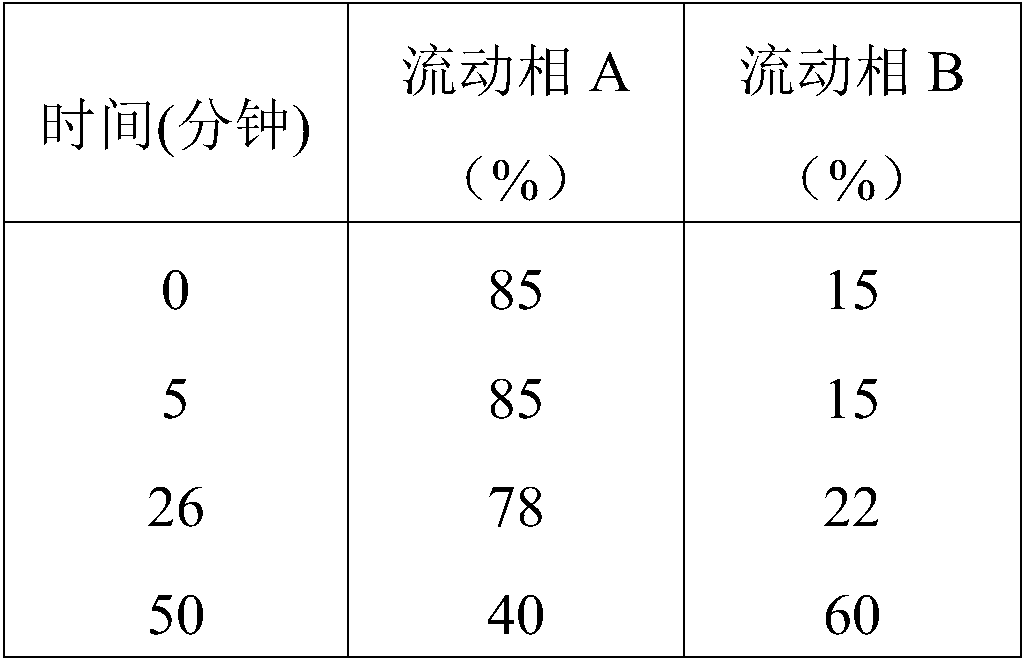
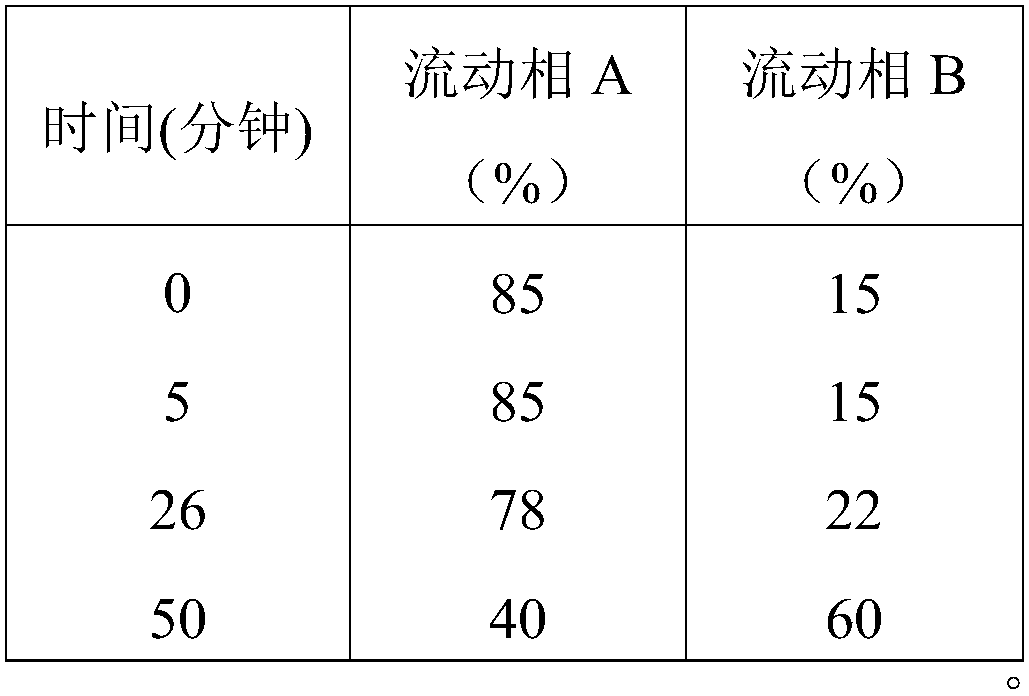
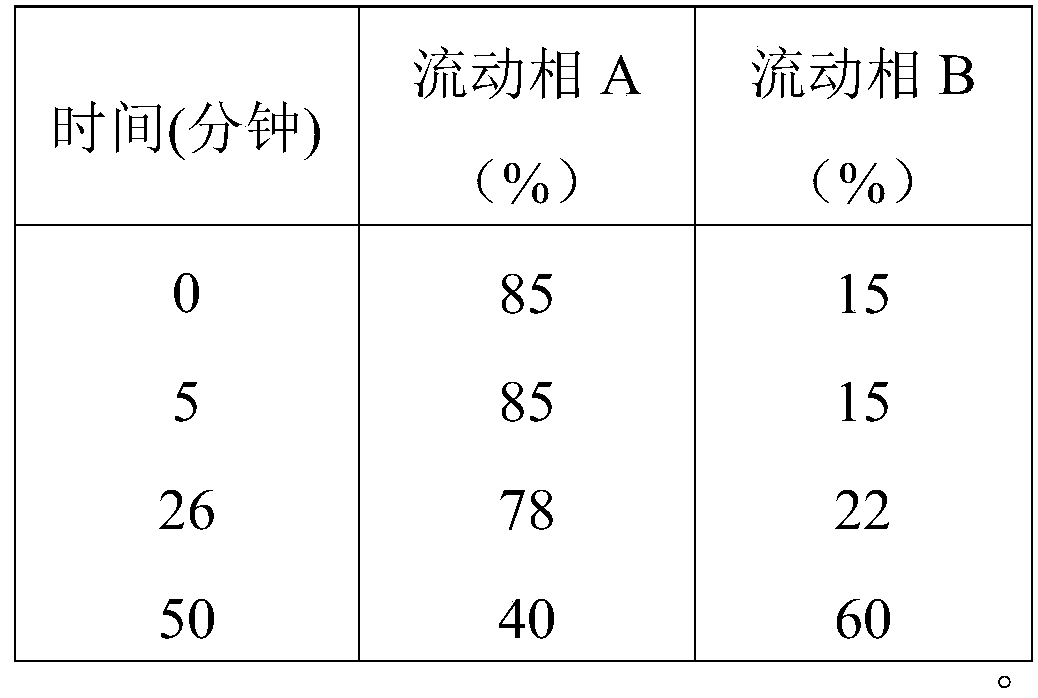
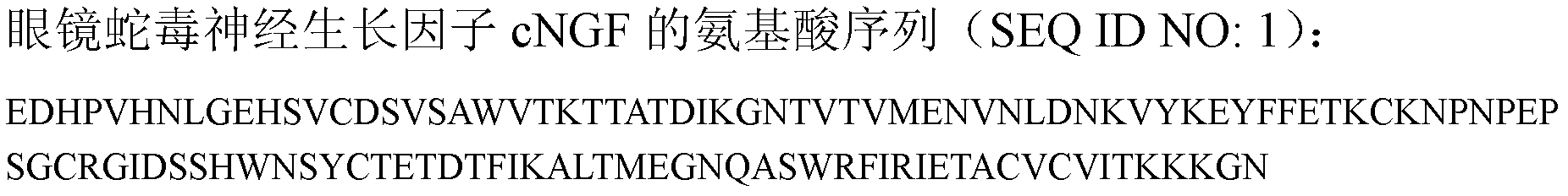Patents
Literature
61 results about "Cobra venom" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Synthetic peptide, inhibitor to DNA viruses
The present invention relates to the identification of the active domain of Herpoxin, a DNA virus-inhibiting-protein which was isolated from cobra venom in U.S. Pat. No. 5,648,339 and has a molecular weight of 13.5 kDa We have isolated a fragment of Herpoxin which contains the active domain and which we have named Herp. Herp mimics the activity of Herpoxin in inhibiting the replication of DNA viruses. A synthetic version of the active fragment was produced having the amino acid sequence Asn-Leu-Tyr-Gln-Phe-Lys-Asn-Met-Ile-Gln. The synthetic version of Herp consisting of ten amino acids inhibits the replication of DNA viruses such as herpes viruses types 1 and 2, cytomegalovirus and varicella zoster virus as well as Tubercle bacilli.
Owner:LIPPS BINIE V +1
Film agent quickly dissolved in oral cavity and preparation method thereof
ActiveCN103211801ALess pain timeAvoid reducing efficacyNervous disorderAntipyreticCobra venomTreatment effect
The invention discloses a film agent quickly dissolved in the oral cavity and a preparation method thereof. The film agent consists of the following components in percentage by weight: 0.1 to 70 percent of medicinal active ingredients, 30 to 90 percent of water-soluble film forming material, 2 to 20 percent of plasticizer, 0 to 5 percent of disintegrating agent, and 1 to 20 percent of water, wherein the medicinal active ingredients are selected from modified or non-modified cobra venom, sumatriptan succinate, ergotamine dihydrogen tartrate and lomerizine hydrochloride or flunarizine hydrochloride. The film agent is quick in response, and avoids decomposition in gastrointestinal tracts due to oral administration to reduce the treatment effect. The preparation method is simple, economic and practical, and facilitates industrialized batch production.
Owner:SUZHOU RENBEN PHARMA
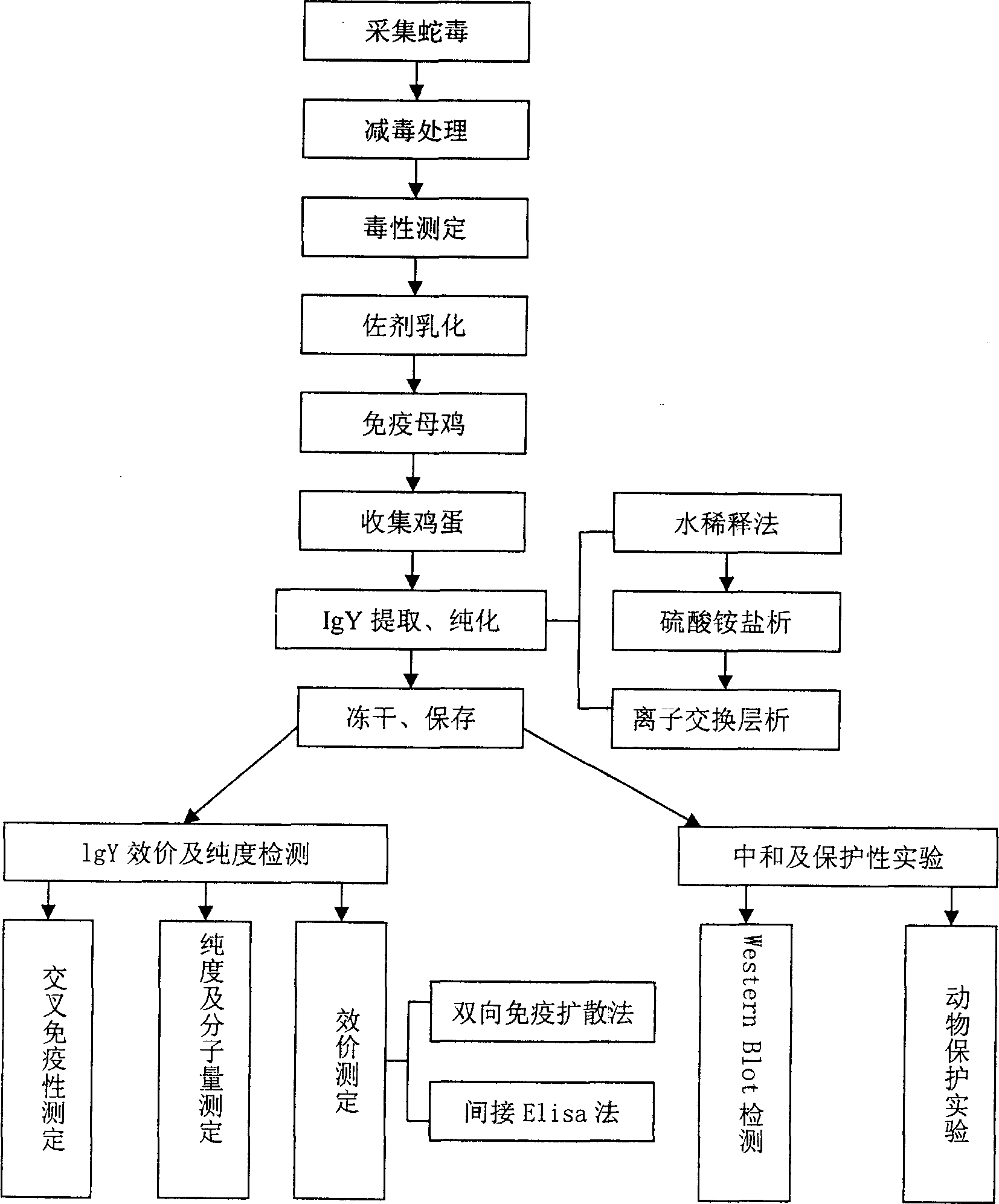
Chicken yoke antibody resisting Bengalese cobra venom and its preparation
InactiveCN1858064AGood treatment effectAvoid harmEgg immunoglobulinsAntinoxious agentsChromatographic separationCobra venom
The present invention is chicken yoke antibody (IgY) resisting Bengalese cobra venom and its preparation process. The preparation process includes water dilution to extract coarse IgY from chicken yoke, salting out IgY with ammonium sulfate, dissolving the precipitate in PBS, ultrafiltering to desalt, concentrating, anion exchanging chromatographic separation for further purifying IgY, ultrafiltering to desalt, concentrating, and freeze drying. The IgY exhibits single tape of molecular weight 200 KD in non-reductant SDS-PAGE and two tapes of molecular weight 65 KD and 35 KD separately in reductant SDS-PAGE, and has at least 13 dyeing strips of molecular weight 100-0.7 KD on PVDF film for Western Blot detection. The chicken yoke antibody (IgY) resisting Bengalese cobra venom may be used in preparing medicine and health food for preventing and / or treating snake bite, and in preparing reagent for detecting relevant snake venom.
Owner:广州医学院
Purification method, extract and preparation of cobra venom neurotoxin
InactiveCN102351951AEasy to separateShorten the timeNervous disorderPeptide/protein ingredientsCobra venomPurification methods
The invention discloses a purification method, an extract and a preparation of cobra venom neurotoxin. The extract of the cobra venom neurotoxin is prepared from cobra crude venom through extraction and purification. A filter membrane filtering method is firstly adopted for removing germs, particles and macromolecular sensitizers in solution, the time and the cost are saved for the subsequent processes, and the safety of products is enhanced; an ion exchange method is adopted for eluting and separating the neurotoxin, and the separation of neurotoxin protein is improved; and finally, acetone is adopted for directly precipitating the cobra venom neurotoxin, and impurities in final products are removed. Compared with the prior art, the extract and the preparation obtained in the invention have the advantages that the product yield, the content and the purity are improved, the adverse reaction of the medicine is effectively reduced, the curative effect is improved, and the quality of products is ensured.
Owner:GUIZHOU YIBAI PHARMA CO LTD
Antineoplastic cytotoxin of snake venom and preparing technique thereof
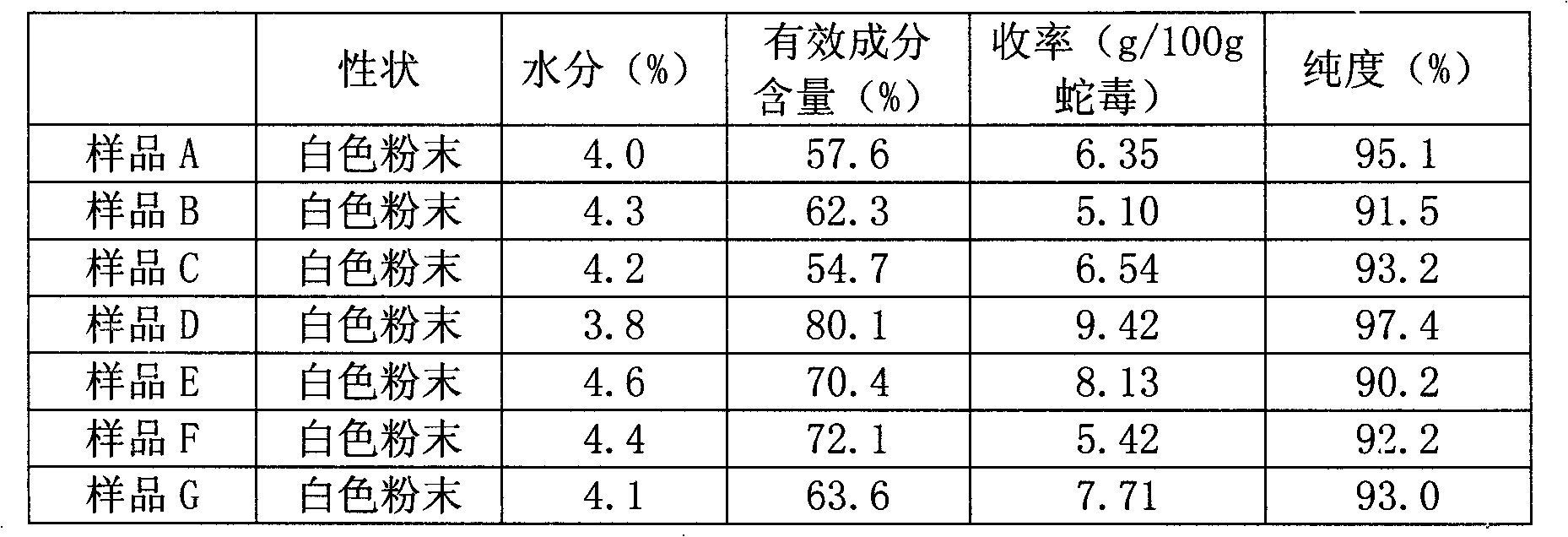
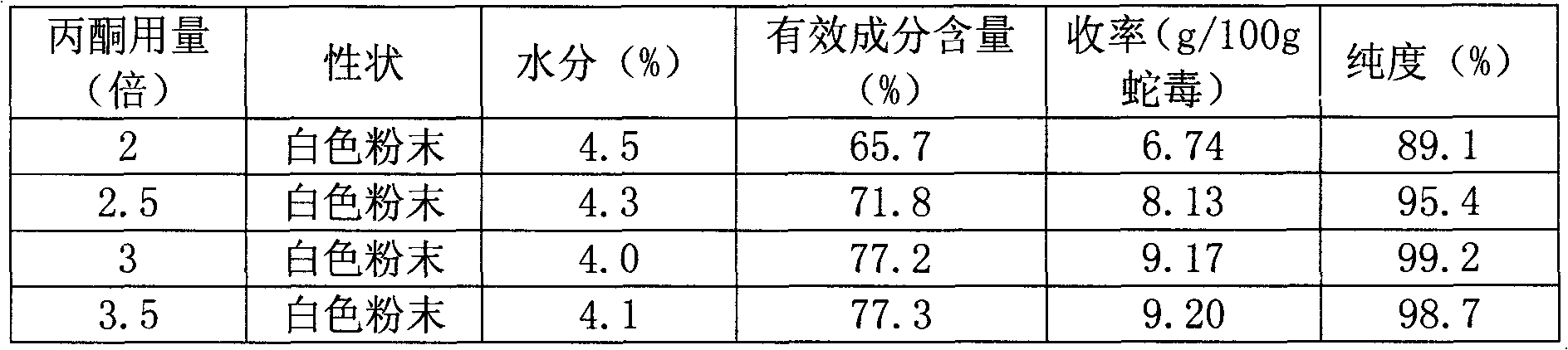
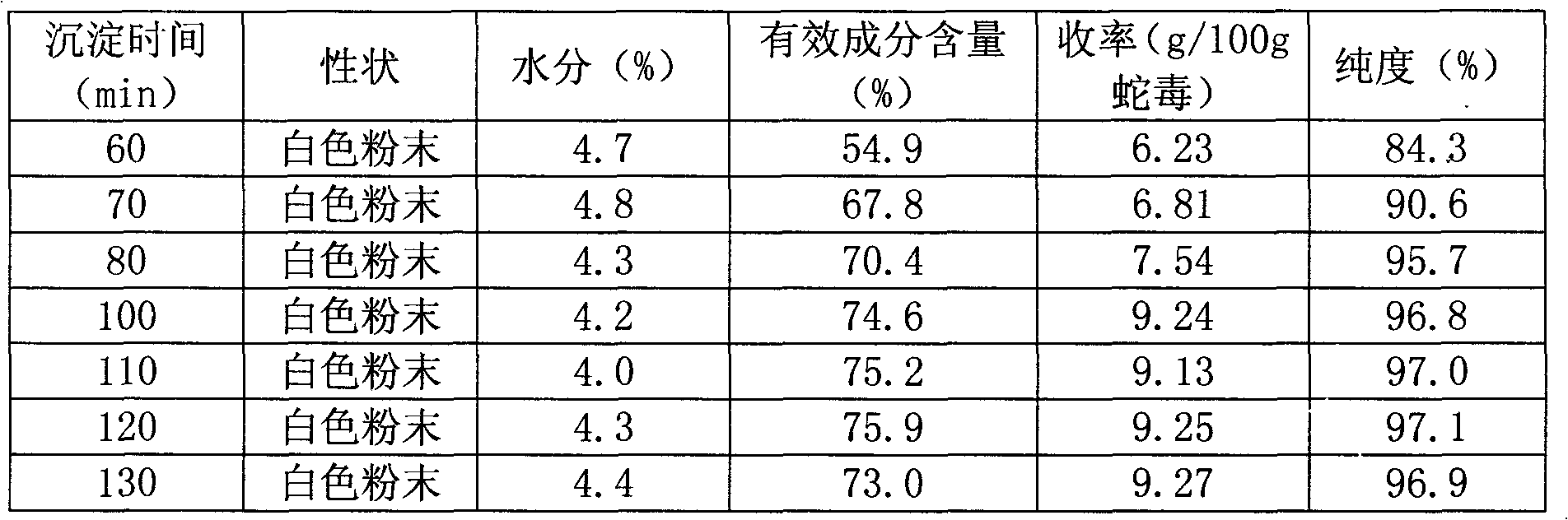
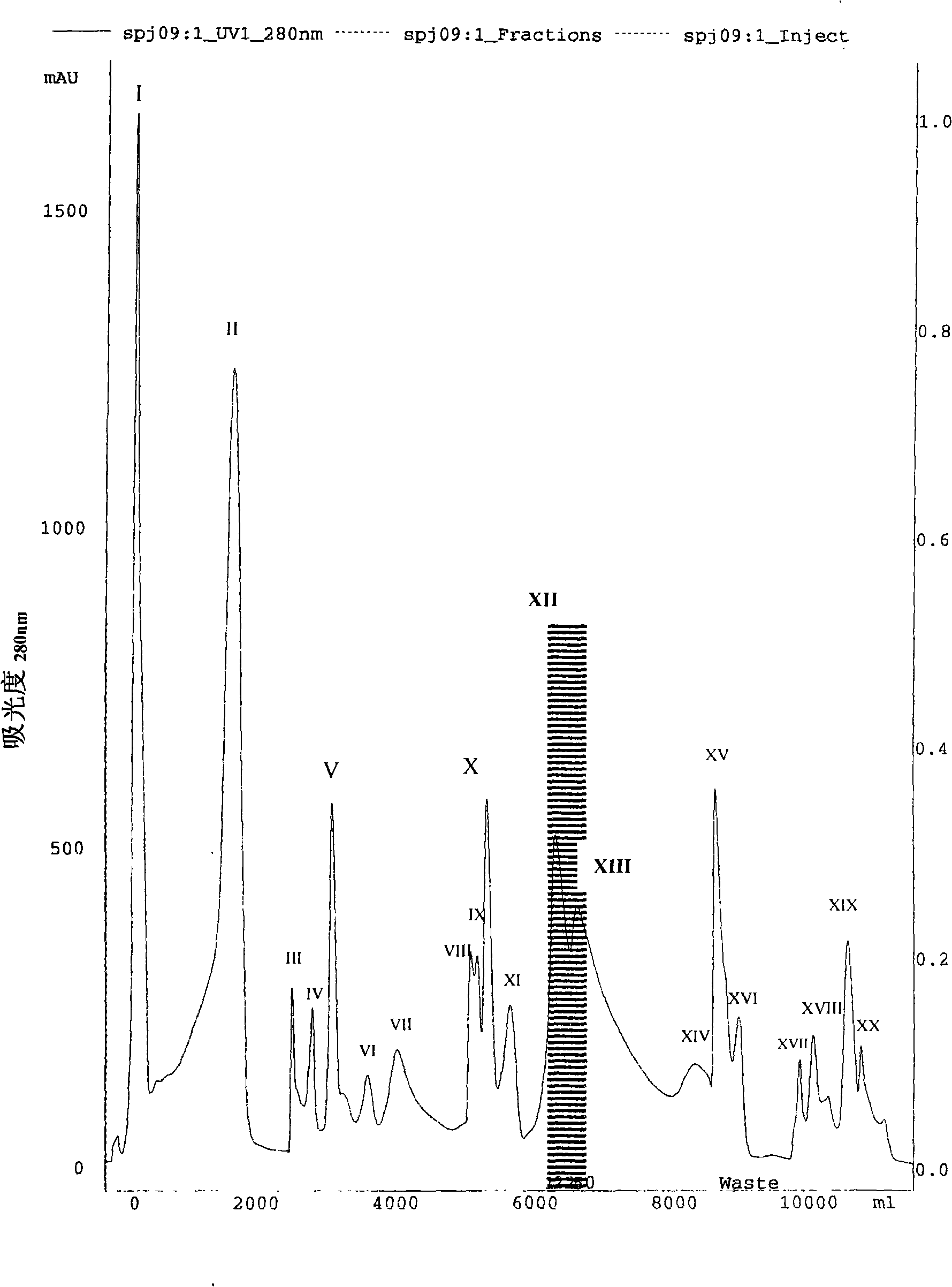
InactiveCN101270159AHigh resolutionGood repeatabilityPeptide preparation methodsAntineoplastic agentsCobra venomPhosphate
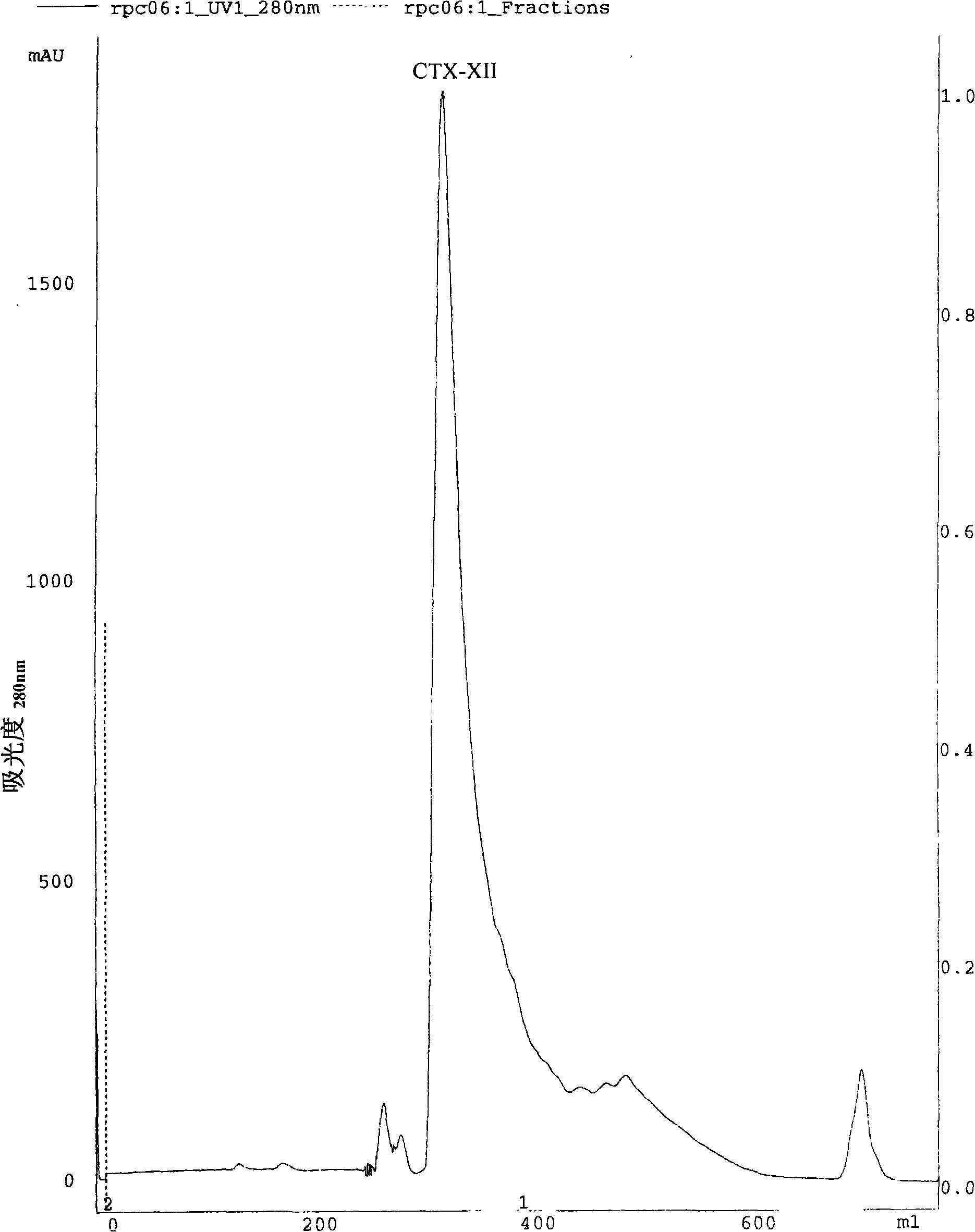
The present invention discloses an anti-tumour snake venom cytotoxin and a production technique thereof, belonging to the fields of biochemistry and biological medicines. The snake venom cytotoxin of the present invention is derived from the prior lyophilized cobra venom powder. In the production technique of the present invention, the lyophilized cobra venom powder is resolved in the known buffer solution, and then on a SP-Sepharose H.P cation exchange column (5.0cm multipled by 29cm), gradiently eluted stage by stage by phosphate buffer and NaC1 at 10ml per tube and the flowing speed of 18ml per minute, a KTA Explorer protein purification system is used to monitor and collect eluting peaks, XII and XIII peaks with cytotoxic effects are collected, and via Source 30RPC reversed phase chromatography, cytotoxin XII and cytotoxin XIII, the molecular weight of which is about 6000 daltons to 7000 daltons and the isoelectric point of which is larger than 10, are respectively obtained. Compared with the purification step of the prior art, the production technique for producing the pure anti-tumour snake venom cytotoxin product without PLA2 has good rapid repeatability and is suitable for expanded production.
Owner:FUJIAN MEDICAL UNIV
Method for separating and purifying cobra neurotoxin protein through dual-ion exchange chromatography, and preparation of cobra neurotoxin protein
ActiveCN103387610AHigh purityIncrease flow ratePeptide/protein ingredientsAntipyreticCobra venomHigh concentration
The invention provides a method for separating and purifying cobra neurotoxin protein through dual-ion exchange chromatography. The method comprises the following steps of: (1) first separation and purification through ion exchange chromatography, namely, a) dissolution of crude cobra venom, and b) separation and purification through an SP-Sephodex-C25 gel column; and (2) second separation and purification through ion exchange chromatography, namely, a) balance of a SourceS(XK5030) column having a column volume of 500 ml by using 1000 ml of liquid A for future use; and b) SourceS(XK5030) column purification, and then collection of the cobra neurotoxin protein purified twice according to a standard purification chromatogram. The method provided by the invention is scientific and rational in process; and no organic reagent, no high-concentration salt and no protein modification method are used in production, so that the biological activity of cobratide can be maintained to an utmost extent. The method provided by the invention is advanced in process and simple to operate; the production period can be greatly shortened, the production time can be saved and the production cost can be reduced; and the method is completely suitable for an industrial production line.
Owner:奔驰生物科技(云南)有限公司
Modified elapid venoms as stimulators of the immune reaction
ActiveUS20080107752A1Avoid seizuresSuppress continued developmentBiocidePeptide/protein ingredientsCobra venomIMMUNE STIMULANTS
Detoxified cobra venom and its constituent neurotoxins have been reported to have potent antiviral activity. Others have reported that snake venoms were generally immune stimulants. Recent research has revealed more specific details on the effects of detoxified venoms and isolated alpha-neurotoxins on cells of the immune system. Exposure of the immune cells to these detoxified proteins yields a strong response in the innate immune reaction that represents the immune systems initial response to infectious agents. Of particular relevance is the marked increase in the expression of genes associated with the production of gamma interferon, a potent antiviral agent and regulator of the immune response. The ability to induce this strong innate response has significant application to those with weakened immune systems where their ability to fight infection has been compromised. It also has the potential application to act as a method to protect individuals from contagious infectious agents as a substitute for anti-viral vaccines.
Owner:RECEPTOPHARM
Venin-sourced demulcent CTXn, and purification method and applications thereof
InactiveCN101717441ARaise the response thresholdGood analgesic effectPeptide/protein ingredientsAntipyreticCobra venomVirulent characteristics
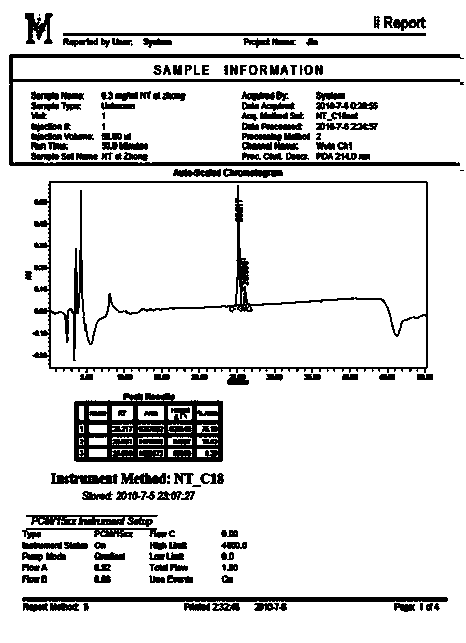
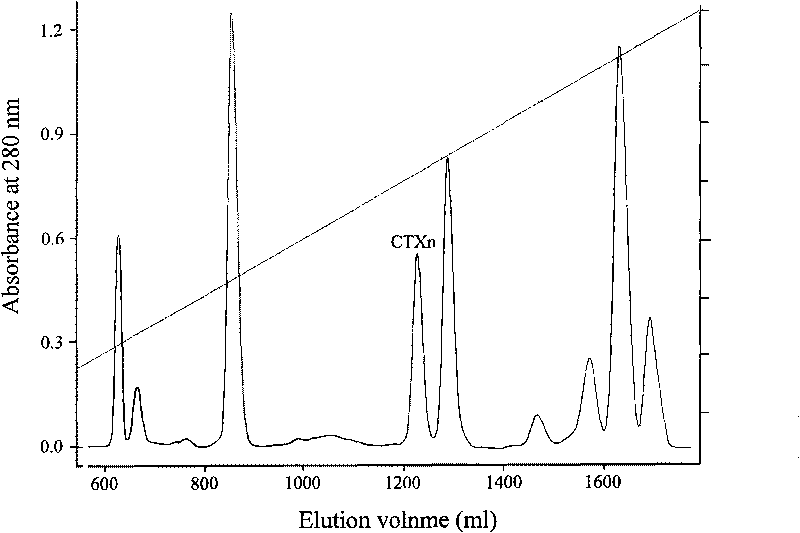
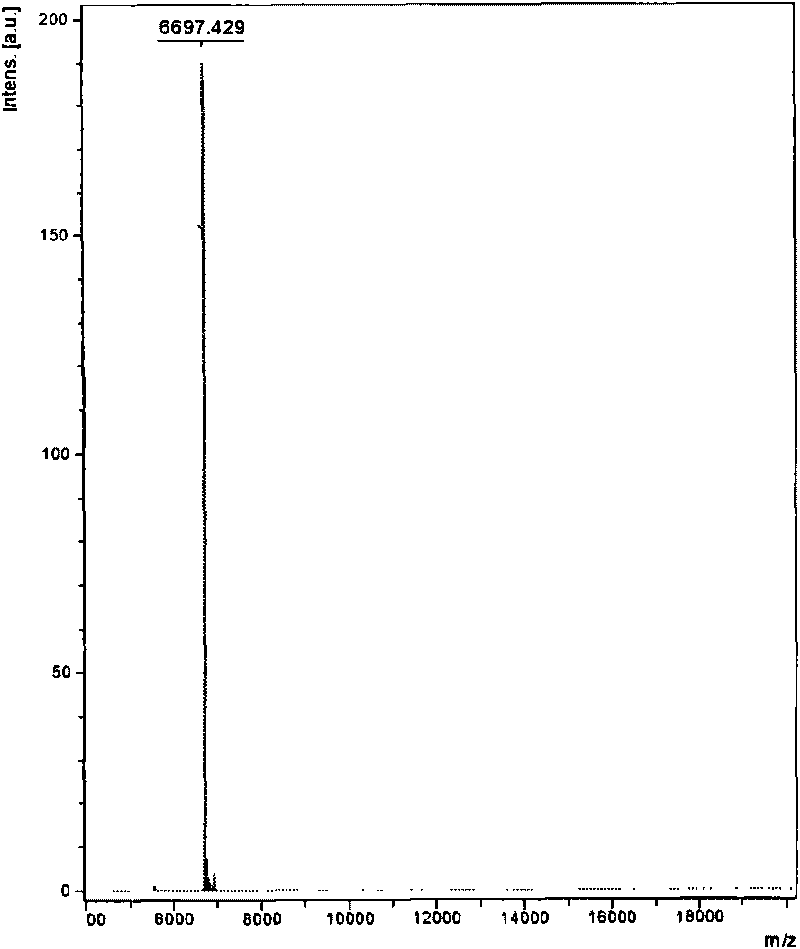
The invention relates to a venin-sourced demulcent CTXn, and a purification method and applications thereof. The invention is characterized in that (1) the molecular weight of the CTXn is 6697.429 Da; (2) and the amino acid sequence of the CTXn is described as the sequence list SEQ ID No.1. The preparation method comprises the following steps of: using a Zhoushan cobra venom sample; carrying out the CTXn separation and purification on the Zhoushan cobra venom sample; primary-screening to select a component III for next step of ion exchange; carrying out the ion exchange on the component III through a quick-purification technique exploitation system by using a filler to obtain a single-virulence polypeptide CTXn, desalting with a G-50 prepacked column, and freeze-drying; and measuring the molecular weight and the amino acid sequence of the CTXn. The CTXn of the invention has favorable demulcent function on somatalgia and visceralgia caused by nociceptive stimulus as well as pains caused by addiction to morphine, has the characteristics of obvious drug effect and low toxic or side effect, is beneficial to large-scale production, and has wide application prospects in the aspect of analgesic development.
Owner:广州医学院
Composition and method for oral delivery of cobra venom
InactiveUS20110311642A1Easy to measure and manageDispersion deliveryAntipyreticCobra venomOral medication
A composition of sterile cobra venom and a method for its oral administration to provide significant analgesic effects to a human and / or animal are disclosed. Such cobra venom compositions comprise a sterilized solution preserved by the addition of one or more suitable food-grade preservatives. The venom composition may be conveniently administered orally by means of a metered spray device.
Owner:REID PAUL
Cobra-venom factor and cobra-venom neurotoxin combined separation and purification method
InactiveCN101747409AImprove utilization efficiencyReduce mutual interferencePeptide preparation methodsAnimals/human peptidesCobra venomPurification methods
The invention discloses a cobra-venom factor and cobra-venom neurotoxin combined separation and purification method which includes the following steps: firstly the cobra-venom crude liquid is processed with centrifugation treatment at low temperature and high speed, and then small molecule substances, low molecular peptides and the like contained in the cobra-venom crude liquid are removed through a 3KDa ultrafiltration membrane. The two chromatography processes respectively adopt a CM-Sephrose. FF chromatographic column and a Superdex.200 gel chromatographic column; and cobra-venom neurotoxin and cobra-venom factor which are two active ingredients can be obtained in one production process. The method has the characteristics that the cobra-venom factor and the cobra-venom neurotoxin can be separated, the application efficiency of the cobra-venom is effectively improved, the purities of the products are improved and the method is suitable for industrial production.
Owner:ORIENTOXIN BIOTECH
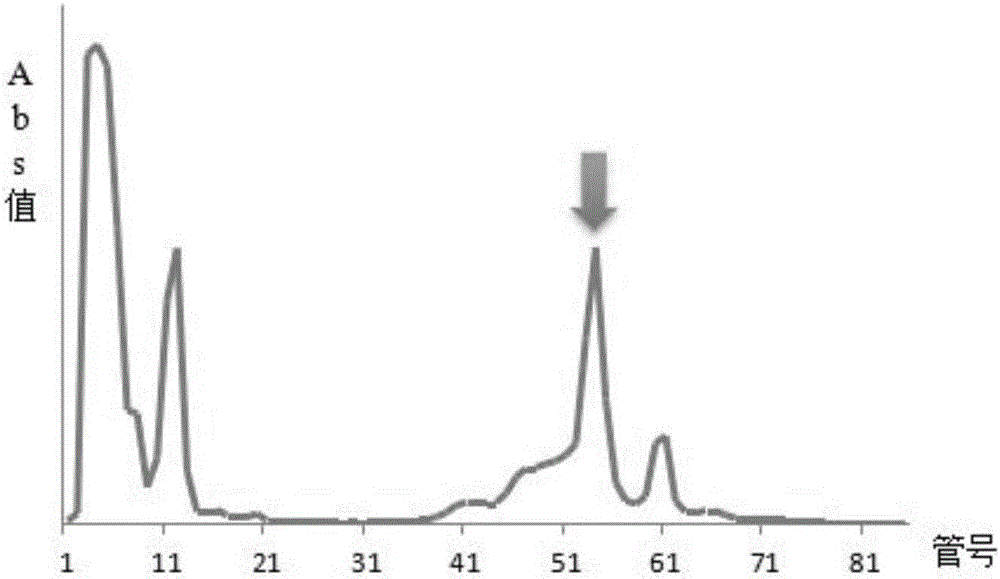
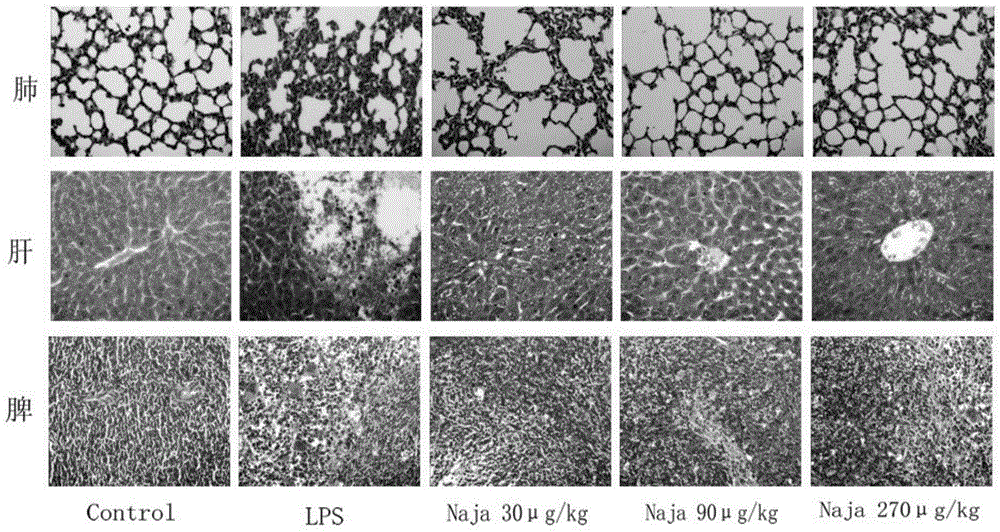
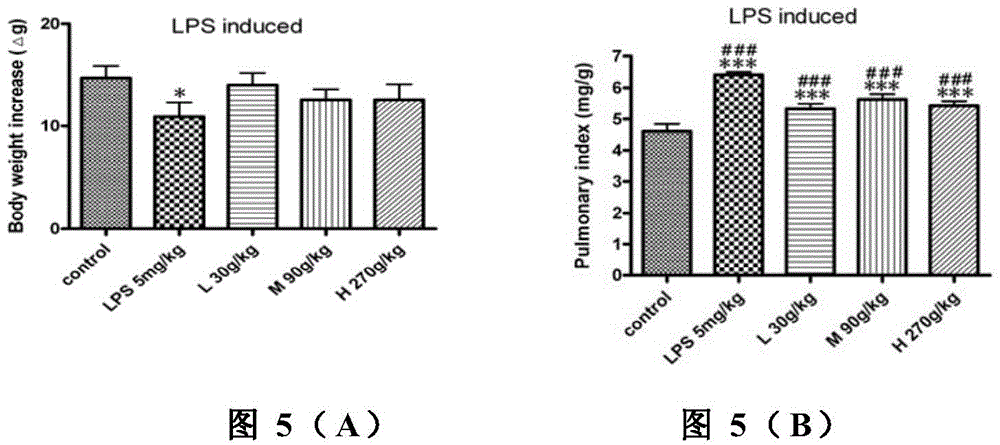
Application of physically-modified cobra venom in preparation of medicament for treating pulmonary fibrosis
InactiveCN102526114ALow toxicityImprove efficacyReptile material medical ingredientsRespiratory disorderDiseaseCobra venom
The invention discloses application of cobra venom in preparation of a medicament for treating lung inflammatory disease and pulmonary fibrosis. Animal experiment proves that Chinese cobra venom can alleviate acute endotoxin poisoning symptom within a range of 10-3000mug / kg, particularly has obvious therapeutic action on lung inflammatory disease caused by endotoxin, and can be used for alleviating inflammatory cell infiltration, alveolar septum thickening, and focal atelectasis and emphysema. Repeated small dose of endotoxin can result in pulmonary fibrosis, the cobra venom can significantly alleviate pulmonary fibrosis symptom and reduce alveoli damage and collagen hyperplasia. Naturally-modified Chinese cobra venom and physically-modified Chinese cobra venom have similar actions, but the physically-modified Chinese cobra venom has better action. Used for treating lung inflammatory disease, the Chinese cobra venom has the advantages of low dose, safety, capability of being orally administrated, injected, and administered through oral mucous membrane and skin and the like.
Owner:SUZHOU RENBEN PHARMA
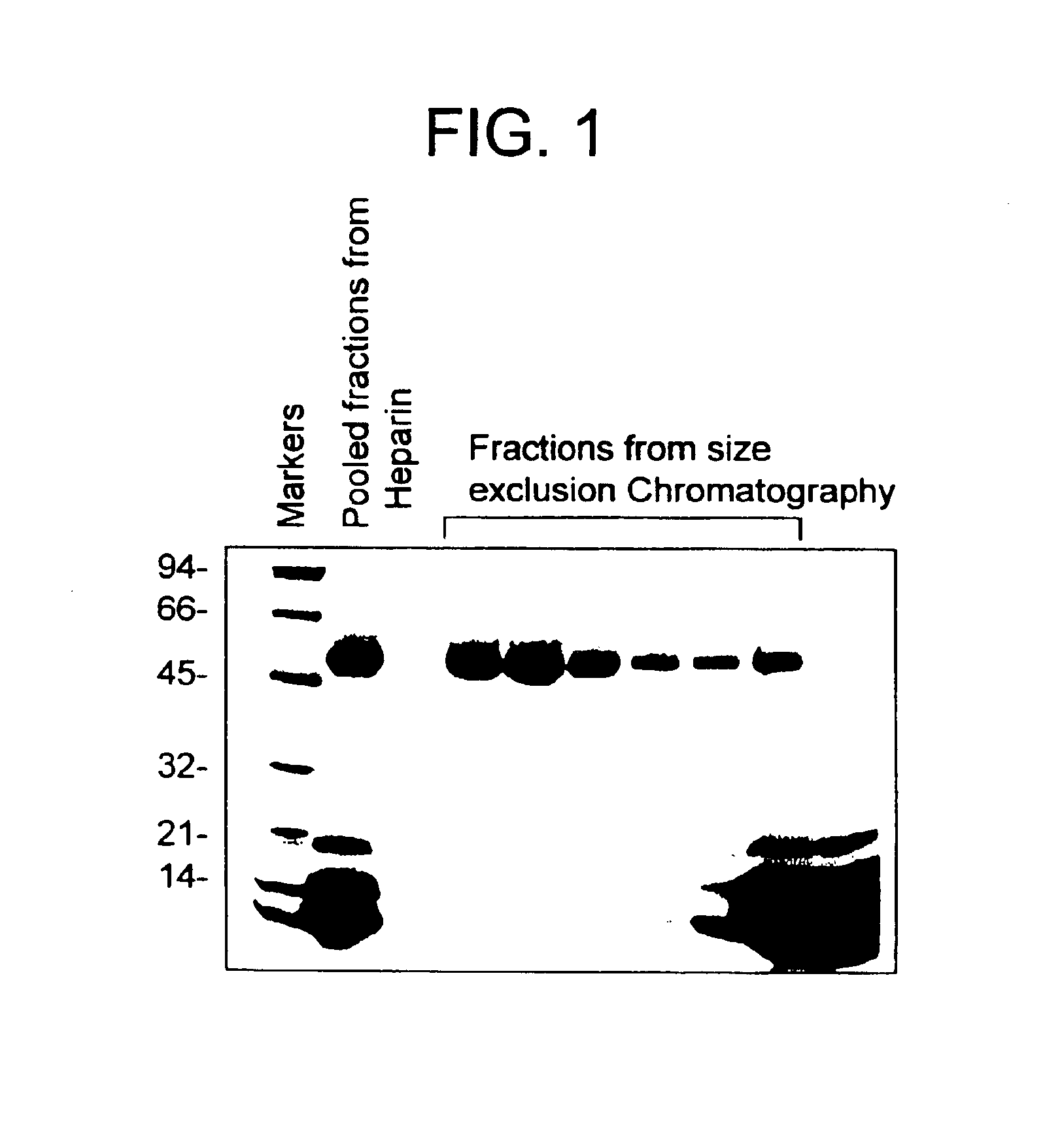
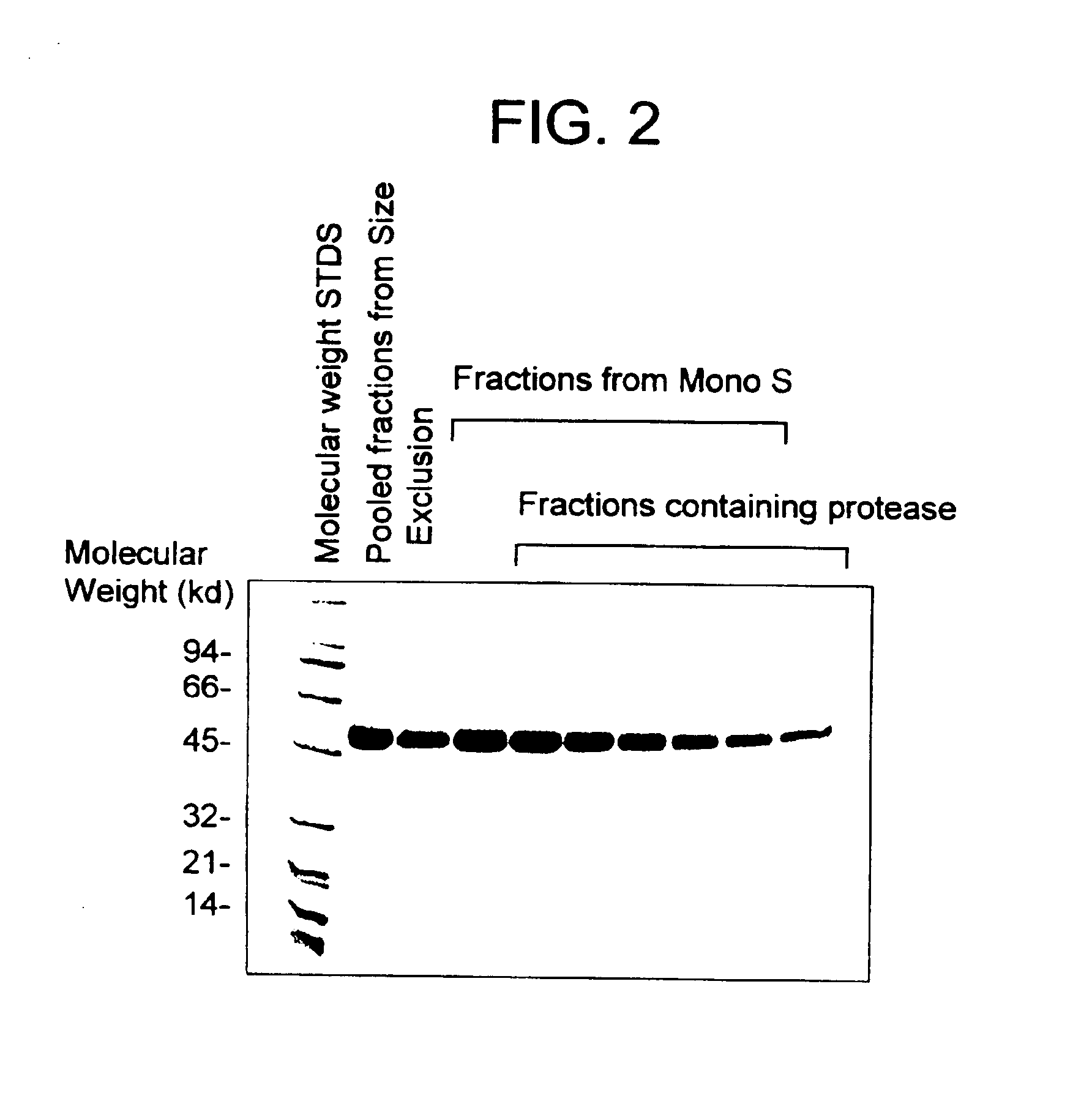
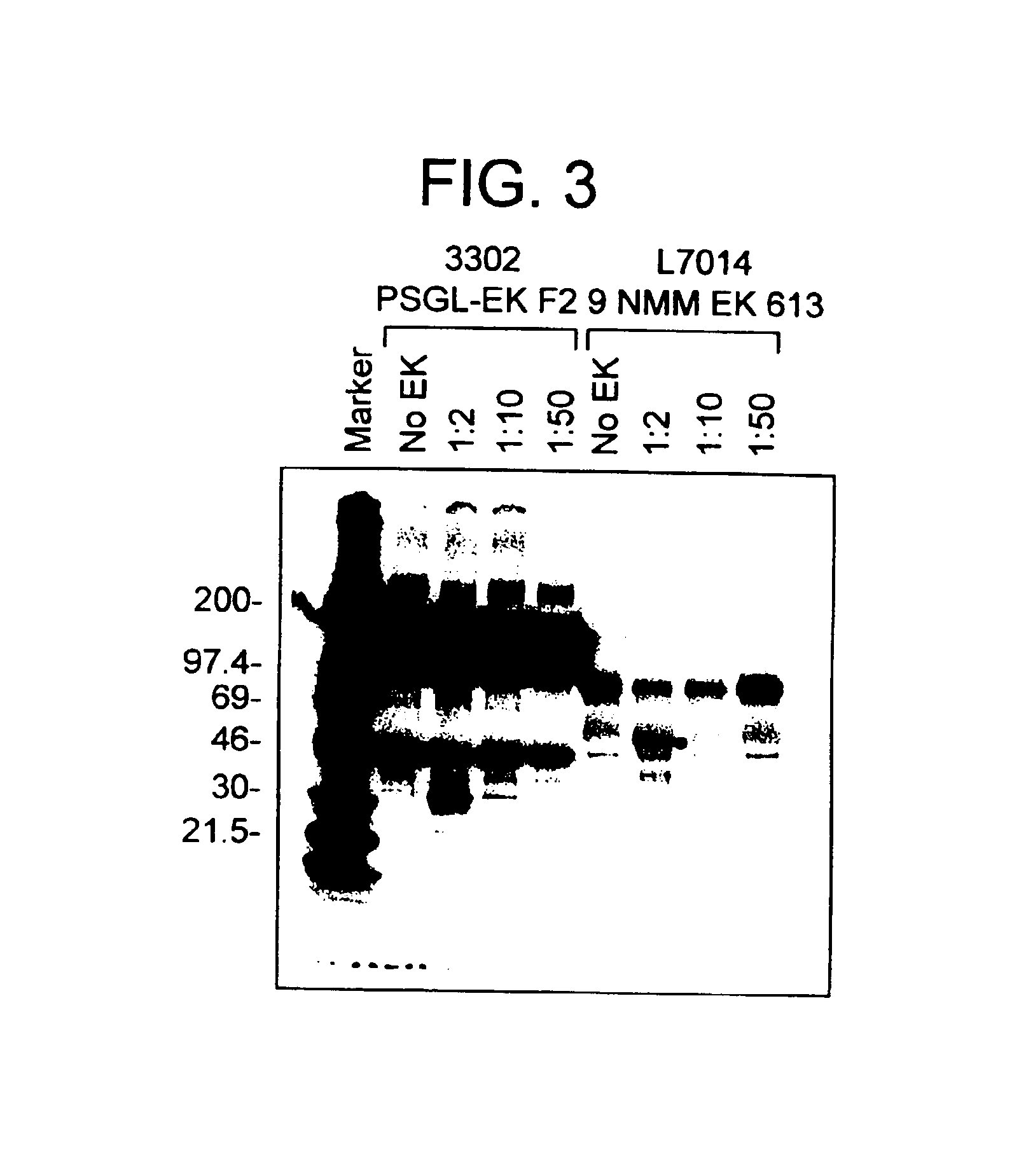
Highly purified mocarhagin, a cobra venom protease, polynucleotides encoding same and related proteases, and therapeutic uses thereof
Highly purified mocarhagin, a cobra venom protease, is disclosed. Pharmaceutical compositions and therapeutic uses of the highly purified protease are also provided. Polynucleotides encoding such protease and related proteases are also disclosed.
Owner:GENETICS INST LLC
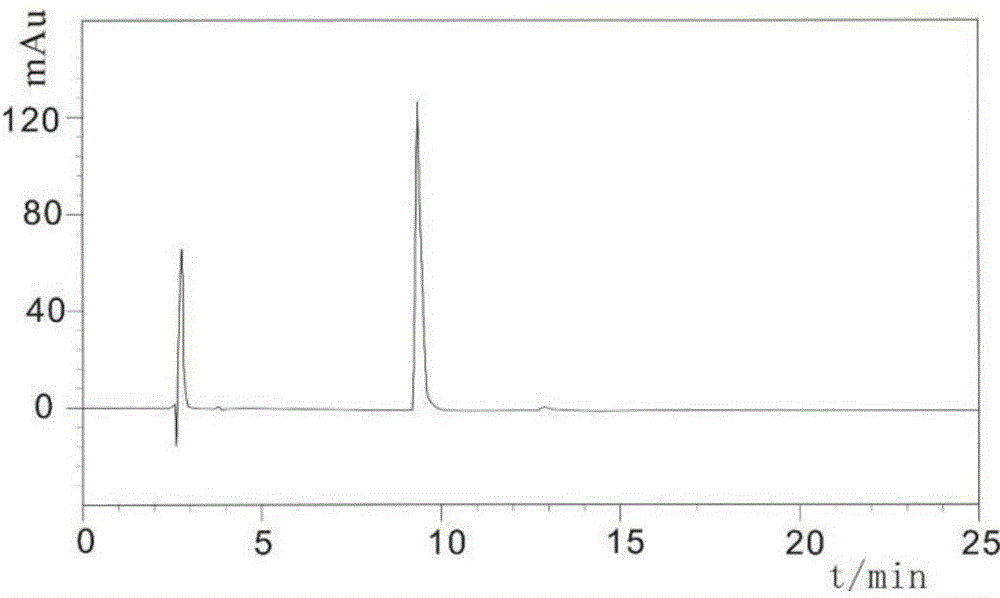
Method for separating and purifying cobra-venom neurotoxin by combining ion exchange and hydrophobic chromatography
ActiveCN104974238ASuitable for industrial productionEasy to operatePeptide preparation methodsAnimals/human peptidesCobra venomPhosphate
The invention particularly relates to a method for separating and purifying cobra-venom neurotoxin by combining ion exchange and hydrophobic chromatography. The method comprises the following steps: (1) weighing 4-5 parts by weight of cobra-venom crude product, adding the cobra-venom crude product into 0.04-0.05 part by volume of water or 0.01-0.05M of phosphate buffer solution with the pH of 5.0-7.0, stirring to dissolve the cobra-venom crude product, centrifuging the solution at the temperature of 0-8 DEG C, discarding precipitates, and filtrating supernatant so as to obtain a test sample solution A for later use; (2) purifying the test sample solution A through ion-exchange chromatography, carrying out gradient elution, carrying out HPLC (High-Performance Liquid Chromatography) detection, and carrying out collecting so as to obtain a test sample solution B for later use; and (3) purifying the test sample solution B through hydrophobic chromatography, carrying out gradient elution, carrying out HPLC detection, carrying out collecting, carrying out concentrating, and carrying out drying thereby obtaining a pure product of cobra-venom neurotoxin, wherein when the unit of parts by weight is g, the unit of parts by volume is correspondingly L. According to the method, operating steps are simplified, the separation and purification cycle is shortened, the yield is relatively high, the purity is relatively high, and thus the method is applicable to the industrial production of cobra-venom neurotoxin.
Owner:SUZHOU RENBEN PHARMA
The mixture of cobra venom and Chinese medical herb extract and its application
InactiveCN1943778ASignificant effectSuit one's needsAnthropod material medical ingredientsInanimate material medical ingredientsCobra venomSnake venom
This invention discloses a mixture of cobra venom with Chinese herb extracts and its applications. , of which the weight portion is 0.004-0.006 and 3-6 respectively. The said Chinese herb extracts are prepared according to the following weight ratios: Succinum (13~17),Caulis Sargentodoxae (8~12),Rhizoma Curculiginis (8~12),Rhizoma erubescens Schott (6~10),Scolopendra (2~6),Dysosma versipellis (6~10 ),Gekko (8~12),Radix Cynanchi Paniculati (6~10),scorpion (8~12),Rhizoma Zingiberis Recens (8~12),Radix Notoginseng (8~12),Herba Lobellae Chinensis (15~25),Ganoderma lucidum (15~25),Cordyceps sinensis (3~7),Radix Glycyrrhizae (25~35),Paris polyphylla (8~12).The mixture of Cobra Venom with Chinese herb extracts in this invention has a remarkable curative effect on cancer. It is able to satisfy people's requirements and worth promoting its application.
Owner:薛忠 +1
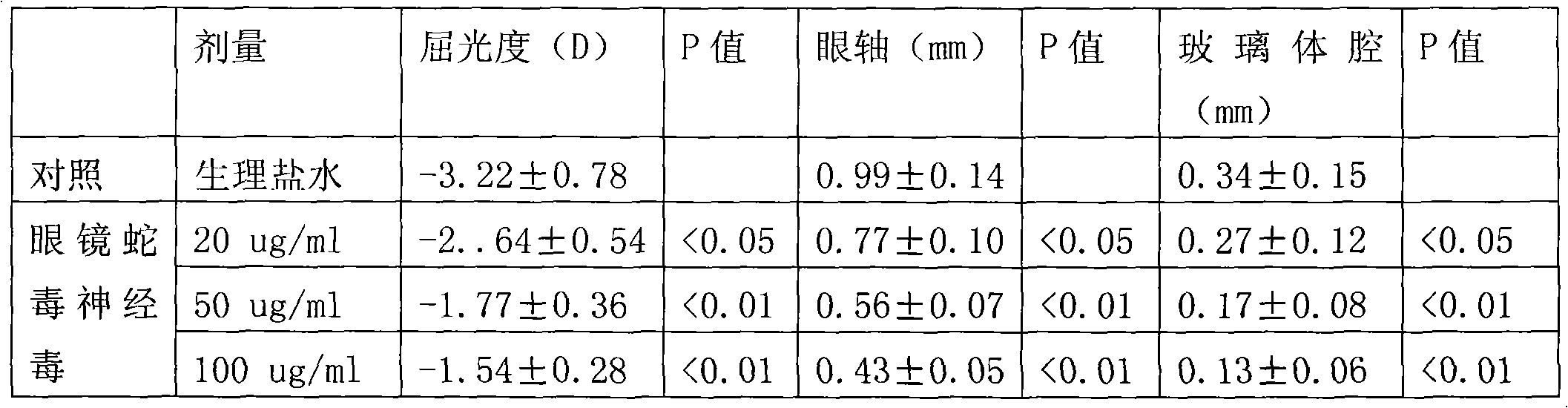
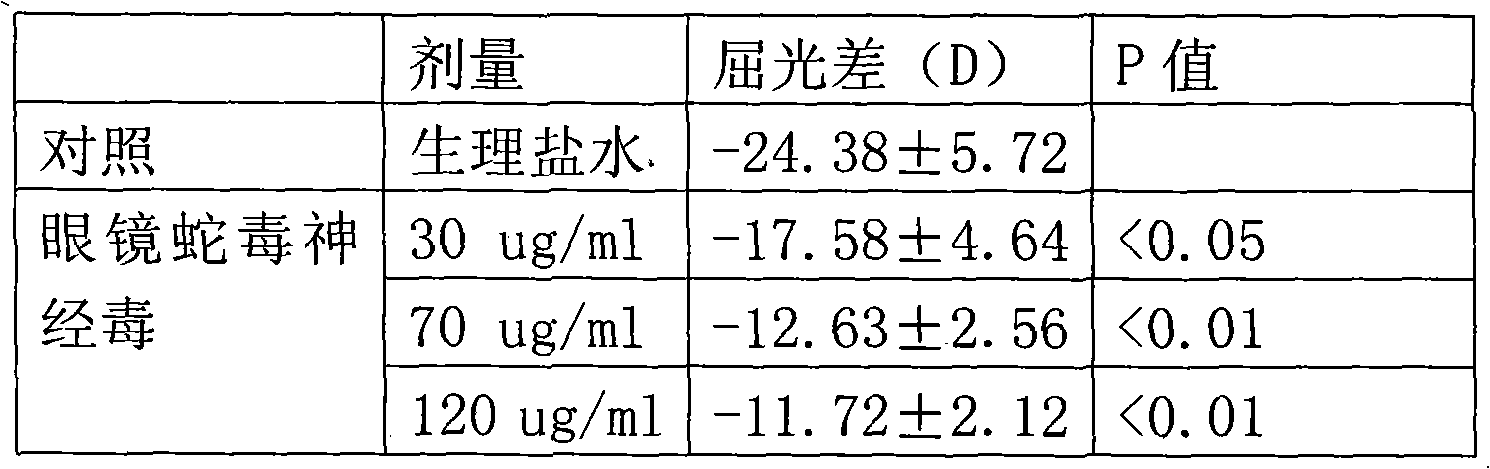
Application of cobra venom neurotoxin in drugs for treating ametropia
The invention relates to application of cobra venom neurotoxin in pharmacy, particularly in drugs for treating ametropia, belonging to technical field of biomedicine. The invention particularly relates to application of cobra venom neurotoxin or a hydrolyzed fragment of the neurotoxin. The neurotoxin or the hydrolyzed fragment of the neurotoxin can be an active ingredient used for preparing drugs for treating ametropia only or together with antibiotics. According to the invention, as an active ingredient for treating ametropia, the cobra venom neurotoxin with low dosage not only can stop signal transduction of neuromuscular junctions, but also can adjust a receptor of the cobra venom neurotoxin to balance a nerve-muscle function, thereby achieving the aim of treating ametropia.
Owner:KUNMING MAOBO BIOLOGICAL TECH
Production method of growth factor supported porous biologic ceramic artificial bone scaffold
InactiveCN105561397APromote proliferationPromote directed differentiationTissue regenerationProsthesisCobra venomOsteoblast
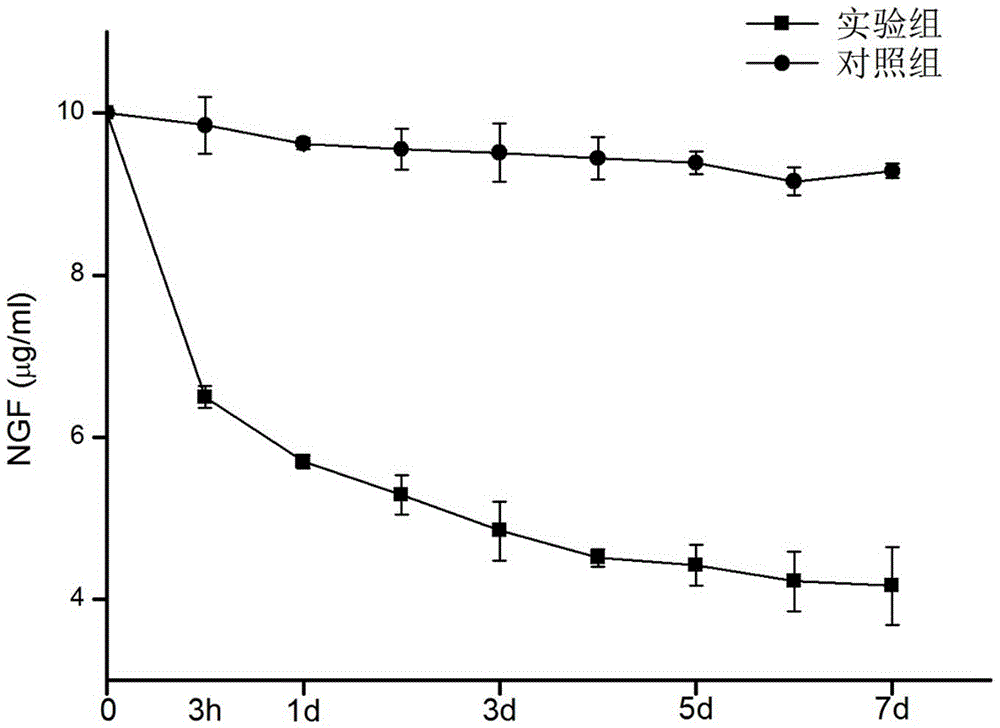
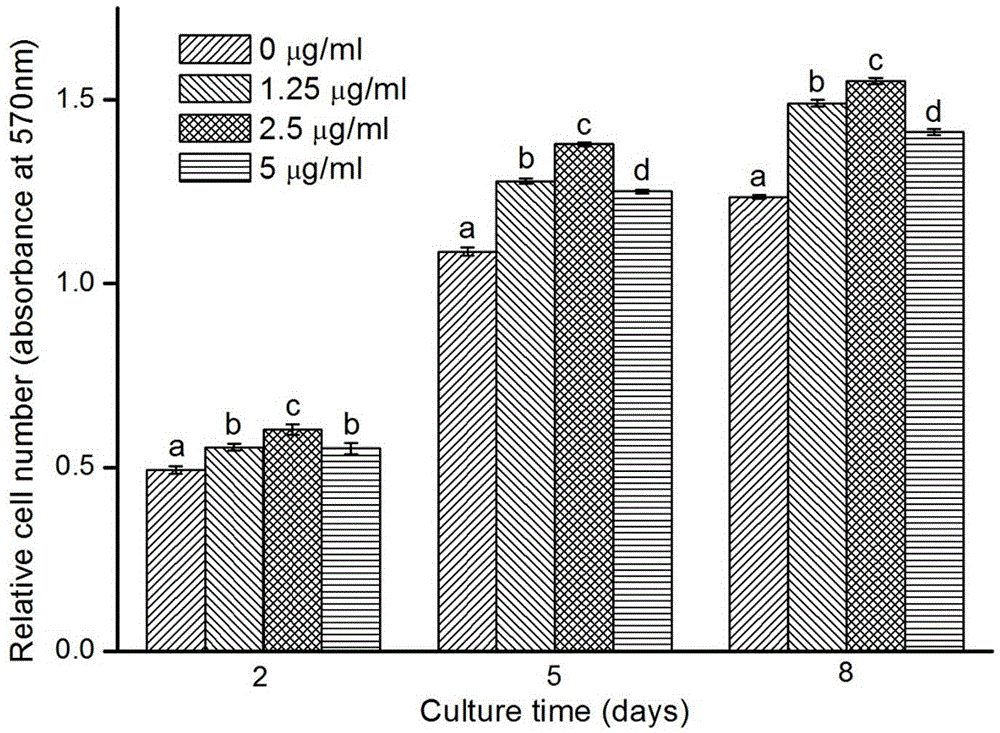
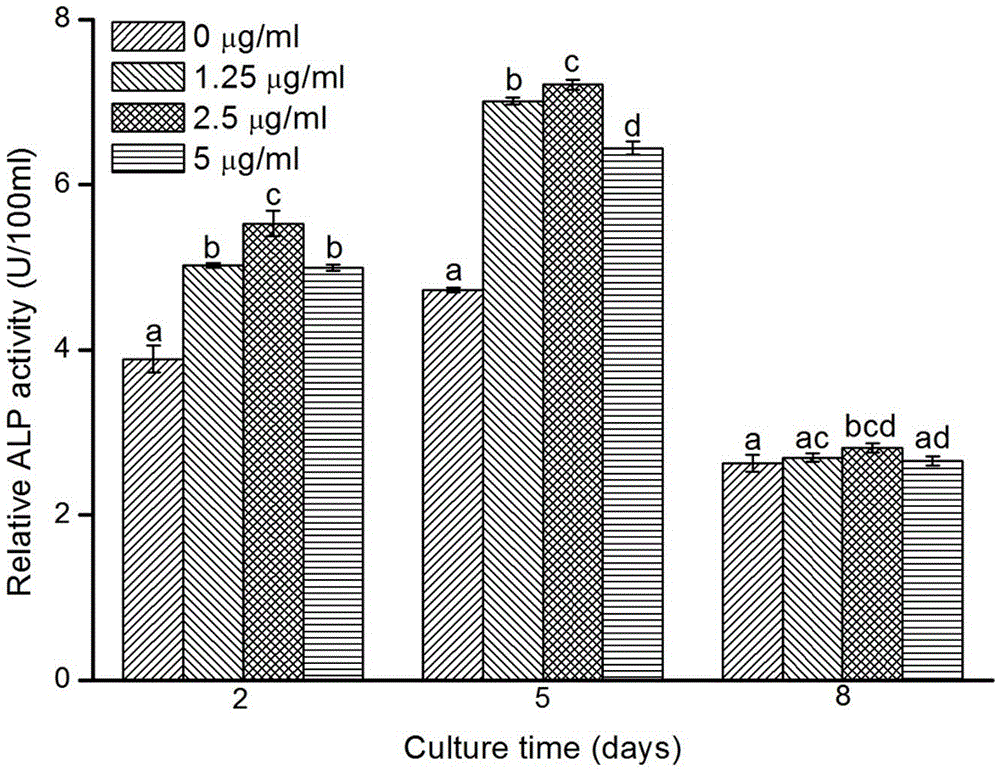
The invention discloses a production method of a growth factor supported porous biologic ceramic artificial bone scaffold, and belongs to the field of medical science and biomedical engineering. Qualitative and quantitative detection of a cobra venom nerve growth factor (NFG) adsorbed through a biphasic calcium phosphate ceramic (BCP) artificial bone scaffold and artificial bone scaffold function detection prove that the NGF supported BCP is in favor of realizing osteoblast proliferation and directed differentiation. The NGP supported BCP has a good ossification promotion effect, provides a possible method for producing osteoblast in vitro culture scaffolds, and makes researches of bioengineering materials be possible.
Owner:GUANGXI MEDICAL UNIVERSITY
Application of cobra venom nerve growth factor in preparing the medicine of preventing and treating liver fibrosis and liver cirrhosis
InactiveCN102552881ASignificant effectSmall toxicityPeptide/protein ingredientsDigestive systemCobra venomSide effect
The invention discloses an application of cobra venom nerve growth factor in preparing the medicine of preventing and treating liver fibrosis and liver cirrhosis. The study indicates that the cobra venom nerve growth factor has the effects of inducing apoptosis in HSC-T6, resisting liver fibrosis at the cellular level and in animals, and effectively inhibiting or reducing the degree of hepatic fibrosis. Taking the cobra venom nerve growth factor as a medicinal main active ingredient, the application has the advantages of remarkable efficacy, fast effect, safety and efficacy, few side effects. The cobra venom nerve growth factor is an ideal medicine of preventing and treating liver fibrosis and liver cirrhosis.
Owner:GUANGXI MEDICAL UNIVERSITY
Cobra venom nerve growth factor purification and preparation method
InactiveCN105732796AHigh activityHigh purityPeptide preparation methodsAnimals/human peptidesCobra venomSephadex
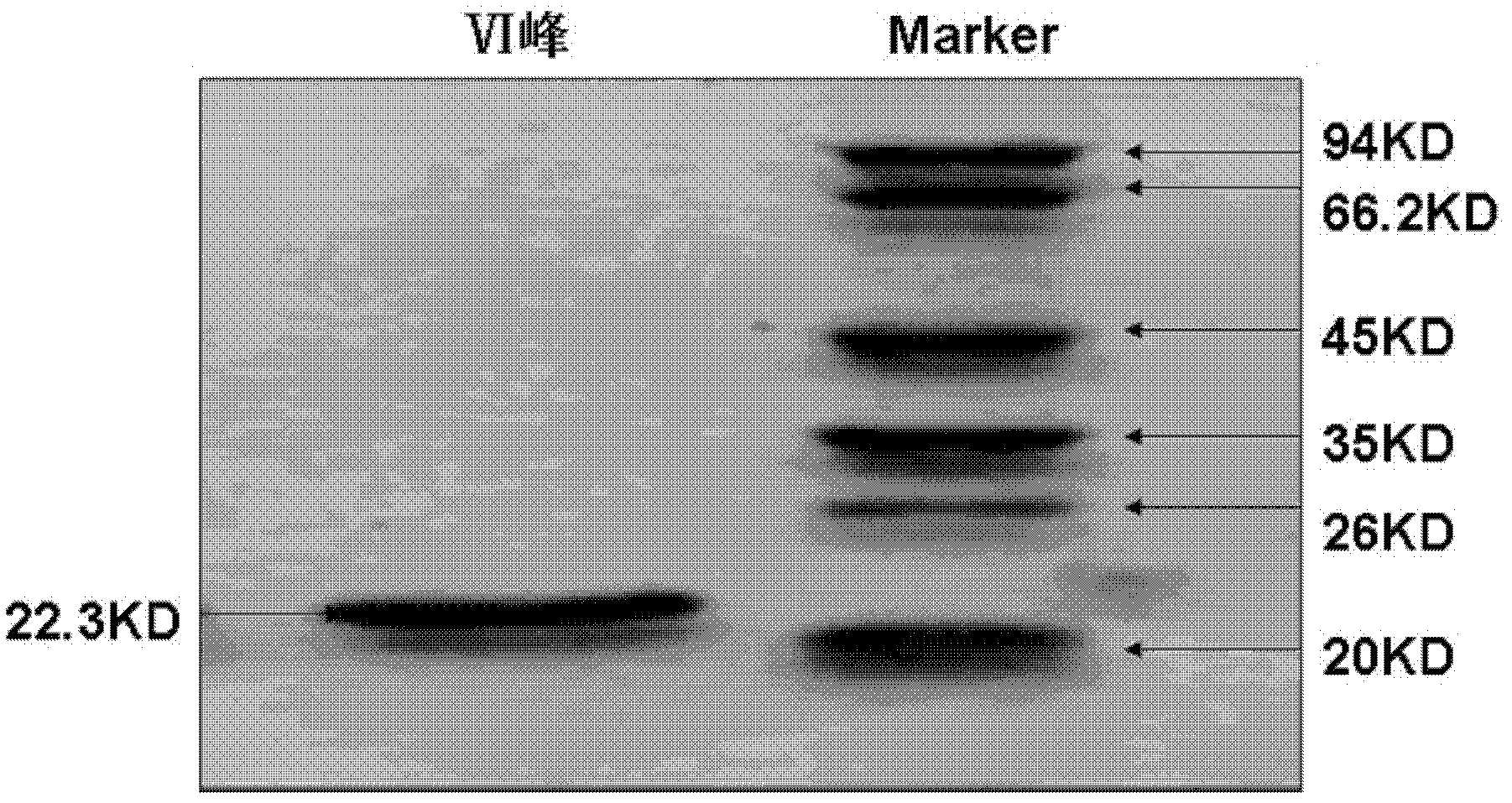
The invention discloses a cobra venom nerve growth factor purification and preparation method. Firstly, a DEAE CL-6B anion exchange column is adopted and then a Sephadex G-50 gel chromatography column, a Macro-prep High S cation exchange column is utilized for separation in sequence, a PC12 cell test method is adopted to dilute collected peaks with ultra-pure water after each time of separation, and then testing is performed to select the peaks having the highest cobra venom nerve growth factor activity as samples for subsequent separation, wherein the minimum concentration of NGF induced PC12 cell differentiation is determined through dilution. An inventor finds peaks having the highest NGF activity in the cobra venom separation process and determines that the minimum concentration of the NGF induced PC12 cell differentiation is up to 0.1 micrograms / ml. Tests show that electrophoresed pure NGF can be obtained by applying the method and have high purity and high activity.
Owner:GUANGXI MEDICAL UNIVERSITY
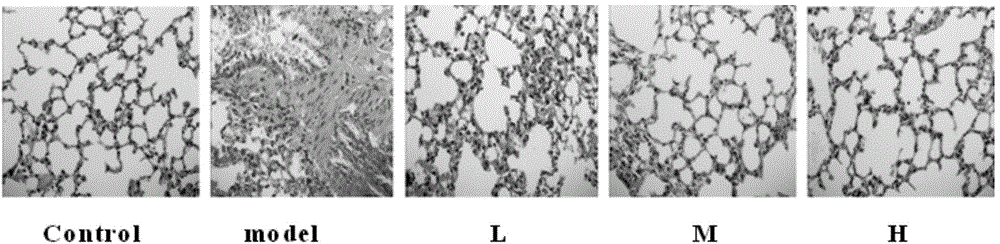
Application of cobra venom in preparation of medicament for treating chronic obstructive pulmonary disease
InactiveCN104434981AGood treatment effectTo achieve the purpose of reducing toxicity and increasing efficiencyPeptide/protein ingredientsUnknown materialsInflammatory factorsCobra venom
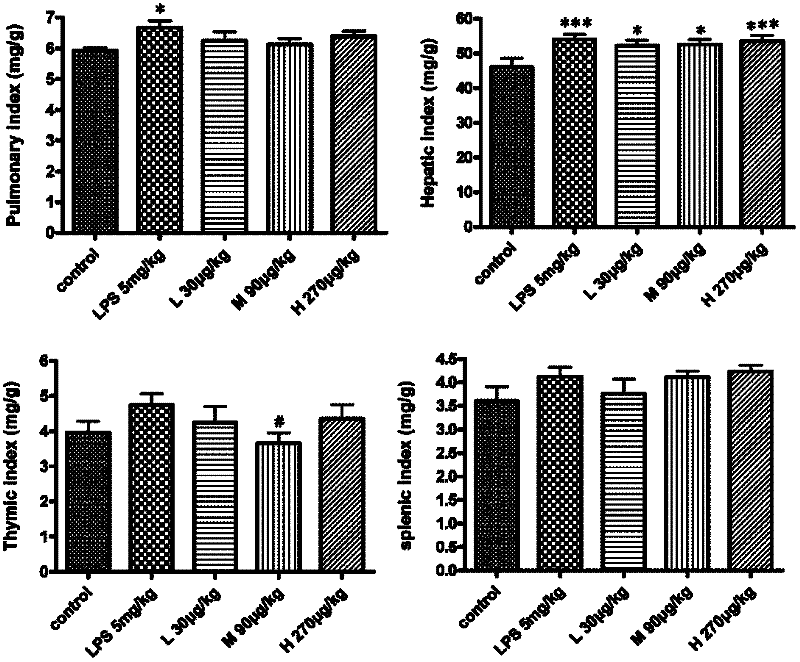
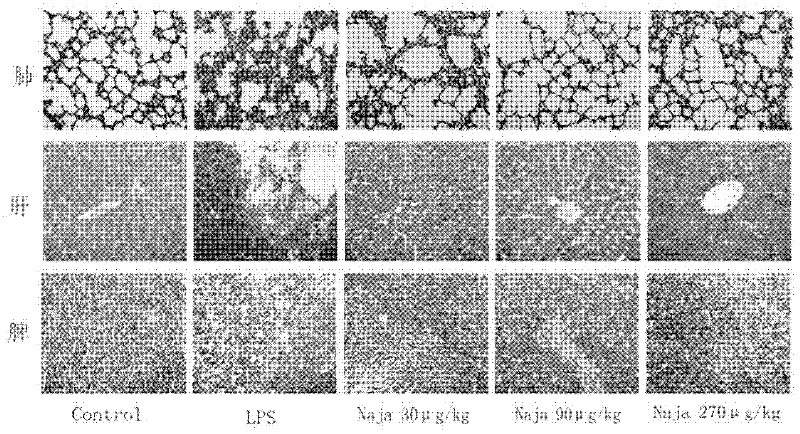
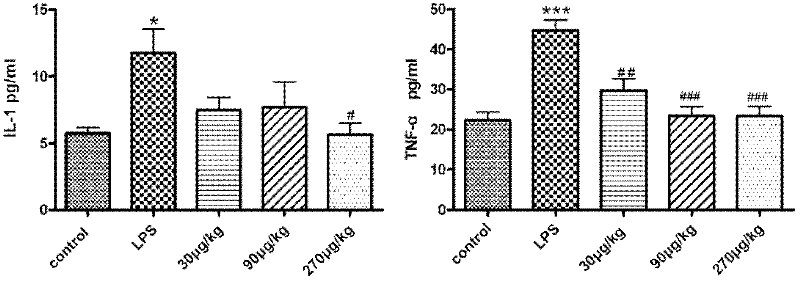
The invention relates to the technical field of medicaments, and particularly relates to an application of cobra venom in preparation of a medicament for treating chronic obstructive pulmonary disease. The cobra venom has an obvious treatment effect on chronic obstructive pneumonia induced by repeatedly contacting LPS (lipopolysaccharide) for a long term, and can effectively improve the pathological injury of lung tissues. The Chinese cobra venom not only can reduce the lung coefficient of a chronic obstructive pneumonia mouse, but also can effectively protect the lung tissues. The cobra venom not only can inhibit the degradation of IkB-alpha in the lung tissues of a sick mouse, but also can lower the expression of TGF-beta1. The cobra venom not only can reduce the TNF-alpha level, but also can reduce the level of an inflammatory factor IL-1.
Owner:SUZHOU RENBEN PHARMA
Natural medical composition for treating hepatitis B
InactiveCN101933957AImprove efficacyEliminate side effectsOrganic active ingredientsDigestive systemCobra venomSodium bicarbonate
The invention discloses a natural medical composition for treating hepatitis B. The medical composition is prepared from the following components in percentage by weight: 10-20g of radix phytolaccae, 0.02-0.03g of cobra-venom, 2-3g of lecithin, 60-80g of cordyceps polysaccharide, 50-60g of honey, 60-70g of syrupus simplex, 0.5-0.6g of hydroxy ethyl and 0.8g of sodium bicarbonate. The medicine of the invention decreases the cost of a traditional hepatitis B treatment scheme, simultaneously lessens the toxic and side effects on the medicine on human bodies, improves the spectral sensitivity and the stability and can solve the problem of instable medical effect of traditional Chinese medicines.
Owner:上海同济环境工程科技有限公司
Purification preparation method and application of cobra venom CTX (cytotoxin)-4N
InactiveCN107098956AHigh activityIncrease concentrationPeptide/protein ingredientsDigestive systemCobra venomElectrophoresis
The invention discloses a purification preparation method of cobra venom CTX (cytotoxin)-4N. According to the method, a DEAE-SepharoseCL-6B anion exchange column is firstly used; then, a SephadexG-50 gel chromatographic column and a Macro-prep High S cation exchange column are used for respective separation; after separation in each time, the peak, with the highest activity, of the cobra venom CTX-4N is selected as a sample of the subsequent separation. Research shows after the method is used, CTX-4N with electrophoresis purity can be obtained; the purity is high; the activity is high. HSC-T6 proliferation inhibition and apoptosis experiments prove that the CTX-4N has a killing effect on HSC-T6 cells; the condition that the CTX-4N has the anti-hepatic fibrosis effect in the hepatic fibrosis development process is prompted; important medicinal values are realized.
Owner:GUANGXI MEDICAL UNIVERSITY
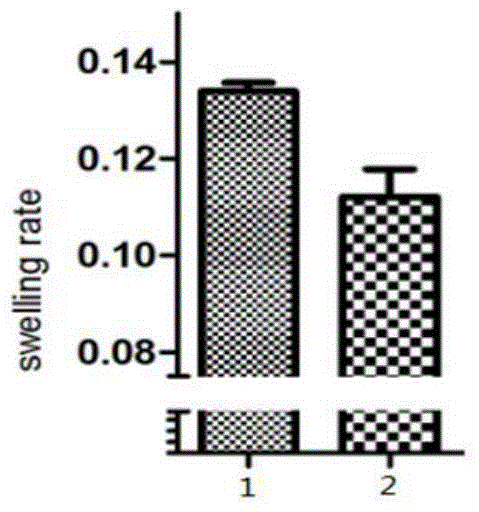
Application of cobra venom or extract thereof to preparation of medicament for reducing uric acid and/or resisting to gouty arthritis
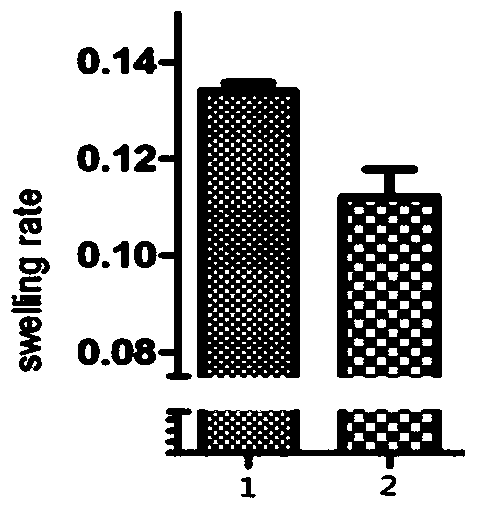
ActiveCN111939179ALower blood uric acid levelsImprove thickeningSkeletal disorderUnknown materialsCartilage cellsCobra venom
The invention relates to the field of medicine, and particularly provides application of cobra venom or an extract thereof to preparation of a medicament for reducing uric acid and / or resisting to gouty arthritis. According to the invention, the cobra venom can effectively reduce the blood uric acid level of a hyperuricemic mouse, meanwhile, has an obvious improvement effect on the gouty arthritis, and can repair the joint injury caused by sodium urate deposition, reduce the joint swelling rate, improve the joint synovial tissue thickening condition, reduce inflammatory cell infiltration, protect cartilage cells; and clinical experiments show that cobra neurotoxin can quickly eliminate subcutaneous gout calculi of gout patients, lumps disappear after the cobra venom is injected into the patients for three days, and no side effect exists. In conclusion, the Chinese cobra venom has the effect of repairing gouty arthritis while reducing uric acid, and can be used as a medicament for treating gout.
Owner:SUZHOU RENBEN PHARMA
Anticoagulant drug based on cobra venom PIII type metalloprotease and applications thereof
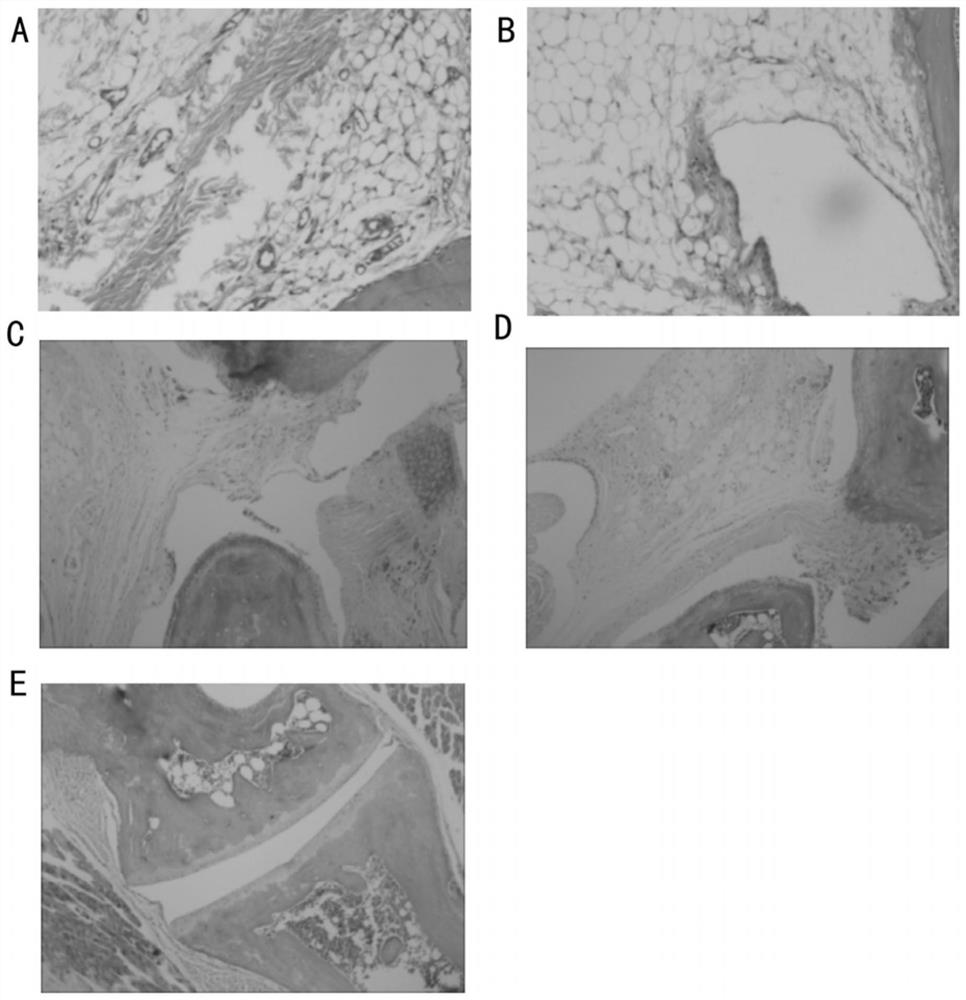
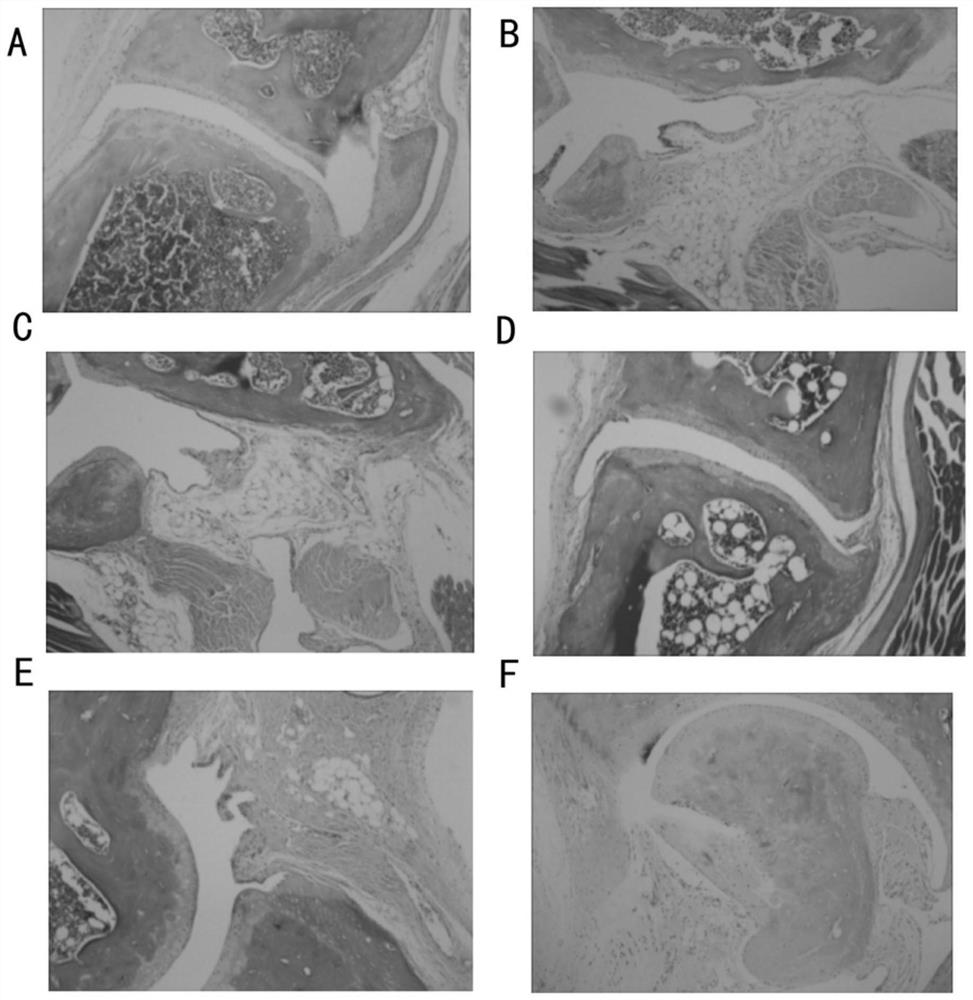
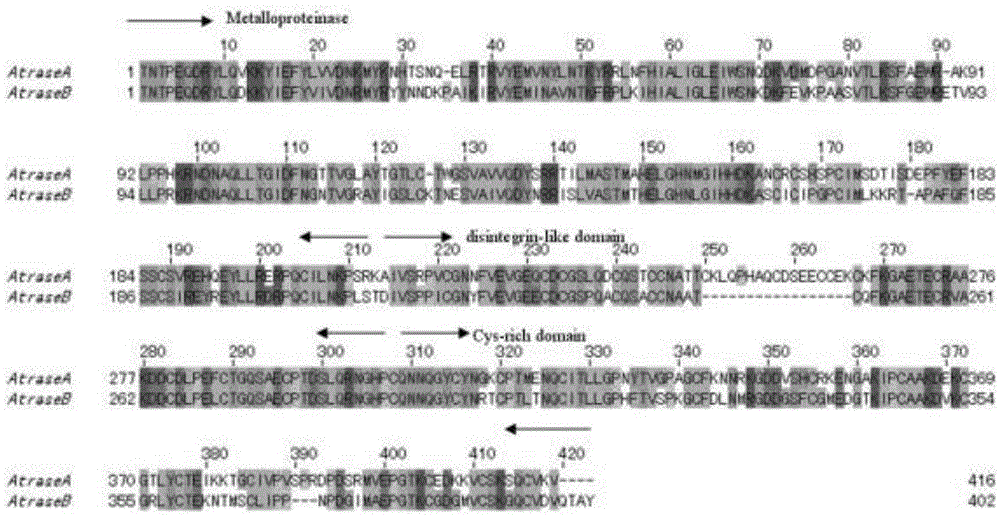
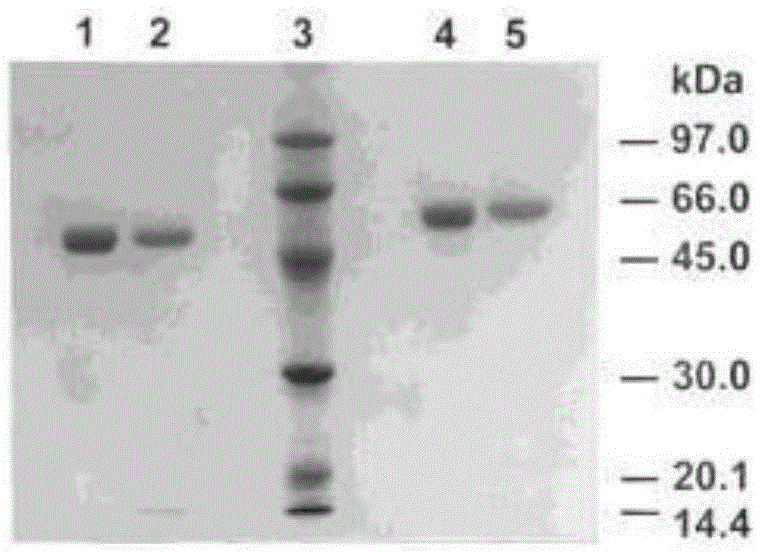
InactiveCN105567666AHas multiple anticoagulant effectsReduce the original levelPeptide/protein ingredientsBlood disorderCystine knotCobra venom
The invention discloses an anticoagulant drug based on cobra venom PIII type metalloprotease. The cobra venom PIII type metalloprotease is extracted from snake venom of naja atra and then purified. The metalloprotease is single chain glycoprotein. The structure analysis shows that metalloprotease has three structural domains: metalloprotease structural domain, disintegrin like structural domain, and cysteine enriched structural domain, and belongs to PIII type cobra venom metalloprotease. The metalloprotease is named as Atrase A and Atrase B; and the sequences of Atrase A and Atrase B are represented by SEQ ID No.1 and SEQ ID No.2 respectively. Target protein and anticoagulant drugs thereof have multiple anticoagulant effects: preventing multiple blood coagulation factors; reducing fibrinogen level; preventing platelet aggregation; and preventing complement so as to relieve abnormal blood coagulation caused by inflammation.
Owner:THE KEY LAB OF CHEM FOR NATURAL PROD OF GUIZHOU PROVINCE & CHINESE ACADEMY OF SCI
Traditional Chinese medicinal composition for treating cobra bite
InactiveCN106038961ASignificant effectGood effectInorganic active ingredientsAntinoxious agentsCobra venomClematis
The invention discloses a traditional Chinese medicinal composition for treating cobra bite and relates to the technical field of traditional Chinese medicinal formula. The traditional Chinese medicinal composition comprises components A: 9 to 12 parts of fructus evodiae, 9 to 12 parts of fritillary bulb, 9 to 12 parts of clematis root, 9 to 12 parts of trogopterus dung, 6 to 9 parts of radix angelicae dahurioae, 6 to 9 parts of herba asari and 6 to 9 parts of realgar; components B: 0 to 10 parts of rhizome coptis, 0 to 3 parts of rhizoma seu radix notopterygii, 0 to 12 parts of radix peucedani decursivi and 0 to 5 parts of herba schizonepetae. Compared with the prior art, the traditional Chinese medicinal composition provided by the invention has excellent curative effect for early-stage patients affected with snake venom, and is remarkable in effect; the influence and the irritation of cobra venom to nerves and a body can be effectively relieved; the traditional Chinese medicinal composition can be safely taken by the patients without toxic or side effects.
Owner:三江县连兴科技有限公司
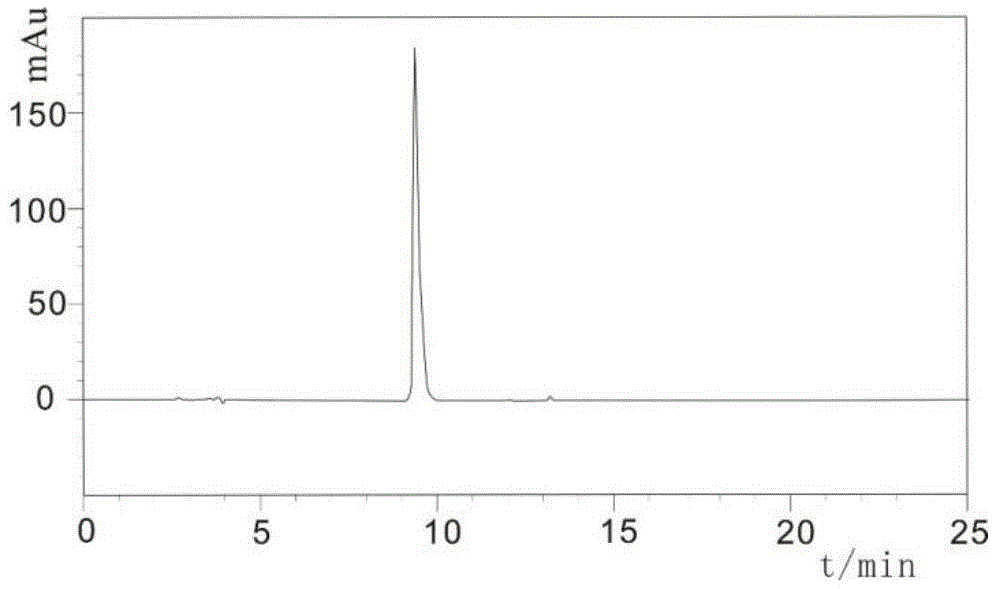
Method for purifying cobra venom and its products
InactiveCN109929020ALow content of related substancesHigh purityNervous disorderPeptide/protein ingredientsCobra venomPurification methods
The invention discloses a method for purifying cobra venom and its products. The invention provides a method for purifying cobra venom, which comprises the following steps: (1) performing cation exchange chromatography on a phosphate buffer containing the cobra venom to obtain an eluent A containing an active ingredient; wherein the phosphate buffer containing the cobra venom includes the cobra venom and a phosphate buffer solution; (2) performing complex mode chromatography on the eluent A containing the active ingredient to obtain an eluent B containing the active ingredient; and (3) desalting the eluent B containing the active ingredient to obtain the active ingredient. The purifying method has the advantages of simple process, easy industrialization, and low cost, and obtained producthas the advantages of high purity and low content of related substances.
Owner:ZHEJIANG JINGXIN PHARMA +1
Anti-acne snake venom compound external application preparation and preparation method thereof
InactiveCN106074611AReduce secretionDelay drug resistanceOrganic active ingredientsPharmaceutical delivery mechanismCobra venomAdditive ingredient
The invention discloses an anti-acne snake venom compound external application preparation and a preparation method thereof. The anti-acne snake venom compound external application preparation contains 1 to 8 percent of active pharmaceutical ingredients, wherein the active pharmaceutical ingredients are obtained by mixing 50 to 70 percent of dry elapidae snake venom powder with 30 to 50 percent of baicalein product, the percentage is by mass. Per 100g of fined dry elapidae snake venom powder is prepared from the following components: 6 to 12g of neurotoxin, 10 to 45g of cardiotoxin, 1.5 to 3g of cobra venom factor and 0.2 to 0.5g of cobra venom nerve growth factor; the content of baicalein in per 100g of the baicalein product is greater than 98g. The neurotoxi, cytotoxin and nerve growth factor of cobra venom have the effects of inhibiting bacteria, resisting inflammation, resisting allergy and relieving skin lesion; by coordinating the effects of the cobra venom with the effects of resisting oxidation, resisting inflammation and reducing grease secretion of the baicalein, the skin irritation and drug resistance of an anti-acne medicament can be reduced, and a more ideal biological agent is provided for clinical acne treatment.
Owner:GUANGZHOU MEDICAL UNIV
Primary structure of snake origin deintegration element mutant anti human platelet aggregation medicine health beneficial protein
InactiveCN1724564AInhibit aggregationInhibition of newbornsFermentationGenetic engineeringCobra venomMutated protein
The invention relates to the primary structure of Yikang protein, a diseased snakes disintegrins mutant anti-human platelet aggregation medicament, which concerns the amino acid residue sequence and the corresponding nucleotide sequence of the disintegrins mutant protein from recombinant agkistrodotoxin of cobra-venom, and the actions for inhibiting human platelet aggregation, preventing and treating thrombus, wherein the protein of low molecular weight consisting of 73 amino acid residues contains 12 cysteine residues and form 6 disulfide bonds. The beneficial effects of the invention include, the action for inhibiting platelet aggregation is achieved, and the suppression to blood vessel neogenesis is avoided.
Owner:赵宝昌
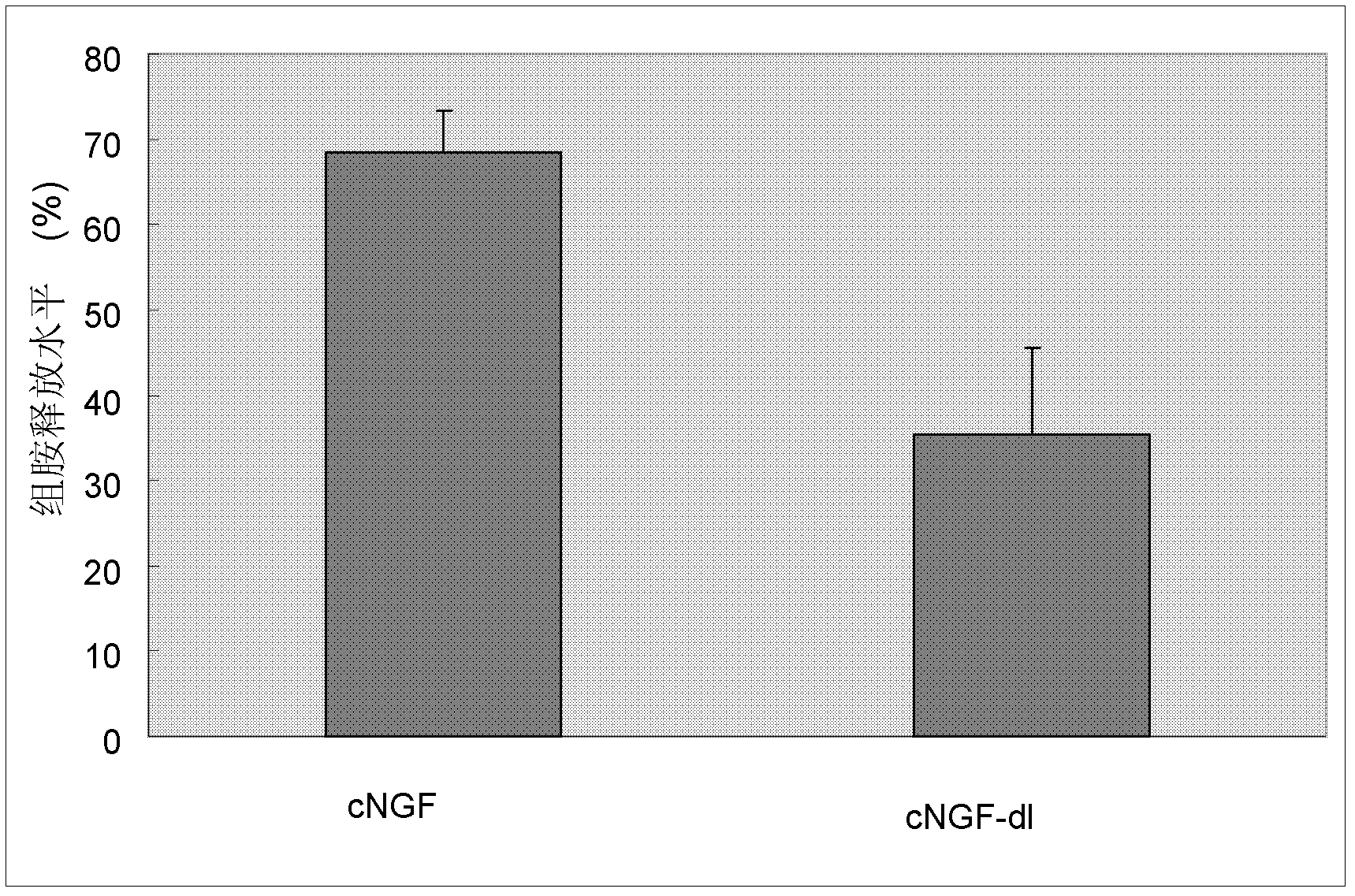
Method capable of reducing immunotoxicity of venom nerve growth factor and enabling immunotoxicity to be drug alternative of mouse origin nerve growth factor
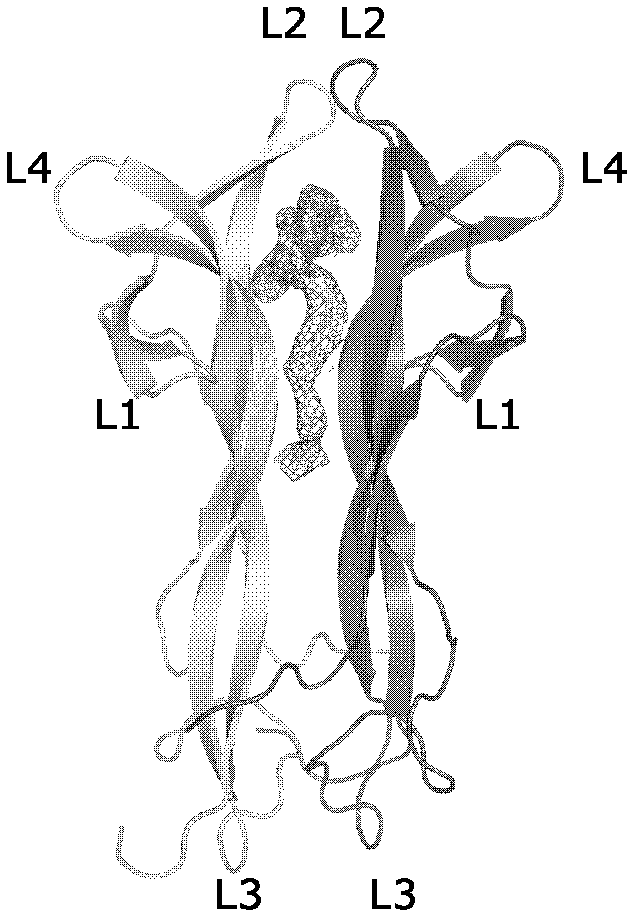
The invention discovers a cobra nerve growth factor (cNGF) specific binding lipid small molecules comes from a cobra is a main source of the immunotoxicity of the cNGF according to studies of structural biology and biochemistry. Therefore, the invention relates to a purification method and a degreasing method of the cobra venom cNGF. Due to the fact that the purified cNGF is processed by means of organic solvent and a special process, the lipid small molecules are successfully eliminated, the immunotoxicity of mouse mastocytoma is reduced by means of the degreased cNGF according to confirmation of a cell experiment, and therefore the immunotoxicity of the cNGF can be used as the drug alternative of the mouse origin nerve growth factor with low side effects and is a drug which has a neurotrophic effect.
Owner:INSITUTE OF BIOPHYSICS CHINESE ACADEMY OF SCIENCES
High-titer anti-cobra neurotoxin serum as well as preparation method and application thereof
InactiveCN106810608AHigh potencyReduce neurotoxicitySerum immunoglobulinsImmunoglobulins against animals/humansCobra venomSerum ige
The invention discloses a high-titer anti-cobra neurotoxin serum and a preparation method thereof. The preparation method comprises the following steps: firstly, reducing neurotoxicity of cobra venom; then, embedding the cobra venom in polyacrylamide gel; and finally, grinding neurotoxin-containing gel, performing immune injection on rabbits to obtain antiserum, and performing cryopreservation. In the invention, different doses of cobra venom are adopted; the neurotoxicity is reduced by heating, SDS and beta-mercaptoethanol treatment; the emulsification is replaced with polyacrylamide gel embedding, so as to ensure healthy survival of immunized animals, reduce production cost of he antiserum, and prepare high-titer antiserum.
Owner:WEST ANHUI UNIV +1
A method for separating and purifying cobra venom neurotoxin in combination with ion exchange and hydrophobic chromatography
ActiveCN104974238BSuitable for industrial productionEasy to operatePeptide preparation methodsAnimals/human peptidesCobra venomTest sample
The invention particularly relates to a method for separating and purifying cobra-venom neurotoxin by combining ion exchange and hydrophobic chromatography. The method comprises the following steps: (1) weighing 4-5 parts by weight of cobra-venom crude product, adding the cobra-venom crude product into 0.04-0.05 part by volume of water or 0.01-0.05M of phosphate buffer solution with the pH of 5.0-7.0, stirring to dissolve the cobra-venom crude product, centrifuging the solution at the temperature of 0-8 DEG C, discarding precipitates, and filtrating supernatant so as to obtain a test sample solution A for later use; (2) purifying the test sample solution A through ion-exchange chromatography, carrying out gradient elution, carrying out HPLC (High-Performance Liquid Chromatography) detection, and carrying out collecting so as to obtain a test sample solution B for later use; and (3) purifying the test sample solution B through hydrophobic chromatography, carrying out gradient elution, carrying out HPLC detection, carrying out collecting, carrying out concentrating, and carrying out drying thereby obtaining a pure product of cobra-venom neurotoxin, wherein when the unit of parts by weight is g, the unit of parts by volume is correspondingly L. According to the method, operating steps are simplified, the separation and purification cycle is shortened, the yield is relatively high, the purity is relatively high, and thus the method is applicable to the industrial production of cobra-venom neurotoxin.
Owner:SUZHOU RENBEN PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com