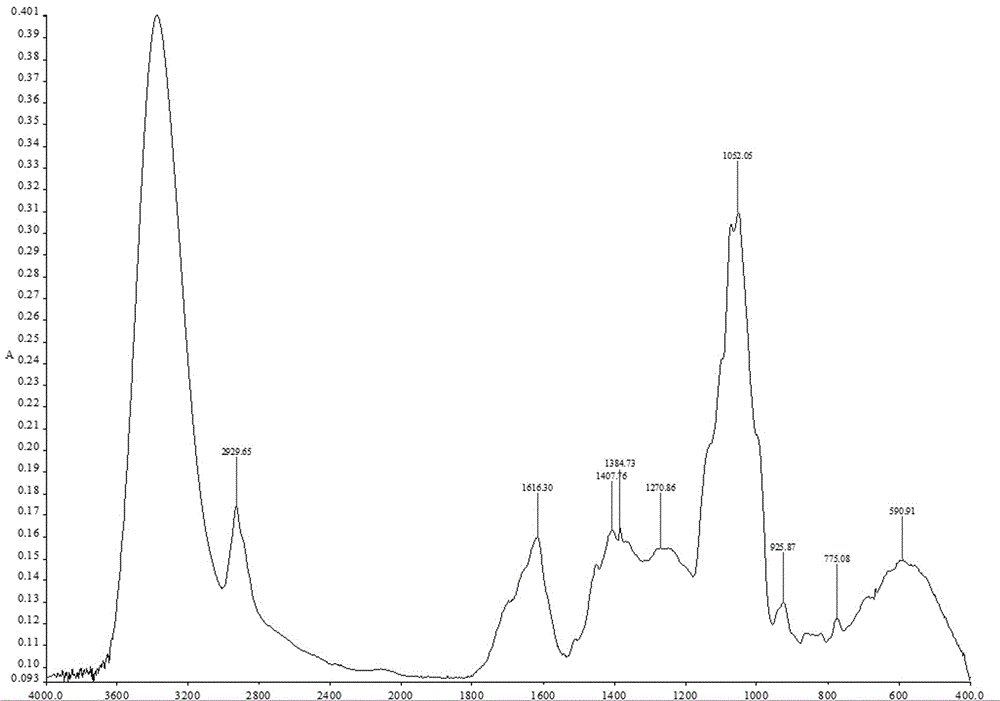
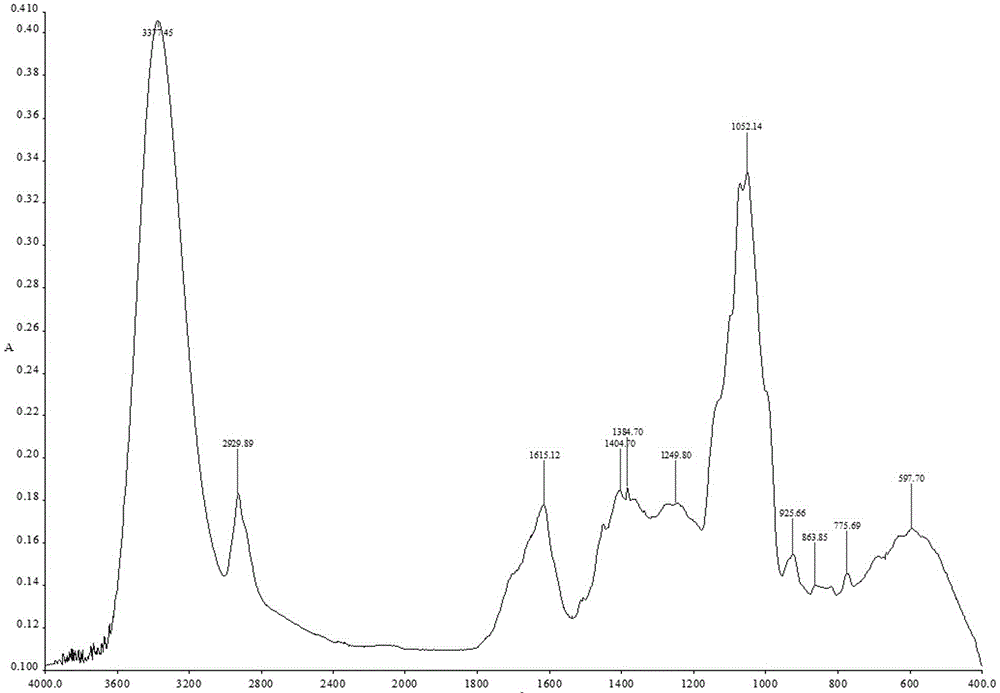
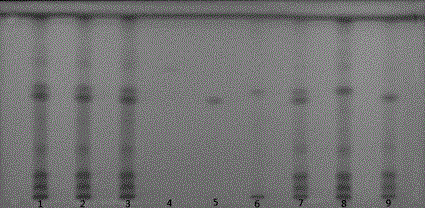
Preparation method and quality control method of Gentiana scabra Bunge liver fire purging formula particles
A technology of Longdan Xiegan Decoction and formula granules, which can be applied to medical formulas, medical preparations containing active ingredients, measuring devices, etc., can solve problems such as lack of quality standards, reduce inspection costs, increase specific surface area, and be easily absorbed Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] A preparation method of Longdan Xiegan Decoction formula granules, comprising the steps of:
[0066] a. Take gentian 250g, gardenia 250g, scutellaria baicalensis 250g, Alisma 250g, rehmannia glutinosa 250g, psyllium 250g, angelica 250g, bupleurum 250g, akeba 250g, licorice 250g, add 25kg of water, decoct for 2 hours , that is, the first decoction; after the first decoction, add 20kg of water to the dregs, and decoct for 1 hour, that is, the second decoction;
[0067] b. Combine the two decoction filtrates, filter, and concentrate the filtrate under reduced pressure under vacuum to a concentrated solution with a relative density of 1.06 at 95°C;
[0068] c. Take the above-mentioned concentrated solution for spray drying, control the inlet air temperature at 175°C-185°C, and the outlet air temperature at 85-95°C to obtain spray-dried powder, which is pulverized into superfine powder by jet mill;
[0069] d. Adding the superfine powder to 10% of the weight of the superfin...
Embodiment 2
[0071] A preparation method of Longdan Xiegan Decoction formula granules, comprising the steps of:
[0072] a. Take gentian 300g, gardenia 300g, scutellaria baicalensis 300g, Alisma 300g, rehmannia glutinosa 300g, psyllium 200g, angelica 200g, bupleurum 200g, akeba 200g, licorice 200g, add 37.5kg of water, decoct 1.5 hour, that is, the first decoction; after the first decoction, add 32.5kg of water to the dregs, and decoct for 1 hour, that is, the second decoction;
[0073] b. Combine the two decoction filtrates, filter, and concentrate the filtrate under reduced pressure under vacuum to a concentrated solution with a relative density of 1.09 at 95°C;
[0074] c. Take the above-mentioned concentrated solution for spray drying, control the inlet air temperature at 175°C-185°C, and the outlet air temperature at 85-95°C to obtain spray-dried powder, which is pulverized into superfine powder by jet mill;
[0075] d. Add the superfine powder to maltodextrin dry granulation accountin...
Embodiment 3
[0079] A preparation method of Longdan Xiegan Decoction formula granules, comprising the steps of:
[0080] a. Take gentian 350g, gardenia 350g, scutellaria baicalensis 350g, Alisma 350g, rehmannia glutinosa 350g, psyllium 150g, angelica 150g, bupleurum 150g, akeba 150g, licorice 150g, add 32.5kg of water, decoct for 1 hours, that is, the first decoction; after the first decoction, add 25kg of water to the dregs, and decoct for 1 hour, that is, the second decoction;
[0081] b. Combine the two decoction filtrates, filter, and concentrate the filtrate under reduced pressure under vacuum to a concentrated solution with a relative density of 1.12 at 95°C;
[0082] c. Take the above-mentioned concentrated solution for spray drying, control the inlet air temperature at 175°C-185°C, and the outlet air temperature at 85-95°C to obtain spray-dried powder, which is pulverized into superfine powder by jet mill;
[0083] d. Adding the superfine powder to maltodextrin dry granulation acc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com