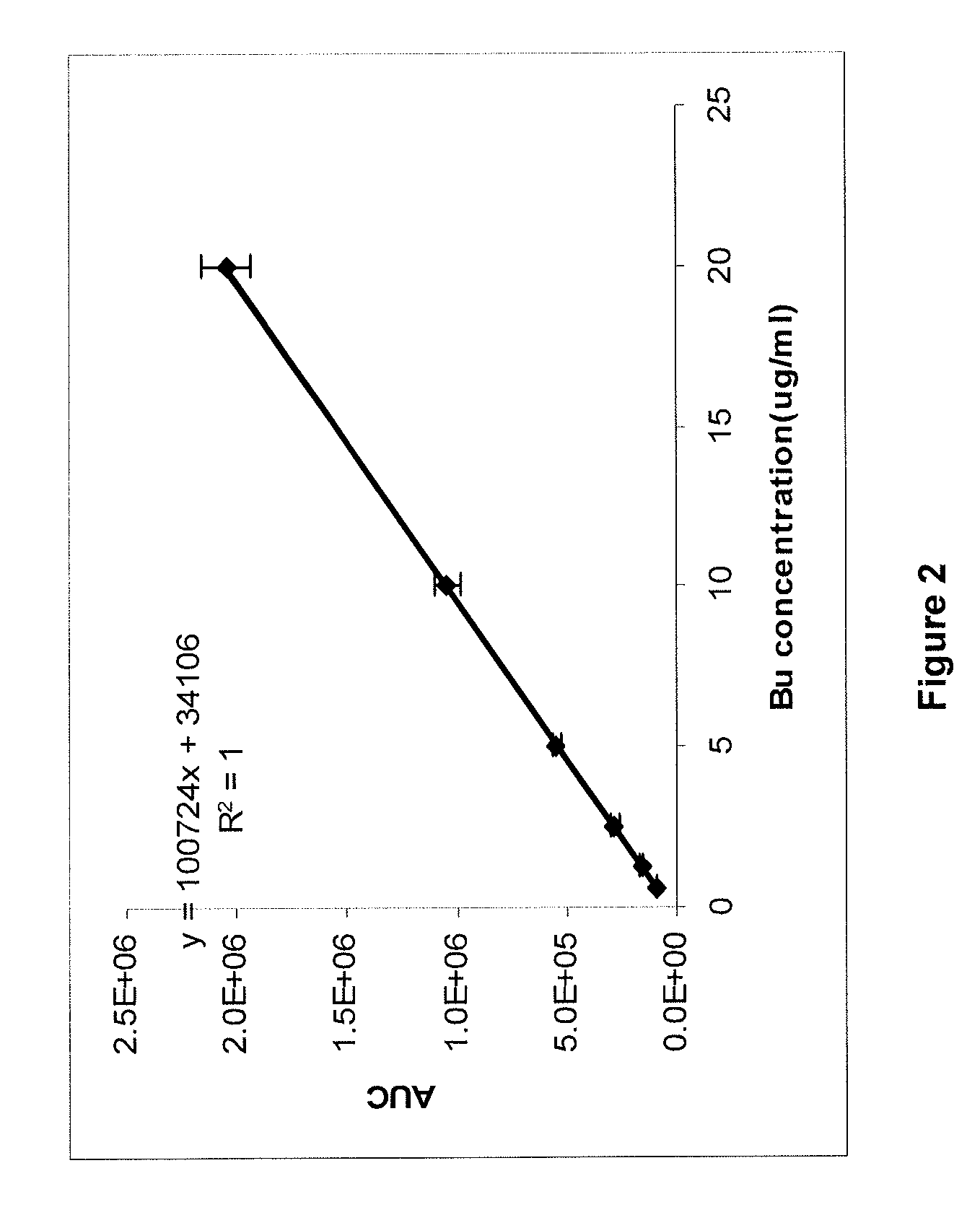
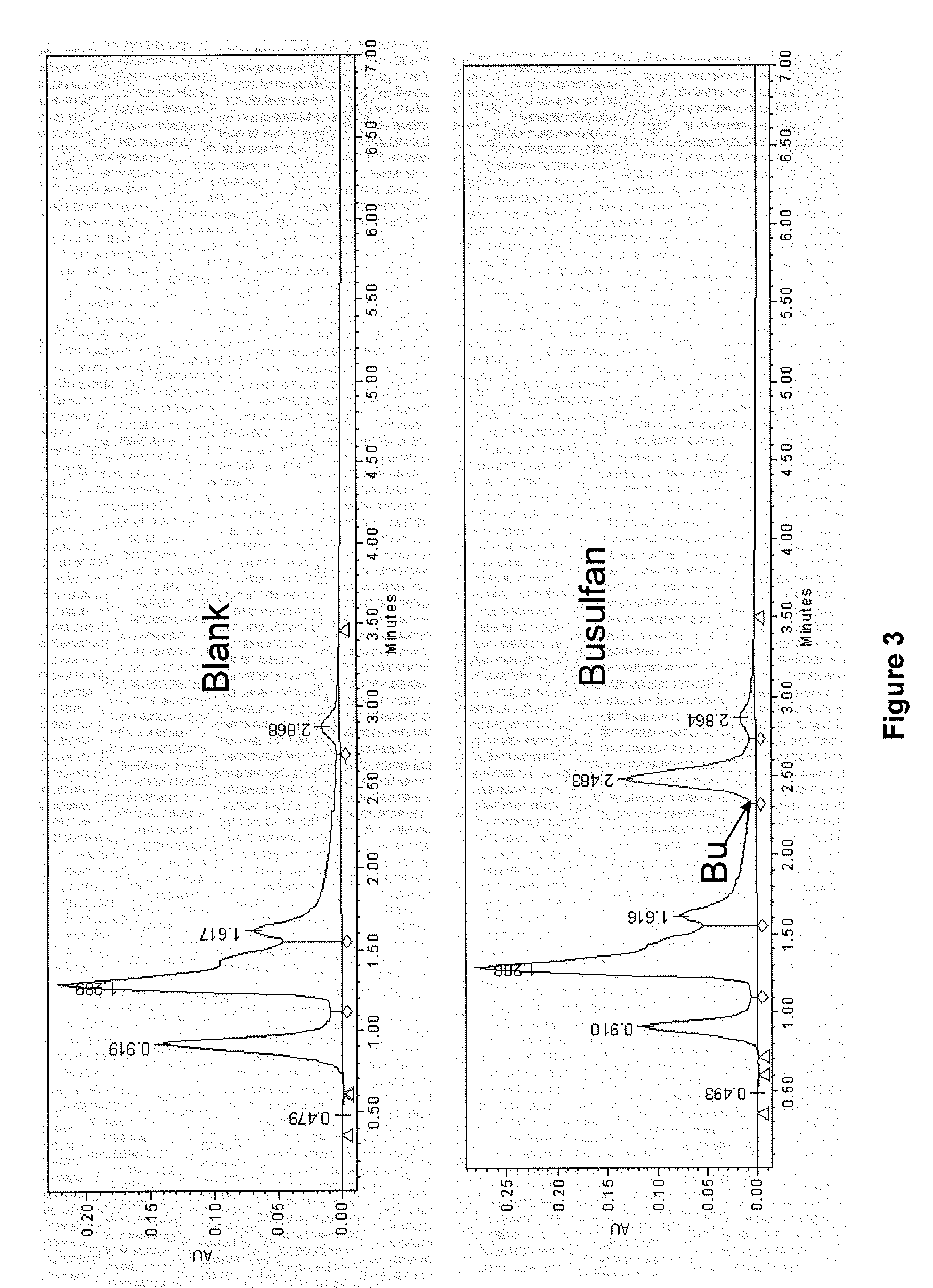
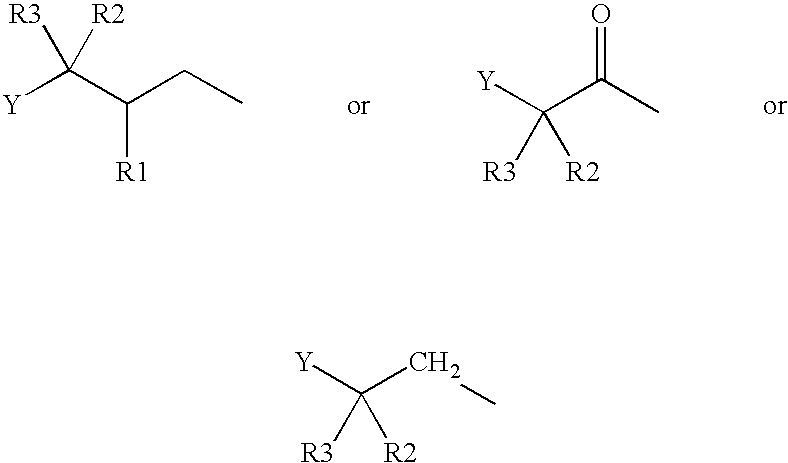
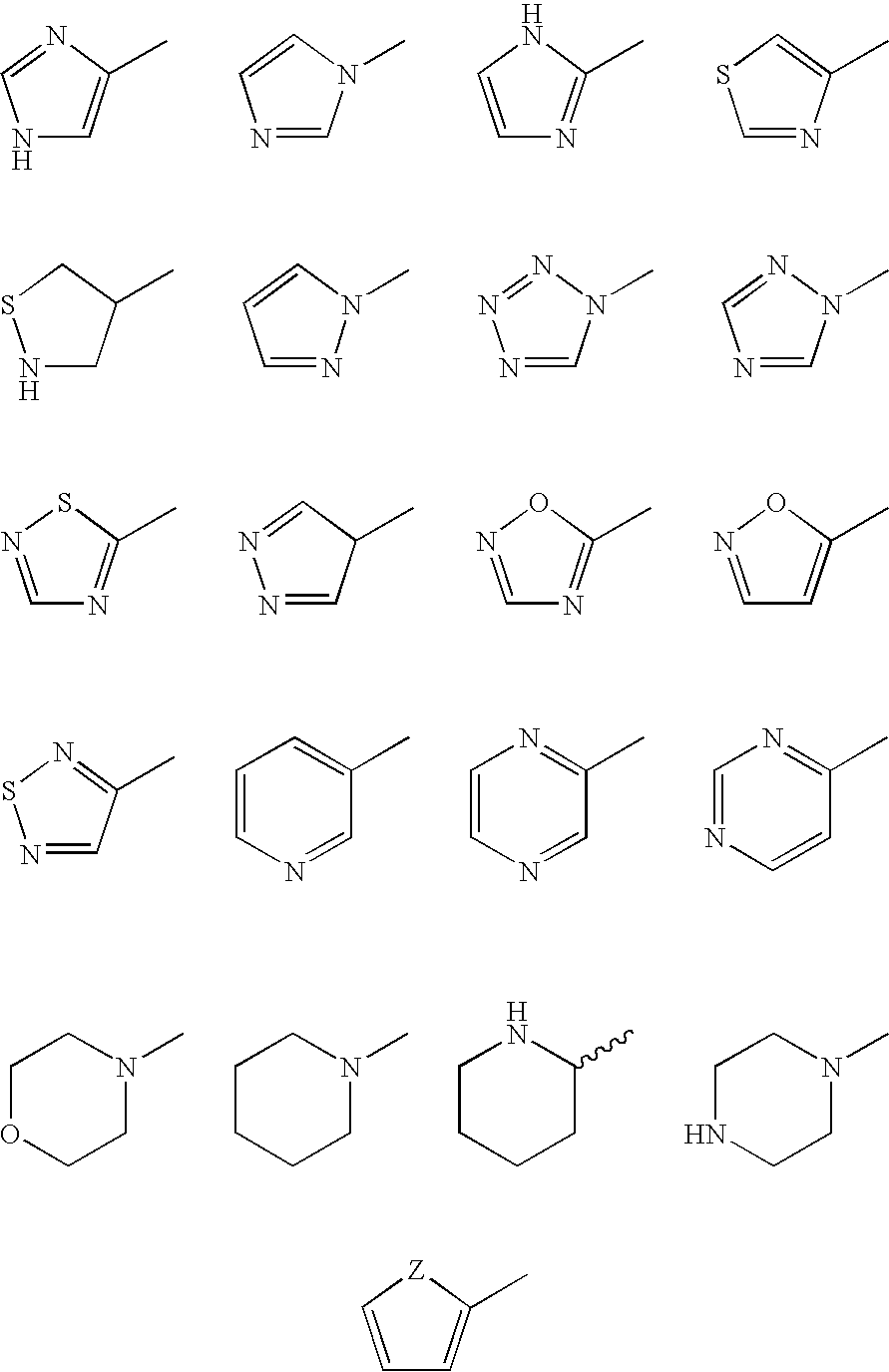
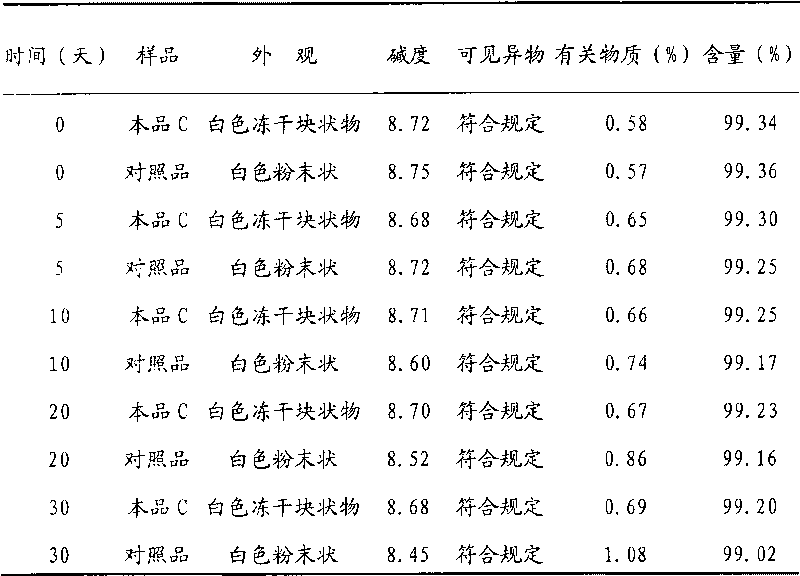
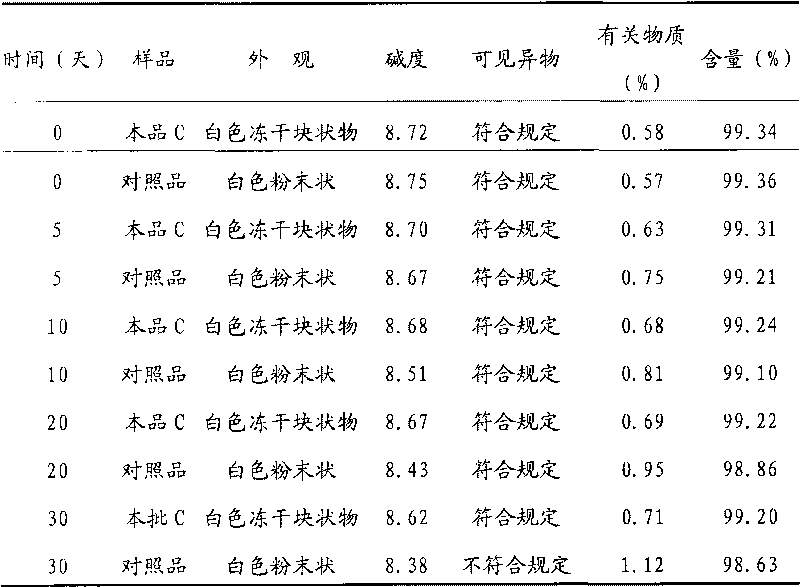
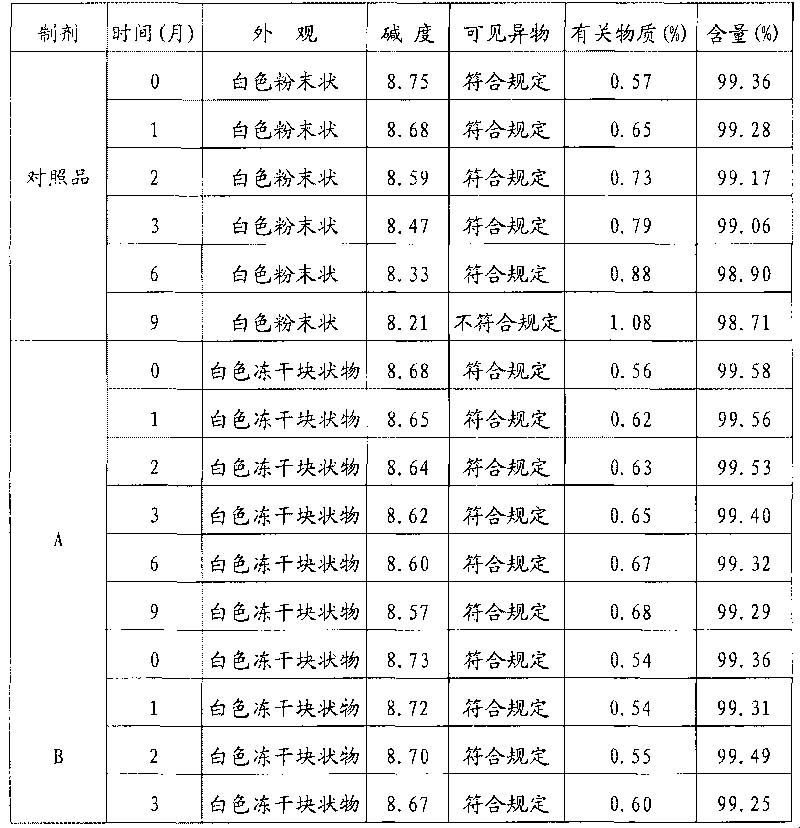
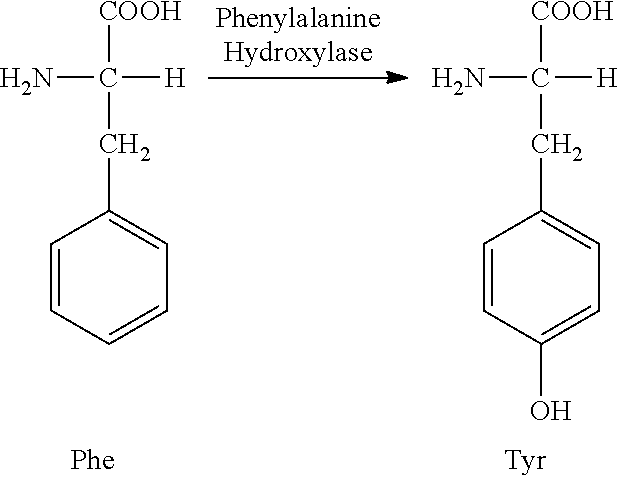
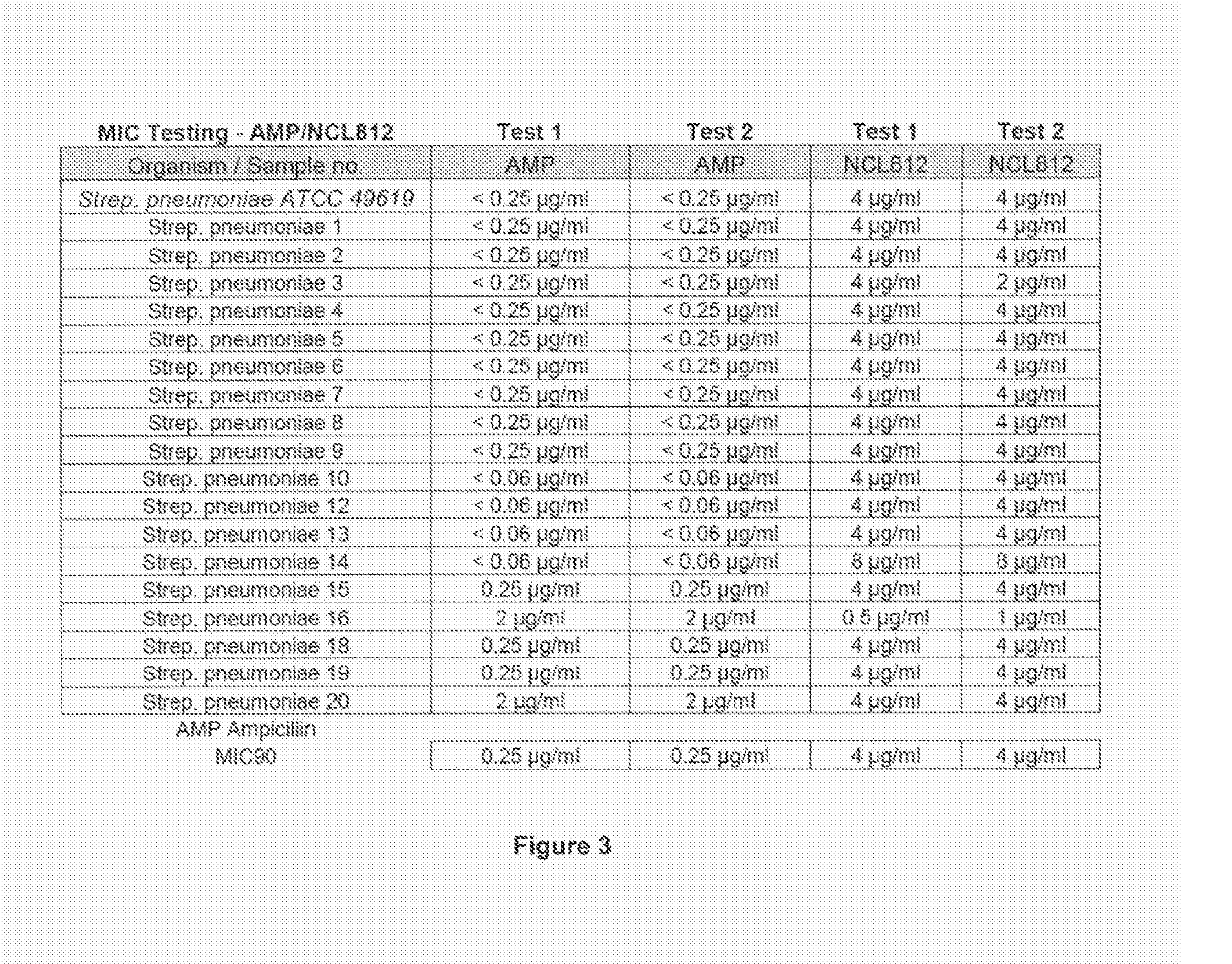Patents
Literature
64results about How to "Improve solubility and stability" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
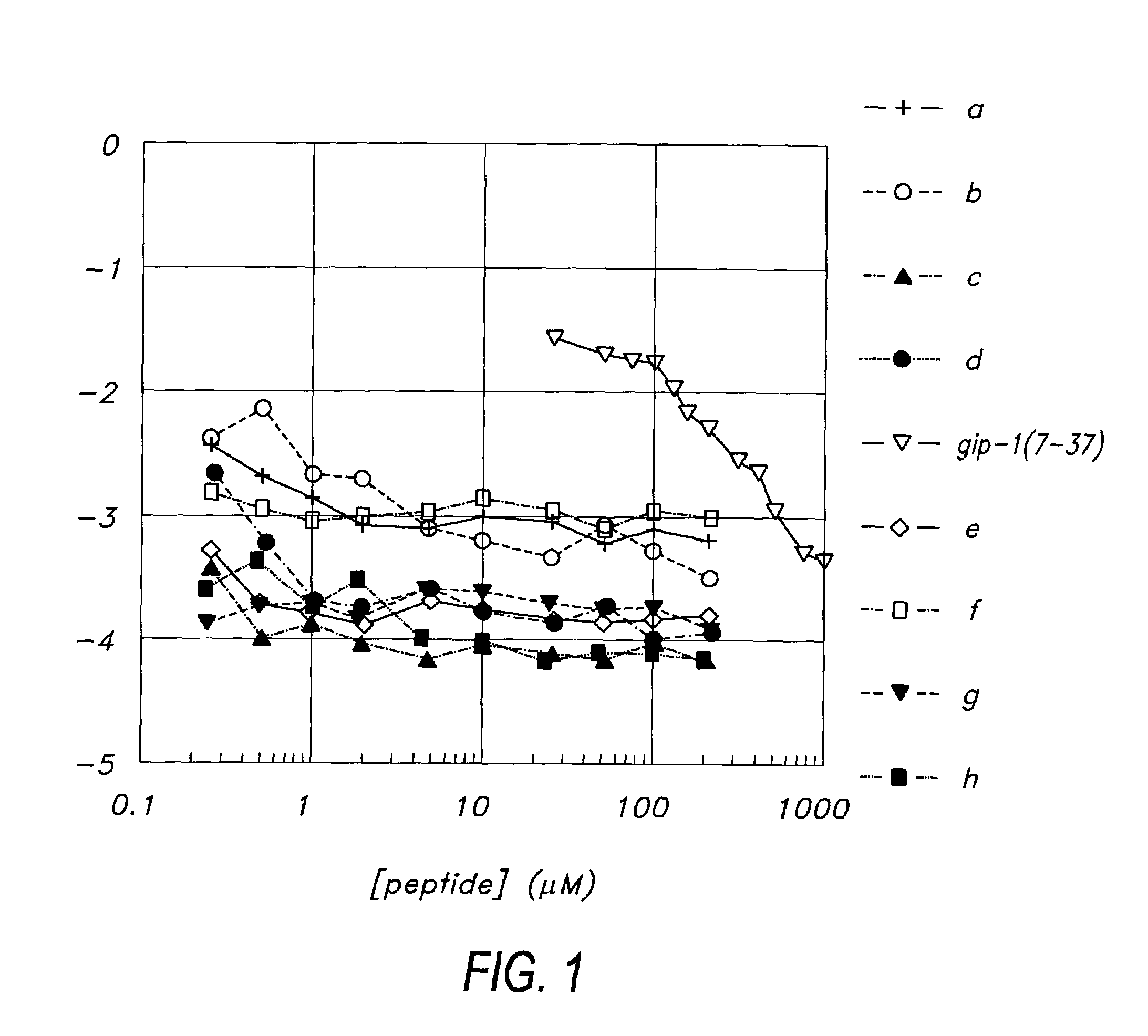
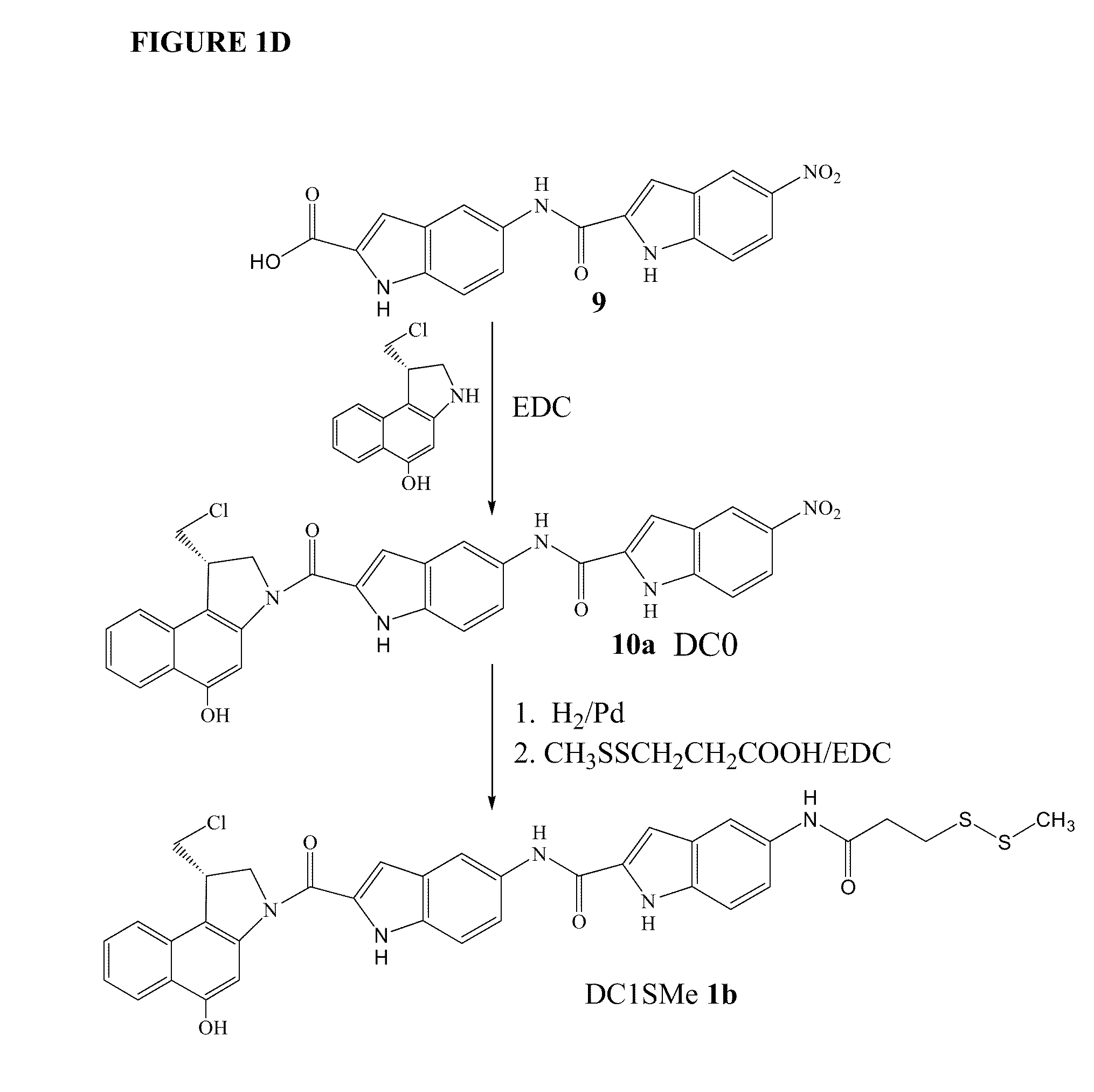
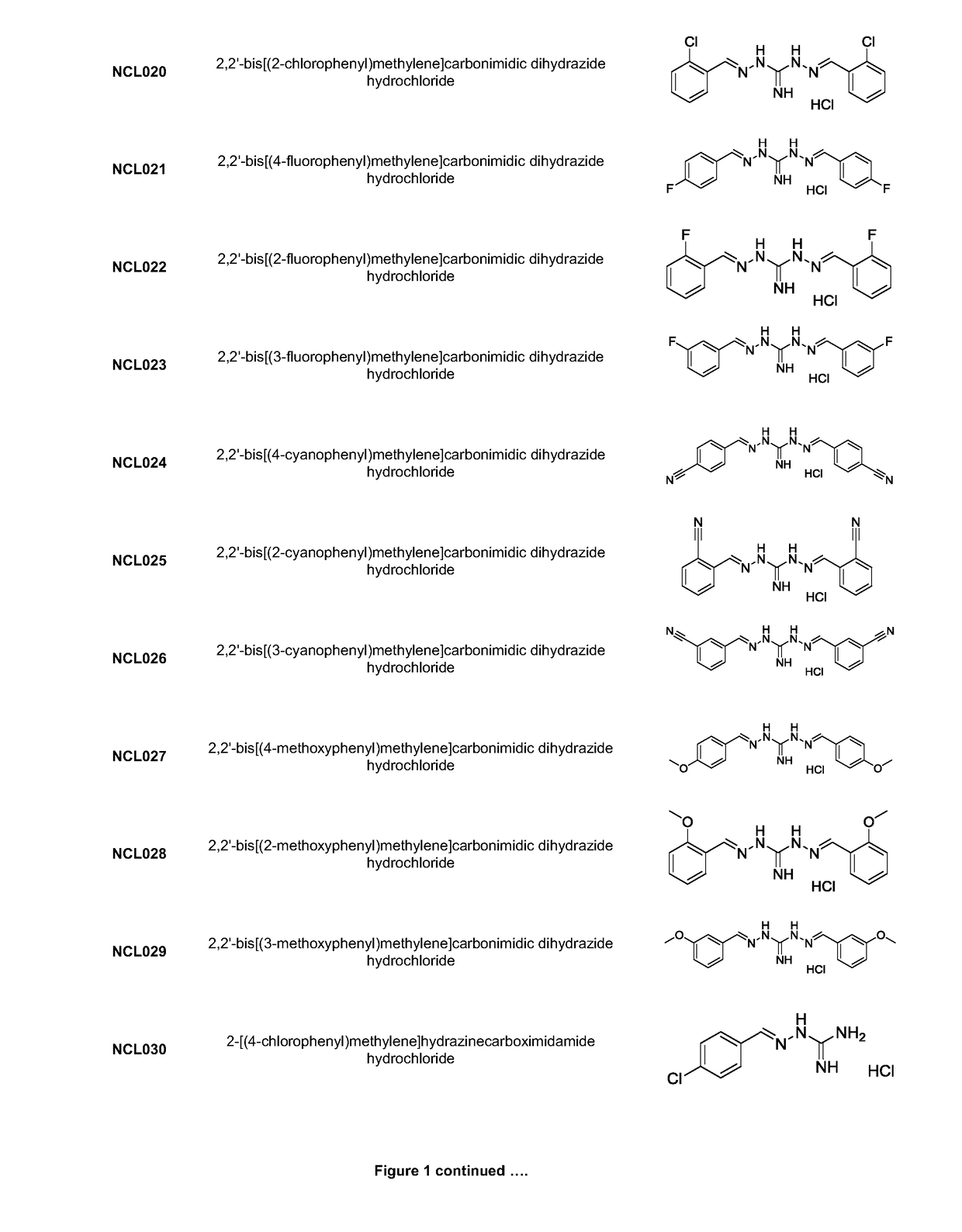
Derivatives of GLP-1 analogs
InactiveUS7235627B2Improve solubility and stabilityReduce capacityOrganic active ingredientsOrganic detergent compounding agentsActive agentSurface-active agents
The present invention relates to a pharmaceutical composition comprising a GLP-1 derivative having a lipophilic substituent; and a surfactant.
Owner:NOVO NORDISK AS
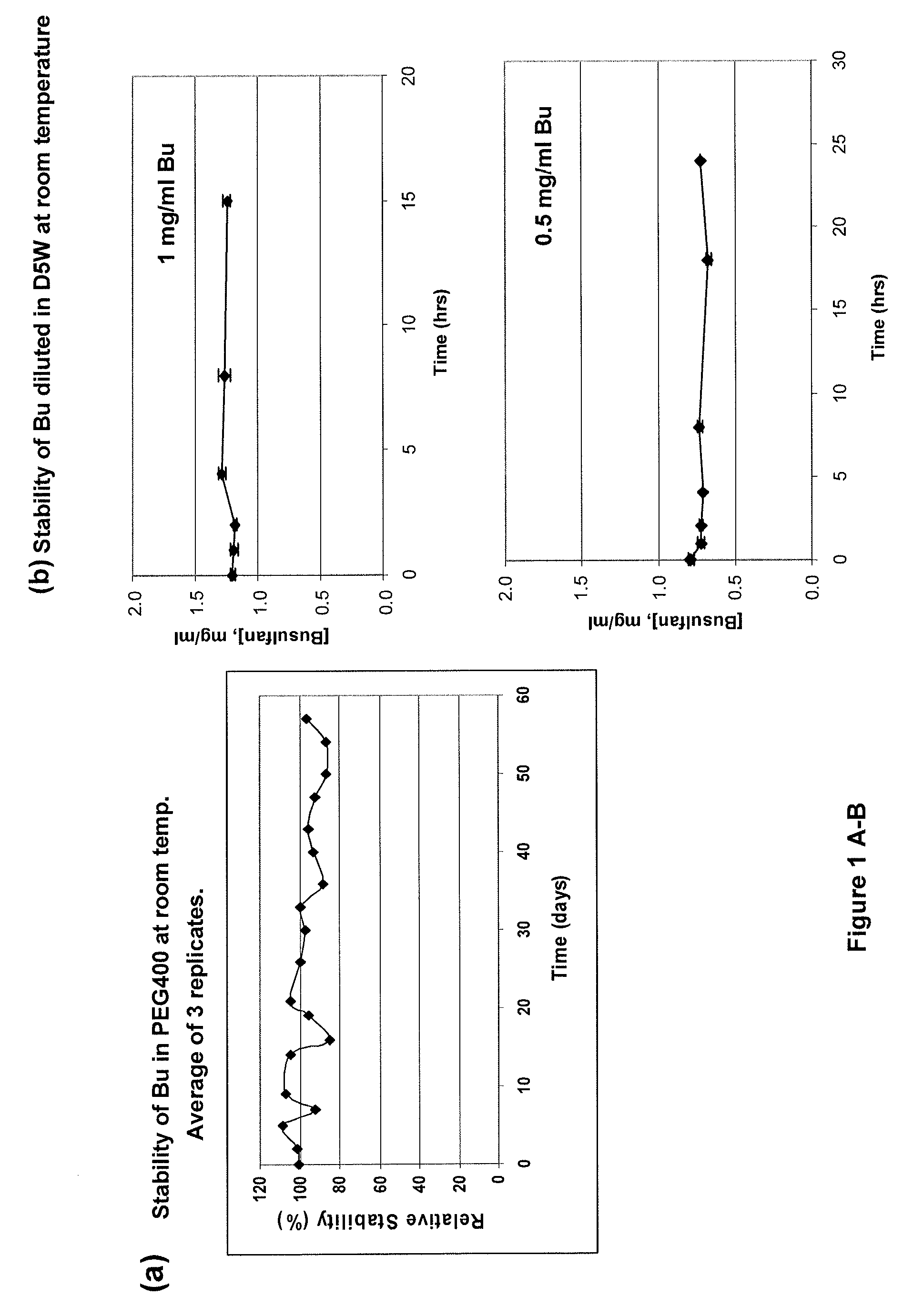
Parenteral formulations of lipophilic pharmaceutical agents and methods for preparing and using the same
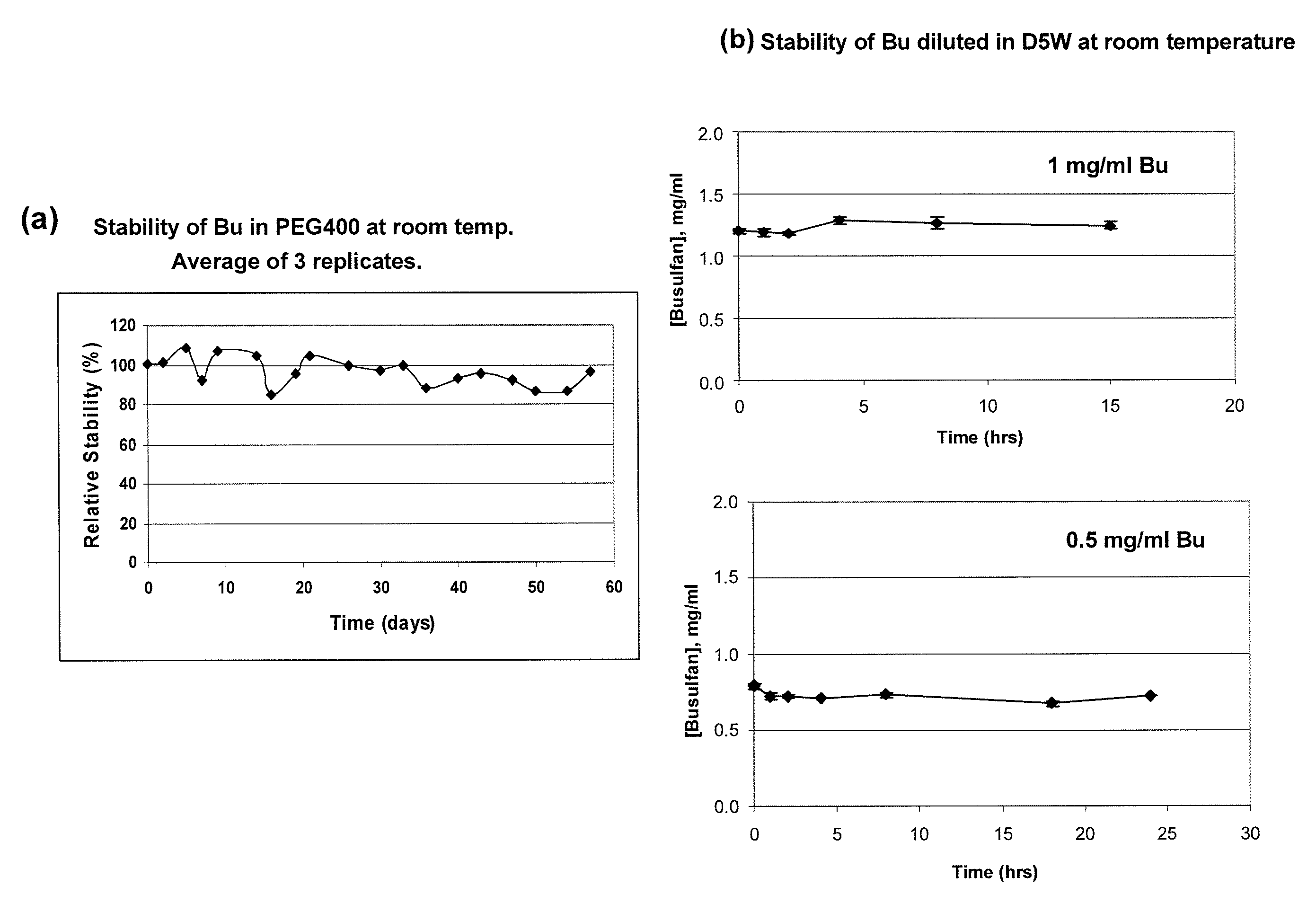
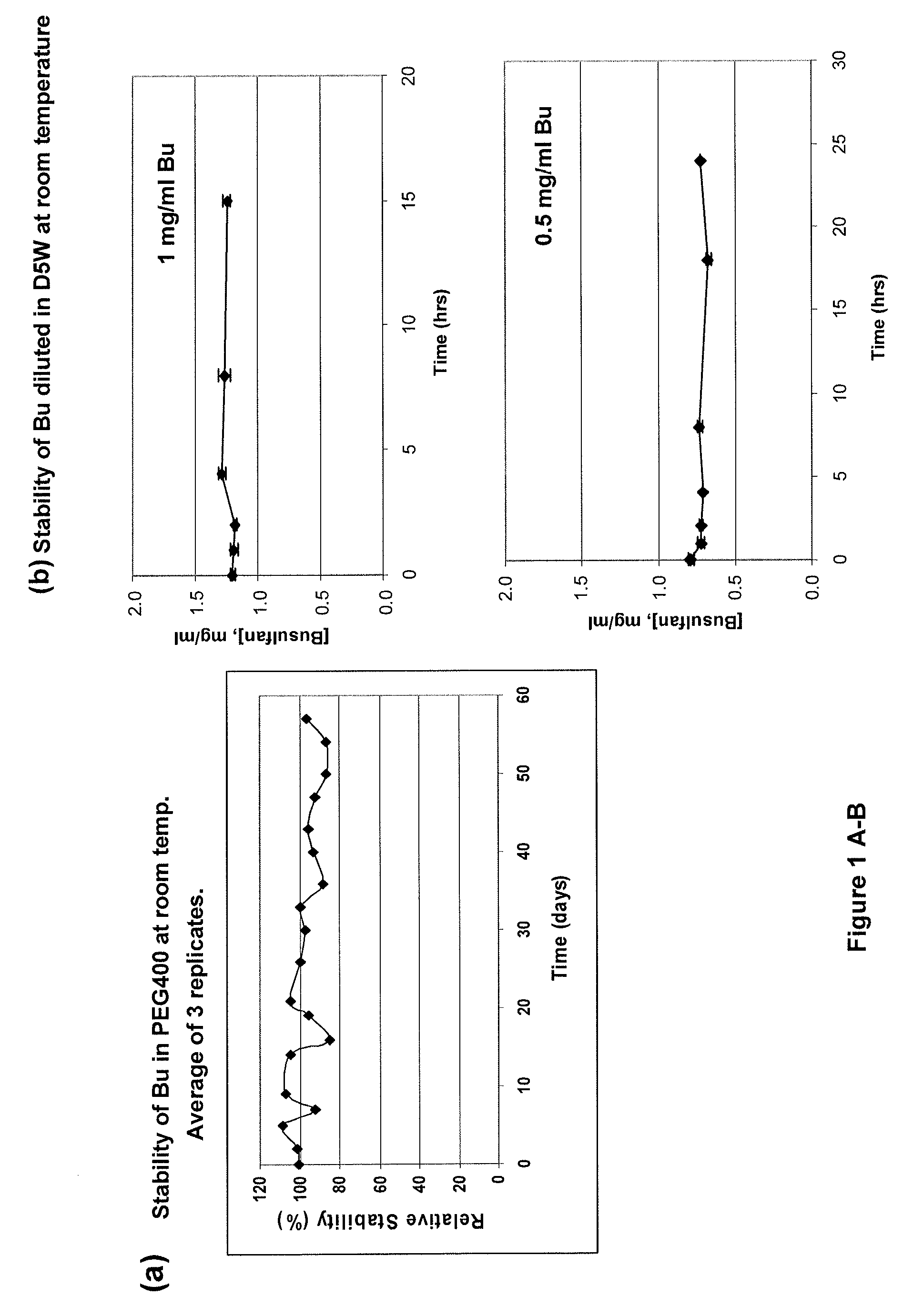
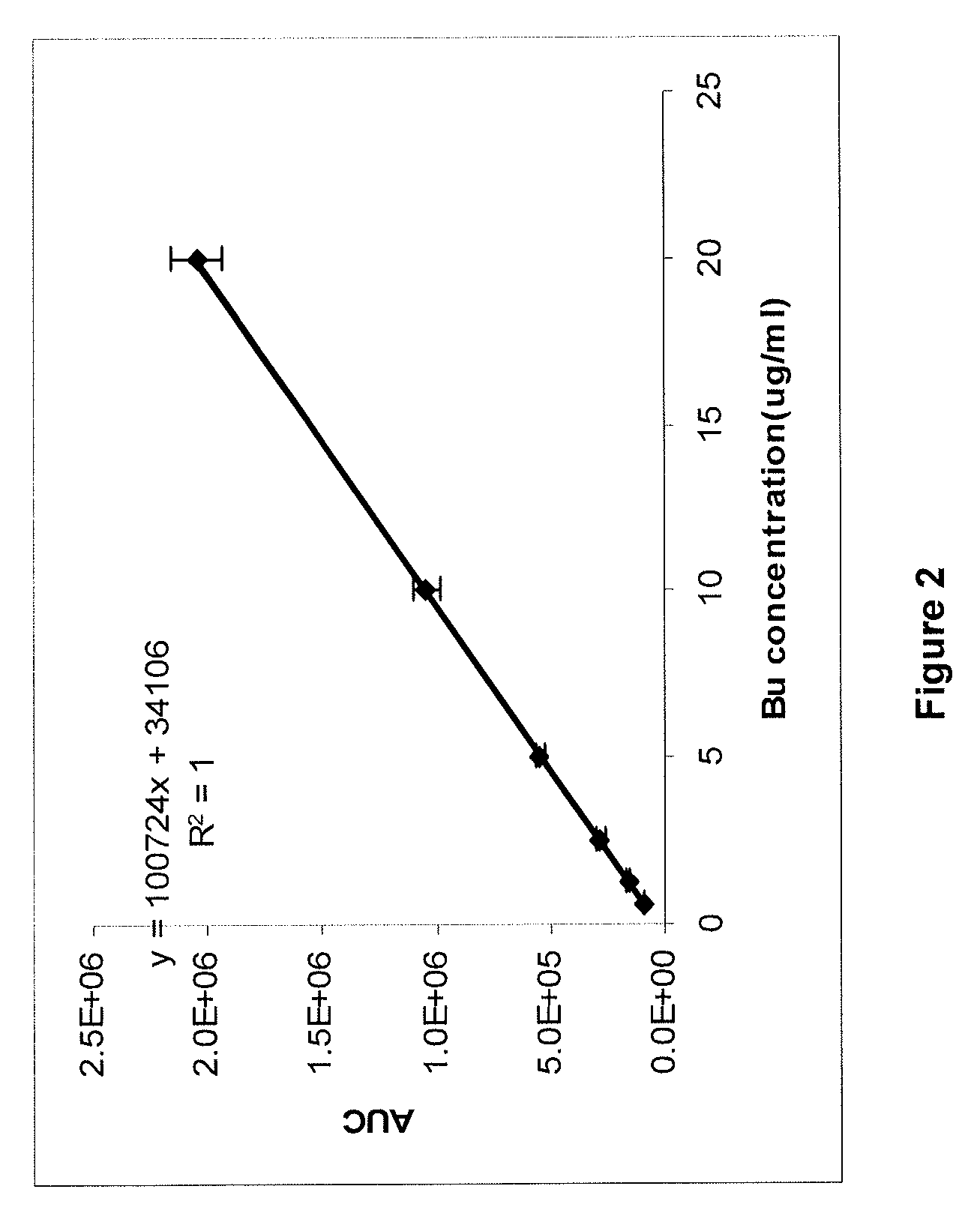
ActiveUS20120277249A1Improve solubilityImprove stabilityAntibacterial agentsOrganic active ingredientsOrganic solventAutoimmune disease
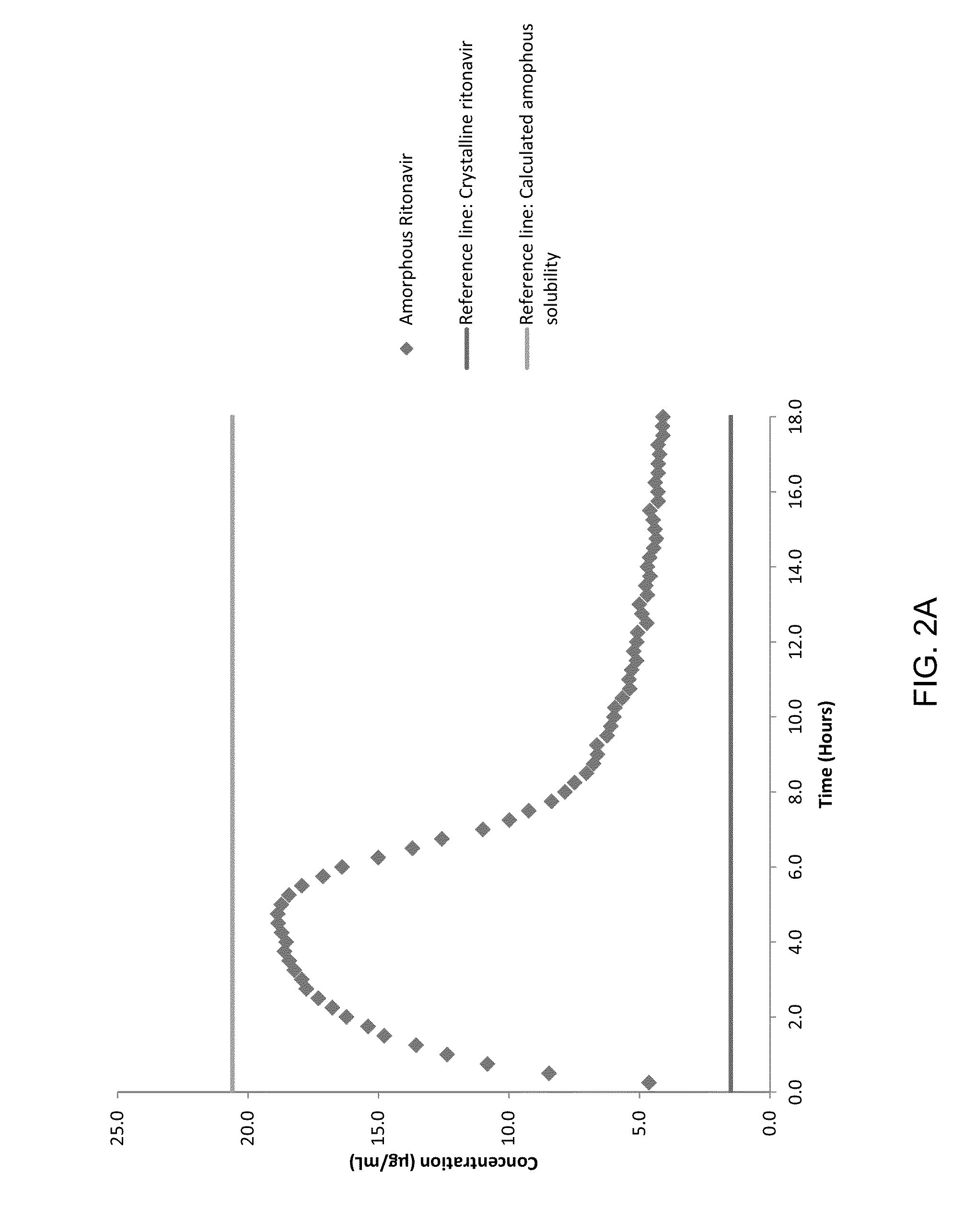
There may be provided compositions of lipophilic pharmaceutical agents with improved solubility and stability. For example, there may be provided a non-aqueous composition that comprises a lipophilic pharmaceutical agent, and an amphiphilic polymeric solvent such as PEG400 but essentially free of organic solvents and non-solubilized particles. The composition may be further diluted with a desired aqueous diluent such as an infusion fluid for parenteral administration to a subject such as a human. The compositions may be useful for the treatment for diseases or conditions that are sensitive to lipophilic agents, such as infectious diseases, malignant or autoimmune diseases.
Owner:GREENJAY THERAPEUTICS INC
Formulation and manufacturing process for coenzyme Q10 soft gel capsules
InactiveUS6855733B2Improve solubility and stabilityPromote absorptionBiocidePeptide/protein ingredientsSoft gel capsuleCo enzyme q10
Owner:SOFT GEL TECHNOLGIES
Derivatives of GLP-1 analogs
InactiveUS20060199763A1Improve solubilityImprove stabilityBiocideOrganic detergent compounding agentsSURFACTANT BLENDSubstituent
The present invention relates to a pharmaceutical composition comprising a GLP-1 derivative having a lipophilic substituent; and a surfactant.
Owner:KNUDSEN LISELOTTE +7
Formulation and manufacturing process for Coenzyme Q10 soft gel capsules
InactiveUS20050037066A1Improve solubility and stabilityPromote absorptionBiocidePeptide/protein ingredientsManufacturing technologyMedicine
A soft gelatine capsule formulation improved manufacturing of Coenzyme Q10, comprising Coenzyme Q10 in a thixotropic gelatine carrier capable of admixing without heating with Coenzyme Q10, and capable of keeping Coenzyme Q10 in suspension at ambient temperature.
Owner:SOFT GEL TECHNOLGIES
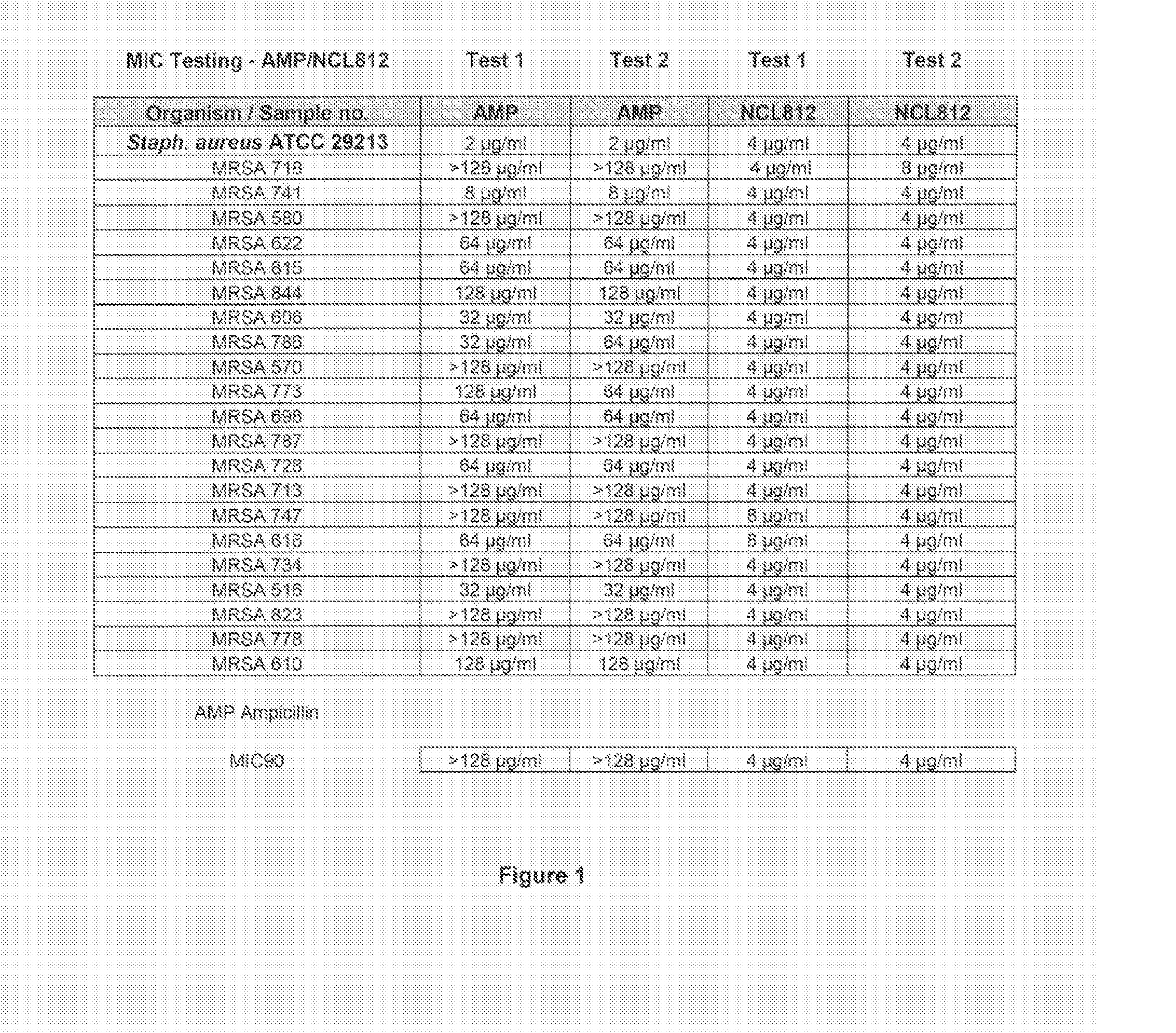
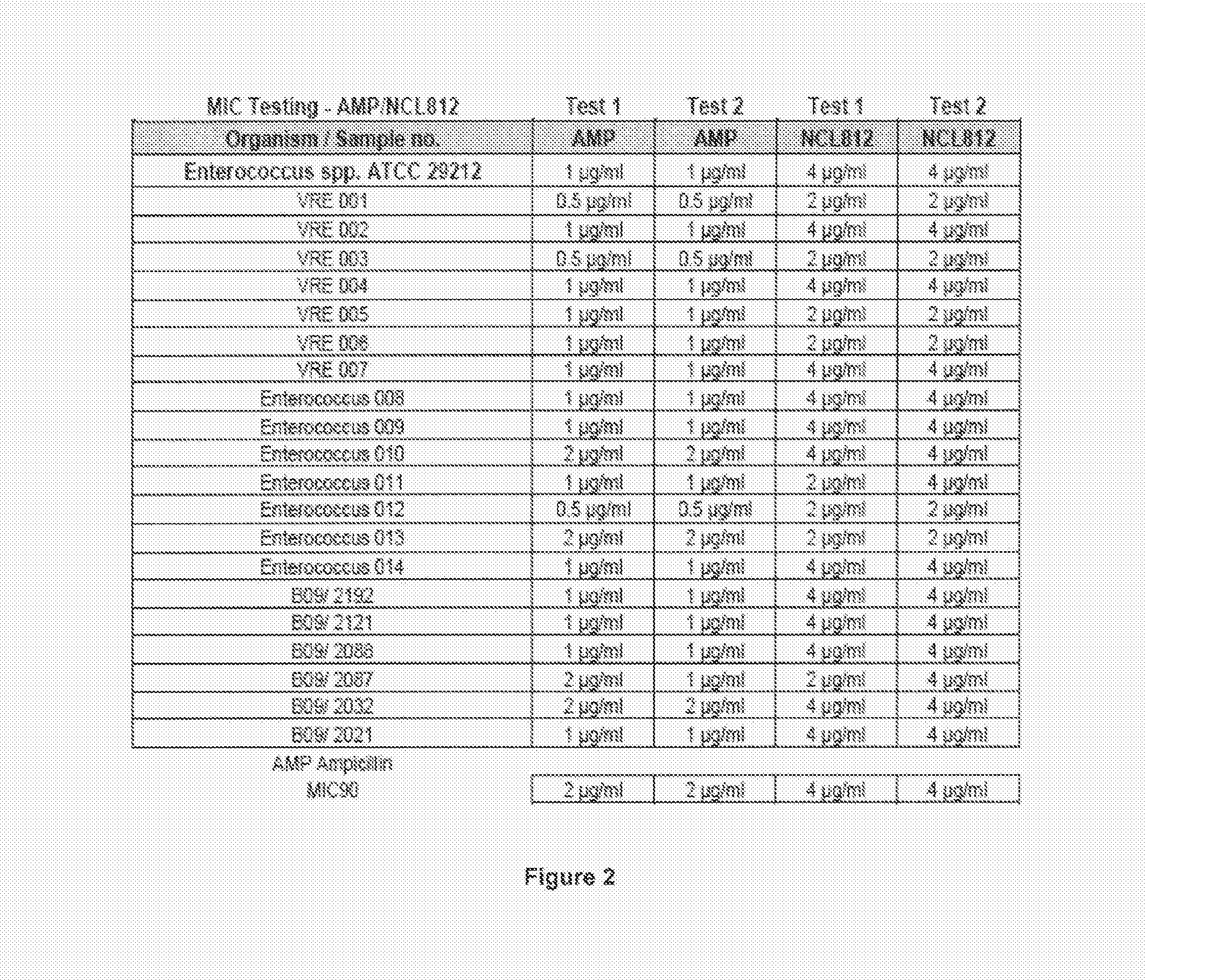
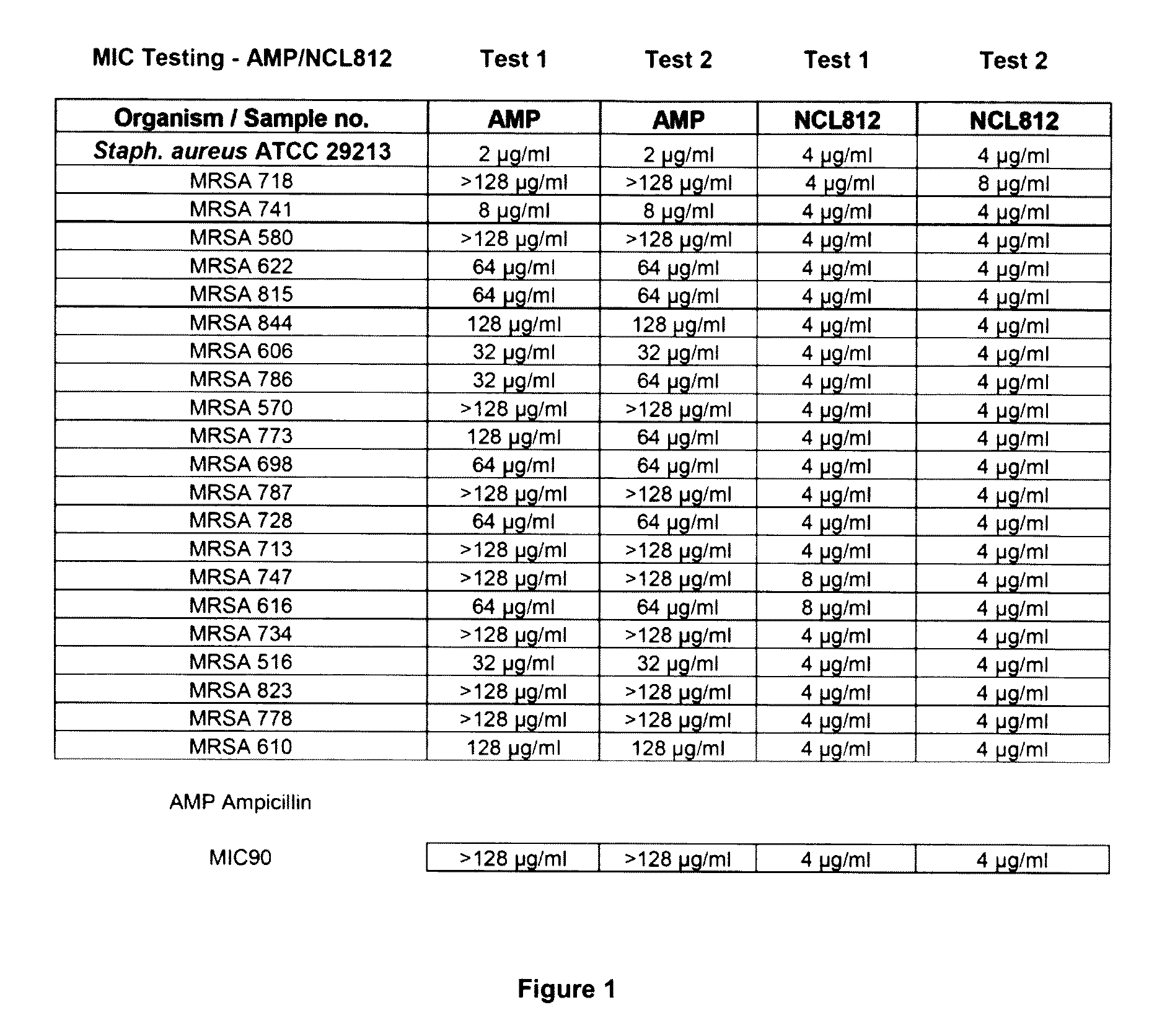
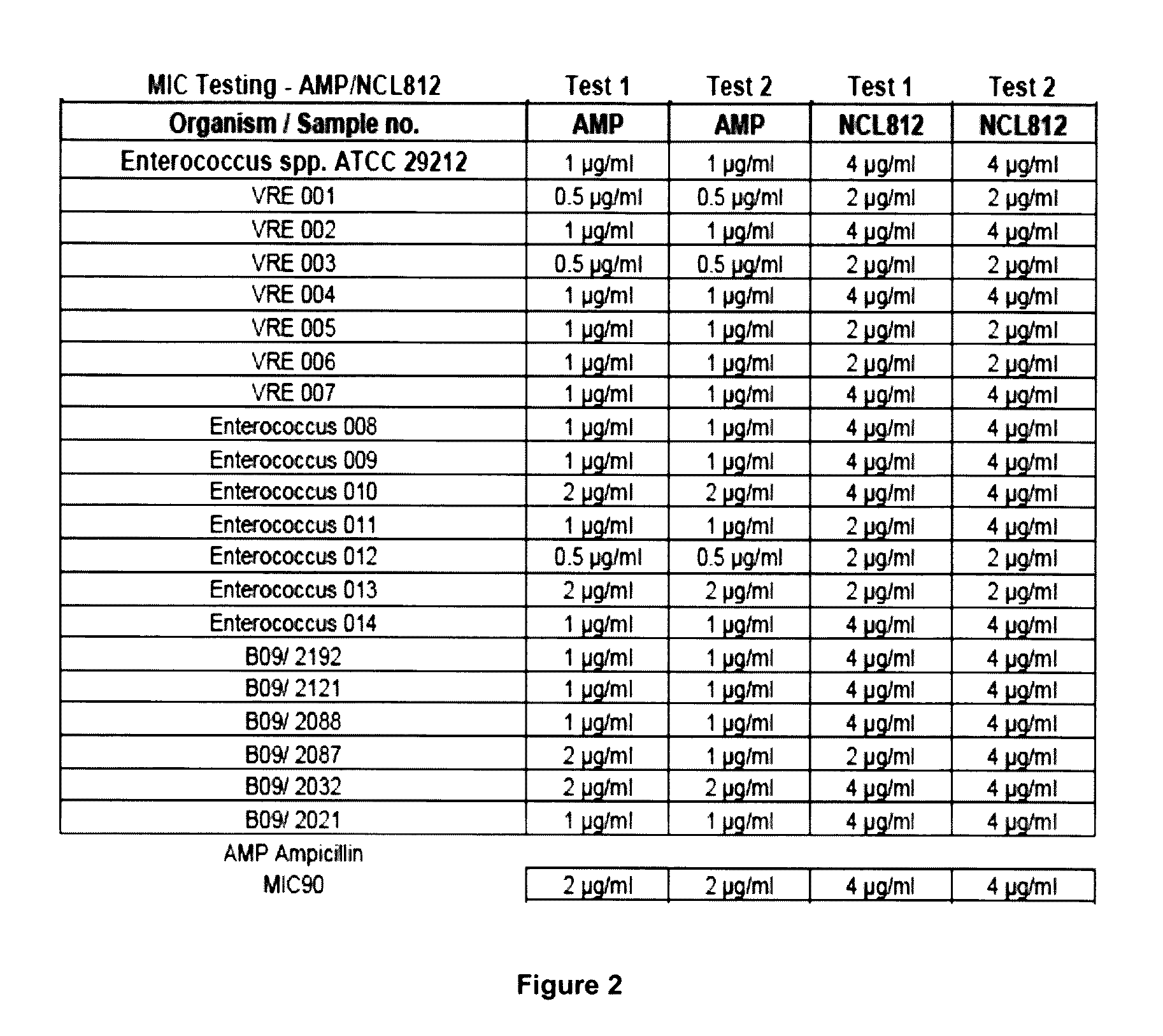
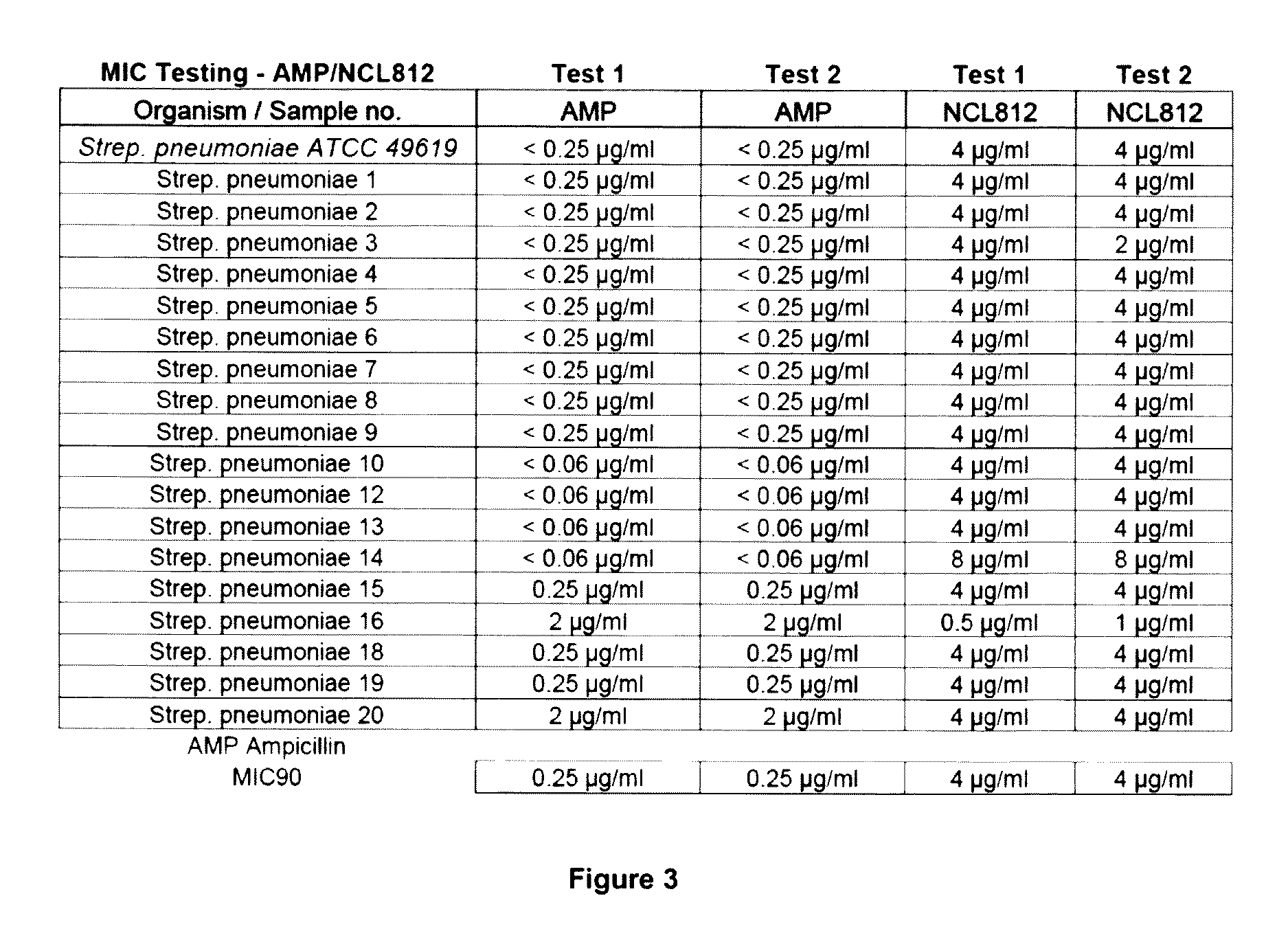
Methods for treating bacterial infections
ActiveUS20160058717A1Improve solubility and stabilityEnhance drug exposureAntibacterial agentsBiocideMedicine
Owner:NEOCULI PTY LTD
Stable solutions of sparingly soluble actives
InactiveUS20110020440A1Stable pharmaceutical compositionComposition is stableBiocideAmide active ingredientsDepressantCombinatorial chemistry
The present invention relates to a stable pharmaceutical composition comprising soft gelatin capsules containing at least one sparingly soluble active drug (singly or in combination with sparingly soluble and / or soluble drugs) and a solvent system, wherein the solvent system comprises of solvent, co-solvent, solubilizer(s), surfactant, aqueous solution of alkali and crystal growth inhibitor. The present invention further relates to process for preparing a stable pharmaceutical composition of sparingly soluble active drug(s) in soft gelatin capsules.
Owner:CADILA PHARMA
Formulation and manufacturing process for Coenzyme Q10 soft gel capsules
InactiveUS20050031681A1Improve solubility and stabilityPromote absorptionBiocidePeptide/protein ingredientsManufacturing technologyMedicine
A soft gelatine capsule formulation improved manufacturing of Coenzyme Q10, comprising Coenzyme Q10 in a thixotropic gelatine carrier capable of admixing without heating with Coenzyme Q10, and capable of keeping Coenzyme Q10 in suspension at ambient temperature.
Owner:SOFT GEL TECHNOLGIES
Cellulose derivatives for inhibiting crystallization of poorly water-soluble drugs
InactiveUS20150004237A1Improve bioavailabilityImprove solubilityPowder deliveryBiocideHydrogen atomWater soluble drug
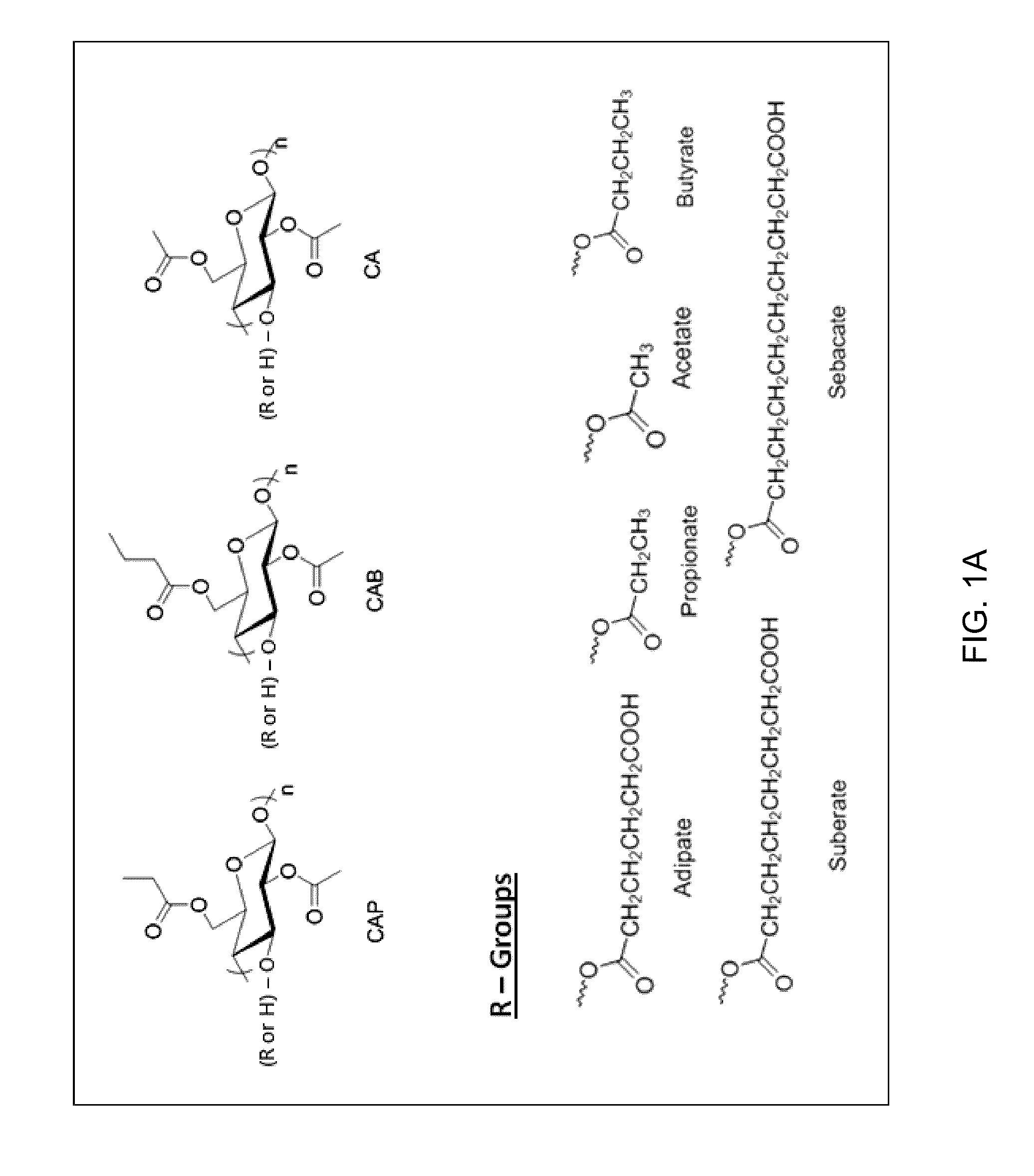
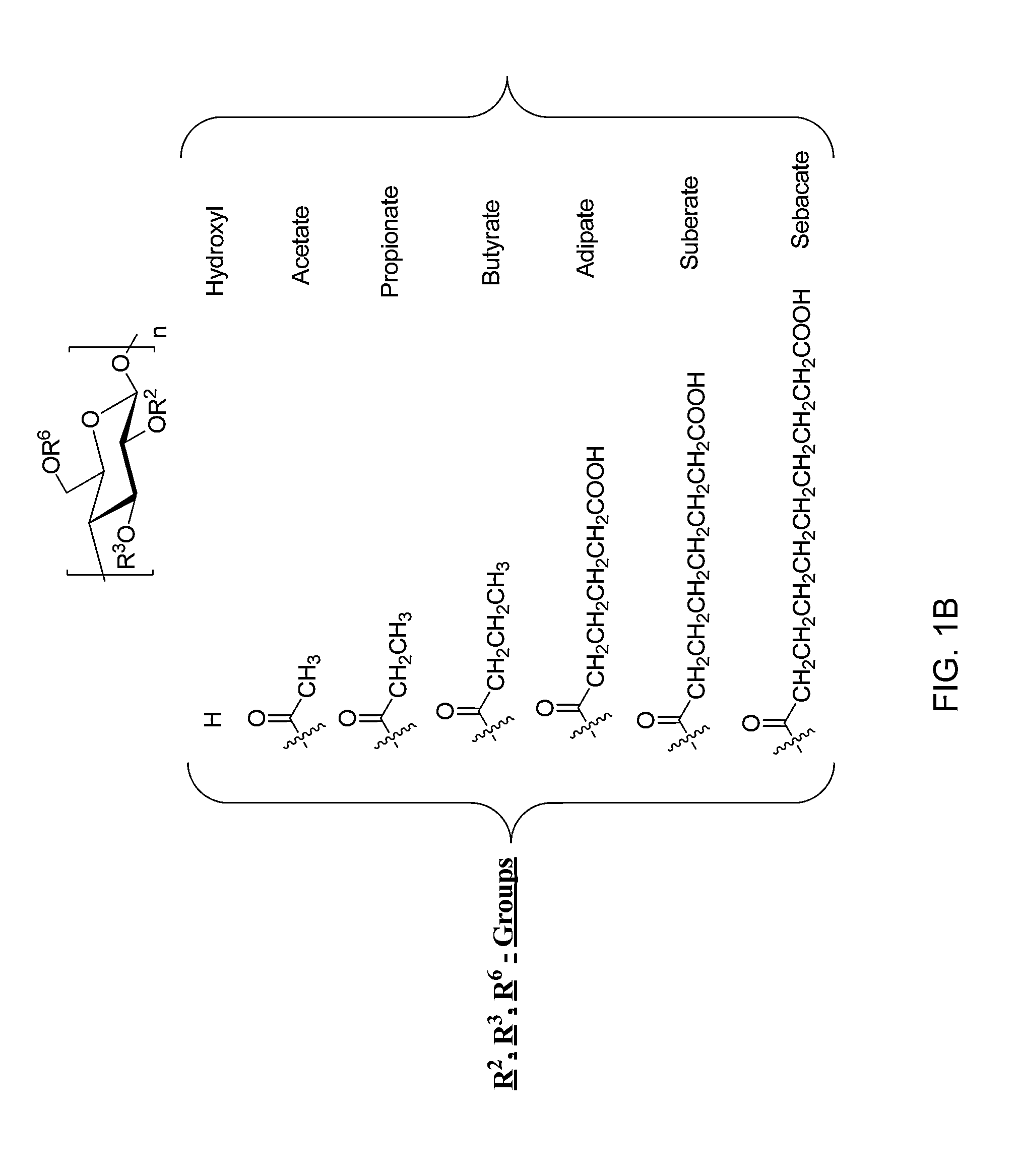
Provided are cellulose esters useful for inhibiting solution crystallization of drugs. Specific polymers include cellulose esters of formula I:wherein n of the ω-carboxyalkanoyl group,is 3, 4, 6, or 8 to provide a ω-carboxyalkanoyl group chosen from succinoyl, glutaroyl, adipoyl, sebacyl, and suberyl groups; and wherein R is chosen from: a hydrogen atom; and an alkanoyl group chosen from acetyl, propionyl, butyryl, valeroyl, hexanoyl, nonanoyl, decanoyl, lauroyl, palmitoyl, and stearoyl groups; wherein there is a total degree of substitution of the alkanoyl group and the ω-carboxyalkanoyl group of at least 2.0; and wherein the polymer comprises m repeating units where n=1 to 1,000,000, or 10 to 100,000, or 100 to 1,000, such as 1 to 6,000. Embodiments further include compositions comprising cellulose esters and poorly water-soluble drugs, which compositions exhibit greater solubility and stability in solution as compared to the drugs alone.
Owner:PURDUE RES FOUND INC +1
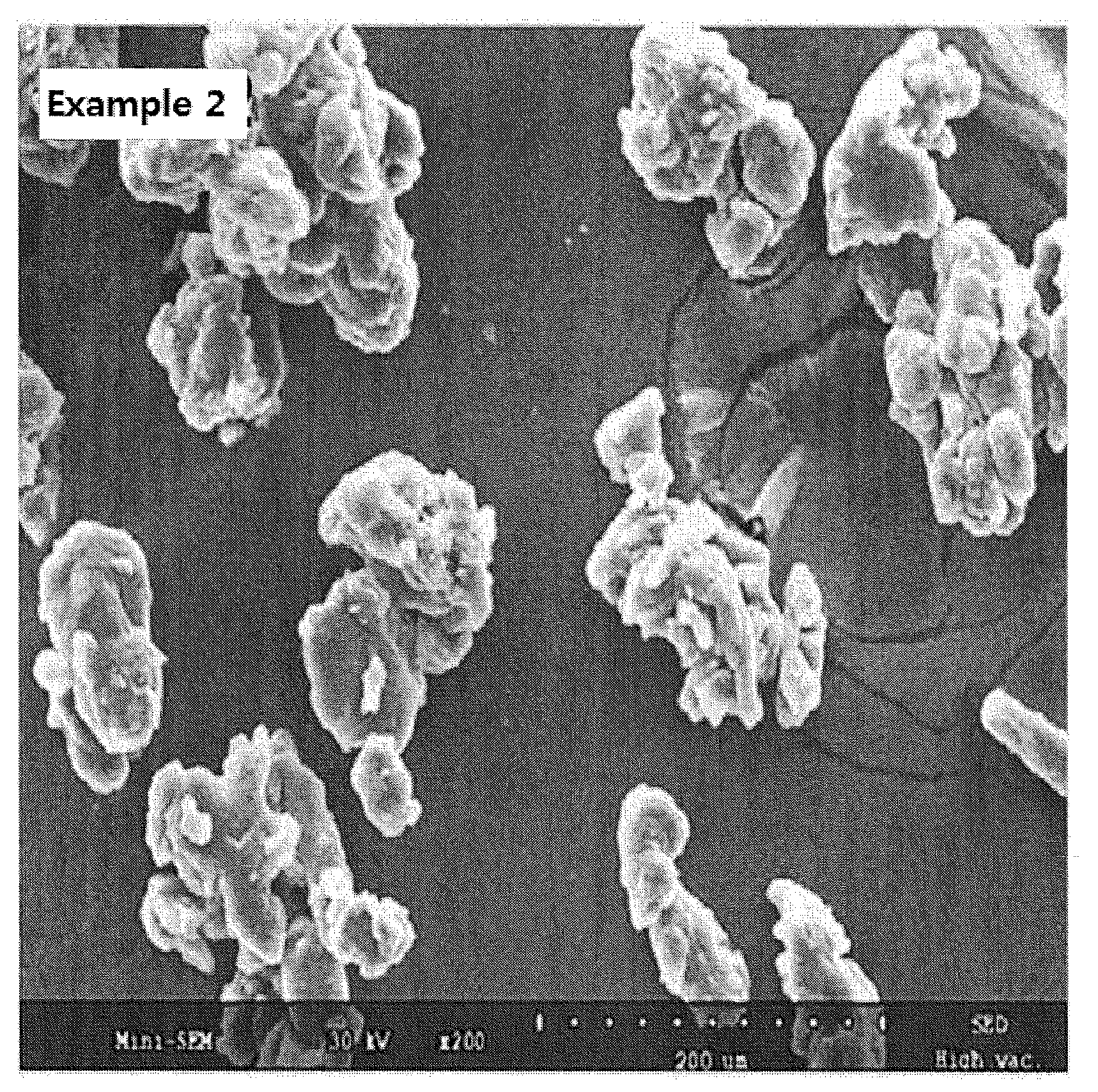
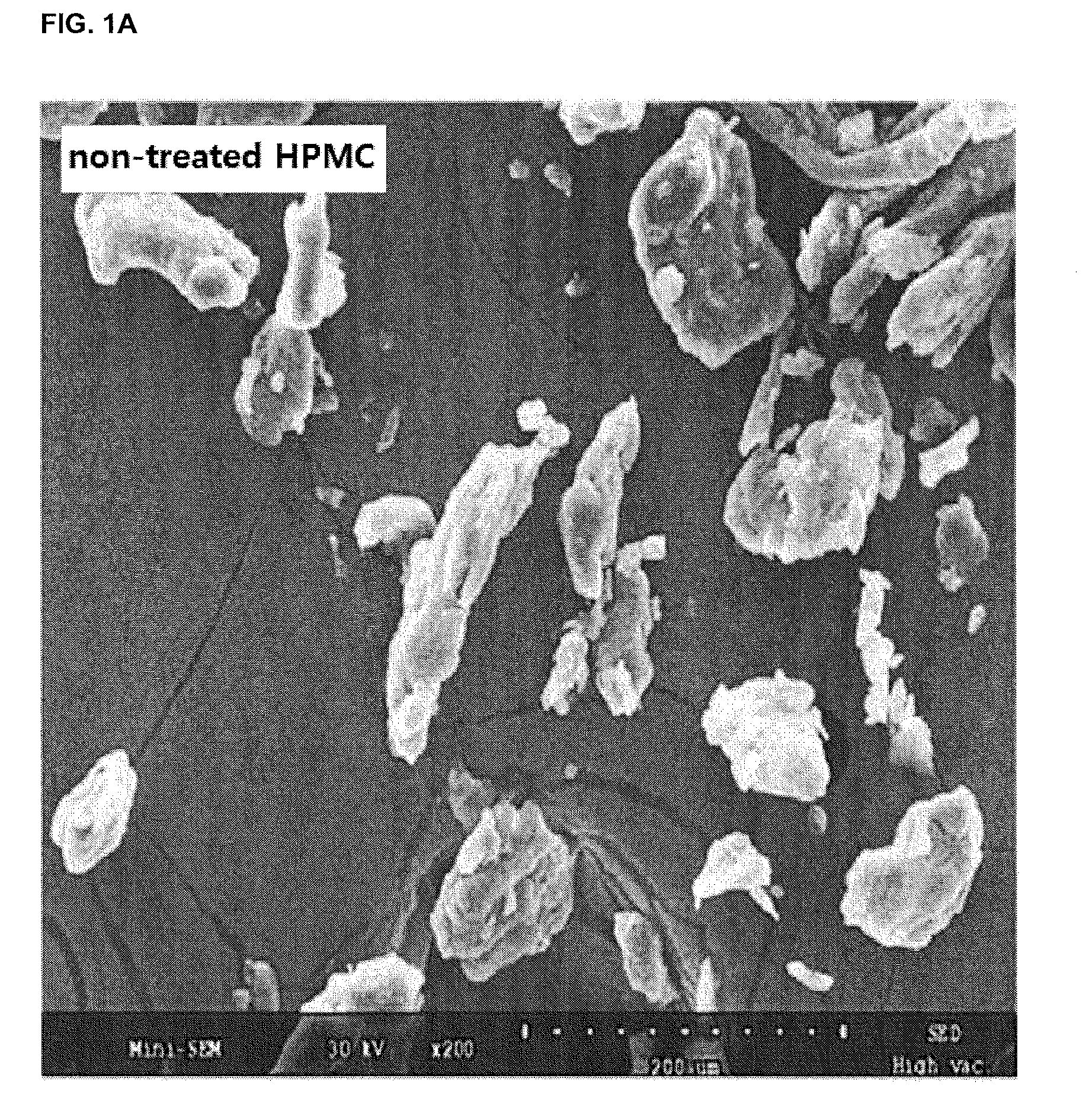
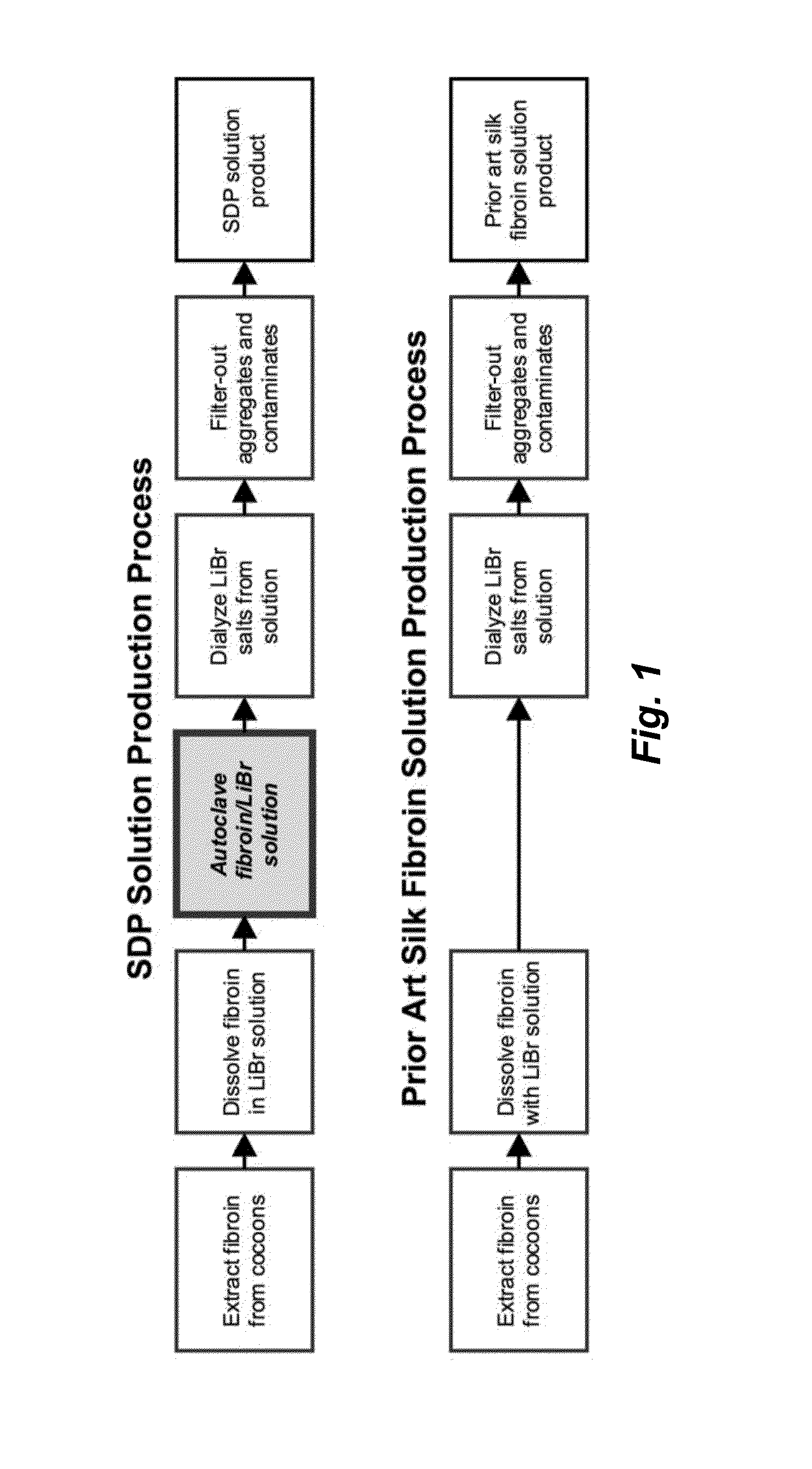
Fibroin-derived protein composition
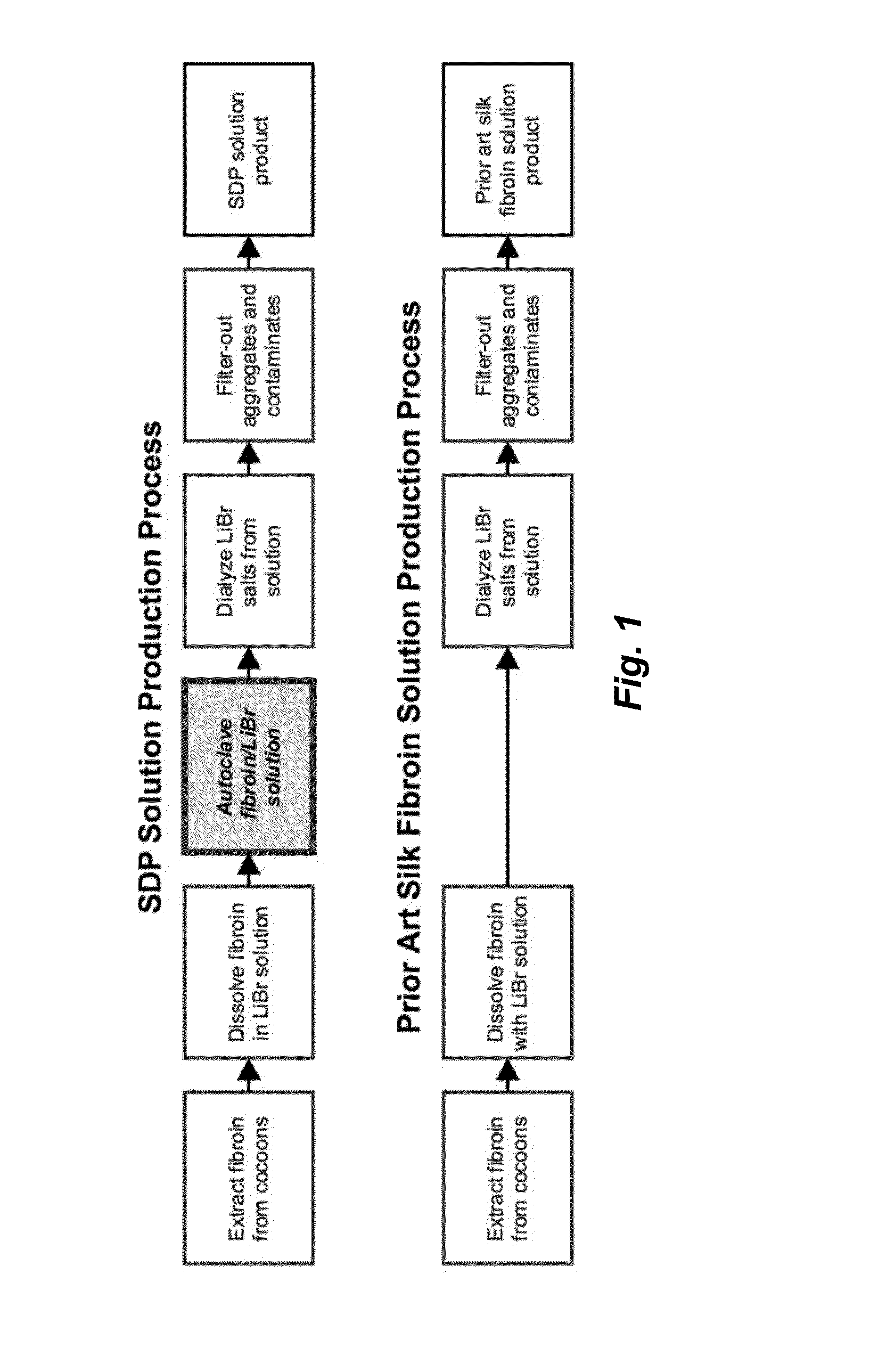
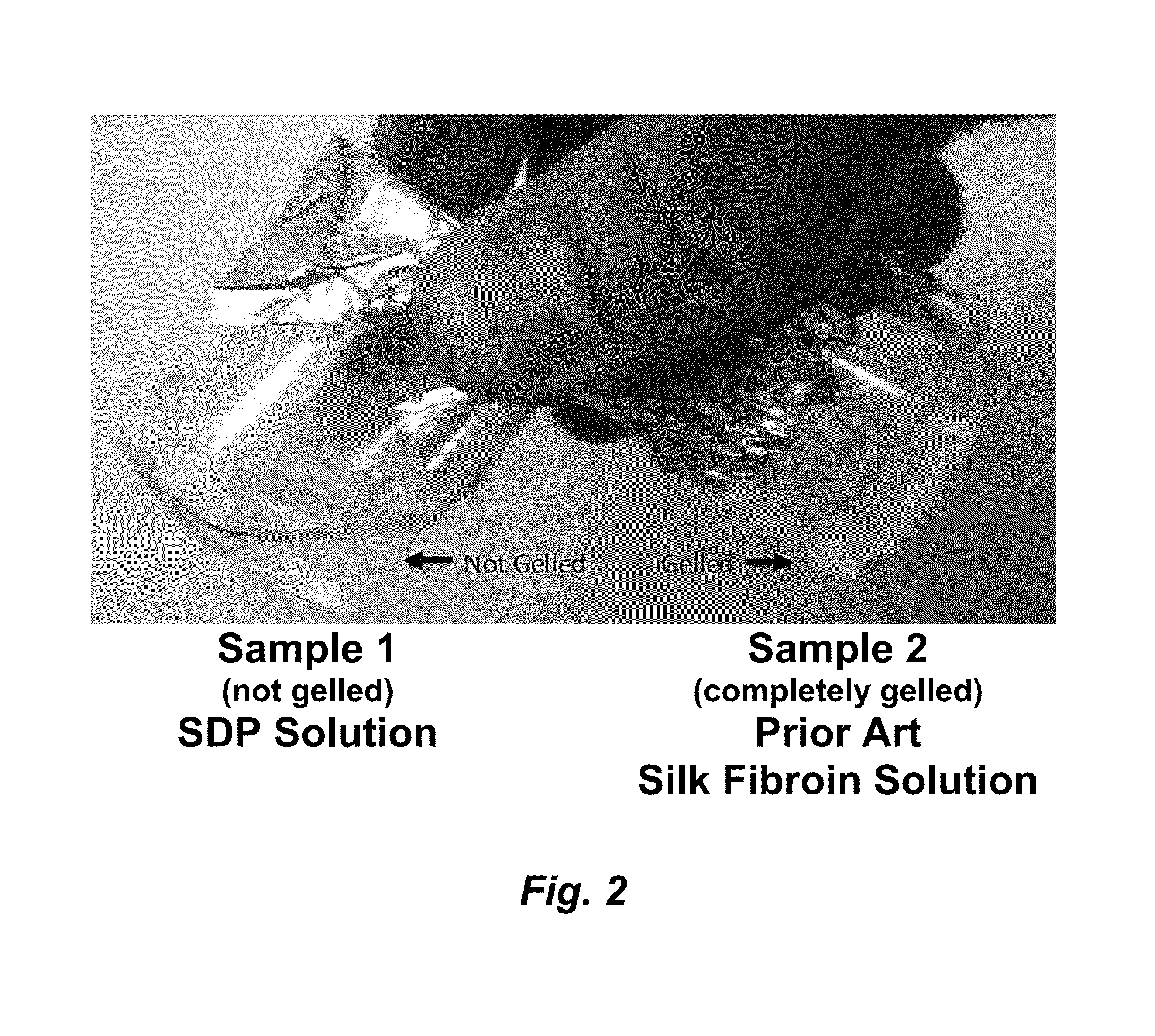
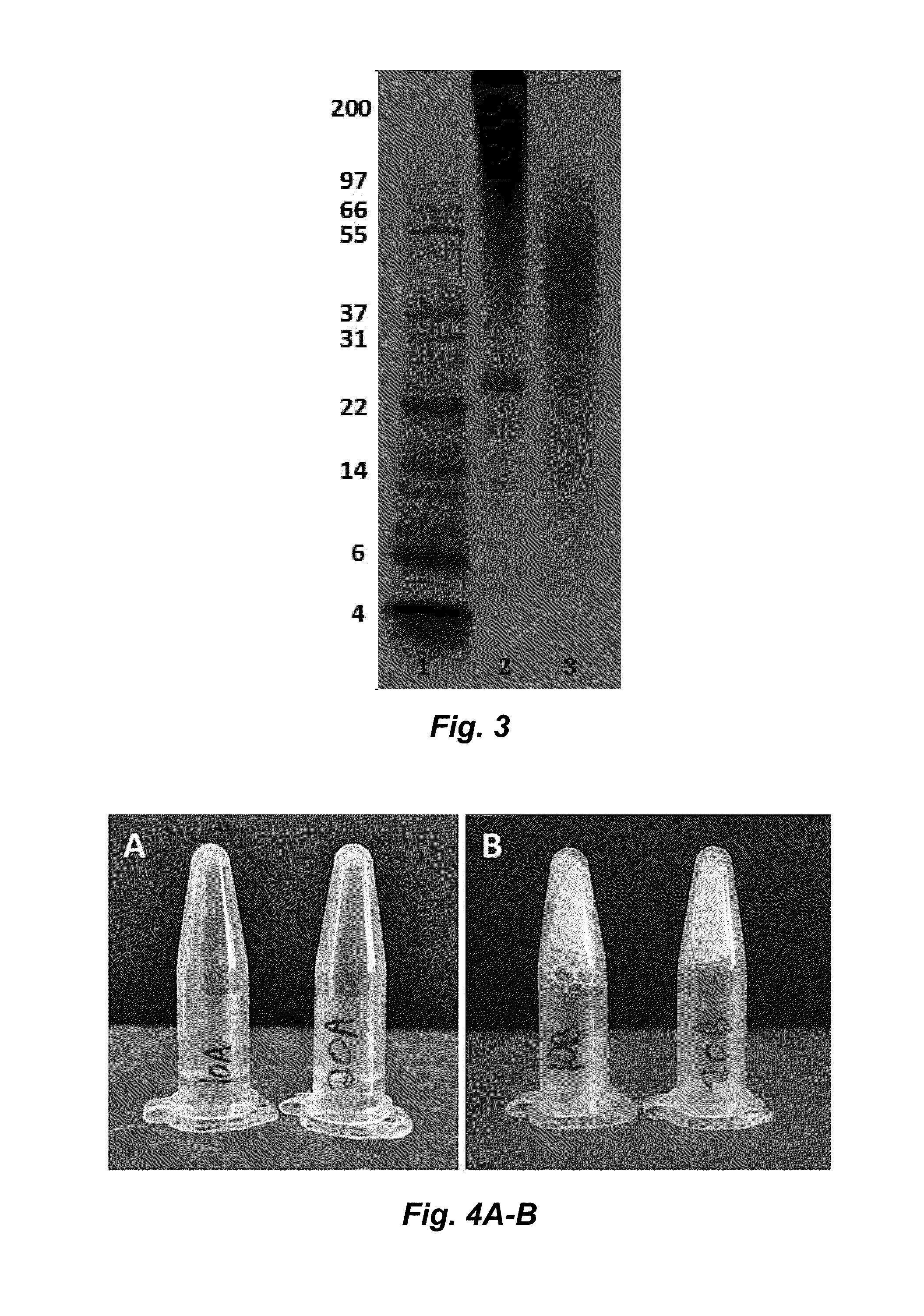
ActiveUS20160096878A1Improve solubilityImprove stabilityConnective tissue peptidesSenses disorderSolubilityProtein composition
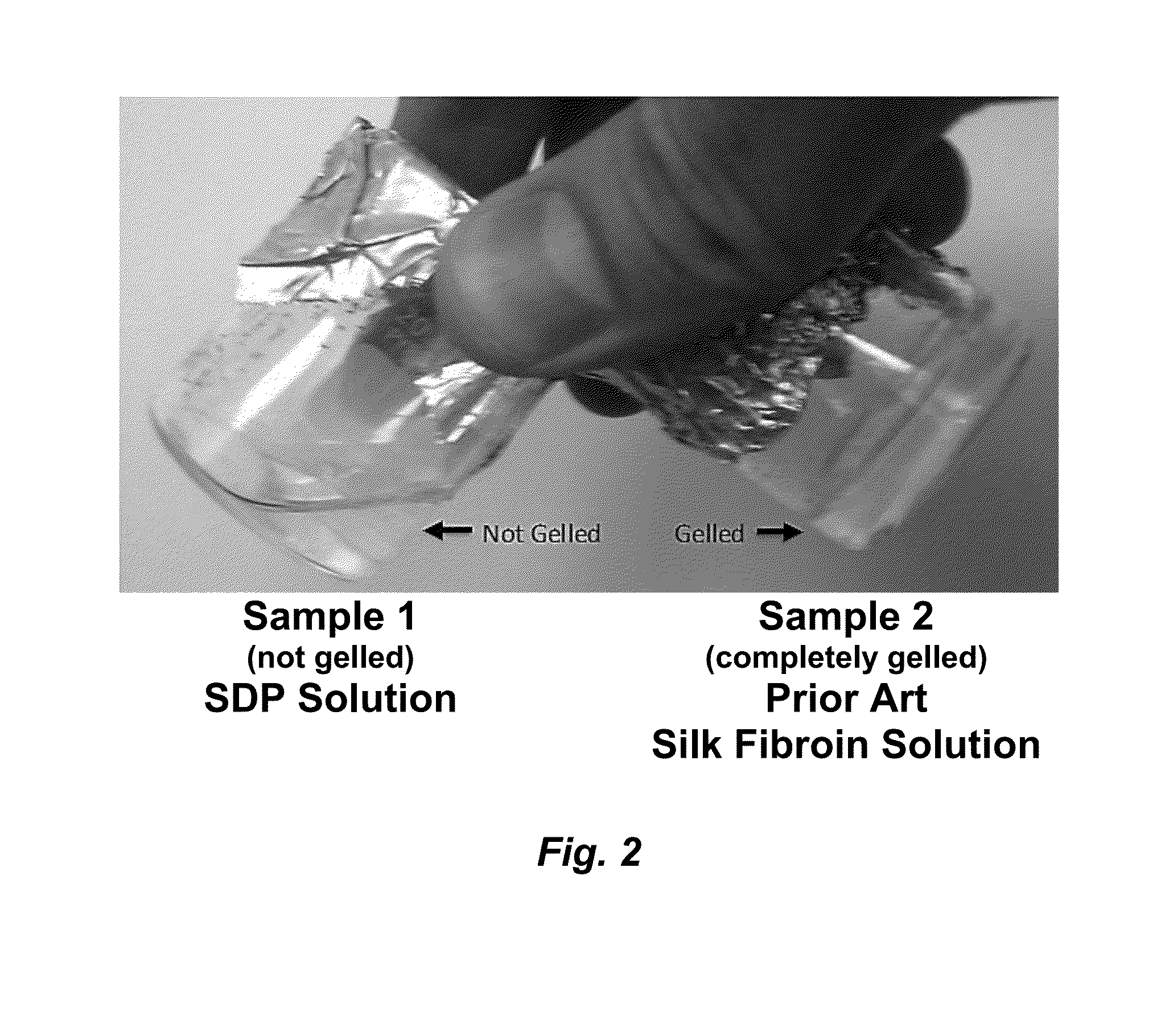
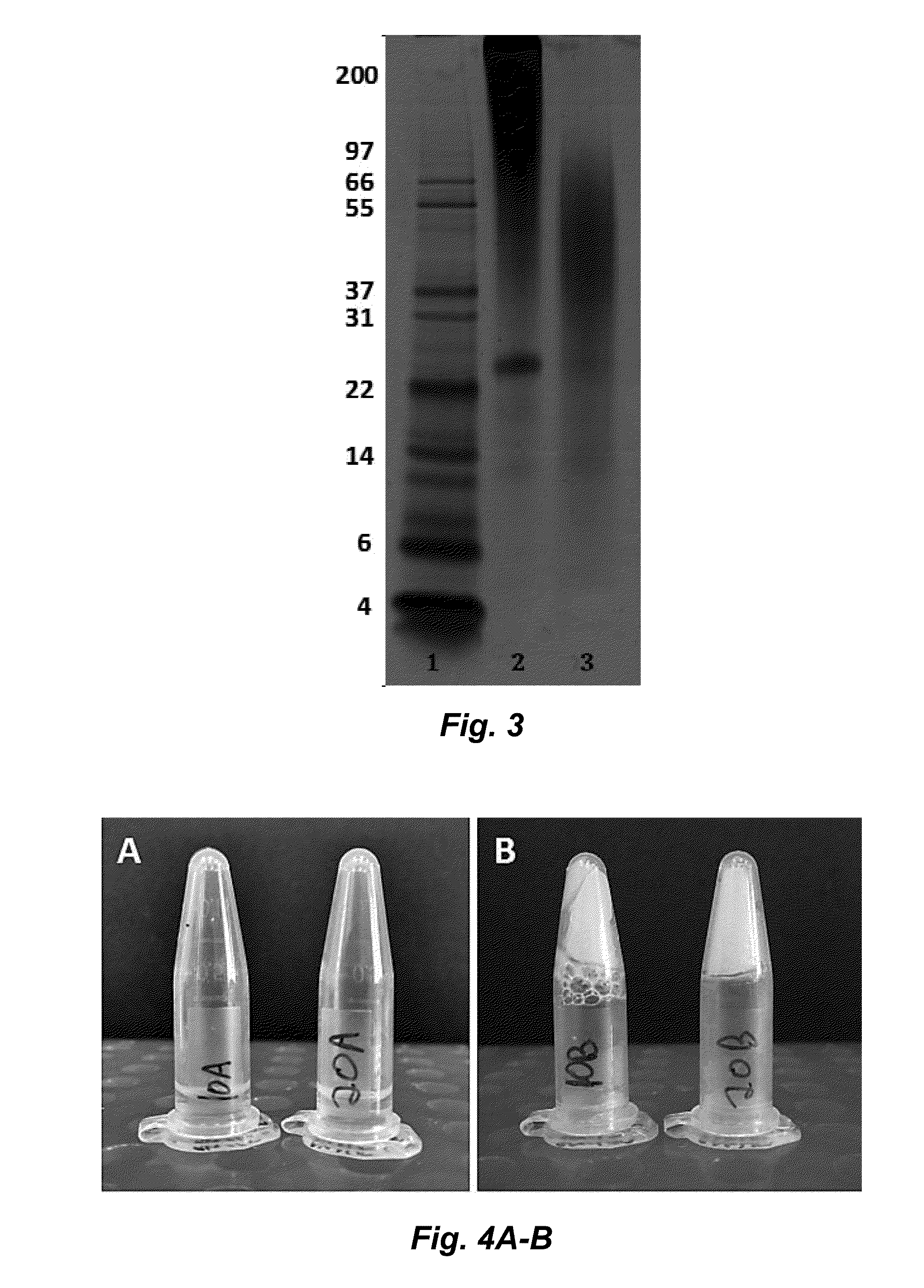
The invention provides a protein composition derived from silk fibroin, which composition possesses enhanced solubility and stability in aqueous solutions. The primary amino acid sequence of native fibroin is modified in the SDP such that cysteine disulfide bonds between the fibroin heavy and fibroin light protein chains are reduced or eliminated. Additionally, the composition can have a serine content that is reduced by greater than 40% compared to native fibroin protein, and the average molecular weight of the SDP is less than about 100 kDa.
Owner:SILK TECH
Prodrugs of cc-a1065 analogs
InactiveUS20090028821A1Improve solubilityGood water solubilityAntibacterial agentsBiocideSolubilityCarbamate
The present invention provides prodrugs of analogs of the anti-tumor antibiotic CC-1065 having a cleavable protective group containing a sulfonic acid containing phenyl carbamate, in which the protecting group confers enhanced water solubility upon the prodrug, and in which the prodrug also has a moiety, such as a sulfide or a disulfide, that can conjugate to a cell binding reagent such as an antibody, and for the therapeutic use of such prodrug and conjugates, and for processes for preparing such prodrugs and conjugates.
Owner:IMMUNOGEN INC
Parenteral formulations of lipophilic pharmaceutical agents and methods for preparing and using the same
ActiveUS9364433B2Improve solubility and stabilityPromote solubilizationAntibacterial agentsBiocideOrganic solventAutoimmune disease
Owner:GREENJAY THERAPEUTICS INC
Hydrophilic drug and hydrophobic drug co-loaded target composite nano-drug preparation and preparation method thereof
InactiveCN102397256AExpand the scope of applicationImprove solubility and stabilityPowder deliveryOrganic active ingredientsCarbon nanotubePaclitaxel
The invention belongs to the fields of biomedical medical polymer materials, nano-biotechnology and pharmaceutics, and relates to a target carbon nano-tube drug carrier and a preparation method thereof, specifically to a hydrophilic drug and hydrophobic drug co-loaded target composite nano-drug preparation and a preparation method thereof. According to the present invention, high-purity carbon nano-tube is adopted as a carrier, wherein the carbon nano-tube is subjected to pegylation; the carbon nano-tube is conjugated with a Fab fragment of HER-2 specific monoclonal antibody, wherein the Fab fragment of the HER-2 specific monoclonal antibody is adopted as the target molecule; two anticancer agents of hydrophilic adriamycin and hydrophobic paclitaxel are loaded on the target carbon nano-tube drug carrier; with the targeting guidance effect of the Fab fragment of the HER-2 specific monoclonal antibody, the enrichment of the two anticancer agents with two different action mechanisms in breast carcinoma tissues with HER-2 positive expression can be achieved, the complementary tumor cell killing ability is provided, the therapeutic effect is increased, the use amount of the drug and the toxic and side-effects are reduced, and the generation of the drug resistance can be delayed.
Owner:FUDAN UNIV
Fulvestrant compositions
InactiveUS20170027958A1Improve solubilityImprove stabilityOrganic active ingredientsOintment deliverySubject matterVitamin
The inventive subject matter provides ready to inject fulvestrant compositions with improved solubility and stability, and methods for preparing the same. Contemplated compositions include fulvestrant at a concentration of greater than 100 mg / ml, and maintain degradation of the fulvestrant at a level of less than 5 wt % when stored over at least three months at 25° C.
Owner:THEMIS MEDICARE LTD +1
Process Of Preparing A Stabilized And Solubilized Formulation Of Sirolimus Derivatives
InactiveUS20130039951A1Improve solubilityImprove stabilityBiocidePowder deliveryWater solubleSolvent
Provided is a process for preparing a solubilized and stabilized formulation of a sirolimus derivative, which comprises the steps of a dissolving a sirolimus derivative in a solvent, and bring a solution of the sirolimus derivative into contact with a water-soluble carrier to disperse the sirolimus derivative in the water-soluble carrier, and a formulation of a sirolimus derivative with improved solubility and stability as prepared by the preparation process as above.
Owner:DONG A PHARMA
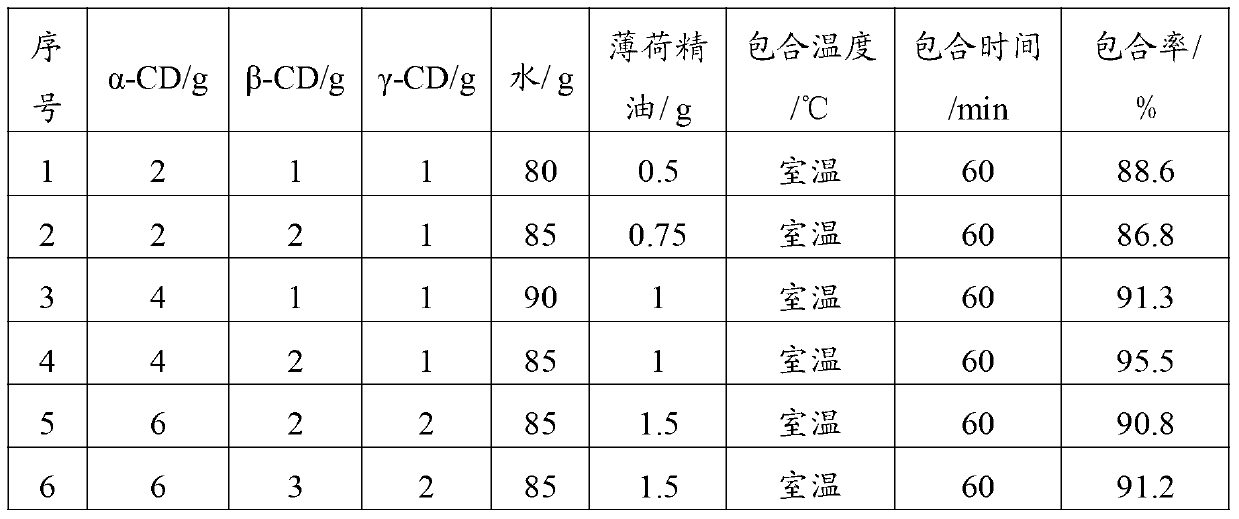
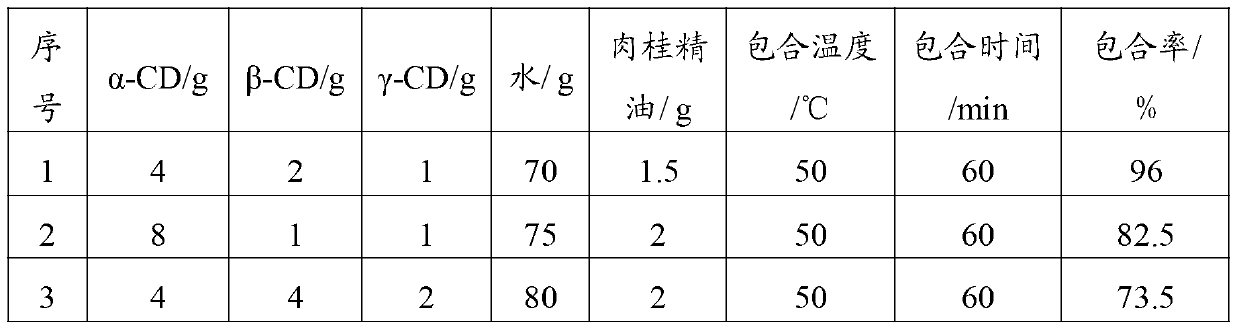
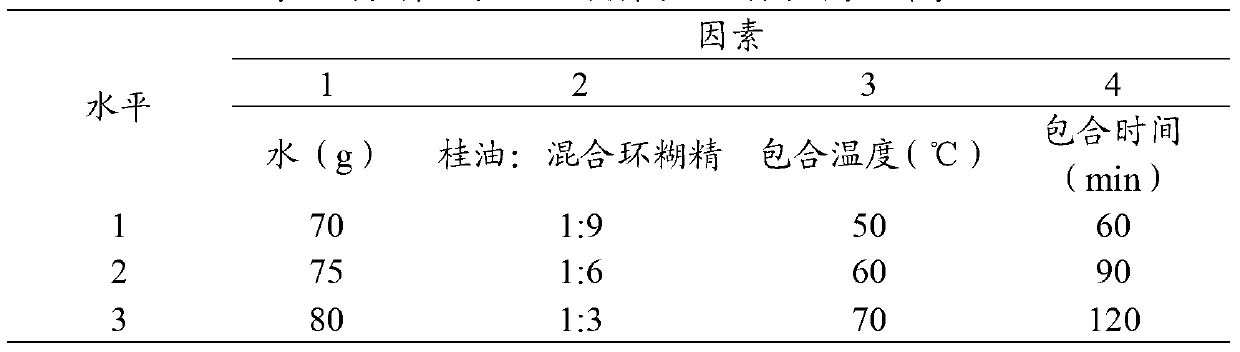
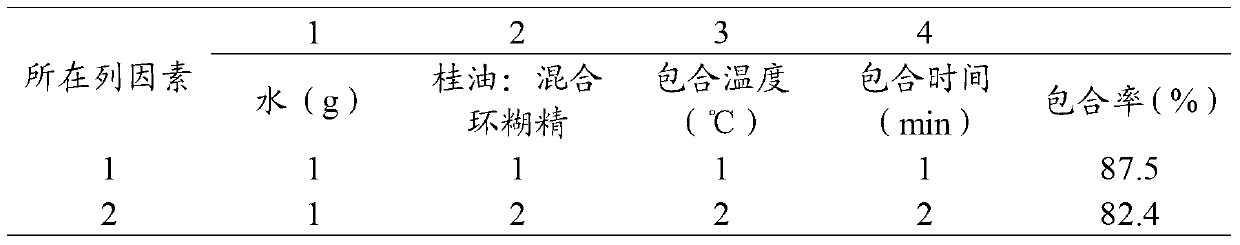
Peppermint essential oil and mixed cyclodextrin inclusion compound and preparation method thereof
InactiveCN103271991AHigh inclusion rateImprove solubility and stabilityCosmetic preparationsSenses disorderPeppermintsChemistry
The invention belongs to the technical field of medicines and in particular relates to a peppermint essential oil and mixed cyclodextrin inclusion compound and a preparation method thereof. According to the technical scheme, the mixture of alpha-cyclodextrin, beta-cyclodextrin and gamma-cyclodextrin and the peppermint essential oil are included in water, so that the inclusion rate is improved, the solubility and stability are improved, and an organic solvent is avoided in the production process.
Owner:江苏丰园生物技术有限公司
Fibroin-derived protein composition
ActiveUS9394355B2Improve solubility and stabilityImprove stabilityConnective tissue peptidesSenses disorderSolubilityProtein composition
The invention provides a protein composition derived from silk fibroin, which composition possesses enhanced solubility and stability in aqueous solutions. The primary amino acid sequence of native fibroin is modified in the SDP such that cysteine disulfide bonds between the fibroin heavy and fibroin light protein chains are reduced or eliminated. Additionally, the composition can have a serine content that is reduced by greater than 40% compared to native fibroin protein, and the average molecular weight of the SDP is less than about 100 kDa.
Owner:SILK TECH
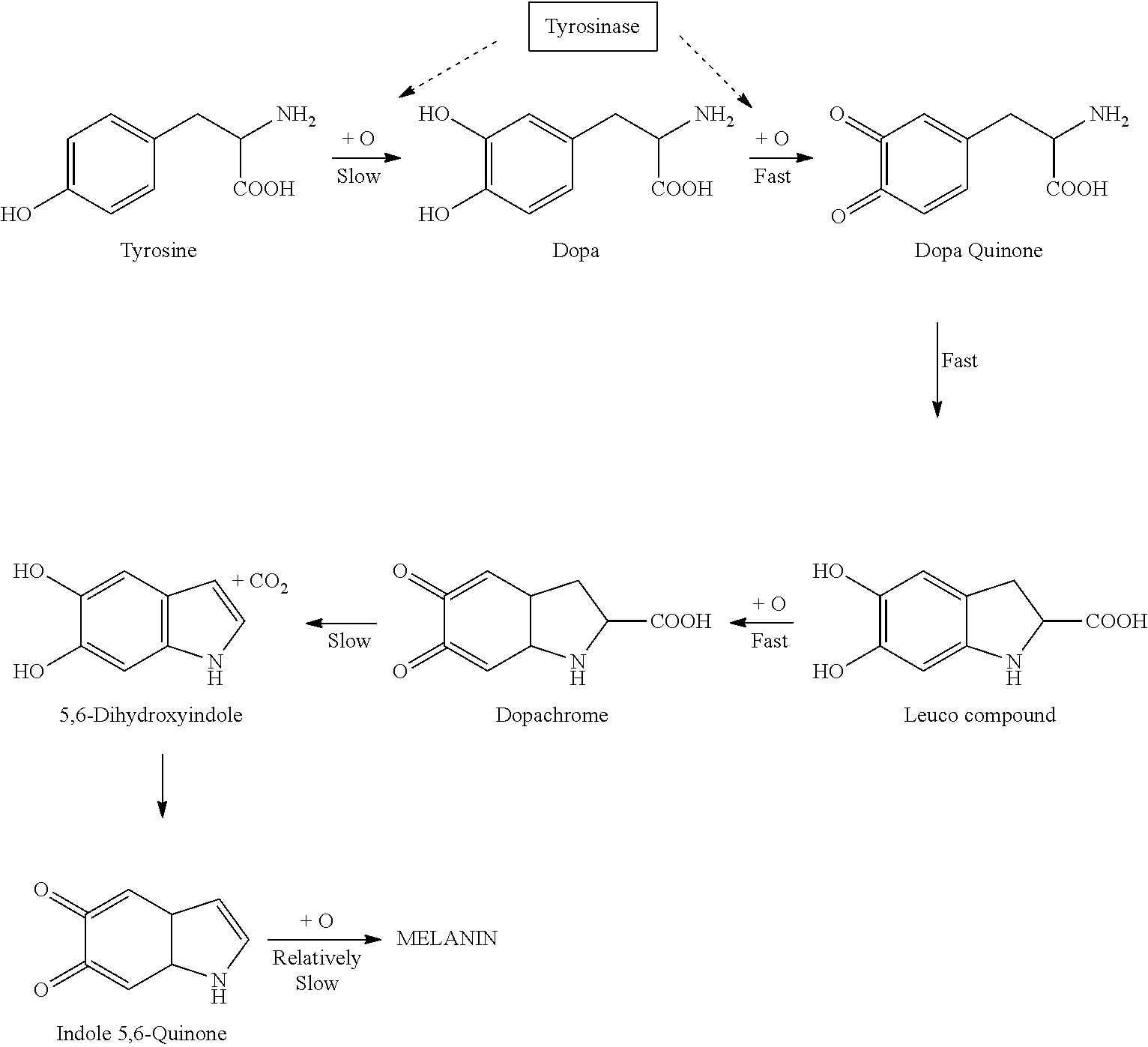
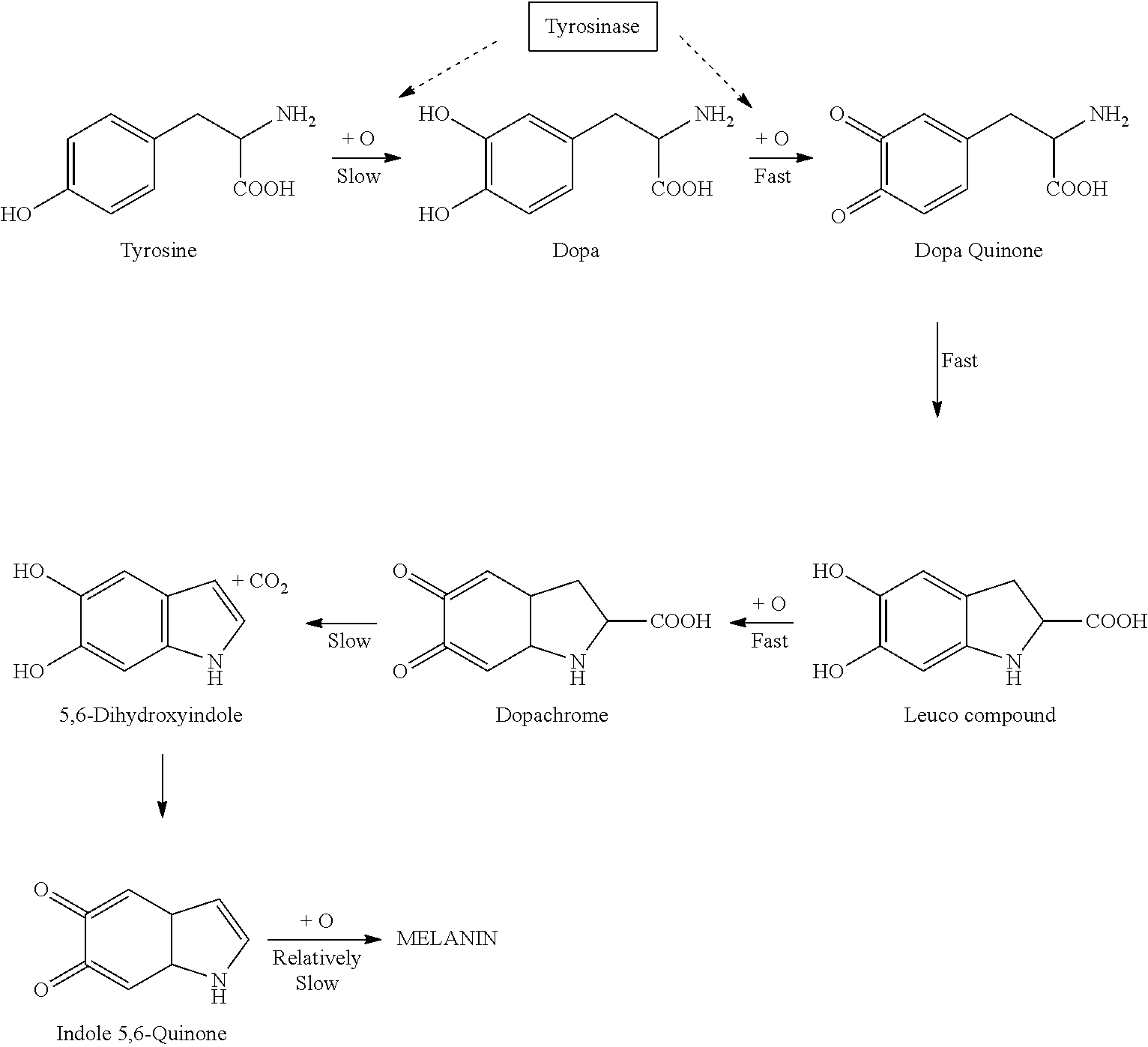
Melanin Promoting Topical Composition
ActiveUS20120134938A1Skin to darkenLess stableCosmetic preparationsToilet preparationsChemical compositionReady to use
The invention provides sunless tanning compositions for application to skin comprising an effective amount of at least one dopamine precursor, an effective amount of curcumin and a dermatologically acceptable carrier. Methods for preparation of said compositions are disclosed. When applied to the skin, the compositions may be used darken skin.
Owner:N V PERRICONE
Creatine phosphate sodium freeze-dried preparation and method for preparing same
InactiveCN101732263AImprove solubility and stabilityFast dissolutionPowder deliveryOrganic active ingredientsChemistryPharmaceutical preservatives
The invention relates to a creatine phosphate sodium freeze-dried preparation and a method for preparing the same. The mass ratio of creatine phosphate sodium to an excipient in the creatine phosphate sodium freeze-dried preparation is 1: 0.05 to 1: 20. The method for preparing the creatine phosphate sodium freeze-dried preparation comprises the steps of: adding active carbon to a solution containing the creatine phosphate sodium and the excipient, stirring, removing pyrogen, then filtering, decarburizing, filtering again, degerming, and packing filtrate in a split charging manner to obtain the creatine phosphate sodium freeze-dried preparation after freeze drying. The preparation, indicated by solubility tests, influencing factor tests, accelerating tests and long term stability tests, has the advantages of quick solution velocity and good stability, compared with the conventional creatine phosphate sodium powdery preparation. In addition, the freeze-dried preparation is produced in a relatively closed environment and the security of products is more reliable.
Owner:杨军
Melanin promoting topical composition
ActiveUS8414869B2Less stableImprove solubility and stabilityCosmetic preparationsToilet preparationsChemical compositionDopamine
The invention provides sunless tanning compositions for application to skin comprising an effective amount of at least one dopamine precursor, an effective amount of curcumin and a dermatologically acceptable carrier. Methods for preparation of said compositions are disclosed. When applied to the skin, the compositions may be used darken skin.
Owner:N V PERRICONE
Method for preparing high-degree polygonatum polysaccharide spirit
InactiveCN101624562AImprove solubility and stabilityImprove solubilityAlcoholic beverage preparationSolubilityPolygonatum
The invention belongs to the technical field of preparing health wine, in particular to a method for preparing a high-degree polygonatum polysaccharide spirit. The method comprises the followings steps: adding powder of polygonatum polysaccharide in a distilled grain spirit of between 53 and 62 degrees, and blending the spirit with auxiliary materials to achieve good taste; and processing the polygonatum polysaccharide spirit by ultrasonic wave. The method solves the difficulties of the dissolubility of the polygonatum polysaccharide in the spirit and the shelf period stability of the product.
Owner:CHENGDU INST OF BIOLOGY CHINESE ACAD OF S
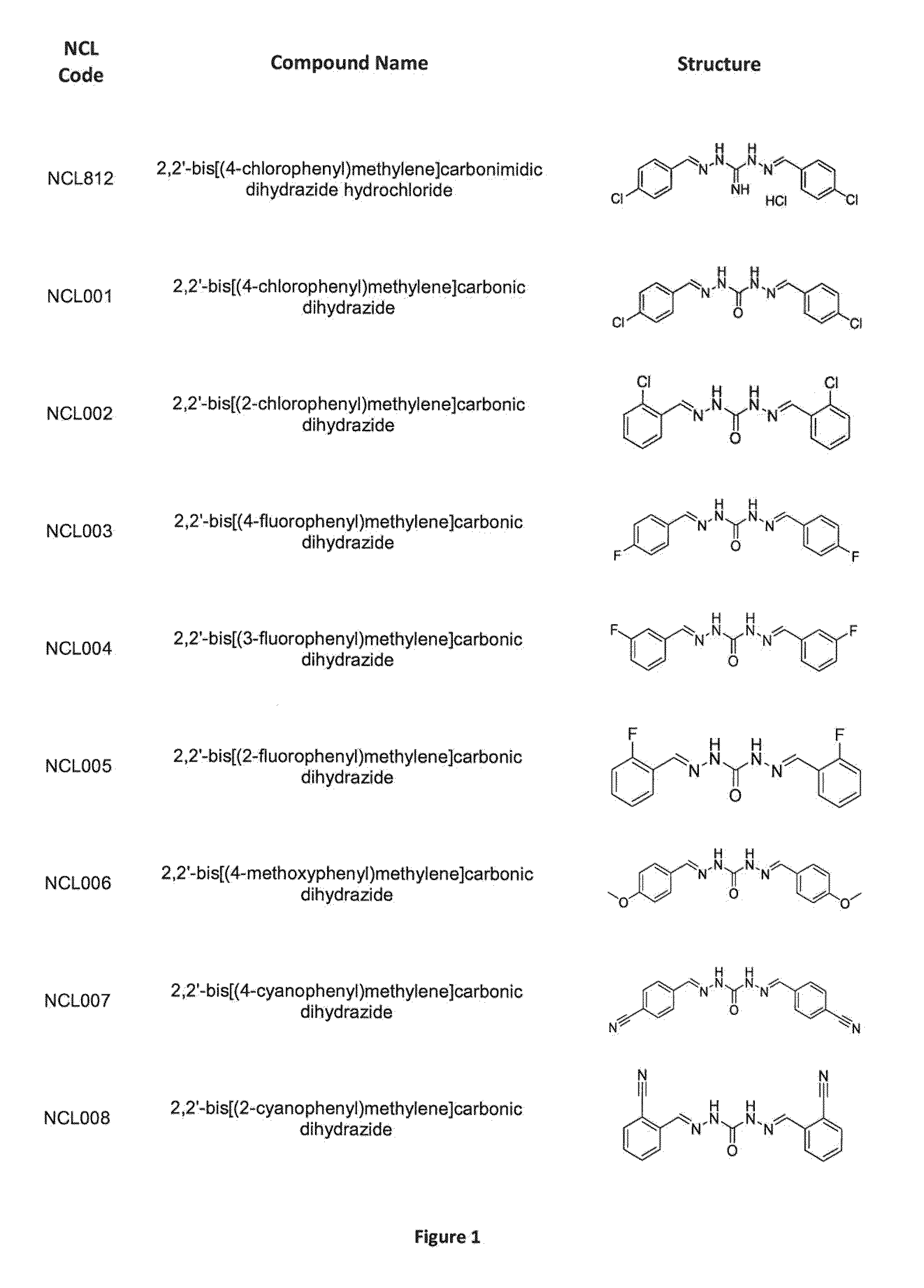
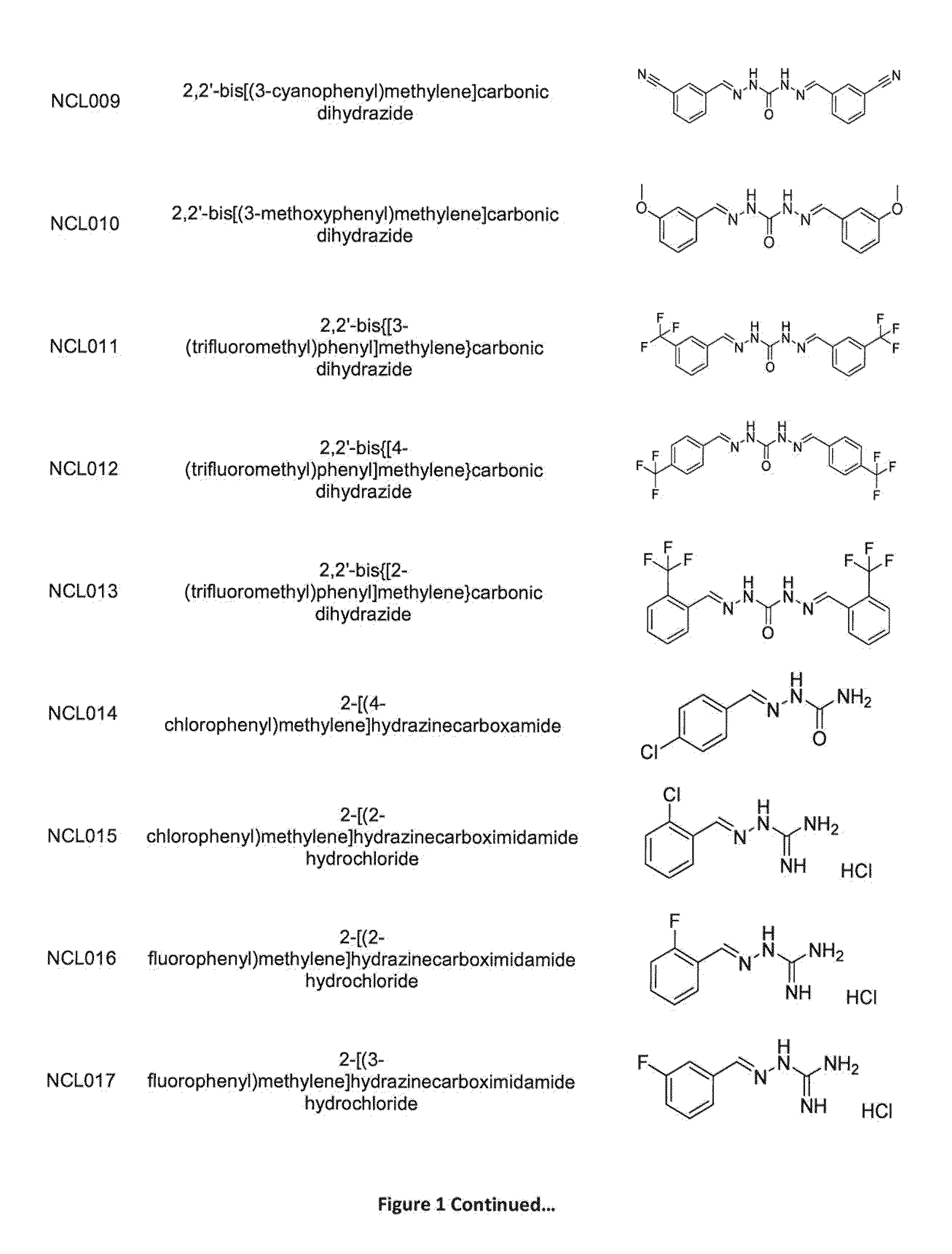
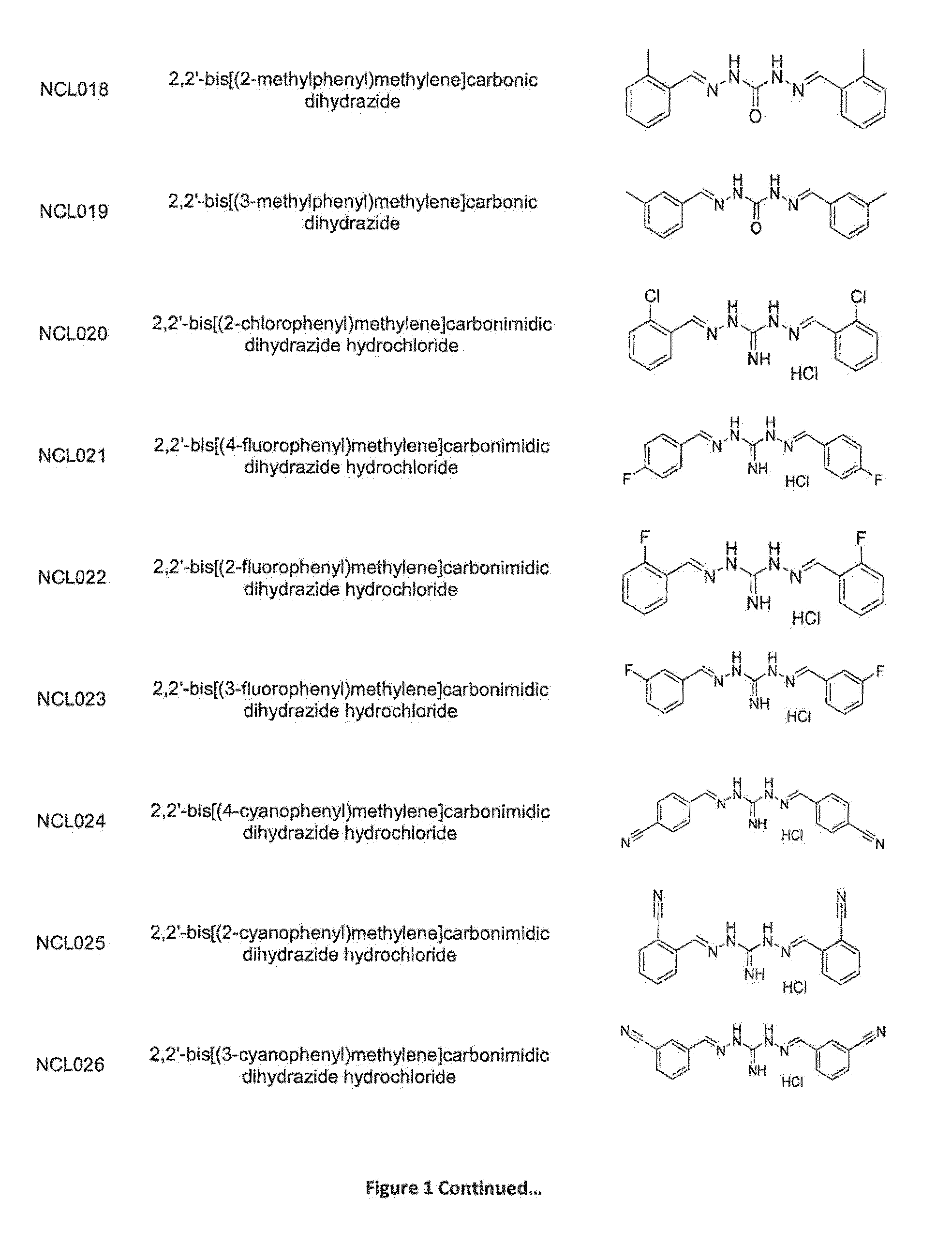
Compounds and methods of treating infections
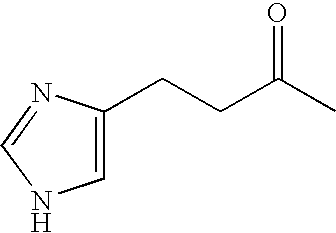
ActiveUS20160075665A1Improve solubility and stabilityEnhance drug exposureAntibacterial agentsBiocideMedicineMedical device
The invention provides compounds of Formula (I), and methods of treating or preventing a bacterial infection in a subject using a compound of Formula (I). The invention also provides the use of a compound of Formula (I) in the manufacture of a medicament for the treatment of a bacterial infection in a subject. The invention further provides a medical device when used in a method of treating or preventing a bacterial infection in a subject and to a medical device comprising the composition of the invention.
Owner:NEOCULI
Cinnamon essential oil-cyclodextrin mixture inclusion compound and preparation method thereof
InactiveCN103271996AHigh inclusion rateImprove solubility and stabilityAntibacterial agentsAntimycoticsChemistrySolubility
The invention belongs to the technical field of medicine, and particularly relates to a cinnamon essential oil-cyclodextrin mixture inclusion compound and a preparation method thereof. The technical scheme, which is implemented by carrying out inclusion on an alpha-cyclodextrin / beta-cyclodextrin / gamma-cyclodextrin mixture and cinnamon essential oil in water, enhances the inclusion rate, increases the solubility and stability, and avoids using an organic solvent in the production process.
Owner:江苏丰园生物技术有限公司
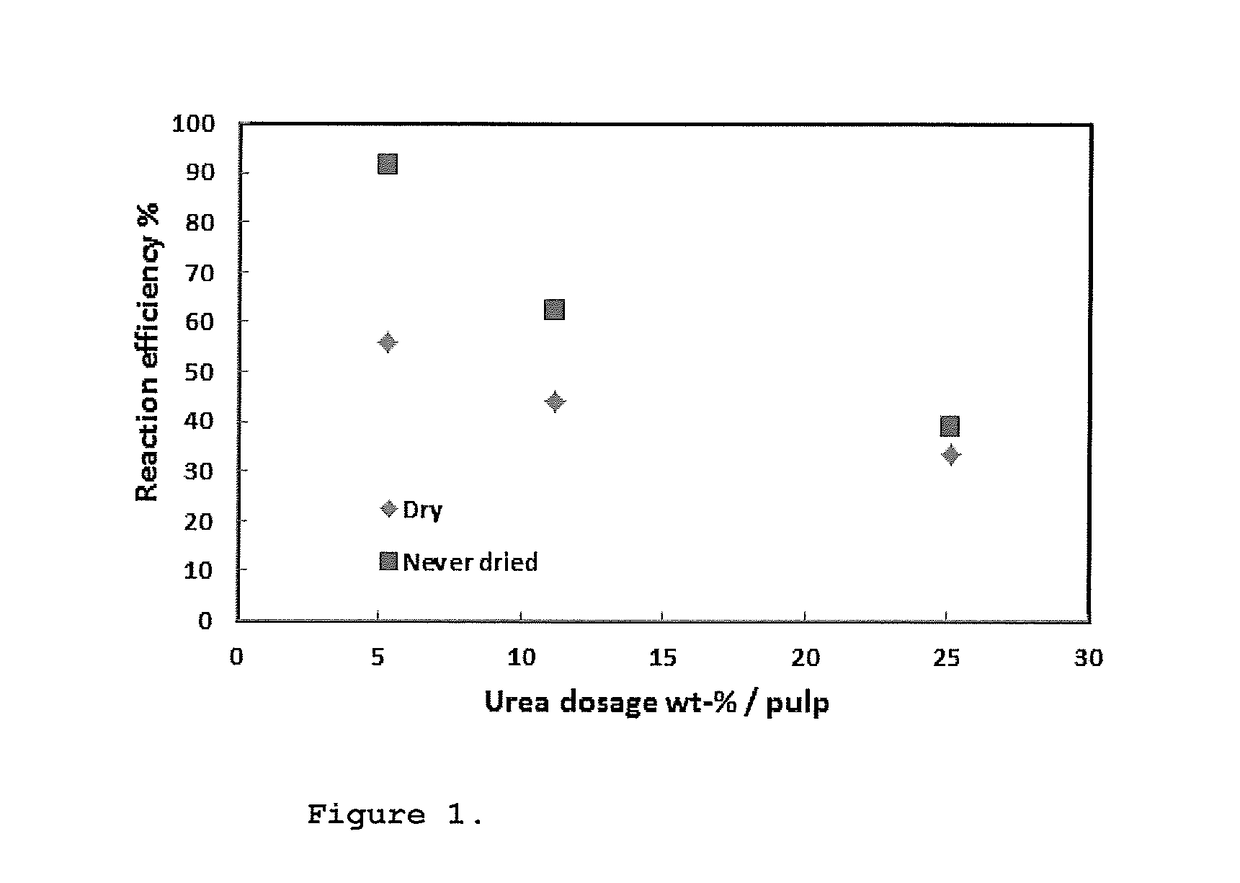
Method for making cellulose carbamate
InactiveUS20170145119A1Easy to handleEfficient and sustainableArtificial filaments from cellulose derivativesVegetable materialCellulosePolymer science
The present invention relates to a method for manufacturing cellulose carbamate which method comprises the following steps: providing a never-dried pulp, adding urea and mixing said pulp with said urea, mechanically treating said mixture, drying the mixture, and heating the relatively dry mixture thus providing a cellulose carbamate. The present invention also relates to a cellulose carbamate obtainable by said method, use of said cellulose carbamate and a dope comprising said cellulose carbamate.
Owner:STORA ENSO OYJ
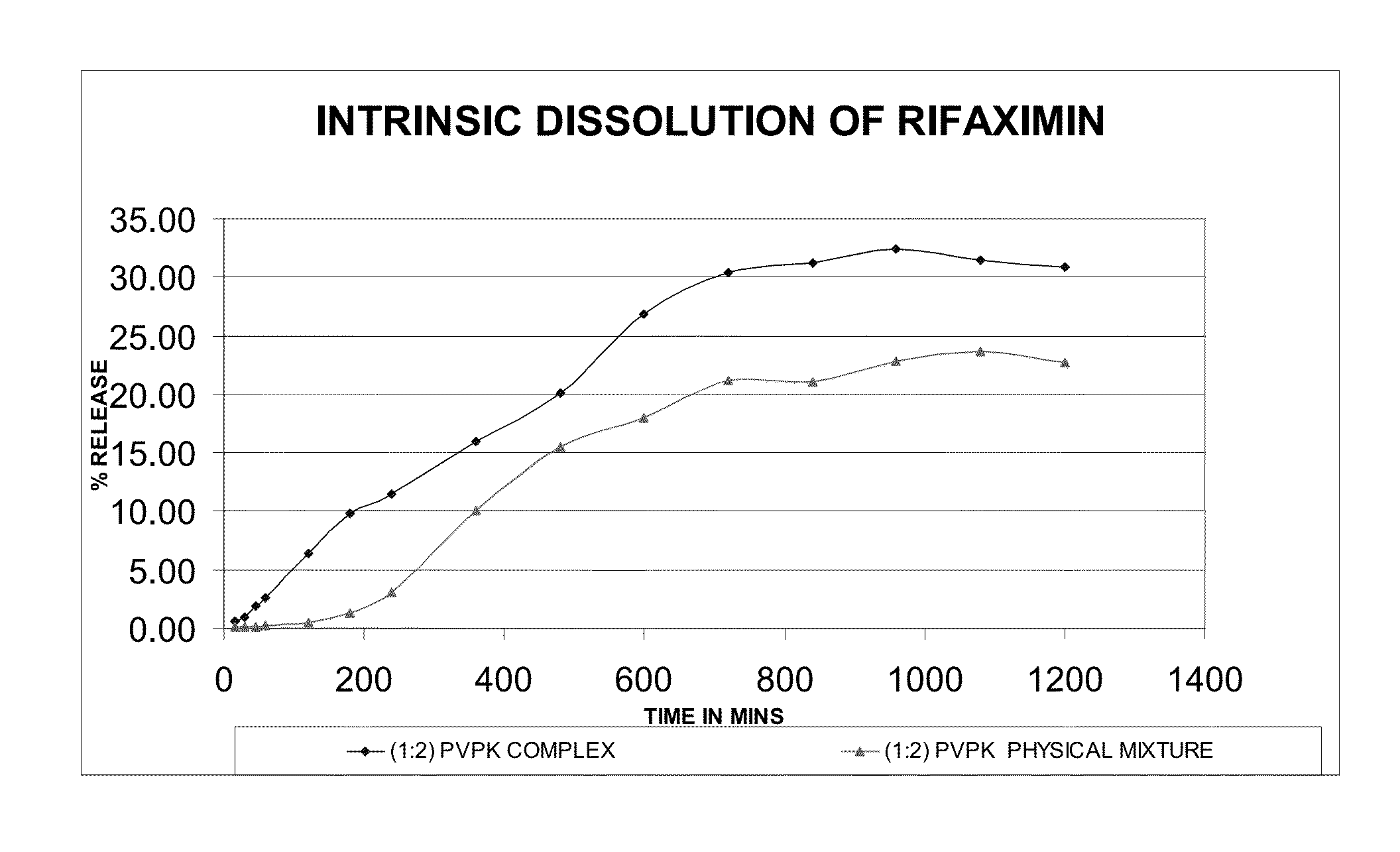
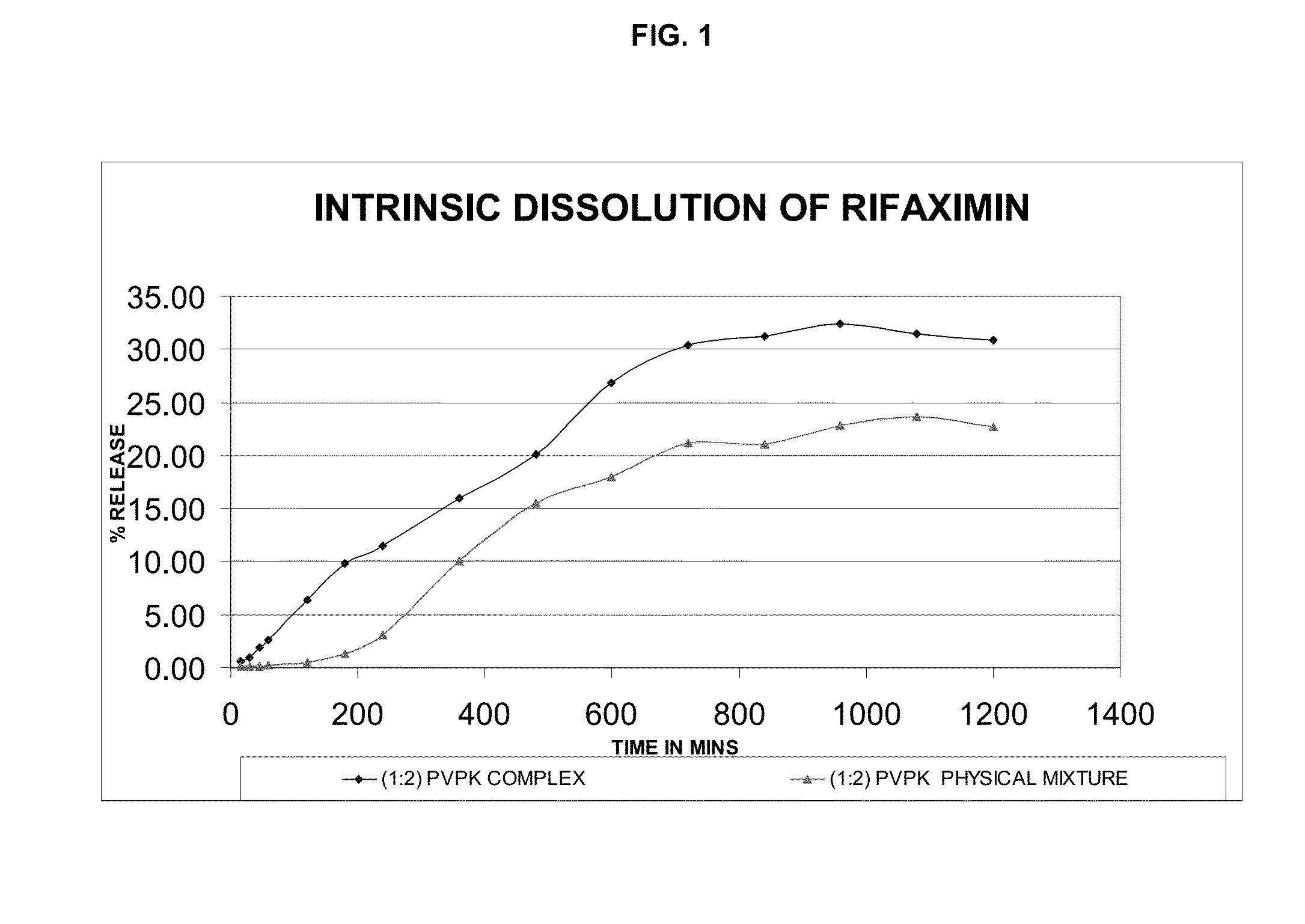
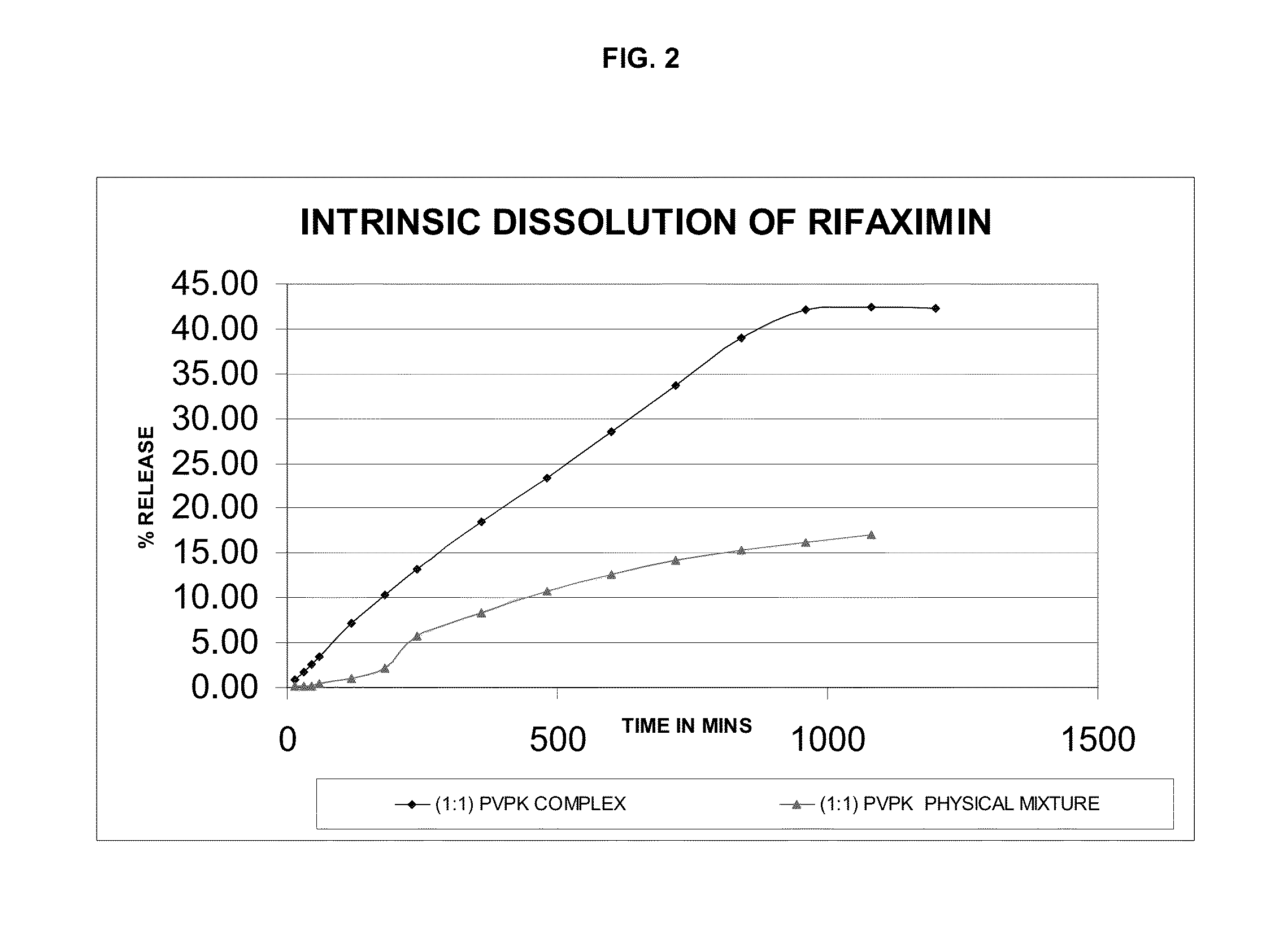
Rifaximin complexes
ActiveUS8916193B2Improve solubility and stabilityImprove stabilityAntibacterial agentsPowder deliveryCyclodextrinPolyvinyl pyrrolidone
There is provided a complex comprising rifaximin and a complexing agent, wherein the complexing agent is a polyvinyl pyrrolidone (PVP) or a cyclodextrin. There is also provided a process for preparing the complex, a pharmaceutical composition including the complex, and therapeutic uses of the complex.
Owner:CIPLA LTD
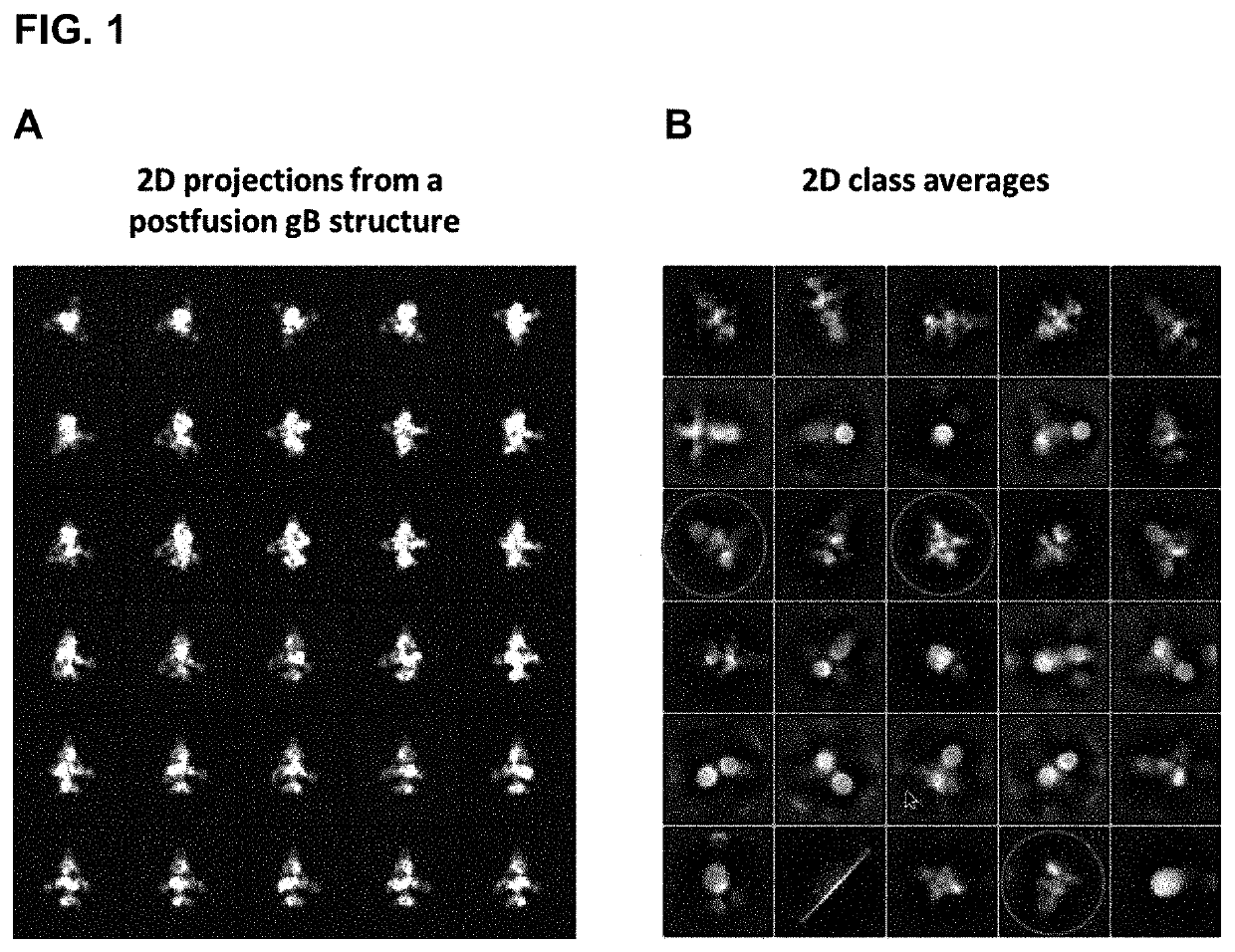
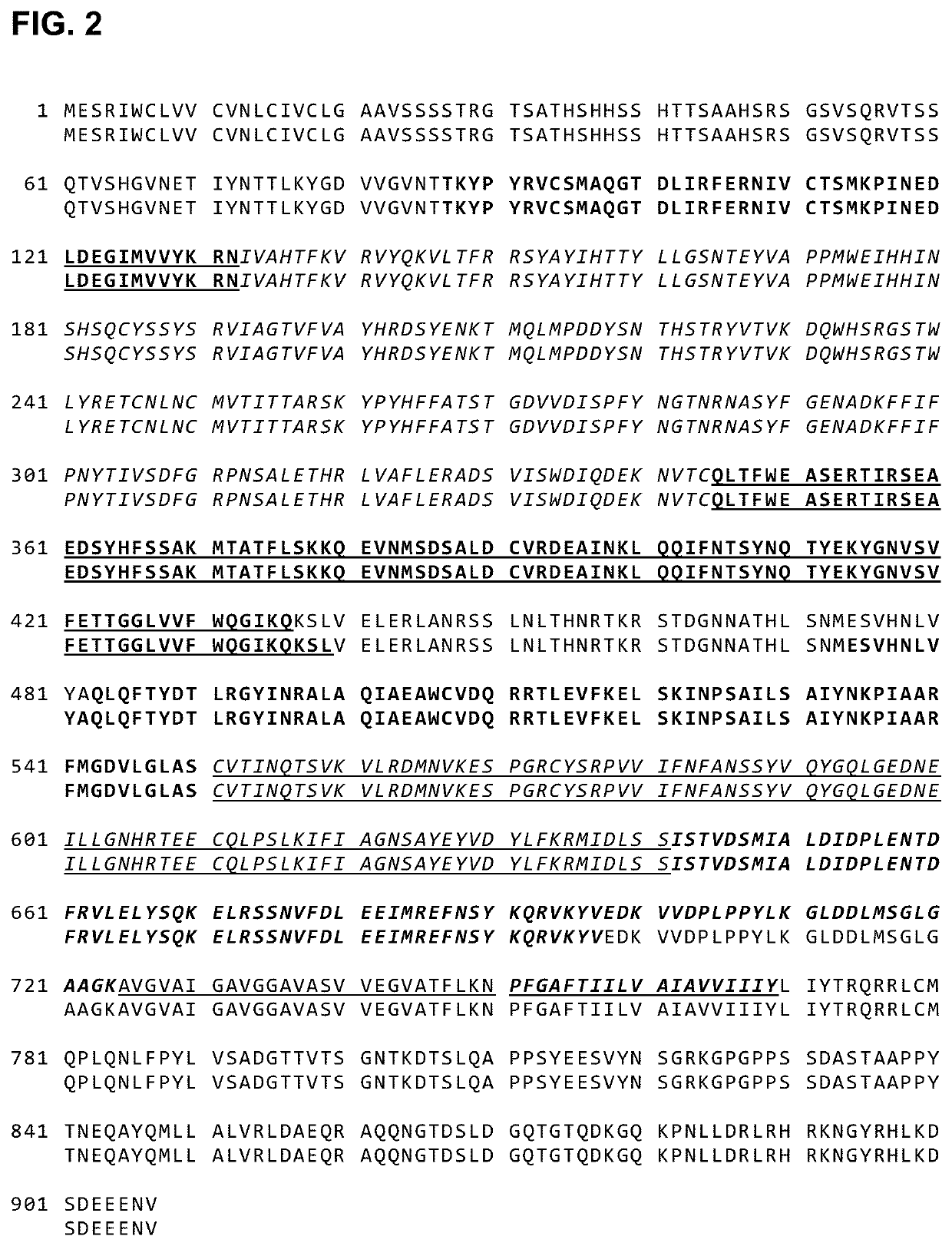
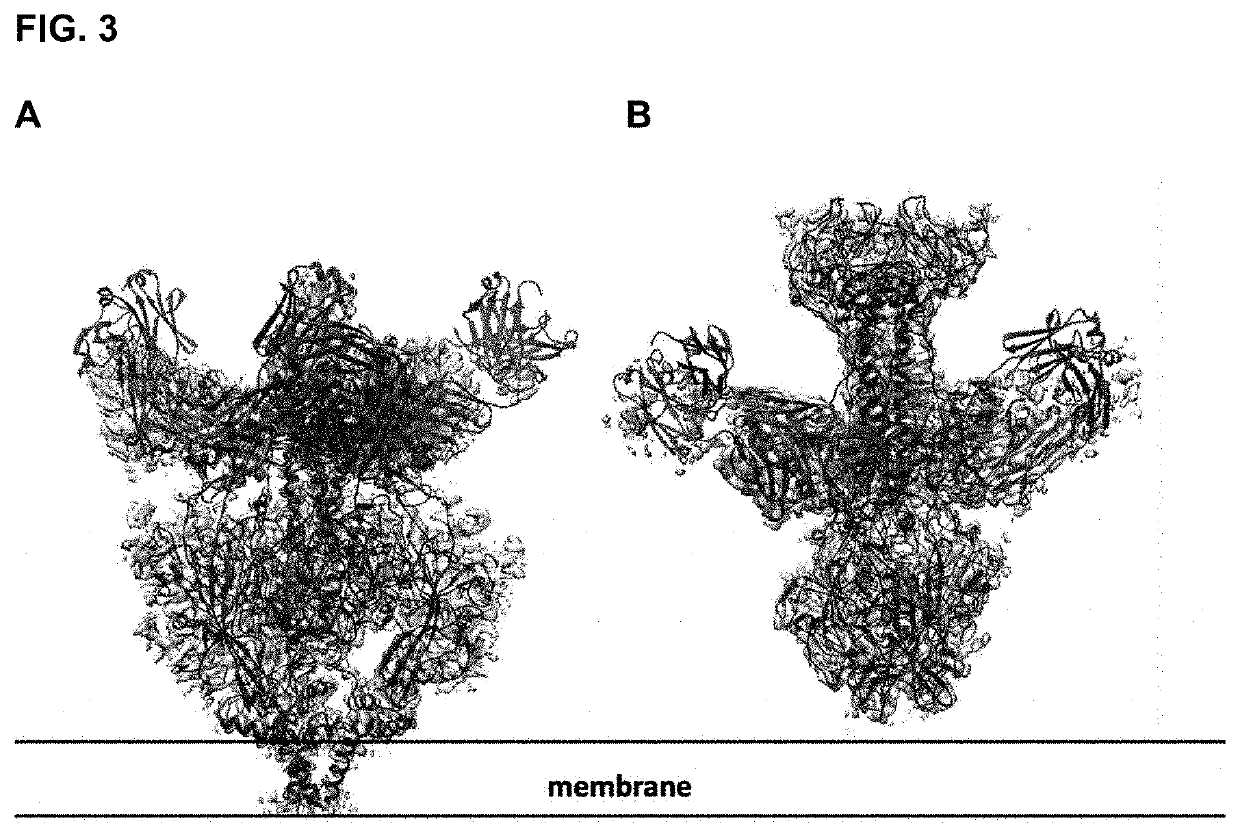
Human cytomegalovirus gb polypeptide
ActiveUS20200247853A1Improve solubility and stabilityViral antigen ingredientsVirus peptidesCytomegalovirus antigenWild type
The present invention relates to polypeptides and cytomegalovirus (CMV) antigens that include at least one introduced amino acid mutation relative to the amino acid sequence of the wild-type HCMV glycoprotein B (gB). In some embodiments, the polypeptide is stabilized in a conformation alternative to the gB postfusion conformation. Also disclosed are compositions including the polypeptides and uses thereof.
Owner:PFIZER INC
Injectable composition of paclitaxel
InactiveUS20050026995A1Low toxicityImprove solubility and stabilityBiocideOrganic active ingredientsSolventSolubility
The disclosure concerns an injectable composition of paclitaxel, more particularly, an injectable composition of paclitaxel having excellent anticancer effect comprising solubilizer such as polyoxyl hydrogenated castor oil, anhydrous ethanol and stabilizer such as N-acetyl amino acid. The injectable compositions of paclitaxel provide a medical effect higher than that of the known compositions showing not only a lower toxicity but also superior solubility of paclitaxel and stability at room temperature, thus enabling venous injection by having fine particles.
Owner:JW PHARMA CORP
Methods for treating protozoan infections
ActiveUS20170291886A1Improve solubility and stabilityEnhance drug exposureHydrazide preparationPharmaceutical delivery mechanismMedical devicePharmacology
The invention provides compounds of Formula (I), and their use in methods for treating or preventing a protozoan infection in a subject using a compound of Formula (I). The invention also provides the use of a compound of Formula (I) in the manufacture of a medicament for the treatment of a protozoan infection in a subject. The invention further provides a medical device when used in a method of treating or preventing a protozoan infection in a subject and to a medical device comprising the composition of the invention.
Owner:NEOCULI
Methods for treating bacterial infections
ActiveUS9539223B2Improve solubility and stabilityEnhance drug exposureAntibacterial agentsPharmaceutical delivery mechanismBiotechnologyPharmaceutical drug
Owner:NEOCULI PTY LTD
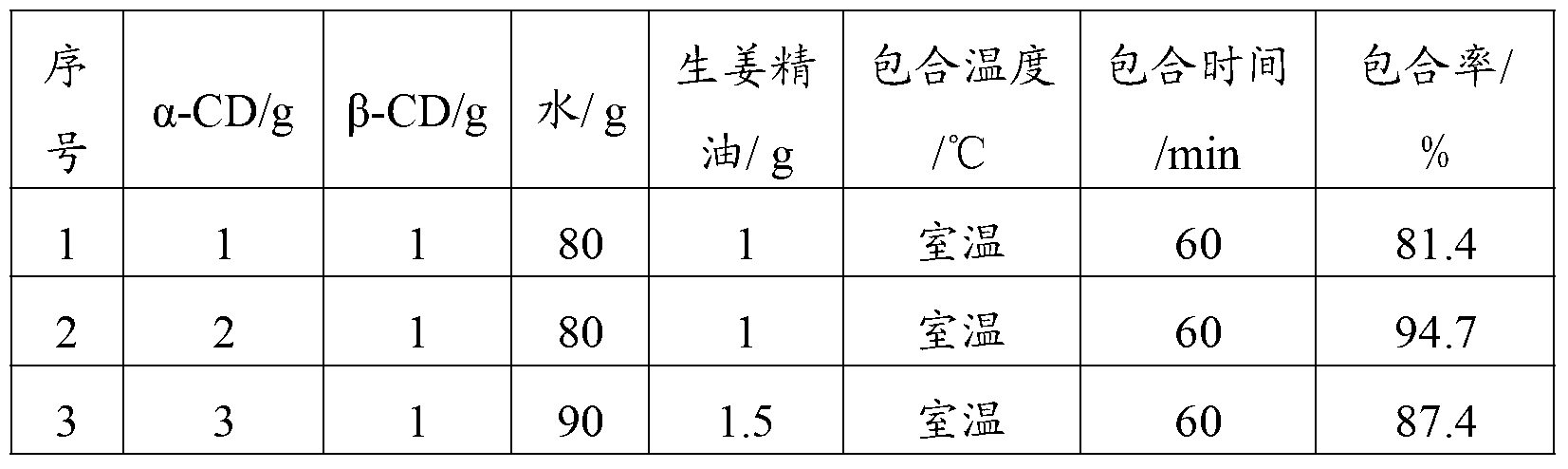
Fresh ginger essential oil and mixed cyclodextrin inclusion compound and preparation method thereof
InactiveCN103263651AHigh inclusion rateImprove solubility and stabilityAntipyreticDigestive systemChemistrySolubility
The invention belongs to the field of a medical technology and particularly relates to a fresh ginger essential oil and mixed cyclodextrin inclusion compound and a preparation method of the fresh ginger essential oil and mixed cyclodextrin inclusion compound. The invention provides a technical scheme of carrying out inclusion on a mixture of alpha-cyclodextrin and beta-cyclodextrin and fresh ginger essential oil in water, so that the inclusion rate is improved, the solubility and the stability are increased and an organic solvent used in a production process is avoided.
Owner:江苏丰园生物技术有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com