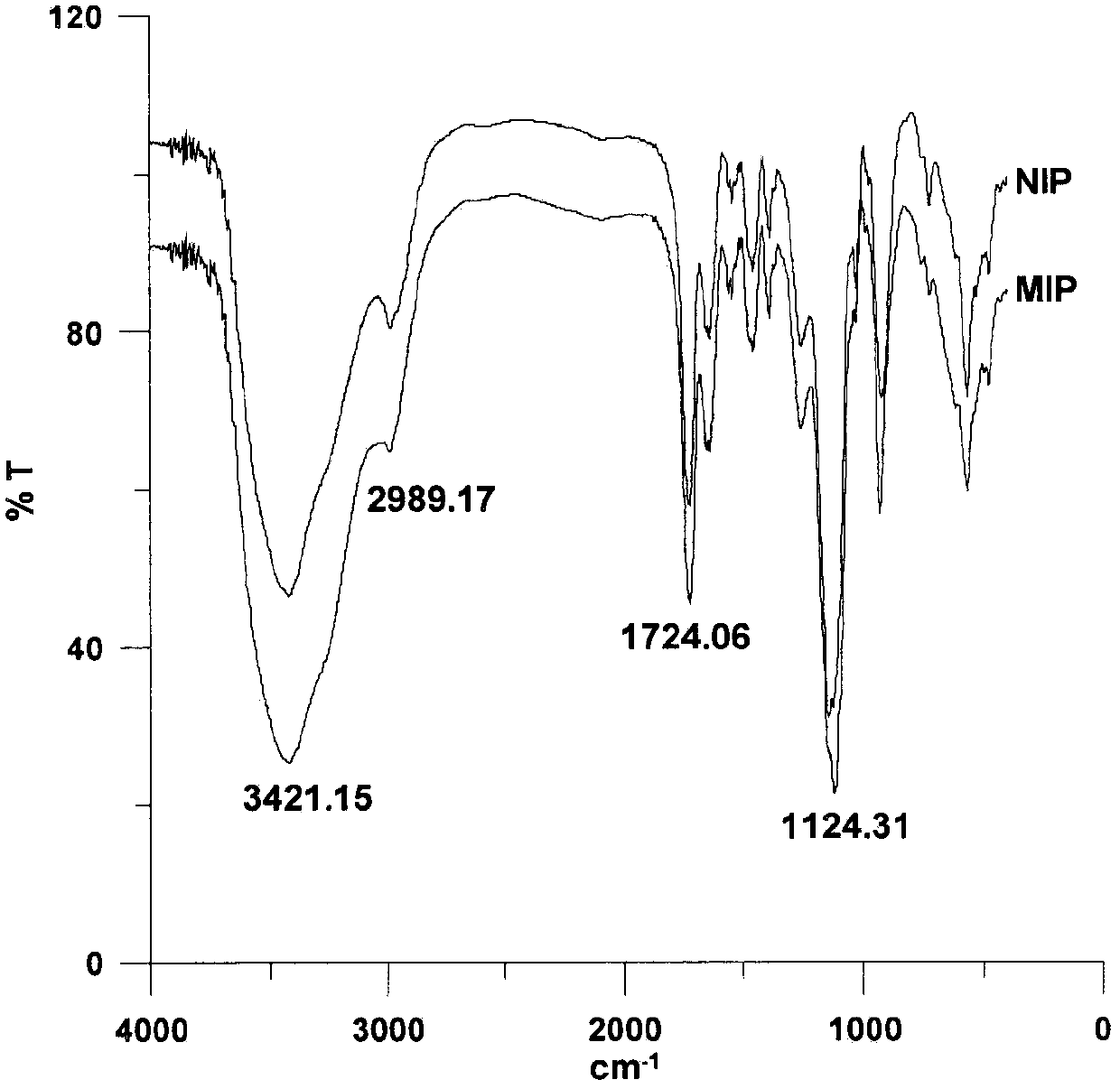
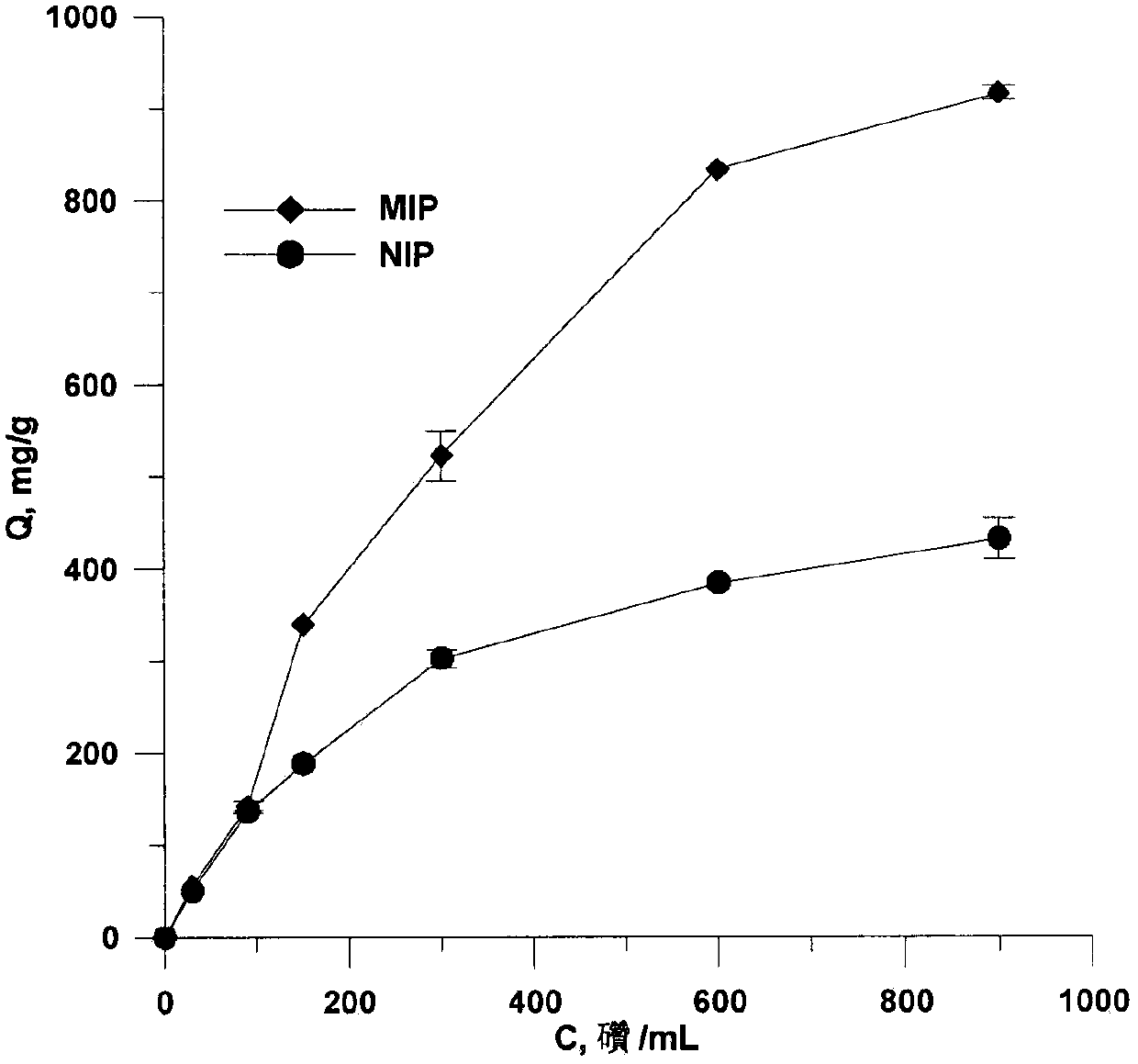
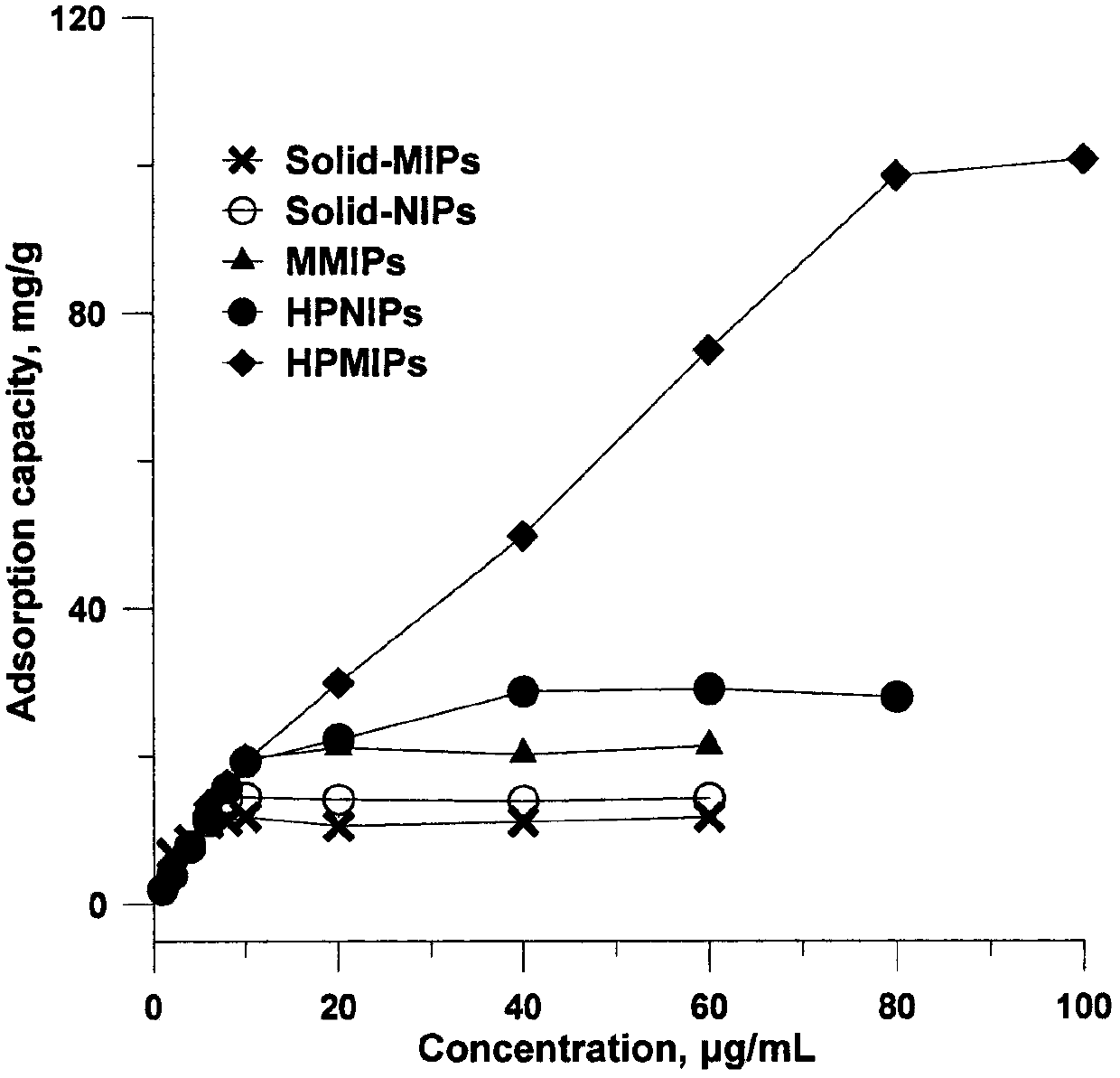
Preparation method and application of thermosensitive macrolide antibiotics molecular-imprinting solid-phase-micro-extracted fibers
InactiveCN107629166AHigh selectivityReduce dosageComponent separationOther chemical processesFiberMacrolide resistance
The invention discloses high-selectivity molecular-imprinting solid-phase-micro-extracted fibers applied to the determination of residual of trace macrolide antibiotics in animal-derived foods and preparation method of the high-selectivity molecular-imprinting solid-phase-micro-extracted fibers. The molecular-imprinting solid-phase-micro-extracted fibers with controllable synthesized coating thickness, disclosed by the invention, are obtained by taking spiramycin as template molecules by adopting a tube-sleeving thermal-initiated polymerization method. The molecular-imprinting solid-phase-micro-extracted fibers have a memory function on three-dimensional structures of the macrolide antibiotics such as the spiramycin, tilmicosin and josamycin. The molecular-imprinting solid-phase-micro-extracted fibers can be used for carrying out high-selectivity enrichment on the macrolide antibiotics such as the spiramycin, the tilmicosin and the josamycin in sample solutions for residual analysis. The preparation method disclosed by the invention is simple, the prepared extracted fibers are good in chemical and mechanical properties and thermal stability, high in extraction capacity, good in selectivity and long in service life, have temperature sensitivity and have a broad application prospect in the fields of analytical chemistry and environmental analysis.
Owner:CHINA PHARM UNIV
Detection method of residual amount of a plurality of macrolide veterinary drugs in casings
InactiveCN102313787AAchieving Simultaneous DetectionHigh recovery rateComponent separationRoxithromycinRelative standard deviation
The invention relates to the fields of analytical chemistry and food safety, and provides a detection method of the residual amount of a plurality of macrolide veterinary drugs in casings. The detection method comprises the following steps of: extracting a sample with methanol or acetonitrile; purifying the obtained extracting solution by a C18 or Oasis HLB (hydrophilic-lipophilic balance) solid-phase extraction column, taking an acetonitrile-methanoic acid aqueous solution with the concentration of 0.15% as a mobile phase, and performing gradient elution by adopting a ZORBAX Eclipse C8 analytical column; and performing electro-spraying, separating by a cationic scanning mode and detecting the residual amount of seven types of macrolide veterinary drugs. The detection lower limit of the seven types of macrolide veterinary drugs is 20 mu g / kg. The recovery rates of spiramycin, tilmicosin, oleandomycin, tylosin, erythrocin, roxithromycin and josamycin are respectively 71.4-78.8, 75.7-85.6, 81.1-85.8, 78.9-83.0, 87.1-89.6, 83.7-85.4 and 75.6-81.8; and the indoor relative standard deviations for the recovery rates of the spiramycin, the tilmicosin, the oleandomycin, the tylosin, the erythrocin, the roxithromycin and the josamycin are respectively 7.7-11.1, 8.0-15.7, 10.5-16.4, 8.1-12.5, 10.5-12.4, 10.6-14.4 and 7.8-18.0. The detection limit, the recovery rate, the precision and the like meet the related requirements at home and abroad.
Owner:林维宣
Macrolide antibiotics sodium hyaluronate eye transfer system
InactiveCN1814299AInvestigate the stay situationAntibacterial agentsOrganic active ingredientsRoxithromycinClarithromycin
This invention relates to an eye use transmission system of macrolide type antibiotic hyaluronic acid natrium. The goal of this invention is to provide a macrolide type antibiotic eye use medicine preparation with carrier is hyaluronic acid or its salt. It is used to cure germ eye region infection. The macrolide type antibiotic in this invention includes antibiotic that has 14 yuan,15yuan, 16yuan large ring construction features , for example, natural antibiotic bring by streptomycete erythromycin, josamycin, spiramycin, medemycin etc. and semisynthesis derivant from by structure modification of natural antibiotic, such as roxithromycin, azithromycin, clarithromycin, rokitamycin, telithromycin.
Owner:无锡康福特药物科技有限公司
Method for reducing impurities of spiramycin by recrystallization
ActiveCN104478973ALow yieldHigh puritySugar derivativesSugar derivatives preparationOxalateMonopotassium phosphate
The invention relates to a method for reducing impurities of spiramycin by recrystallization. The method comprises the following steps: (1) dissolving monopotassium phosphate in water and then adjusting the pH value to 2-3 by oxalic acid; (2) putting solid spiramycin into a mixed solution obtained the step (1) and stirring to dissolve at 25-40 DEG C; (3) adding a sodium hydroxide solution with mass concentration of 12-15% into the spiramycin solution obtained in the step (2), raising the temperature for 60-90 minutes and then keeping the temperature to 50-65 DEG C and stirring to crystallize; and (4) centrifugalizing and separating to obtain wet powder, repeating the steps (1), (2) and (3) on the wet powder, and drying the wet powder obtained again to obtain the low-impurity spiramycin. According to the method provided by the invention, by changing the addition of monopotassium phosphate and oxalic acid and then re-dissolving the spiramycin finished product into the solution of monopotassium phosphate and oxalic acid to be re-crystalized so as to further remove related substances, the product quality is further improved.
Owner:TOPFOND PHARMA CO LTD
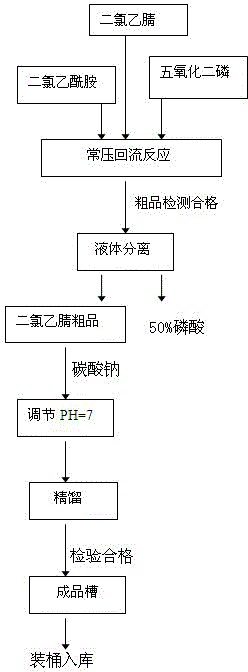
Novel production process for dichloroacetonitrile
InactiveCN106278945AReduce the temperatureLower reaction conditionsCarboxylic acid nitrile purification/separationReaction temperatureBacterial disease
The invention relates to a novel production process for dichloroacetonitrile, specifically to a novel synthetic process for a key intermediate dichloroacetonitrile of a fine chemical engineering product, i.e., florfenicol. Dichloroacetonitrile is a key intermediate of florfenicol, and florfenicol is a novel special wide-spectrum veterinary chloramphenicol antibiotic successively developed in later 80s and is used as a feed additive for a pig for prevention and treatment of swine bacterial diseases. When same dosages of florfenicol and spiramycin are used for treatment of respiratory diseases, the cure rate (91%) of florfenicol is substantially higher than the cure rate (41%) of spiramycin; and when 50 ppm of florfenicol is mixed with a feed for treatment of man-induced swine actinobacillus pleuropneumonia, a cure rate reaches 100%. The process provided by the invention mainly overcomes the problems of severe reaction conditions, high danger, high reaction temperature, slow reaction rate, low yield, poor controllability and the like of conventional processes. According to the process, dichloroacetamide and dichlorine pentoxide are subjected to a reflux reaction in a liquid environment with dichloroacetonitrile so as to obtain a crude product, and the crude product undergoes rectification so as to obtain a qualified product. The process has the advantages of mild reaction conditions, no pollution, high yield, high content, high added value of a byproduct and high economic benefits and environmental protection benefits.
Owner:HUBEI UNIV OF ARTS & SCI
Preparation and application method of macrolide antibiotic molecularly imprinted magnetic metal organic framework composite material
PendingCN114011388AHigh adsorption selectivitySuperparamagneticWater treatment parameter controlOther chemical processesMetal-organic frameworkClarithromycin
The invention belongs to the technical field of adsorption materials, and relates to a preparation and application method of a macrolide antibiotic molecularly imprinted magnetic metal organic framework composite material. The material combines the excellent pore characteristics of a metal organic framework and the high selectivity of a molecularly imprinted polymer, can specifically adsorb erythromycin, clarithromycin, roxithromycin, acetylspiramycin, korubicin and tylosin in an aqueous solution, and is relatively large in adsorption capacity and relatively short in equilibrium time, and the material has relatively high removal efficiency on low-concentration macrolide antibiotics. The material has superparamagnetism, can quickly realize solid-liquid separation, and can be repeatedly used for multiple times. The material is used as a solid-phase extraction adsorbent and is combined with high performance liquid chromatography, so that trace macrolide antibiotics in a complex environmental water sample can be analyzed and detected.
Owner:GUANGDONG UNIV OF TECH
Biopesticide complex inoculant for root disease prevention and control of crops like cotton and preparation method thereof
The invention discloses a biopesticide complex inoculant for root disease prevention and control of crops like cotton and a preparation method thereof. The biopesticide complex inoculant is microbial pesticide utilizing a powerful microbial combination and having efficient effect on root diseases of the crops like cotton. Specifically, microorganisms like white hook streptomyces generating gentamicin, streptomyces ambofaciens generating spiramycin and Congomycin, streptomyces cellulosae generating streptomycin and actinomycin, streptomyces hygroscopicus Jingang variant generating Jingangmycin and preventing and controlling rice sheath blight, streptomyces microaureus generating kasugamycin and preventing and controlling rice blast, streptomyces generating staphylomycin and anti-G+cell, streptomyces Jingyangensis generating long-acting stimulin and streptomyces rimosus generating tetraenes antibiotics are subjected to fermenting cultivation, and an improved culture medium formula is adopted to prepare 2 billion per ml biological unit. The biopesticide complex inoculant is used for seed soaking, seed mixing or being blended in biological seed fertilizer before seeding the crops like cotton to play a role in preventing and controlling root diseases of the crops like cotton, and can be used for root irrigation when cotton and other crops are infected by the diseases to prevent and control crop root diseases, and effective rate reaches 65-90%.
Owner:段一皋 +1
A vegetable resistance organic fertilizer
InactiveCN104513080AFertilizer effect time is longImprove fertilizer efficiencyMagnesium fertilisersAlkali orthophosphate fertiliserNitrohumic acidRoot growth
A vegetable resistance organic fertilizer is disclosed. The fertilizer is characterized by comprising following raw materials: 20-30% of silkworm excrement, 10-20% of air plant powder, 15-24% of innocently-treated spiramycin dreg, 10-15% of urea, 6-13% of diammonium phosphate, 6-14% of potassium type inorganic salts, 2-3% of calcium ammonium nitrate, 5-10% of trace element compounds and 10-15% of nitrohumic acid. The fertilizer is long in water retention and fertilizer efficiency time, good in air permeability, capable of facilitating root growth and phosphorous absorption and utilization of plants, effectively preventing loss of ammonia and potassium and promoting plant growth, small in fertilizer using amount, low in cost, and high in fertilizer efficiency, and overcomes environment pollution caused by heavy use of fertilizers and pesticides. The fertilizer is insect-preventing, disease-resistant, pollution-free, soil-improving and hardening-eliminating, and good in fertilizing effects and has a good development and application prospect.
Owner:SHANDONG ENBAO BIOTECH
Method for separating and purifying spiramycin by utilizing alkylphenol polyoxyethylene polyoxypropylene ether NPE-108
ActiveCN112375109ANo deflagrationNon-volatileSugar derivativesSugar derivatives preparationAlkylphenolEther
The invention relates to a method for separating and purifying spiramycin. Alkylphenol polyoxyethylene polyoxypropylene ether NPE-108 can be used for forming a two-aqueous-phase system to separate thespiramycin. The method is characterized in that during first forward extraction, NPE-108 and spiramycin fermentation liquor are mixed according to the volume ratio of 1: 5, the pH value is adjusted to be 9.0, the two-aqueous-phase system is formed through induction at the temperature of 55 DEG C, and after distribution equilibrium is achieved, the spiramycin in the fermentation liquor is distributed to an NPE-108 phase (a lower phase), an upper phase is separated, and the upper phase is used for second forward extraction; lower phases of the two times of forward extraction are mixed, pure water with 0.25 times of volume is added, the pH value is adjusted to be 5.8, distribution is condcuted at 55 DEG C, and the spiramycin enriched in the NPE-108 phase is reversely extracted into a water phase; the spiramycin can be separated from the fermentation liquor through two-stage forward extraction and one-stage reverse extraction; and the NPE-108 can be recovered through temperature adjustment, and the recovery rate reaches 98.8%. The method has the advantages of mild operation conditions, efficient recovery, safety and environmental protection and the like.
Owner:EAST CHINA UNIV OF SCI & TECH +1
Oral composition of spiramycin and preparation method thereof
ActiveCN102397284ALow costSimple prescriptionAntibacterial agentsOrganic active ingredientsWaxMedicine
The invention discloses an oral composition of spiramycin, which comprises the following components in percentage by weight: 30 to 70 percent of spiramycin or active components of medical spiramycin, 10 to 35 percent of wax lipidLazhi (with white wax as the main component thereof) materials, and 10 to 50 percent of water insoluble materials (except the wax lipidLazhi materials). The oral composition of the spiramycin has the positive effects that: the taste masking effect of the spiramycin particles is good, medicaments can be quickly released in stomachs and intestinal tracts, and the bioavailability of the medicament is not affected.
Owner:ZHUOHE PHARM GRP CO LTD
Waste bacteria treatment method and waste bacteria recycling method in waste bacterial production
ActiveCN108277161APrevent outflowAvoid pollutionMicroorganism lysisMicroorganism based processesProteinase activityNitrogen source
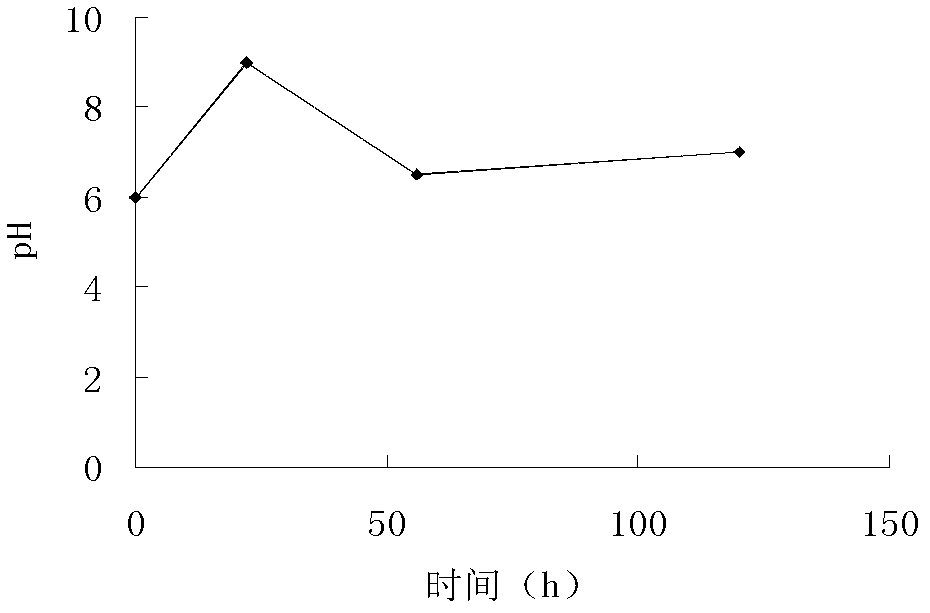
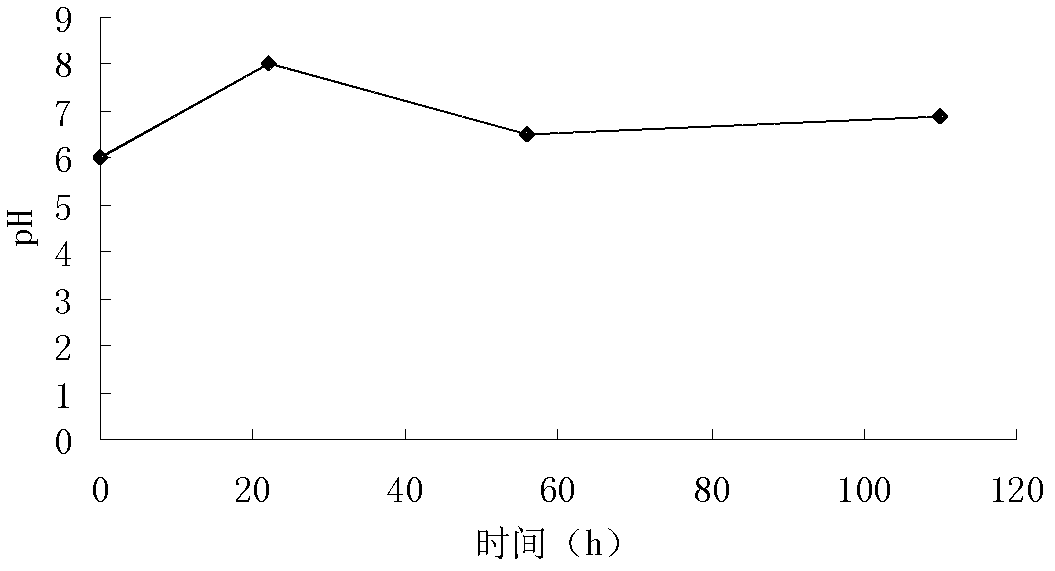
The invention discloses a waste bacteria treatment method and a waste bacteria recycling method in waste bacterial production. The waste bacteria treatment method comprises: a, adjusting the pH valueof a spiromycin production fermentation broth to 4.0-6.5, and filtering by using a ceramic membrane to obtain a concentrated liquid retained by the ceramic membrane; b, adding lysozyme to the concentrated liquid to obtain a lysozyme reaction solution; c, adding protease to the lysozyme reaction solution to obtain a protease reaction solution; and d, carrying out spray drying on the protease reaction solution to obtain waste bacteria powder. The waste bacteria recycling method comprises: replacing the organic nitrogen source in fermentation production of spiramycin with the obtained waste bacteria powder. With the methods of the present invention, the waste bacteria can achieve the fermentation raw material condition, and can be reused for the production of the original product so as to avoid the external flow of the waste bacteria.
Owner:TOPFOND PHARMA CO LTD
Anti-toxoplasma composition drug and screening method thereof
ActiveCN104998277AGood against Toxoplasma gondiiClean up thoroughlyOrganic active ingredientsAntiparasitic agentsSulfamonomethoxineSulfanilamide
The invention discloses an anti-toxoplasma composition drug and a screening method thereof, and belongs to the field of medicine. According to the anti-toxoplasma composition drug and the screening method thereof, mouse test toxoplasmosis animal models are established, the ten drugs of a sulfadiazine sodium injection, a compound sulfamethoxazole, a lincomycin hydrochloride injection, a sulfamonomethoxine sodium injection, a florfenicol injection, acetylspiramycin, pyrimethamine, roxithromycin, artemisinin and radix sophorae flavescentis are selected out, two compositions which have the best anti-toxoplasma effect are screened out, one composition comprises the sulfamonomethoxine, the pyrimethamine and TMP, and the other composition comprises the compound sulfamethoxazole and the acetylspiramycin; animal models in mice and pigs are established, it is verified that the two compositions are both effective on the treatment of artificially infected toxplasmosis in pigs, and the treatment effect on the pigs is in accordance with that of the mouse models. By means of the anti-toxoplasma composition drug and the screening method thereof, it is proved that the the feasible method is achieved by utilizing the mouse test toxoplasmosis models to evaluate the efficacy of drugs on treating toxplasmosis in pigs, and meanwhile two anti-toxoplasma drug compositions with good effects are provided.
Owner:SOUTH CHINA AGRI UNIV
Method for treating spiramycin zymophyte dreg
InactiveCN103849654AAvoid wastingLow costGas production bioreactorsWaste based fuelBiochemical engineeringSlurry
The invention provides a method for treating spiramycin zymophyte dreg by virtue of resourceful treatment. The method comprises the following steps: (a) activating outside a tank body; (b) activating inside the tank body; (c) after the activation of slurry in the tank body is ended, replenishing a spiramycin zymophyte dreg liquor through the bottom of a first-grade tank body every day, subsequently sending biogas slurry with the same volume at the top of the first-grade tank body to a second-grade tank body through the bottom of the second-grade tank body, and the like, and finally discharging a feed liquor which has the same volume and is treated by virtue of a multi-stage anaerobic system from the top outlet of a last-grade tank body. The method is simple to operate, low in operating cost and capable of tolerating high organic load and also obviously degrading residual bioactive substances in the zymophyte dreg.
Owner:SHANGHAI INST OF PHARMA IND +1
Pediococcus acidilactici P3-4, screening and identification method thereof and application of pediococcus acidilactici P3-4 to degrading spiramycin
ActiveCN104946559AImproving the Efficiency of Treating Antibiotic PollutionHigh pollution efficiencyBacteriaContaminated soil reclamationMicroorganismScreening method
The invention discloses pediococcus acidilactici collected in the China General Microbiological Culture Collection Center (CGMCC), with the collection number of CGMCC No.10574. The pediococcus acidilactici has the technical effects that a new method for screening lactobacillus is established by adopting the mode of combining a microbiological method with a chemical method; lactobacillus with a spiramycin degrading function is screened from soil through preliminary screening and secondary screening of an antibiotic selective medium; a reasonable and effective screening method of a spiramycin degrading strain is established to obtain lactobacillus meeting the needs; the safe and effective spiramycin degrading strain is screened through separation, purification and screening of lactobacillus and preliminary screening and secondary screening of spiramycin degrading lactobacillus; when applied to control over spiramycin pollution, the pediococcus acidilactici has the advantages of no secondary pollution, high treatment efficiency, wide range of application and low cost; the pediococcus acidilactici provides more choices for microbiological control over antibiotic pollution of complex environments.
Owner:山东中创健康科技集团有限公司
Ureaplasma urealyticum/mycoplasma hominis combined rapid culture and drug sensitivity detection kit
InactiveCN104988206AResolve detectionResolve accuracyMicrobiological testing/measurementMicroorganism based processesPenicillinArginine
Owner:姜洪波 +1
A kind of preparation method and application of macrolide antibiotic hollow porous molecularly imprinted polymer
InactiveCN107722178BHigh selectivityReduce dosageOther chemical processesFunctional monomerMesoporous silica
The invention discloses a high-selectivity hollow porous molecularly imprinted polymer for measuring amount of trace macrolide antibiotics residues in animal-derived foods and a preparation method thereof. The preparation method comprises the following steps of using spiramycin as a template molecule, using methacrylic acid as a functional monomer, and using MCM-41 mesoporous silica gel as a sacrifice support template; adopting a thermal induced polymerization method, so as to obtain the hollow porous molecularly imprinted polymer with a memory function on a stereo structure of the macrolide antibiotics. The high-selectivity hollow porous molecularly imprinted polymer is used as a dispersing solid-phase extracting adsorbent, and is used for enriching and selectively separating macrolide antibiotics medicines; by combining with HPLC-MS (high performance liquid chromatography-mass spectrometry) / MS detection, the better effect is obtained. The preparation method is simple. The prepared hollow porous molecularly imprinted material has the advantages that the chemical and mechanical properties and thermal stability are good, the extraction capacity is high, the mass transfer speed is quick, the selectivity is good, the service life is long, and the broad application prospect is realized in the fields of analytical chemistry and environmental analysis.
Owner:CHINA PHARM UNIV
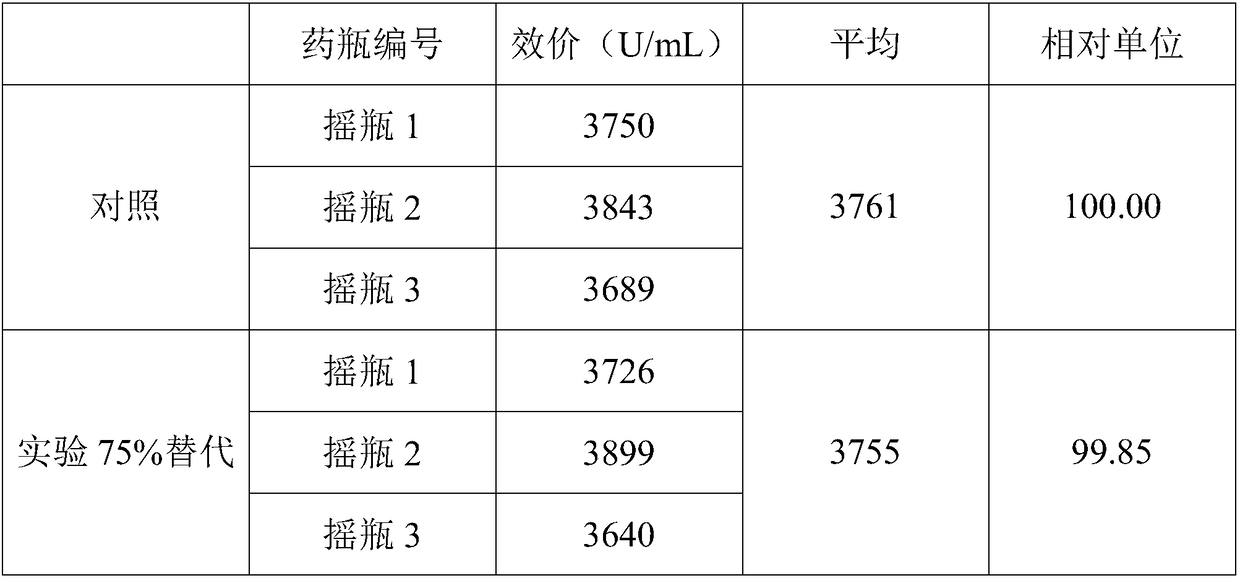
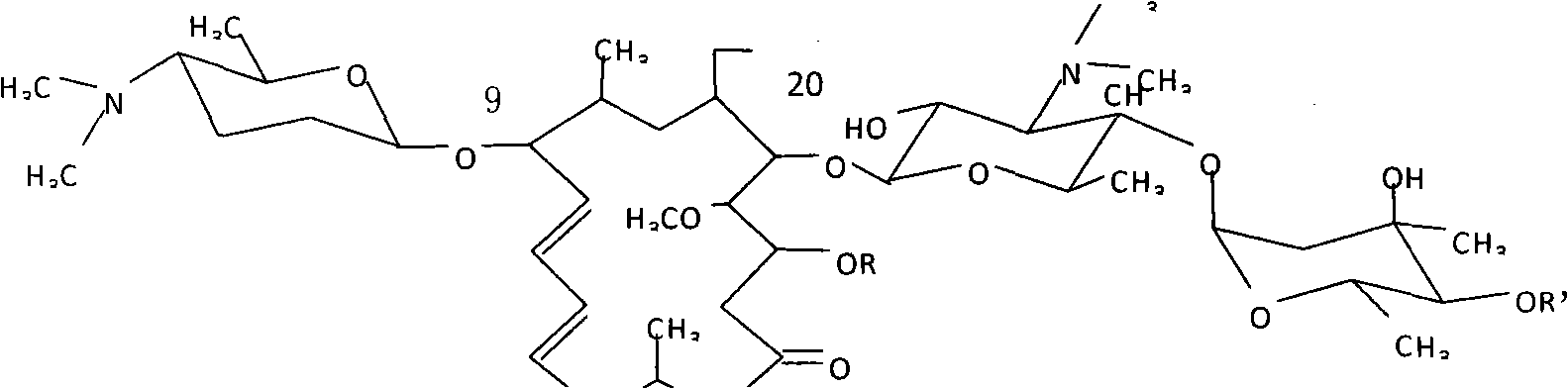
Gene engineering bacteria containing high isovaleryl spiramycin principal component
The invention relates to gene engineering bacteria containing a high-antibiotic content principal component constructed by utilizing a regulator gene. The gene engineering bacteria are products that an acyB2 regulator gene and an isovaleryl transferase gene ist are linked and co-expressed in a spiramycin producing strain. Experimental study shows that the strain can obviously improve the ratio ofthe principal component isovaleryl spiramycin by 213 to 232 percent and provide important guarantee for industrial production and clinical application.
Owner:SHENYANG TONGLIAN GRP CO LTD
Advertising art design wrapping paper printing ink
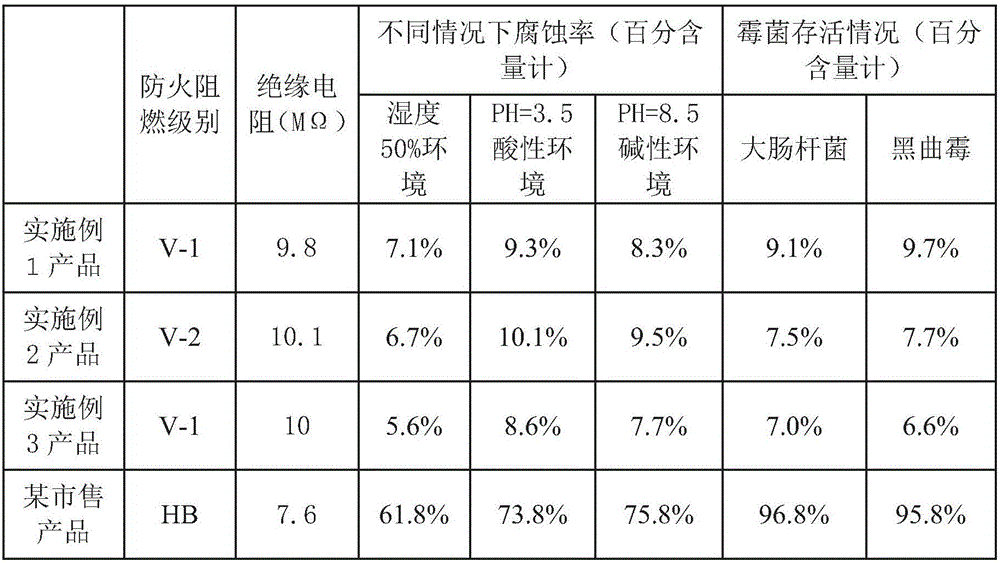
InactiveCN105949877AThe preparation process is simpleImprove flame retardant performanceInksPhosphatePotato starch
The invention relates to an advertising art design wrapping paper printing ink which is composed of trioctyl trimellitate, zinc oxide, alkyl benzene sulfonate, carbon black, triethanolamine, a polyamide resin, triethylenetetramine, diethyl fumarate, chitin, isothiazolinone, decadebromodiphenylethane, coumarone, polyvinylpyrrolidone, acetyl spiramycin, basalt powder, shell powder, guanylurea phosphate, titanium dioxide powder, diethylenetriamine, chlorinated polyethylene, polydimethylsiloxane, potato starch, urea, disodium hydrogen phosphate, sodium taurate, sodium hypochlorite, methyl methacrylate, itaconic acid, sodium sulfamate, acrylate and water. The advertising art design wrapping paper printing ink is simple in preparation technique. The product has the advantages of favorable flame retardancy, favorable insulating property, acid / alkali resistance, favorable mildew resistance and favorable antibacterial property.
Owner:王义金
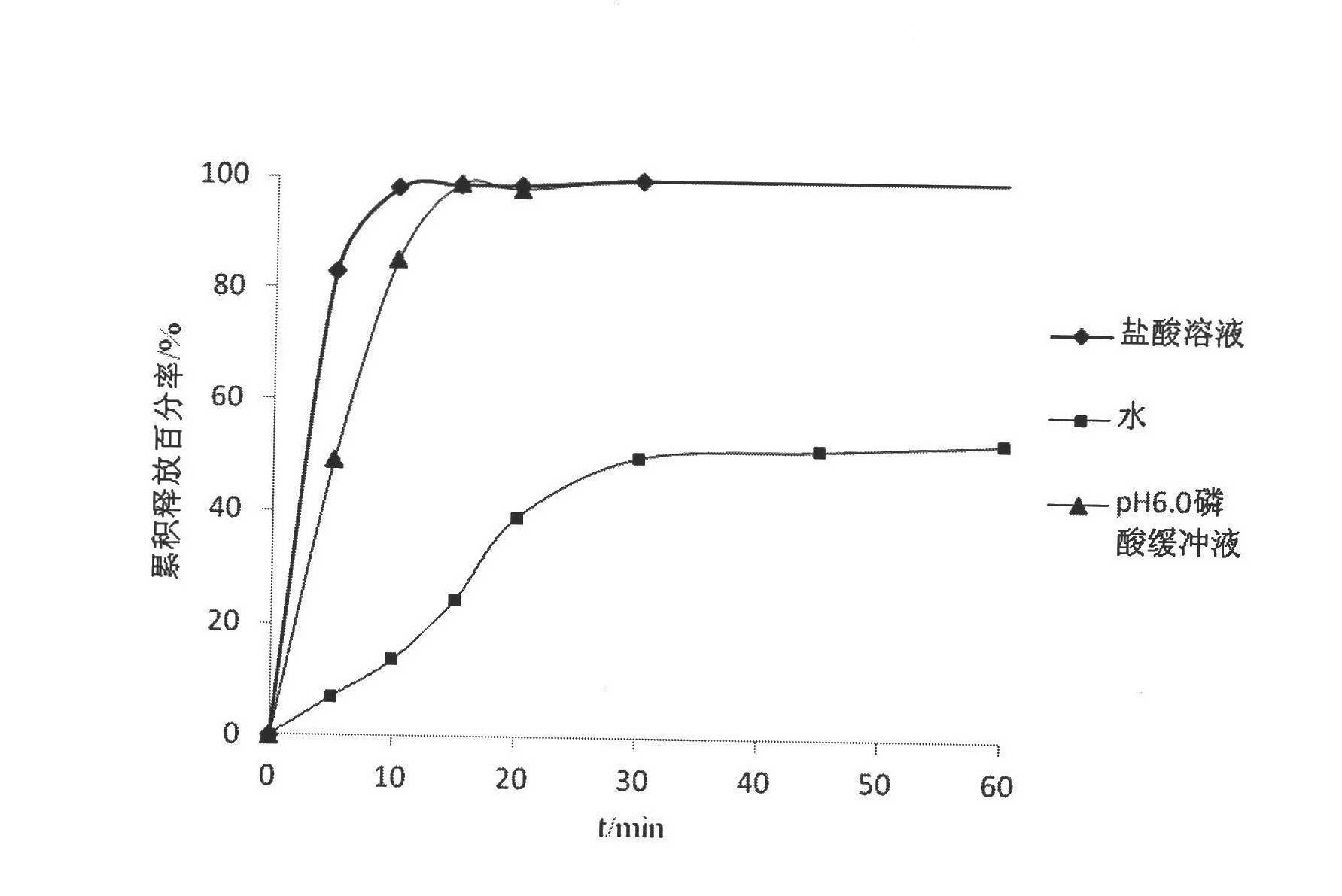
Method for removing spiramycin in water in nanofiltration mode
InactiveCN103130304AEfficient removalWater/sewage treatment bu osmosis/dialysisEnvironmental engineeringNanofiltration
The invention relates to a method for removing spiramycin in water in a nanofiltration mode. By means of the characteristic that the nanofiltration membrane separation technique can be used for effectively removing 200-1000 organics, the method for removing the spiramycin in water in a nanofiltration mode can well retain spiramycin with more than 800 molecular weight in water. According to the method for removing the spiramycin in water in a nanofiltration mode, spiramycin solutions with different concentrations are treated as research objects, different nanofiltration membranes are selected, the operating pressure and the operation temperature are changed, a nanofiltration separation performance test is carried out, and the research on the removal of the spiramycin is investigated. The method for removing the spiramycin in water in a nanofiltration mode provides a feasible control technology for effectively removing the spiramycin in water, and has wide application prospects.
Owner:RES CENT FOR ECO ENVIRONMENTAL SCI THE CHINESE ACAD OF SCI
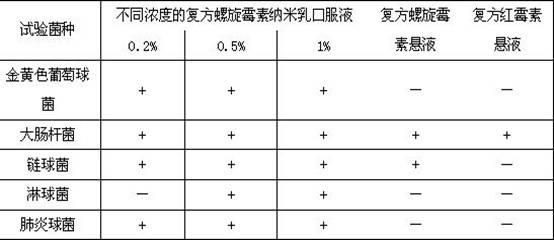
Compound spiramycin nanoemulsion oral liquid and preparation method thereof
InactiveCN102499935AIncrease fat solubilityReduce intakeAntibacterial agentsOrganic active ingredientsTrimethoprimumTrimethoprim
The invention discloses a compound spiramycin nanoemulsion oral liquid. The particle size of the oral liquid is 1-100nm, and the compound spiramycin nanoemulsion oral liquid comprises the following raw materials in percentage by weight: 15%-35% of surfactant, 3.95%-17.5% of cosurfactant, 0.2%-10% of oil phase, 0.2%-10% of spiramycin, 0.05%-2.5% of trimethoprim and the balance of deionized water, and the sum of the percentages by weight of all the raw materials is 100%. The content of the spiramycin in the nanoemulsion oral liquid can be up to 0.20%-10.00%, the appearance of the compound spiramycin nanoemulsion oral liquid is of colorless or light yellow transparent liquid, the bioavailability of the spiramycin can be significantly improved, and a strong in-vivo antibacterial effect and anantibacterial after effect can be further played. Simultaneously, the stability of a spiramycin medicament is improved, the shortcoming of bitter taste during oral administration of the spiramycin issolved, and the compound spiramycin nanoemulsion oral liquid further has the advantages of high safety, simple preparation and low energy consumption.
Owner:NORTHWEST A & F UNIV
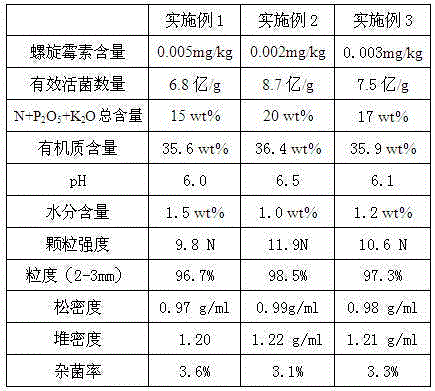
Deep-ploughing-free fertilizer with spiramycin fermentation residues and preparation method thereof
ActiveCN105016897AImprove breathabilityImprove water retentionFertilizer mixturesCrosslinked chitosanActivated sludge
The invention provides deep-ploughing-free fertilizer with spiramycin fermentation residues and a preparation method thereof. The deep-ploughing-free fertilizer comprises the spiramycin fermentation residues, chicken manure, straw powder, TPA polymerization peptide, activated sludge, ethylene glycol diglycidyl ether crosslinked chitosan, scenedesmus obliquus, the root bark of white mulberry, coptis chinensis extract, organic carbon, kieselguhr, sepiolite, phenyl phosphonic acid and fermented compound inoculants. The deep-ploughing-free fertilizer with the spiramycin fermentation residues has the advantages that the spiramycin content is 0.002-0.005 mg / kg, the number of effective living bacteria is 0.68-0.87 billion / g, the total content of N+P2O5+K2O is 15-20wt%, the organic content is 35.6-36.4wt%, pH is 6.0-6.5, and the moisture content is 1.0-1.5wt%. The deep-ploughing-free fertilizer can achieve deep scarification of soil, increase soil breathability and water retaining capacity and improve crop output and nutrient content.
Owner:SHANDONG WOKEQI IND CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com