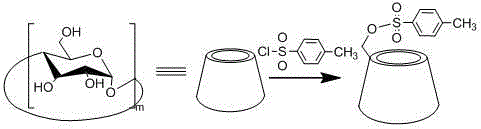Clathrate compound of artemisinin series and alkaline cyclodextrin and method for preparing same
A cyclodextrin inclusion complex and cyclodextrin technology are applied in the directions of pharmaceutical combinations, non-active components of polymer compounds, medical preparations containing active components, etc. The effect of mild reaction conditions and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
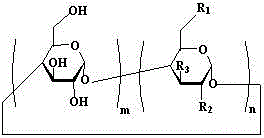
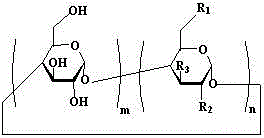
[0045] Example 1: The clathrates of artemisinin series and basic cyclodextrin, including artemisinin and mono-[6-(ethylenediamine)-6-deoxy]-β-cyclodextrin, artemisinin and mono The weight ratio of -[6-(ethylenediamino)-6-deoxy]-β-cyclodextrin is 1:3.
[0046] During preparation, proceed as follows:
[0047] 1. Preparation of sulfonylated cyclodextrin
[0048] Take 210g of recrystallized β-cyclodextrin and dissolve it in 1300mL of distilled water. After fully stirring, the solution turns into a white emulsion. Add sodium hydroxide solution (17.2g, 50mL) and stir for 1.5h. Weigh 26.0g of p-toluenesulfonyl chloride, dissolve it in 80mL of acetonitrile solution, slowly add the solution dropwise to β-cyclodextrin lye, stir at room temperature for 2 hours, remove a small amount of insoluble matter by suction filtration, and adjust the pH value of the filtrate with 2M hydrochloric acid To 7.5, at this time a large amount of precipitation occurs, and the filtrate is removed by suc...
Embodiment 2
[0054] Example 2: The inclusion complexes of artemisinin series and alkaline cyclodextrin, including artemisinin and mono-[6-(diethylenetriamine)-6-deoxy]-β-cyclodextrin, artemisinin and The weight ratio of mono-[6-(diethylenetriamine)-6-deoxy]-β-cyclodextrin is 1:98.
[0055] Preparation: Dissolve 1g of artemisinin in 30mL of methanol to form a solution; dissolve 98g of mono-[6-(diethylenetriamine)-6-deoxy]-β-cyclodextrin in 500mL of water to prepare a solution Mix the above two solutions under stirring conditions, stir at 40°C for 9h, filter the resulting solution, concentrate under reduced pressure at 40°C, and dry to obtain artemisinin clathrates, which can be dissolved in water at 25°C The solubility is 38mg / mL (calculated based on the amount of artemisinin).
[0056]
Embodiment 3
[0057] Example 3: In the clathrate compound of artemisinin series and alkaline cyclodextrin, the weight ratio of artemisinin to mono-[6-(amino)-6-deoxy]-β-cyclodextrin is 1:50.
[0058] 1g artemisinin was dissolved in 20mL dimethyl sulfoxide to form a solution; 50g mono-[6-(amino)-6-deoxy]-β-cyclodextrin was dissolved in 200mL water to prepare a solution; Mix the above two solutions under stirring conditions, stir at 20°C for 18 hours, filter the obtained solution, concentrate under reduced pressure at 40°C, and dry to obtain artemisinin clathrate. The solubility of clathrate in water at 25°C is 22mg / mL (calculated based on the amount of artemisinin).
[0059]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com