Clathrate compound, curing catalyst, composition for forming cured resin, and cured resin
A technology of epoxy curing resin and compound, applied in the direction of organic chemistry, can solve the problems of rapid curing and poor stability of single liquid, achieve effective curing and improve the stability of single liquid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
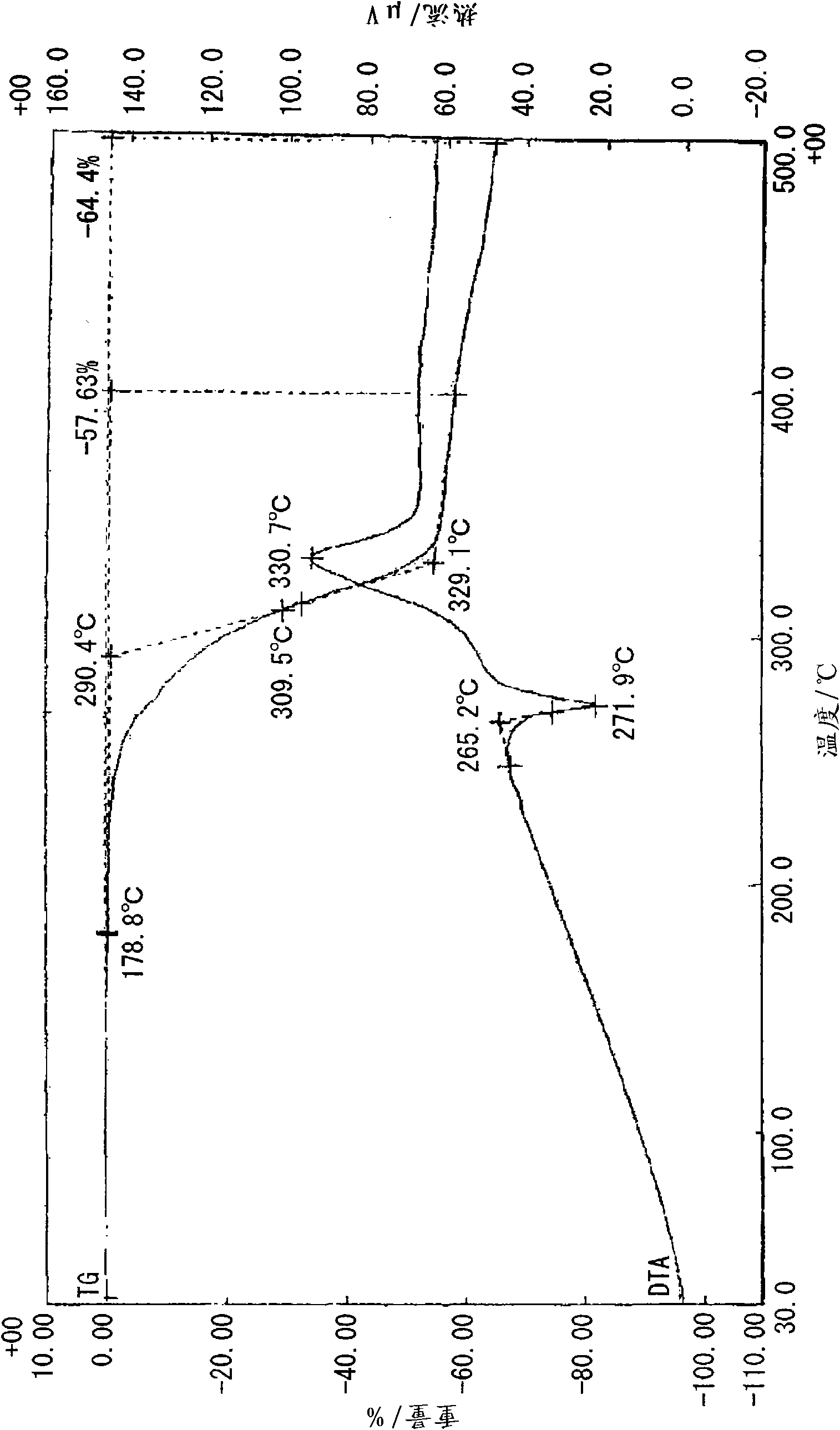
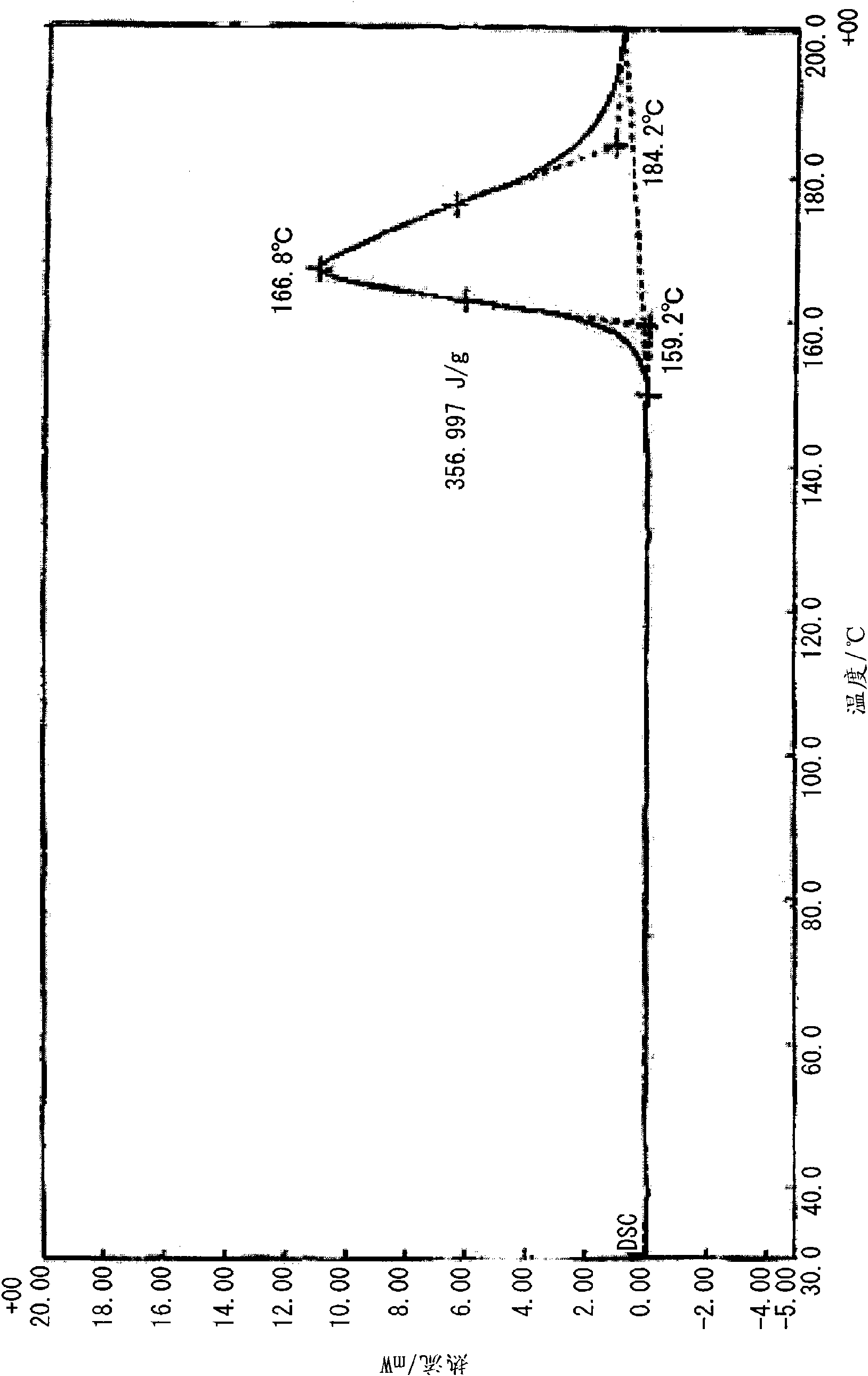
[0100] Under heating and reflux, 20 ml of a methanol solution of 10 mmol (1.10 g) of 2-ethyl-4-methylimidazole was added to 20 ml of a methanol solution of 5 mmol (1.05 g) of 5-nitroisophthalic acid while stirring. Then, crystals were precipitated immediately after heating was stopped, and clathrate (0.5 g, 33%) was obtained by filtering and vacuum drying after standing overnight at room temperature. Analysis of the obtained clathrate by NMR revealed that it was a 1:1 clathrate crystal. The obtained clathrate (5-NO2IPA-2E4MZ) 1 HNMR spectra and X-ray diffraction patterns are shown in Figure 18 and Figure 19 . For comparison, the X-ray diffraction pattern of 5-nitroisophthalic acid (5-NO2-IPA) is shown in Figure 19 . The thermal analysis (TG / DTA) diagram of the clathrate crystal obtained is shown in figure 1 . In addition, the thermal analysis (DSC) diagram based on the temperature change of the obtained clathrate crystal is shown in figure 2 , the thermal analysis ...
Embodiment 2
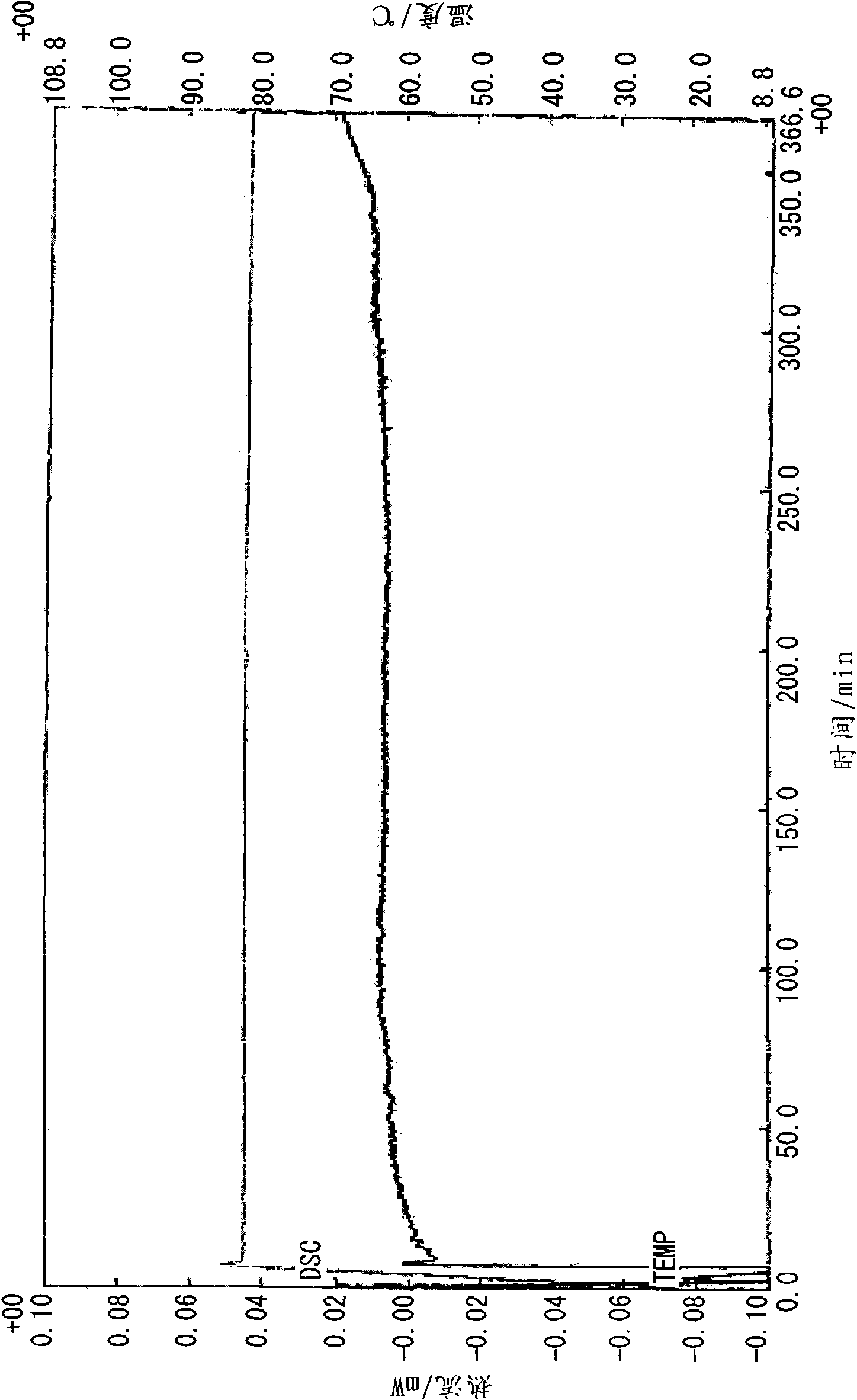
[0102] Add 15mmol (3.33g) of 5-tert-butylisophthalic acid and 18mmol (1.98g, 1.2eq.) of 2-ethyl-4-methylimidazole into 60ml of methanol, stir in an eggplant-shaped flask, and carry out Heat to reflux for 30 minutes to dissolve the crystals. Then, the precipitated crystal was filtered and vacuum-dried at room temperature to obtain an inclusion compound (2.34 g, 47%). Analysis of the obtained clathrate by NMR revealed that it was a 1:1 clathrate crystal. The thermal analysis (TG / DTA) diagram of the clathrate crystal obtained is shown in Figure 4 . In addition, the thermal analysis (DSC) diagram based on the temperature change of the obtained clathrate crystal is shown in Figure 5 , the thermal analysis (DSC) diagram at a fixed temperature (80 °C) is shown in Figure 6 .
Embodiment 3
[0104] Except having made 2-ethyl-4-methylimidazole into 16.5 mmol (1.81g, 1.1eq.), it carried out similarly to Example 2 (2.08g, 42%). The obtained clathrate was analyzed by NMR, and the result was a 1:1 clathrate crystal, and the thermal analysis (TG / DTA) chart was also consistent with the clathrate obtained in Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com