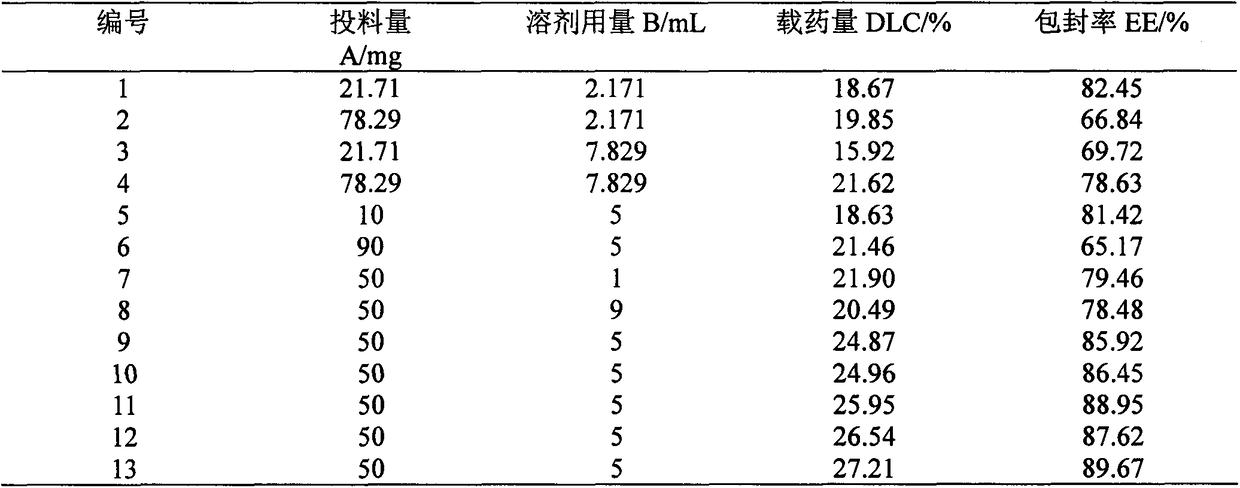
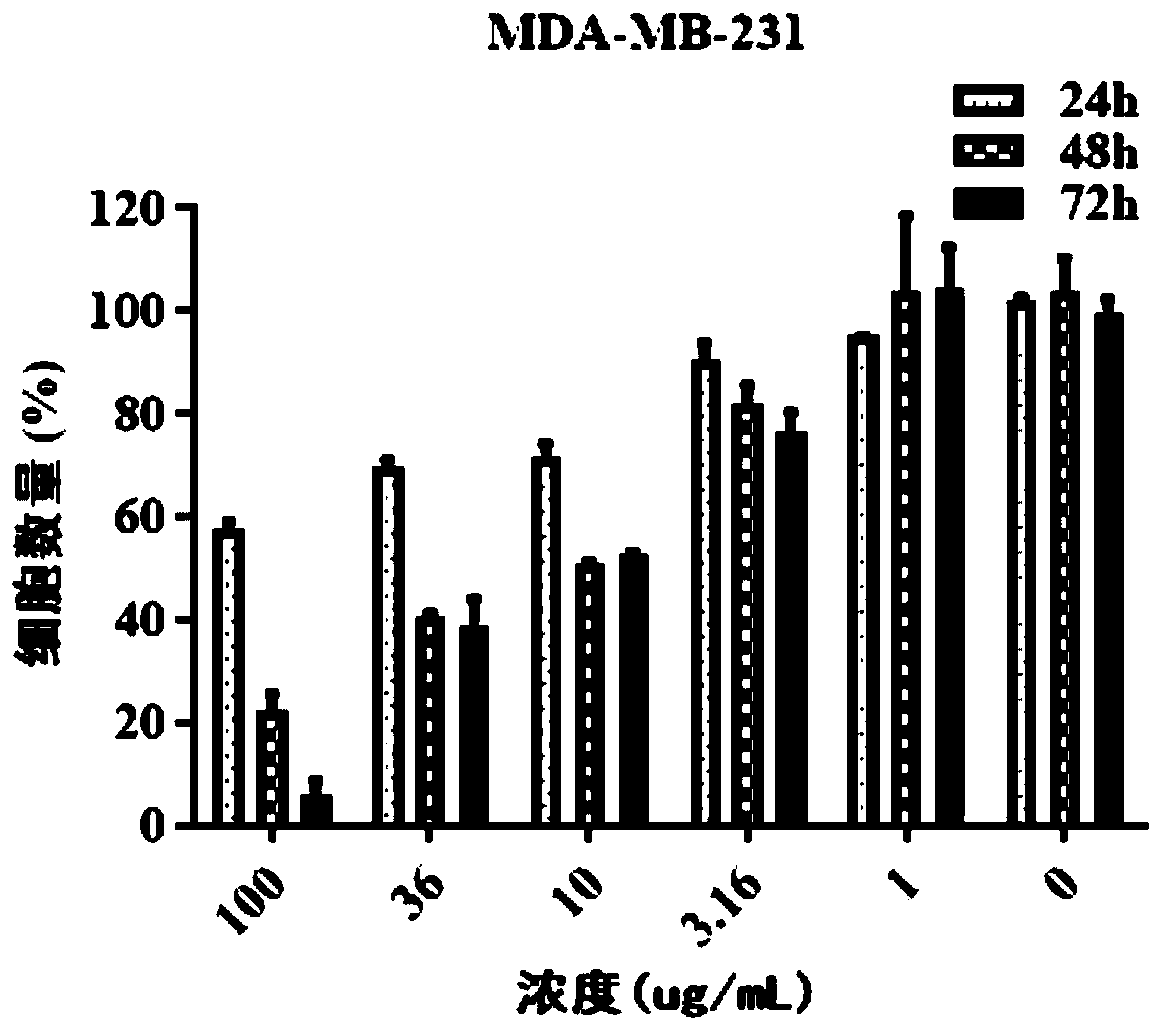
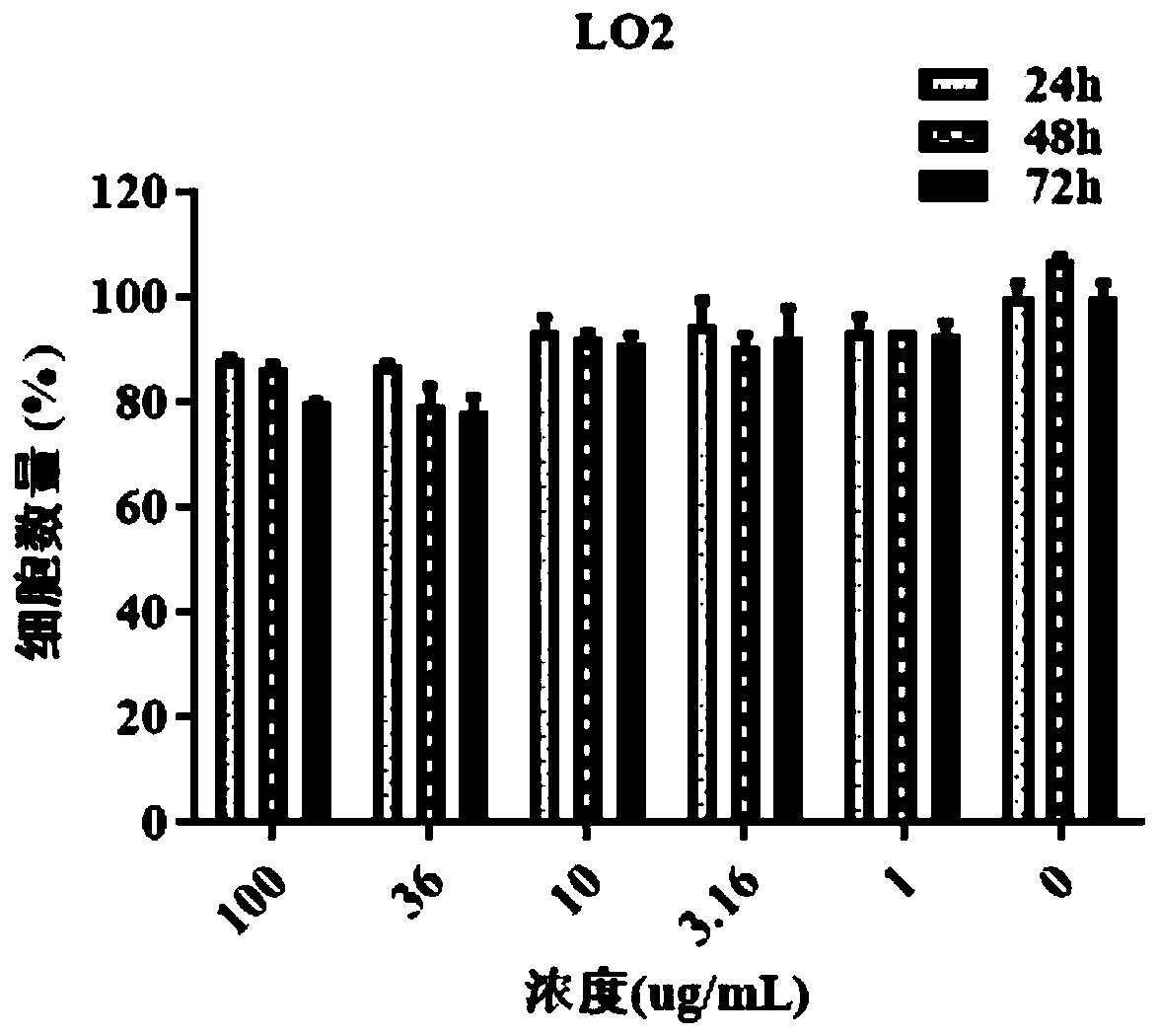
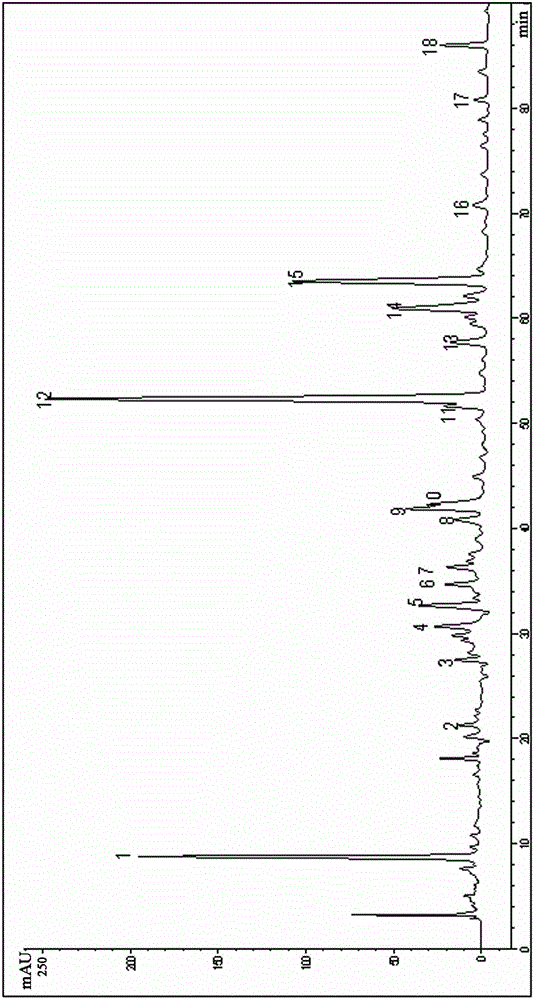
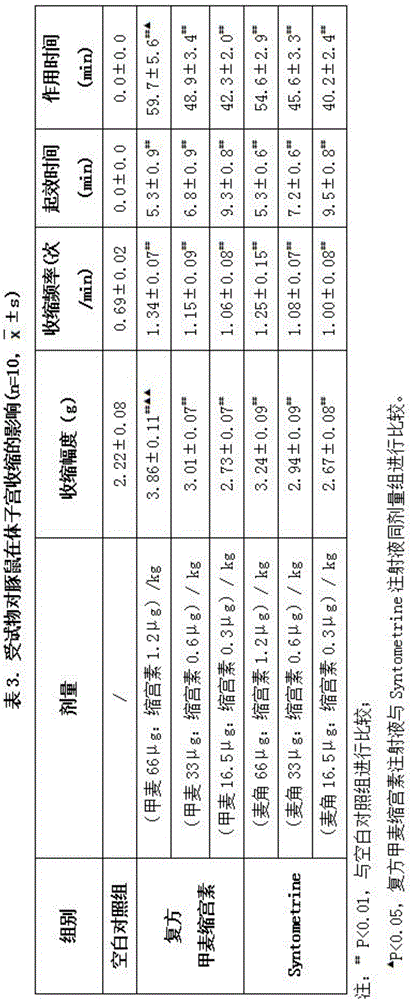
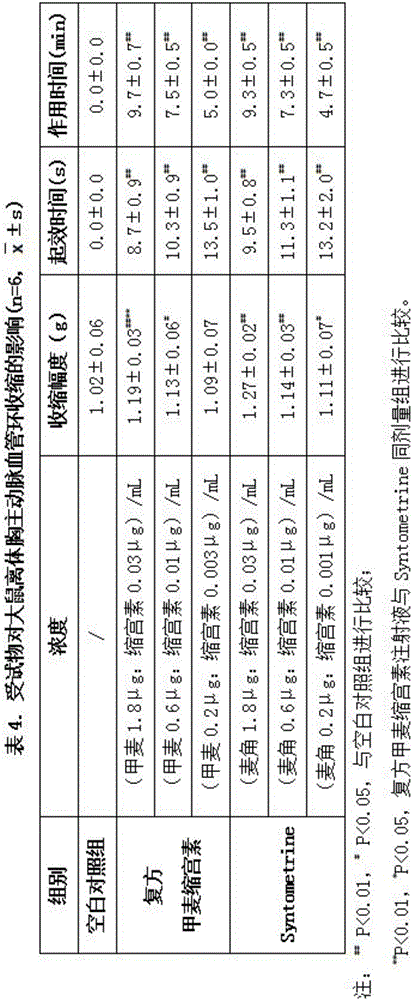
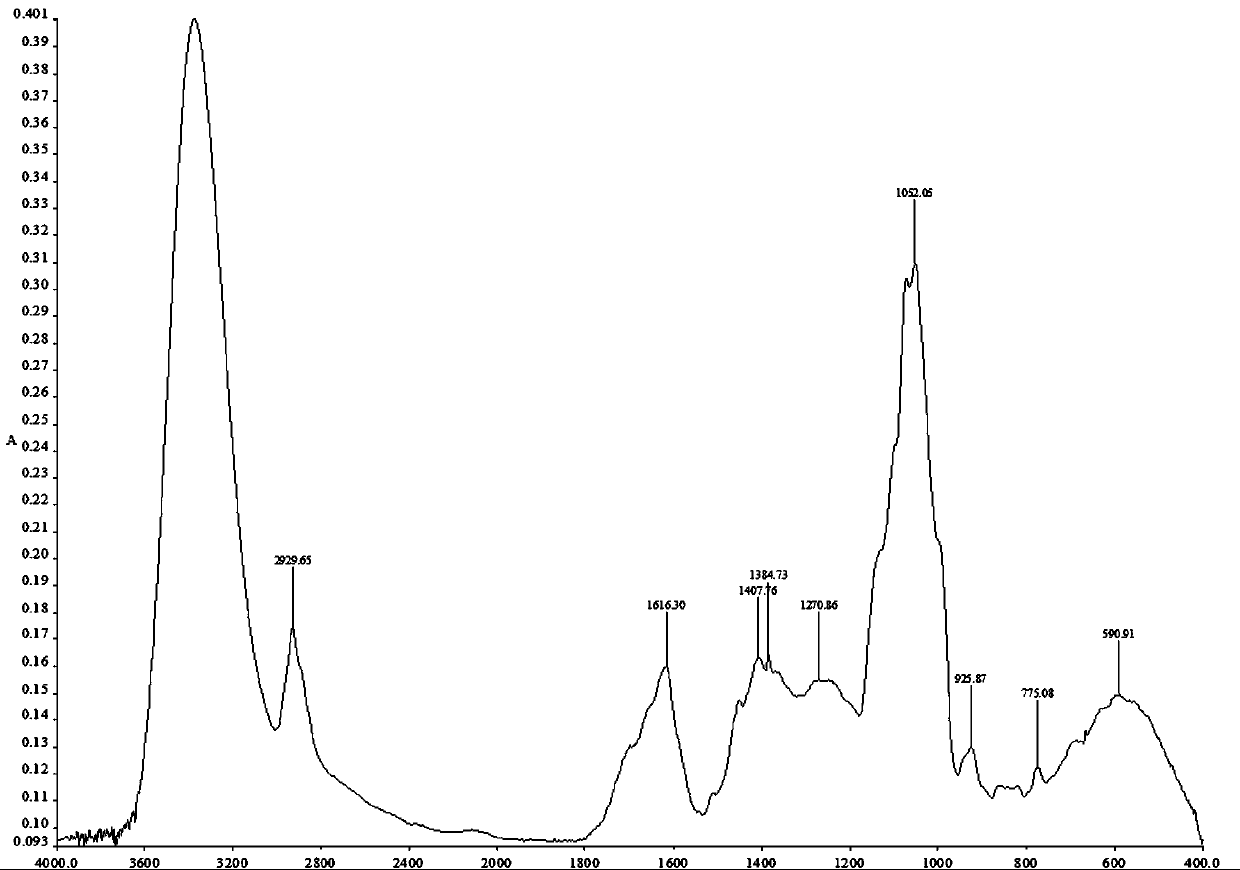
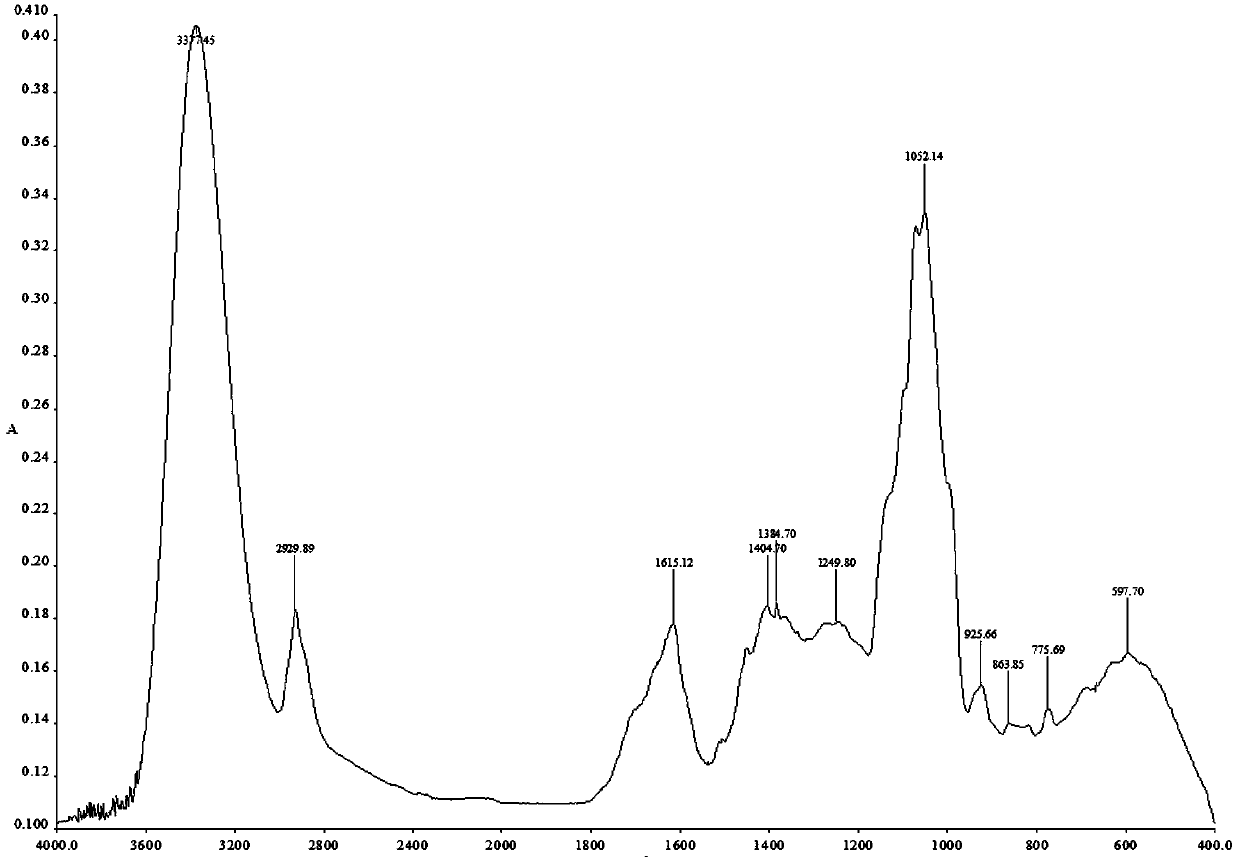
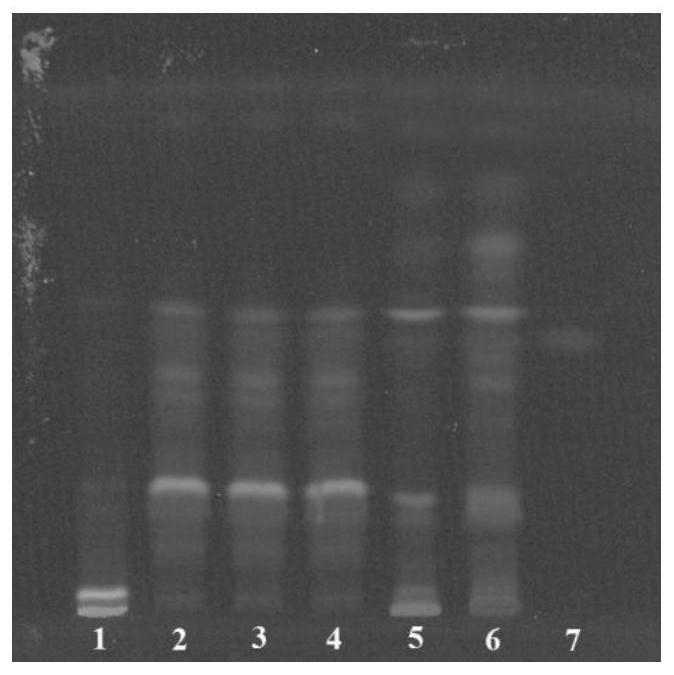
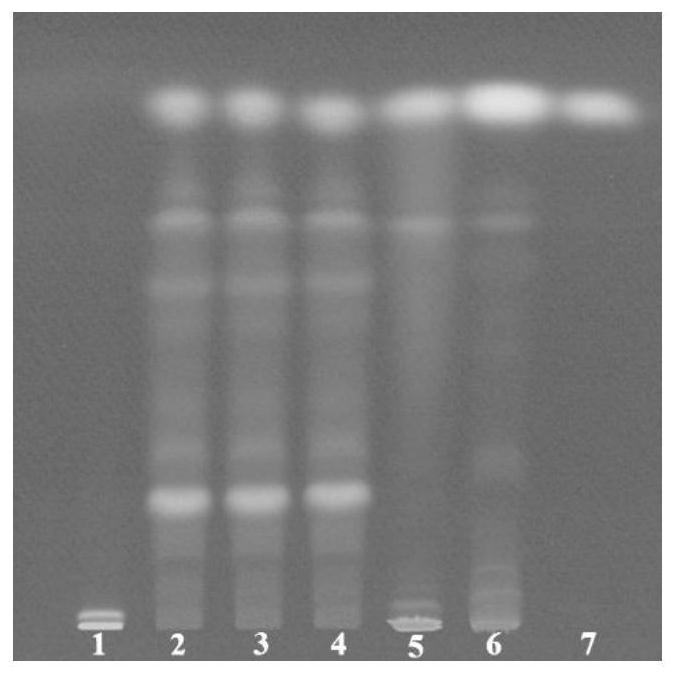
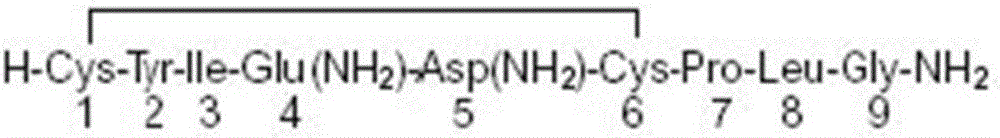Patents
Literature
32results about How to "To achieve the purpose of reducing toxicity and increasing efficiency" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Peony and liquorice soup formula granule, preparation method and detection method of peony and liquorice soup formula granule
InactiveCN103330758AEasy to storeEasy to carry and useComponent separationAntipyreticLiquoricesPaeoniflorin
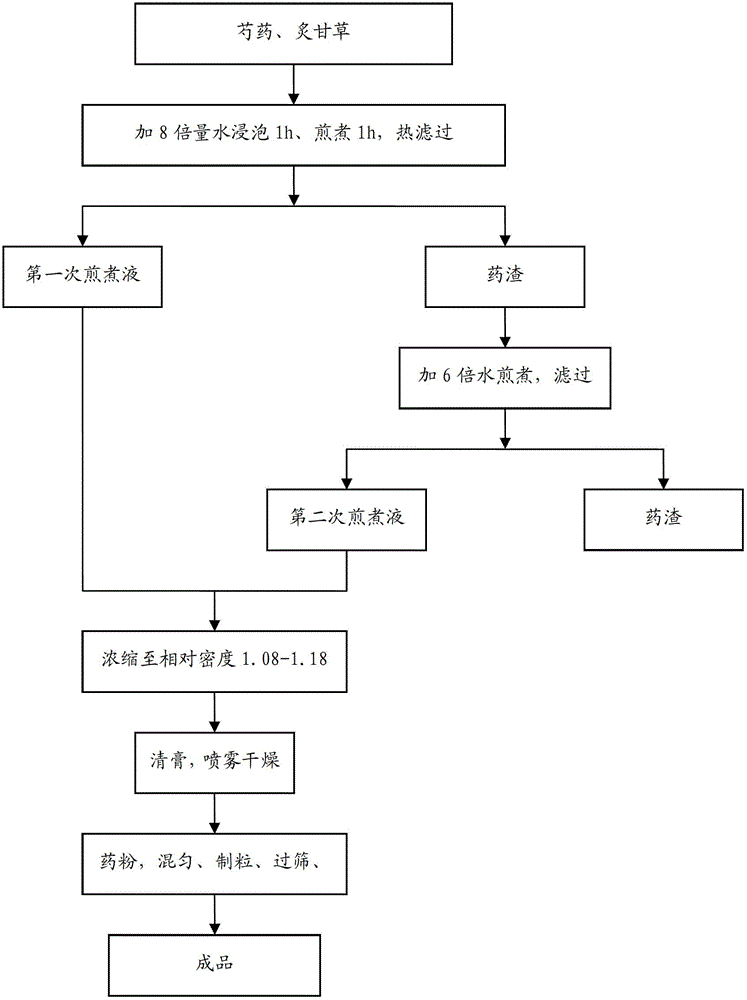
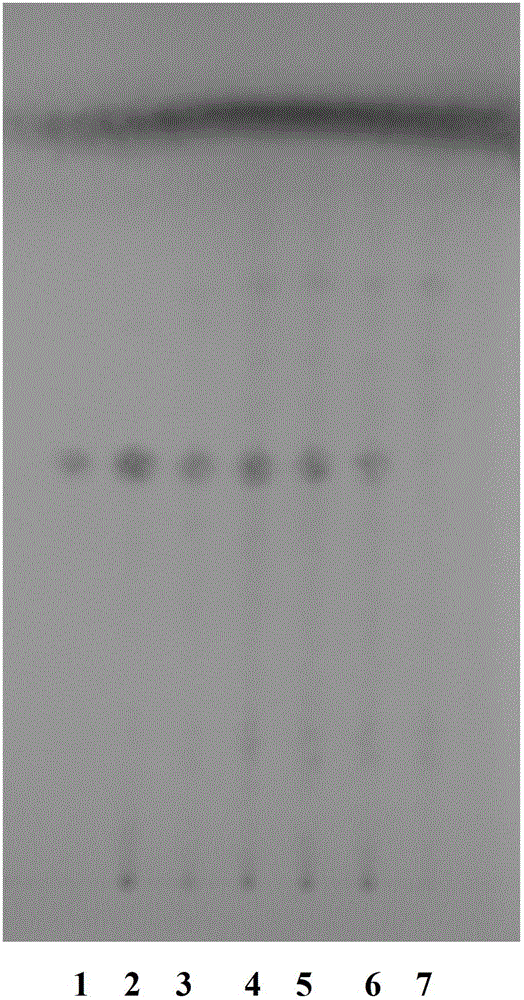
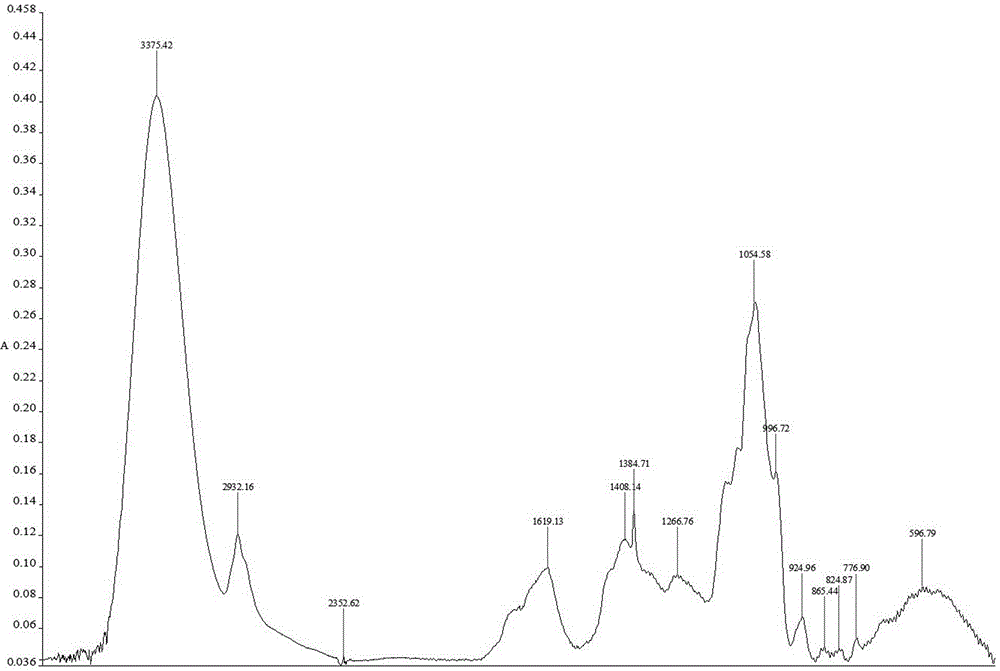
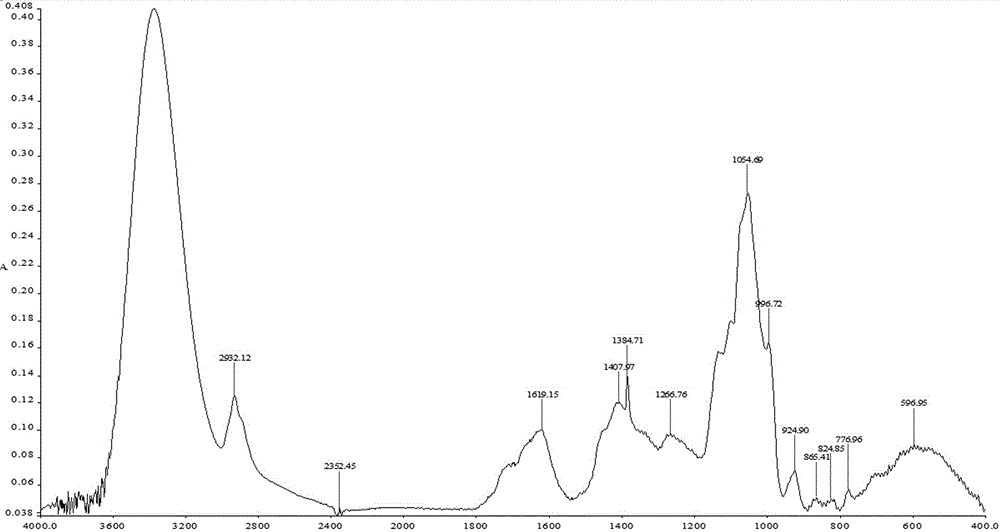
The invention provides a peony and liquorice soup formula granule, and a preparation method and a detection method of the peony and liquorice soup formula granule. The preparation method comprises the steps of weighing peony and liquorice at a mass ratio of 1:1, adding water for decocting and extracting, merging filtrate, decompressing and condensing the filtrate to obtain a concentrated solution, drying the concentrated spray, adding water for granulating and sieving, and obtaining the peony and liquorice soup formula granule. The detection method comprises the steps of identifying the peony and liquorice soup formula granule by a thin-layer chromatography, and measuring the heavy metal content, the organo-chlorine pesticide residual amount, the fingerprint spectrum, the extract content and the paeoniflorin content of the peony and liquorice soup formula granule. The peony and liquorice soup formula granule inherits advantages of a traditional Chinese medicine single-formula granule, fully considers interaction during decocting (or other processing) of a decoction piece, and has a higher value, and the detection method allows the quality of the peony and liquorice soup formula granule to be controlled more effectively, so that the peony and liquorice soup formula granule is safer to use.
Owner:KANGMEI PHARMA +1
Preparation method of pubescent angelica and mistletoe decoction formula granules and quality control method thereof
ActiveCN105477166AEmbody conceptTo achieve the purpose of reducing toxicity and increasing efficiencySenses disorderNervous disorderAdditive ingredientAngelica Sinensis Root
The invention discloses a preparation method of pubescent angelica and mistletoe decoction formula granules and a quality control method thereof. The preparation method includes the steps that radix angelicae pubescentis, asarum, cassia twigs, radices sileris, ligusticum wallichii and radix angelica sinensis are extracted for obtaining volatile oil, and the volatile oil is subjected to clathration of beta-cyclodextrin; water with the weight 5-15 times the weight of total feeding amount is added to herbal residues and other nine types of medicine, and decoction and extraction are conducted twice; filtrate is subjected to vacuum decompression concentration; a concentrated solution is subjected to spray drying, spray-drying powder is obtained, and the spray-drying powder is crushed into submicron powder through an airflow pulverizer; maltodextrin and a beta-cyclodextrin clathrate compound are added to the submicron powder to be mixed uniformly, granulation is conducted through a dry method, and the pubescent angelica and mistletoe decoction formula granules are obtained. The quality control method includes the steps of infrared fingerprint spectra, thin-layer qualitative identification and HPLC content measuring. By means of the method, the advantage of drug matching can be fully played, and effective ingredients are maintained to the maximum degree; a perfect quality standard is completed, a once-measurement multi-evaluation technology of thin-layer chromatography is adopted, quality control is conducted in combination with infrared spectroscopy and chromatography, and the quality of the formula granules can be effectively controlled.
Owner:GUANGDONG YIFANG PHARMA
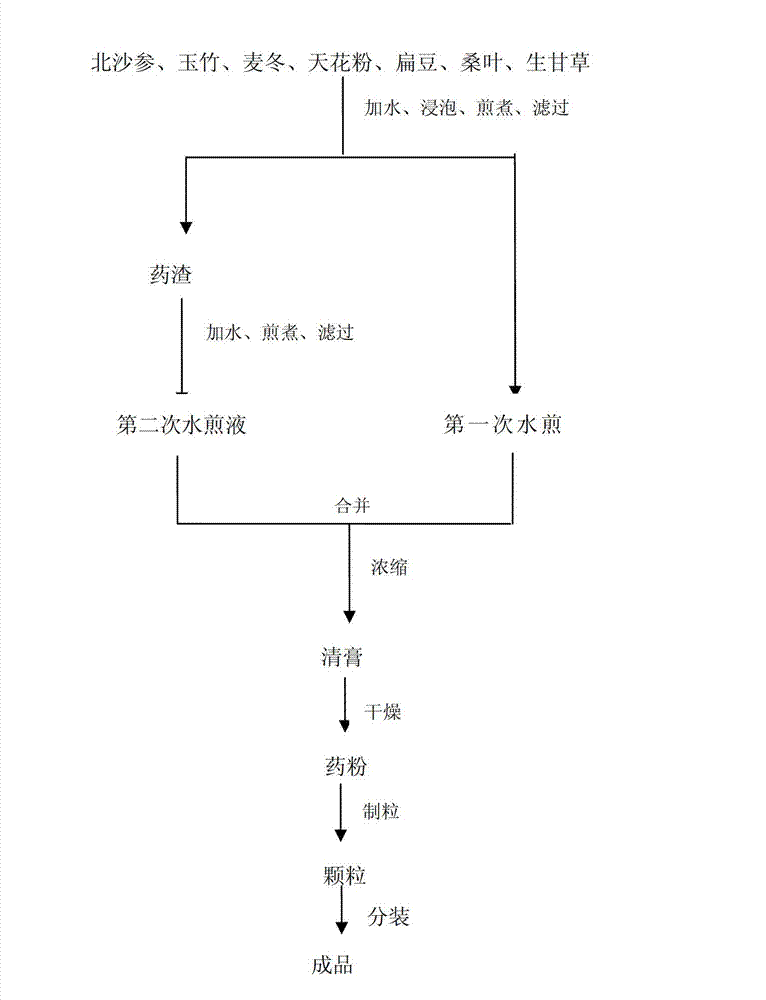
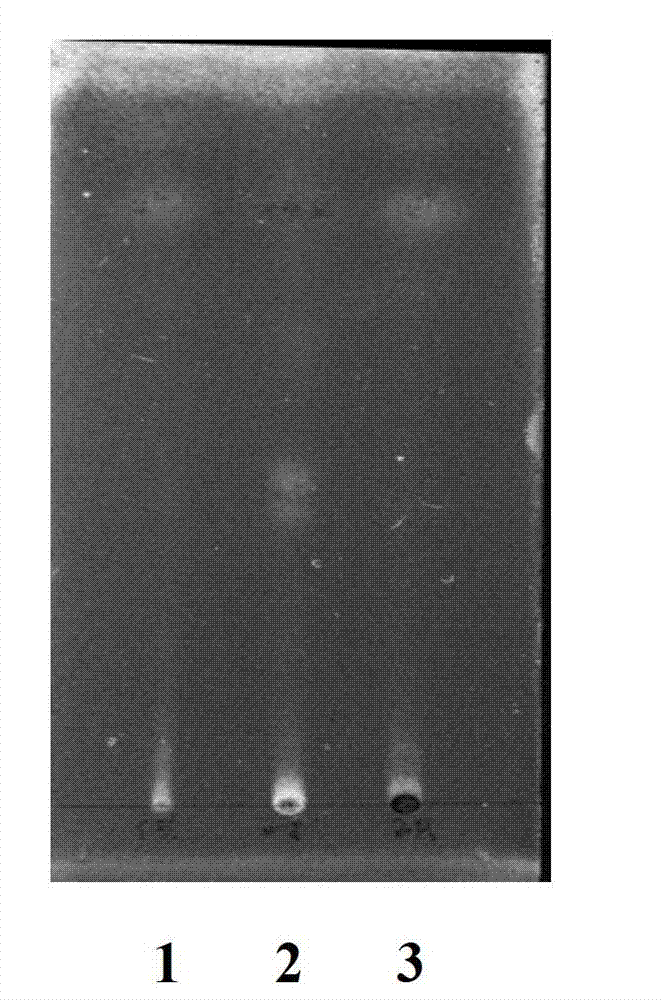
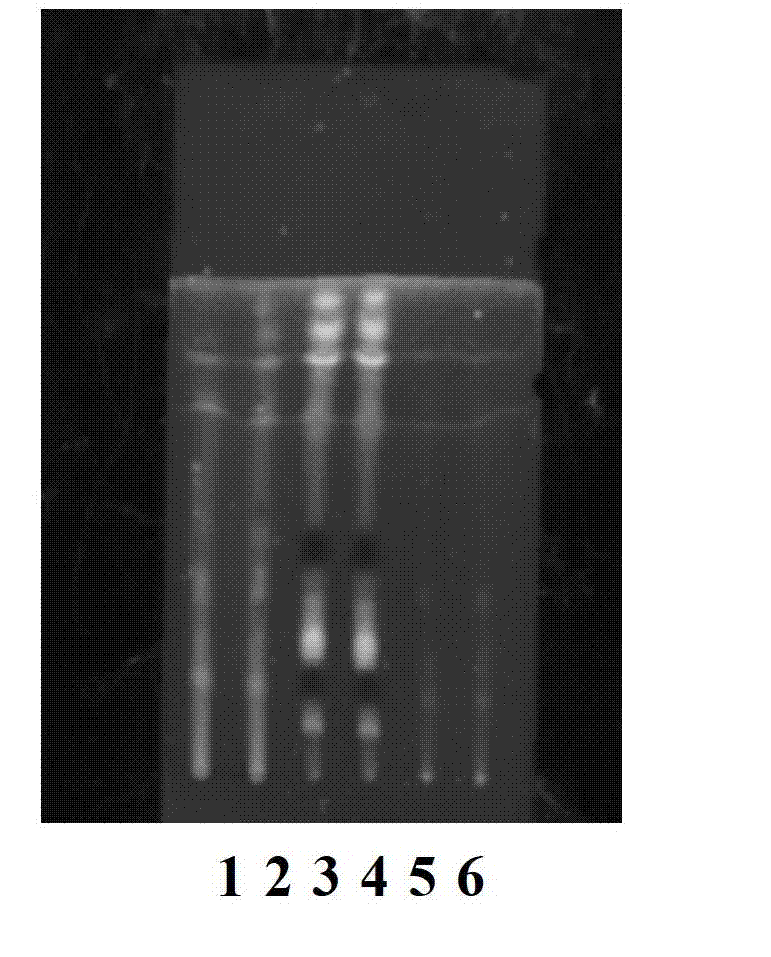
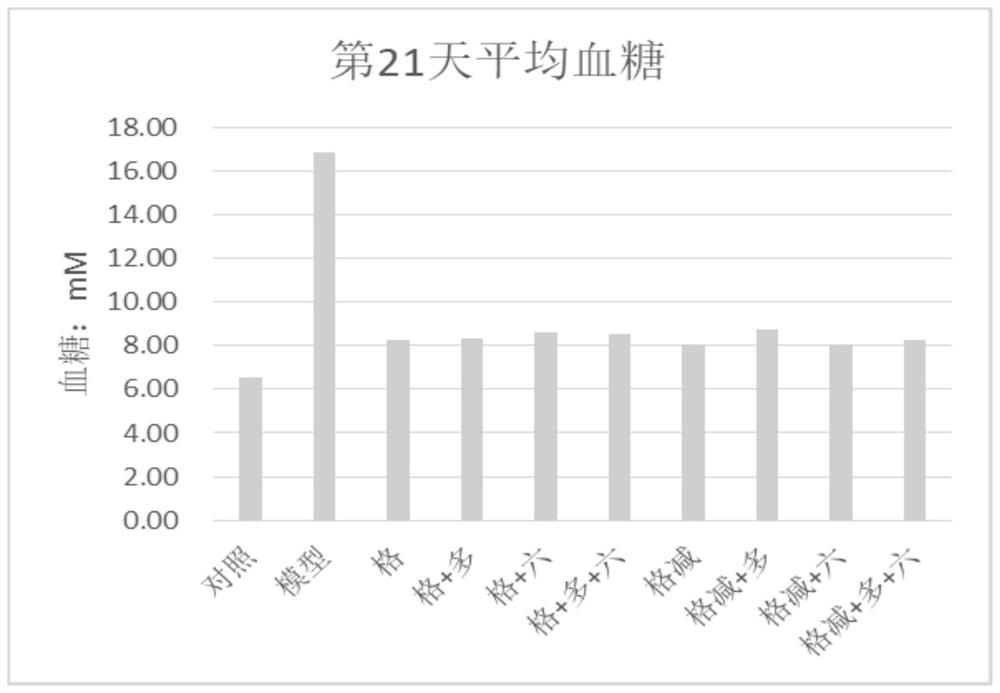
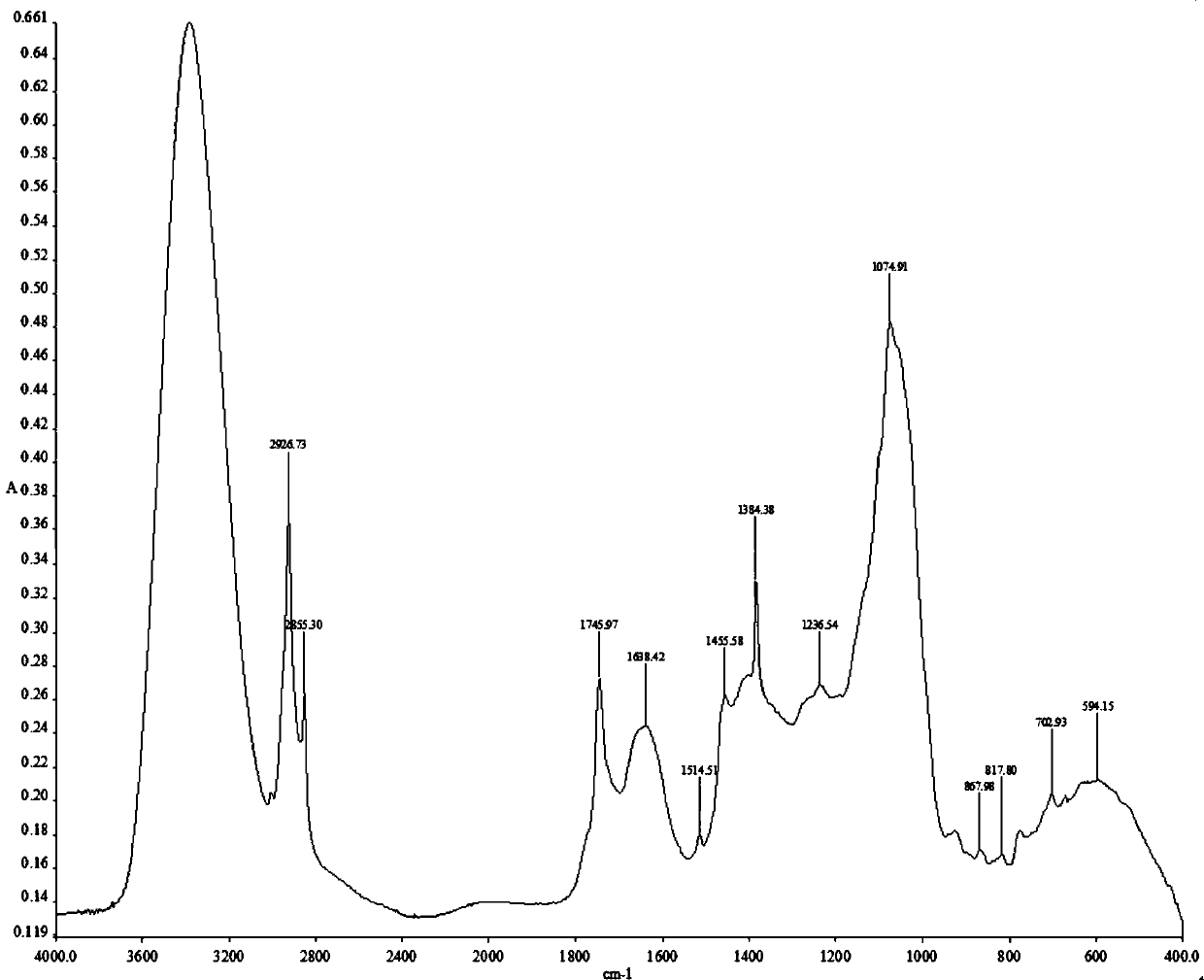
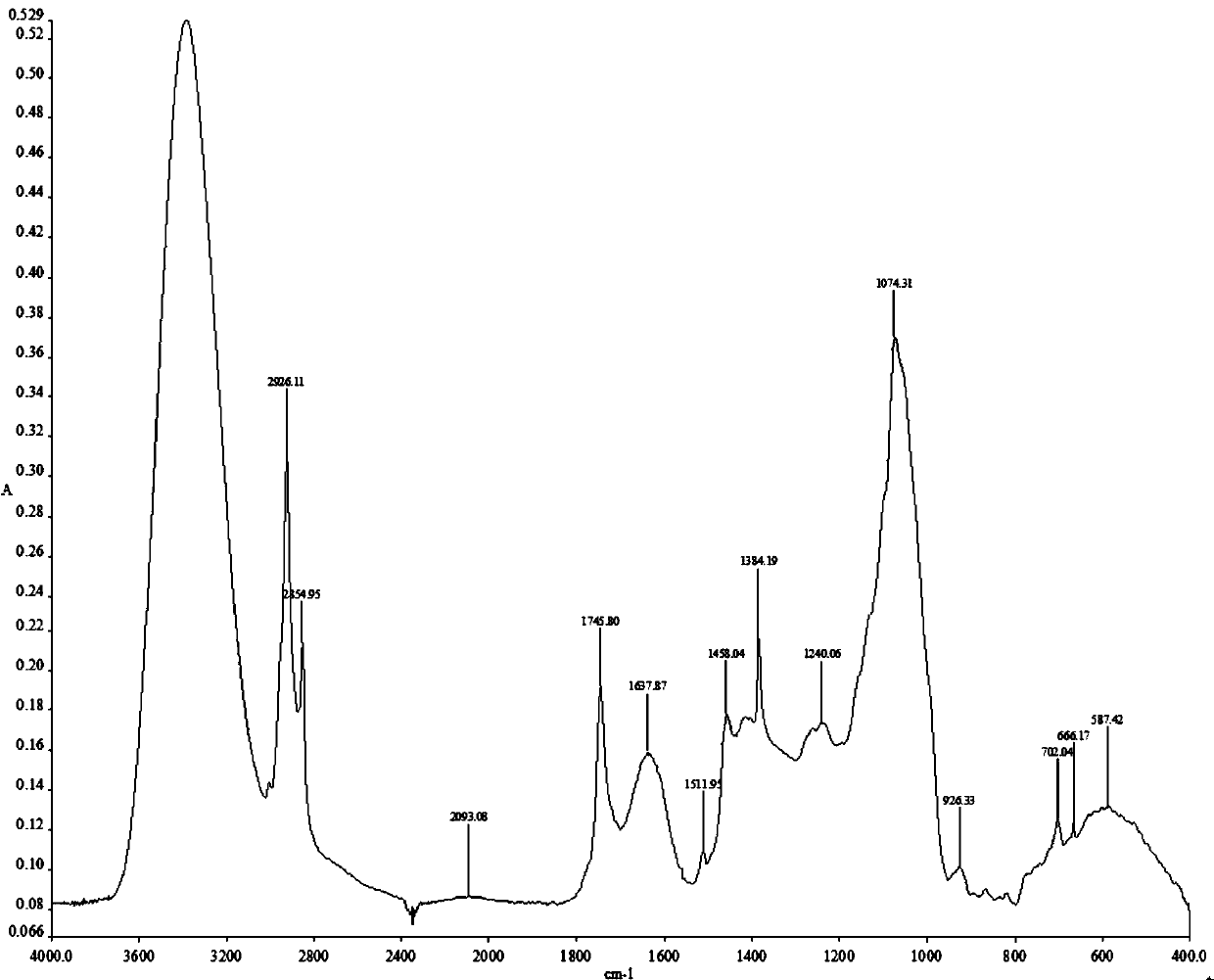
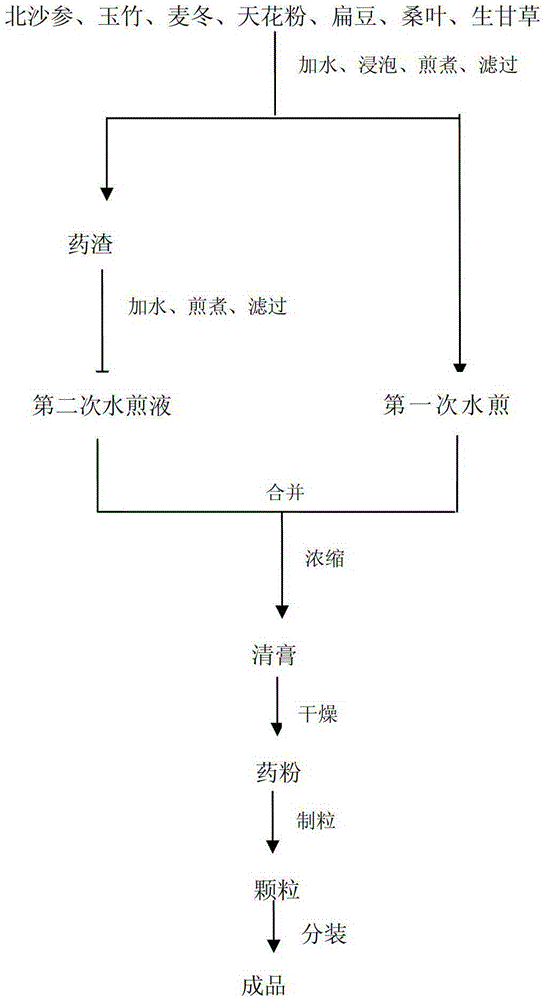
Adenophora radix ophiopogonis soup formula granules as well as preparation method and detection method thereof
ActiveCN103230517ATo achieve the purpose of reducing toxicity and increasing efficiencyQuality improvementComponent separationGranular deliveryPesticide residueOfficinalis
The invention provides adenophora radix ophiopogonis soup formula granules as well as a preparation method and a detection method thereof. The granules comprises the raw materials as follows: radix glehniae, radix polygonati officinalis, radix ophiopogonis, radices trichosanthis, hyacinth beans, mulberry leaves and raw liquorice in the mass ratio of (0.5-2):(0.5-2):(0.5-2):(1-3):(0.5-2):(0.1-1.2):(0.1-1), and the raw materials are respectively immersed for multiple times, boiled and filtered, so as to obtain cream after filter liquors are mixed and concentrated, and the cream is dried to be prepared into granules. The detection method comprises the following steps of: identifying the adenophora ophiopogonis soup formula granules through a using thin-layer chromatography; measuring the content of organic chloride pesticide residue in the adenophora ophiopogonis soup formula granules through using a pesticide residue measuring method; measuring the content of heavy metal in the adenophora ophiopogonis soup formula granules through using a residue on ignition examination method; measuring the content of extractum of the adenophora ophiopogonis soup formula granules through using a hot dipping method; and measuring the content of substances in the adenophora ophiopogonis soup formula granules through a using high-efficiency liquid chromatography.
Owner:KANGMEI PHARMA +1
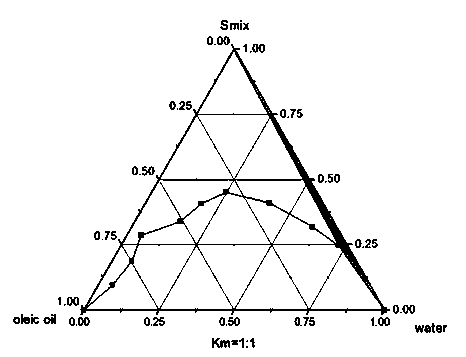
Tripterygium wilfordii-caulis sinomenii microemulsion gel and preparation method thereof
ActiveCN104306447AImprove securityAvoid gastrointestinal reactionsAntipyreticAerosol deliveryTransdermal absorptionEfficacy
The invention belongs to the technical field of medicines, and specifically discloses a tripterygium wilfordii-caulis sinomenii microemulsion gel and a preparation method thereof. A prescription of the tripterygium wilfordii-caulis sinomenii microemulsion gel comprises caulis sinomenii extracts, tripterygium wilfordii extracts, a surfactant, a cosurfactant, an oil phase, a gel matrix material and water. The preparation method of the tripterygium wilfordii-caulis sinomenii microemulsion gel comprises the steps of dissolving medicines in the oil phase, mixing uniformly with the surfactant, the cosurfactant and water to obtain microemulsion; adding the gel matrix material into the microemulsion for swelling; regulating a pH value; and stirring uniformly. The tripterygium wilfordii-caulis sinomenii microemulsion gel is administered through skins, increases permeation rate through the skins, promotes transdermal absorption of the tripterygium wilfordii and caulis sinomenii, overcomes the problem of strong mobility of the microemulsion, achieves the object of sustained release and long-term efficacy, reduces toxicity of tripterygium wilfordii, and improves a curative effect of caulis sinomenii. The tripterygium wilfordii-caulis sinomenii microemulsion gel has a significant curative effect for treating rheumatoid arthritis, has small toxic or side effect, and has good product stability; and the preparation process is simple and suitable for large-scale production.
Owner:GUANGZHOU UNIVERSITY OF CHINESE MEDICINE +1
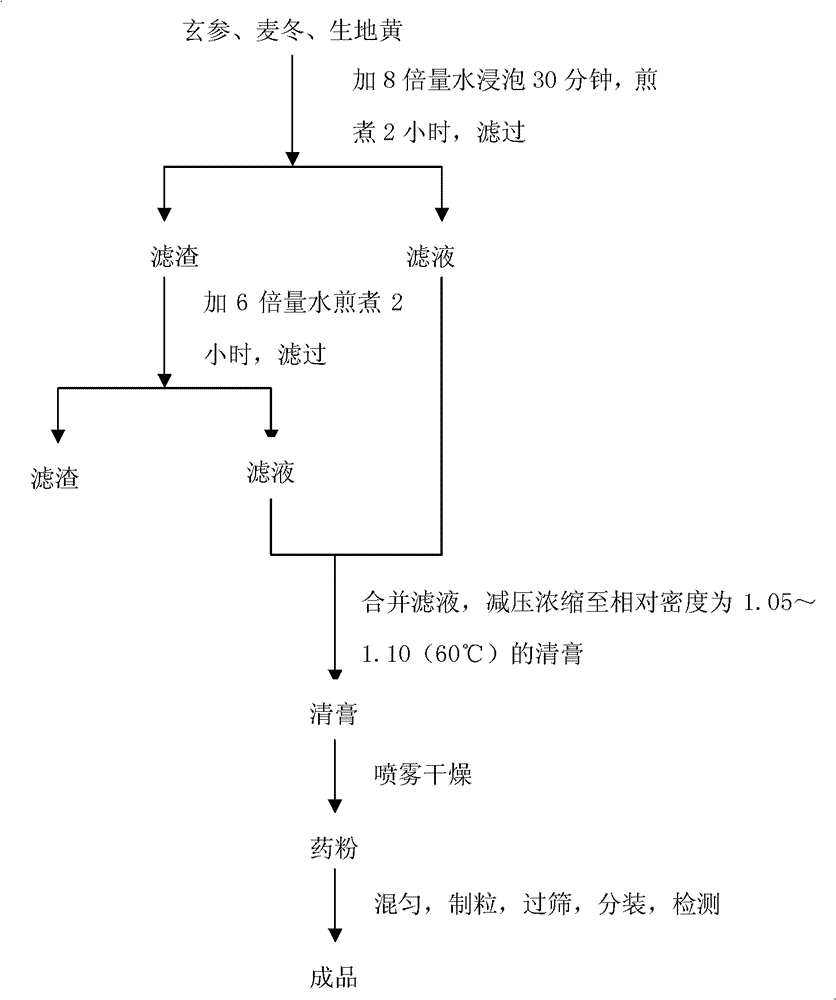
Fluid-increasing decoction formula granules and preparation method, application and detection method thereof
ActiveCN103169864AOvercome the disadvantage of lack of co-fryingPerfect quality control methodSenses disorderComponent separationMedicineRadix Ophiopogonis
The invention provides fluid-increasing decoction formula granules. The fluid-increasing decoction formula granules contain extracts prepared by the step of decocting radix scrophulariae, radix ophiopogonis and radix rehmanniae recen. The invention also provides a preparation method, application and a detection method of the fluid-increasing decoction formula granules. According to the fluid-increasing decoction formula granules, the defect that various herbal medicines are simply added when the conventional single-component formula granules are taken is overcome, the compatibility advantages of traditional Chinese medicine are fully exerted, the holistic thinking of traditional Chinese medicine is reflected, the aim of reducing toxicity and enhancing efficacy is ensured, the fluid-increasing decoction formula granules are quasi steady and controllable in quality and convenient to store and carry by patients, and the decoction trouble is avoided. The preparation method of the fluid-increasing decoction formula granules is simple, the herbal medicines are decocted together according to the traditional method, and the characteristics of the traditional Chinese medicine are met. According to the detection method of the fluid-increasing decoction formula granules, the perfect quality standard of the fluid-increasing decoction formula granules is established, and the quality of the formula granules can be effectively controlled.
Owner:KANGMEI PHARMA +1
Method for preparing siwu decoction formula granules and quality control method thereof
ActiveCN105287875AEmbody conceptTo achieve the purpose of reducing toxicity and increasing efficiencyComponent separationMaterial analysis by optical meansBiotechnologyFormulary
The invention discloses a method for preparing siwu decoction formula granules and a quality control method thereof. The method for preparing the siwu decoction formula granules comprises steps: prepared rehmannia roots, angelica sinensis, radix paeoniae alba and ligusticum wallichii medicinal slices are added with water which is 5-15 times of the weight of total inventory, are extracted for two times, aromatic water is collected and is combined with two frying filter liquids, are decompressed and concentrated in vacuum, a concentrated solution is added with beta (Beta)-cyclodextrin and silicon dioxide to uniformly stir and obtains a clear paste, and the aromatic water and the aromatic water are sprayed and dried after being uniformly mixed, and are pelletized through a dry method to prepare the siwu decoction formula granules. The quality control method of the siwu decoction formula granules comprises qualitative identification of an infrared fingerprint spectrum and a thin layer and content determination of a high performance liquid chromatography (HPLC). The method for preparing siwu decoction formula granules and the quality control method thereof decoct in a combined mode according to a traditional method, can perfectly take full advantages of drug matching compared with an existing method that various medicinal odours are added when single formula particles are taken, reflects the overall concept of Chinese medicine, guarantees to achieve the purpose of reducing toxicity and enhancing efficacy, supplies novel selection for clinical medication, builds a perfect quality standard, controls quality by combining a power-spectral method and a chromatography, and can effectively control quality of complex granules from the overall to the more specific.
Owner:GUANGDONG YIFANG PHARMA
Preparation method and quality control method of Gentiana scabra Bunge liver fire purging formula particles
ActiveCN105640893AEmbody conceptTo achieve the purpose of reducing toxicity and increasing efficiencyComponent separationDigestive systemLicorice rootsCrusher
The invention discloses a preparation method of Gentiana scabra Bunge liver fire purging formula particles. The preparation method comprises the following steps: taking decoction pieces of Gentiana scabra Bunge, Fructus Gardeniae, Radix Scutellariae, Rhizoma Alismatis, glutinous rehmannia, Semen plantaginis, Chinese angelica root, radix bupleuri, Caulis Clematidis armandii and licorice root, adding water with the weight 5-15 times the total weight of the decoction pieces, and carrying out decocting extraction twice; mixing above obtained two decoction filtrates, and carrying out vacuum reduced pressure concentration on the obtained mixed filtrate; and carrying out spray drying the obtained concentrate to obtain spray dried powder, crushing the spray dried powder by using an airflow crusher to form ultrafine powder, adding the ultrafine powder to maltodextrin, and carrying out dry granulation to prepare the Gentiana scabra Bunge liver fire purging formula particles. A quality control method of the particles comprises infrared fingerprint, thin layer qualitative discrimination and HPLC content determination. The method fully performs advantages of traditional Chinese medicine compatibility, and maximally reserves effective components; and quality control is carried out through establishing complete quality standards, adopting a one-measurement and multiple-evaluation technology of thin layer chromatography and combining infrared spectroscopy with chromatography, so the quality of the compound particles is effectively controlled.
Owner:GUANGDONG YIFANG PHARMA
"Sanhuang" heart-fire removing decoction formula granules and preparing method and detection method thereof
ActiveCN104069200ATo achieve the purpose of reducing toxicity and increasing efficiencyQuality improvementSenses disorderAntipyreticSan-Huang-Xie-Xin-TangProcess conditions
The invention provides "sanhuang" heart-fire removing decoction formula granules and a preparing method and a detection method thereof. The granules are prepared by adding water into rheum officinale, root of baikal skullcap and Chinese goldthread in a weight ratio of 2:1:1, decocting, filtering, and concentrating and drying filtrate. The detection method includes: identifying the granules by a thin-layer chromatography method; detecting a fingerprint spectrum of the granules by a high performance liquid chromatography method; detecting the extract content of the granules by a hot dipping method; and detecting the contents of compounds in the granules by a high performance liquid chromatography method. The "sanhuang" heart-fire removing decoction is prepared by mixed-decoction according to traditional methods, advantages of traditional Chinese medicine compatibility are fully exerted, the holism concept of traditional Chinese medicines is reflected, and effects of toxicity reducing and efficacy enhancing are achieved. Disadvantages brought by simple addition of medicines in a taking process of single-medicine formula granules at present are overcome. Process conditions and complete quality standards of the granules are established.
Owner:KANGMEI PHARMA +1
Traditional Chinese medicine for treating deficiency of qi and yin after ovarian cancer chemotherapy
InactiveCN104324356AImprove immunityEnhance physical fitnessPowder deliveryAnthropod material medical ingredientsRadix OphiopogonisFructus psoraleae
The invention discloses a traditional Chinese medicine for treating deficiency of qi and yin after ovarian cancer chemotherapy. The traditional Chinese medicine is prepared from the following medicinal materials: codonopsis pilosula, astragalus membranaceus, rhizoma atractylodis macrocephalae, radix trichosanthis, schisandra chinensis, radix paeoniae alba, asparagus cochinchinensis, radix ophiopogonis, cortex moutan, radix adenophorae, poria cocos, radix rehmanniae, radix scrophulariae, colla corii asini, angelica sinensis, ligusticum wallichii, rehmannia glutinosa, fructus psoraleae, eclipta alba, fingered citron, rhizoma smilacis glabrae, radix aucklandiae, cornu cervi degelatinatum, arillus longan, solanum lyratum, dysosma versipellis, terfezia leonis, fruit of Chinese wolfberry, sulfur bacteria, spirulina, fried rice sprout, roasted malt, orange peel, cortex lycii radicis, felt covered agaric, caulis spatholobi, catharanthus roseus, cephalotaxus oliveri, fructus amomi, medicated leaven, endothelium corneum gigeriae galli, turtle shell, tortoise plastron, stir-baked squama manitis, raw oyster shell, ground beetle, pinellia ternate, caulis bambusae in taeniam, ginger and liquorice. Clinic practice proves that traditional Chinese medicine is capable of safely and effectively treating the deficiency of qi and yin after ovarian cancer chemotherapy.
Owner:李福荣
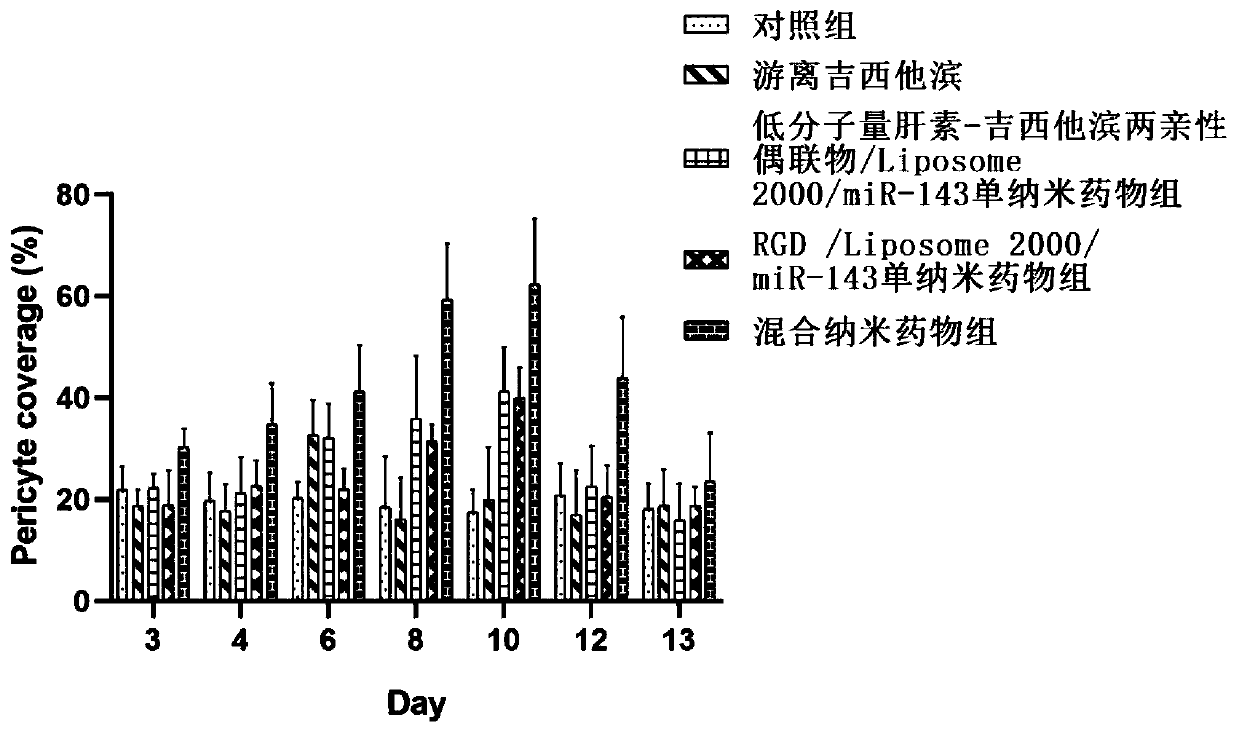
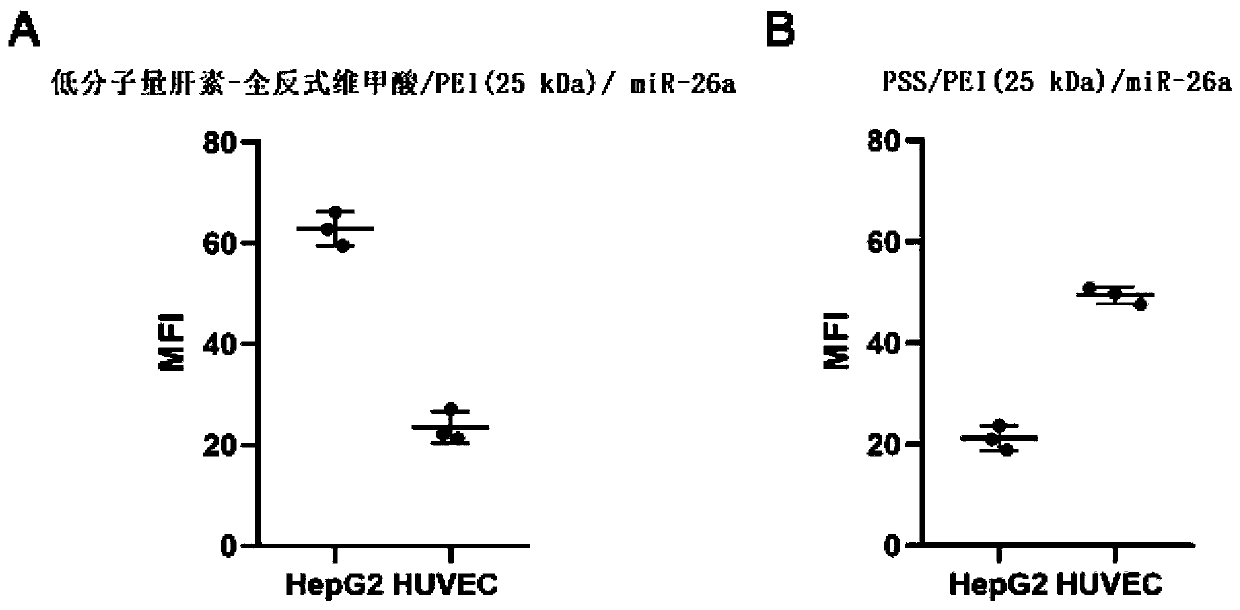
Preparation and application of hybrid nano-formulation based on tumor extensive metabolism regulation
ActiveCN111135312ASignificant metabolic regulationSignificantly normalized therapeutic effectPowder deliveryMaterial nanotechnologyVascular endotheliumTumor vessel
The invention discloses a hybrid nano-formulation for promoting normalization of tumor blood vessels based on tumor extensive metabolism regulation and application thereof. A nano self-assembly technology based on electrostatic interaction and hydrogen bonding is used to obtain the hybrid nano-formulation. Heparin polysaccharide macromolecules that having VEGF inhibitory activity and derivatives and active biological macromolecules thereof, or active substance targeting vascular endothelial cells are assembled with non-viral gene carriers and metabolic regulation related gene drugs to construct stable nano-drugs targeting tumor cells and tumor vascular endothelial cells, separately. After the hybrid nano-formulation formed only by physically mixing the two ternary assembled nano-drugs areadministered in the manner of beat, significant metabolic regulation effects of tumor cells and tumor vascular endothelial cells, promoting normalization of tumor blood vessels, and the multiple therapeutic effects of tumor proliferation inhibition can be obtained. The three effects are synergistic and complement each other to enhance the effectiveness.
Owner:CHINA PHARM UNIV
A kind of double rattan microemulsion gel and preparation method thereof
ActiveCN104306447BTo achieve the purpose of reducing toxicity and increasing efficiencyImprove securityAntipyreticAerosol deliverySide effectTripterospermum taiwanense
The invention belongs to the technical field of medicines, and specifically discloses a tripterygium wilfordii-caulis sinomenii microemulsion gel and a preparation method thereof. A prescription of the tripterygium wilfordii-caulis sinomenii microemulsion gel comprises caulis sinomenii extracts, tripterygium wilfordii extracts, a surfactant, a cosurfactant, an oil phase, a gel matrix material and water. The preparation method of the tripterygium wilfordii-caulis sinomenii microemulsion gel comprises the steps of dissolving medicines in the oil phase, mixing uniformly with the surfactant, the cosurfactant and water to obtain microemulsion; adding the gel matrix material into the microemulsion for swelling; regulating a pH value; and stirring uniformly. The tripterygium wilfordii-caulis sinomenii microemulsion gel is administered through skins, increases permeation rate through the skins, promotes transdermal absorption of the tripterygium wilfordii and caulis sinomenii, overcomes the problem of strong mobility of the microemulsion, achieves the object of sustained release and long-term efficacy, reduces toxicity of tripterygium wilfordii, and improves a curative effect of caulis sinomenii. The tripterygium wilfordii-caulis sinomenii microemulsion gel has a significant curative effect for treating rheumatoid arthritis, has small toxic or side effect, and has good product stability; and the preparation process is simple and suitable for large-scale production.
Owner:GUANGZHOU UNIVERSITY OF CHINESE MEDICINE +1
Warm light drink compound formula granule, as well as preparation method as well as detection method thereof
InactiveCN103083458ATo achieve the purpose of reducing toxicity and increasing efficiencyQuality improvementNervous disorderAntipyreticScutellaria baicalensis extractMedicine
The invention provides a warm light drink compound formula granule comprising angelica sinensis extract, ligusticum wallichii extract, scutellaria baicalensis extract, prepared rehmannia root extract, coptis chinensis extract, golden cypress extract, gardenia extract and radix paeoniae alba extract, wherein the mass ratio of the raw herbal materials of the above components is 1:1:1:1:1:1; the invention further provides a preparation method and a detection method of the above light drink compound formula granule. By adopting the formula granule, the preparation method and the detection method, the shortage caused by the simple addition of each medicine when the existing unit formula granule is taken is overcome, the technical condition and the complete quality standard of the light drink compound formula granule are created, the quality of the formula granule can be effectively controlled, and the medicine is convenient to store and carry by a user.
Owner:KANGMEI PHARMA +1
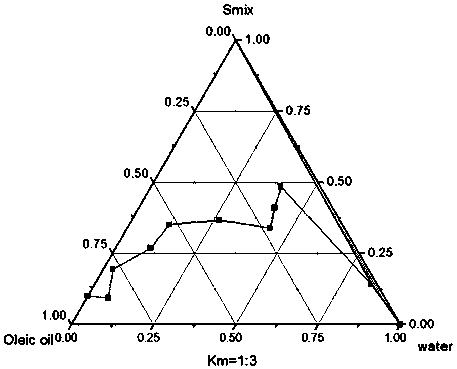
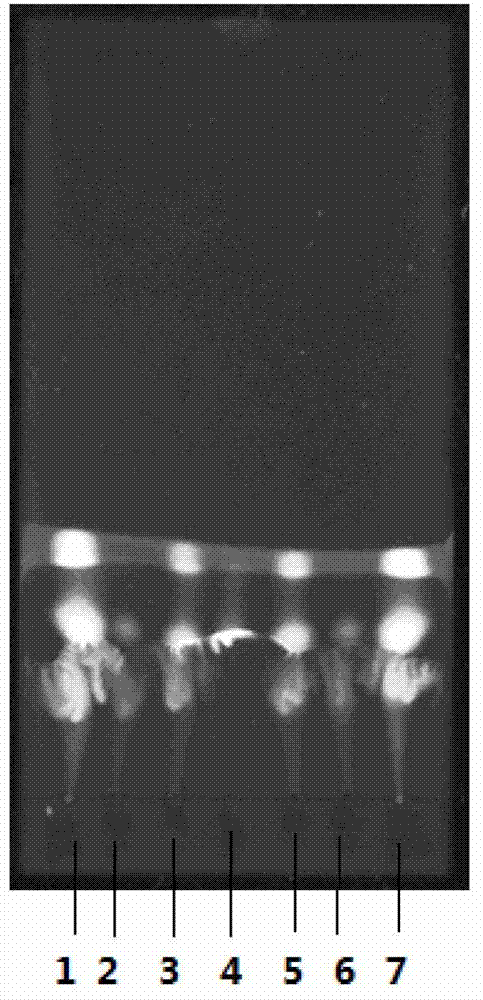
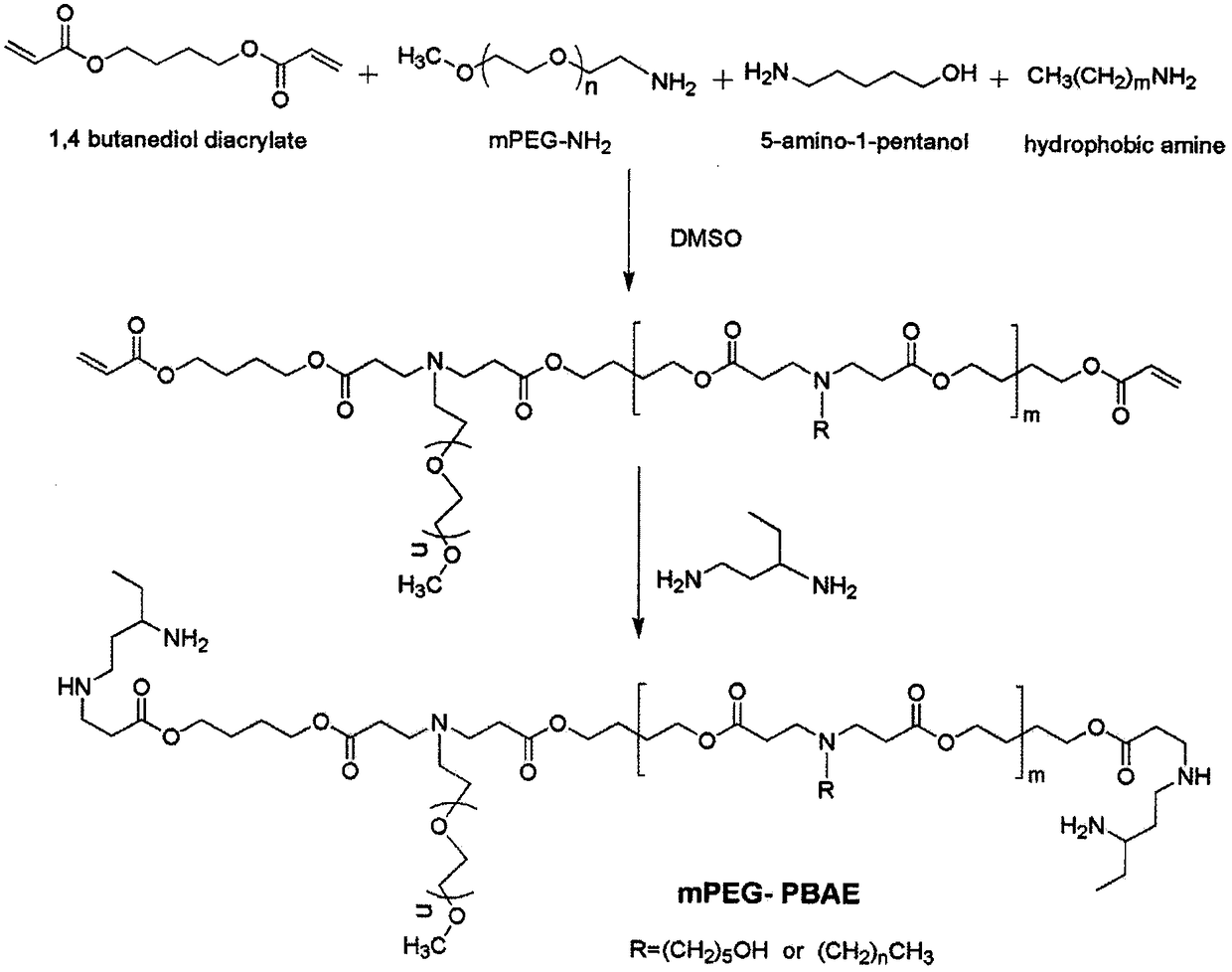
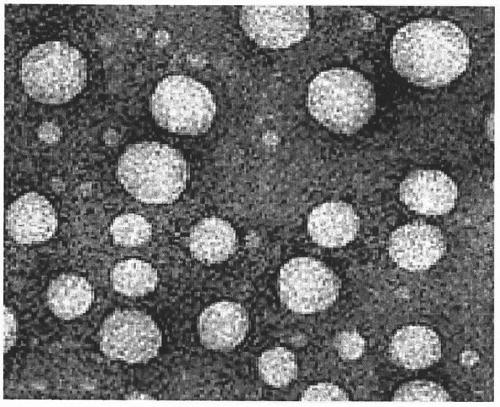
Preparation method of urushiol-loading pH responsive amphiphilic copolymer micelle
ActiveCN109303768ALow CMC valueImprove stabilityHydroxy compound active ingredientsPharmaceutical non-active ingredients1-PentanolPolyethylene glycol
The invention relates to a preparation method of urushiol-loading pH responsive amphiphilic copolymer micelle. The preparation method comprises the following steps: firstly, dissolving a certain amount of mPEG, hydrophobic amine monomer, 5-amino-1-pentanol and 1,4-butanediol diacrylate in DMSO to prepare a reaction solution with a certain concentration, performing polyreaction for 20-48h, then adding a certain amount of 1,3-pentanediamine, and continuing the reaction for 24h to synthesize polyethylene glycol-poly-beta-amino ester amphiphilic copolymer (mPEG-PBAE); and then taking and dissolving a certain amount of mPEG-PBAE and urushiol in DMF, performing uniform ultrasonic mixing, and performing dialyzing, centrifuging and filtering to obtain the urushiol-loading pH responsive amphiphiliccopolymer micelle. The drug-loading micelle has an average particle diameter of 140-160nm, good stability, obvious pH responsive drug-release property, good biocompatibility, and good targeting anti-tumor activity, can be applied to clinical targeting anti-tumor drugs, has very high added value, and can become a new technique for clinically developing urushiol targeting anti-tumor drugs.
Owner:INST OF CHEM IND OF FOREST PROD CHINESE ACAD OF FORESTRY
Preparation method and quality control method of rhizoma cyperi four-material granules
ActiveCN106728651AEmbody conceptTo achieve the purpose of reducing toxicity and increasing efficiencyComponent separationAntipyreticIcing sugarWater vapor
The invention discloses a preparation method and a quality control method of rhizoma cyperi four-material granules. The preparation method comprises the steps of extracting volatile oil from four medicinal materials including angelica, Szechuan lovage rhizome, rhizoma cyperi and costus root by adopting a steam distillation method, performing grinding inclusion on the volatile oil by adopting beta-cyclodextrin, adding prepared rehmannia root, white peony root and rhizoma corydalis into the residue obtained after the volatile oil is extracted, adding water for decocting and extracting, precipitating the extract by using 95% ethanol, standing, filtering, concentrating the supernate under reduced pressure, drying to obtain a dry extract, adding powdered sugar and dextrin, mixing uniformly, granulating, drying, shaping, adding an inclusion compound, and mixing uniformly, thus obtaining the four-material granules. The quality control method comprises thin-layer qualitative identification and HPLC (High Performance Liquid Chromatography) content measurement. The volatile oil is separately extracted and included, so that double effects of reducing the consumption of accessories and exerting the treatment effect are achieved; compared with the conventional single-formula granules, the four-material granules having various medicinal flavors can sufficiently exert the advantage of compatibility of Chinese medicaments; and a perfect quality standard is established, so that the quality of the compound granules can be effectively controlled.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Prepared technology for nutmeg
InactiveCN101164605AQuality improvementEasy to operatePlant ingredientsBrown colourPharmacological action
The present invention relates to a preparation method of nutmeg mix-fried with wheat bran. Said preparation method includes the following steps: selecting raw material nutmeg, cleaning its, removing its worm-holed portion and tainted portion, adding proper quantity of water and soaking the cleaned nutmeg for 2-24hr, then taking out, cooling and drying to remove its surface water content, placing said dried nutmeg and wheat bran into a medicine-frying pan or a rotary medicine-frying machine whose rotating speed is 20rpm, bran-frying temperature is 130-200deg.C, mixing ratio of medicine and wheat bran is 100:40-60, and the bran-frying time is 15-35min, when its surface is brown colour, has cracks and produces intense fragrance, taking out and sieving to remove wheat bran, cooling so as to obtain the invented nutmeg mix-fried wheat bran. As compared with raw nutmeg material the chemical components and pharmacological action of said nutmeg produce obvious change, in which the effective component methyl syringophenol and methyl isozyringophenol contents are increased and the toxic component nutmeg ether and sassafras ether are reduced.
Owner:LIAONING UNIV OF TRADITIONAL CHINESE MEDICINE
A preparation method and quality control method of Siwutang formula granules
ActiveCN105287875BEmbody conceptTo achieve the purpose of reducing toxicity and increasing efficiencyComponent separationMaterial analysis by optical meansBiotechnologyFormulary
Owner:GUANGDONG YIFANG PHARMA
Detoxification processing technology of aconitum sinomontanum nakai
ActiveCN104491035AIncrease contentReduce contentPlant ingredientsAconitum carmichaeliSteroidal alkaloid
The invention discloses a detoxification processing technology of aconitum sinomontanum nakai. The detoxification processing technology comprises the steps: washing and selecting aconitum sinomontanum nakai medicinal materials; softening; chopping into sections; drying in the shade to obtain crude decoction pieces; uniformly stirring by using licorice juices; steaming for 3-5 hours at the temperature of 115-127 DEG C under the pressure of 0.10-0.15 MPa; taking out and drying to obtain the aconitum sinomontanum nakai. An experiment proves that the processed aconitum sinomontanum nakai has 4.98-7.75 percent of the content of total alkaloids and 1.17-1.48 percent of the content of lappaconitine. Relative to the aconitum sinomontanum nakai medicinal material, the content of the total alkaloids is increased by 130.65-141.75 percent, and the content of the lappaconitine is reduced by 46.62-55.56 percent; because the total alkaloids of the aconitum sinomontanum nakai is greatly increased, the content of the lappaconitine in the aconitum sinomontanum nakai medicinal materials is greatly reduced while the pharmacological effect is maintained; therefore, the toxicity is greatly reduced, the aim of reducing toxicity and increasing efficiency is truly fulfilled, and the safety and the efficiency of clinical medication are guaranteed.
Owner:GANSU UNIV OF CHINESE MEDICINE
Intestinal tract slow-release bovine colostrum and sea cucumber peptide chewable tablet and preparation method thereof
ActiveCN111821421AIncrease peristalsisEnhance fruityNervous disorderHydrolysed protein ingredientsNutritionPhosphopeptide
The invention provides an intestinal tract slow-release bovine colostrum and sea cucumber peptide chewable tablet and a preparation method thereof. The chewable tablet comprises the following components: casein phosphopeptide, sea cucumber peptide, bovine colostrum powder, soybean peptide, concentrated whey protein, lutein ester, vitamin C, fruit and vegetable powder, resistant dextrin, lactose, erythritol, sorbitol, DL-malic acid, xylooligosaccharide and magnesium stearate; and the chewable tablet also comprises a targeted slow-release biocompatible microcapsule which comprises a polycarbonate film, bovine serum albumin freeze-dried powder, amino acid, chitosan oligosaccharide, lecithin and poly(2-ethyl-2-oxazoline) grafted glucan nanoparticles. The intestinal tract slow-release bovine colostrum and sea cucumber peptide chewable tablet prepared by using the preparation method and the components provided by the invention can release active peptides with quality and nutrition effects inintestinal tracts, and can penetrate through a blood brain barrier to act on brain cells to repair cardiovascular and cerebrovascular diseases and injuries after oxidative stress; and the bovine colostrum powder and the concentrated whey protein are digested in the stomach in an auxiliary manner, so the human immunity is enhanced.
Owner:SHANGHAI JIAO TONG UNIV +1
A detection method for formula granules of Maxing Shigan Decoction
ActiveCN105147800BEmbody conceptTo achieve the purpose of reducing toxicity and increasing efficiencyComponent separationMaterial analysis by optical meansBiotechnologyLicorice roots
The invention discloses a preparation method and a quality control method of formula granules containing ephedra, semen armeniacae amarae, gypsum and licorice root. The preparation method comprises the following steps: boiling water which is 5-15 times weight of the total amount of materials; putting ephedra, semen armeniacae amarae, and honey-fried licorice root slices; putting gypsum slices; decocting and extracting twice, mixing two decoctions, and filtering; concentrating the filtrate in vacuum at reduced pressure; drying the concentrate by spray; and granulating by a dry method. The quality control method comprises infrared fingerprint, thin-layer qualitative identification and HPLC (high performance liquid chromatography) content determination. According to the preparation method, effective ingredients in the compound can be furthest maintained, advantages of traditional Chinese medicine compatibility can be sufficiently achieved, the whole concept of traditional Chinese medicine can be reflected, a thorough quality standard of the formula granules containing ephedra, semen armeniacae amarae, gypsum and licorice root can be established, and the quality of compound granules can be effectively controlled.
Owner:GUANGDONG YIFANG PHARMA
A kind of Shashen Maidongtang formula granule and preparation method and detection method thereof
ActiveCN103230517BTo achieve the purpose of reducing toxicity and increasing efficiencyQuality improvementComponent separationGranular deliveryPesticide residueOfficinalis
The invention provides adenophora radix ophiopogonis soup formula granules as well as a preparation method and a detection method thereof. The granules comprises the raw materials as follows: radix glehniae, radix polygonati officinalis, radix ophiopogonis, radices trichosanthis, hyacinth beans, mulberry leaves and raw liquorice in the mass ratio of (0.5-2):(0.5-2):(0.5-2):(1-3):(0.5-2):(0.1-1.2):(0.1-1), and the raw materials are respectively immersed for multiple times, boiled and filtered, so as to obtain cream after filter liquors are mixed and concentrated, and the cream is dried to be prepared into granules. The detection method comprises the following steps of: identifying the adenophora ophiopogonis soup formula granules through a using thin-layer chromatography; measuring the content of organic chloride pesticide residue in the adenophora ophiopogonis soup formula granules through using a pesticide residue measuring method; measuring the content of heavy metal in the adenophora ophiopogonis soup formula granules through using a residue on ignition examination method; measuring the content of extractum of the adenophora ophiopogonis soup formula granules through using a hot dipping method; and measuring the content of substances in the adenophora ophiopogonis soup formula granules through a using high-efficiency liquid chromatography.
Owner:KANGMEI PHARMA +1
Application of pharmaceutical composition in preparation of drugs for treating cancers
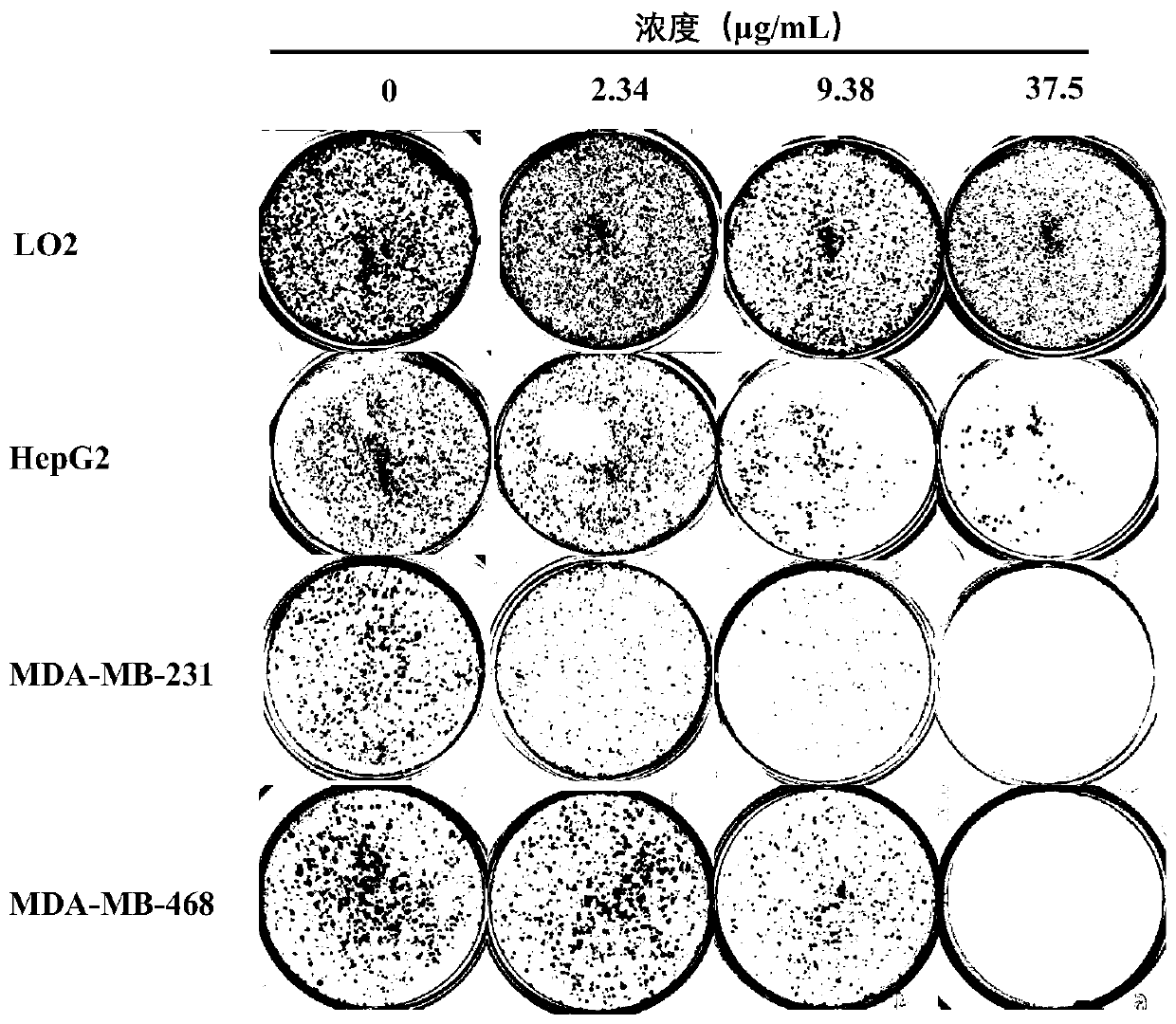
ActiveCN111544479AGrowth inhibitionInhibitory therapyOrganic active ingredientsAntineoplastic agentsCancer cellSide effect
The invention discloses a new application of a pharmaceutical composition, and concretely relates to an application of the pharmaceutical composition in the preparation of cancer treatment medicines,and the pharmaceutical composition comprises a Shuganning preparation. The pharmaceutical preparation can effectively inhibit the growth of multiple cancer cells, reduce the formation of clone numberof cancer cells, induce cancer cell death, block the cancer cell period in the S phase, effectively inhibit the growth of transplanted tumor of nude mice, does not influence the weight of nude mice, can effectively treat cancer, and has low toxic and side effects. The Shuganning preparation in the traditional Chinese medicine composition can selectively kill cancer cells, does not influence the growth of normal cells, and has a synergistic anti-tumor effect (wherein synergistic index, CI is less than 1) when being combined with 5-fluorouracil for use.
Owner:杜晶晶
Zengyetang formula granules and its preparation method, application and detection method
ActiveCN103169864BOvercome the disadvantage of lack of co-fryingPerfect quality control methodSenses disorderComponent separationTraditional medicineRadix Ophiopogonis
The invention provides fluid-increasing decoction formula granules. The fluid-increasing decoction formula granules contain extracts prepared by the step of decocting radix scrophulariae, radix ophiopogonis and radix rehmanniae recen. The invention also provides a preparation method, application and a detection method of the fluid-increasing decoction formula granules. According to the fluid-increasing decoction formula granules, the defect that various herbal medicines are simply added when the conventional single-component formula granules are taken is overcome, the compatibility advantages of traditional Chinese medicine are fully exerted, the holistic thinking of traditional Chinese medicine is reflected, the aim of reducing toxicity and enhancing efficacy is ensured, the fluid-increasing decoction formula granules are quasi steady and controllable in quality and convenient to store and carry by patients, and the decoction trouble is avoided. The preparation method of the fluid-increasing decoction formula granules is simple, the herbal medicines are decocted together according to the traditional method, and the characteristics of the traditional Chinese medicine are met. According to the detection method of the fluid-increasing decoction formula granules, the perfect quality standard of the fluid-increasing decoction formula granules is established, and the quality of the formula granules can be effectively controlled.
Owner:KANGMEI PHARMA +1
A kind of Sanhuang Xiexin Tang formula granule and its preparation method and detection method
ActiveCN104069200BTo achieve the purpose of reducing toxicity and increasing efficiencyQuality improvementComponent separationPreparing sample for investigationBiotechnologyFluid phase
The invention provides a formula granule of Sanhuang Xiexin Decoction and its preparation method and detection method. The formula granule of Sanhuang Xiexin Decoction is decocted with rhubarb, scutellaria baicalensis and Coptis chinensis in a mass ratio of 2:1:1, and filtered. , and then concentrated and dried the filtrate; the detection method includes identifying the Sanhuang Xiexin Decoction formula granules by thin-layer chromatography; determining the fingerprint of the Sanhuang Xiexin Decoction formula granules by high performance liquid chromatography; Determination of the extract content of the Sanhuang Xiexin Decoction granules by hot soaking method; determination of the substance content in the Sanhuang Xiexin Decoction formula granules by high performance liquid chromatography. The Sanhuang Xiexin Decoction of the present invention is decocted according to the traditional method, which gives full play to the advantages of Chinese medicine compatibility, embodies the overall concept of Chinese medicine, ensures that it achieves the purpose of reducing toxicity and increasing efficiency, and overcomes the simple taste of each medicine when taking the current single-flavor formula granules. The shortcomings brought about by the addition established the technological conditions and perfect quality standards of Sanhuang Xiexin Tang formula granules.
Owner:KANGMEI PHARMA +1
A kind of preparation and preparation method of ginseng flower
ActiveCN104825750BSymptoms improvedGrowth QiMetabolism disorderAntipyreticBiotechnologyActive ingredient
The invention relates to a preparation and preparation method of ginseng flower. Honey, onion and ginseng flower are scientifically processed into a preparation, which retains the active ingredients of ginseng flower, enhances the effect of ginseng flower for nourishing qi, and changes the coldness of ginseng flower. Coolness, after adding refined honey, it can increase the moistness of ginseng, reconcile the medicinal properties of the present invention, improve the taste and be more conducive to human body absorption, and improve the tonic effect of ginseng. At the same dose, the purpose of reducing toxicity and increasing efficiency can be achieved. It is of great significance to protect scarce medicinal resources.
Owner:JILIN BEIYU AMERICAN GINSENG RES
A compound pharmaceutical composition for childbirth and its application
ActiveCN104189886BEasy to shrinkFacilitate strippingOrganic active ingredientsPeptide/protein ingredientsCompounding drugsBULK ACTIVE INGREDIENT
The invention discloses a compound drug composition for delivery and an application of the compound drug composition. The compound drug composition for the delivery comprises the following active ingredients: oxytocin and methylergonovine, wherein methylergonovine is present in a form of free or pharmaceutically acceptable salt. The compound drug composition for the delivery can achieve purposes of attenuation and synergism; compared with the prior art, the compound drug composition has stronger activity, takes effect more quickly and has longer duration and smaller adverse reactions; and the composition can be prepared into the drug composition drug administered to mammals including human beings, and is used for treating massive haemorrhage caused by active intervention at the third stage of labor and after the delivery.
Owner:WUHAN DOCAN PHARMA
A preparation method and quality control method of Longdan Xiegan Decoction formula granules
ActiveCN105640893BEmbody conceptTo achieve the purpose of reducing toxicity and increasing efficiencyComponent separationDigestive systemLicorice rootsCrusher
The invention discloses a preparation method of Gentiana scabra Bunge liver fire purging formula particles. The preparation method comprises the following steps: taking decoction pieces of Gentiana scabra Bunge, Fructus Gardeniae, Radix Scutellariae, Rhizoma Alismatis, glutinous rehmannia, Semen plantaginis, Chinese angelica root, radix bupleuri, Caulis Clematidis armandii and licorice root, adding water with the weight 5-15 times the total weight of the decoction pieces, and carrying out decocting extraction twice; mixing above obtained two decoction filtrates, and carrying out vacuum reduced pressure concentration on the obtained mixed filtrate; and carrying out spray drying the obtained concentrate to obtain spray dried powder, crushing the spray dried powder by using an airflow crusher to form ultrafine powder, adding the ultrafine powder to maltodextrin, and carrying out dry granulation to prepare the Gentiana scabra Bunge liver fire purging formula particles. A quality control method of the particles comprises infrared fingerprint, thin layer qualitative discrimination and HPLC content determination. The method fully performs advantages of traditional Chinese medicine compatibility, and maximally reserves effective components; and quality control is carried out through establishing complete quality standards, adopting a one-measurement and multiple-evaluation technology of thin layer chromatography and combining infrared spectroscopy with chromatography, so the quality of the compound particles is effectively controlled.
Owner:GUANGDONG YIFANG PHARMA
A kind of preparation method of Xiangfu Siwu granules and its quality detection method
ActiveCN106728651BEmbody conceptTo achieve the purpose of reducing toxicity and increasing efficiencyAntipyreticComponent separationMedicinal herbsIcing sugar
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
A detoxification process of aconite
The invention discloses a detoxification processing technology of aconitum sinomontanum nakai. The detoxification processing technology comprises the steps: washing and selecting aconitum sinomontanum nakai medicinal materials; softening; chopping into sections; drying in the shade to obtain crude decoction pieces; uniformly stirring by using licorice juices; steaming for 3-5 hours at the temperature of 115-127 DEG C under the pressure of 0.10-0.15 MPa; taking out and drying to obtain the aconitum sinomontanum nakai. An experiment proves that the processed aconitum sinomontanum nakai has 4.98-7.75 percent of the content of total alkaloids and 1.17-1.48 percent of the content of lappaconitine. Relative to the aconitum sinomontanum nakai medicinal material, the content of the total alkaloids is increased by 130.65-141.75 percent, and the content of the lappaconitine is reduced by 46.62-55.56 percent; because the total alkaloids of the aconitum sinomontanum nakai is greatly increased, the content of the lappaconitine in the aconitum sinomontanum nakai medicinal materials is greatly reduced while the pharmacological effect is maintained; therefore, the toxicity is greatly reduced, the aim of reducing toxicity and increasing efficiency is truly fulfilled, and the safety and the efficiency of clinical medication are guaranteed.
Owner:GANSU UNIV OF CHINESE MEDICINE
Compound and application thereof
ActiveCN114344262AIncrease intakeImproved multidrug resistanceOrganic active ingredientsDigestive systemCholic acidToxicity reduction
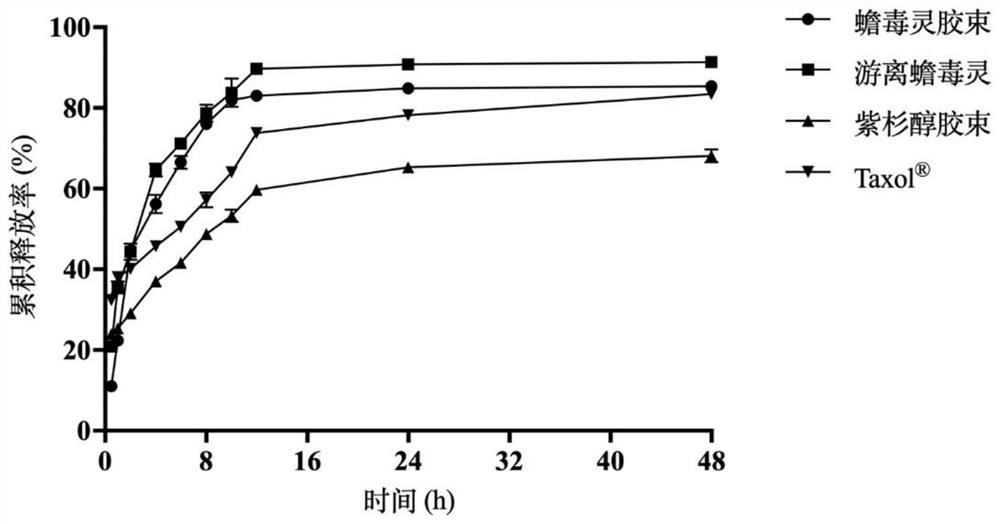
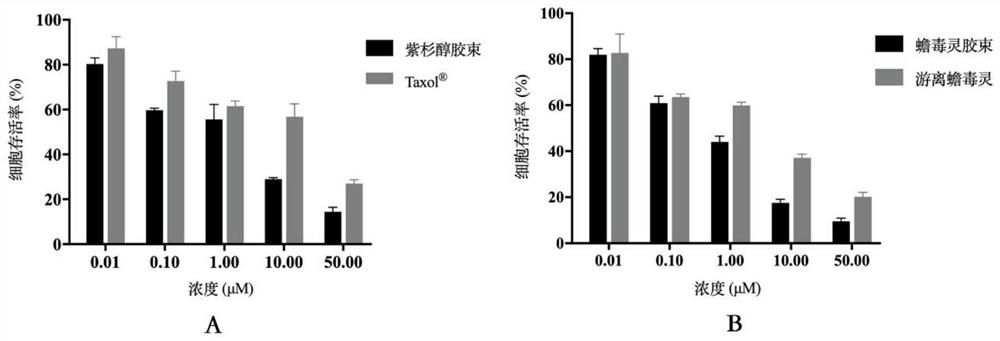
The invention relates to the field of new dosage forms and new technologies of medicines, in particular to a compound and application thereof, and the compound comprises two medicines, namely paclitaxel and bufalin. As the two drugs are poor in water solubility, the two drugs are respectively encapsulated in carriers of cholic acid-polyethylene glycol / vitamin E polyethylene glycol succinate to form mixed polymer micelles, and then the mixed polymer micelles are combined to treat liver cancer. The combined application of the paclitaxel micelles and the bufalin micelles improves the problems of drug resistance of paclitaxel and high toxicity of bufalin, and results of toxicity reduction and efficacy enhancement are generated.
Owner:CHINA PHARM UNIV
A preparation method and quality control method of Duhuo Jisheng Decoction formula granules
ActiveCN105477166BEmbody conceptTo achieve the purpose of reducing toxicity and increasing efficiencySenses disorderNervous disorderAdditive ingredientBeta-Cyclodextrins
Owner:GUANGDONG YIFANG PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com