Levorotary isovaleryl spiramycin II as well as preparation, preparation method and application thereof
A technology of L-isovalerylspiramycin and Isovalerylspiramycin, which is applied in the field of macrolide genetic engineering new antibiotics, and can solve the problems that it is difficult to meet the quality control standards of chemicals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Example 1 Separation and preparation of L-isovalerylspiramycin II
[0085] (1) Biological fermentation: the helicinase-producing bacteria clone strain WSJ-195 containing 4″-isovaleryltransferase gene was added to soybean meal containing 2%, glucose 1%, starch 3%, CaCO 3 On a slant medium of 0.5%, 0.4% NaCl, and 2% agar, culture for 8-15 days at pH 6.5-7.5, temperature 28-38°C, and inoculate it with 1.5% soybean meal powder, 3.0% starch, NaCl 0.4%, CaCO 3 0.5%, fish peptone 0.3% and KH 2 PO 4 0.05% seed culture medium, cultured at pH 6.5~7.5, 25~30℃ for 40~80 hours, inoculated with 0.1~20% inoculum containing 0.5% glucose, 6.0% starch, 0.5% yeast powder, Fish meal 2.0%, NH 4 NO 3 0.6%, NaCl 1.0%, CaCO 3 0.5%, KH 2 PO 4 0.05%, MgSO 4 Fermentation medium containing 0.1%, 0.5% soybean oil and 0.02% defoamer is cultured for 72-120 hours at pH 6.5-7.5 and 26-30°C to obtain fermentation broth;
[0086] Among them, through the adjustment and optimization of culture and ferment...
Embodiment 2
[0098] Example 2: Separation and preparation of L-isovalerylspiramycin II
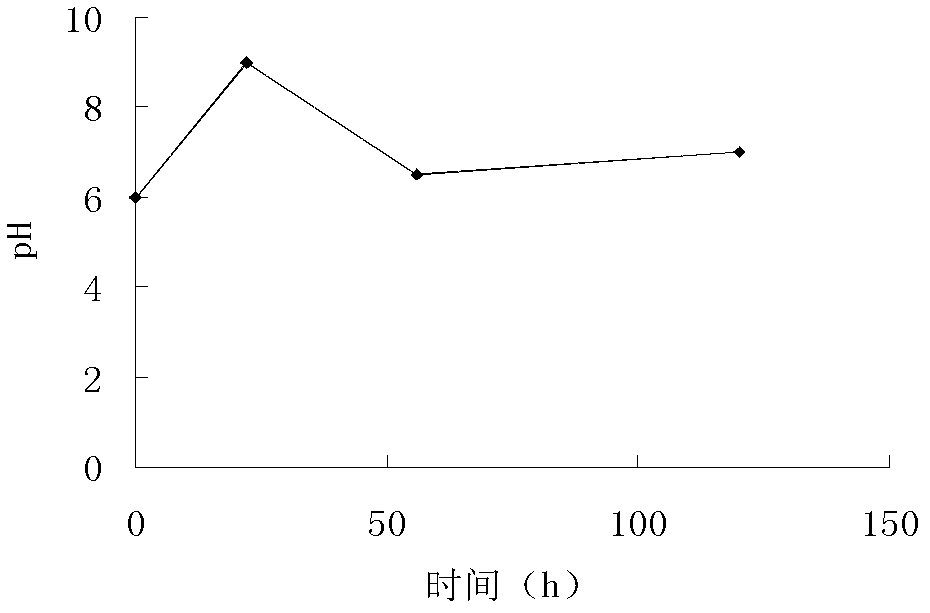
[0099] (1) Biological fermentation: the helicinase-producing bacteria clone strain WSJ-195 containing 4″-isovaleryltransferase gene was used to contain soybean meal 2%, glucose 1%, starch 3%, CaCO3 0.5%, NaCl On the slant medium of 0.4% and 2% agar, cultured for 12 days at pH 7.2 and temperature 32°C, inoculated into soybean meal containing 1.5% starch, 3.0% starch, 0.4% NaCl, and CaCO 3 0.5%, fish peptone 0.3% and KH 2 PO 4 A 0.05% seed culture medium was cultured at pH 7.2 and 27°C for 70 hours, and 12% inoculum was seeded with 0.5% glucose, 6.0% starch, 0.5% yeast powder, fish meal 2.0%, NH4NO3 0.6%, Fermentation medium containing NaCl 1.0%, CaCO3 0.5%, KH2PO4 0.05%, MgSO4 0.1%, soybean oil 0.5% and defoamer 0.02%, cultured for 100 hours under the conditions of pH 6.0~9.0 and 26℃ to obtain fermentation broth ; Fermentation is carried out under the condition of pH 6.0~8.0, the fermentation time is 110h...
Embodiment 3
[0111] Example 3 Separation and preparation of L-isovalerylspiramycin II
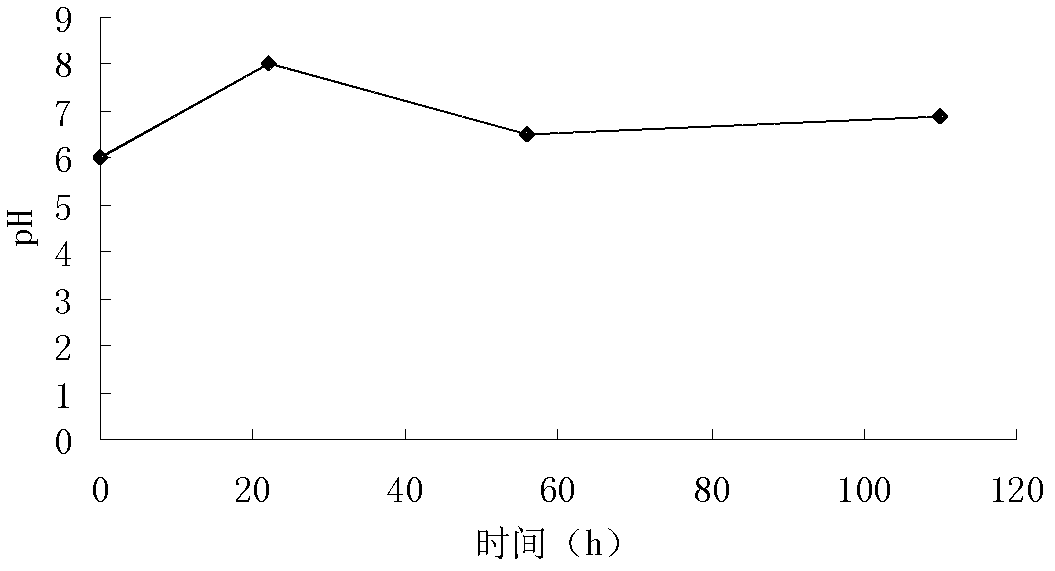
[0112] (1) Cultivation and fermentation: the spiramycin-producing bacteria clone strain WSJ-195 containing the 4″-isovaleryltransferase gene is cultivated on a slant medium, and then inoculated into a seed medium. After cultivation, the It is inoculated into the fermentation medium, and the fermentation process is controlled by glucose and citric acid. The fermentation is carried out under the condition of pH 6.0-7.5. The fermentation time is 115h, and the pH curve with time shows three consecutive stages. One stage satisfies the equation y 1 =0.0682x 1 +6.0, where 01 2 = -0.0294x 2 +8.147, where 223 =0.0078x 3 +6.06, where 563 Figure 4 , Obtain the fermentation broth.
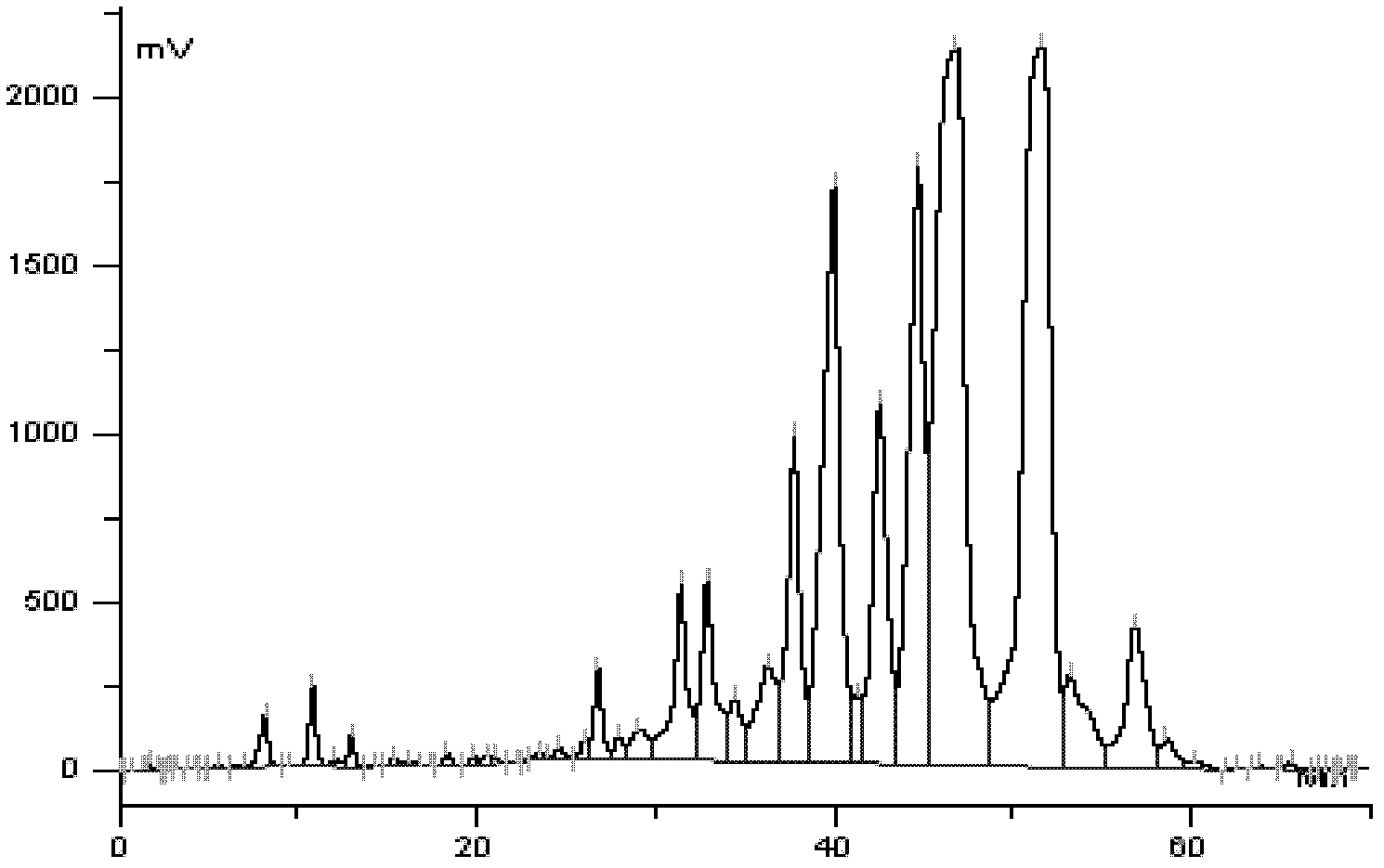
[0113] (2) Extraction of biological fermentation broth: the fermentation broth obtained in step (1) is treated with aluminum sulfate to obtain the filtrate, adjusted to pH 8.6, and extracted with butyl acetate, and the butyl acetate extrac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific rotation | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| optical rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com