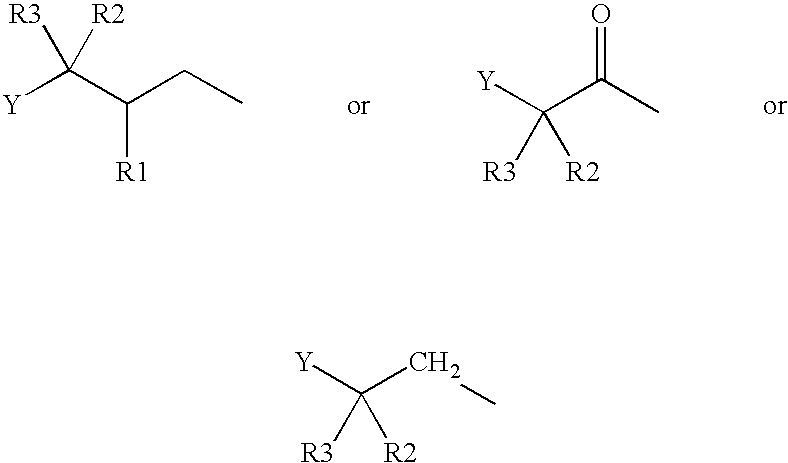
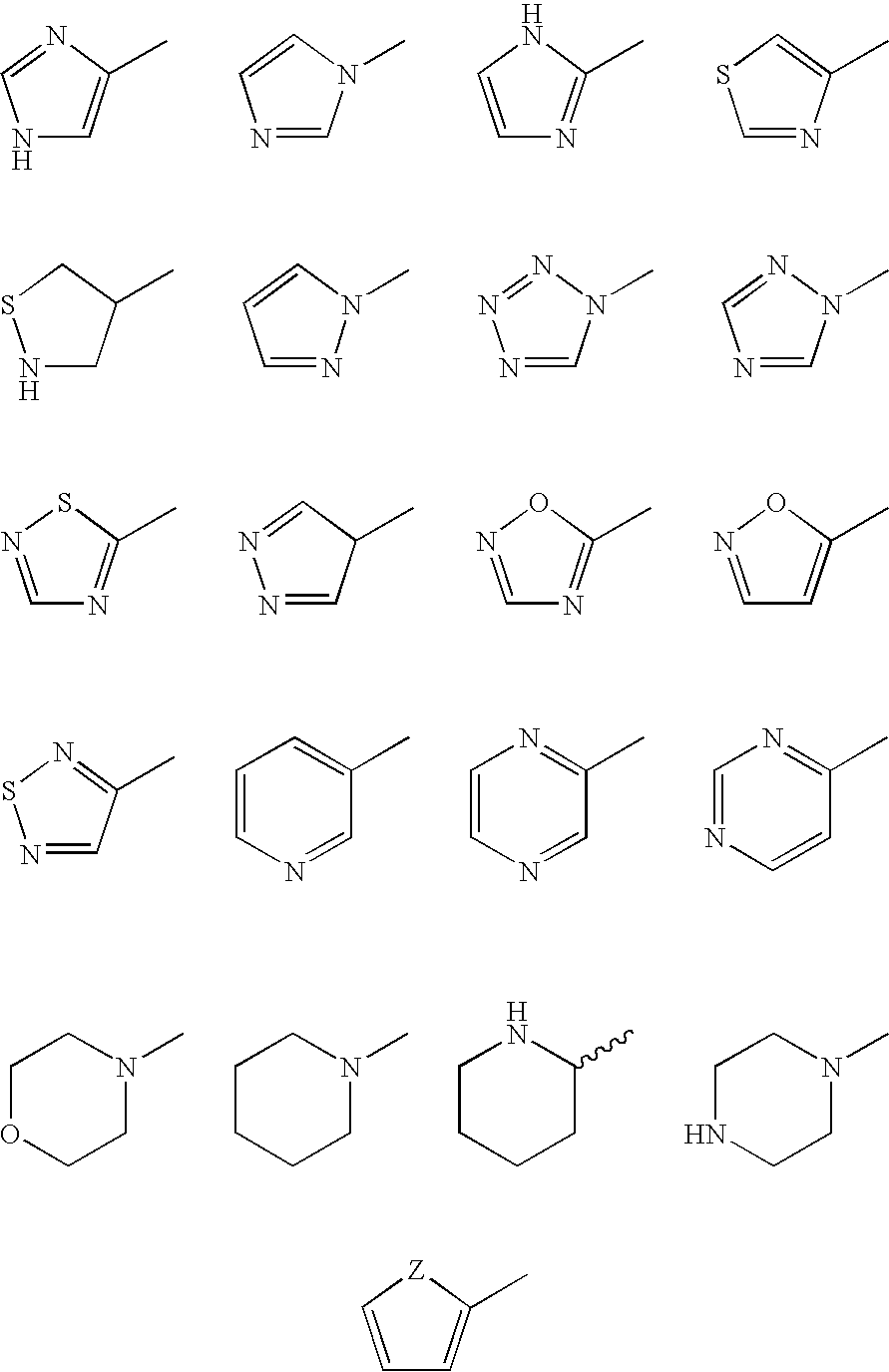
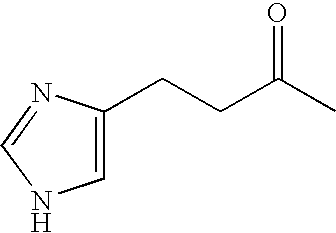
Derivatives of GLP-1 analogs
a technology of glucagonlike peptide and derivative, which is applied in the field of derivatives of human glucagonlike peptide1, can solve the problems of inconvenient high clearance of therapeutic agents, inapplicability, and the effect of improving solubility and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Lys26(N?-tetradecanoyl)-GLP-1(7-37)
[1306] The title compound was synthesised from GLP-1(7-37). A mixture of GLP-1(7-37) (25 mg, 7.45 μm), EDPA (26.7 mg, 208 μm), NMP (520 ?l) and water (260 ?l) was gently shaken for 5 min. at room temperature. To the resulting mixture was added a solution of Myr-ONSu (2.5 mg, 7.67 μm) in NMP (62.5 ?l), the reaction mixture was gently shaken for 5 min. at room temperature and then allowed to stand for 20 min. An additional amount of Myr-ONSu (2.5 mg, 7.67 μm) in NMP (62.5 ?l) was added and the resulting mixture gently shaken for 5 min. After a total reaction time of 40 min. the reaction was quenched by the addition of a solution of glycine (12.5 mg, 166 μmol) in 50% aqueous ethanol (12.5 ml). The title compound was isolated from the reaction mixture by HPLC using a cyanopropyl column (Zorbax 300SB-CN) and a standard acetonitrile / TFA system, yield: 1.3 mg (corresponding to 4.9% of the theoretical yield). The column was heated to 65?C and...
example 2
Synthesis of Lys34(N?-tetradecanoyl)-GLP-1(7-37)
[1308] The title compound was isolated by HPLC from the reaction mixture described in Example 1. PDMS analysis yielded a protonated molecular ion at m / z 3567.7±3. The molecular weight was found to be 3566.7±3 amu (theoretical value: 3565.9 amu). The acylation site was determined on the basis of the fragmentation pattern.
example 3
Synthesis of Lys26,34-bis(N?-tetradecanoyl)-GLP-1(7-37)
[1309] The title compound was isolated by HPLC from the reaction mixture described in Example 1. PDMS analysis yielded a protonated molecular ion at m / z 3778.4±3. The molecular weight was found to be 3777.4±3 amu (theoretical value: 3776.1 amu).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com