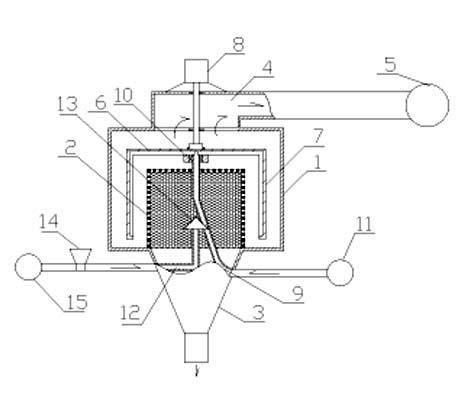
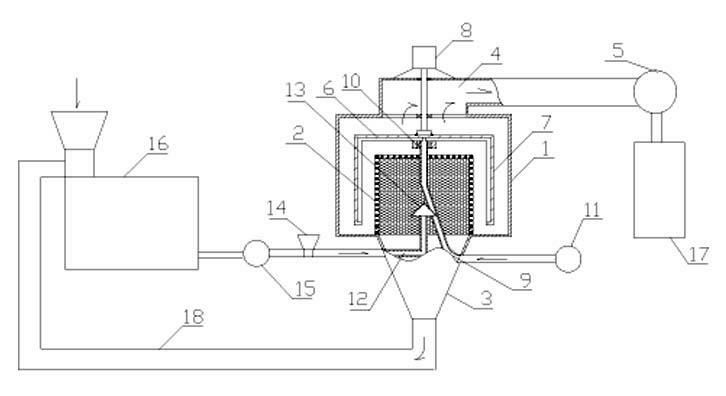
Patents
Literature
119results about How to "Quality standards are easy to control" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Separation and preparation of isovaleryl-spiramycin I and application thereof
InactiveCN101785778ASimple production processQuality standards are easy to controlAntibacterial agentsOrganic active ingredientsAntibiotic YAntibacterial activity
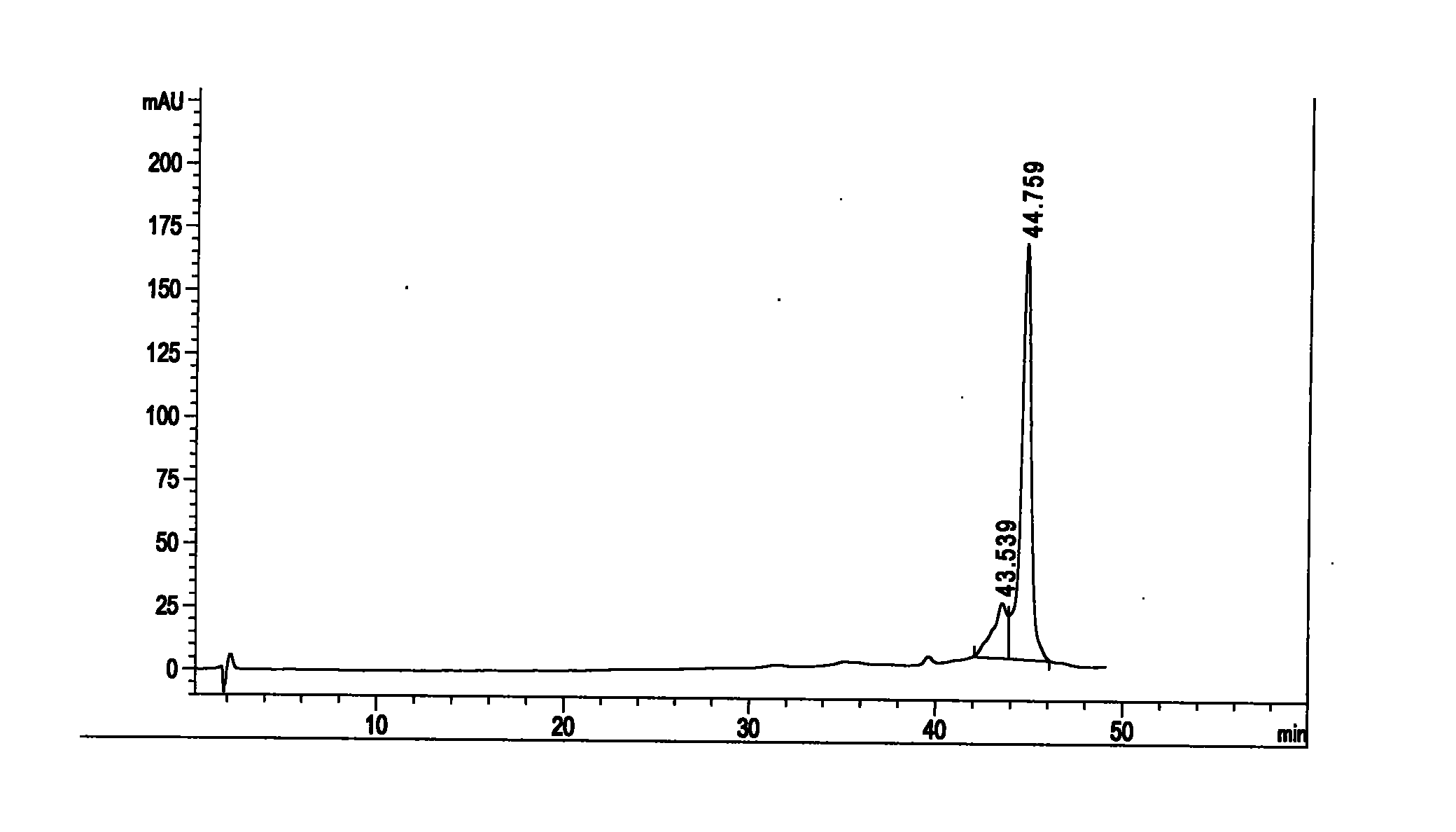
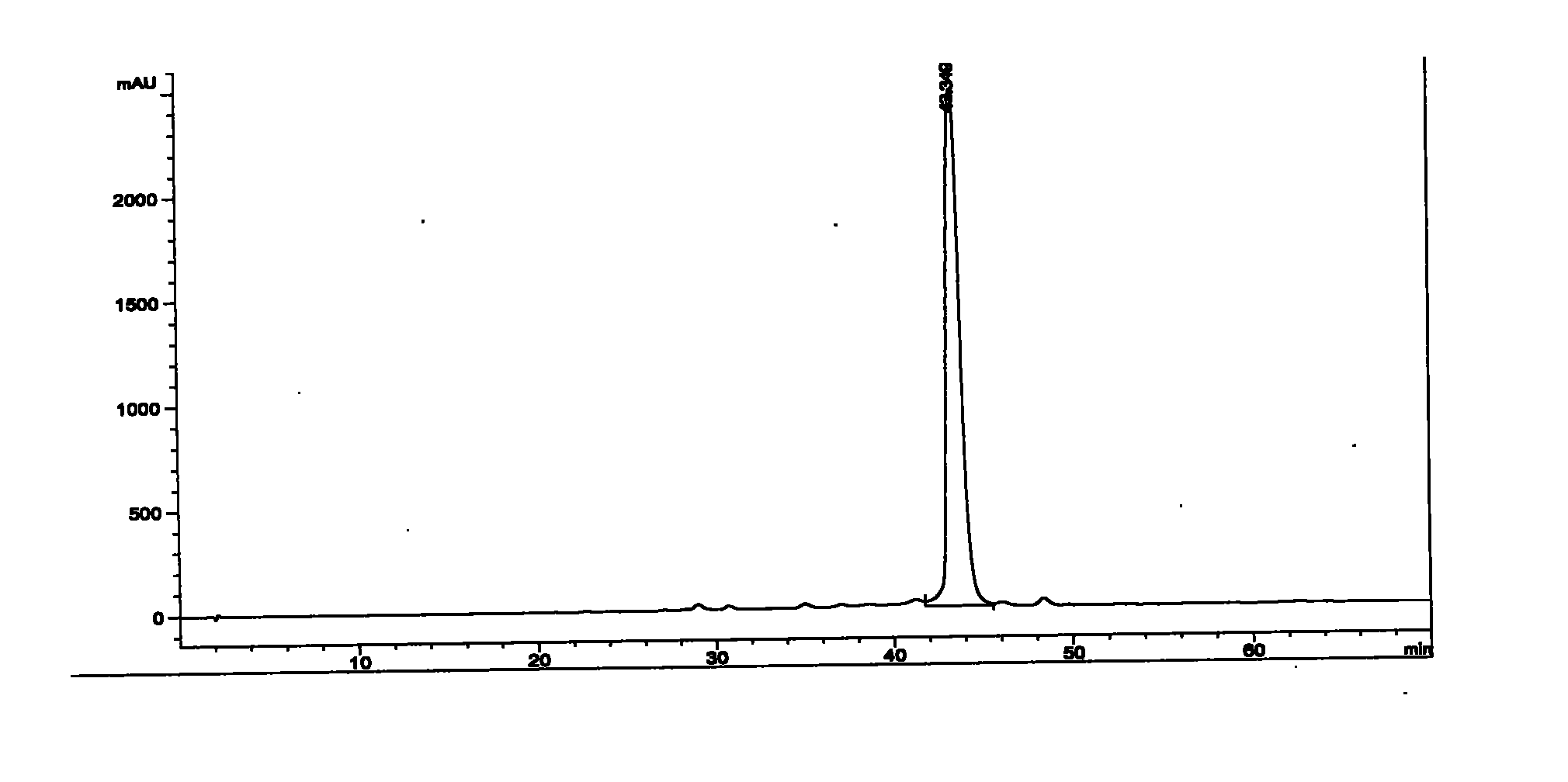
The invention relates to the separation of antibiotic and the application of the antibiotic in resisting infectious diseases, in particular to an isovaleryl-spiramycin I single-component compound. A kelimycin sample is separated by HPLC (high performance liquid chromatography) after crude separation, high-efficiency purification and post-processing, so as to obtain the pure isovaleryl-spiramycin I compound. Biological experiment results show that the antibacterial activity of the isovaleryl-spiramycin II compound is better than that of kelimycin and is much better than that of a control group. The invention lays the foundation for developing the clinical and effective isovaleryl-spiramycin II single-component antibiotic.
Owner:SHENYANG TONGLIAN GRP CO LTD
Normal-temperature preparation method for ultrafine coccidia powder and special bilateral airflow sieving machine thereof
ActiveCN102512514AImprove smellGreat tastePowder deliveryGas current separationChinese cinnamonEngineering
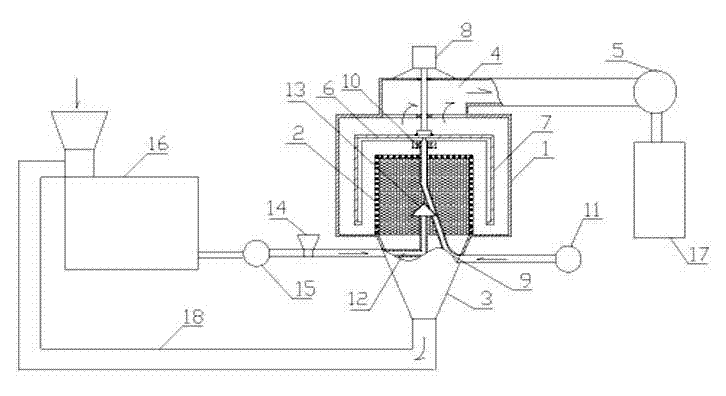
The invention discloses a normal-temperature preparation method for ultrafine coccidia powder. The method comprises the following steps of: selecting decoction pieces of sweet wormwood, hairyvein agrimony, tuber fleeceflower root, Chinese pulsatilla root and cinnamon according to parts by weight, drying and smashing to obtain coarse powder of which the granularity is 80 meshes; putting the obtained coarse powder into a rod mill for smashing, feeding into the special bilateral airflow sieving machine through wind for sieving with a 500-mesh sieve, sieving to obtain power of which the particle diameter is less than or equal to 25 micrometers, conveying the powder which is obtained by sieving into a cyclone aggregator through airflow generated by a draught fan for collecting to obtain finished ultrafine coccidia powder; and collecting powder which is not sieved with the 500-mesh sieve with a funnel, and conveying to the rod mill through a pipeline for smashing circularly once again. The method has the advantages that: the entire preparation process is performed at the normal temperature without low temperature or special additional conditions; the particle diameter of a prepared finished medicament is less than or equal to 25 micrometers, and the cell-wall breaking rate and the biological availability are greatly increased; and due to the adoption of the bilateral airflow sievingmachine, the preparation process of the ultrafine coccidia powder is simplified, and the aim of controlling the quality standard of Chinese medicinal powder is fulfilled.
Owner:河南省康星常笑动物药业有限公司
Submicron powder bazheng powder normal temperature preparation method and special bidirectional airflow sieving machine thereof
ActiveCN102512545AImprove smellGreat tastePowder deliveryGas current separationPolygonum aviculareMetallurgy
The invention discloses a submicron powder bazheng powder normal temperature preparation method, which comprises choosing technical materials of akebiaquinata, dianthus superbus, polygonum aviculare, liquorice, fried jasmine, rheum officinale, juncos communis medicinal slices, soapstone and plantain seed raw materials in proportion; drying and smashing to obtain coarse powder; extracting plantainseed oil in the plantain seed coarse powder to be reserved; evenly mixing the rest of coarse powder and the plantain seed dregs in a rod mill to be smashed to be sent into a special bidirectional airflow sieving machine for 500 sieves to obtain powder materials with the grain diameter equal to or smaller than 25 micrometers; achieving bazheng powder submicron powder by collecting powder materialsconveyed to a cyclone material collector by air airflow generated by an air induced fan; collecting the powder material not sieved for 500 sieves to be conveyed into the rod mill through a pipeline to be smashed repeatedly; and evenly mixing the collected bazheng powder submicron powder and the plantain seed oil to obtain submicron powder bazheng powder end products. The submicron powder bazheng powder normal temperature preparation method has the advantages of performing the whole process at the normal temperature. The special bidirectional airflow sieving machine simplifies preparation process of the submicron powder bazheng powder.
Owner:HENAN KANGXING PHARMA
Colloid bismuth pectin compound and quality control method of pharmaceutical compositions thereof
InactiveCN102507381AStrong selective adhesionGood selective adhesionMaterial analysis by observing effect on chemical indicatorDirect flow property measurementClinical efficacyBismuth / pectin
The invention relates to a colloid bismuth pectin compound and a quality control method of pharmaceutical compositions of the colloid bismuth pectin compound, and specifically provides a colloid bismuth pectin compound, new quality control indexes of pharmaceutical compositions of the colloid bismuth pectin compound and a detection method of the colloid bismuth pectin compound and the pharmaceutical compositions of the colloid bismuth. In the prior art, the quality of products having poor efficacy can not be well controlled because the efficacy-related indexes are not controlled strictly in the existing quality control methods of colloid bismuth pectin and pharmaceuticals thereof. Based on the existing quality control methods of colloid bismuth pectin and pharmaceuticals thereof, the following detection items and indexes are added: intrinsic viscosity, gel property, uniformity and galacturonic acid content. The invention can effectively ensure clinical efficacy of products, make the product quality standards more scientific, reasonable and controllable, and has great significance in quality control of the colloid bismuth pectin and the pharmaceutical preparation of colloid bismuth pectin.
Owner:于学敏
Separation and preparation of isovaleryl-spiramycin II and application thereof
ActiveCN101785779ASimple production processQuality standards are easy to controlAntibacterial agentsOrganic active ingredientsAntibiotic YAntibacterial activity
The invention relates to the separation of antibiotic and the application of the antibiotic in resisting infectious diseases, in particular to an isovaleryl-spiramycin II single-component compound. A kelimycin sample is separated by HPLC (high performance liquid chromatography) after crude separation, high-efficiency purification and post-processing, so as to obtain the pure isovaleryl-spiramycin II compound. Biological experiment results show that the antibacterial activity of the isovaleryl-spiramycin II compound is better than that of kelimycin and is much better than that of a control group. The invention lays the foundation for developing the clinical and effective isovaleryl-spiramycin II single-component antibiotic.
Owner:SHENYANG TONGLIAN GRP CO LTD
Method for separating effertive components of Chinese medicine by adopting membrane technique
InactiveCN1476892AAvoid destructionReduce lossesOrganic compounds purification/separation/stabilisationUnknown materialsMembrane technologyUltrafiltration
The method for separating effective component of Chinese medicine by using membrane technology is characterized by that firstly, the liquid medicine can be filtered to remove impurity component, thenaccording to the molecular mass of effective component of Chinese medicine required for separation and extraction from liquid medicine, from large to small, the ultrafiltration membrane and nanofiltration membrane can be selected to make one stage or multistage, stage-to-stage entrapment of effective component of Chinese medicine so as to implement separation of effective component of Chinese medicine.
Owner:范兆科
Chinese medicinal composition for clearing heat, dispersing phlegm, ventilating the lung and relieving asthma and process for preparing the same
ActiveCN1733110AProlonged drug-induced asthma incubation periodSignificant antiasthmatic effectPowder deliveryPill deliveryMedicineHouttuynia
The invention relates to a Chinese medicinal composition for clearing heat, dispersing phlegm, ventilating the lung and relieving asthma and process for preparation, wherein the composition comprises Chinese ephedra, licorice root, bitter apricot seed, radish seeds, lepidium seed, purple perilla seed, baikal skullcap root, mulberry bark, clerodendroa cyrtophyllum, cordate houttuynia and gypsum.
Owner:RONGCHANG PHARM ZIBO CO LTD
Levo-isovaleryl spiramycin I and preparation, preparation method and application thereof
ActiveCN102229634AImprove antibacterial propertiesImprove pharmacological activityAntibacterial agentsOrganic active ingredientsDiseaseFreeze-drying
The invention relates to levo-isovaleryl spiramycin I and a preparation, a preparation method and application thereof. The preparation consists of the levo-isovaleryl spiramycin I and pharmaceutically acceptable carriers and / or excipient, wherein the purity of the levo-isovaleryl spiramycin I is over 90 weight percent, preferably over 95 weight percent and further preferably over 98 weight percent. The levo-isovaleryl spiramycin I has good antimicrobial activity; the preparation of the levo-isovaleryl spiramycin I comprises water injection, powder injection or freeze dried powder injection; the preparation fills the blank of the single component preparation of the isovaleryl spiramycin I at present market, and provides a new path with quick response for treating anti-infective diseases; and the production process for the preparation of single component of the isovaleryl spiramycin I is stable, standard and easily controlled in quality and suitable for large-scale industrialized production.
Owner:SHENYANG TONGLIAN GRP CO LTD
L-isovalerylspiramycin iii, its preparation, preparation method and application
ActiveCN102260308AImprove antibacterial propertiesImprove pharmacological activityAntibacterial agentsOrganic active ingredientsFreeze-dryingAntibacterial activity
The invention relates to levorotatory isovaleryl spiramycin III, and also relates to a preparation, a preparation method and application thereof. The preparation consists of the levorotatory isovaleryl spiramycin III and a pharmaceutically acceptable carrier and / or auxiliary materials, and the purity of the levorotatory isovaleryl spiramycin III is over 90 weight percent, preferably over 95 weight percent and further preferably over 98 weight percent. The levorotatory isovaleryl spiramycin III has good antibacterial activity; the preparation of the levorotatory isovaleryl spiramycin III comprises water injection for injection, powder injection for injection and freeze-dried powder injection, fills up a blank of a single-component preparation of the isovaleryl spiramycin III in the current market and provides a new quick-response way for treating infectious diseases. The single-component preparation of the isovaleryl spiramycin III has the advantages of stable production process, easily controlled quality standard, and suitability for large-scale industrial production.
Owner:SHENYANG TONGLIAN GRP CO LTD
Method for processing sauced marinated duck
The invention relates to a method for processing a sauced marinated duck, and the method comprises the steps of taking a fresh duck or a duck embryo as a raw material, carrying out rapid salinization for a certain time by adopting the high pressure method, boiling for 20-30 minutes in marinade which is prepared by soy sauce, fine salt, liquor and white sugar, further boiling for 100-120 minutes in the marinade juice which is prepared by adding ginger, five spice powder, pepper and dried chilli in the marinade, fishing out, draining, cooling and then adopting UV irradiation for sterilization. The sauced marinated duck is pure gold, bright, fresh, tender and delicious, thereby being applicable to the requirements of scale production.
Owner:JIANGXI HUANGSHANGHUANG GROUP FOOD
General flavanone capsule of desmodium styracifolium and preparation method and application thereof
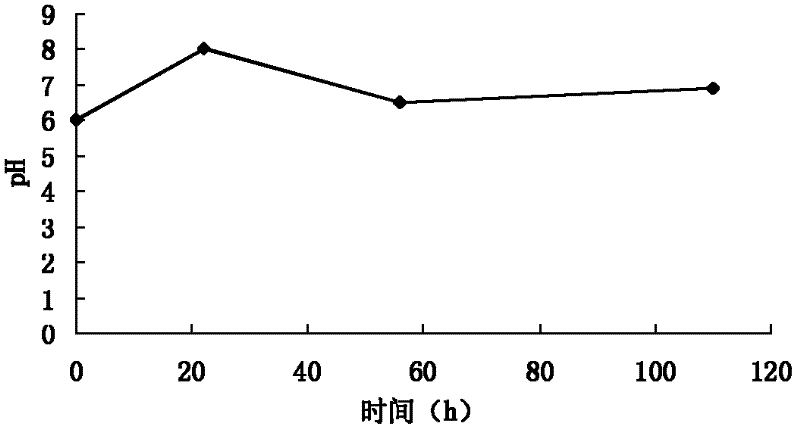
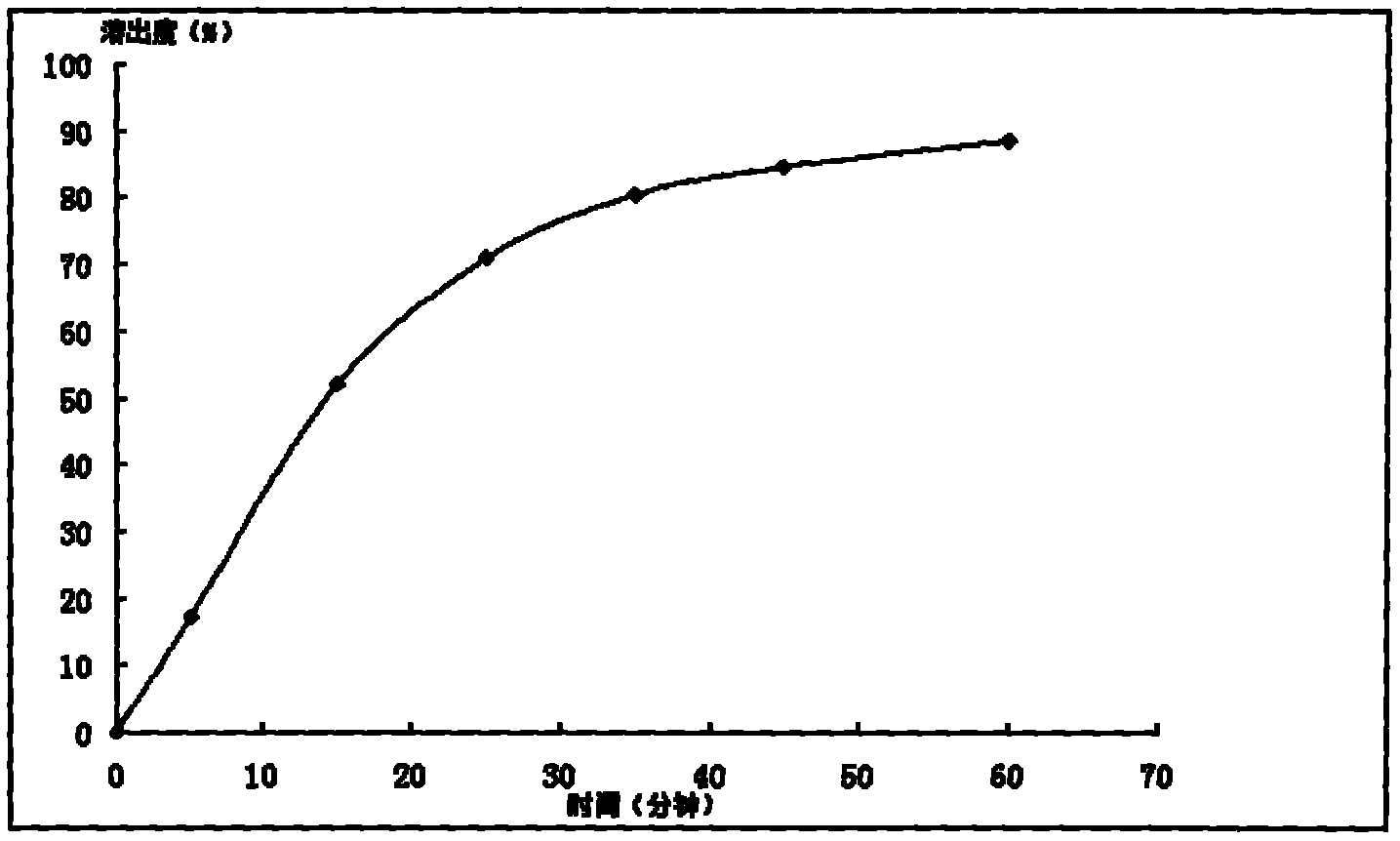
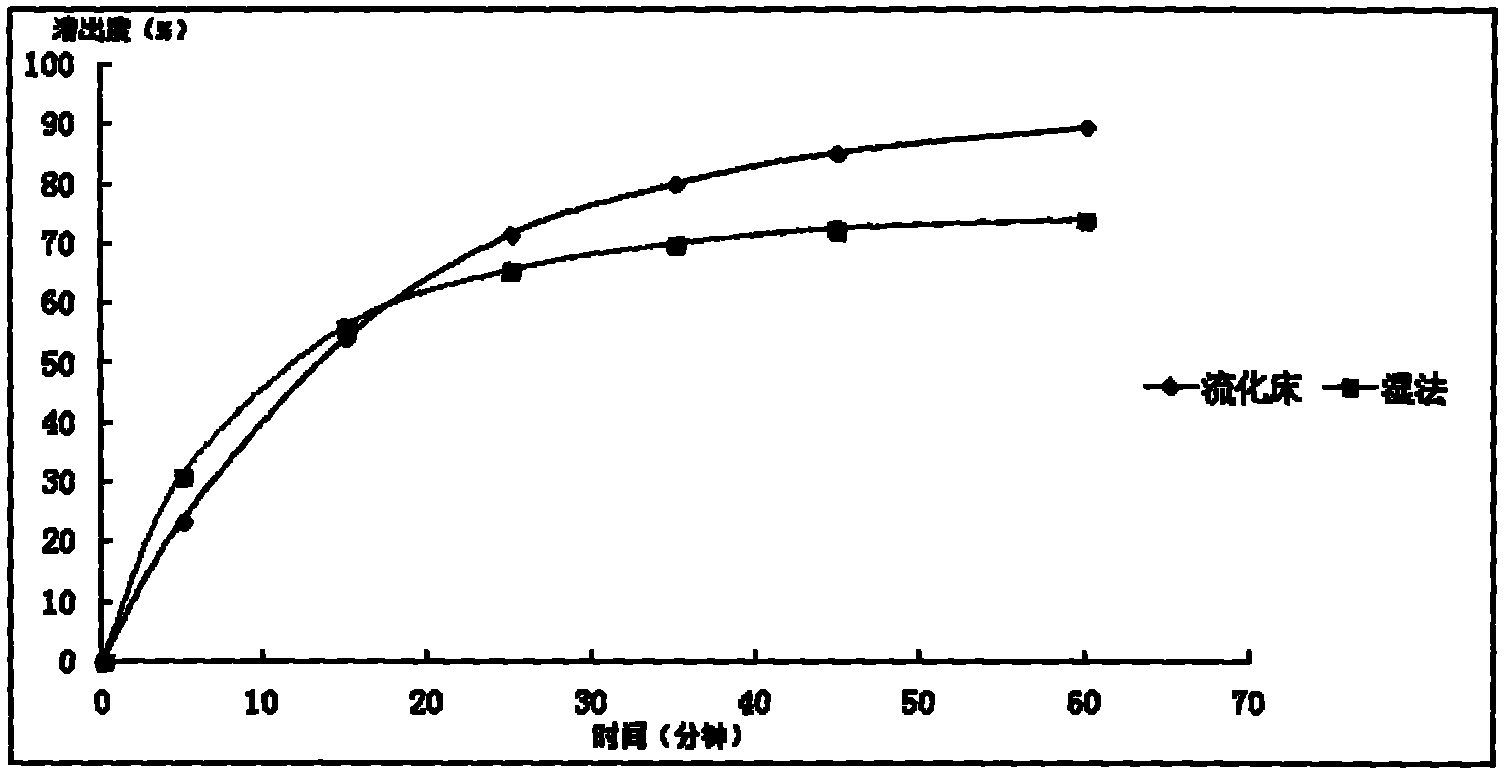
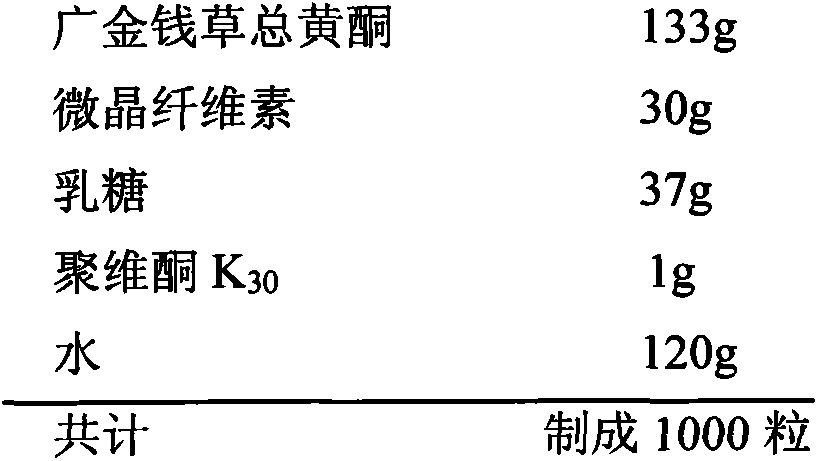
InactiveCN103893246AHigh dissolution rateGood quality and stabilityUrinary disorderGranular deliveryAlcoholMedicine
The invention provides a general flavanone capsule of desmodium styracifolium and a preparation method and application thereof, wherein the general flavanone capsule of the desmodium styracifolium comprises total flavanone of the desmodium styracifolium, which is an alcohol extract of the desmodium styracifolium, and pharmaceutically acceptable medicinal excipients. The general flavanone capsule of the desmodium styracifolium disclosed by the invention has the characteristics of being explicit in effective material basis, controllable in quality standard, good in drug dissolution degree, good in quality stability, significant in pharmacology and drug efficacy, less in dosage, safe and convenient to take, and completely applicable to industrial massive production.
Owner:HUMANWELL HEALTHCARE GRP +1
Medicine for external application in orthopedics, production method and application method thereof
InactiveCN101983710AWide range of medicinesLow pricePowder deliveryAerosol deliveryShiny bugleweedRhizome
A medicine for external application in orthopedics, a production method and an application method thereof. The external application in orthopedics is composed of following raw materials by weight: 4-10 parts of rhubarb root and rhizome, 3-7 parts of amur cork-tree bark; phellodendron bark, 4-8 parts of scutellaria root, 2-8 parts of angelica root, 4-8 parts of garden burnet root, 5-7 parts of red peony root, 4-8 parts of tree peony bark, 4-8 parts of Cape jasmine fruit, 3-9 parts of Safflower, 3-7 parts of hirsute shiny bugleweed herb, 4-8 parts of Chinese angelica, 2-6 parts of combined spicebush root, 2-6 parts of nutgrass galingale rhizome, 3-9 parts of corydalis tuber, 3-9 parts of turmeric root tuber, 2-6 parts of Himalayan teasel root, and 2-6 parts of notoptetygium root. The raw materials are prepared to external application powder in traumatology, external application ointment in orthopedics or external application hot application powder in orthopedics. Three medicines are combined for treatment in different dosage forms according to different time sequences in early stage, middle stage and late stage of patient's condition of fracture. The invention can rapidly treat the fracture, having an effective rate of 100%; the medicine is flexible to administrate, has strong targeting function, is able to effectively achieve the fracture position and better plays effects, thus greatly reducing unfavourable reactions of medicine, preventing waste of the medicine resource and shortening period of healing of fracture.
Owner:株洲市中医伤科医院
Submicron powder Jinfang Baidu San normal temperature preparation method and special two-way airflow screen grader
ActiveCN102397370AImprove smellGreat tastePowder deliveryAntiviralsHeracleum hemsleyanumSaposhnikovia
The invention discloses a submicron powder Jinfang Baidu San normal temperature preparation method, which comprises the following steps that: herba schizonepetae, divaricate saposhnikovia root, notopterygium root, heracleum hemsleyanum michaux, radix bupleuri, peucedanum root, bitter orange, tuckahoe, balloon flower, rhizoma ligustici wallichii, liquorice and field mint are selected, dried and pulverized into coarse powder, the coarse powder is uniformly blended according to a proportion and is placed into a rod mill to be pulverized, the pulverized powder is blown into a special two-way airflow screen grader to pass through a 500-mesh sieve, the powder passing the sieve and having the granularity being less than or equal to 25 micrometers is conveyed into a cyclone collector to collect by airflow produced by a draught fan to obtain the submicron powder Jinfang Baidu San finished product; and powders which cannot pass through the 500-mesh sieve are collected by a funnel and are conveyed into the rod mill through a pipeline to be circularly pulverized. The submicron powder Jinfang Baidu San has advantages that: the entire preparation process is carried out under the normal temperature, no low temperature or other special additional conditions are required; the granularity of the prepared finished product medicine is less than or equal to 25 micrometers, so the cell wall breaking rate and the bioavailability are greatly improved; and the two-way airflow screen grader utilized in the method can simplify the preparation process of the submicron powder Jinfang Baidu San.
Owner:HENAN KANGXING PHARMA
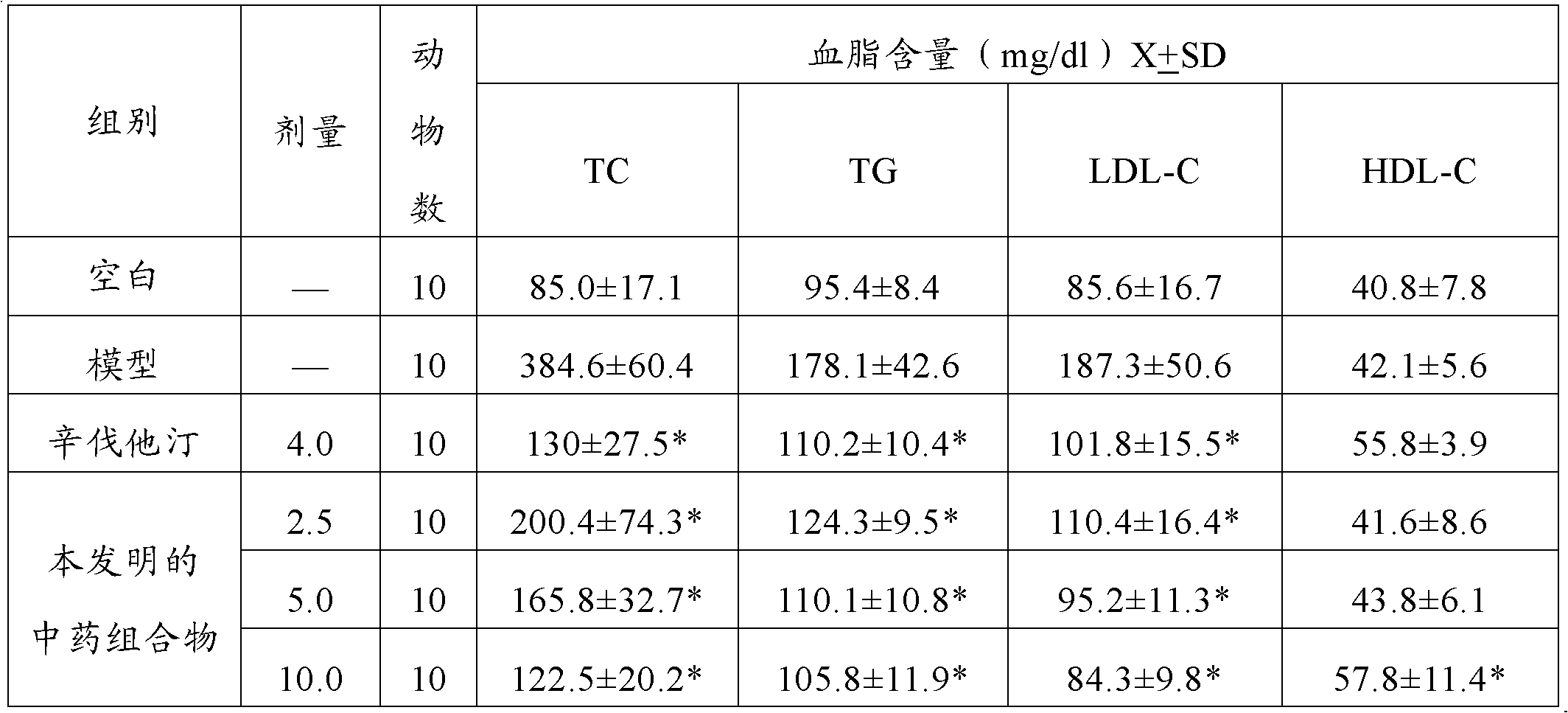
Hypolipidemic traditional Chinese medicine composition and preparation method and application thereof
ActiveCN103182009AMedication requirementsRaw materials are easy to getOrganic active ingredientsMetabolism disorderMedicineRhizome
The invention provides a hypolipidemic traditional Chinese medicine composition, and a preparation method and an application thereof. The traditional Chinese medicine composition comprises an extract of a mixture of oriental waterplantain tuber and largehead atractylodes rhizome. The composition is characterized in comprising 0.5-2wt% of 23-acetyl alisol B and 0.1-1wt% of atractylenolide B. The invention also provides a preparation method of the traditional Chinese medicine composition, an application of the traditional Chinese medicine composition in preparing hypolipidemic medicines, and medicines comprising the traditional Chinese medicine composition.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Levorotary isovaleryl spiramycin II as well as preparation, preparation method and application thereof
ActiveCN102311471AImprove antibacterial propertiesImprove pharmacological activityAntibacterial agentsOrganic active ingredientsFreeze-dryingAntibacterial activity
The invention relates to a levorotary sovaleryl spiramycin II, and also relates to a preparation, a preparation method and an application thereof. The preparation is composed of the levorotary sovaleryl spiramycin II and a pharmaceutically acceptable carrier and / or auxiliary material, and the purity of the levorotary sovaleryl spiramycin II is greater than 90wt%, preferably 95wt%, and further preferably 98wt%. The levorotary sovaleryl spiramycin II has good antibacterial activity; the preparation comprises a water injection, a powder injection, and a freeze-dried powder injection; the preparation provided by the invention fills up the blank of an isovaleryl spiramycin II individual component preparation in the markets at present, thereby providing a rapid-acting way for treating anti-infection diseases; and the isovaleryl spiramycin II individual component preparation provided by the invention has the advantages that the production process is stable, the quality standard is easy to control, and the preparation is suitable for large-scale industrial production.
Owner:SHENYANG TONGLIAN GRP CO LTD
Preparation method of exosome and stem cell proliferation reagent containing exosome
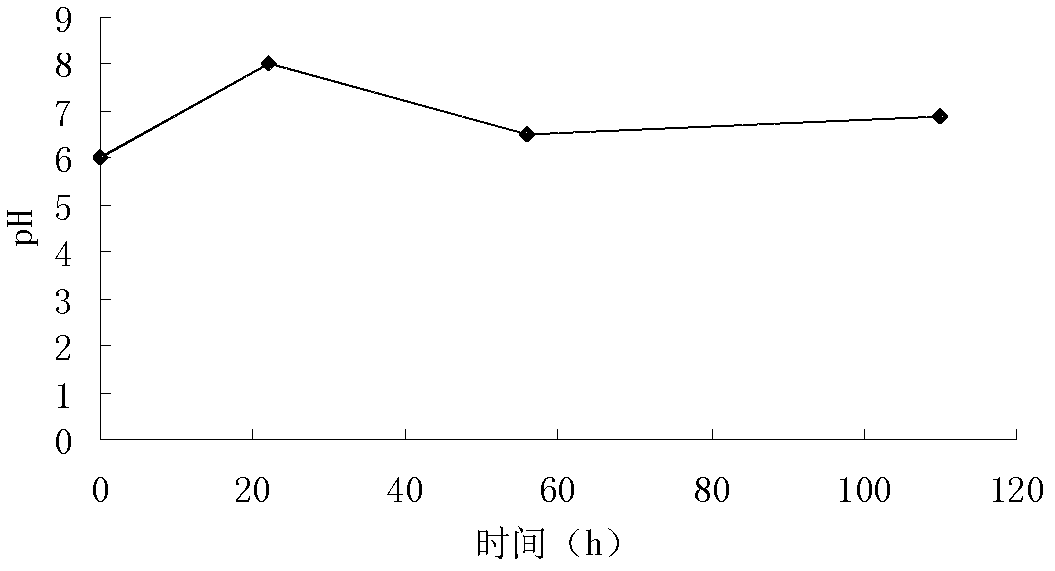
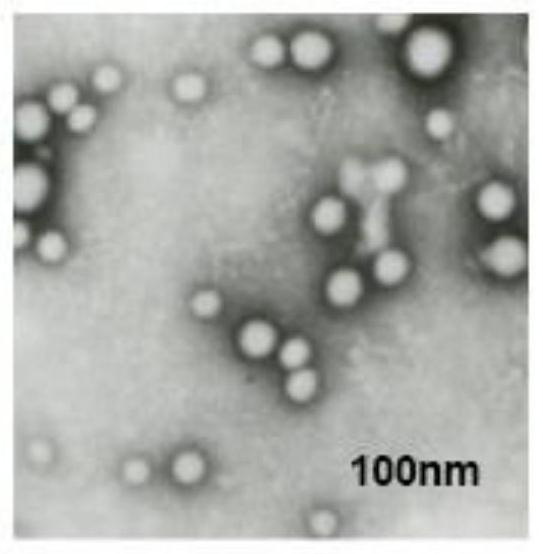
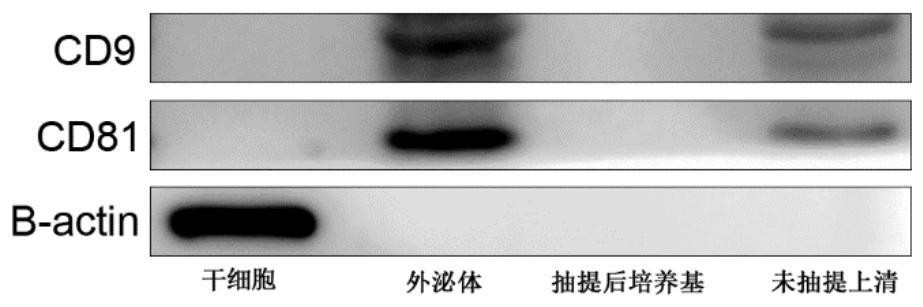
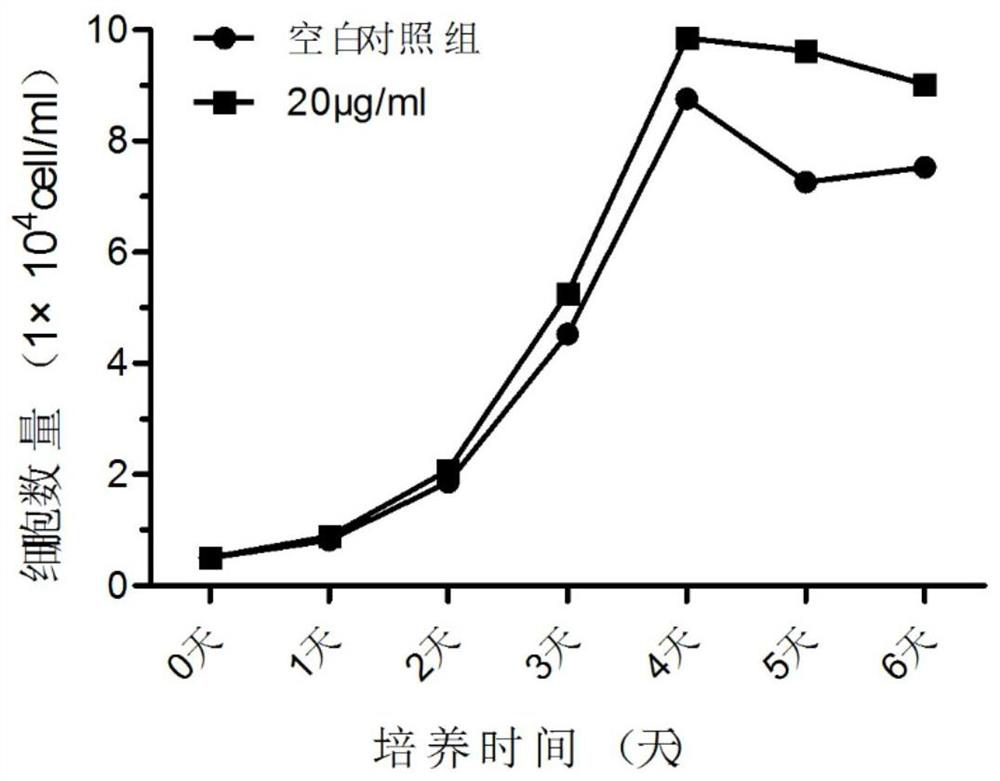
InactiveCN112280734APromote proliferation and survivalPromote absorptionCell dissociation methodsBiological material analysisDead cellExosome
The invention discloses a preparation method of an exosome and a stem cell proliferation reagent containing the exosome. The preparation method of the exosome comprises the following steps: step 1, adding a culture medium without exosome serum into bone marrow mesenchymal stem cells, diluting the bone marrow mesenchymal stem cells into a cell suspension, inoculating the cell suspension into a cellculture bottle for culture, collecting cultured supernatant, and extracting the exosome; and step 2, removing cell debris, dead cells and impurities in the supernatant, and after removal of the supernatant, adding a phosphate buffer salt solution (PBS) to dissolve and collect an exosome precipitate, conducting filtering by using a sterile filter membrane, sub-packaging the obtained exosome precipitate into sterile centrifugal tubes, and storing the exosome precipitate for later use. According to the method, in-vitro experiments prove that co-culture of exosome secreted by early-generation bone marrow mesenchymal stem cells and human umbilical cord mesenchymal stem cells can significantly promote the proliferative activity and related functions of the mesenchymal stem cells; and the methodis an effective strategy for potentially improving cell functions in a large-scale cell culture process.
Owner:广东佰鸿干细胞再生医学有限公司
Scorpion venom polypeptide for promoting cell proliferation, preparation method and medicinal application thereof
InactiveCN101979409APromote proliferationSingle ingredientPeptide/protein ingredientsPeptide preparation methodsVenomX ray irradiation
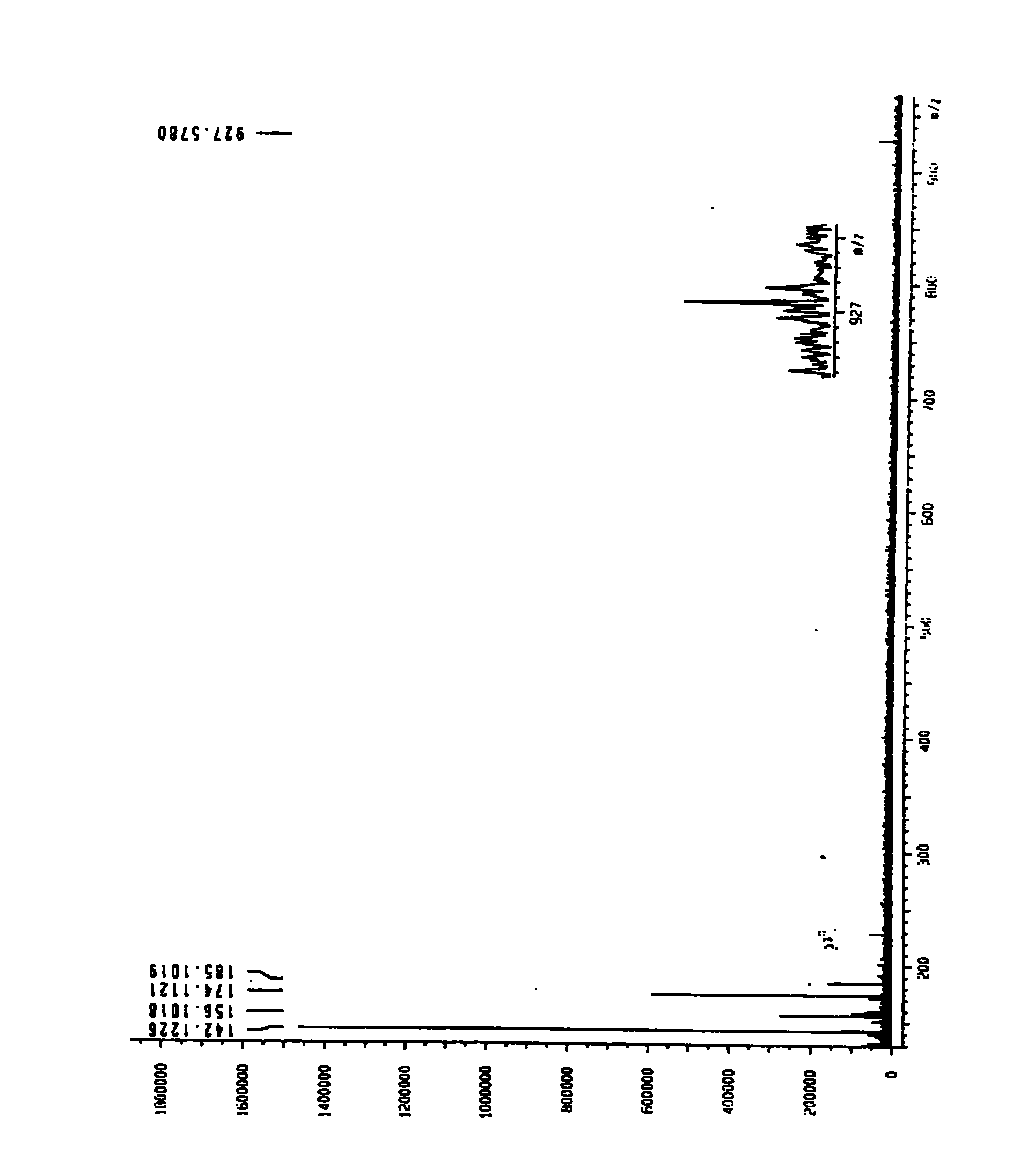
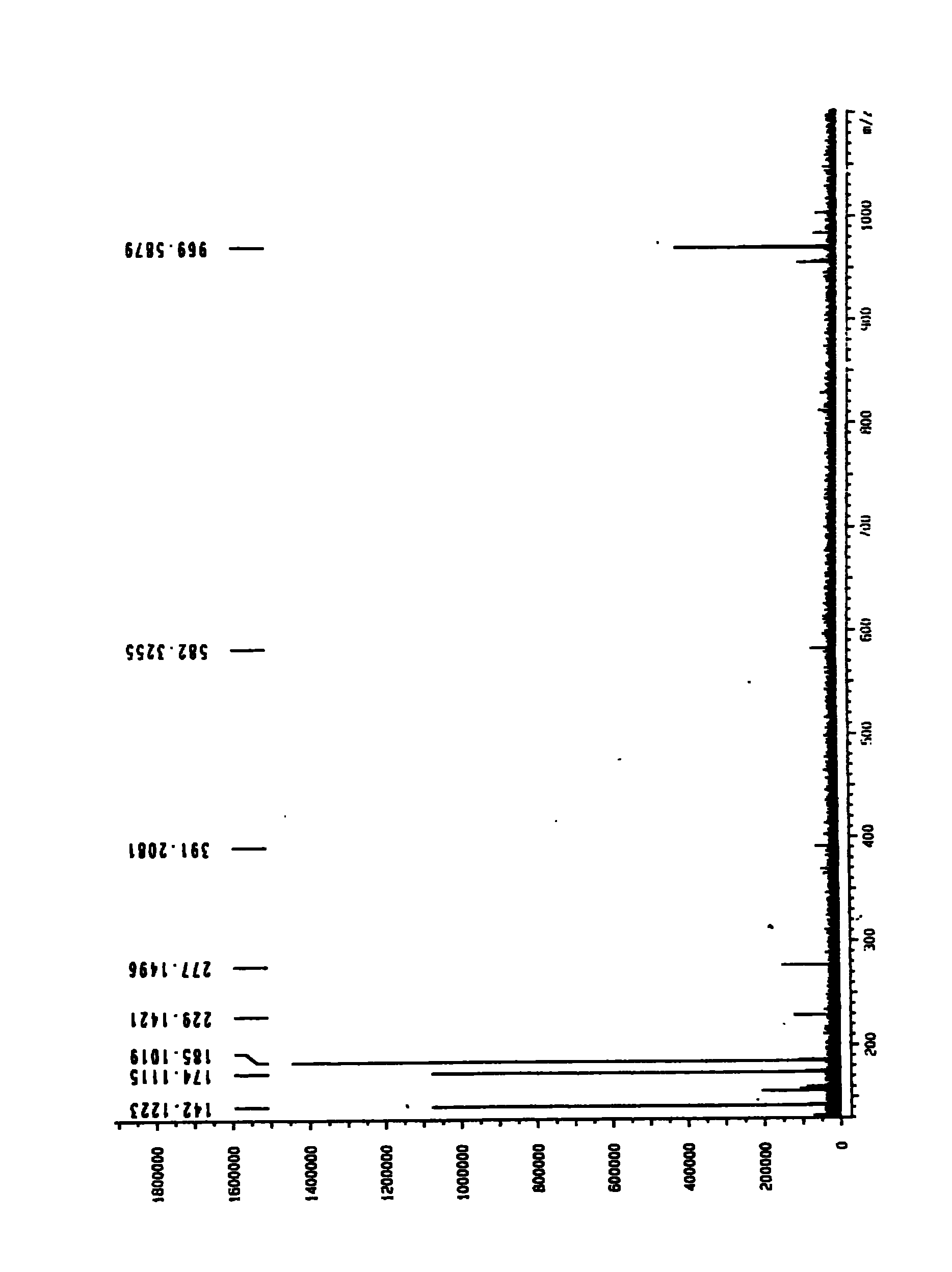
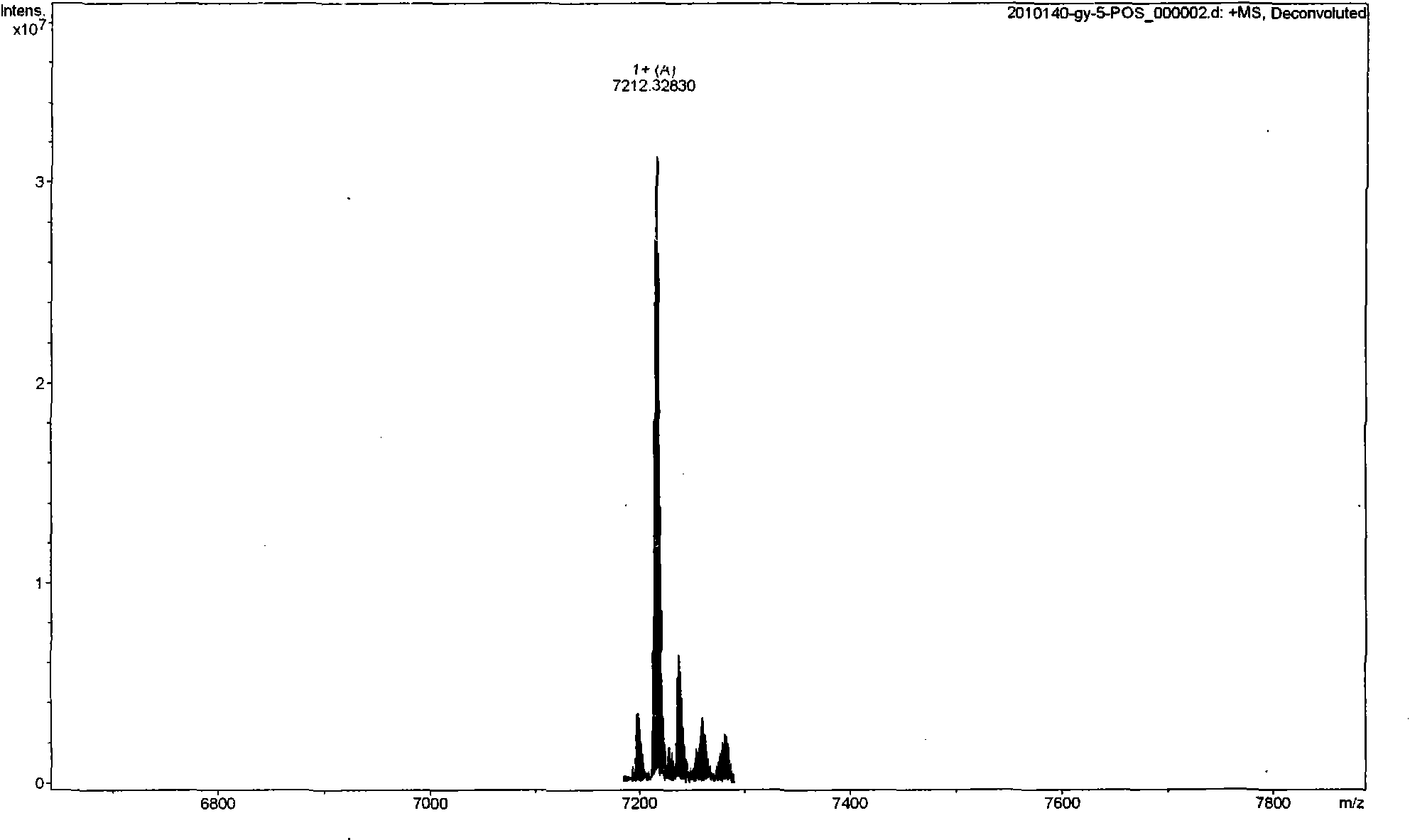
The invention relates to a scorpion venom polypeptide for promoting cell proliferation, a preparation method and medicinal application thereof. The scorpion venom polypeptide for promoting cell proliferation is characterized in that: the peptide is separated from scorpion venom of Buthus martensii Karsch and purified; and a sequence of 30 amino acid residues at an N end of the scorpion venom polypeptide for promoting cell proliferation is shown as a sequence table SEQ ID No:1, wherein the molecular weight of the corpion venom polypeptide is 7212.32830Da. The scorpion venom polypeptide for promoting cell proliferation is a novel peptide derived from the scorpion venom of the Buthus martensii Karsch, and has the effect of promoting the proliferation of normal or radiated hematopoietic cells. The Buthus martensii Karsch polypeptide (BmKpp) has a single component, precise molecular weight, clear 30 amino acid residues at the N end, obvious effect of promoting cell proliferation, and standard and controllable quality in the process of purification, and thus contributes to scale production. The scorpion venom polypeptide for promoting cell proliferation has the prominent advantages and the wide application prospect in development of medicaments for promoting cell proliferation and preventing cell irritability damage (such as radiation and the like), and can promote colony formation of mouse bone marrow hematopoietic cell after the cell is irradiated by gamma rays or X rays and proliferation of M-NFS-60 cell.
Owner:广州医学院
Preparation method for superfine bupleurum powder under normal temperature and special bidirectional airflow sieving machine
ActiveCN102488803AImprove cell wall breaking rateEnhance pharmacological effectsPowder deliverySenses disorderProcess engineeringBupleurum fruticescens
The invention discloses a preparation method for superfine bupleurum powder under a normal temperature; the preparation method comprises the following steps of: firstly selecting radix bupleuri, radix scutellariae, pinellia, dangshen and liquorice, cleaning and cutting the radix bupleuri, the radix scutellariae, the pinellia, the dangshen and the liquorice into sections; drying the sections, and then respectively grinding the sections to obtain crude traditional Chinese medicine powders; uniformly mixing the crude traditional Chinese medicine powders according to proportions of 3:3:2:3:1, then placing the mixed crude traditional Chinese medicine powder in a rod mill to be ground, leading the ground powder to enter a special bidirectional airflow sieving machine through air supply to pass through a sieve with 500 meshes, sieving the ground powder to obtain powder with the particle size of less than or equal to 25 micrometers, conveying the powder into a cyclone material collecting device through air currents generated by a draught fan to be collected, thereby obtaining the superfine bupleurum powder finished product; and collecting and conveying the powder which is not sieved by the sieve with 500 meshes into the rod mill for secondary cyclic grinding. The preparation method has the advantages that the whole preparation process is carried out under the normal temperature, and low temperature or special auxiliary conditions are not needed; the particle size of the prepared medicine finished product is less than or equal to 25 micrometers, and the wall-broken rate of cells and the bioavailability are greatly increased; and the bidirectional airflow sieving machine adopted in the preparation method simplifies the preparation process of the superfine bupleurum powder.
Owner:HENAN KANGXING PHARMA
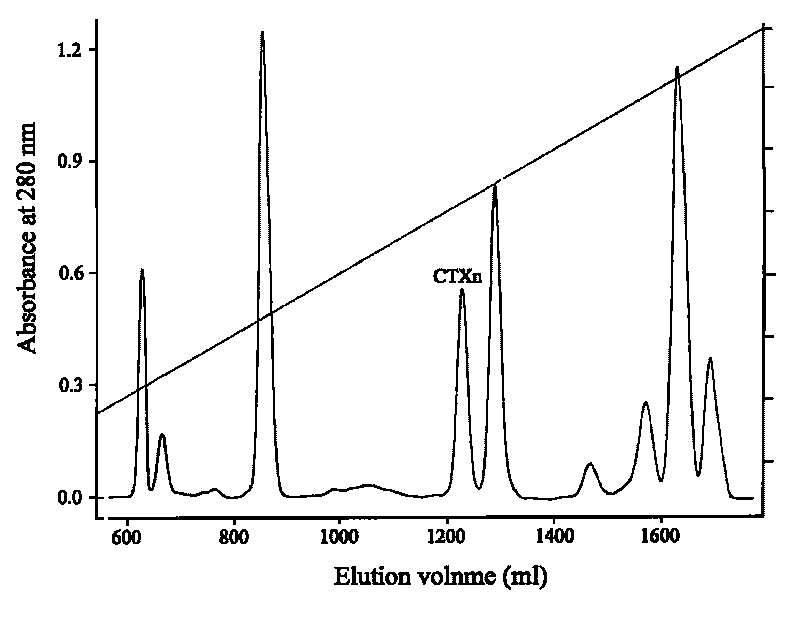
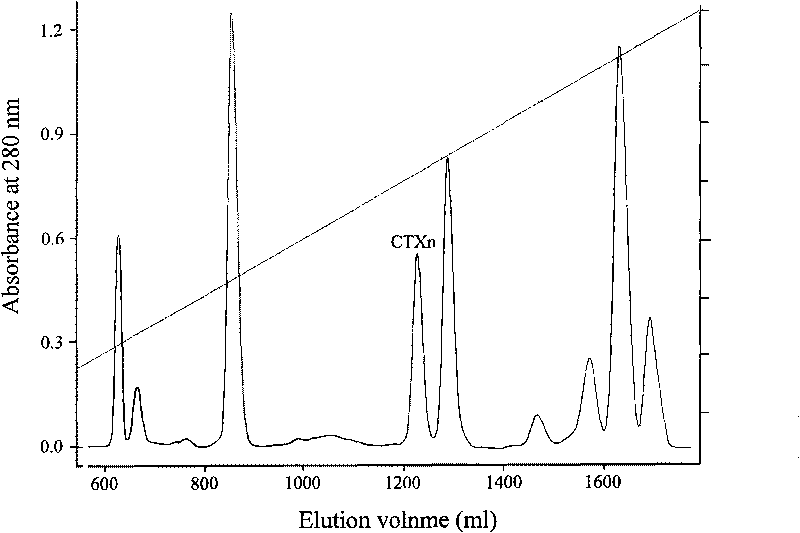
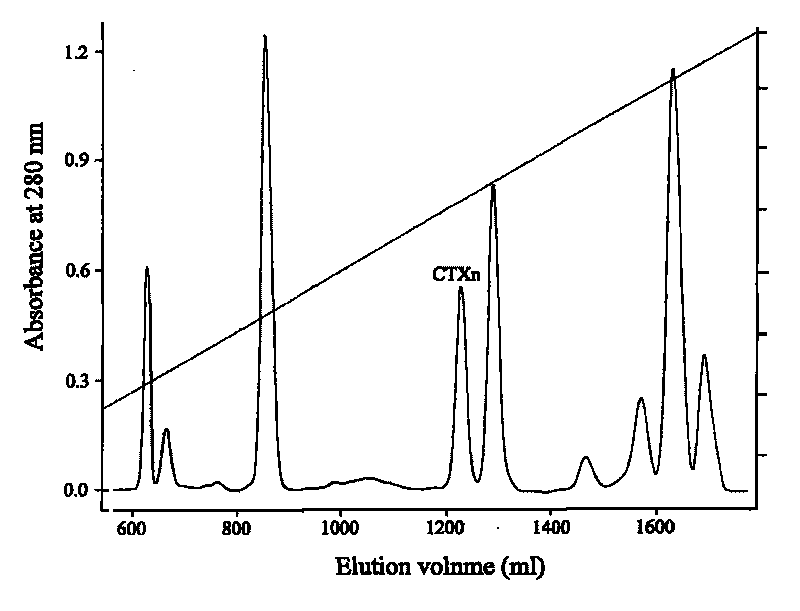
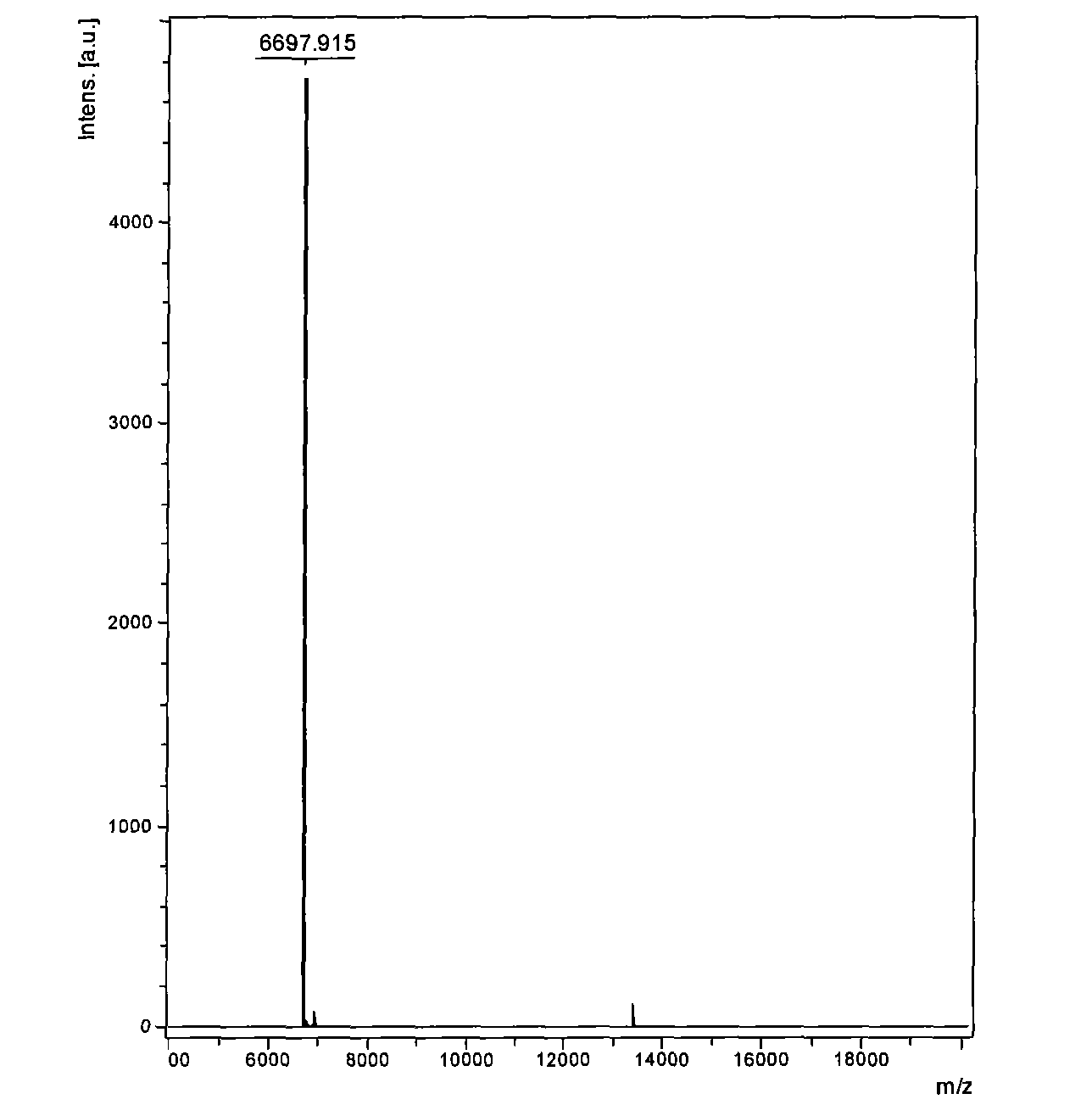
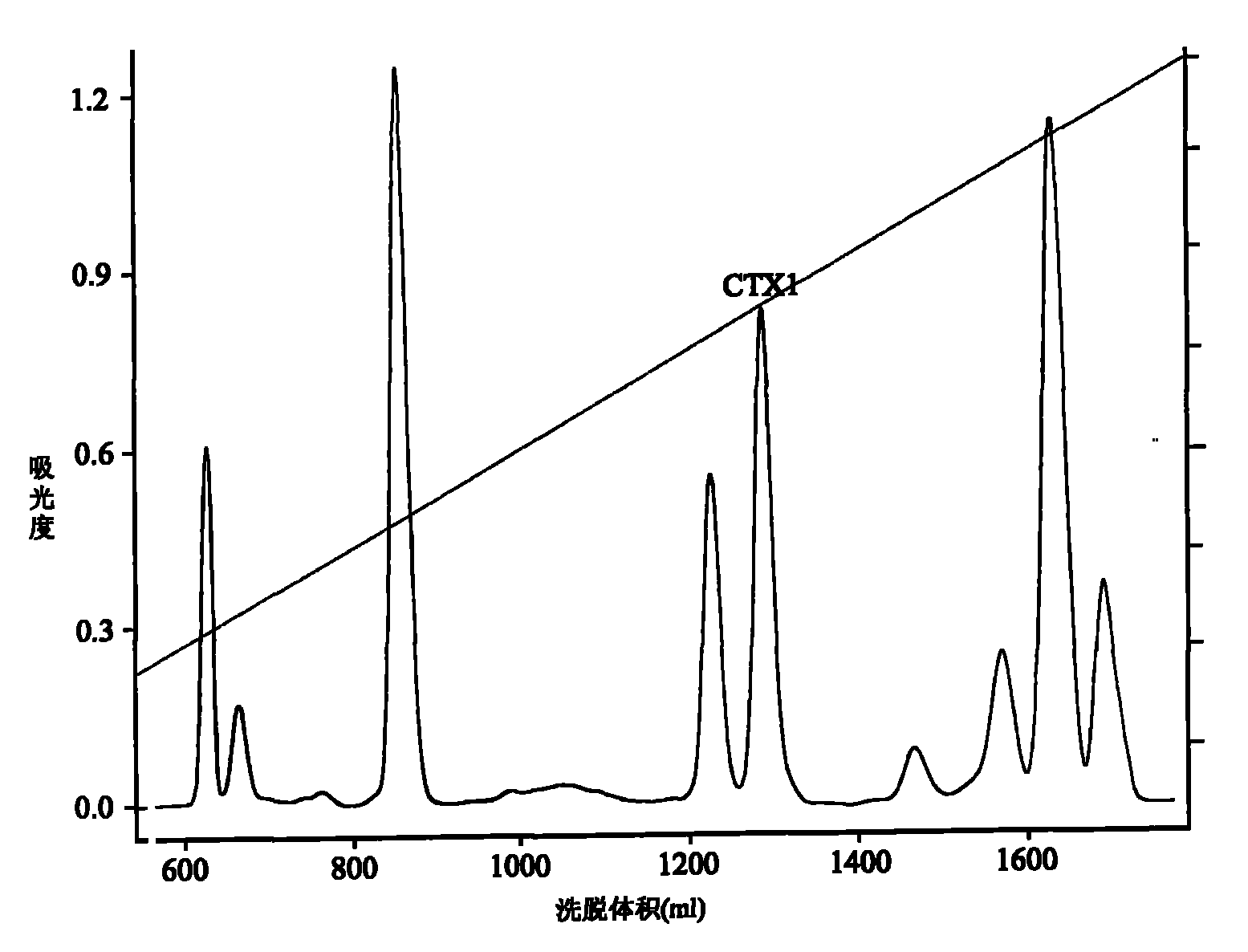
Venin-derived cytotoxin CTXn for drug rehabilitation and purification method and application thereof
InactiveCN101717439ACPP inhibitionSignificant detoxification effectNervous disorderPeptide/protein ingredientsPurification methodsSide effect
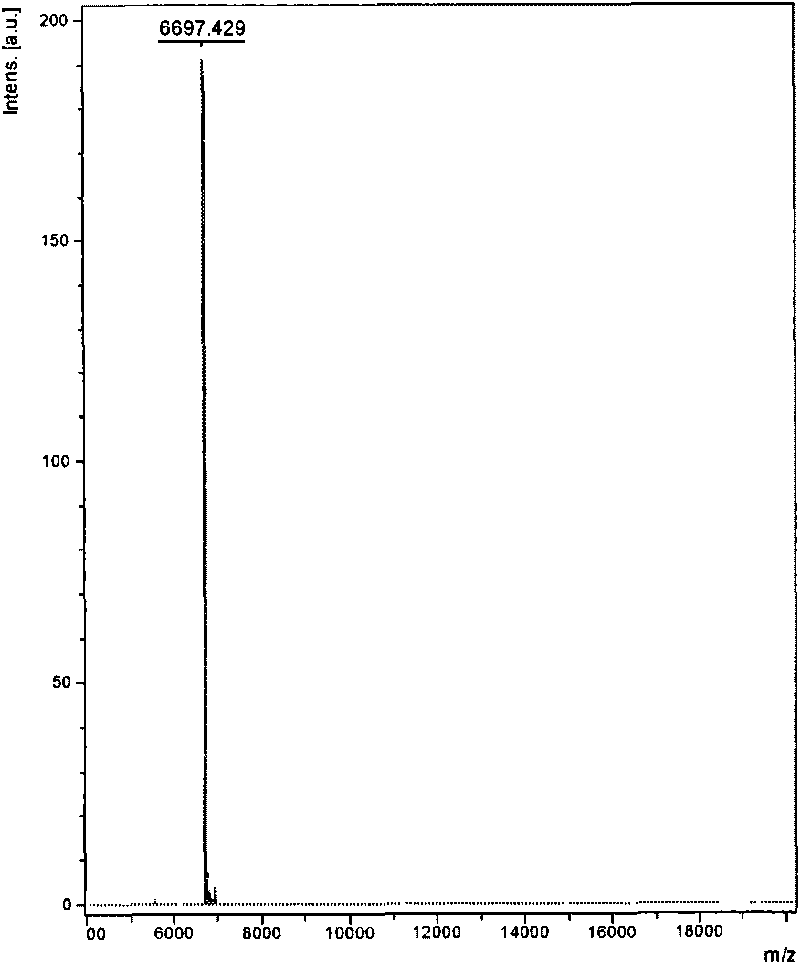
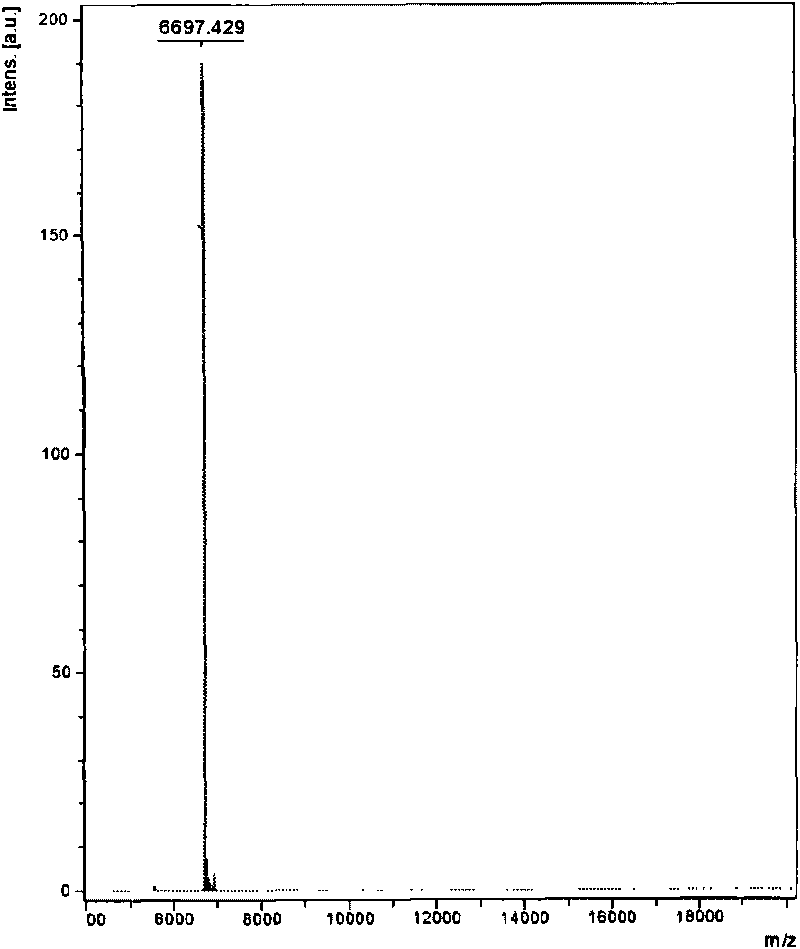
The invention relates to venin-derived cytotoxin CTXn for drug rehabilitation and a purification method and application thereof, which is characterized in that: 1. the molecular weight of the CTXn is 6697.429Da; 2. the amino acid sequence of the CTXn is LKCNQLIPPF YKTCAAGKNLCYKMFMVAAP KVPVKRGCID VCPKSSLLVK YVCCNTDRCN. A preparation method comprises the following steps of: collecting a Zhoushan cobratoxin specimen outside; carrying out CTXn separation and purification for the Zhoushan cobratoxin specimen, firstly carrying out gel filtration, secondly selecting a component III for the next step of ion exchange through primary screening, and thirdly carrying out ion exchange which adopts a rapid purification process development system, obtaining single toxic polypeptide CTXn, desalting with a G-50 prepacked column, and freezing to dryness for standby; and measuring the molecular weight of the CTXn, the amino acid sequence thereof and the amino acid molecular weight. The venin-derived cytotoxin CTXn can eliminate the drug thirsting behaviors and withdrawal symptoms of addictive rats for morphine, has the characteristics of remarkable drug effect and small toxic or side effect, is favorable for large-scale production, and has great social meaning and economic value for preventing drug harms.
Owner:广州医学院
Pharmaceutical composition as well as preparation method and application thereof
InactiveCN103893247AEffective treatmentSpontaneous activity influencePowder deliveryOrganic active ingredientsActive componentDesmodium styracifolium
The invention provides a medicinal composition as well as a preparation method and application thereof. The pharmaceutical composition is a medicinal preparation for clinical administration, which is prepared from desmodium styracifolium general flavone extract serving as an active component and pharmaceutically acceptable medicinal auxiliaries. The composition can be used for preparing clinical treatment medicament for clearing damp-heat, promoting diuresis and expelling stone (damp-heat stagnation).
Owner:HUMANWELL HEALTHCARE GRP +1
Venin-sourced demulcent CTXn, and purification method and applications thereof
InactiveCN101717441ARaise the response thresholdGood analgesic effectPeptide/protein ingredientsAntipyreticCobra venomVirulent characteristics
The invention relates to a venin-sourced demulcent CTXn, and a purification method and applications thereof. The invention is characterized in that (1) the molecular weight of the CTXn is 6697.429 Da; (2) and the amino acid sequence of the CTXn is described as the sequence list SEQ ID No.1. The preparation method comprises the following steps of: using a Zhoushan cobra venom sample; carrying out the CTXn separation and purification on the Zhoushan cobra venom sample; primary-screening to select a component III for next step of ion exchange; carrying out the ion exchange on the component III through a quick-purification technique exploitation system by using a filler to obtain a single-virulence polypeptide CTXn, desalting with a G-50 prepacked column, and freeze-drying; and measuring the molecular weight and the amino acid sequence of the CTXn. The CTXn of the invention has favorable demulcent function on somatalgia and visceralgia caused by nociceptive stimulus as well as pains caused by addiction to morphine, has the characteristics of obvious drug effect and low toxic or side effect, is beneficial to large-scale production, and has wide application prospects in the aspect of analgesic development.
Owner:广州医学院
Method for preparing ultrafine powder Qiangzhuang San at normal temperature and special bidirectional airflow sieving machine
ActiveCN102430089AImprove cell wall breaking rate and bioavailabilityEnhance pharmacological effectsPowder deliveryGas current separationWater contentMicrometer
The invention discloses a method for preparing ultrafine powder Qiangzhuang San at normal temperature, which comprises the following steps of: selecting Chinese herbal medicine decoction pieces such as pilose asiabell root, medicated leaven, malt, stir-fried hawthorn fruit, astragalus, tuckahoe, largehead atractylodes rhizome and katsumade galangal seed; cleaning, and drying until water content is less than or equal to 10 percent; grinding the Chinese herbal medicines at normal temperature to obtain coarse powder with the particle size of 60 to 120 meshes; uniformly mixing the obtained coarsepowder in a ratio, putting into a rod mill for grinding, feeding into a special bidirectional airflow sieving machine through air, sieving with a 500-mesh screen, conveying powder which is obtained by sieving and has the particle size of less than or equal to 25 micrometers to a cyclone collector through airflow generated by a draught fan, and collecting to obtain a finished ultrafine powder Qiangzhuang San product; and collecting powder which does not pass through the 500-mesh screen by using a funnel, conveying to the rod mill through a pipeline, and performing cyclic grinding. The method has the advantages that: the whole preparation process is performed at normal temperature, the particle size of the prepared finished product medicine is less than or equal to 25 micrometers, and the cell wall breaking rate and bioavailability are greatly improved; and by the bidirectional airflow sieving machine used in the method, the preparation process for the ultrafine powder Qiangzhuang San is simplified.
Owner:HENAN KANGXING PHARMA
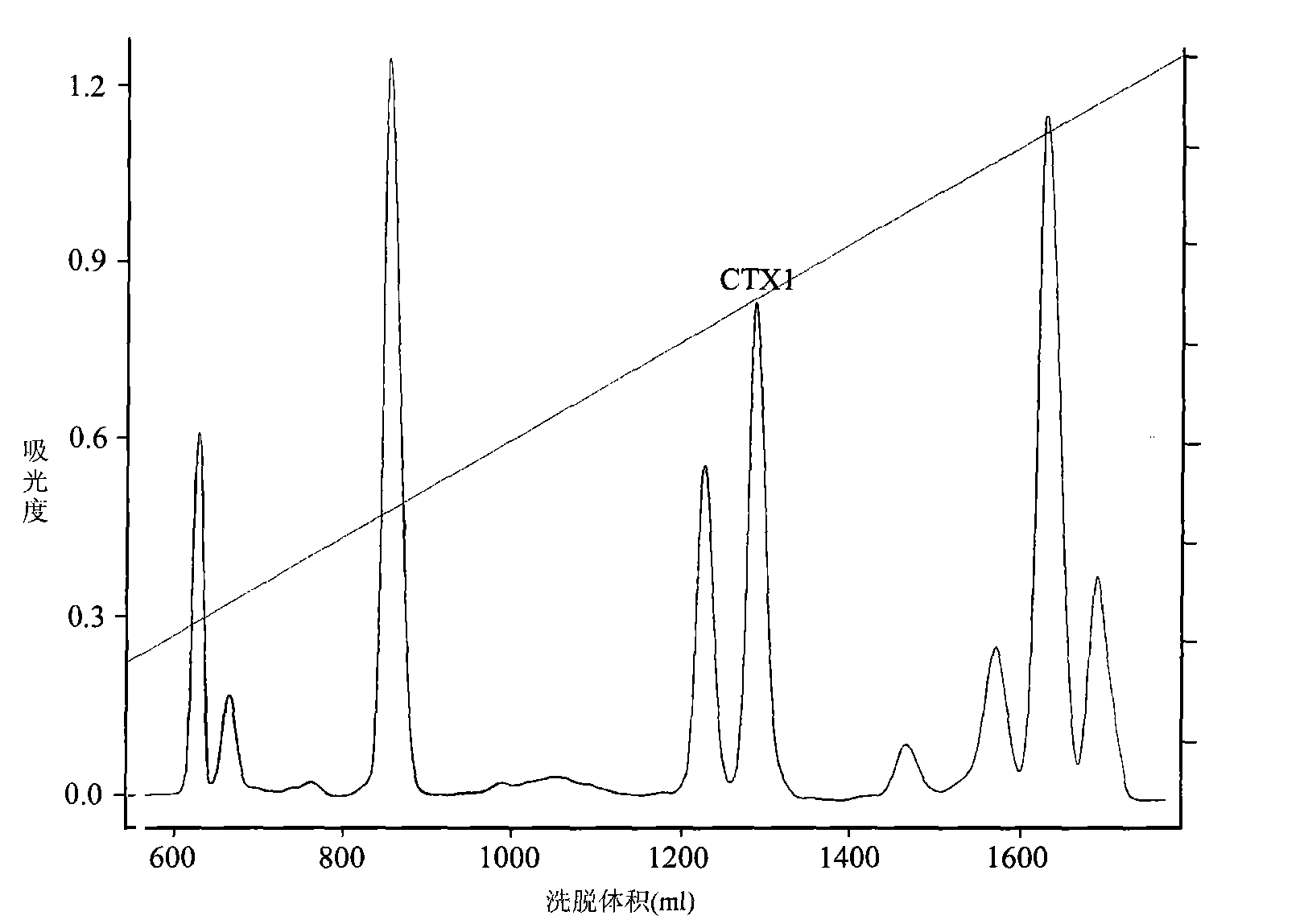
Application of cytotoxin (CTX1) from snake venom to preparation of medicament for rehabilitating
InactiveCN101926980ADesensitization of behaviorEliminate withdrawal symptomsNervous disorderPeptide/protein ingredientsDrug withdrawal symptomsThirst
The invention discloses application of cytotoxin (CTX1) from snake venom to preparation of a medicament for rehabilitating. Proved by the experiment, the CTX1 can better eliminate the behavioral sensitization of addict rats by morphine, eliminate the medicament thirst behavior and the abstinence syndrome of rats and has favorable rehabilitating effect. In addition, the invention can improve the function state, the diet and the activities of the addict rats and remarkably increase the weight, thereby recovering normal physiological activity.
Owner:SUN YAT SEN UNIV
A kind of method utilizing potassium mixed salt to prepare potassium chloride
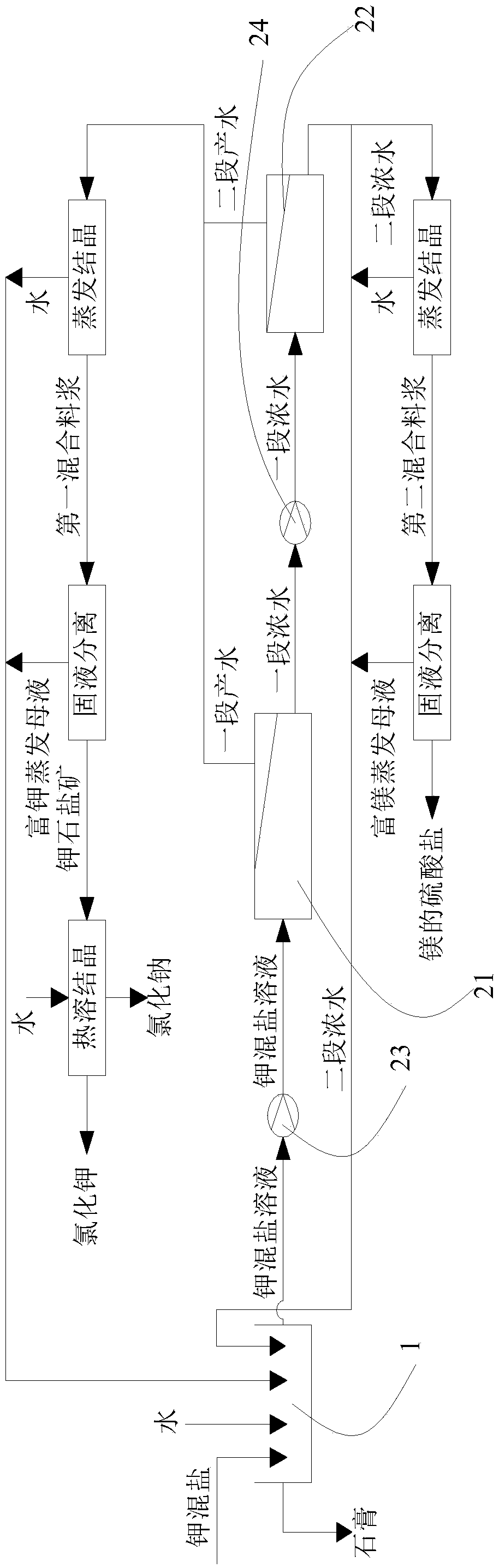
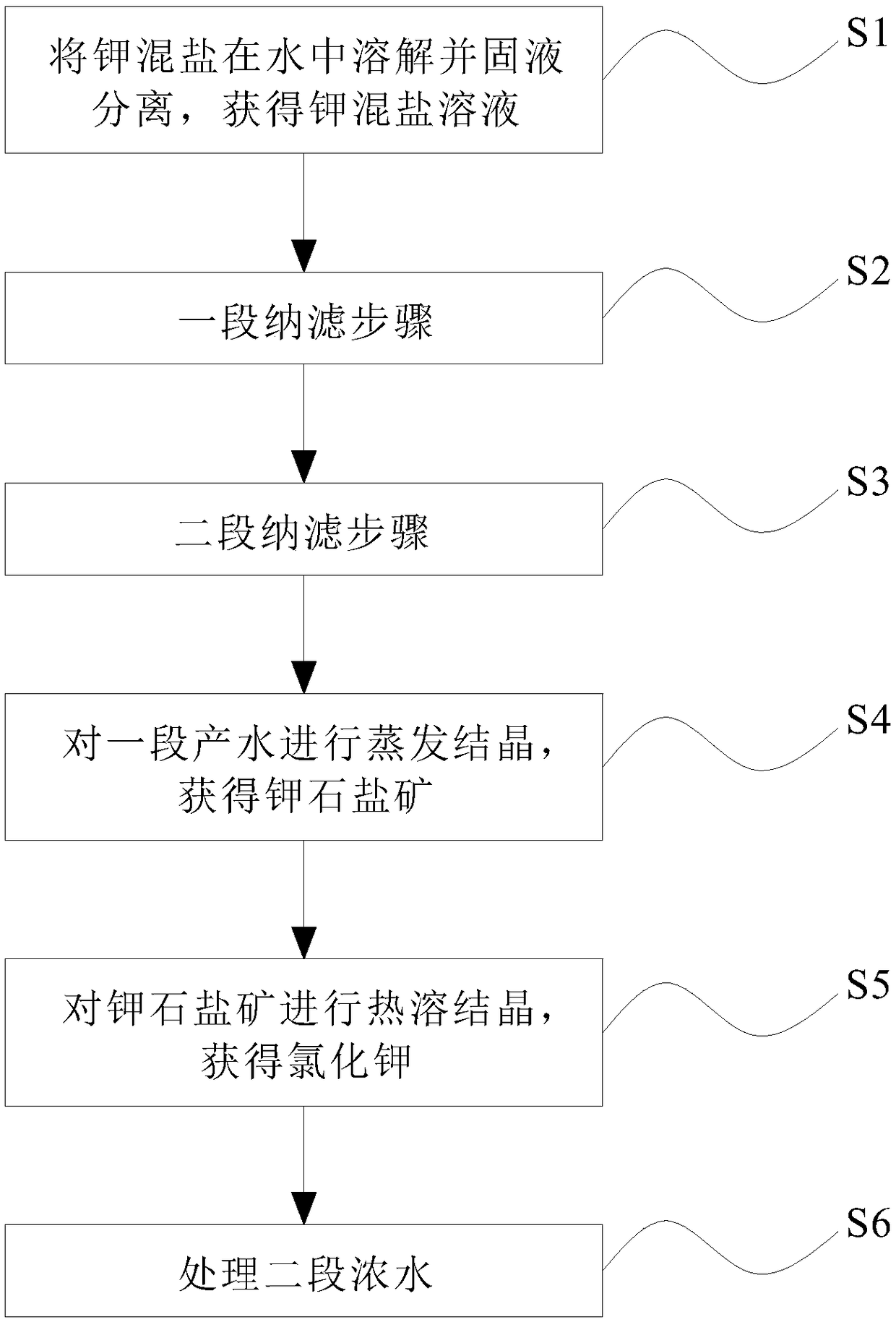
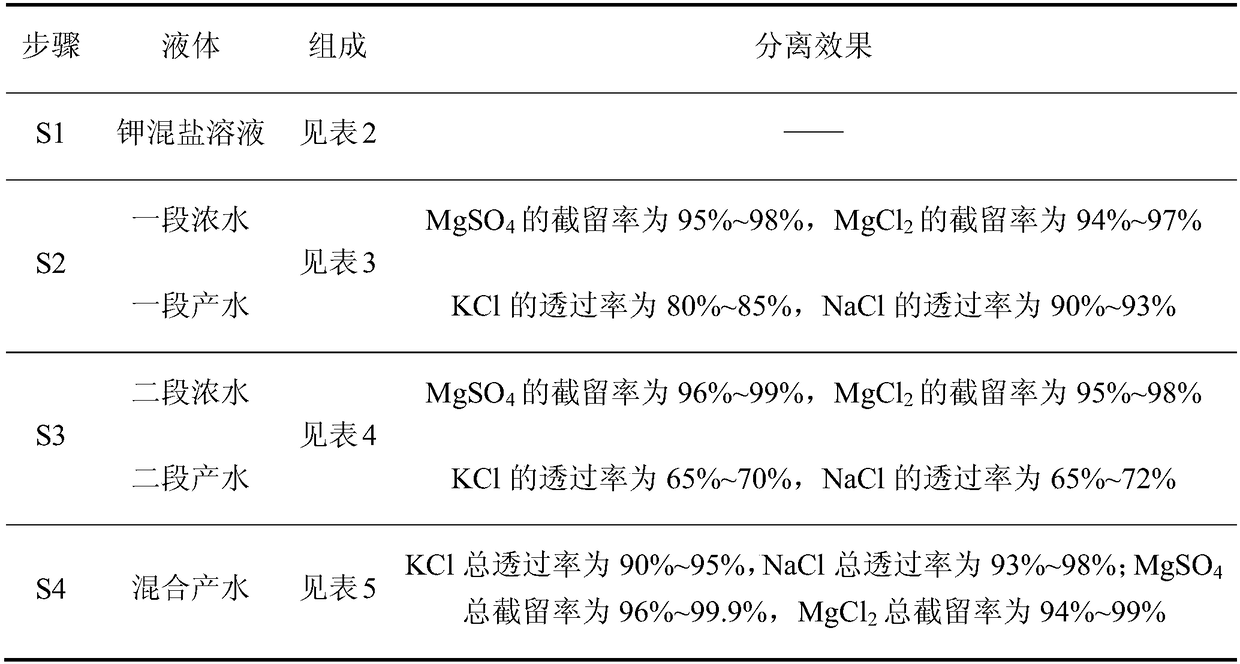
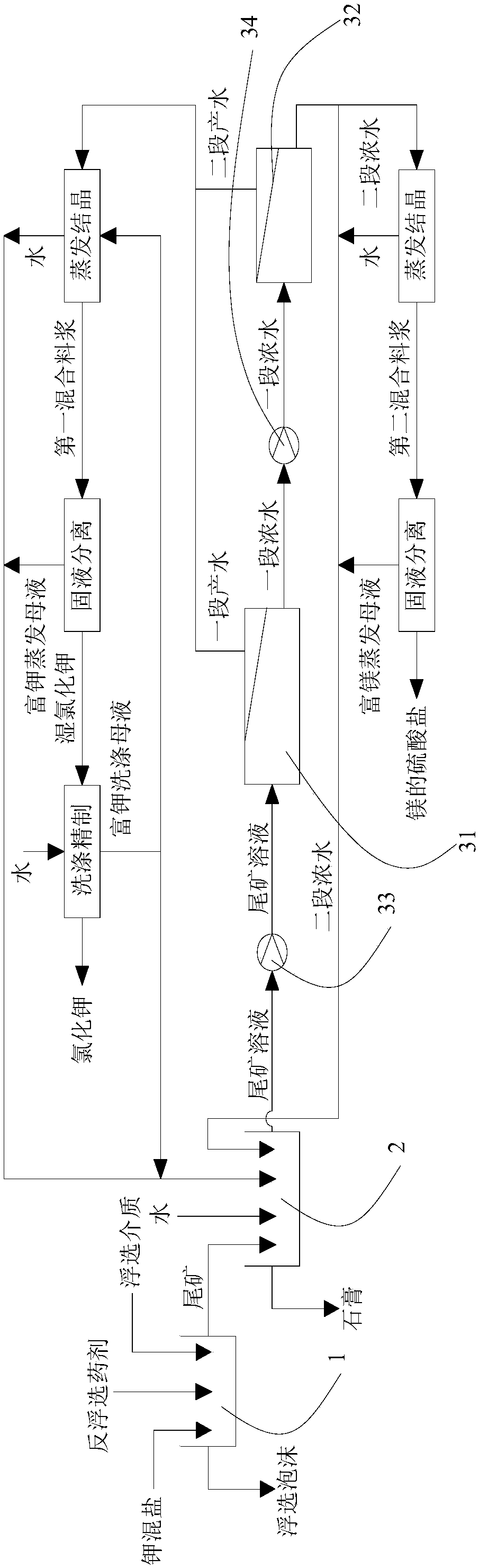
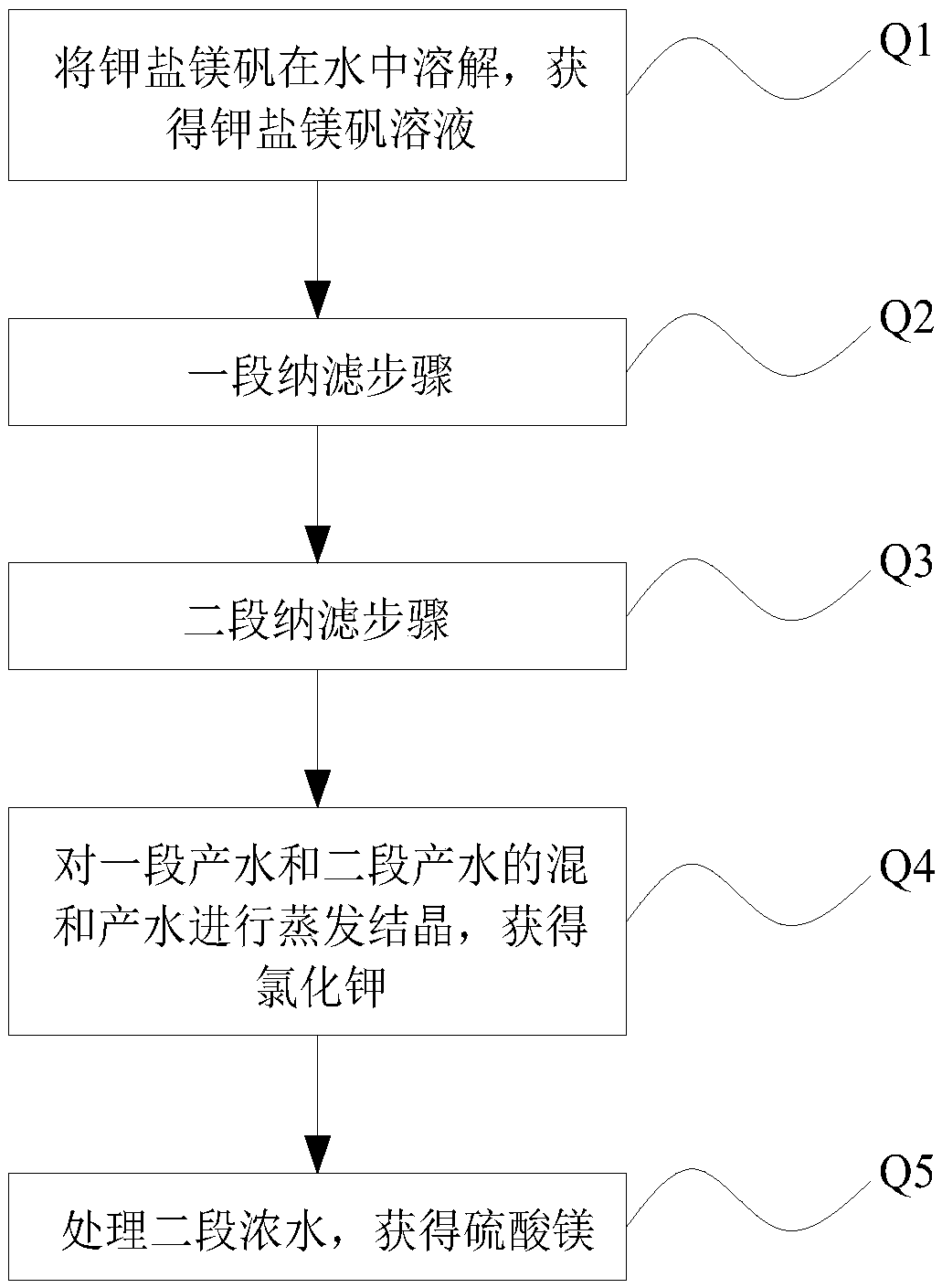
ActiveCN106517251BHigh purityLarge particlesAlkali metal chloridesAlkali metal halide purificationChloride potassiumChemistry
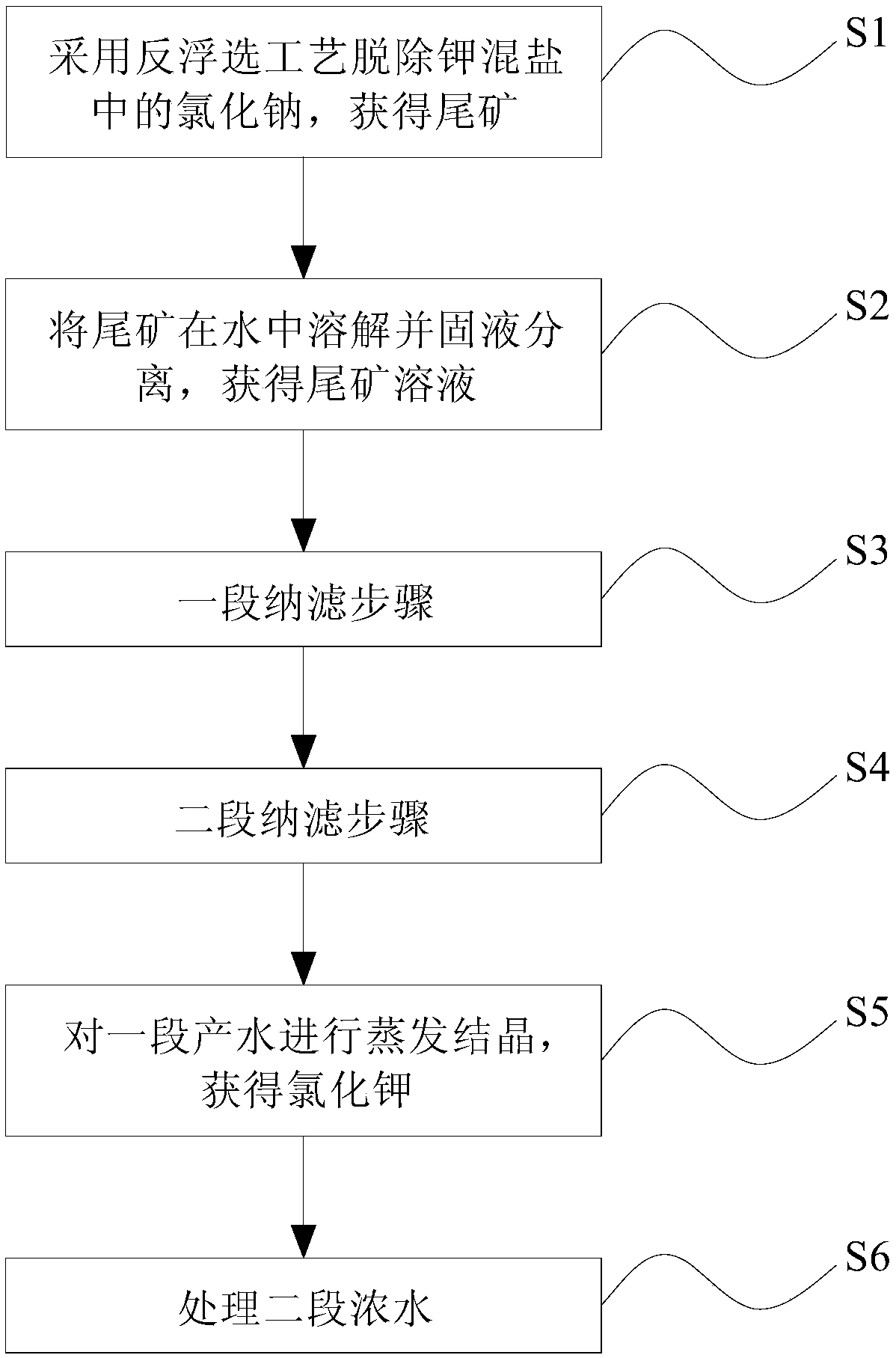
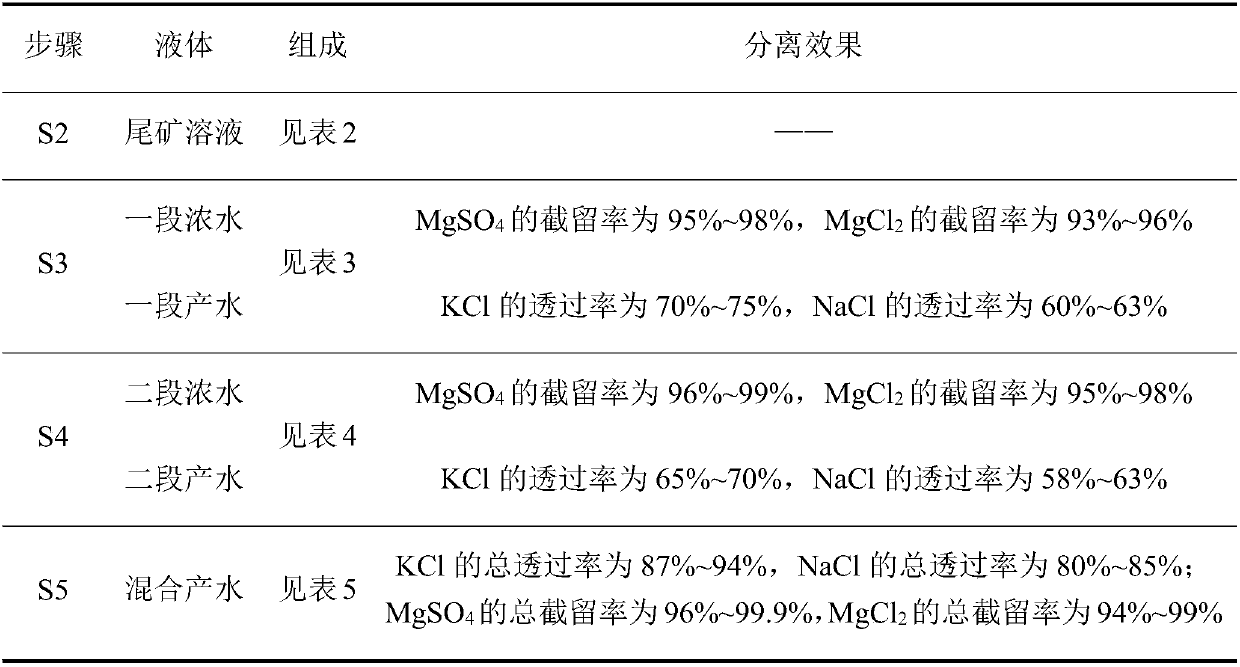
The invention discloses a method for preparing potassium chloride from potassium mixed salt. The method comprises the steps that the potassium mixed salt is dissolved in water, solid-liquid separation is carried out, and a potassium mixed salt solution is obtained; a first-section nanofiltration step is carried out, wherein a nanofiltration membrane system is adopted for carrying out first-section nanofiltration treatment on the potassium mixed salt solution, first-section concentrated water and first-section produced water are obtained, the nanofiltration system comprises a first nanofiltration membrane assembly, a first concentrated water box and a first produced water box, and the first concentrated water box and the first produced water box are connected to the first nanofiltration membrane assembly; the first-section concentrated water is stored in the first concentrated water box, and the first-section produced water is stored in the first produced water box; the first-section produced water is subjected to evaporative crystallization, and sylvinite ore is obtained; the sylvinite ore is subjected to flash melting crystallization, and potassium chloride is obtained. The novel method is used for producing potassium chloride from the potassium mixed salt and has the advantages of being simple in process, high in product purity, large in particle and high in yield.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI
Utilize potassium mixed salt to prepare the method for potassium chloride
ActiveCN106430248BGuaranteed yieldEasy to separateReverse osmosisAlkali metal chloridesNanofiltrationMethods preparation
The invention discloses a method for preparing potassium chloride by utilizing potassium mixed salt. The method comprises the steps that a reverse flotation process is adopted to remove sodium chloride in the potassium mixed salt, and a tailing is obtained; the tailing is dissolved in water, and solid-liquid separation is performed to obtain a tailing solution; a section of nanofiltration step is executed, wherein a nanofiltration membrane system is adopted to conduct a section of nanofiltration treatment on the tailing solution to obtain a section of concentrated water and a section of produced water and comprises a first nanofiltration membrane module and a first concentrated water tank and a first produced water tank connected to the first nanofiltration membrane module, the section of concentrated water is stored in the first concentrated water tank, and the section of produced water is stored in the first produced water tank; evaporative crystallization is conducted on the section of produced water to obtain the potassium chloride. The invention provides a novel method for producing potassium chloride by using potassium mixed salt as a raw material. The method has the advantages of being simple in process, high in product purity and high in yield.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI
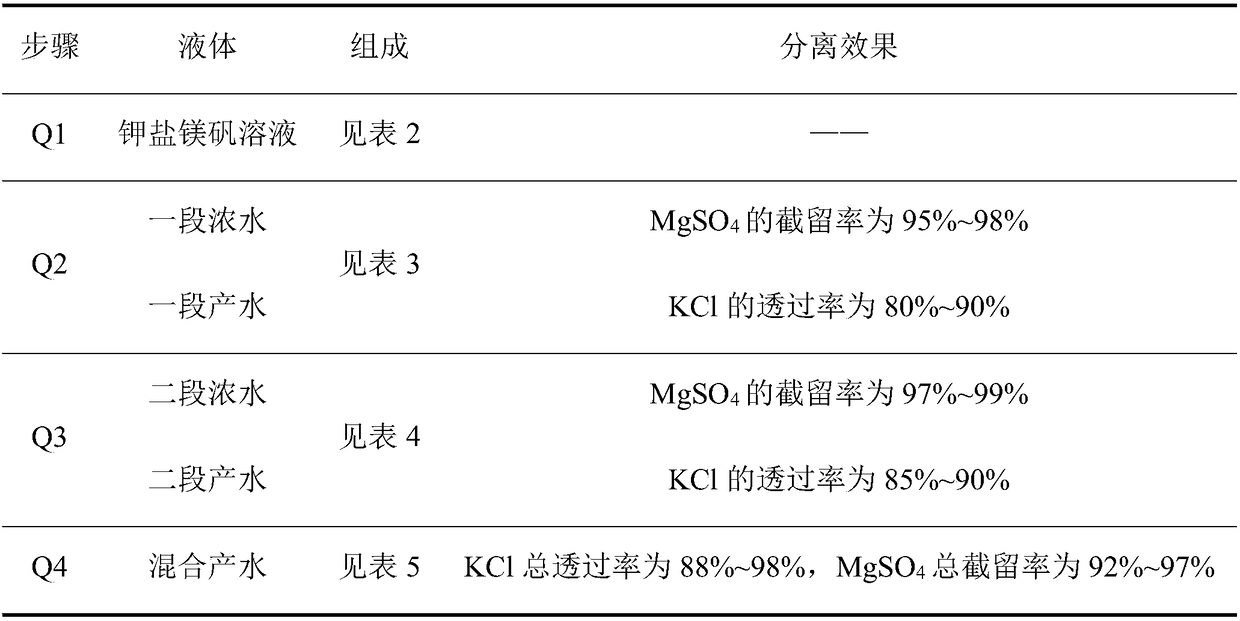
The method for preparing potassium chloride and magnesium sulfate by using kainite
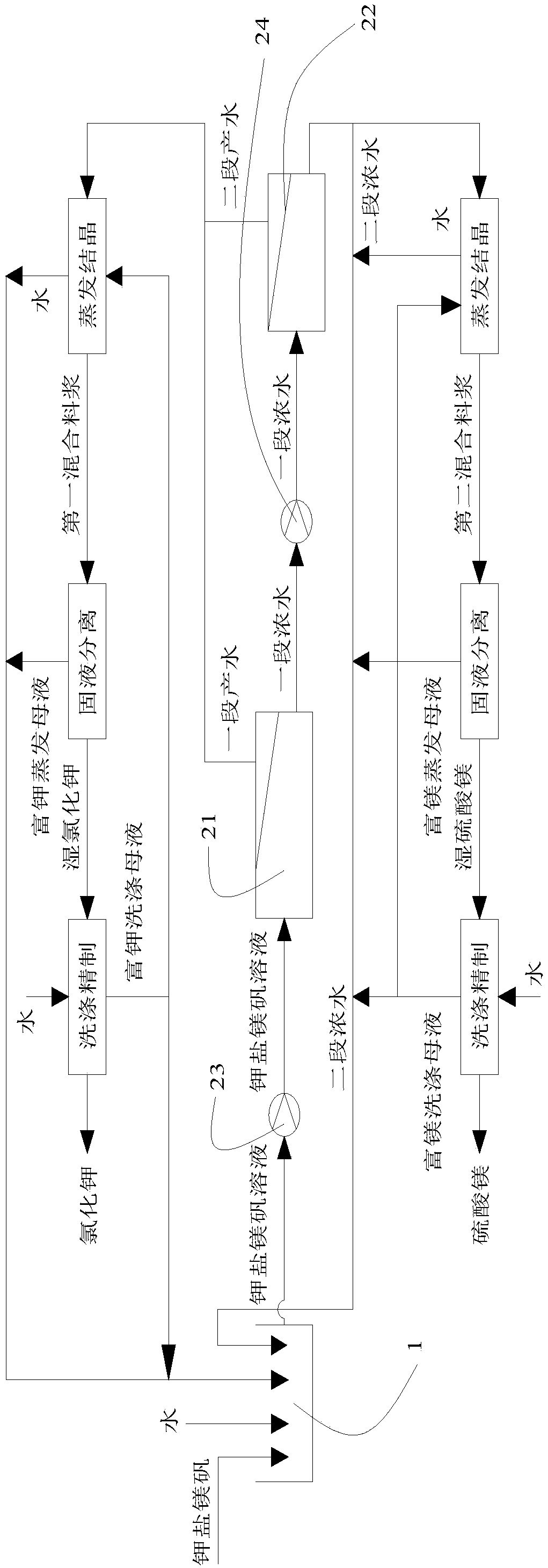
ActiveCN106517250BAvoid excessive consumptionHigh separation yieldMagnesium sulfatesAlkali metal chloridesStrontiumChloride
The invention discloses a method for preparing potassium chloride and magnesium sulfate from kainite. The method comprises the following steps: dissolving the kainite in water to obtain a kainite solution, and performing primary nanofiltration: performing primary nanofiltration treatment on the kainite solution by adopting a nanofiltration membrane system to obtain primary concentrated water and primary producing water, wherein the nanofiltration membrane system comprises a first nanofiltration membrane assembly and a first concentrated water tank and a first producing water tank which are connected to the first nanofiltration membrane assembly, the primary concentrated water is stored in the first primary concentrated water tank, and the primary producing water is stored in the first producing water tank; performing evaporative crystallization on the primary producing water to obtain potassium chloride; performing evaporative crystallization on the primary concentrated water to obtain magnesium sulfate, or performing secondary nanofiltration to obtain secondary concentrated water, and performing evaporative crystallization on the secondary concentrated water to obtain the magnesium sulfate. The invention provides a novel method for producing the potassium chloride and the magnesium sulfate from the kainite serving as a raw material; and furthermore, the method has the advantages of simple process, high product purity and high yield.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI
Medicament for treating colonitis
ActiveCN101590216ADetermine the nature of the lesionQuality assuranceDigestive systemAluminium/calcium/magnesium active ingredientsSal ammoniacChronic colitis
The invention discloses a medicament for treating colonitis, which is prepared from the following bulk drugs by weight: 200 portions of calcite (calcined), 40 portions of halitum (calcined), 400 portions of clematis aethusifolia (calcined), 400 portions of rhododendron micranthum (calcined), 5 portions of nutmeg, 5 portions of coriander fruit, 5 portions of rhizoma zingiberis, 5 portions of long pepper, 5 portions of dwarf lilyturf root, 1 portion of pepper, 5 portions of dried white turnip, 5 portions of sal ammoniac, 5 portions of purple sal ammoniac, 5 portions of elecampane, and 10 portions of potassium nitrate. The formulation is a pure Chinese medicament preparation, has small toxic side effect, is suitable for the characteristic of chronic colonitis recurrent attacks, can be taken for a long time, and has remarkable curative effect; besides the formulation adopts a dosage form of enteric-coated granules to avoid the first pass effect in stomach absorption and act on affected parts directly, and each medicinal material in the formulation adopts the principle of relieving diarrhea with astringents and treating both principal and secondary aspects of a disease to achieve the aim of treatment, thus the medicament for treating colonitis is surely a classic anaesthetic worthy of development in clinical application.
Owner:INNER MONGOLIA TIANQI HAN&MONGOLIA PHARMA CO
Antiviral influenza drug and preparation method thereof
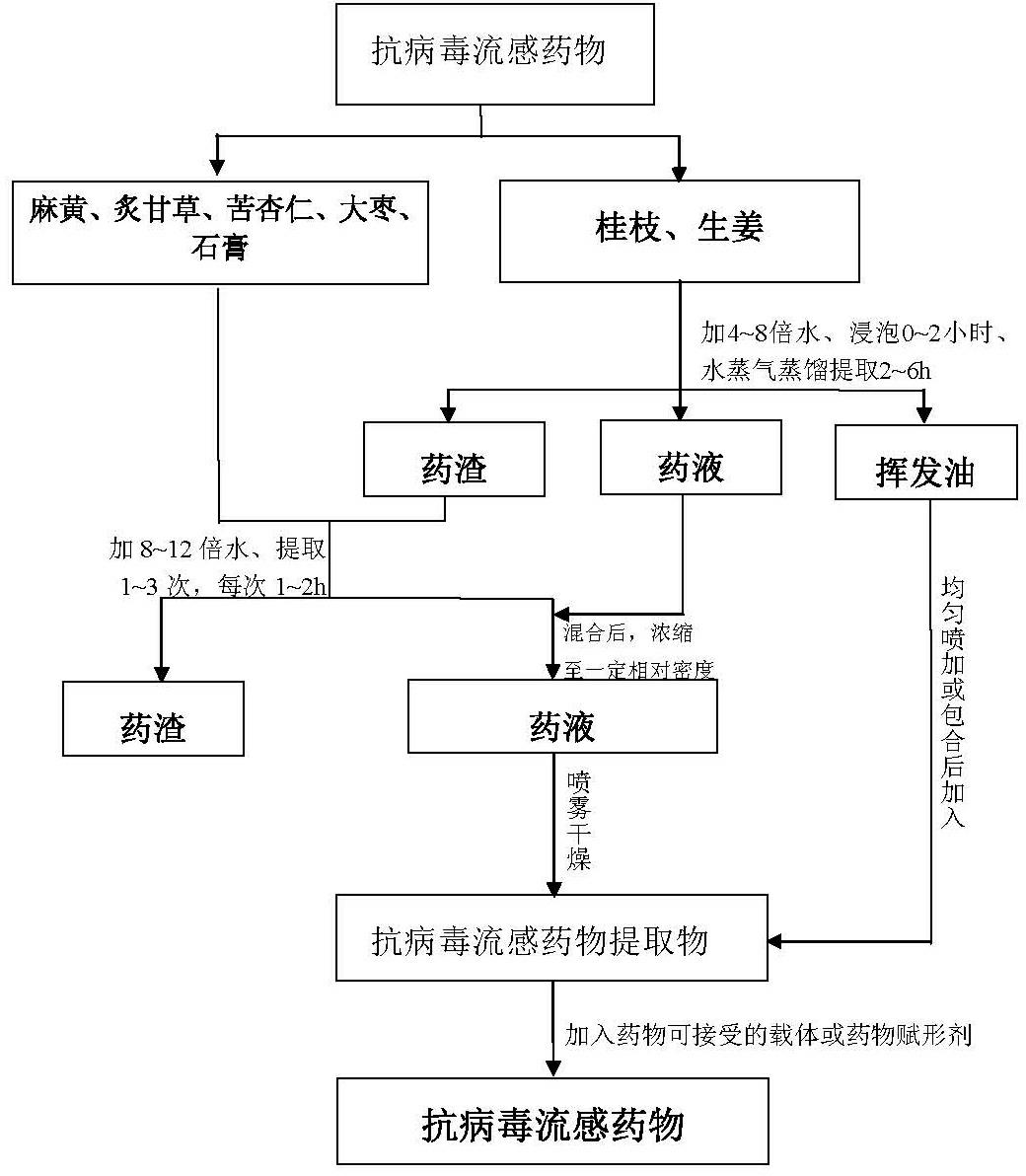
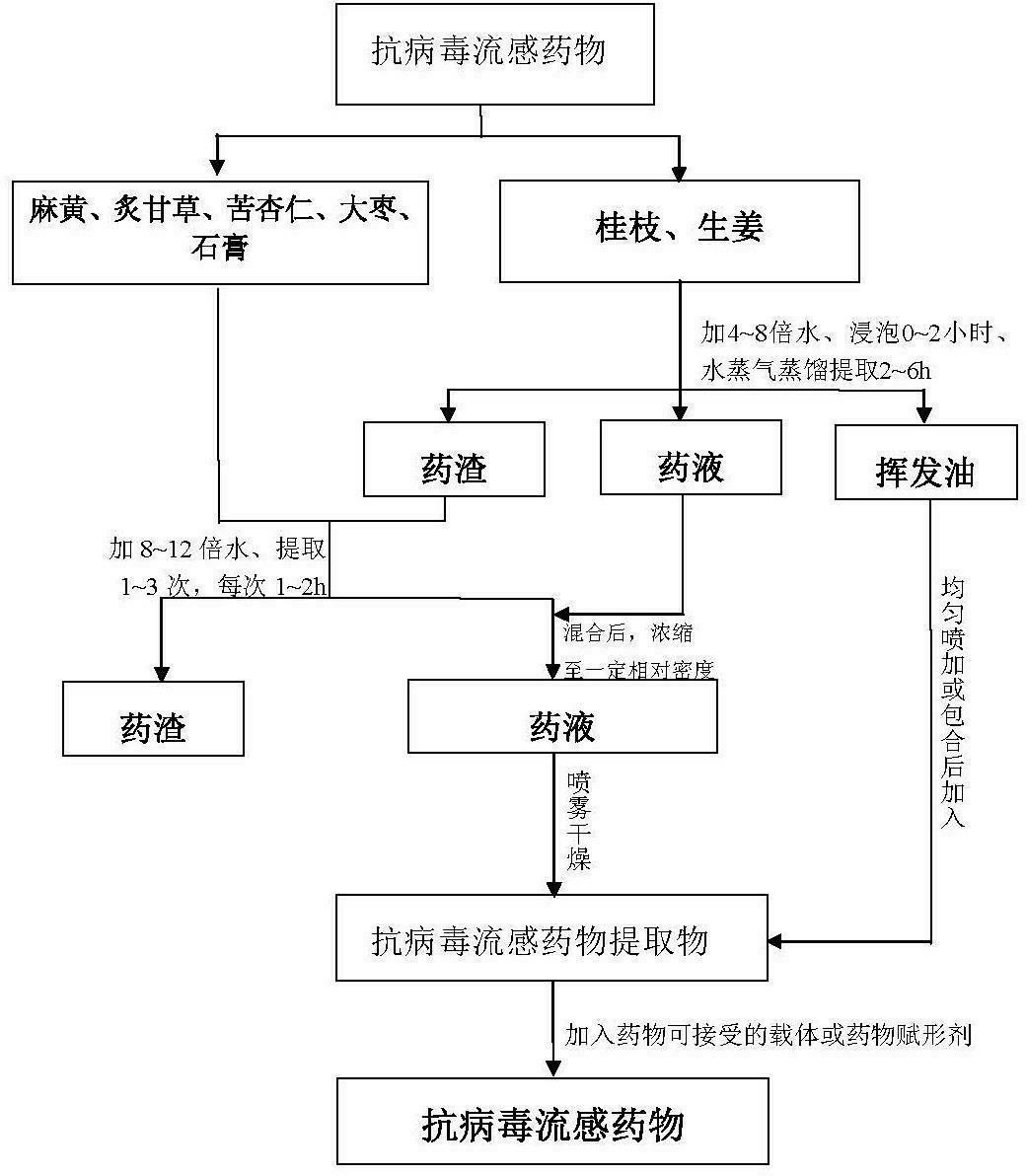
InactiveCN102631658ASignificant effectEffectivenessAntiviralsAluminium/calcium/magnesium active ingredientsLicorice rootsTwo step
The invention relates to an antiviral influenza drug and a preparation method of the antiviral influenza drug. The drug comprises the following ingredient by weight: 8-12 parts of ephedra, 8-12 parts of cassia twig, 5-7 parts of processed licorice root, 8-12 parts of semen armeniacae amarae, 8-12 parts of ginger, 18-22 parts of Chinese-date and 18-22 parts of gypsum. The preparation method comprises the steps of soaking the cassia twig and the ginger, extracting to obtain volatile oil and liquid medicine for standby application; then mixing the drug dregs and other crude drugs, extracting to obtain liquid medicine, mixing the liquid medicines obtained from the above two steps, then concentrating, spraying and drying to obtain a drug extract; and finally, mixing the extract with a pharmaceutically acceptable carrier or a drug excipient according to proportion, pelletizing and drying, and adding the standby volatile oil to prepare the antiviral influenza drug. The antiviral influenza drug has obvious curative effect, can be used in large range, and is convenient to take, accurate in curative effect, controllable in quality, simple in manufacturing process flow, controllable in quality standard, energy-saving in energy resources, free from environment pollution, small in potential safety hazard, and suitable for industrial production.
Owner:东莞市沙田医院
Oral solid preparation containing desmodium styracifolium general flavone and application thereof
ActiveCN103893258ASmall amount of clinical useEffective substance basis is clearPowder deliveryPill deliveryPharmacologyQuality standard
The invention provides an oral solid preparation containing desmodium styracifolium general flavone and application thereof, wherein the oral solid preparation containing the desmodium styracifolium general flavone comprises desmodium styracifolium general flavone which is an alcohol extract of desmodium styracifolium, and medicinal auxiliary materials. The oral solid preparation containing the desmodium styracifolium general flavone disclosed by the invention has the characteristics of being explicit in effective material basis, controllable in quality standard, good in drug dissolution degree, high in bioavailability, good in quality stability, significant in pharmacology and drug efficacy, less in dosage, safe and convenient to take, and completely applicable to industrial massive production.
Owner:HUMANWELL HEALTHCARE GRP +1
Preparation method and application of anticoccidial traditional Chinese medicine composition
InactiveCN108815222AThe preparation process is simple and reliableQuality standards are easy to controlAntiparasitic agentsAlkali/alkaline-earth metal chloride active ingredientsCoccidiosisAnimal food
The invention discloses a preparation method and application of an anticoccidial traditional Chinese medicine composition. Dried dandelions and dried fructus cnidii are obtained and put into a traditional Chinese medicine grinding machine for grinding for 10-20 minutes, the materials are sieved through a 100-mesh screen, and dandelion powder and fructus cnidii powder are obtained; the dandelion powder, the fructus cnidii powder and salt are mixed in the mass ratio of (50-60):(30-40):(1-10), and the anticoccidial traditional Chinese medicine composition is obtained. The preparation process is simple and reliable, the medicine materials in use come from plants, planting and processing of the medicine materials are environmentally friendly, the prepared medicine is small in toxic and side effect on animals in use and free of irritation, antibiotic residues in animal food are avoided, and food safety is guaranteed. The prepared traditional Chinese medicine composition has the effect of resisting infection of one kind of eimeria coccidium and mixed infection of various kinds of eimeria coccidium, and is novel traditional Chinese medicine powder for preventing coccidiosis. Pharmacodynamical research shows that the traditional Chinese medicine composition has an obvious prevention and treatment effect on chick coccidiosis.
Owner:SICHUAN AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com