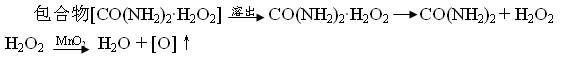Patents
Literature
123results about How to "High inclusion rate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
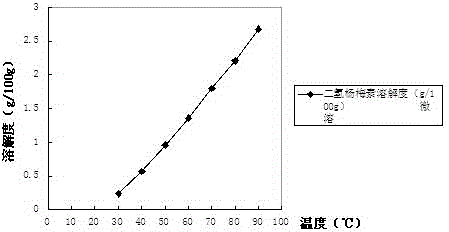
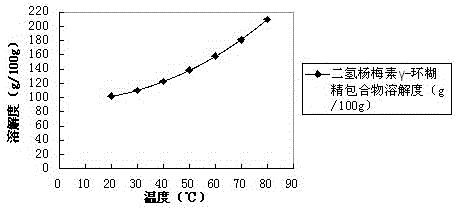
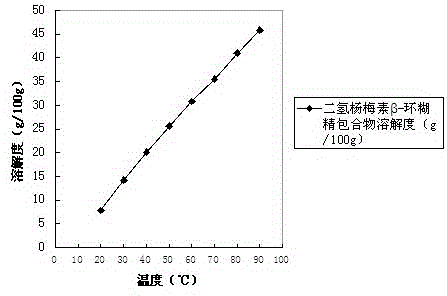
Dihydromyricetin cyclodextrin inclusion compound and preparation method thereof
InactiveCN104666293AImprove solubilityImprove stabilityAntibacterial agentsOrganic active ingredientsSolubilityNatural substance
The invention discloses a dihydromyricetin cyclodextrin inclusion compound, which comprises cyclodextrin and dihydromyricetin, wherein the dihydromyricetin is embedded into a cyclodextrin cavity; and the inclusion compound employing the cyclodextrin as a host molecule and dihydromyricetin as a guest molecule is formed. The dihydromyricetin cyclodextrin inclusion compound has the beneficial effects that 1 the dihydromyricetin cyclodextrin inclusion compound is embedded by the cyclodextrin, and the solubility, the stability and the bioavailability of the dihydromyricetin are improved; 2 the preparation method of the dihydromyricetin cyclodextrin inclusion compound disclosed by the invention is simple in process and suitable for industrialized production; 3 a spray drying process is adopted, the process from spray drying to dust collection is finished within 20 seconds, the problem of product oxidation in the drying process is avoided, the method is simple in process, simple and convenient to operate; the production conditions are easy to control, and the inclusion rate of the dihydromyricetin is high; and 4 the dihydromyricetin used by the dihydromyricetin cyclodextrin inclusion compound is extracted from vitaceae ampelopsis, and the dihydromyricetin in the vitaceae ampelopsis is high in content, and is a natural substance and free of toxicity.
Owner:江苏丰园生物技术有限公司
Preparation method of rose essence beta-cyclodextrin polymer microsphere inclusion compound
InactiveCN102703221ARetain cavity structureImprove hydrophobicityCosmetic preparationsToilet preparationsSolubilityMicrosphere
A preparation method of a rose essence beta-cyclodextrin polymer microsphere inclusion compound comprises the following steps: preparing a beta-cyclodextrin polymer microsphere by an inverse emulsion polymerization method; dissolving rose essential oil in a small amount of anhydrous ethanol; including the solution with the beta-cyclodextrin polymer microsphere by a saturated aqueous solution method; and after completion of inclusion, eluting and performing vacuum decompressed drying to obtain an inclusion product. The preparation method of the rose essence beta-cyclodextrin polymer microsphere inclusion compound takes the beta-CD (cyclodextrin) as a matrix which is crosslinked by epichlorohydrin, and uses the beta-CD microsphere which is synthesized by the inverse emulsion polymerization method, so that not only the original cavity structure and the slow-release and controlled-release and identification capabilities of the beta-CD are kept, but also the hydrophobicity of a beta-CD cavity is improved, the inclusion is promoted and the inclusion rate is improved; and due to intermolecular synergistic inclusion after beta-cyclodextrin polymerization, so that rose essence and a substance with similar structure and solubility to the rose essence can be firmly clamped, thus playing a relatively good slow-release effect.
Owner:SHANGLUO UNIV
Skinniness hormone packing matter water solution and application thereof
InactiveCN101199855AUniform inclusionLow moisture requirementsOrganic active ingredientsSenses disorderWater solubleCyclodextrin derivative
The invention relates to a preparation of antibiotic and cortical hormone, in particular to an eye-used or ear-used preparation combining cortical-hormone inclusion compound and antibiotic. Cyclodextrin derivative is used to prepare insoluble cortical hormone into water-soluble inclusion compound, and then the inclusion compound solution, along with antibiotic is used to prepare pellucid medicinal dropping liquid or transparent gelatine solution preparation, for local eye or ear treatment. The preparation, which is based on cortical-hormone inclusion compound, has the advantage of stable liquid, high inclusion efficiency, simple preparation process, high yield and good adaptability. The invention can obtain the dropping liquid and gelatine solution prepared jointly by cortical-hormone inclusion compound and antibiotic.
Owner:TIANJIN PHARMA GROUP CORP
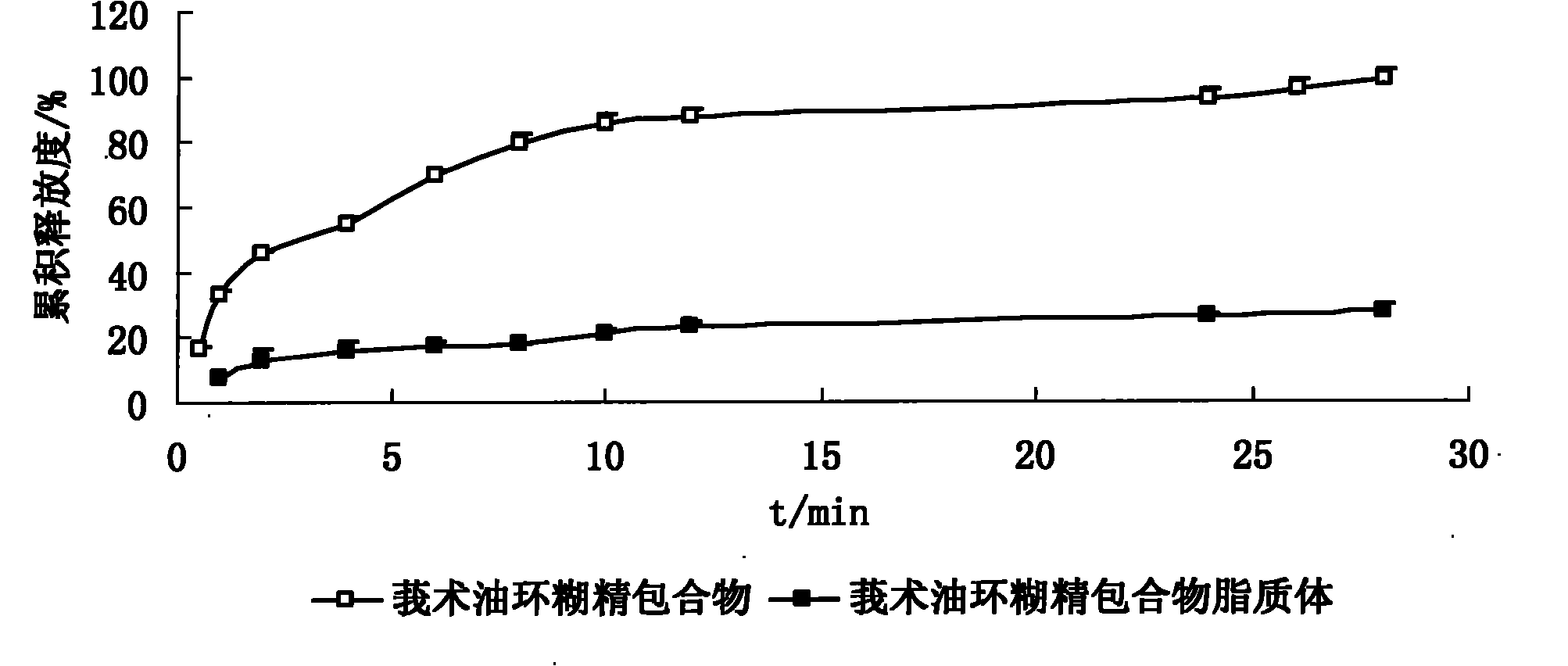
Hydroxypropyl-beta-cyclodextrin inclusion liposome of zedoary turmeric oil and preparation method thereof
InactiveCN101926962ANo hemolytic toxicityObvious slow-release and long-actingPharmaceutical non-active ingredientsAntineoplastic agentsHemolysisEthanol Injection
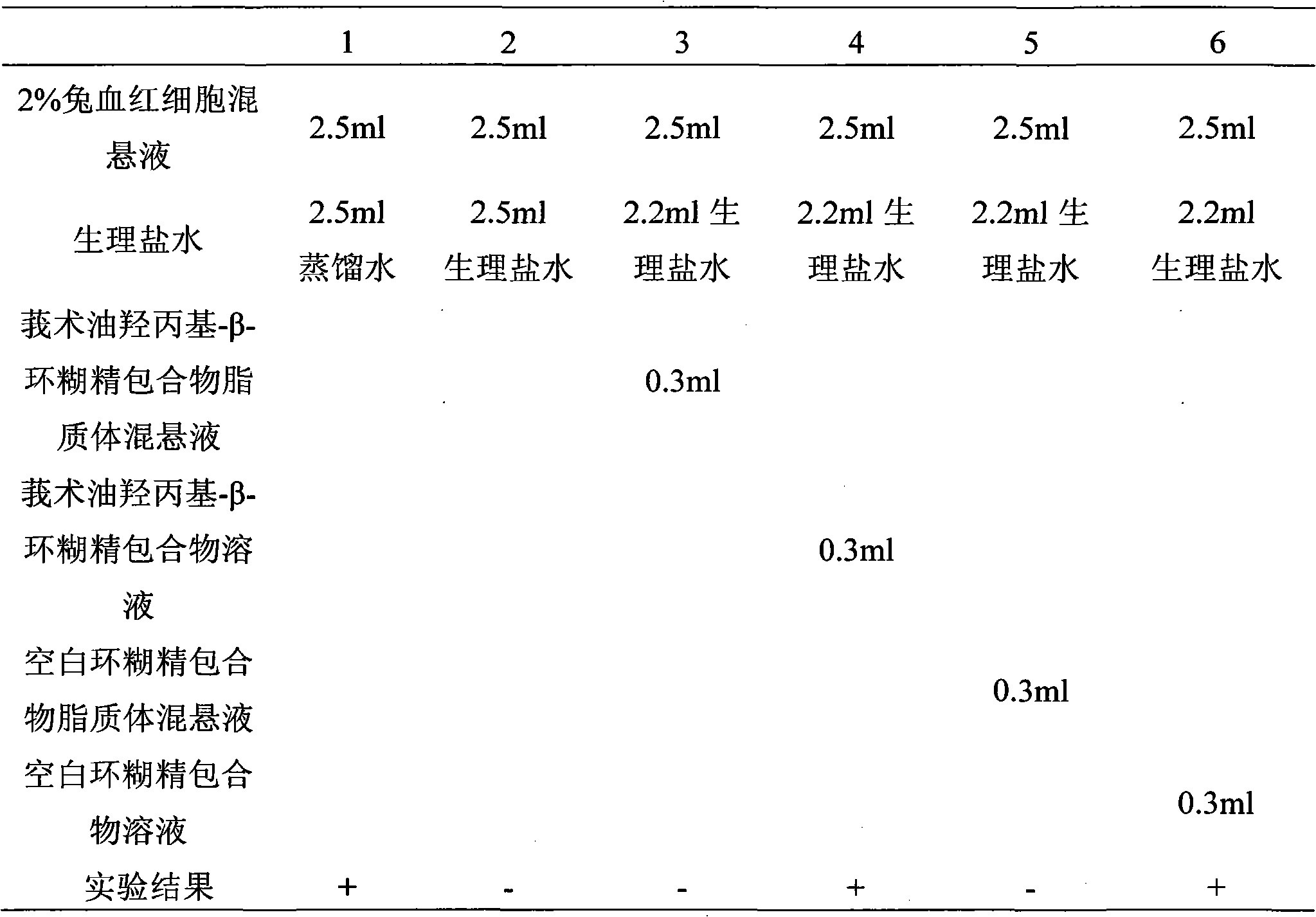
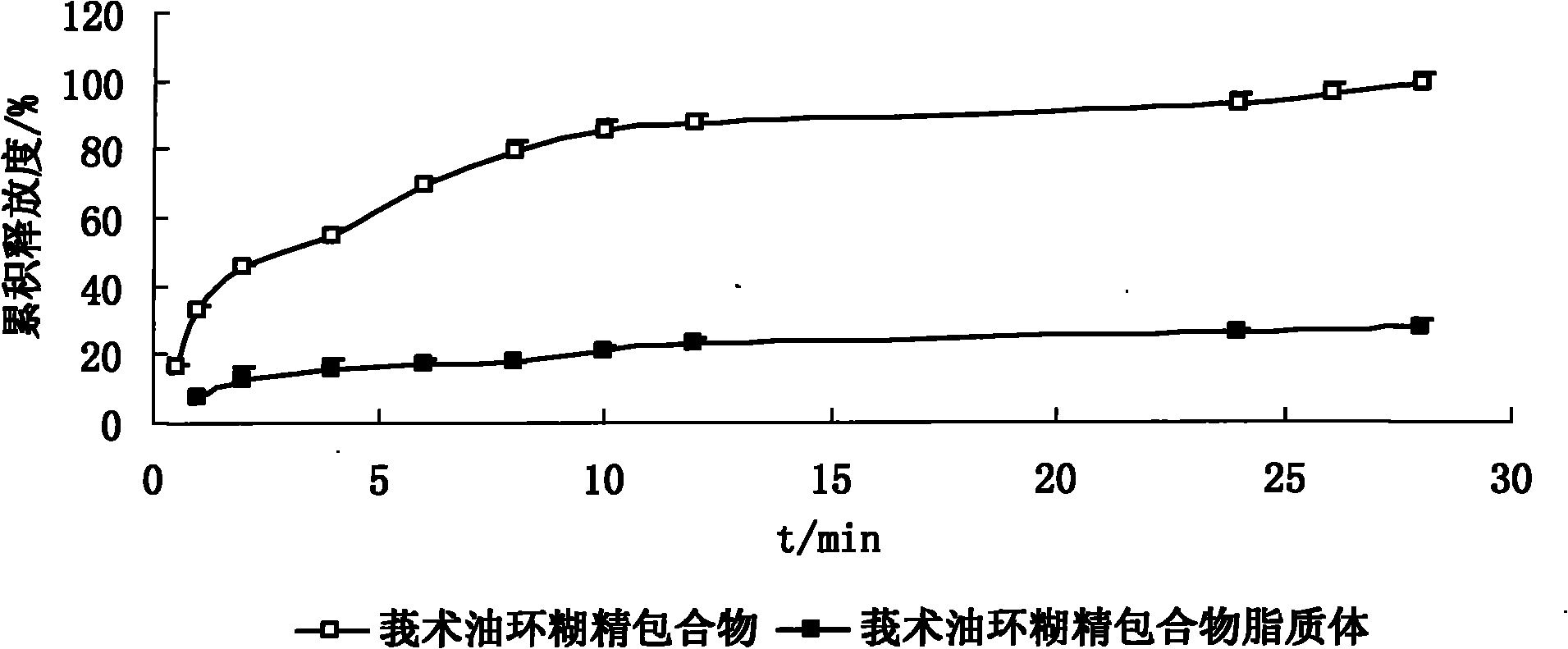
The invention discloses a hydroxypropyl-beta-cyclodextrin inclusion liposome of zedoary turmeric oil and a preparation method thereof. The liposome is prepared by the following steps of: preparing a hydroxypropyl-beta-cyclodextrin inclusion of the zedoary turmeric oil from the zedoary turmeric oil and hydroxypropyl-beta-cyclodextrin through an inclusion process; and preparing the hydroxypropyl-beta-cyclodextrin inclusion liposome of the zedoary turmeric oil from the hydroxypropyl-beta-cyclodextrin inclusion, phospholipid and cholesterol through an ethanol injection method. Experimental results show that: the hydroxypropyl-beta-cyclodextrin inclusion liposome of the zedoary turmeric oil has the advantages of good sustained release and long action, high loading rate, particularly no untoward effect such as hemolysis, and higher safety.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Oxygen-increasing slow-release fertilizer
ActiveCN103304310AIncrease oxygen contentImprove survival rateBulk chemical productionFertilizer mixturesResearch resultFertilizer
The invention relates to an oxygen-increasing slow-release fertilizer. The fertilizer is prepared from a urea peroxide-beta-cyclodextrin inclusion compound, urea peroxide (Perurea: CO(NH2)2.H2O2) and beta-cyclodextrin which are in effective quantities, wherein the urea peroxide-beta-cyclodextrin inclusion compound takes urea peroxide as a kernel and takes beta-cyclodextrin as an outer layer. According to the slow-release fertilizer disclosed by the invention, the oxygen content is high and can reach 17-20%; and after the slow-release fertilizer disclosed by the invention is used, the oxygen release is sustained, mild, stable and controllable and can sustain 300 hours per 100g of urea peroxide in net content. The slow-release fertilizer disclosed by the invention has the advantages that the oxygen release manner is slow, mild and stable, is stronger in activity and strong in disinfection and can be used for easily eliminating diseases and pests from potted landscapes, so that the normal survival ratio of potted plants is increased effectively; and meanwhile, the slow-release fertilizer disclosed by the invention has effects on increasing, disinfecting and cleaning indoor oxygen and has incomparable superiority compared with currently reported relevant research results and new products on the market.
Owner:新胜利工业集团有限公司
Isoquercitrin clathrate and preparation thereof
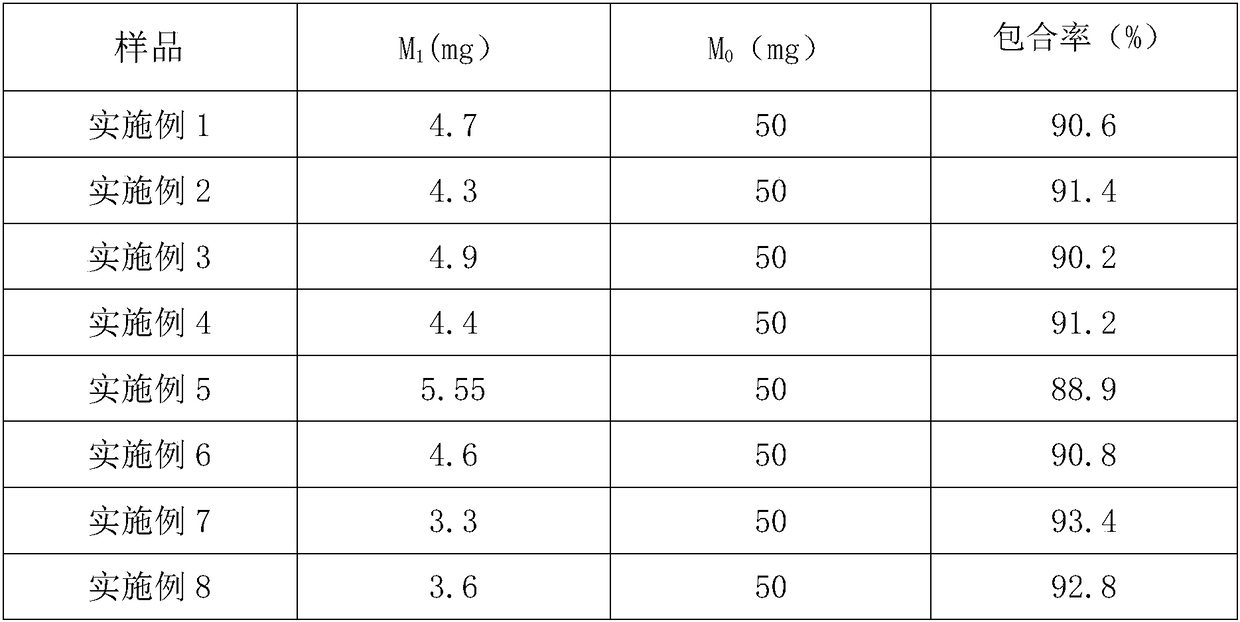
InactiveCN101301477ASimple manufacturing processHigh inclusion rateOrganic active ingredientsPharmaceutical non-active ingredientsFood additiveSolubility
The present invention provides a clathrate compound formed by isoquercitrin and [beta]-cyclodextrin or derivatives thereof. The weight ratio of the isoquecitrin and the [beta]-cyclodextrin or the derivatives thereof is 1:2 to 20. The preparation method comprises the following steps of: dissolving the [beta]- cyclodextrin or the derivatives thereof into distilled water; putting the isoquercitrin into an organic dissolvant to dissolve; adding the isoquercitrin slowly into the water solution of the [beta]- cyclodextrin or the derivatives thereof, controlling the temperature and stirring; keeping stand, pumping-filtrating or directly condensing, vacuum drying, and obtaining the isoquercitrin clathrate compound. The solubility of the obtained isoquercitrin clathrate compound is significantly improved, and the isoquercitrin clathrate compound can be further developed into multiple solid dosage forms or liquid dosage forms which are suitable for medicines or food additives.
Owner:SHANXI UNIV +1
Sustained-release microsphere containing short chain deoxyribonucleic acid or short chain ribonucleic acid and method of producing the same
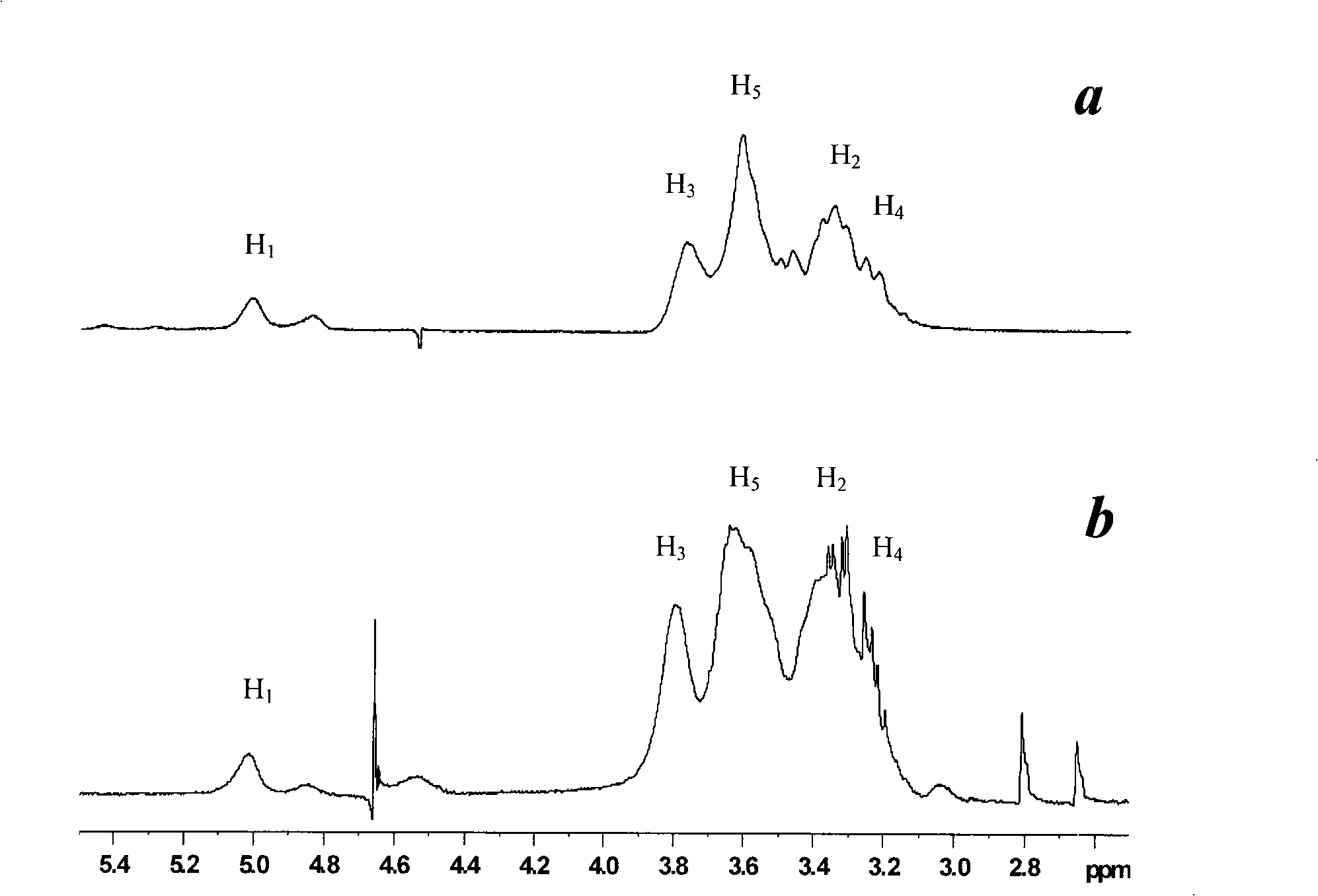
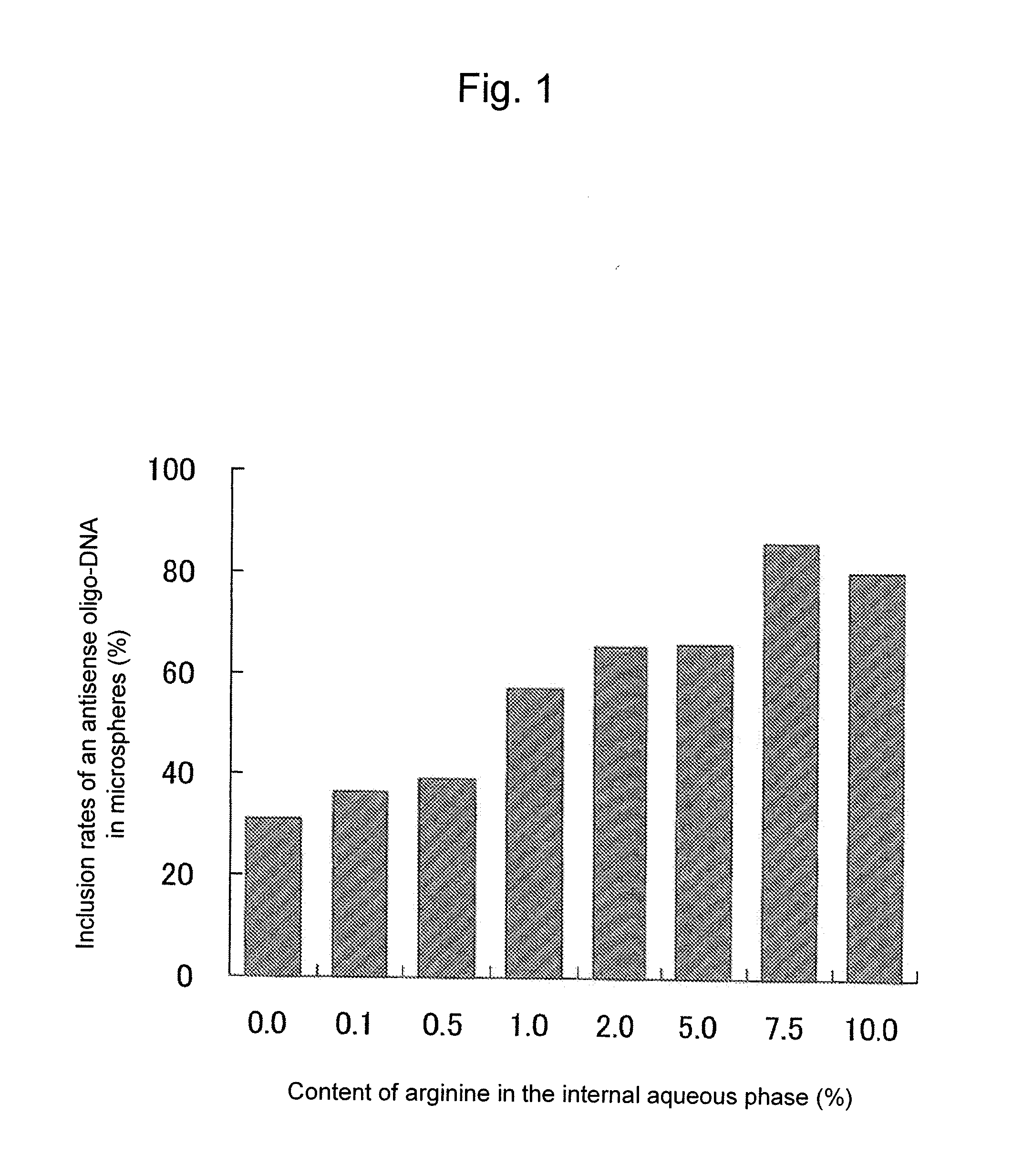
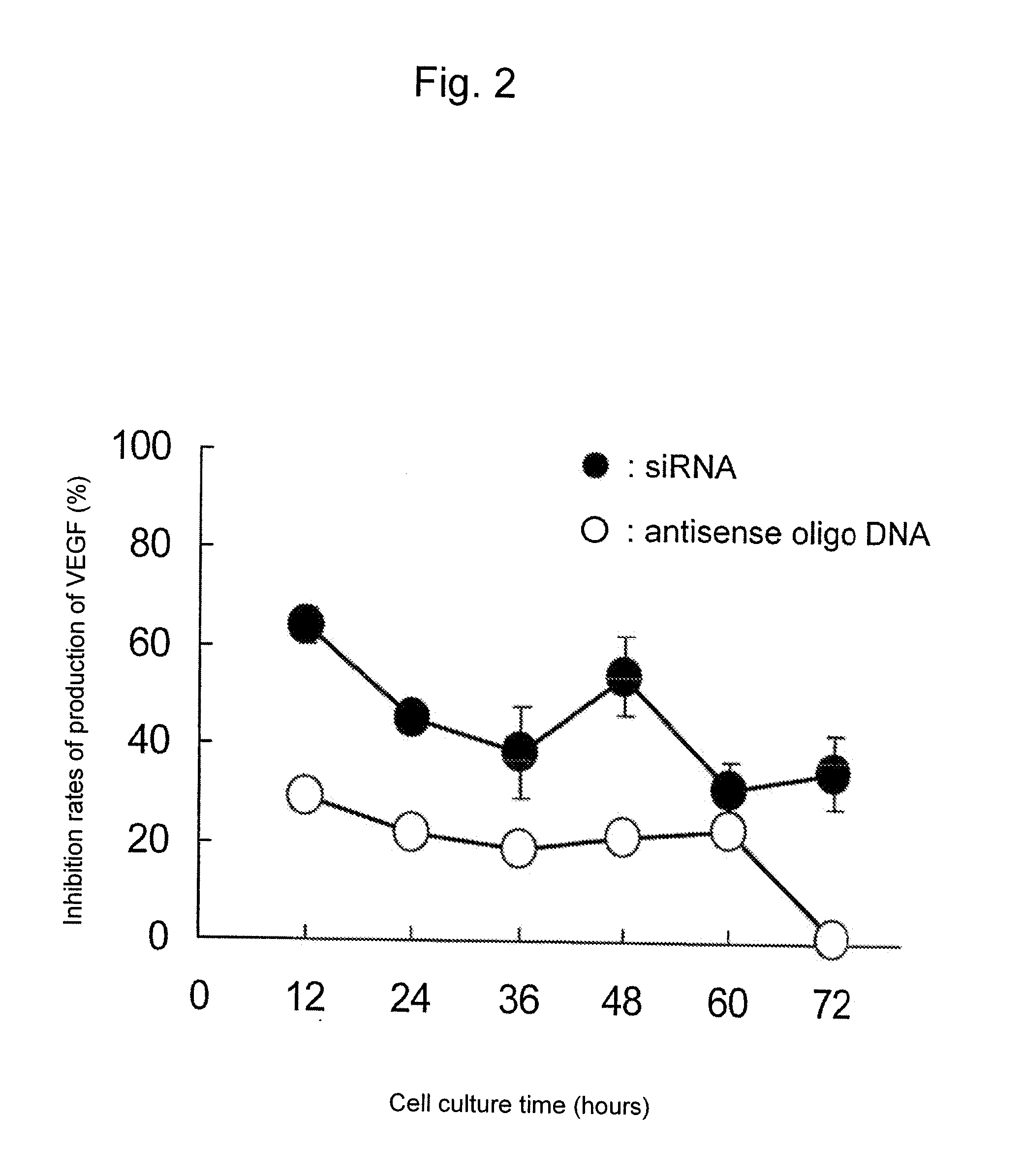
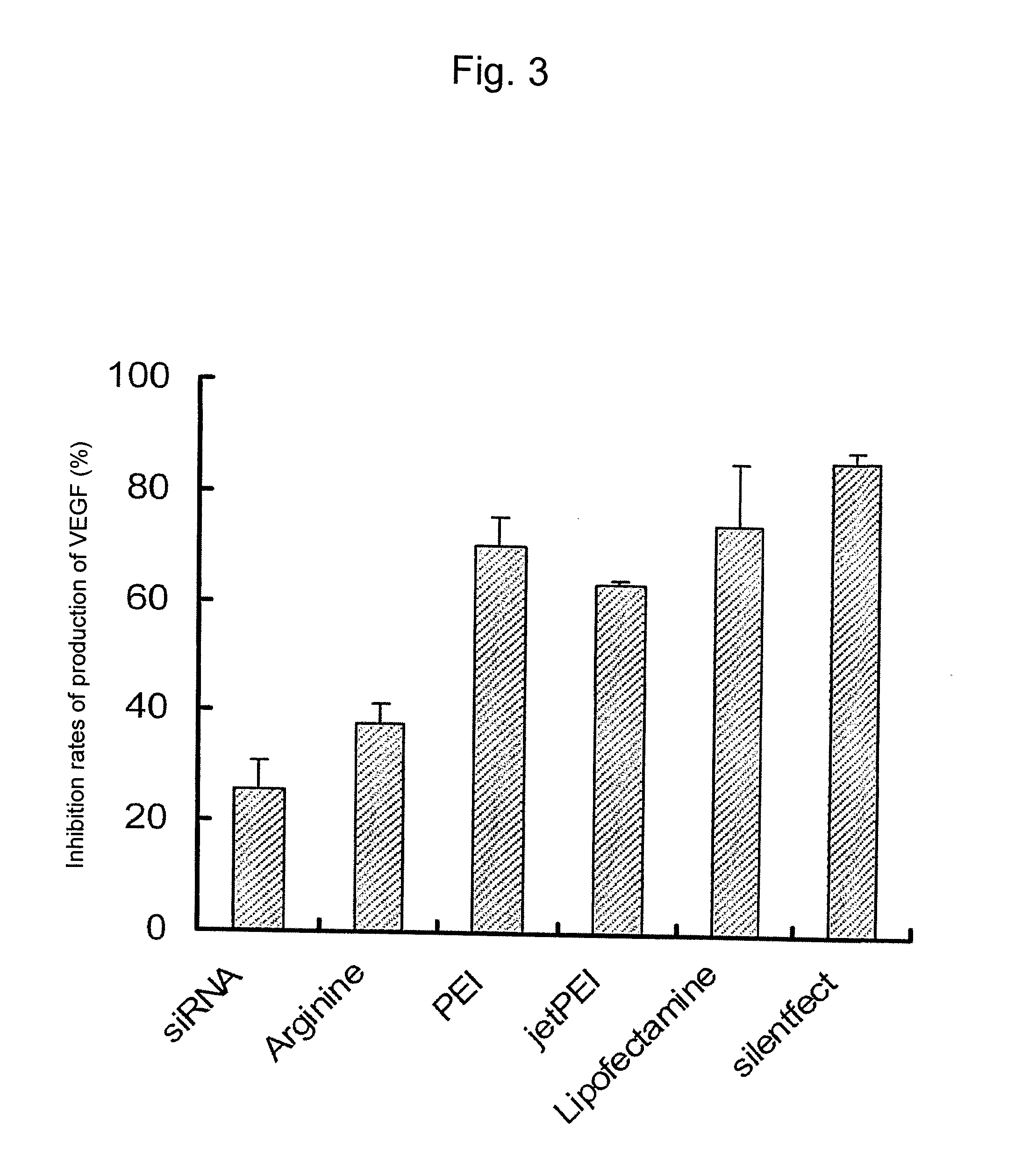
InactiveUS20100310670A1Inhibit expressionHigh inclusion ratePowder deliveryOrganic active ingredientsDiseaseArginine
A sustained-release microsphere formulation containing a short chain deoxyribonucleic acid or a short chain ribonucleic acid as an active ingredient, which has improved sustained-release properties and long-lasting efficacy, is provided. A fine particle formulation, encapsulating stably a short chain deoxyribonucleic acid or a short chain ribonucleic acid, being capable of inhibiting, for a long period, expression of a specific protein related to a disease, and which can be administered by injection or transmucosally, and a production method of the same are provided. A sustained-release microsphere formulation containing a short chain deoxyribonucleic acid or a short chain ribonucleic acid, particularly siRNA, as an active ingredient, especially a sustained-release microsphere prepared through a w1 / o / w2 type emulsion, is characterized in that a positively charged basic substance, such as arginine, polyethylenimine, a cell permeable peptide, poly-L-lysine or poly-L-ornithine, is included in an in vivo degradable polymer.
Owner:TAKEDA PHARMA CO LTD
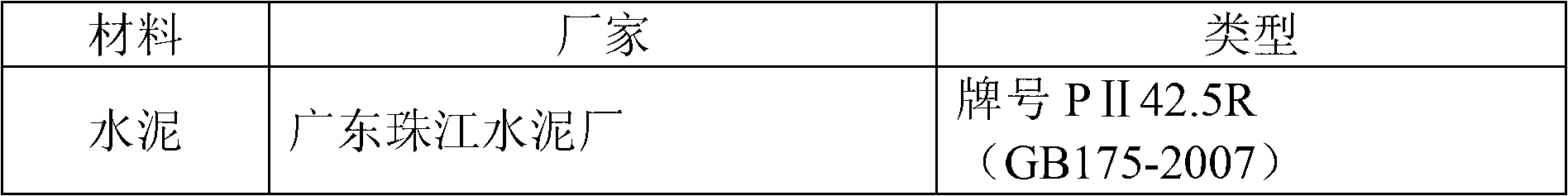
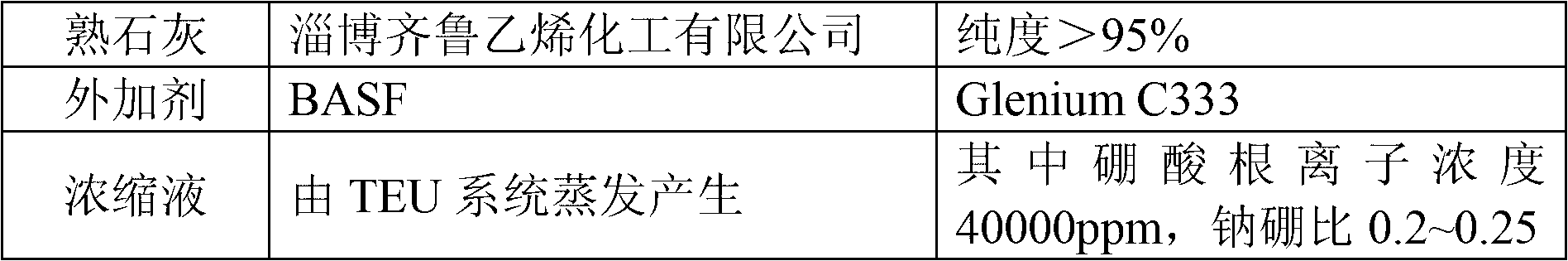
Cement solidification method of nuclear power wastes
ActiveCN102800377AReduce volumeHigh inclusion rateSolid waste managementRadioactive decontaminationSulfate radicalsPhosphate ion
The invention discloses a cement solidification method of nuclear power wastes. The cement solidification method comprises the steps of taking cement, lime and an additive as raw materials of a curing agent; detecting the density of concentrated solution, the concentration of boric acid radical ions, total salt content, the concentration of phosphate radical ions, and the concentration of sulfate radical ions in nuclear power waste concentrated solution; determining the weight of the needed cement, additive and lime; sequentially adding the additive, the lime and the cement in the nuclear power waste concentrated solution, and stirring; and standing and curing slurry to obtain the cement solidification body. According to the cement solidification method of the nuclear power wastes provided by the invention, the volume of the cement solidification body is reduced, the containing rate of the nuclear power waste is increased, and the processing process is safe and reliable.
Owner:中广核工程有限公司 +1
Preparation method of toltrazuril-cyclodextrin inclusion compound
InactiveCN101518652AImprove solubilityQuality improvementPharmaceutical non-active ingredientsAntiparasitic agentsHigh volume manufacturingOrganic solvent
The invention relates to a preparation method of a toltrazuril-cyclodextrin inclusion compound. The method comprises the following steps: the toltrazuril is dissolved in the dilute alkaline solution and performs inclusion with cyclodextrin or the derivative thereof at the temperature of 20 to 80 DEG C, the entire system is neutralized with acid, the resultant is dried and crushed and then the toltrazuril-cyclodextrin inclusion compound is obtained. The invention does not utilize the organic solvent for dissolving and has the advantages of simple process, easy control, low cost and mass production; and the prepared inclusion compound has high security, good stability and good dissolvability and is convenient to use.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
Radioactive concentrated liquor solidifying formula
InactiveCN103886926AInhibition of precipitation rateHigh inclusion rateRadioactive decontaminationWaste treatmentPrecipitation
The invention belongs to the technical field of radioactive concentrated liquor solidification of pressure water reactor nuclear power stations, and particularly relates to a radioactive concentrated liquor solidifying formula. The problem that the radioactive solid waste volume ratio in each waste treatment package in the cement solidification formula in the prior art is low is solved. The radioactive concentrated liquor solidifying formula comprises Portland cement, spent resin, zeolite, lime, water reducing agents and water; and the Portland cement is 900kg, concentrated liquor is 450L, the zeolite is 55kg, the lime is 110kg, and the water reducing agents are 3kg. The precipitation rate of radioactive elements is restrained by adding the zeolite in the existing formula; a stirring process is smooth by adding the water reducing agents in the existing formula; sand in the original formula is removed; the ratio of various components in the formula is reasonably adjusted; the radioactive concentrated liquor in each cement barrel can be increased to 450 litres from 342 litres; the radioactive spent resin solid volume ratio in each cement barrel is increased to 52.5% from 39.9%; and the solid volume ratio is obviously increased.
Owner:CNNC NUCLEAR POWER OPERATION MANAGEMENT
Tanshinone inclusion fluid as well as preparation method and application thereof
InactiveCN104027815AHigh inclusion rateGood solubilization effectOrganic active ingredientsPharmaceutical non-active ingredientsMilk cow'sPharmacology
The invention discloses a tanshinone inclusion fluid. The host molecule of the tanshinone inclusion fluid is beta-cyclodextrin, and the guest molecule of the tanshinone inclusion fluid is tanshinone. The invention also provides a preparation method of the tanshinone inclusion fluid and an application of the tanshinone inclusion fluid in preparing a cow breast injection agent. The tanshinone inclusion fluid disclosed by the invention has the beneficial effects as follows: a tanshinone beta-cyclodextrin inclusion compound solution is prepared, and preparation process parameters of the tanshinone beta-cyclodextrin inclusion compound solution are optimized; a tanshinone cyclodextrin inclusion solution with minimum cyclodextrin dosage, highest tanshinone inclusion rate, relatively good solubilization effect and relatively stable quality can be obtained by virtue of investigation of physical and chemical properties of tanshinone and single-factor experiment and orthogonal experimental designs by focusing on the studies on each process parameter which influences the inclusion effects of the inclusion solution.
Owner:LANZHOU INST OF ANIMAL SCI & VETERINARY PHARMA OF CAAS
Peppermint essential oil and mixed cyclodextrin inclusion compound and preparation method thereof
InactiveCN103271991AHigh inclusion rateImprove solubility and stabilityCosmetic preparationsSenses disorderPeppermintsChemistry
The invention belongs to the technical field of medicines and in particular relates to a peppermint essential oil and mixed cyclodextrin inclusion compound and a preparation method thereof. According to the technical scheme, the mixture of alpha-cyclodextrin, beta-cyclodextrin and gamma-cyclodextrin and the peppermint essential oil are included in water, so that the inclusion rate is improved, the solubility and stability are improved, and an organic solvent is avoided in the production process.
Owner:江苏丰园生物技术有限公司
Cyclodextrin inclusion compound containing antibiotic components, a compounding method thereof and feed additive
InactiveCN105639120AImprove palatabilityImprove performanceAntibacterial agentsAntiviralsSolubilityAdditive ingredient
The invention provides a cyclodextrin inclusion compound containing antibacterial components and a compounding method of the cyclodextrin inclusion compound. The cyclodextrin inclusion compound containing the antibacterial components is prepared from an inclusion agent and the antibacterial components, wherein the inclusion agent is cyclodextrin and / or a derivative of the cyclodextrin; a weight ratio of the inclusion agent and a component antibacterial agent is (0.5 to 500) : 1. The compounding method of the cyclodextrin inclusion compound comprises the following steps of mixing and stirring the inclusion agent, the component antibacterial agent and a solvent; completing inclusion; distilling to remove the solvent, thus obtaining the cyclodextrin inclusion compound. The cyclodextrin inclusion compound provided by the invention is used in the aspects of feed, a feed additive and the like and is used for replacing the functions of health protection, growth promotion, oxidation resistance and the like of existing feed antibiotic antibacterial drugs; according to the cyclodextrin inclusion compound provided by the invention, the problems of poor palatability, strong pungency, poor water solubility, easiness in volatilization, easiness in oxidation, instability, strong smell and the like caused by the existence of a single antibacterial component are solved, and a new path is developed for wide application of the component antibacterial agent; the controlled release effect is good.
Owner:HUNAN JINGTIAN TECH IND CO LTD
Method for preparing florfenicol-beta-cyclodextrin inclusion compound through ultrasonic-centrifugal drying
ActiveCN107693801AImprove solubilityHigh yield and inclusion rateAntibacterial agentsOrganic active ingredientsChemistrySolubility
The invention belongs to the field of veterinary drugs and discloses a method for preparing a florfenicol-beta-cyclodextrin inclusion compound through ultrasonic-centrifugal drying. The method comprises the following steps: florfenicol and beta-cyclodextrin are taken, water is added, the solution is heated and dissolved at the constant temperature of 90-110 DEG C, and a feed liquid A is obtained,wherein the mass ratio of florfenicol to beta-cyclodextrin is 1:(2-9), and the ratio of the mass of the water to the total mass of florfenicol and beta-cyclodextrin is (2-4):1; the feed liquid A is ultrasonically treated with the ultrasonic frequency being 40-60 HZ, and a feed liquid B is obtained; the feed liquid B is centrifugally dried, and a dried product is the florfenicol-beta-cyclodextrin inclusion compound; in terms of centrifugal drying parameters, the rotating rate of a centrifugal pan is 20,000-24,000 rpm, the air intake temperature is 140-160 DEG C, and the feeding amount is 200-400 kg / h. The water solubility of the florfenicol-beta-cyclodextrin inclusion compound is as high as 5,000 ppm at the water temperature of 25 DEG C, so that the water solubility of the florfenicol is improved substantially; the yield of the florfenicol-beta-cyclodextrin inclusion compound is 98% or higher, and the inclusion rate is 95% or higher.
Owner:HENAN SOAR VETERINARY PHARMA
Method for preparing docetaxel/beta-cyclodextrin clathrates
InactiveCN103505737AHigh inclusion rateImprove solubilityOrganic active ingredientsMacromolecular non-active ingredientsDocetaxelChemistry
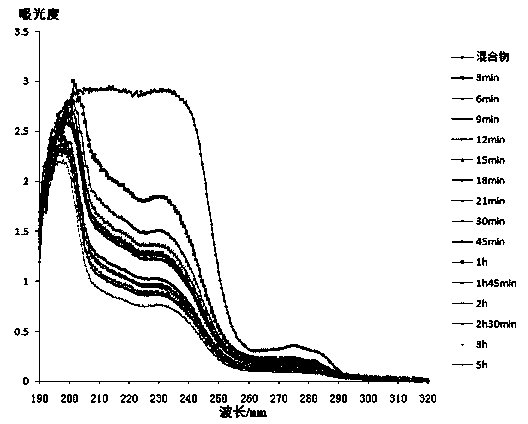
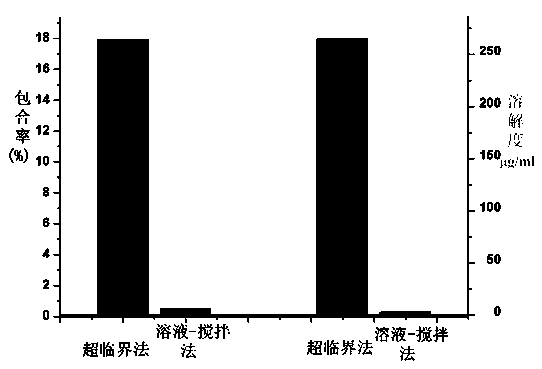
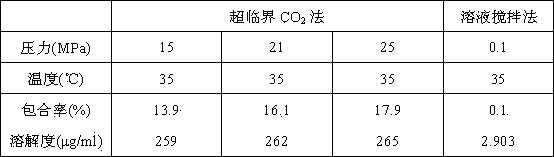
The invention discloses a method for preparing docetaxel / beta-cyclodextrin clathrates by a supercritical carbon dioxide fluid. The docetaxel / beta-cyclodextrin clathrates with different clathration rates can be synthesized by changing preparation pressure. Compared with the a general solution-stirring method for preparing the docetaxel / beta-cyclodextrin clathrates, the method prepared by the invention, by regulating and controlling the temperature and pressure of the supercritical carbon dioxide fluid, substantially raises the clathration rates of the docetaxel in the docetaxel / beta-cyclodextrin clathrates. Furthermore, the solubility of the docetaxel / beta-cyclodextrin clathrates prepared by the supercritical carbon dioxide method in water is higher than that of the docetaxel / beta-cyclodextrin clathrates prepared by the general solution-stirring method.
Owner:EAST CHINA UNIV OF SCI & TECH
Prepn process of combined delayed releasing agent for preventing and treating animal's tinea pedis and mite disease
InactiveCN1526406AHigh inclusion rateConstant releasePowder deliveryOrganic active ingredientsDiseaseAbamectin
The present invention is the preparation process of combined delayed releasing agent for preventing and treating animal's tinea pedis and mite disease. The obviously raised inclusion rate of the medicine in beta-cyclodextrin makes the new medicine preparation form efficient and stable in preventing and treating animal's dermatosis. The technological scheme is that griseofulvin, abamectin or ivermectin and beta-cyclodextrin in reasonable proportion as main materials are prepared into the combined delayed releasing agent powder or injection via certain technological process including mixing, drying, crushing, packing and other steps. The said medicine is used mainly in preventing and treating tinea pedis and mite disease of rabbit, pig, sheep, dog, cat, ox and other animals.
Owner:ZHEJIANG ACADEMY OF AGRICULTURE SCIENCES
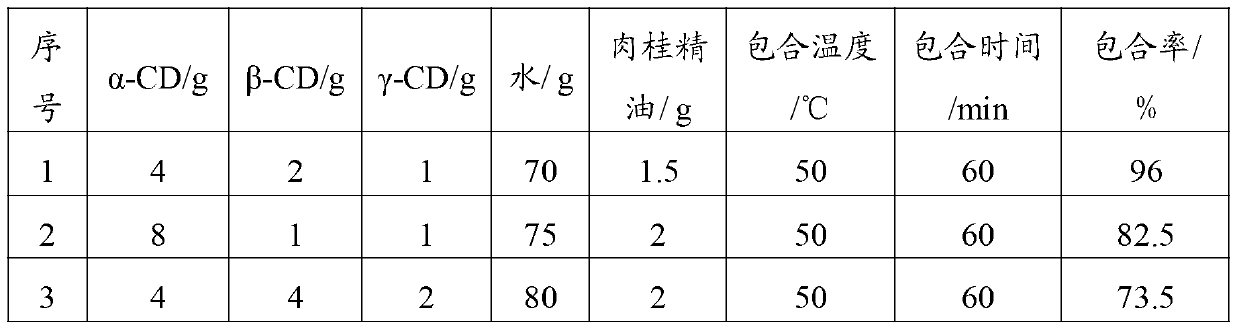
Cinnamon essential oil-cyclodextrin mixture inclusion compound and preparation method thereof
InactiveCN103271996AHigh inclusion rateImprove solubility and stabilityAntibacterial agentsAntimycoticsChemistrySolubility
The invention belongs to the technical field of medicine, and particularly relates to a cinnamon essential oil-cyclodextrin mixture inclusion compound and a preparation method thereof. The technical scheme, which is implemented by carrying out inclusion on an alpha-cyclodextrin / beta-cyclodextrin / gamma-cyclodextrin mixture and cinnamon essential oil in water, enhances the inclusion rate, increases the solubility and stability, and avoids using an organic solvent in the production process.
Owner:江苏丰园生物技术有限公司
Stable butylphthalide sodium chloride injection as well as preparation method and application thereof
PendingCN112386571AImprove solubilityGood water solubilityOrganic active ingredientsInorganic non-active ingredientsSodium Chloride InjectionCyclodextrin
The invention discloses a stable butylphthalide sodium chloride injection as well as a preparation method and application thereof, belongs to the technical field of pharmaceutical preparations, and solves the problems of poor safety and stability of the butylphthalide sodium chloride injection in the prior art. The stable butylphthalide sodium chloride injection comprises a sulfobutyl betacyclodextrin sodium compound, sodium chloride and water. The pH value of the butylphthalide sodium chloride injection is 4.0-5.0. The preparation method comprises the following steps of weighing a prescription amount of sulfobutyl betacyclodextrin sodium, and adding water for dissolving to prepare an auxiliary material solution; weighing a prescription amount of butylphthalide, adding the butylphthalide into the auxiliary material solution, stirring to enable the sulfobutyl betacyclodextrin sodium to include the butylphthalide, and adjusting the pH value of the solution to 4.0-5.0 after the inclusionof the butylphthalide is completed; and filtering, filling and sterilizing to obtain the product. The stable butylphthalide sodium chloride injection as well as the preparation method and applicationthereof are scientific in design and ingenious in thought, and the butylphthalide sodium chloride injection has good stability and safety.
Owner:CHENGDU SHIBEIKANG BIOLOGICAL MEDICINE TECH CO LTD
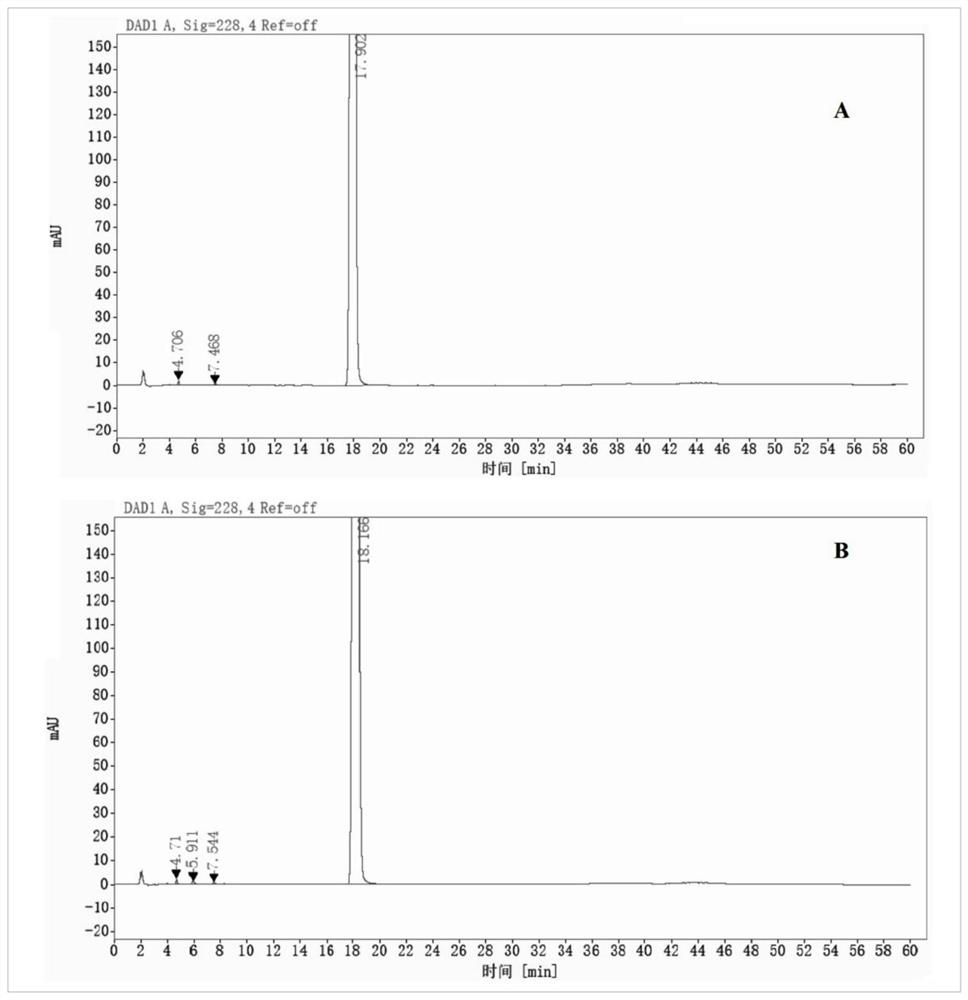
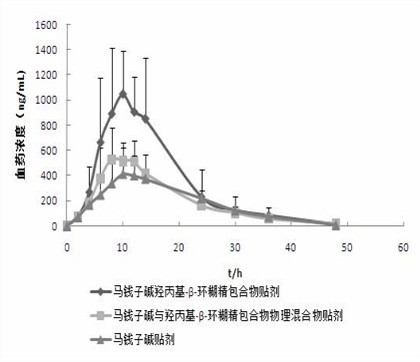
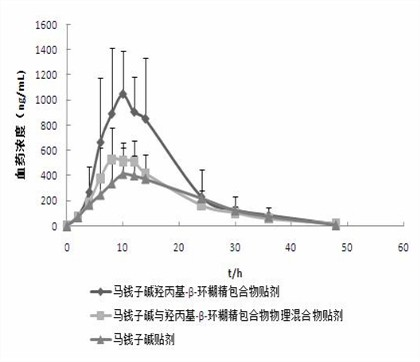
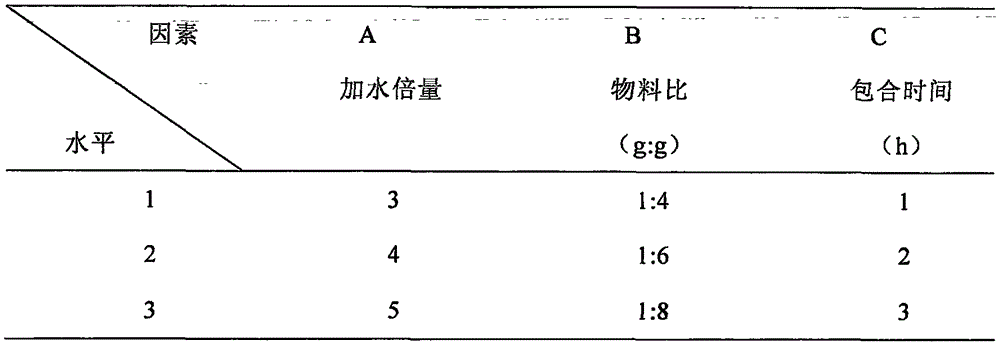
Hydroxypropyl-beta-cyclodextrin inclusion of strychnine and preparation method thereof
InactiveCN102008476AImprove securityGood water solubilityOrganic active ingredientsAntipyreticWater solubleBioavailability
The invention discloses a hydroxypropyl-beta-cyclodextrin inclusion of strychnine and a preparation method thereof. The inclusion is prepared from the strychnine and hydroxypropyl-beta-cyclodextrin in weight ratio of 1: (1-100) by using the preferential inclusion process. Experimental results show that the hydroxypropyl-beta-cyclodextrin inclusion of the strychnine has the advantages of high inclusion rate, great drug loading and good stability, and can significantly improve the water solubility of the strychnine, lead the solubility in water to be more than 20 times that of the strychnine, significantly improve the bioavailability after administration of the strychnine, play a role in reducing the toxicity of the strychnine and lead the clinical application to be safer.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Method for solidifying boron-containing nuclear waste liquid by phosphate polymer
InactiveCN109903875AReduce energy consumptionSimple processRadioactive decontaminationAluminium chlorohydratePhosphate
The invention discloses a method for solidifying a low-level radioactive nuclear waste liquid by a polymer, and concretely relates to a method for solidifying a boron-containing nuclear waste liquid by a phosphate polymer. The method mainly includes the following steps: (1) mixing a concentrated phosphoric acid solution with a concentrated boron-containing nuclear waste liquid according to a certain ratio to prepare a mixed solution; (2) preparing a polymer precursor by using water glass and polyaluminum chloride as raw materials and hydrochloric acid as an additive; and (3) mixing the mixed solution obtained in step (1) with the polymer precursor obtained in step (2) according to a certain ratio, and performing uniform stirring to prepare a solidified boron-containing nuclear waste liquid. The method has the advantages of large capacity for the boron-containing nuclear waste liquid, no need of alkali addition for neutralization, overcoming of the defect of strong retardation of a cement solidification process, good stability of the final solidified body, simple preparation process, low cost, no secondary pollution, and easiness in engineer application.
Owner:SOUTHWEAT UNIV OF SCI & TECH
Xingnaojing powder injection and preparing method
InactiveCN1616020AAvoid degradationReduce moisture contentPowder deliveryNervous disorderMedicineDecomposition
The present invention features that main medicine material including musk, curcuma root and cape jasmine extract and borneol are prepared into aromatic water solution, and the aromatic water solution is included with hydroxypropyl-beta-cyclodextrin and freeze driedor spray dried to prepare Xingnaojing powder for intravenous injection. The Xingnaojing powder for intravenous injection is superior to Xingnaojing injection in that it has the advantages of no high temperature decomposition of medicine component, high stability and less pollutant in product.
Owner:BEIJING BOERDA BIO TECH DEV
Preparation method of menthol inclusion compound
ActiveCN105664172AHigh inclusion rateSmall granularityHydroxy compound active ingredientsPharmaceutical non-active ingredientsMentholSolubility
The invention belongs to the field of pharmaceutical technology, and discloses a preparation method of a menthol inclusion compound. Superfine grinding is carried out for menthol into menthol ultrafine powder; ultrasonic mixing is carried out for (2-hydroxypropyl)-beta-cyclodextrin and water for uniformly mixing, and a saturated aqueous solution of (2-hydroxypropyl)-beta-cyclodextrin is prepared; finally, the menthol ultrafine powder is slowly added into the saturated aqueous solution of (2-hydroxypropyl)-beta-cyclodextrin, continuously ultrasonic inclusion is carried out, mixed liquor is permitted to stand, pumping filtration is carried out, vacuum drying is carried out till the liquor is dry, and the inclusion compound of menthol with (2-hydroxypropyl)-beta-cyclodextrin is obtained. The menthol inclusion compound prepared by the method has the advantages of short inclusion time of the menthol inclusion compound, high inclusion rate, good dissolvability between menthol and the inclusion agent, and substantially improved utilization rate of menthol.
Owner:宁夏金太阳药业有限公司
Oxygen-increasing controlled-release fertilizer as well as preparation method and application thereof
ActiveCN102219609AIncrease oxygen contentImprove survival rateFertilising methodsBulk chemical productionDiseaseControlled release
The invention relates to an oxygen-enriched controlled-release fertilizer which is characterized by consisting of a urea peroxide-beta-cyclodextrin complex with an effective quantity, urea peroxide (Perurea: CO(NH2)2.H2O2) and beta-cyclodextrin, wherein in the urea peroxide-beta-cyclodextrin complex, urea peroxide is used as an inner core, and beta-cyclodextrin is used as an outer layer. The oxygen-containing activity amount of the controlled-release fertilizer is high and can reach 17%-20%; and after being applied, the oxygen-enriched controlled-release fertilizer can continuously, moderately, stably and controllably release oxygen, and 100g of the fertilizer (the net content of Perurea) can continuously release oxygen for 300 hours. The controlled-release fertilizer has the advantages of slow, moderate and stable oxygen-enrichment mode, strong activity degree and strong antiseptic property, and is easy to eliminate the diseases in bonsai, thereby effectively improving the normal survive rate of a bonsai plant; and simultaneously, the oxygen-enriched controlled-release fertilizer has the effects of indoor oxygen enrichment, cleaning and disinfection; and compared with the relatedresearch results reported at present and novel products supplied by a market, the oxygen-enriched controlled-release fertilizer has incomparable superiority.
Owner:杨季冬
Preparation method for antibacterial hemostatic microspheres based on cellulose
ActiveCN106421877AKeep hydrophilicMaintain hemostatic effectSurgical adhesivesPharmaceutical delivery mechanismMicrosphereHemostatics
The invention relates to a preparation method for antibacterial hemostatic microspheres based on cellulose, and belongs to the field of functional polymer materials. The preparation method for the antibacterial hemostatic microspheres comprises the steps: firstly, dissolving amphiphilic cellulose derivatives in an organic solvent, dissolving alginate in water, and mixing the two solutions; then adding an oil-in-water type emulsifier and tea tree oil into the mixed solution, and then carrying out heating and stirring reaction for 1-10 h under a condition of the temperature of 30-80 DEG C; and finally, separating microspheres by centrifugation, washing with a washing auxiliary agent, and filtering and drying to obtain the microsphere product. The method avoids toxic effects generated from microsphere crosslinking with a chemical crosslinking agent, and the obtained microspheres have certain hydrophilicity and have good hemostatic and antibacterial effects.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Norfloxacin capsules and preparation method thereof
InactiveCN106389376AHigh inclusion rateImprove stabilityAntibacterial agentsOrganic active ingredientsPolyethylene glycolNorfloxacin
The invention relates to the technical field of pharmaceutical preparations, and in particular to norfloxacin capsules and a preparation method thereof. Every 1000 pieces of the norfloxacin capsules consist of the following components: 45g of norfloxacin, 64-66g of beta-cyclodextrin, 8-12g of polyethylene glycol, 28-32g of mannitol, 25-35g of lactose, 14-18g of microcrystalline cellulose, 8-11g of low-substituted hydroxypropyl cellulose, 18-22g of sodium carboxymethyl starch and 10-14g of a lubricant. The norfloxacin capsules provided by the invention are high in dissolution and release speeds, high in bioavailability and stable in drug performance, and the dissolution rate of the norfloxacin capsules, which are preserved for a long time, cannot be changed.
Owner:合肥美利康医药技术股份有限公司
Glass substrate composition for incineration ash of combustible wastes with low and medium-level radioactivity, and glass curing body prepared from glass substrate composition
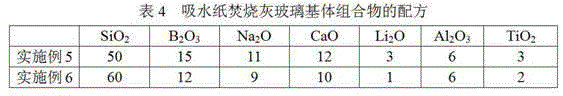
ActiveCN104310781AHigh inclusion rateImprove performanceGlass shaping apparatusRadioactive wasteNuclear facilities
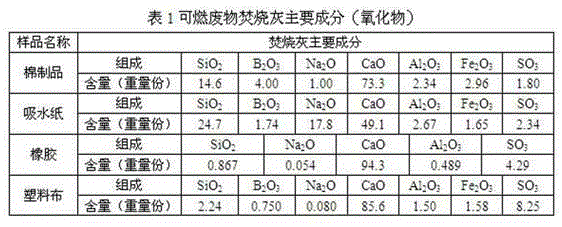
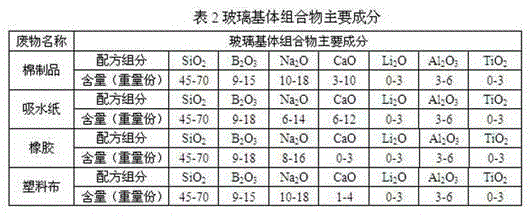
The invention discloses a glass substrate composition for incineration ash of combustible wastes with low and medium-level radioactivity, and a glass curing body prepared from the glass substrate composition. Aiming at the special physicochemical properties of the combustible wastes such as absorbent paper, cotton products, plastic cloth, rubber and the like which have low and medium-level radioactivity and are produced by nuclear facilities, the invention provides the glass substrate composition which is respectively suitable for the combustible wastes such as the absorbent paper, the cotton products, the plastic cloth and the rubber. The glass curing body can meet the requirements of weight loss ratio of a radioactive waste curing body, the density after soaking, the compressive strength, the shock strength and the irradiation resistance.
Owner:NUCLEAR POWER INSTITUTE OF CHINA
Coenzyme Q10 inclusion compound and preparation technology thereof
ActiveCN108719988AReduce the ratioHigh inclusion rateOrganic active ingredientsAntinoxious agentsSolid-stateCo enzyme q10
The invention relates to a coenzyme Q10 inclusion compound and a preparation technology thereof, and belongs to the fields of food, medicines and health care products. The coenzyme Q10 inclusion compound is prepared from the following components in parts by weight, 0.5-8 parts of coenzyme Q10, 1-10 parts of cyclodextrin and 50-600 parts of hydrophilic diluting agent. The coenzyme Q10 inclusion compound and the preparation technology thereof have the advantages that a reasonable dosage of the hydrophilic diluting agent is used for making non-polar coenzyme Q10 molecules be repelled by the hydrophilic diluting agent and remain in cavities of the cyclodextrin better, the proportion of the coenzyme Q10 molecules which are detached from the cyclodextrin in an inclusion compound drying process is reduced, the inclusion rate of the coenzyme Q10-cyclodextrin inclusion compound is improved, and the stability of the coenzyme Q10 in the solid state is further improved.
Owner:北京素维生物科技有限公司
Preparation method of aromatic decocting-free traditional Chinese medicine agent
InactiveCN105168261AImprove protectionAvoid residueAntipyreticAnalgesicsMedicinal herbsAdditive ingredient
The invention belongs to the technical field of traditional Chinese medicine preparation and particularly relates to a preparation method of an aromatic decocting-free traditional Chinese medicine agent. According to the preparation method, effective ingredients of volatile oil contained in aromatic traditional Chinese medicines are extracted with a supercritical CO2 extraction technology, extraction is performed with an ultrasonic-microwave synergic extraction technology, an extraction solution is filtered through a microfiltration technology, filtrate is concentrated and mixed with the volatile oil after inclusion, the mixture is dried and smashed, and the aromatic decocting-free traditional Chinese medicine agent is prepared from extract fine powder. The problems that the utilization rate of raw materials is low, effective ingredients of the volatile oil are not completely extracted and are severely damaged, the extraction efficiency is low and the like during extraction of traditional Chinese medicinal materials with a conventional method are solved; the whole technological process is simple to control, green and environment-friendly, the technological steps have synergistic interaction, accordingly, the extraction efficiency of the preparation method is higher, the effective ingredients are effectively protected, and the utilization rate of the aromatic traditional Chinese medicinal materials is increased.
Owner:HENAN XINGZHI PATENT SERVICE CO LTD
Hinokitiol clathrate compound and preparation method thereof
PendingCN107661505AImprove light discolorationPromote degradationCosmetic preparationsOrganic active ingredientsSolubilityFreeze-drying
The invention relates to hinokitiol clathrate compound and a preparation method thereof and specifically relates to the hinokitiol clathrate compound which can obviously improve hinokitiol water solubility and completely solve illumination color change and degradation of the hinokitol and the preparation method thereof. The hinokitiol clathrate compound disclosed by the invention is preferably clathrate compound prepared from the hinokitiol and cyclodextrin or derivative of the cyclodextrin, wherein the cyclodextrin or the derivative thereof is chosen from alpha-cyclodextrin or derivative thereof, beta-cyclodextrin or derivative thereof and gamma-cyclodextrin or derivative thereof, and a mass ratio of the hinokitiol to the cyclodextrin or the derivative thereof is (1 to 8) to (1 to 1000).The preparation method of the hinokitiol clathrate compound is chosen from a precipitation and coprecipitation method, a grinding method, an ultrasonic method, a freeze drying method, a spray drying method, a liquid-liquid method or a liquid-gas method, a solid phase clathration method and the like.
Owner:SHANGHAI JIANHE PHARM & TECH CO LTD +1
Resveratrol-sulfobutyl ether-beta-cyclodextrin inclusion compound and preparing method thereof
InactiveCN105903033AGood water solubilityImprove stabilityPowder deliveryHydroxy compound active ingredientsSolubilityHemolysis
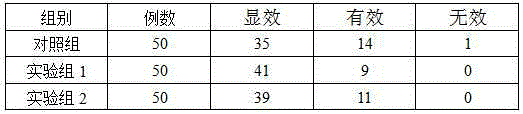
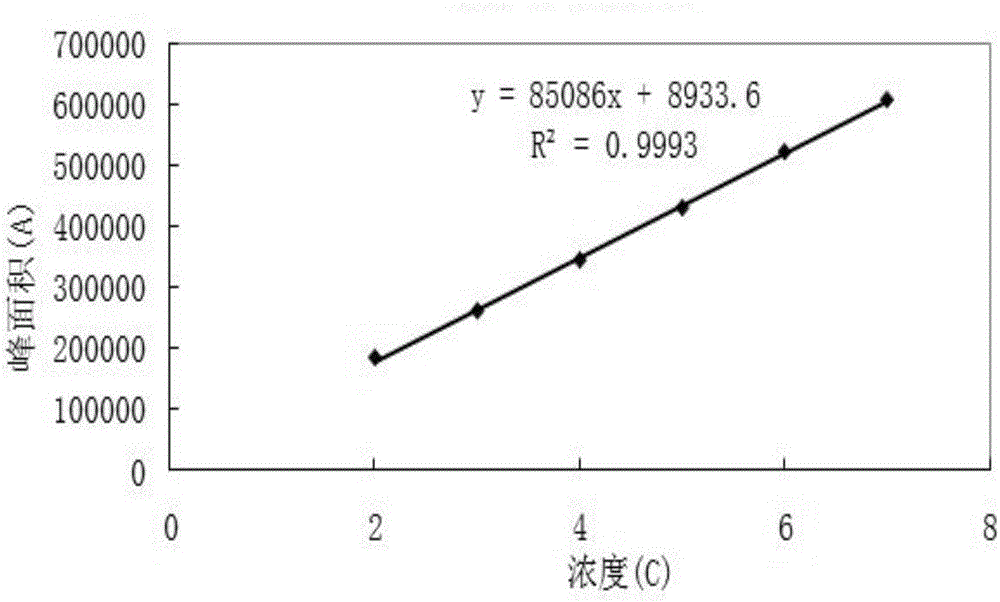
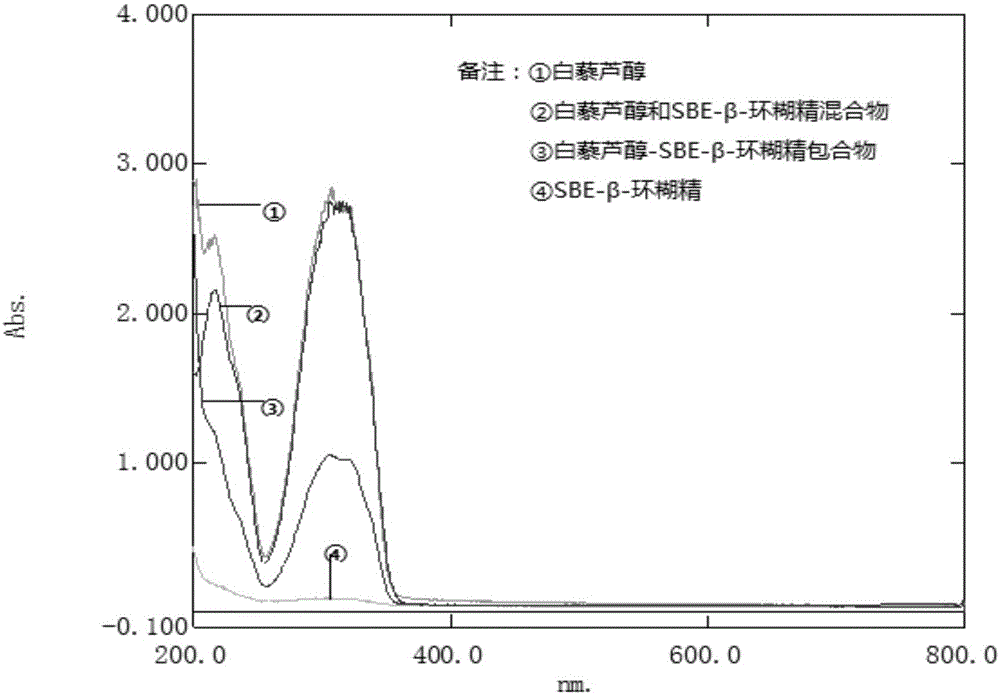
The invention discloses a resveratrol-sulfobutyl ether-beta-cyclodextrin inclusion compound and a preparing method thereof. In the resveratrol-sulfobutyl ether-beta-cyclodextrin inclusion compound, the molar ratio of resveratrol to sulfobutyl ether-beta-cyclodextrin is 1:2. The resveratrol-SBE-beta-CD inclusion compound belongs to a new dosage form. By using the SBE-beta-CD as an auxiliary material, water solubility and stability of resveratrol are improved, and the application range is broadened. Beta-CD or hydroxypropyl-beta-CD is adopted in the prior art mostly, by comparison, as SBE-beta-CD is adopted as the auxiliary material, all the properties of the inclusion compound are remarkably improved, the inclusion compound has better water solubility and a higher inclusion rate, the side effect is small, and adverse reactions such as renal toxicity and hemolysis can be reduced to the lowest degree. By means of the method, the satisfied inclusion rate can be obtained, the problem that resveratrol can not be dissolved into water easily and is poor in stability is solved, and the method can be applied to production and clinical needs.
Owner:CHONGQING MEDICAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com