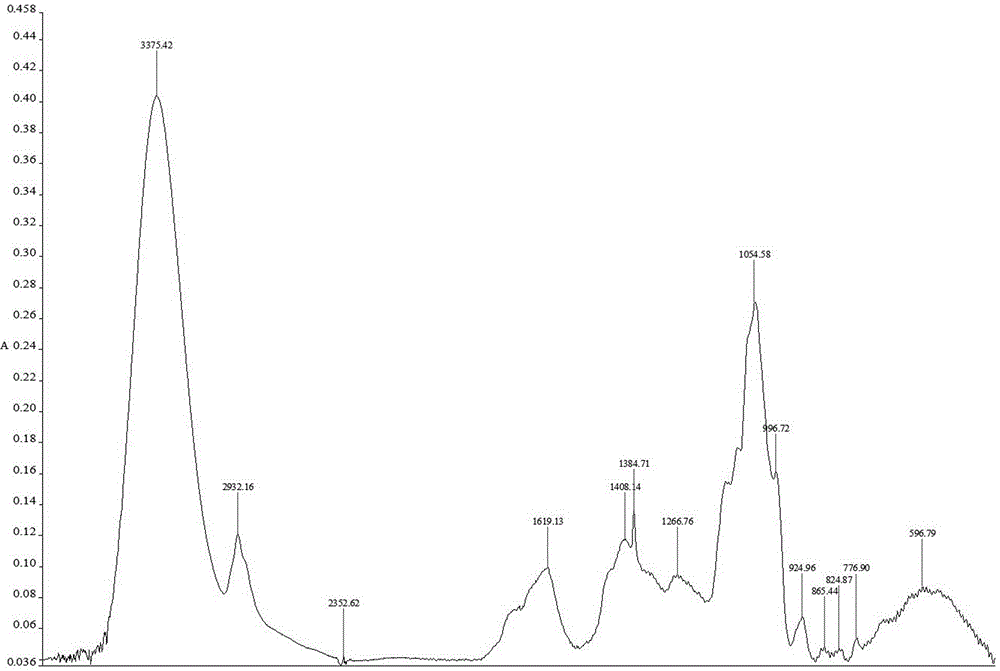
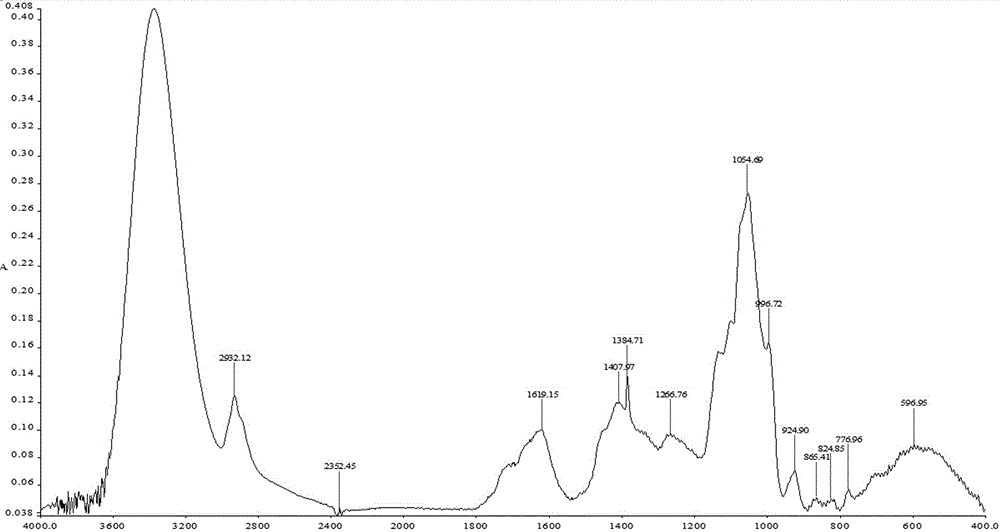
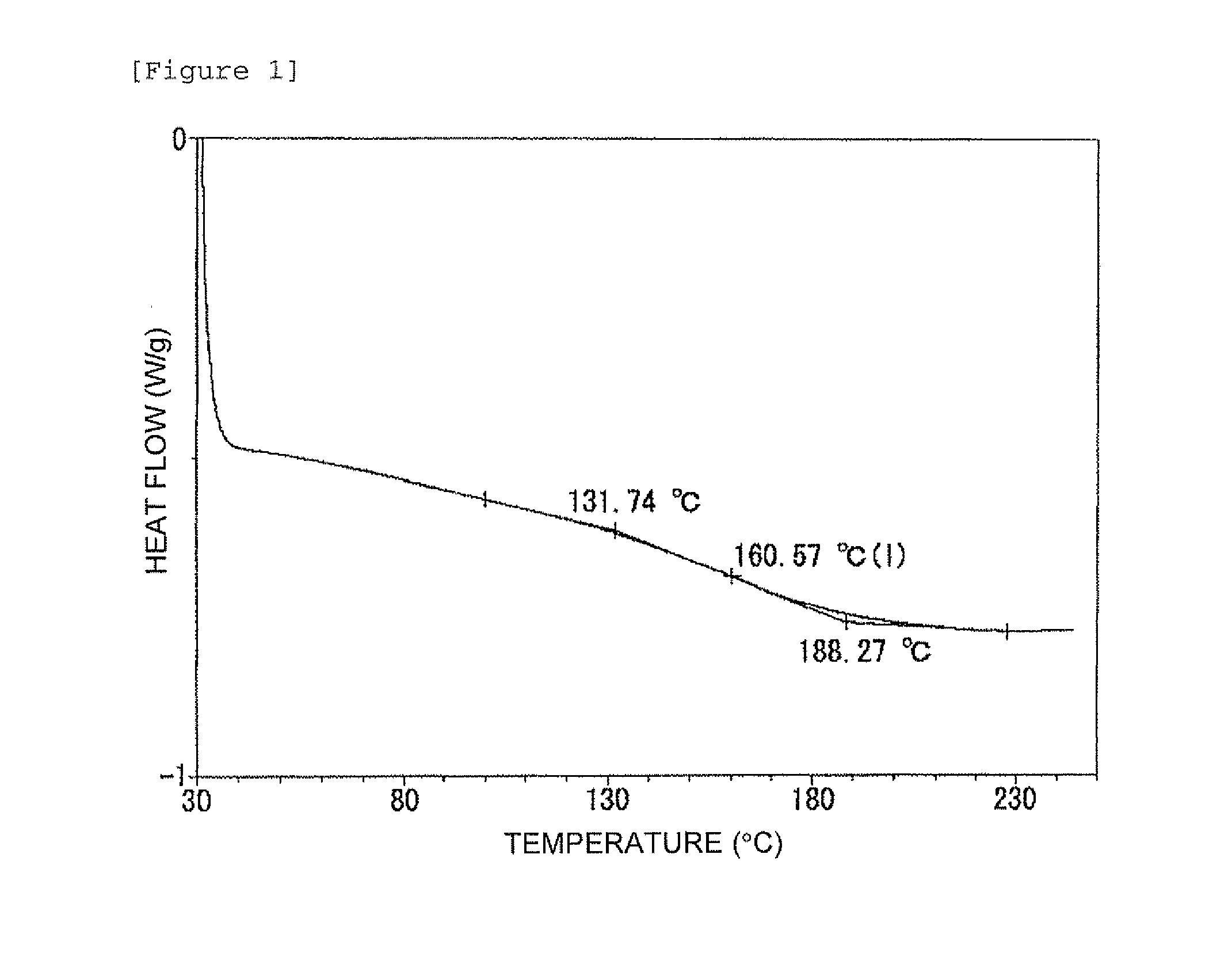
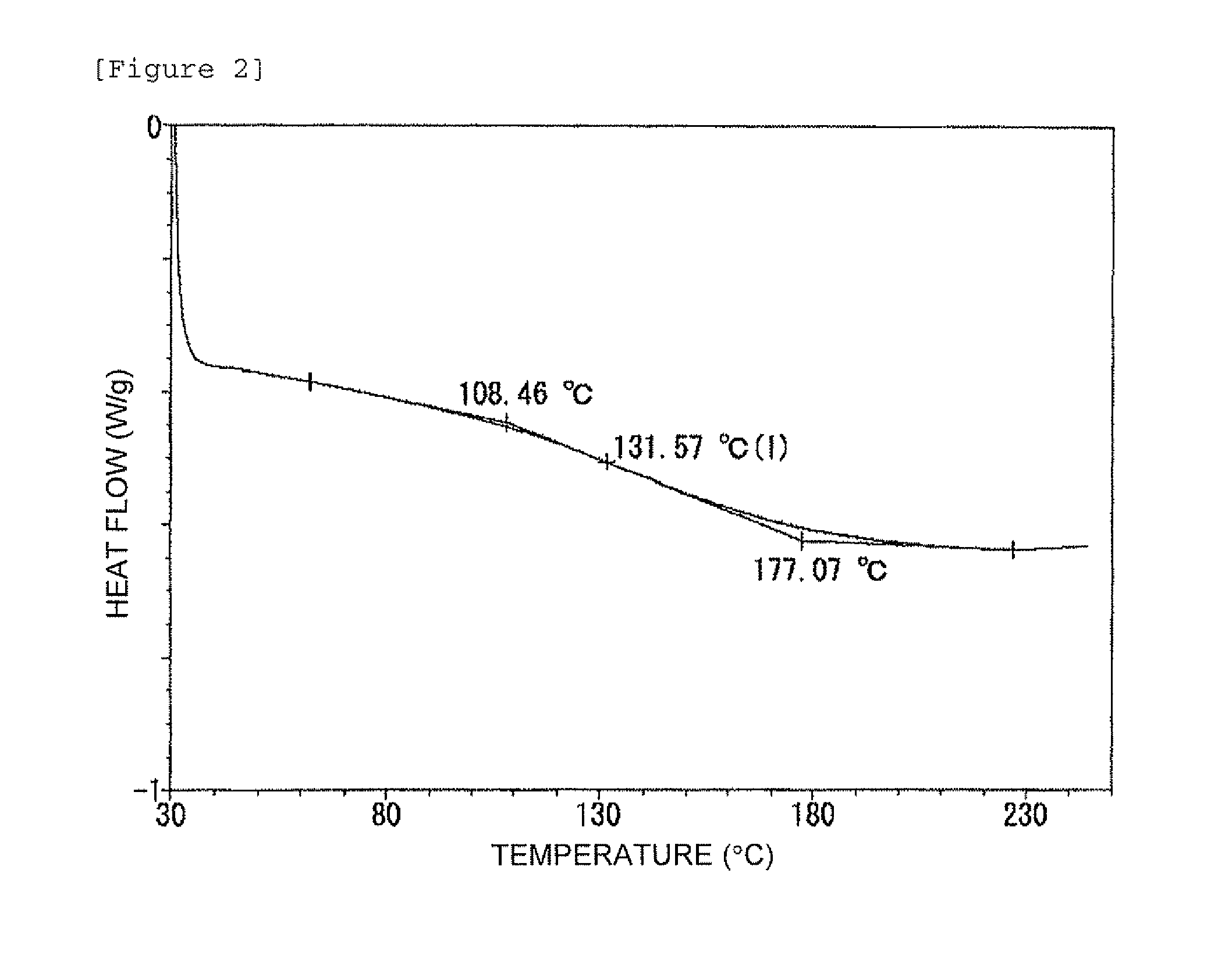
Patents
Literature
429 results about "Clathrate compound" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
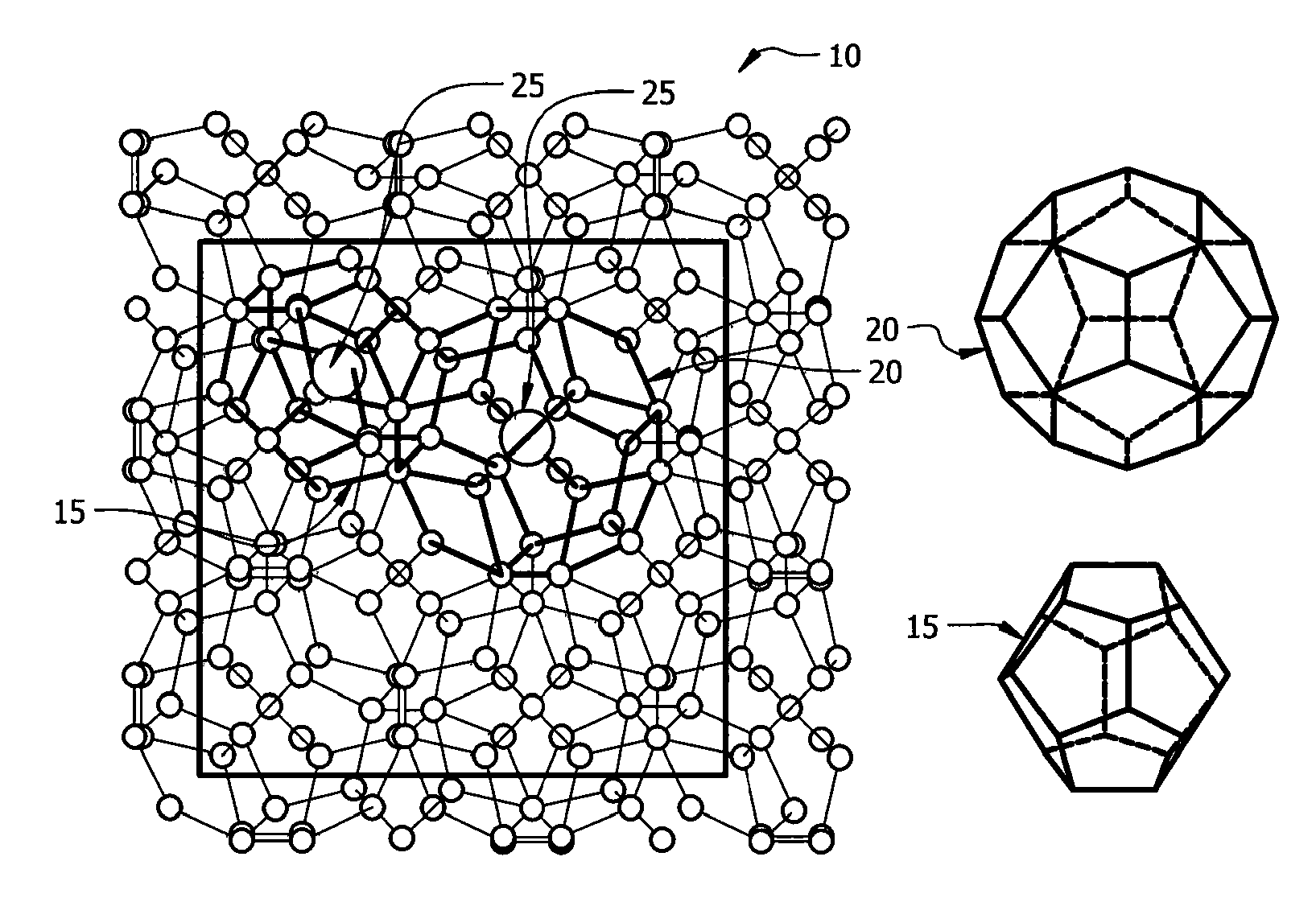
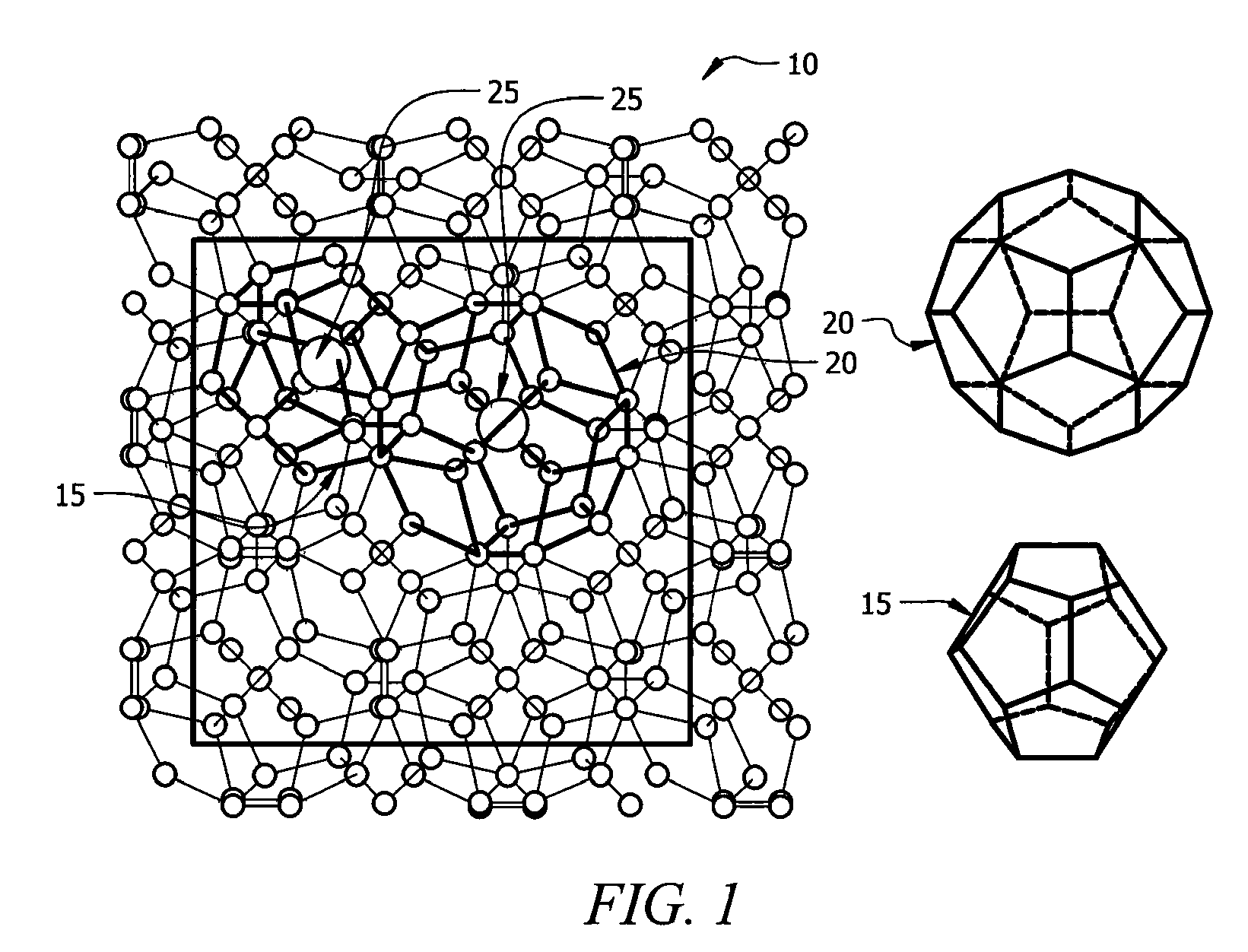
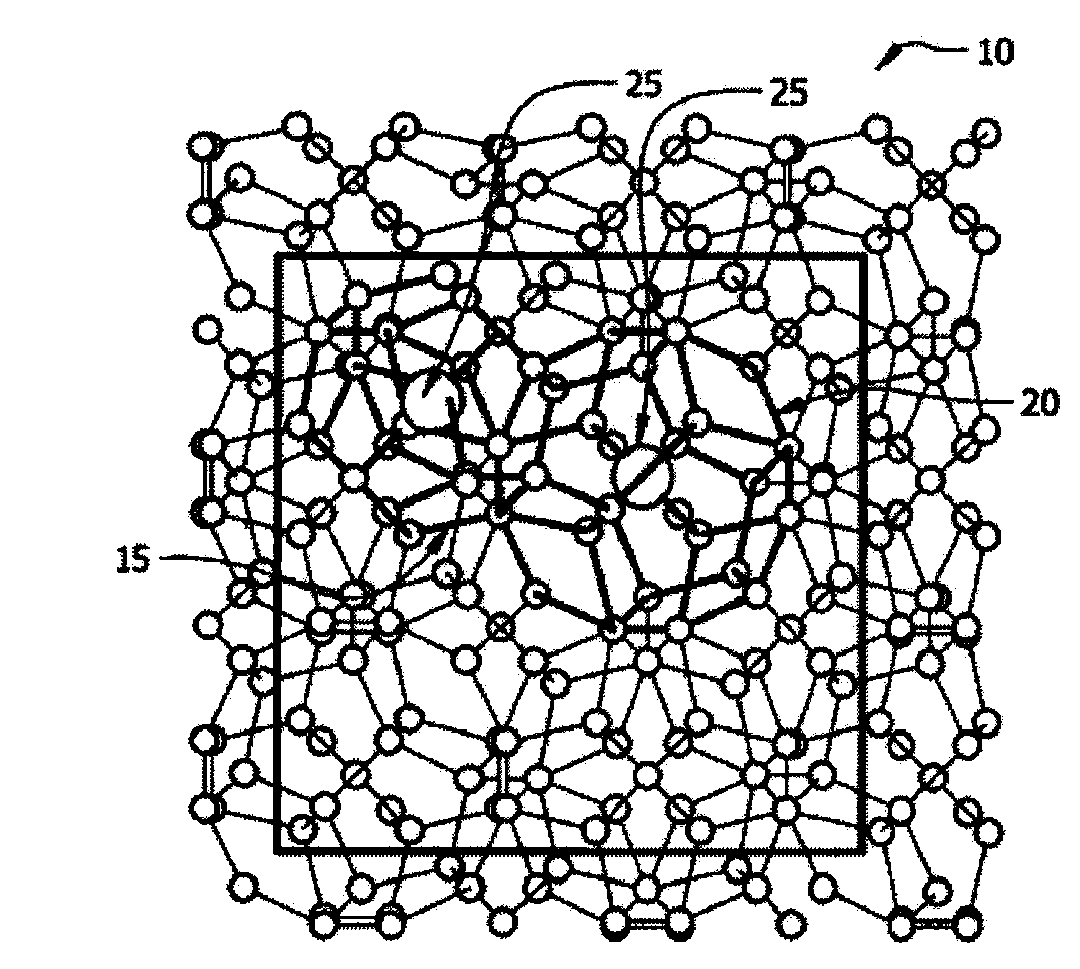
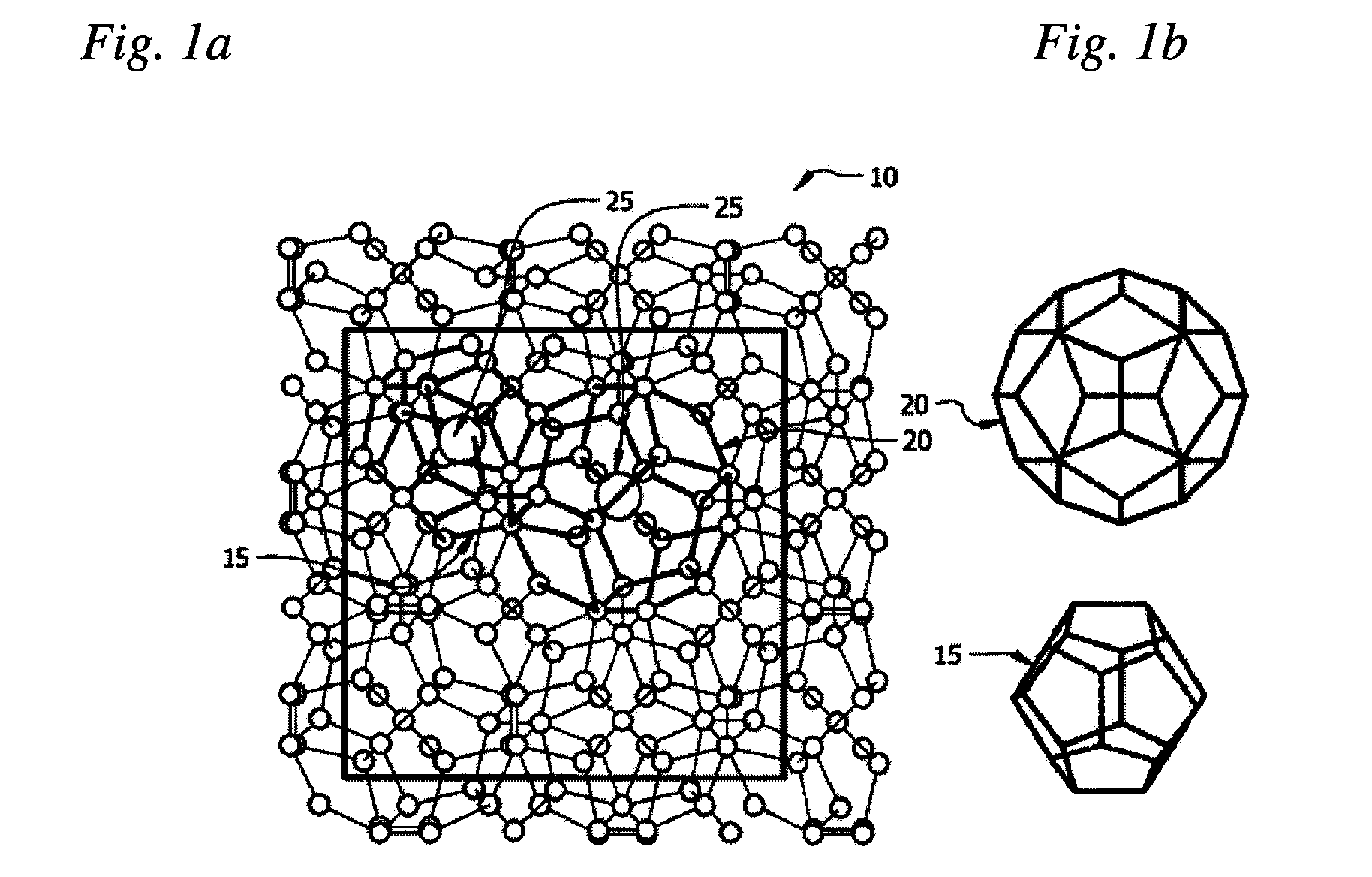
A clathrate is a chemical substance consisting of a lattice that traps or contains molecules. The word clathrate is derived from the Latin clatratus meaning with bars or a lattice. Traditionally, clathrate compounds are polymeric and completely envelop the guest molecule, but in modern usage clathrates also include host–guest complexes and inclusion compounds. According to IUPAC, clathrates are "Inclusion compounds in which the guest molecule is in a cage formed by the host molecule or by a lattice of host molecules."
Nitrogen stabilizing agent and application thereof
InactiveCN102746073AImprove stabilizerStabilizer holdAgriculture gas emission reductionFertilizer mixturesControl releaseNitrogen
The invention relates to a nitrogen stabilizing agent and application of the nitrogen stabilizing agent. The nitrogen stabilizing agent is a clathrate compound formed by mechanically mixing urease inhibitors, nitrification inhibitors and beta-cyclodextrin or the beta-cyclodextrin and zeolite or the beta-cyclodextrin and attapulgite clay at normal temperature for 1-2 hours, and comprises the following raw materials by weight: 1-20 parts of the beta-cyclodextrin or the beta-cyclodextrin and the zeolite or the beta-cyclodextrin and the attapulgite clay, 1-10 parts of the urease inhibitor and 0.5-10 parts of the nitrification inhibitor, wherein the zeolite is 50-90% of total using amount of the beta-cyclodextrin and the zeolite, and the attapulgite clay is 50-90% of total using amount of the beta-cyclodextrin and the attapulgite clay. The nitrogen stabilizing agent and nitrogenous fertilizers are mixed to produce long-acting controlled release fertilizers for use, wherein the using amount of the nitrogen stabilizing agent is 0.5wt%-10wt% of nitrogen element total amount of the nitrogenous fertilizers. Nitrogen using ratio of the nitrogenous fertilizers is improved, and nitrogen loss is reduced.
Owner:赵晓斌
Slime remover and slime preventing/removing agent containing a clathrate compound
InactiveUS6927199B2Reduce skin irritationReduce solubilityBiocideDead animal preservationMicroorganismMetabolite
Owner:NIPPON SODA CO LTD
Clathrate compounds, manufacture thereof, and thermoelectric materials, thermoelectric modules, semiconductor materials and hard materials based thereon
InactiveUS20030197156A1High hardnessImprove applicabilityConductive materialMetal silicidesThermoelectric materialsSemiconductor materials
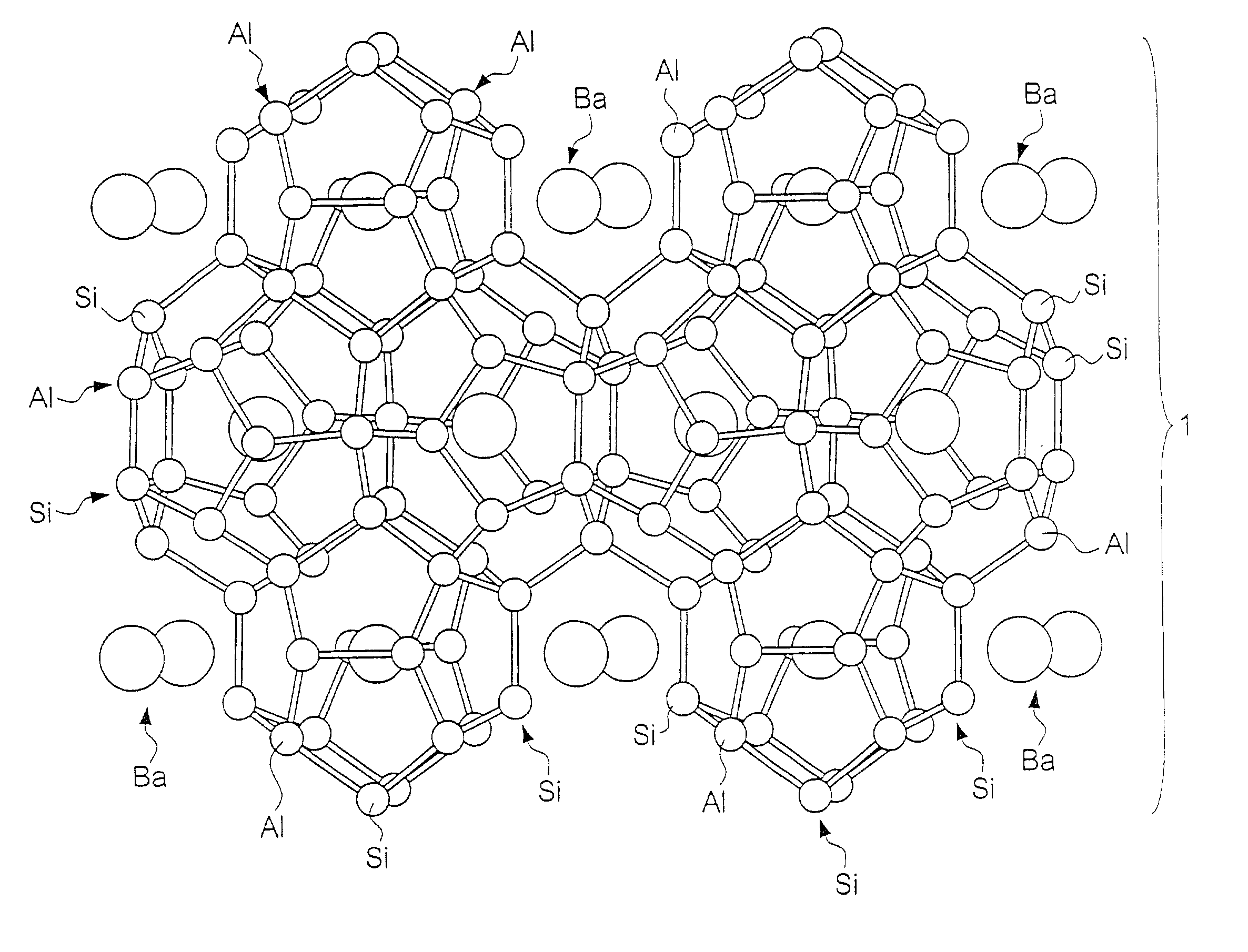
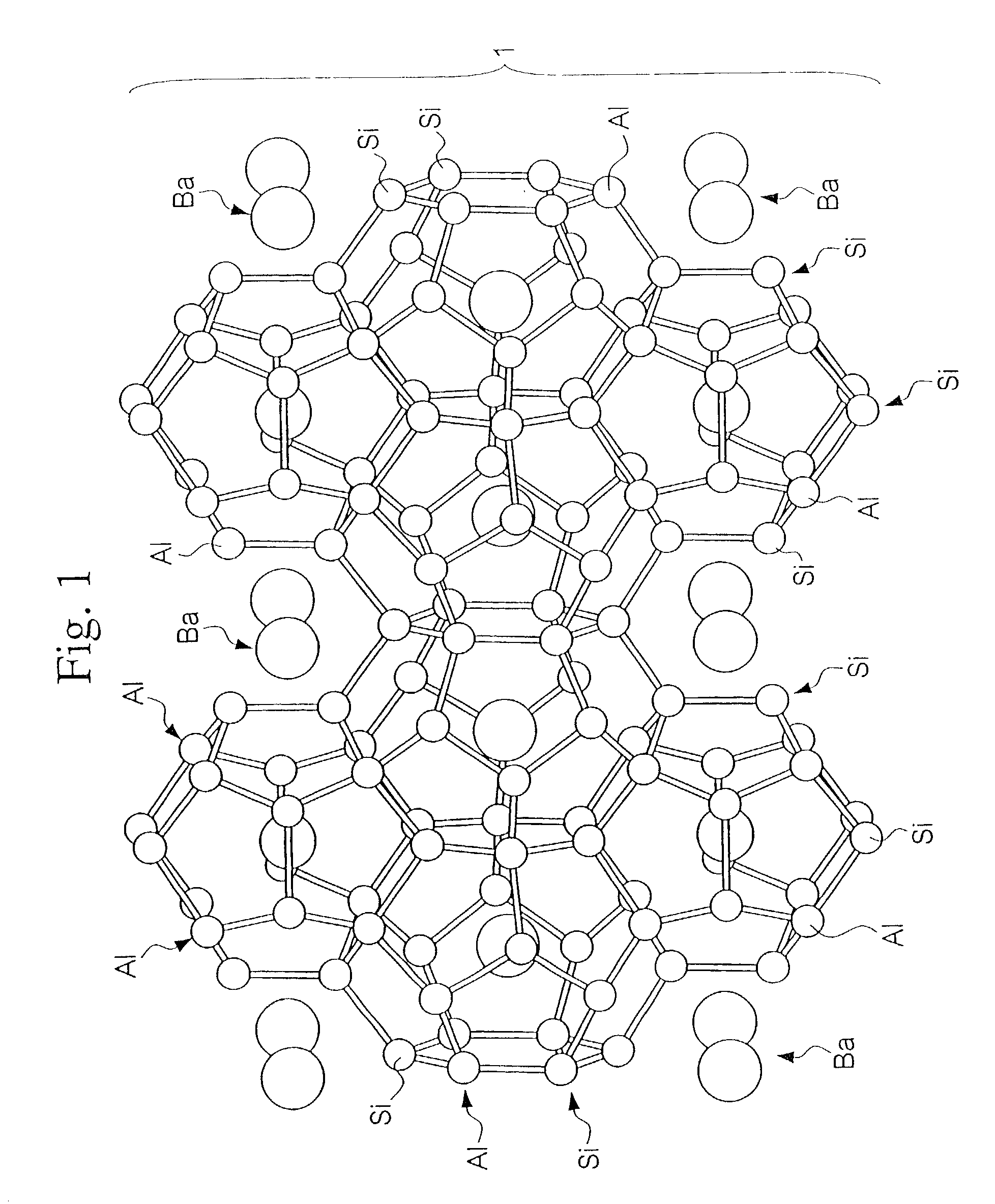
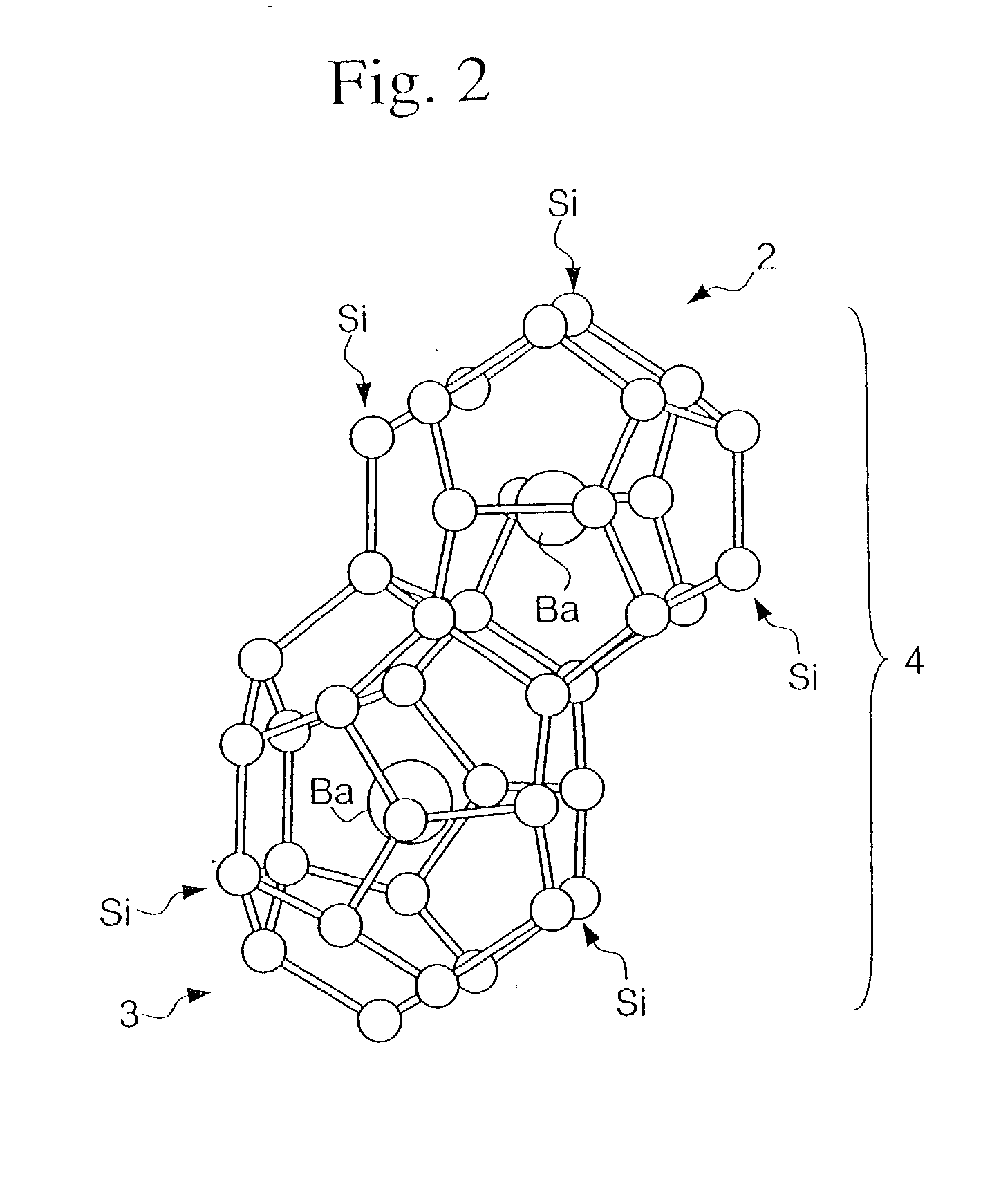
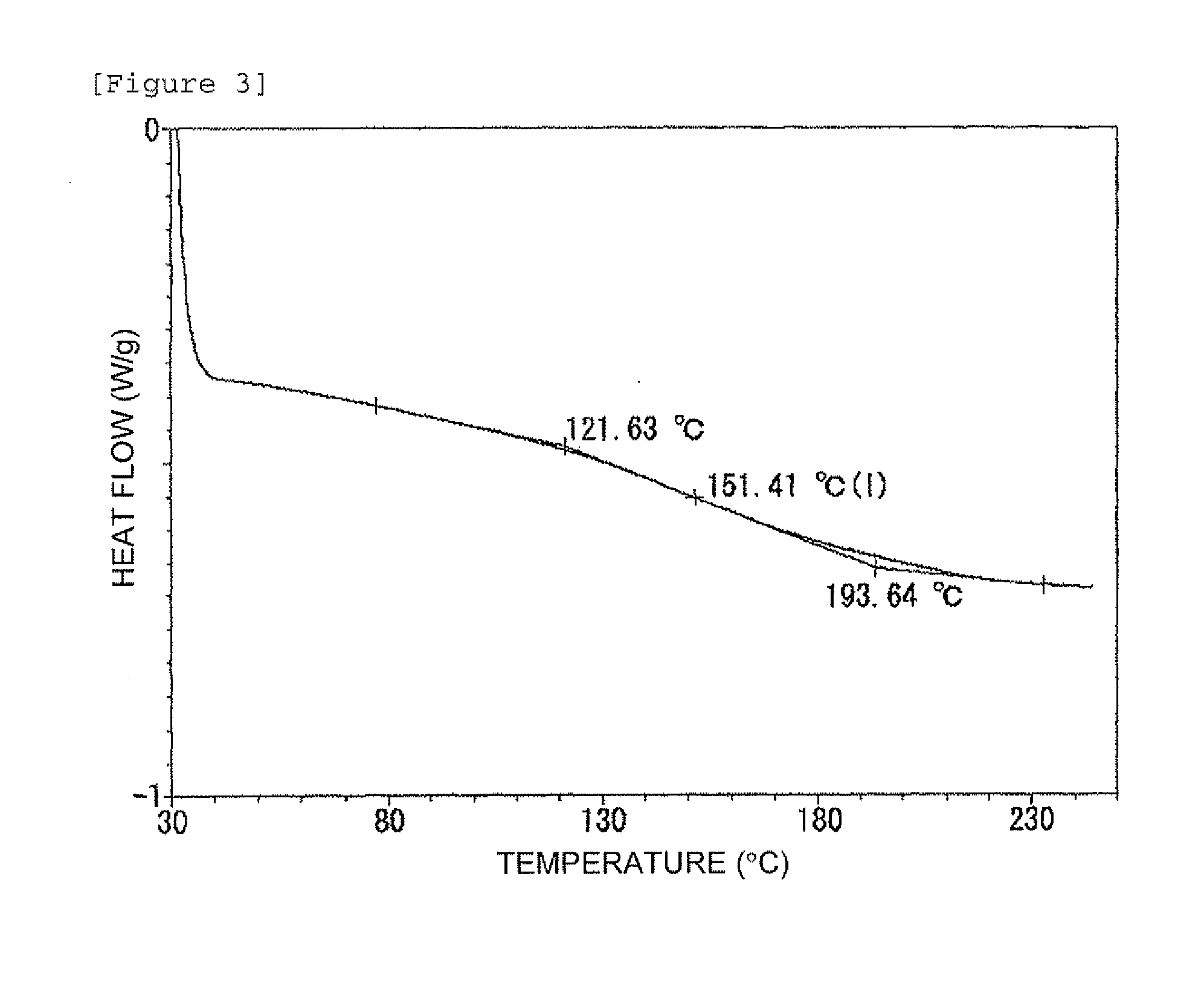
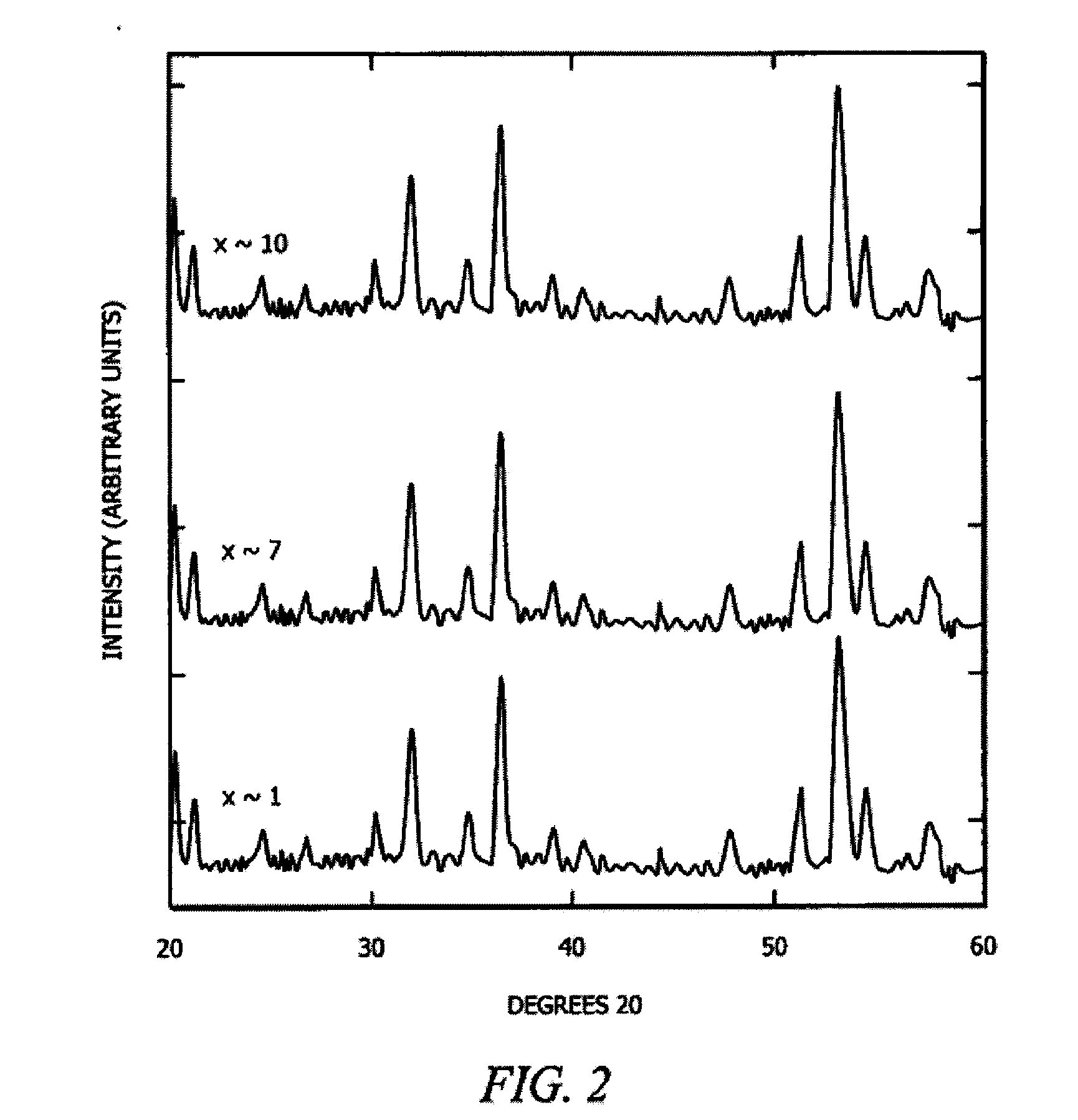
In high-tech fields such as electronics, the development of new high performance materials which differ from conventional materials has received much attention. An object of the present invention is to provide a clathrate compound which can be used as a thermoelectric material, a hard material, or a semiconductor material. Atoms of an element from group 4B of the periodic table are formed into a clathrate lattice, and a clathrate compound is then formed in which specified doping atoms are encapsulated within the clathrate lattice, and a portion of the atoms of the clathrate lattice are substituted with specified substitution atoms. Suitable doping atoms are atoms from group 1A, group 2A, group 3A, group 1B, group 2B, group 3B, group 4A, group 5A, group 6A, and group 8, and suitable substitution atoms are atoms from group 1A, group 2A, group 3A, group 1B, group 2B, group 3B, group 5A, group 6A, group 7A, group 5B, group 6B, group 7B, and group 8 of the periodic table. Suitable manufacturing methods include melt methods and sintering methods, and moreover intercalant intercalation compounds or the like may also be used as raw materials.
Owner:IHI CORP +1
Reinforcement treatment of bisphenol waste water
InactiveCN1778704AImprove photodegradation efficiencyIncrease reaction rateWater/sewage treatment by irradiationWastewaterUltraviolet lights
A fortified treatment of bisphenol waste water is carried out by adding ª‰-cyclodextrin into waste-water containing bisphenol substances in proportion of 5:1í½10:1, forming stable clathrate compound, and light degrading at normal temperature and pressure with ultraviolet light as light source. It can improve bisphenol substance light degradation efficiency by 40-60% and reaction speed ratio 2.3-11.0 times. It is cheap, simple and has no secondary pollution.
Owner:WUHAN UNIV
Clathrate compound of artemisinin series and alkaline cyclodextrin and method for preparing same
InactiveCN102716491APromote formationEasy to prepareOrganic active ingredientsAntiparasitic agentsArtemisininsCarboxyl radical
The invention discloses a clathrate compound of artemisinin series (artemisinin, dihydroartemisinin and artesunate) and alkaline cyclodextrin and a method for preparing the same. The alkaline cyclodextrin in the clathrate compound refers to an amido-substituted cyclodextrin; and due to the amido substituting of the cyclodextrin, an alkaline environment is formed in an aqueous solution apart from clathration between the cavity of the cyclodextrin and the artemisinin series, and forms ionic interaction with hydroxyls or carboxyls on the artemisinin series; and therefore, the artemisinin series can be dissolved in water to form a solution within an extremely wide concentration range so that liquid artemisinin series preparations can be formed. The clathrate compound provided by the invention is high in stability, high in bioavailability, simple in preparation, easy for operation, moderate in condition, and suitable for industrial production.
Owner:KUNMING UNIV OF SCI & TECH
Pentacyclic triterpenoid cyclodextrin clathrate compound proliposome and preparation method thereof
ActiveCN102871848ALess irritatingEasy to useCosmetic preparationsBody powdersCholesterolBetulonic acid
Owner:西安雅芝生物科技有限公司
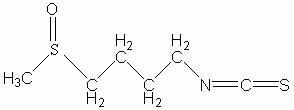
Chinese radish sulfane clathrate compound and preparation method thereof
InactiveCN102423492ASolve instabilityIncrease production capacityPharmaceutical non-active ingredientsEster active ingredientsFreeze-dryingDistilled water
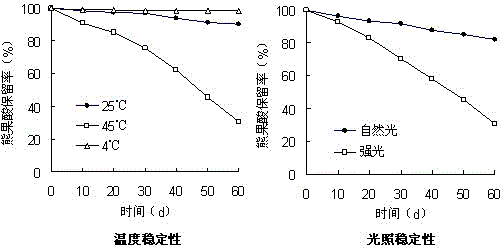
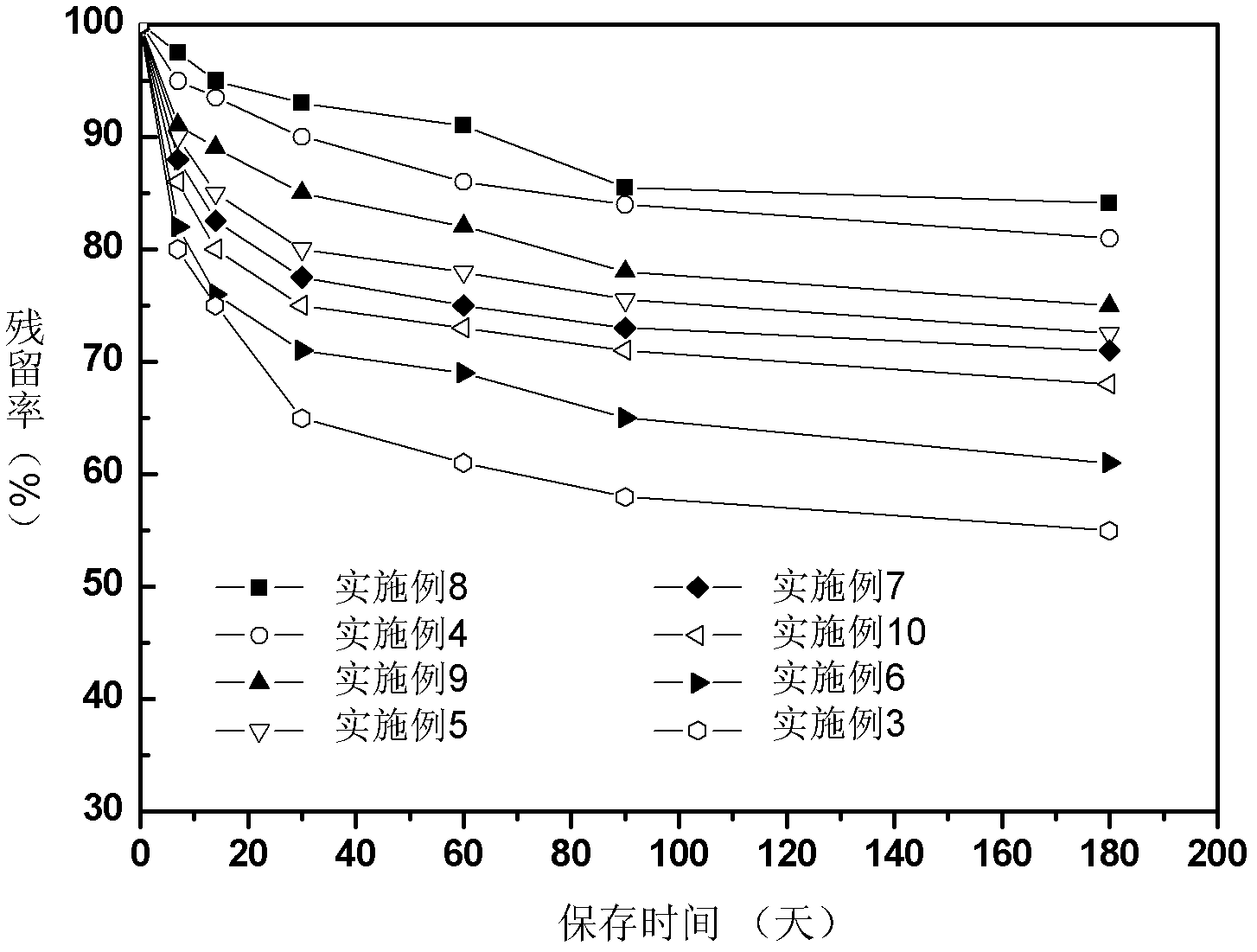
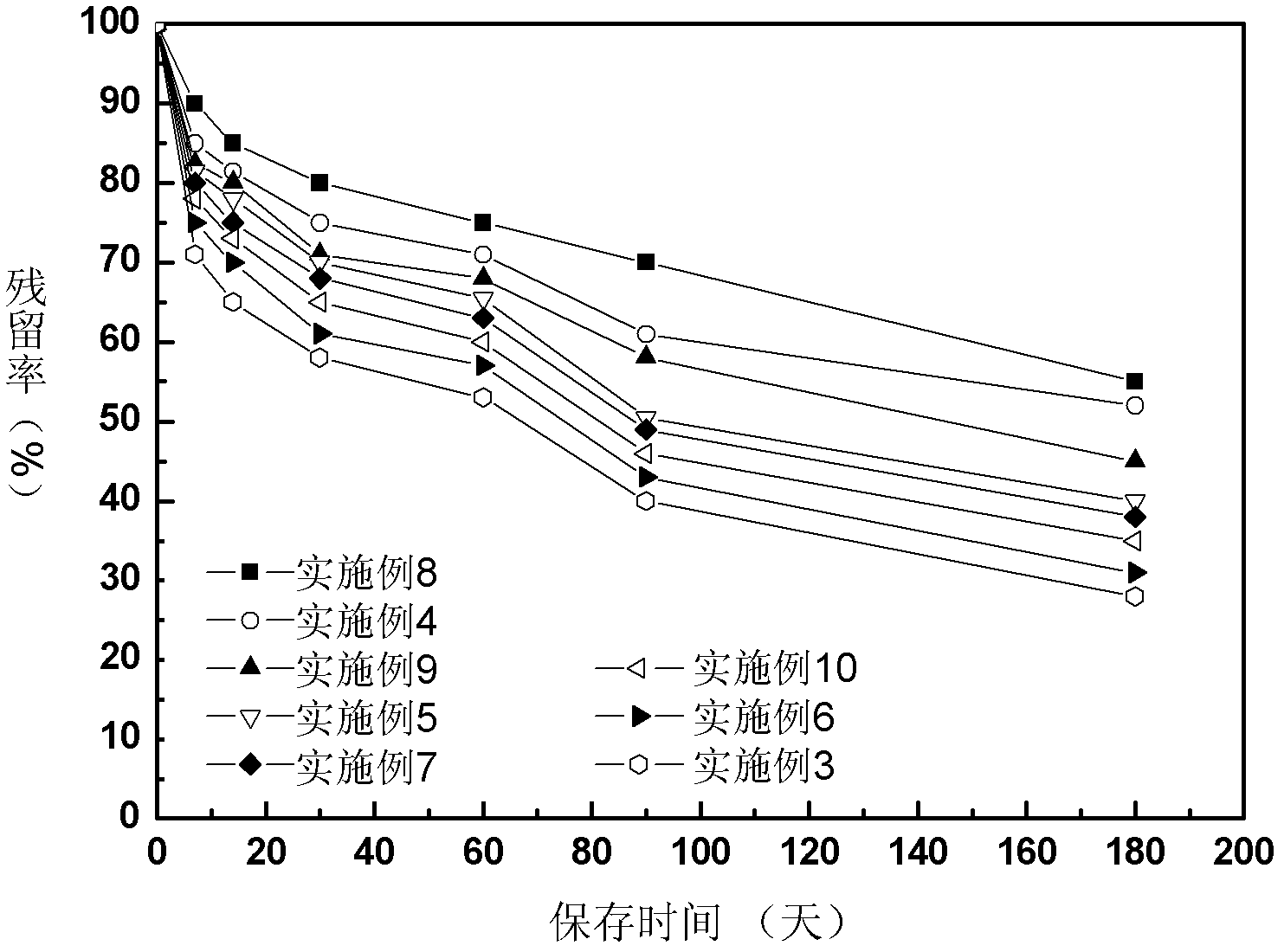
The invention relates to a Chinese radish sulfane clathrate compound and a preparation method thereof. The invention solves the problem that the Chinese radish sulfane is not stable when being stored at room temperature, and can not be easily produced or applied to practice. The goal of the invention is implemented by forming a clathrate compound from Chinese radish sulfane and propenyl-beta-cyclodextrin. The implementation process comprises the following steps: reacting beta-cyclodextrin with bromopropylene under alkaline conditions, and processing to obtain propenyl-beta-cyclodextrin; and dissolving the propenyl-beta-cyclodextrin in distilled water, dropwisely adding corresponding chemical amount of Chinese radish sulfane ethanol solution into the propenyl-beta-cyclodextrin solution, and processing to obtain freeze-dried powder. After the Chinese radish sulfane propenyl-beta-cyclodextrin clathrate compound is stored at room temperature for half a year, the residue rate is up to 80%.
Owner:肖广惠
Preparation method of pubescent angelica and mistletoe decoction formula granules and quality control method thereof
ActiveCN105477166AEmbody conceptTo achieve the purpose of reducing toxicity and increasing efficiencySenses disorderNervous disorderAdditive ingredientAngelica Sinensis Root
The invention discloses a preparation method of pubescent angelica and mistletoe decoction formula granules and a quality control method thereof. The preparation method includes the steps that radix angelicae pubescentis, asarum, cassia twigs, radices sileris, ligusticum wallichii and radix angelica sinensis are extracted for obtaining volatile oil, and the volatile oil is subjected to clathration of beta-cyclodextrin; water with the weight 5-15 times the weight of total feeding amount is added to herbal residues and other nine types of medicine, and decoction and extraction are conducted twice; filtrate is subjected to vacuum decompression concentration; a concentrated solution is subjected to spray drying, spray-drying powder is obtained, and the spray-drying powder is crushed into submicron powder through an airflow pulverizer; maltodextrin and a beta-cyclodextrin clathrate compound are added to the submicron powder to be mixed uniformly, granulation is conducted through a dry method, and the pubescent angelica and mistletoe decoction formula granules are obtained. The quality control method includes the steps of infrared fingerprint spectra, thin-layer qualitative identification and HPLC content measuring. By means of the method, the advantage of drug matching can be fully played, and effective ingredients are maintained to the maximum degree; a perfect quality standard is completed, a once-measurement multi-evaluation technology of thin-layer chromatography is adopted, quality control is conducted in combination with infrared spectroscopy and chromatography, and the quality of the formula granules can be effectively controlled.
Owner:GUANGDONG YIFANG PHARMA
Compound rhodiola rosea chewing tablet and preparing method thereof
InactiveCN103977056APromote dissolutionIncrease surface areaOrganic active ingredientsAnthropod material medical ingredientsAdjuvantRHODIOLA ROSEA ROOT
The invention provides a compound rhodiola rosea chewing tablet and a preparing method thereof. The tablet comprises following raw materials by weight: 1-2% of a rhodiola rosea clathrate compound that is the main medicine, 3.6-4.6% of adjuvants, 76-86% of a filling agent, 7.4-17.5% of a corrigent, 0.7-1% of a lubricant and a proper amount of a wetting agent. The tablet has characteristics of good dispersion state, short disintegration time, rapid medicine digestion, rapid absorption, high bioavailability and taking convenience.
Owner:凌春生
Composition for formation of cured epoxy resin, and cured products thereof
An object of the present invention is to provide a composition for the formation of a cured epoxy resin, wherein the composition can suppress a curing reaction at a low temperature to thereby enhance one-pack stability, and can also be subjected to a heating treatment to thereby effectively cure a resin. The present invention provides a composition for the formation of a cured epoxy resin, the composition comprising the following components (A), (B) and (C):(A) an epoxy resin;(B) a clathrate compound of a carboxylic acid derivative represented by formula (I):R(COOH)n (I);and an imidazole compound represented by formula (II);and (C) a tetrakisphenol type compound represented by formula (III).
Owner:NIPPON SODA CO LTD +1
Clathrate compounds and method of manufacturing
InactiveUS20080226836A1Improve efficiencyEasily integrated into current electronics technologyRadiation applicationsVacuum evaporation coatingElectricityElectron
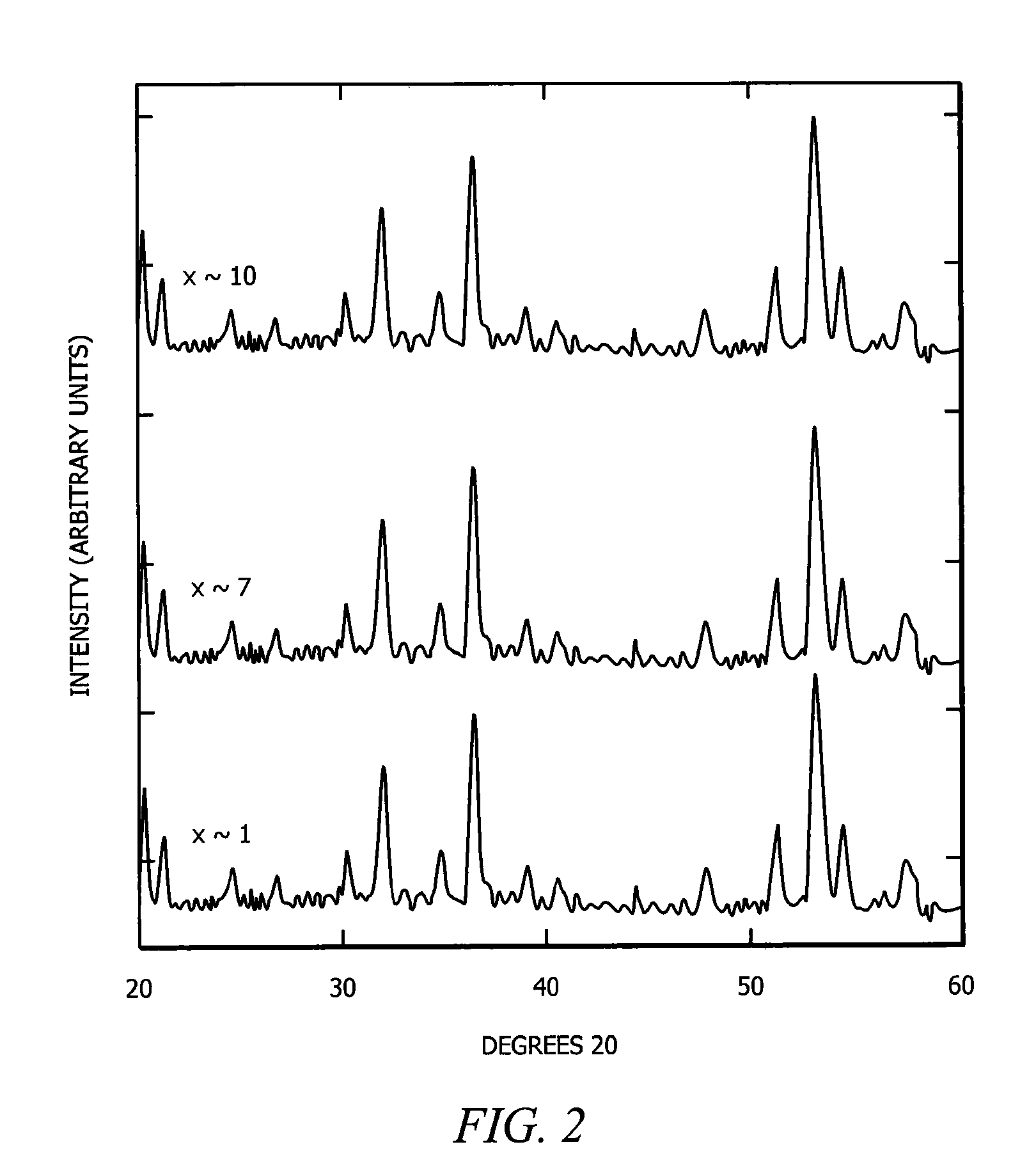
The present invention comprises new materials, material structures, and processes of fabrication of such that may be used in technologies involving the conversion of light to electricity and / or heat to electricity, and in optoelectronics technologies. The present invention provide for the fabrication of a clathrate compound comprising a type II clathrate lattice with atoms of silicon and germanium as a main framework forming lattice spacings within the framework, wherein the clathrate lattice follows the general formula Si136-yGey, where y indicates the number of Ge atoms present in the main framework and 136-y indicates the number of Si atoms present in the main framework, and wherein y>0.
Owner:UNIV OF SOUTH FLORIDA
Curcumin cyclodextrin clathrate compound and preparation method thereof
InactiveCN104873983AAvoid degradationReduce usageAntipyreticMetabolism disorderSolubilityOrganic solvent
The invention discloses a curcumin cyclodextrin clathrate compound which is composed of the following components in parts by mass: 1 part of curcumin and 1-100 parts of cyclodextrin. The invention further discloses a preparation method of the curcumin cyclodextrin clathrate compound. Curcumin directly dissolves in alkaline solution in a lucifugal environment, and the clathration process adopts the low-temperature technology, so that the degradation of curcumin is avoided, and the application of organic solvent is also avoided; the cyclodextrin has the cannular molecular structure, and the curcumin comprising the liposoluble constituent is included in cyclodextrin to form a molecular capsule, so that the water solubility and biocompatibility are enhanced; the clathration of cyclodextrin is utilized to include curcumin so as to improve the stability and bioavailability.
Owner:福建省力菲克药业有限公司
Allicin cyclodextrin clathrate compound, formulation and its preparation method
InactiveCN1565430ASolve the problem of water solubilityResolve irritationOrganic active ingredientsDigestive systemCurative effectWater soluble
The invention discloses a garlicin cyclodextrin clathrate compound, preparation and the process for preparing same, wherein the allicin, cyclodextrin or their derivatives (such as beta-cyclodextrin, hydroxypropyl-beta-cyclodextrin) can be used for preparing garlicin cyclodextrin clathrate compound, which can be used for preparing various preparations of different oral preparation, suppositorium, spray, and injection.
Owner:毛友昌
Nano particles of taxane cyclodextrin inclusion compound and preparation method thereof
ActiveCN1879612AOrganic active ingredientsMacromolecular non-active ingredientsMedicinePharmaceutical formulation
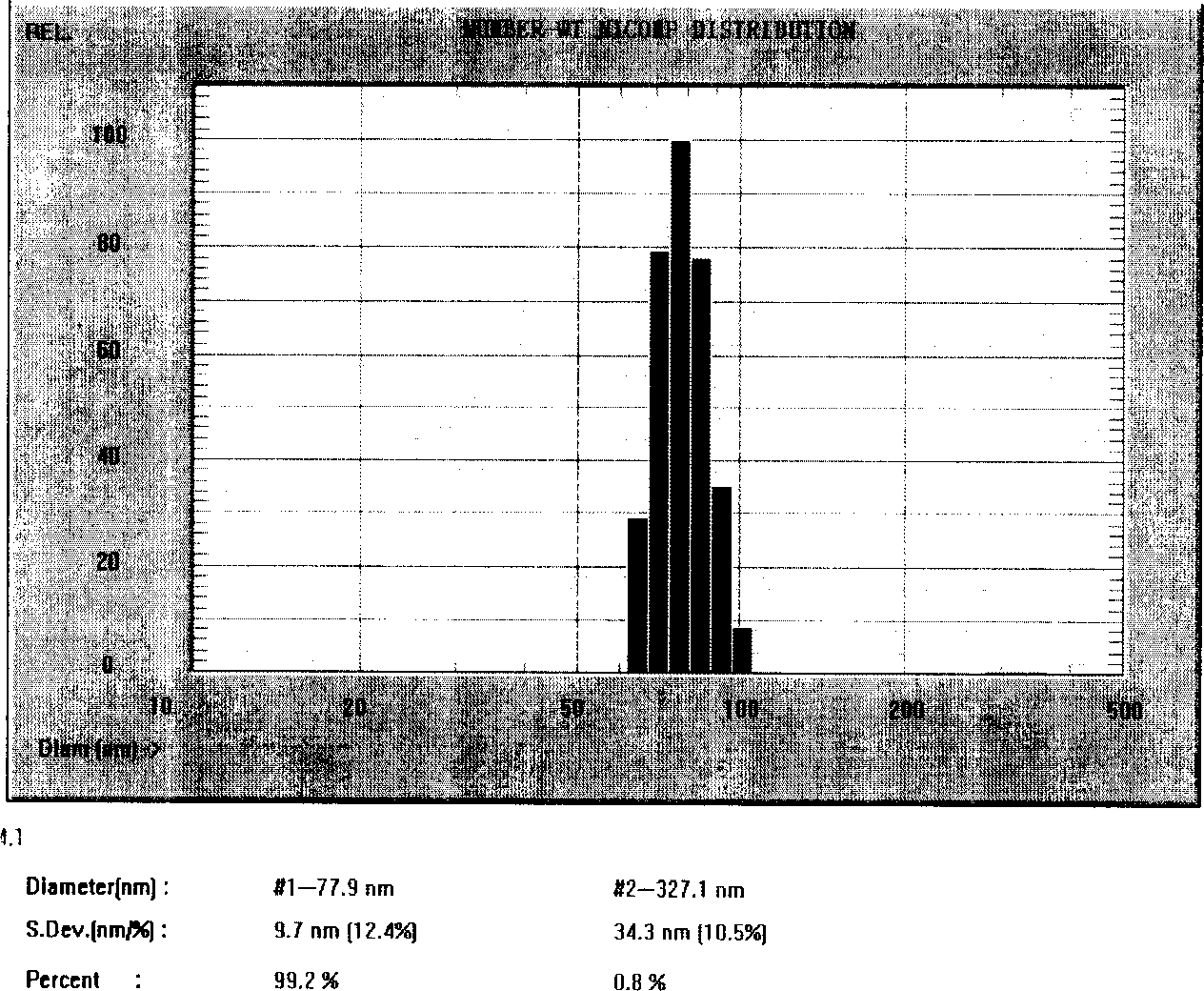
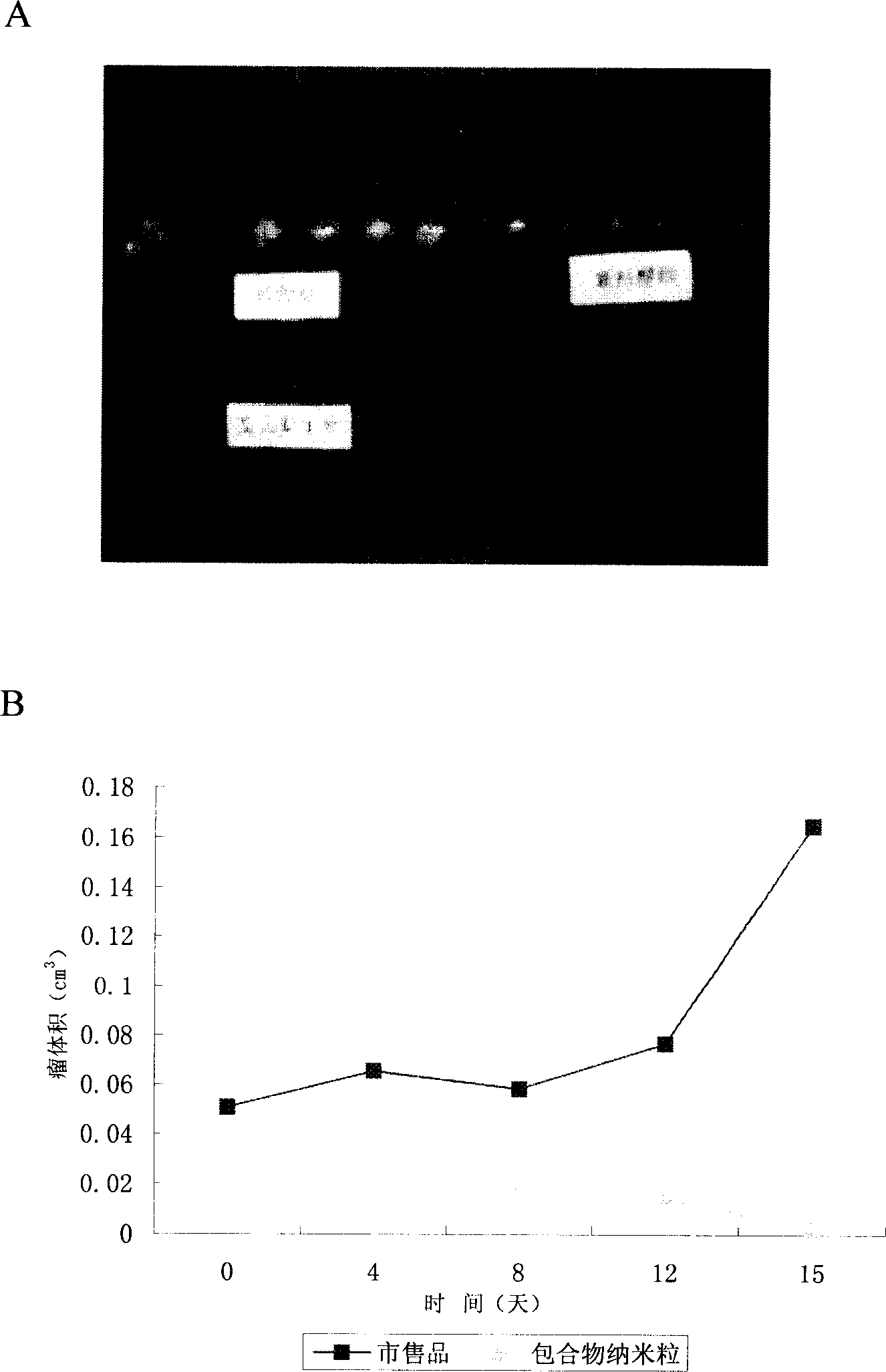
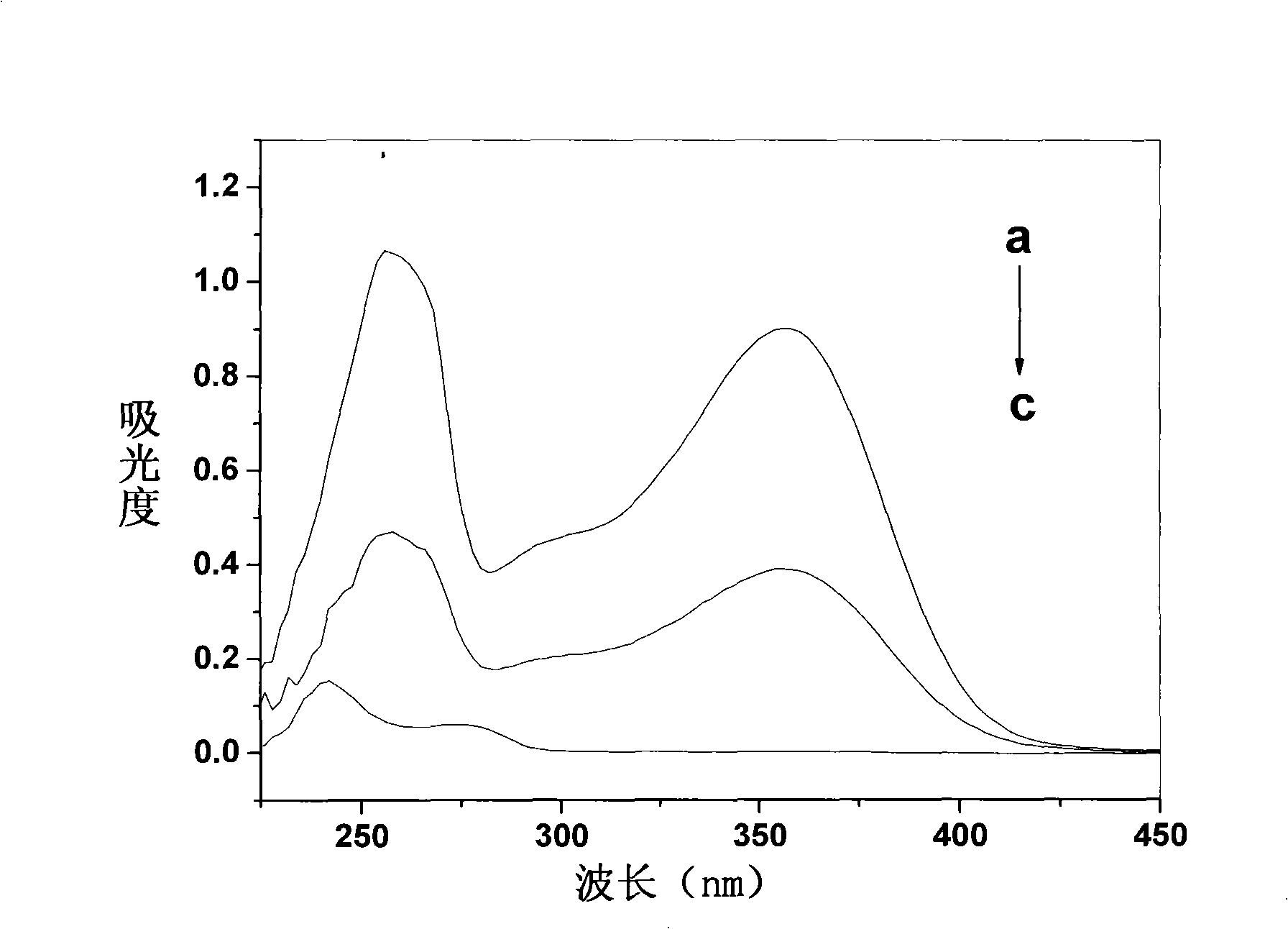
The invention relates to a Paclitaxel cyclodextrin clathrate compound nanometer grain and relative medicine agent, wherein it uses hydroxypropyl-beta-cyclodextrin as carrier and PVP as stabilizer to prepare Paclitaxel clathrate compound nanometer grain, whose diameter is 40-200nm, to improve the dissolvability of Paclitaxel as 100-50times. The animal experiment has proven that: compared with present Paclitaxel, its resistant agent is improved 2.67tims with better cancer resistant effect. The invention uses general material and simple condition, with stable quality and the application to be processed into oral agent.
Owner:SHANGHAI ALLIST PHARM CO LTD
Isoquercitrin clathrate and preparation thereof
InactiveCN101301477ASimple manufacturing processHigh inclusion rateOrganic active ingredientsPharmaceutical non-active ingredientsFood additiveSolubility
The present invention provides a clathrate compound formed by isoquercitrin and [beta]-cyclodextrin or derivatives thereof. The weight ratio of the isoquecitrin and the [beta]-cyclodextrin or the derivatives thereof is 1:2 to 20. The preparation method comprises the following steps of: dissolving the [beta]- cyclodextrin or the derivatives thereof into distilled water; putting the isoquercitrin into an organic dissolvant to dissolve; adding the isoquercitrin slowly into the water solution of the [beta]- cyclodextrin or the derivatives thereof, controlling the temperature and stirring; keeping stand, pumping-filtrating or directly condensing, vacuum drying, and obtaining the isoquercitrin clathrate compound. The solubility of the obtained isoquercitrin clathrate compound is significantly improved, and the isoquercitrin clathrate compound can be further developed into multiple solid dosage forms or liquid dosage forms which are suitable for medicines or food additives.
Owner:SHANXI UNIV +1
Developing solution for photolithography, method for forming resist pattern, and method and apparatus for producing developing solution for photolithography
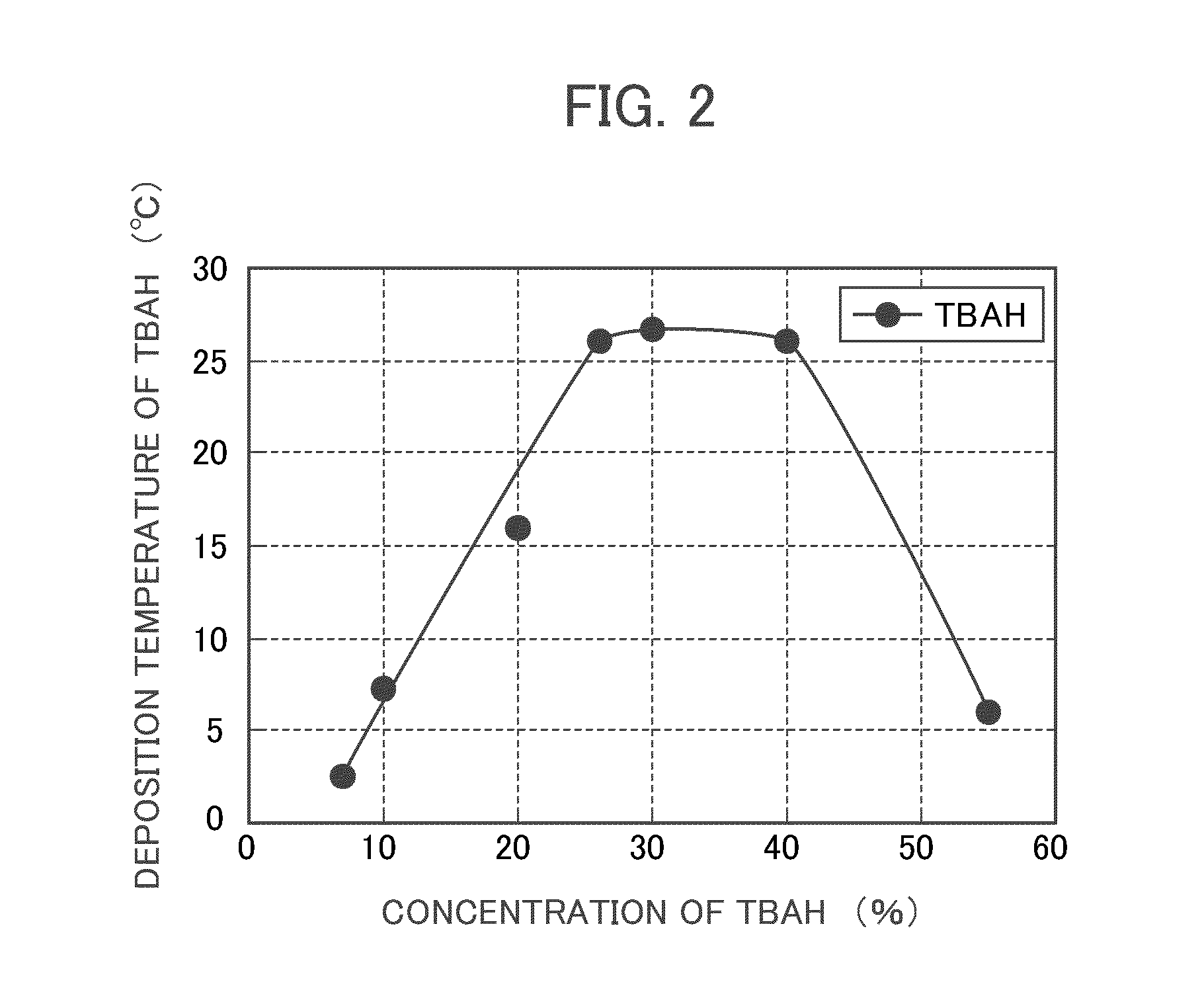
InactiveUS20110159447A1Deposition is suppressedDeposition of TBAH in the developing solution can be suppressedRotary stirring mixersTransportation and packagingResistOrganic solvent
Firstly, to provide a developing solution for photolithography in which tetrabutylammonium hydroxide (TBAH) is used as an alkaline agent of the developing solution and deposition of TBAH is suppressed. Secondary, to provide a method for producing a developing solution for photolithography capable of suppressing TBAH deposition when producing the developing solution by diluting a concentrated developing solution containing TBAH and a production apparatus used for the production method. The present invention is firstly a developing solution for photolithography comprising tetrabutylammonium hydroxide (A), and at least one selected from the group consisting of a water-soluble organic solvent (B1), a surfactant (B2), and a clathrate compound (B3). The present invention is secondary characterized by maintaining the temperature of liquid at 27° C. or higher during dilution.
Owner:TOKYO OHKA KOGYO CO LTD
Preparation method of ceftiofur acid long-acting injection
InactiveCN103230364ALong injection half-lifeImprove solubilityAntibacterial agentsOrganic active ingredientsHalf-lifeDissolution
The invention belongs to the technical field of preparation of medicines and in particular relates to a preparation method of ceftiofur acid long-acting injection. The preparation method comprises the followings steps of: adding ceftiofur acid and 2-hydroxypropyl-beta-cyclodextrin in a molar ratio of 1:(1-2) to a ball mill for uniformly mixing; sufficiently grinding under the room temperature, and sufficiently uniformly and sieving to obtain a ceftiofur acid clathrate compound; dissolving sodium alginate in sterile water and adding poloxamer 407 and poloxamer 188, storing for 12-24 hours under the temperature condition of 4 DEG C, so that the poloxamer 407 and the poloxamer 188 are completely dissolved and sterilized under the temperature of 121 DEG C, carrying out ice-bath cooling to obtain a transparent solution; magnetically stirring under the temperature condition of 4 DEG C and the rotation speed condition of 150r / min; adding the ceftiofur acid clathrate compound in a weight ratio of the sodium alginate to the ceftiofur acid clathrate compound of (0.1-0.3):(5-10), so that the ceftiofur acid clathrate compound is sufficiently dispersed uniformly to obtain the ceftiofur acid long-acting injection. According to the preparation method of the ceftiofur acid long-acting injection, the preparation process is simple, the product intramuscular injection half-life period is long, the dissolution degree of the ceftiofur acid is high, the production and treatment cost is low, the cure rate is high and the environment is friendly.
Owner:QINGDAO AGRI UNIV
Clathrate compounds and methods of manufacturing
The present invention comprises new materials, material structures, and processes of fabrication of such that may be used in technologies involving the conversion of light to electricity and / or heat to electricity, and in optoelectronics technologies. The present invention provide for the fabrication of a clathrate compound comprising a type II clathrate lattice with atoms of silicon and germanium as a main framework forming lattice spacings within the framework, wherein the clathrate lattice follows the general formula Si136-yGey, where y indicates the number of Ge atoms present in the main framework and 136-y indicates the number of Si atoms present in the main framework, and wherein y>0.
Owner:UNIV OF SOUTH FLORIDA
Preparing method and usage of vitamin E clathrate
InactiveCN1568969APromote absorptionImprove solubilityOrganic active ingredientsMetabolism disorderSolubilityCyclodextrin derivative
The invention provides the cyclodextrin of vitamin E or cyclodextrin derivative clathrate compound and their preparing process, and their use in preparing pharmaceutical preparations and cosmetics. By utilizing the bundling action of the cyclodextrin or cyclodextrin derivative to vitamin E, the stability and water-solubility of the vitamin E can be increased.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Clathrate compound, curing catalyst, composition for forming cured resin, and cured resin
The present invention provides a curing catalyst (clathrate compound) for which the curing reaction is suppressed at low temperatures, allowing an improvement in the one-pot stability, but which can effectively cure a resin upon heat treatment. The clathrate compound comprises at least an isophthalic acid compound represented by a formula (1) [wherein R1 represents a C1 to C6 alkyl group or the like] and an imidazole compound represented by a formula (II) [wherein R2 represents a hydrogen atom or a C1 to C10 alkyl group or the like, and R3 to R5 each independently represents a hydrogen atom or a nitro group or the like].
Owner:NIPPON SODA CO LTD
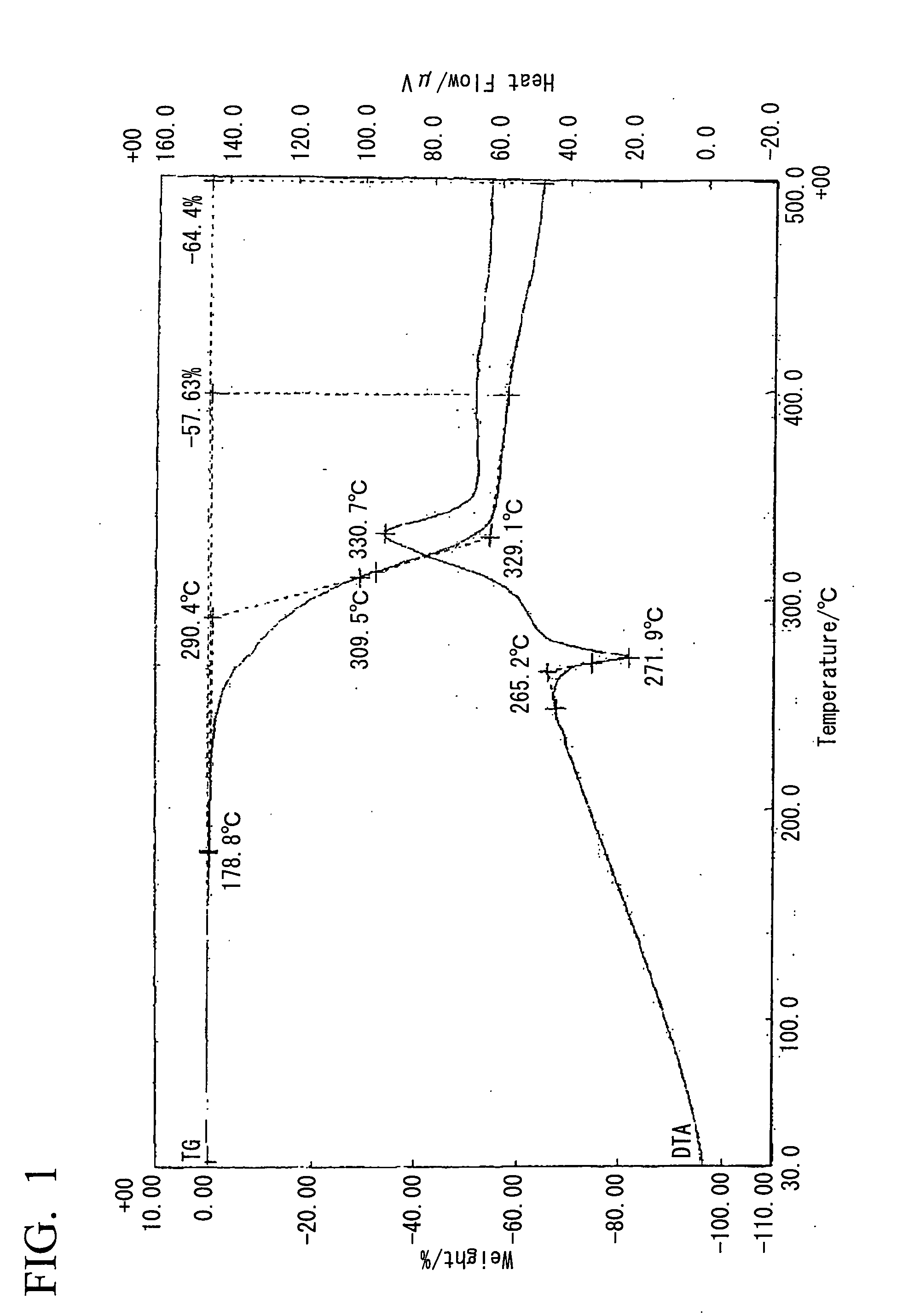
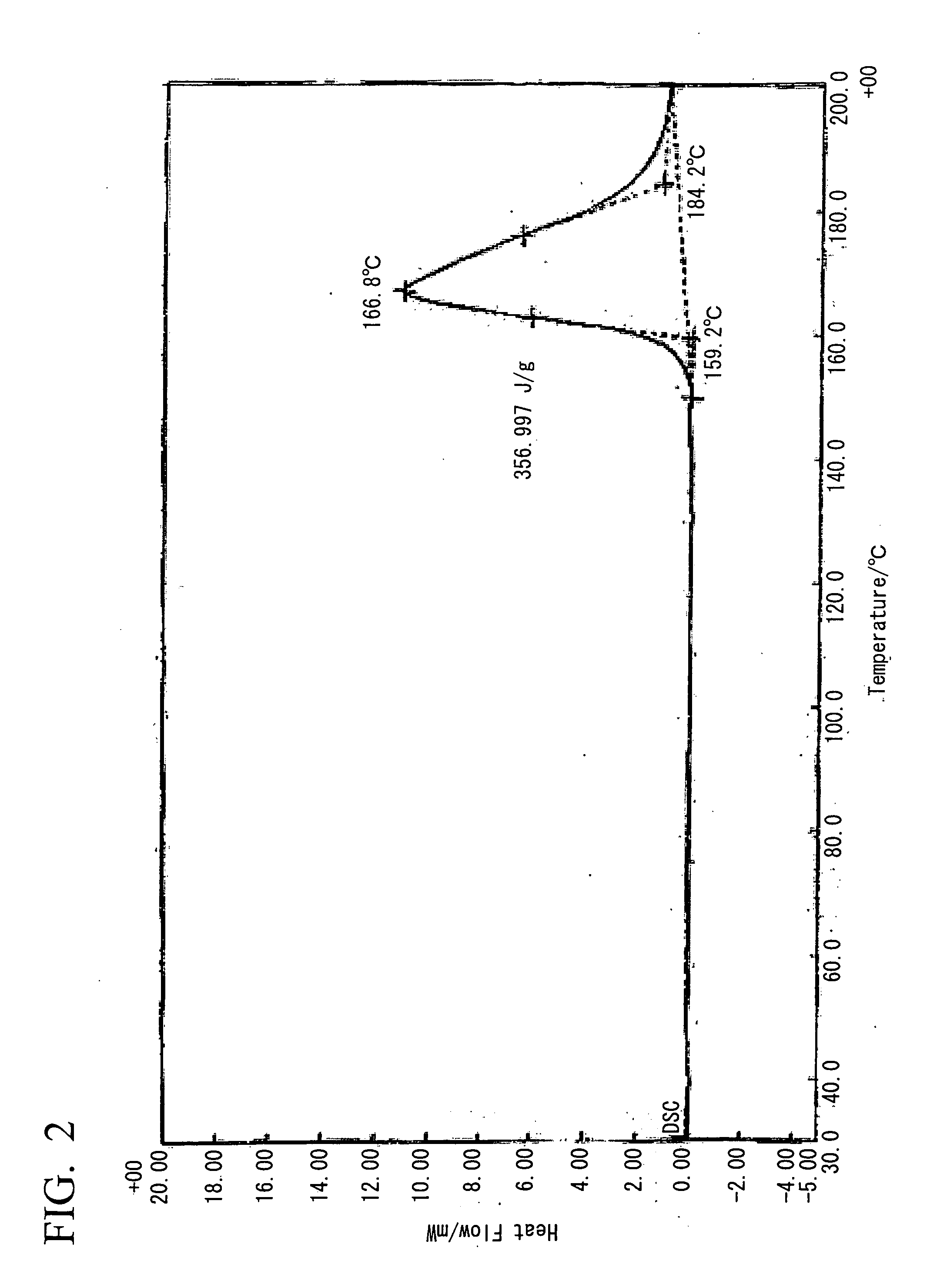
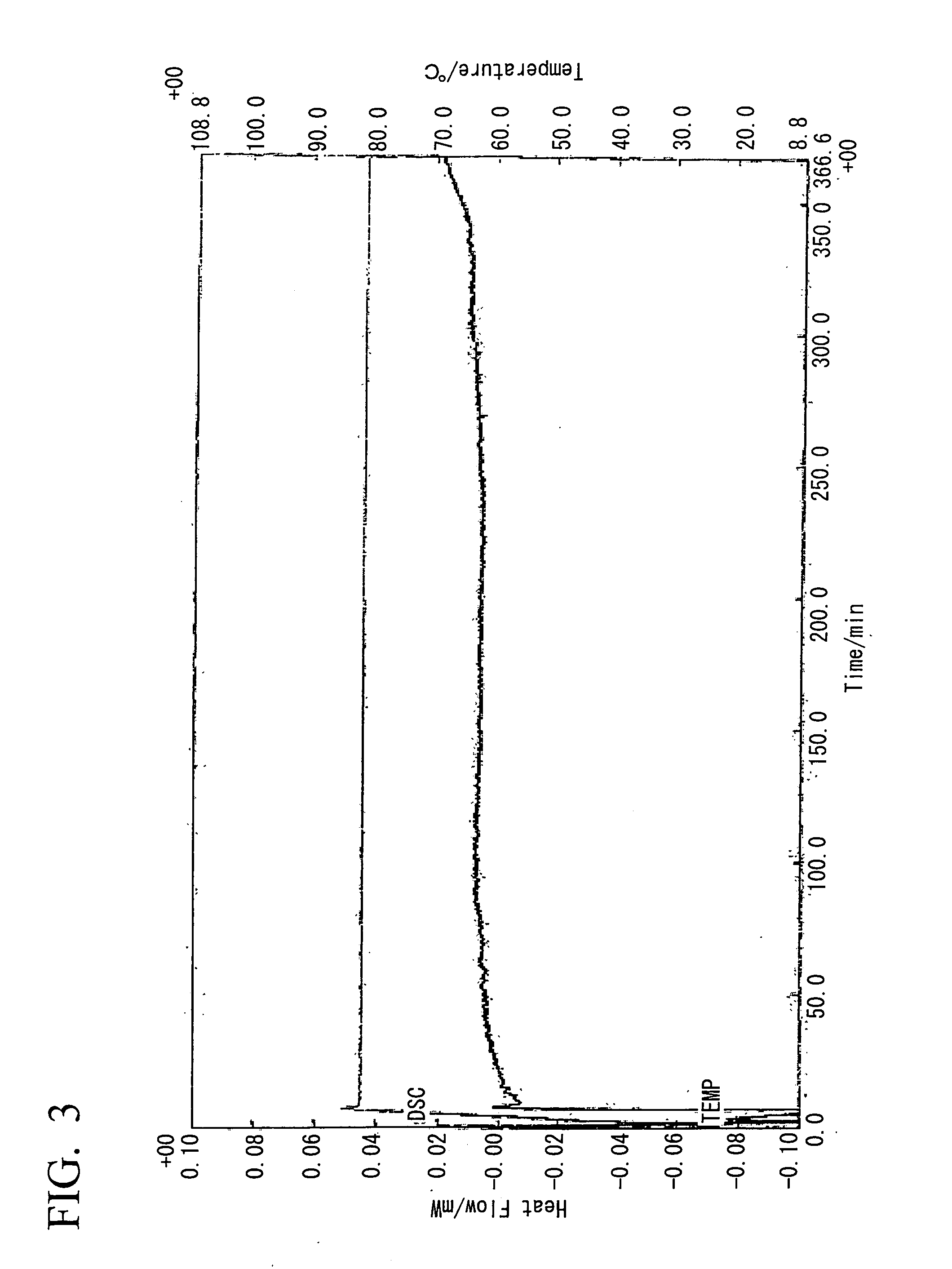
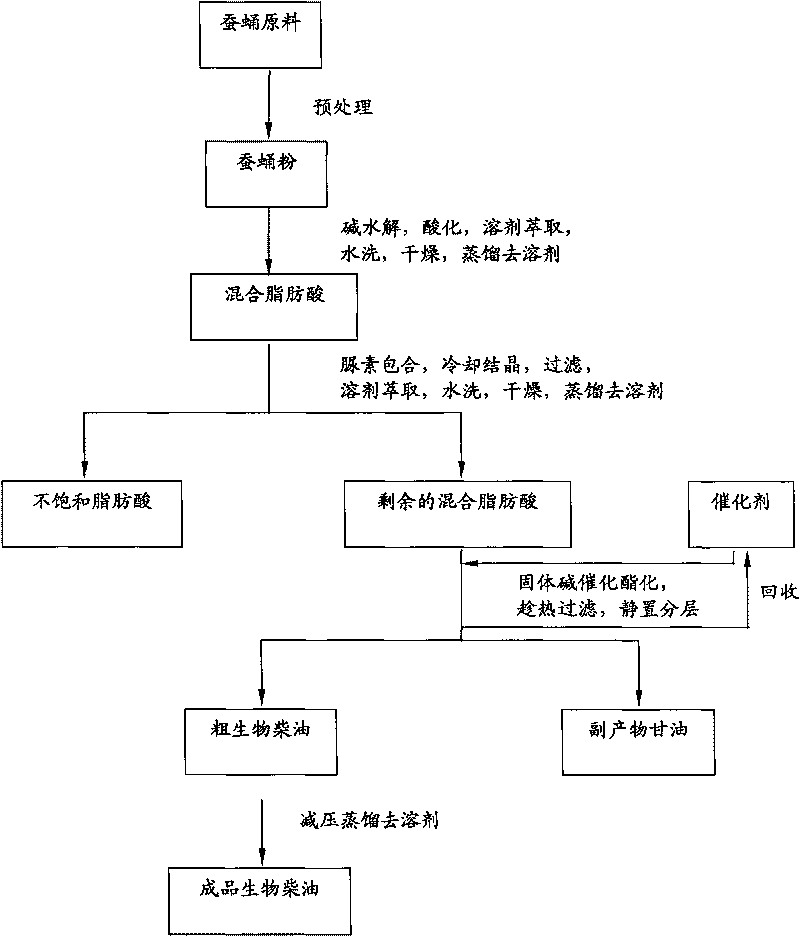
Method for preparing biodiesel by taking pupal oil as production raw material
InactiveCN101691519ABroaden source channelsAvoid pollutionFatty acid esterificationBiofuelsWater bathsBiodiesel
The invention provides a method for preparing biodiesel by taking pupal oil as a production raw material, comprising the following steps: extracting crude pupal oil from pupal powder by petroleum ether or normal hexane through a heat refluxing method; mixing the crude pupal oil with the solution of NaOH and ethanol; stirring in water bath; adding petroleum ether or normal hexane; adding hydrochloric acid into a saponified fluid for acidification to obtain non-esterified fatty acid; extracting fatty acid by petroleum ether or normal hexane; standing and layering; mergering petroleum ether layers or normal hexane layers; washing by water to be neutral; drying; filtering to obtain the mixed fatty acid; stirring the mixed fatty acid with low alcohol saturated solution of urea to obtain clathrate compound; cooling, crystallizing and filtering the obtained clathrate compound; filtering; extracting the obtained filtrate by petroleum ether or normal hexane; washing by water and drying the extract; carrying out reduced pressure distillation on solvent to obtain unsaturated fatty acid; placing the rest pupal fatty acid into a reactor, adding solid base catalyst and stirring, adding methanol, filtering the reaction liquid after reaction, standing and laying the reaction liquid; and carrying out reduced pressure distillation on crude biodiesel to obtain the biodiesel product.
Owner:JIANGSU UNIV OF SCI & TECH
Slow-release essence microcapsule and preparation method thereof
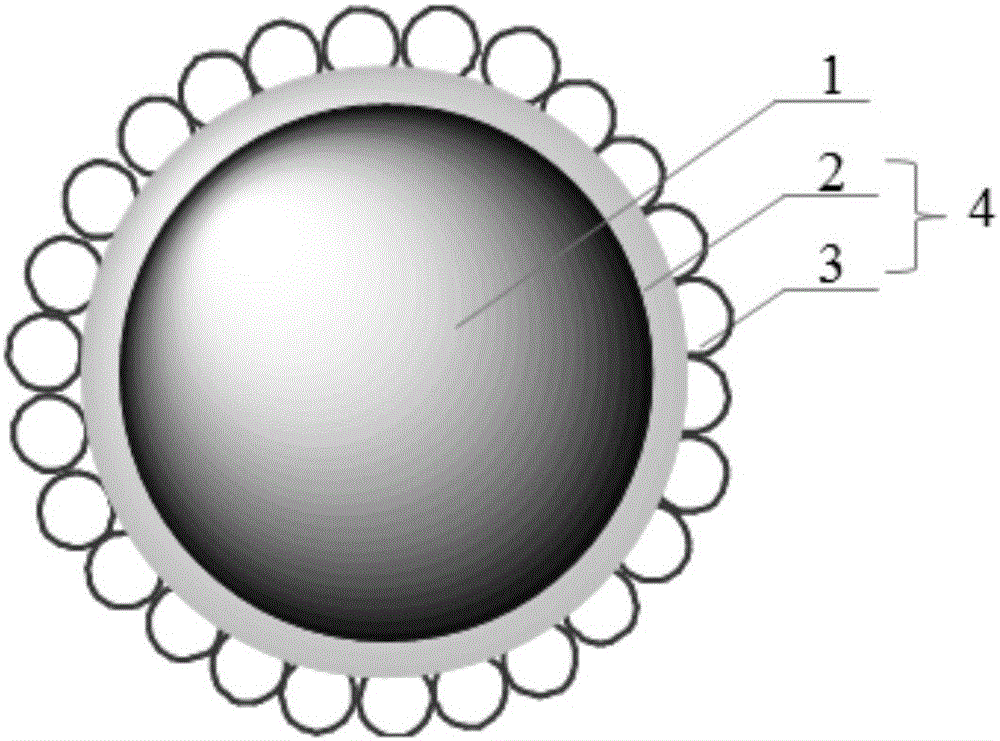
ActiveCN105838501ASlow down fast volatile lossAvoid heat deteriorationEssential-oils/perfumesMicroballoon preparationWaxHeat resistance
The invention relates to a slow-release essence microcapsule and a preparation method thereof. The slow-release essence microcapsule comprises a core material (1), polyacrylate (2) and an emulsifier (3). The emulsifier (3) is carried by the outer wall of the polyacrylate (2). The polyacrylate (2) and the emulsifier (3) form a continuous capsule composite wall and the composite wall wraps the core material (1) so that the coated essence microcapsule is obtained. The core material (1) comprises essence, an organic carrier and initiator residues. The emulsifier (3) is a clathrate compound of cyclodextrin and wax. The polyacrylate (2) is prepared from an acrylate monomer and an initiator through polymerization. A mass ratio of cyclodextrin to wax is 1: (0.1-0.2). A mass ratio of the organic carrier, cyclodextrin, essence, acrylate monomer to initiator is 1: (0.2-0.6): (0.01-1): (0.5-2): (0.004-0.02). The slow-release essence microcapsule effectively improves fragrance endurance and feel comfortableness and has the characteristics of environmental friendliness, no toxicity, good mechanical strength and good heat resistance.
Owner:NANJING UNIV OF TECH
Clathrate compounds and method of manufacturing
The present invention comprises new materials, material structures, and processes of fabrication of such that may be used in technologies involving the conversion of light to electricity and / or heat to electricity, and in optoelectronics technologies. The present invention provide for the fabrication of a clathrate compound comprising a type II clathrate lattice with atoms of silicon and germanium as a main framework forming lattice spacings within the framework, wherein the clathrate lattice follows the general formula Si136-yGey, where y indicates the number of Ge atoms present in the main framework and 136-y indicates the number of Si atoms present in the main framework, and wherein y>0.
Owner:UNIV OF SOUTH FLORIDA
Traditional Chinese medicine lavipeditum preparation and preparation method thereof
ActiveCN104984315AImprove sleepingPromote moisturizing and whiteningNervous disorderHydroxy compound active ingredientsStephaniaRhizome
The invention relates to the technical field of health maintenance and health care products, in particular to a traditional Chinese medicine lavipeditum preparation and a preparation method thereof. The traditional Chinese medicine lavipeditum preparation comprises, by weight, 3-10 parts of tea powder, 5-20 parts of folium artemisiae argyi, 1-5 parts of white atractylodes rhizome, 8-20 parts of stephania terandra, 5-10 parts of rhizoma zingiberis, 5-15 parts of eucommia ulmoides, 1-10 parts of bidentate achyranthes roots, 1-5 parts of rhizoma acori graminei, 5-10 parts of rhizoma alismatis, 1-10 parts of angelica sinensis, 1-5 parts of rhizoma cyperi, 1-10 parts of ligusticum wallichii, 1-8 parts of astragalus membranaceus, 1-2 parts of safflower carthamus, 1-5 parts of morinda officinalis, 2-5 parts of dogwood, 1-5 parts of cinnamon, 2-6 parts of kudzu vine roots, 1-2 parts of schisandra chinensis, 2-5 parts of sophora flavescens, 3-8 parts of aloe, 0-2 parts of essential oil, 0-5 parts of menthol, 0-5 parts of borneol and 20-75 parts of auxiliary materials. The preparation method of the traditional Chinese medicine lavipeditum preparation comprises the steps of picking materials, conducting extraction and preparing extract powder, mixing a volatile oil clathrate compound and an essential oil clathrate compound and the like. By means of the traditional Chinese medicine lavipeditum preparation and the preparation method thereof, the problems that lavipeditum products are rough, the action effect is not obvious, the experience sense is not great, and a majority of the products are chemical preparations are solved.
Owner:IDEALITY TECH GRP
Clathrate compound, curing catalyst, composition for forming cured resin, and cured resin
InactiveCN101563326AImprove stabilityEfficient curingOrganic chemistryHydrogen atomMedicinal chemistry
The present invention provides a curing catalyst (inclusion compound) for which the curing reaction is suppressed at low temperatures, allowing an improvement in the one-pot stability, but which can effectively cure a resin upon heat treatment. The inclusion compound comprises at least an isophthalic acid compound represented by a formula (I) [wherein R1 represents a C1 to C6 alkyl group or the like] and an imidazole compound represented by a formula (II) [wherein R2 represents a hydrogen atom or a C1 to C10 alkyl group or the like, and R3 to R5 each independently represents a hydrogen atom or a nitro group or the like].
Owner:NIPPON SODA CO LTD
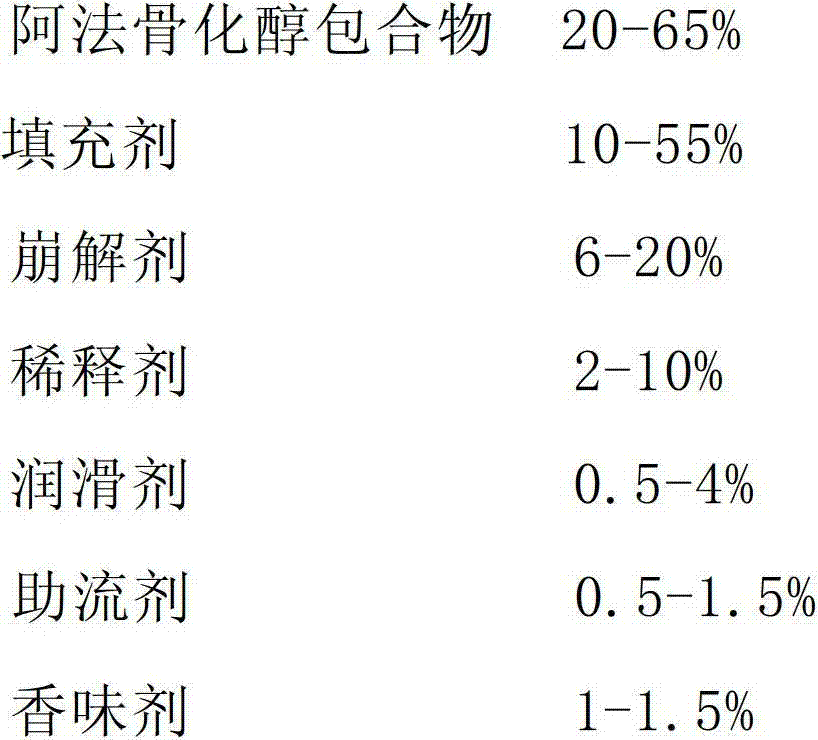
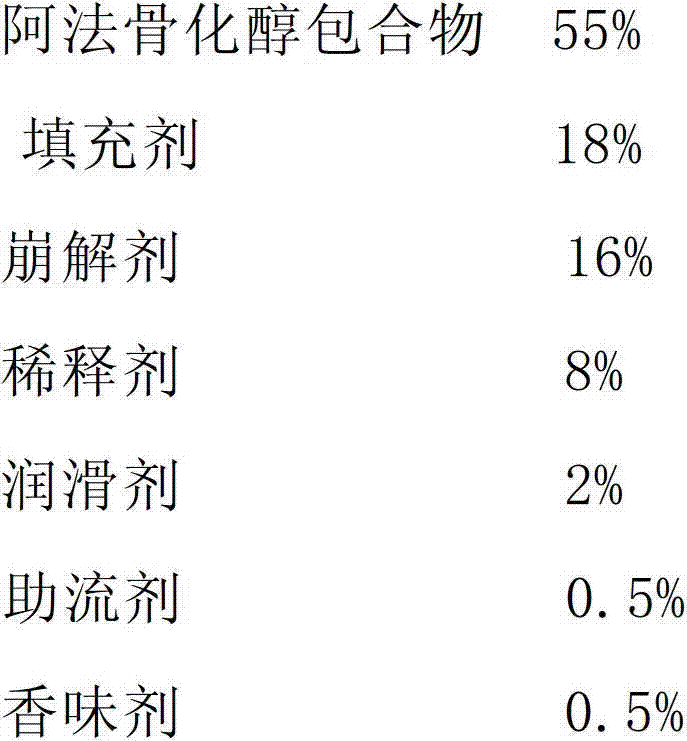
Alfacalcidol dispersible tablet and preparation method thereof
ActiveCN103110598APromote dissolutionFast absorptionOrganic active ingredientsMetabolism disorderFlavouring agentMedicine
The invention discloses an alfacalcidol dispersible tablet and a preparation method of the alfacalcidol dispersible tablet. The alfacalcidol dispersible tablet is prepared from an alfacalcidol clathrate compound and a preparation supplementary material, wherein the preparation supplementary material comprises a filling agent, a disintegrating agent, a diluting agent, a lubricating agent, a flow aid and a flavouring agent; and the alfacalcidol clathrate compound is prepared by carrying out clathration on alfacalcidol and 2,6-dimethyl-Beta-cyclodextrin based on the molar ratio of 4: 1 to 0.5: 3. The alfacalcidol dispersible tablet has the advantages of being quick to dissolve out, fast to absorb, high in bioavailability, excellent in stability, convenient to take, etc.
Owner:CP PHARMA QINGDAO CO LTD
Method for solubilizing camptothecin compound
InactiveCN102000080AImprove solubilityPrevent precipitationOrganic active ingredientsPharmaceutical non-active ingredientsSolubilityOrganic solvent
The invention discloses a method for solubilizing a camptothecin compound, which comprises the following steps of: dissolving the camptothecin compound into alkaline aqueous solution to prepare solution of camptothecin; dissolving a water-soluble cyclodextrin derivative into acid aqueous solution to prepare solution of cyclodextrin derivative; and mixing the solution of camptothecin with the solution of cyclodextrin derivative, stirring till the solution is clarified and transparent and thus, obtaining cyclodextrin clathrate compound solution of the camptothecin compound. The method is simple and practicable without using an organic solvent or special preparation equipment, and the solubility of the compound can be successfully improved by over 100 times by using the water-soluble cyclodextrin derivative through simple acid-base neutralization.
Owner:JIANGSU SIMCERE PHARMACEUTICAL R & D CO LTD
Method for preparing azelaic acid hydroxypropyl betacyclodextrin clathrate compound
InactiveCN108187070AGood compatibilitySimple production processAntibacterial agentsOrganic active ingredientsSolubilityWater soluble
The invention discloses a method for preparing azelaic acid hydroxypropyl betacyclodextrin clathrate compound. The method comprises the steps that firstly hydroxypropyl betacyclodextrin is added intoa reaction kettle, then purified water is added, the mixture is stirred to make hydroxypropyl betacyclodextrin being dissolved, lastly azelaic acid is added, heating is started, and stirring and heating are simultaneously performed; the temperature is risen to 80 DEG C and is kept for 0.5 hour, then the mixture is cooled to 50 DEG C, spray drying is carried out, the air inlet temperature is controlled to 165 DEG C-205 DEG C, the air outlet temperature is 60 DEG C-100 DEG C, and drying is carried out for 2 hours, and the azelaic acid hydroxypropyl betacyclodextrin clathrate compound is obtained. The prepared azelaic acid hydroxypropyl betacyclodextrin clathrate compound avoids the defects of the azelaic acid during the use of cosmetics, the functions of the azelaic acid are maintained, thewater solubility is improved, the irritation is reduced, the prepared azelaic acid hydroxypropyl betacyclodextrin clathrate compound is more conductive to using in skin drugs, cosmetics and nursing products, and greatly expands the application of the azelaic acid.
Owner:SHANDONG BINZHOU ZHIYUAN BIO TECH CO LTD
Clathrate compound containing ginsenoside Rg2 and method of preparing the same
The present invention discloses an inclusion complex containing the panaxsaponin Rg2 and the preparation method. The inclusion complex of the invention consists of the panaxsaponin Rg2 and the hydroxypropyl-Beta-cyclodextrin. The preparation method of the present invention is as following. The hydroxypropyl-Beta-cyclodextrin water solution is prepared; the hydroxypropyl-Beta-cyclodextrin solution is kept in the water bath; the extraction powder of the panaxsaponin Rg2 or the extraction of the liquid-typed Rg2 dissoluted by the solvent is added before mixing and complex; therefore the liquid inclusion complex is obtained and the inclusion complex after drying. The inclusion complex of the present invention has functions of increasing the heart perfusion and the blood flow, expanding the diameter of the vein and the artery, improving the microcirculation disturbance, improving the myocardial ischemia and anti-thrombosis effect. The present invention can significantly reduce the acute toxicity of the panaxsaponin Rg2.
Owner:GUANGZHOU TIANAN MEDICAL TECH
Clathrate compound of beta-cyclodextrin or derivatives of beta-cyclodextrin for Nifuroxazide and preparation method for preparation of clathrate compound
ActiveCN102671210AImprove bioavailabilityImprove and increase bioavailabilityAntibacterial agentsOrganic active ingredientsPerylene derivativesAqueous solubility
The invention relates to a clathrate compound of beta-cyclodextrin or derivatives of the beta-cyclodextrin for Nifuroxazide and a preparation method for a preparation of the clathrate compound. The clathrate compound consists of the following raw materials in weight ratio: 1 percent of Nifuroxazide, 0.125-0.25 percent of povidone K30 and 5-25 percent of beta-cyclodextrin or derivatives of the beta-cyclodextrin. The invention aims to solve the problems that traditional market products are low in water solubility and dissolution rate generally, sediments are often generated and colors are darkened during the application process and the like. According to the invention, clathration is performed on the Nifuroxazide by adopting the beta-cyclodextrin or the derivatives of the beta-cyclodextrin,so that the Nifuroxazide can be easily dissolved in water, and meanwhile, the stability of the Nifuroxazide is improved; the form of the preparation is expanded; the route of administration is enlarged; and an application effect is promoted.
Owner:SHANGHAI E K M BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com