Application of cobra venom in preparation of medicament for treating chronic obstructive pulmonary disease
A technology for chronic obstructive pulmonary disease and cobra snake venom, which is applied in drug combinations, respiratory diseases, and pharmaceutical formulations. effect of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
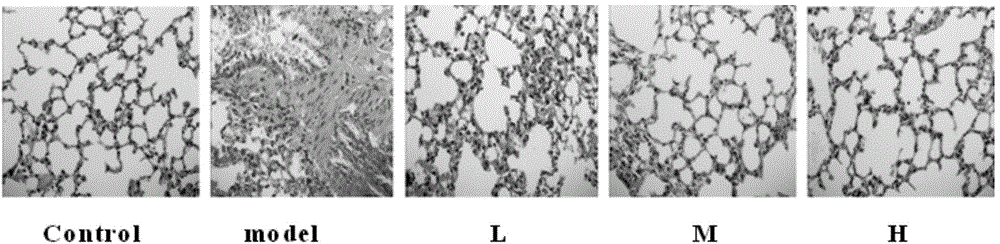
[0036] The animal pharmacodynamic experiment of embodiment 1 cobra venom
[0037] Effects of cobra snake venom on acute pneumonia in mice induced by initial exposure to lipopolysaccharide:
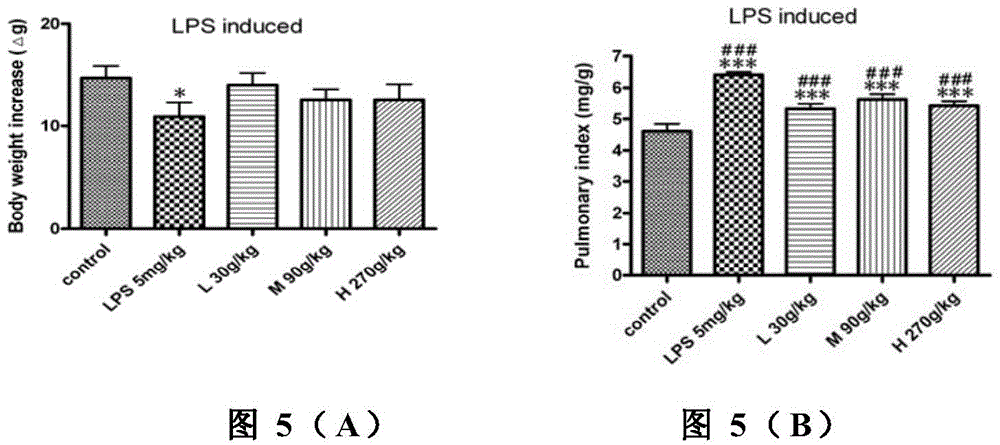
[0038] Lipopolysaccharide (LPS) is a component in the cell wall of Gram-negative bacteria, which can cause various inflammatory reactions in the body. A single administration of lipopolysaccharide 5mg / kg can make a mouse model of acute pneumonia. Kunming mice were randomly divided into 5 groups (n=10) half and half female, body weight 18-22g: normal saline control group, model group, small dose of Chinese cobra snake venom heating group (30 μg / kg), medium dose group (90 μg / kg), high dose group ( 270 μg / kg). After administration of lipopolysaccharide (5 mg / kg), observe the animal's activity state and respiratory rate. In the test of cobra snake venom in treating lipopolysaccharide-induced acute lung injury in mice, the embodiment adopts intragastric administration, and the dosage is 10-3000...
Embodiment 2
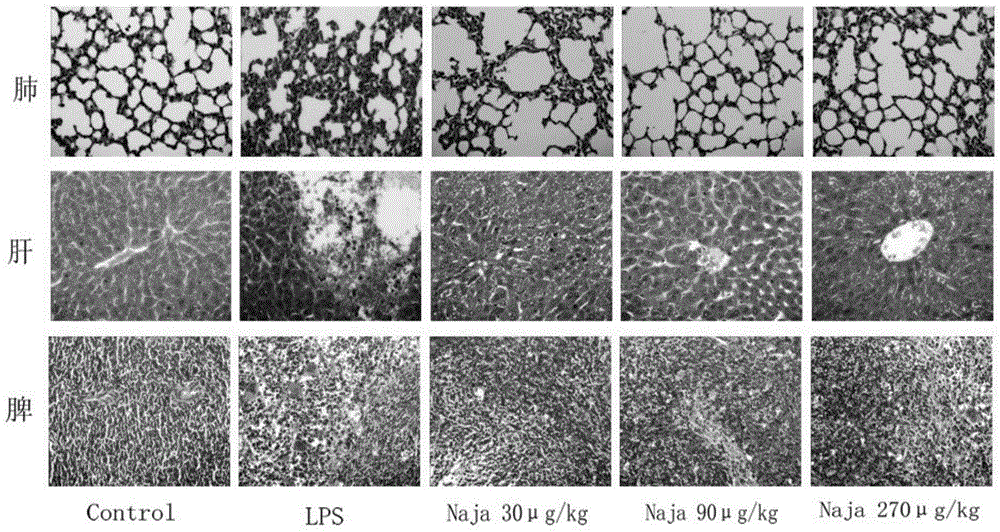
[0045] Example 2 Cobra snake venom effect experiment on chronic obstructive pneumonia induced by repeated administration of LPS
[0046] In the experiment, lipopolysaccharide 5mg / kg was given once a week, and the repeated administration was repeated for 8 weeks to make a mouse model of chronic obstructive pneumonia. The Kunming mice were randomly divided into 5 groups (n=10) with half and half females, weighing 18-22g : Physiological saline control group, model group, low-dose Chinese cobra venom heating group (30 μg / kg), medium-dose group (90 μg / kg), high-dose group (270 μg / kg). During the long-term administration, the activity state and respiratory rate of the animals were observed. In the experiment of treating lipopolysaccharide-induced chronic obstructive pneumonia in mice with Chinese cobra venom, the embodiment adopts intragastric administration, and the dosage is 10-3000 μg / kg, once a day.
[0047] Eight weeks after the experiment, the mice were removed from the eyeba...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com