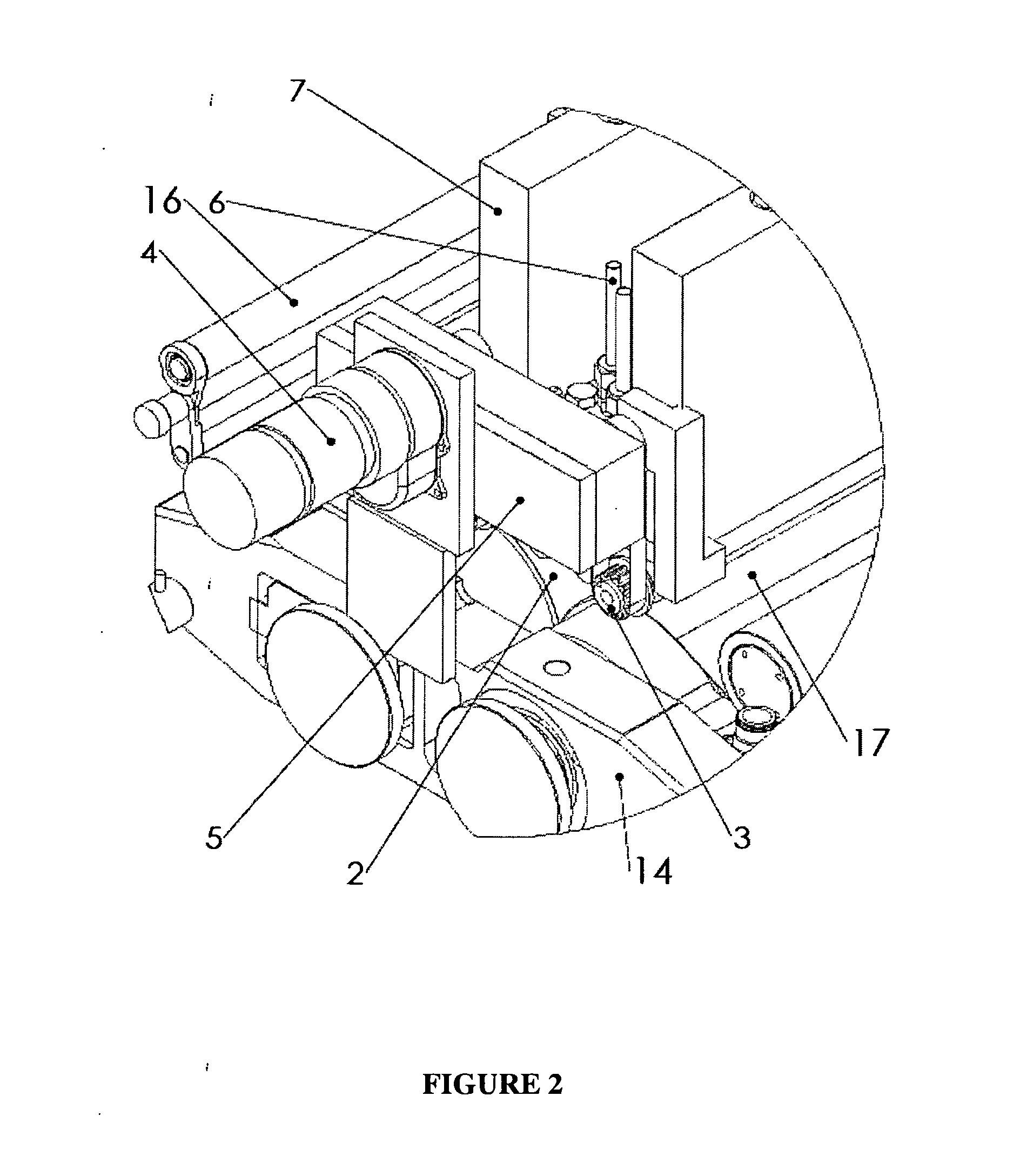
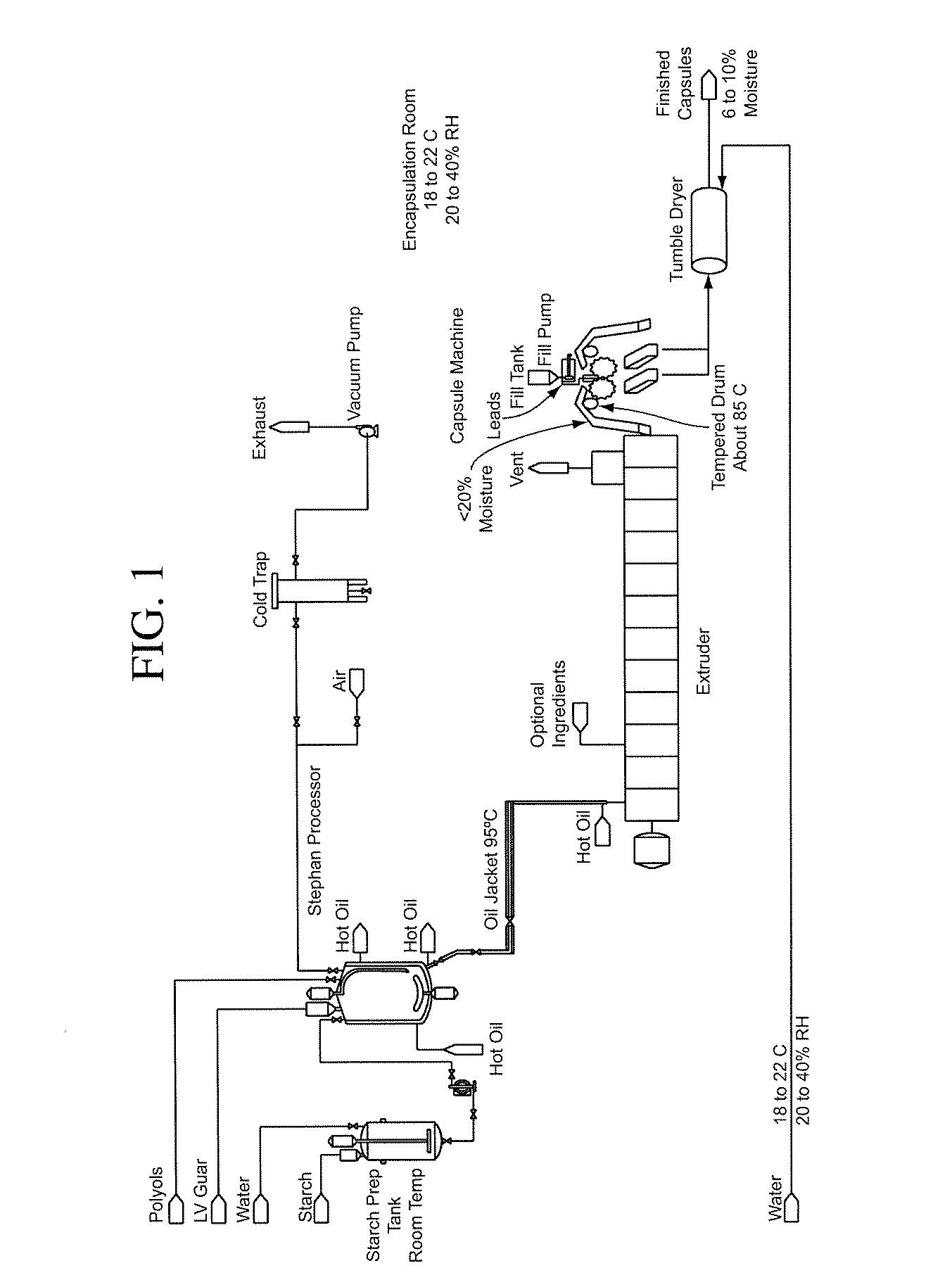
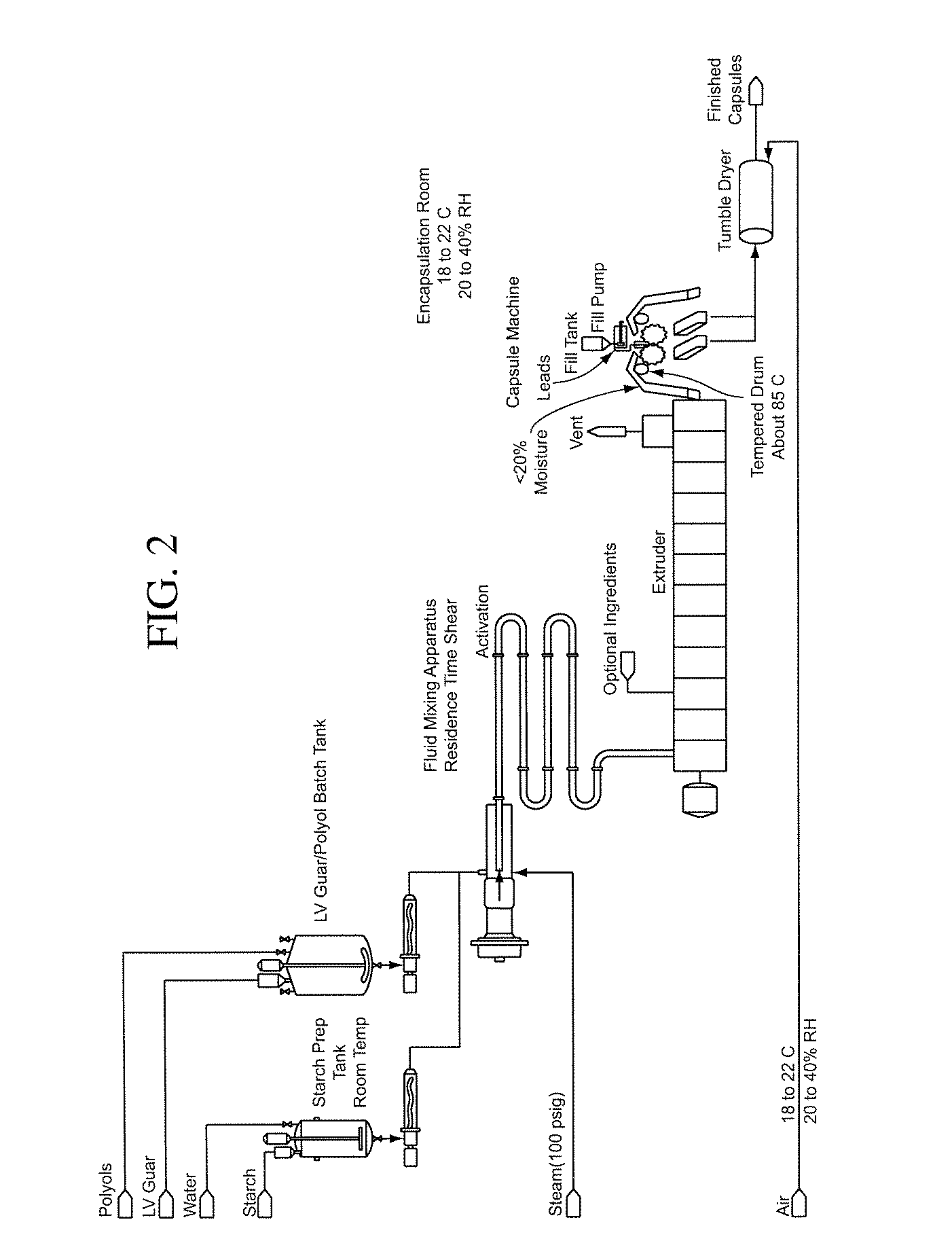
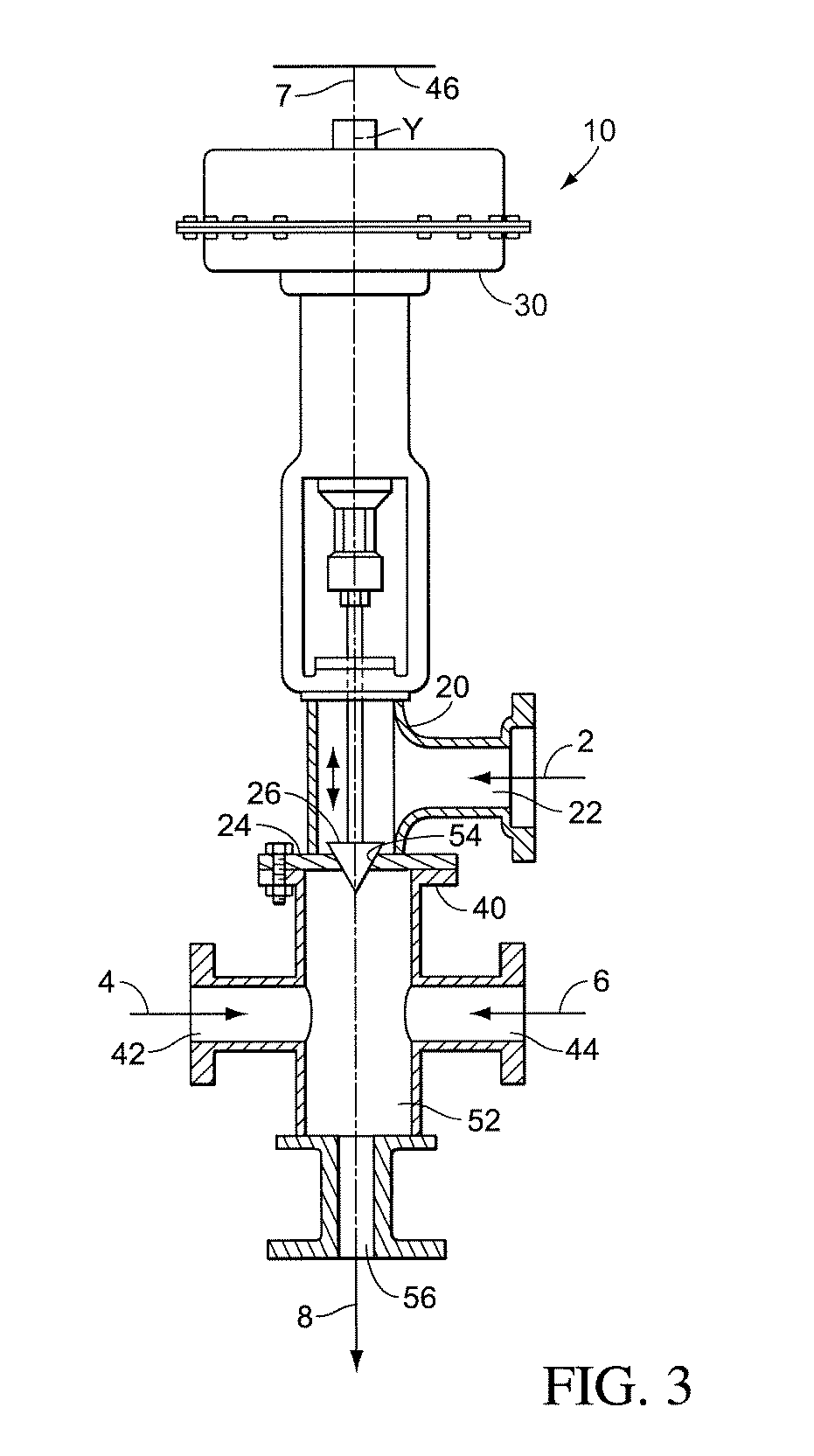
Patents
Literature
576 results about "Soft gel capsule" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Oral liposomal delivery system
A liposome-capsule dosage unit system for the delivery of a biologically active material is formed by encapsulating a biologically active materials in liposomes and then placing the liposome encapsulated material into a capsule. The capsule is typically a soft gel capsule or a two piece capsule capable of tolerating a certain amount of water. A less water tolerant capsule can be employed if the liposomes are dehydrated prior to placement within the capsule. Biologically active material include drugs, nutritional supplements, vitamins, minerals, enzymes, hormones, proteins and polypeptides. The system is especially suited for the delivery of materials with poor oral solubility, materials that are not absorbed or are poorly absorbed from the gastrointestinal tract, and materials that have conventionally been given by an invasive route. The system can be administered orally, intra-occularly, intranasally, rectally, or vaginally.
Owner:BIOZONE LAB
Oral liposomal delivery system
A liposome-capsule dosage unit system for the delivery of a biologically active material is formed by encapsulating a biologically active materials in liposomes and then placing the liposome encapsulated material into a capsule. The capsule is typically a soft gel capsule or a two piece capsule capable of tolerating a certain amount of water. A less water tolerant capsule can be employed if the liposomes are dehydrated prior to placement within the capsule. Biologically active material include drugs, nutritional supplements, vitamins, minerals, enzymes, hormones, proteins and polypeptides. The system is especially suited for the delivery of materials with poor oral solubility, materials that are not absorbed or are poorly absorbed from the gastrointestinal tract, and materials that have conventionally been given by an invasive route. The system can be administered orally, intra-occularly, intranasally, rectally, or vaginally.
Owner:BIOZONE LAB
Fatty acid compositions and methods of use
InactiveUS20090011012A1Easy to carryConvenient travelBiocideCapsule deliveryCyclosporine toxicityAntioxidant
The invention relates to highly concentrated DHA and EPA formulations in a soft gel capsule. A capsule may contain at least 80% omega-3 fatty acids, salts or derivatives thereof, where EPA and DHA are present in relative amounts of greater than or equal to 3:1 or less than or equal to 1:3, and constitute at least 75% to greater than 95% of the total fatty acids present in the capsule. Capsules of the invention may be provided in a blister package so as to provide clean and protected oils that are easy to travel with. Compliance is improved with one-pill-a-day dosing and the days of the week imprinted on the foil packing. Anitoxidant protection may be provided by rosemary and vitamin C. The invention also provides a methods of treatment, modulation or prophalaxis of coronary disease, altering serum LDL-cholesterol and / or HDL-cholesterol, lowering serum triglycerides, lowering blood pressure, pulse rate, altering the activity of the blood coagulation factor VII complex, mild hypertension, protection from cyclosporine toxicity in kidney transplant, rheumatoid arthritis, development and progression of retinopathy, hypertriglyceridemia, and neurological disorders in a subject.
Owner:BAUM SETH J
Soft gel capsules containing polymethoxylated flavones and palm oil tocotrienols
ActiveUS20060003947A1High riskImprove bioavailabilityBiocideHydrocarbon active ingredientsPlant sterolBULK ACTIVE INGREDIENT
The present invention is directed to soft gel compositions, methods of delivery and packaged nutraceuticals of the soft gel compositions that include at least one polymethoxylated flavone and, optionally, at least one tocotrienol. Optional active ingredients include a phytosterol, DHA, EPA, coenzyme Q-10 or an analog thereof and mixtures thereof.
Owner:SOFT GEL TECHNOLGIES
Solubilized CoQ-10
Owner:SOFT GEL TECHNOLGIES
Solubilized CoQ-10
Owner:SOFT GEL TECHNOLGIES
Solubilized CoQ-10
Owner:SOFT GEL TECHNOLGIES
Enteric composition for the manufacture of soft capsule wall
ActiveUS8685445B2Loss of strengthLoss of viscosityOrganic active ingredientsPeptide/protein ingredientsHard CapsuleBiomedical engineering
Owner:PATHEON SOFTGELS INC
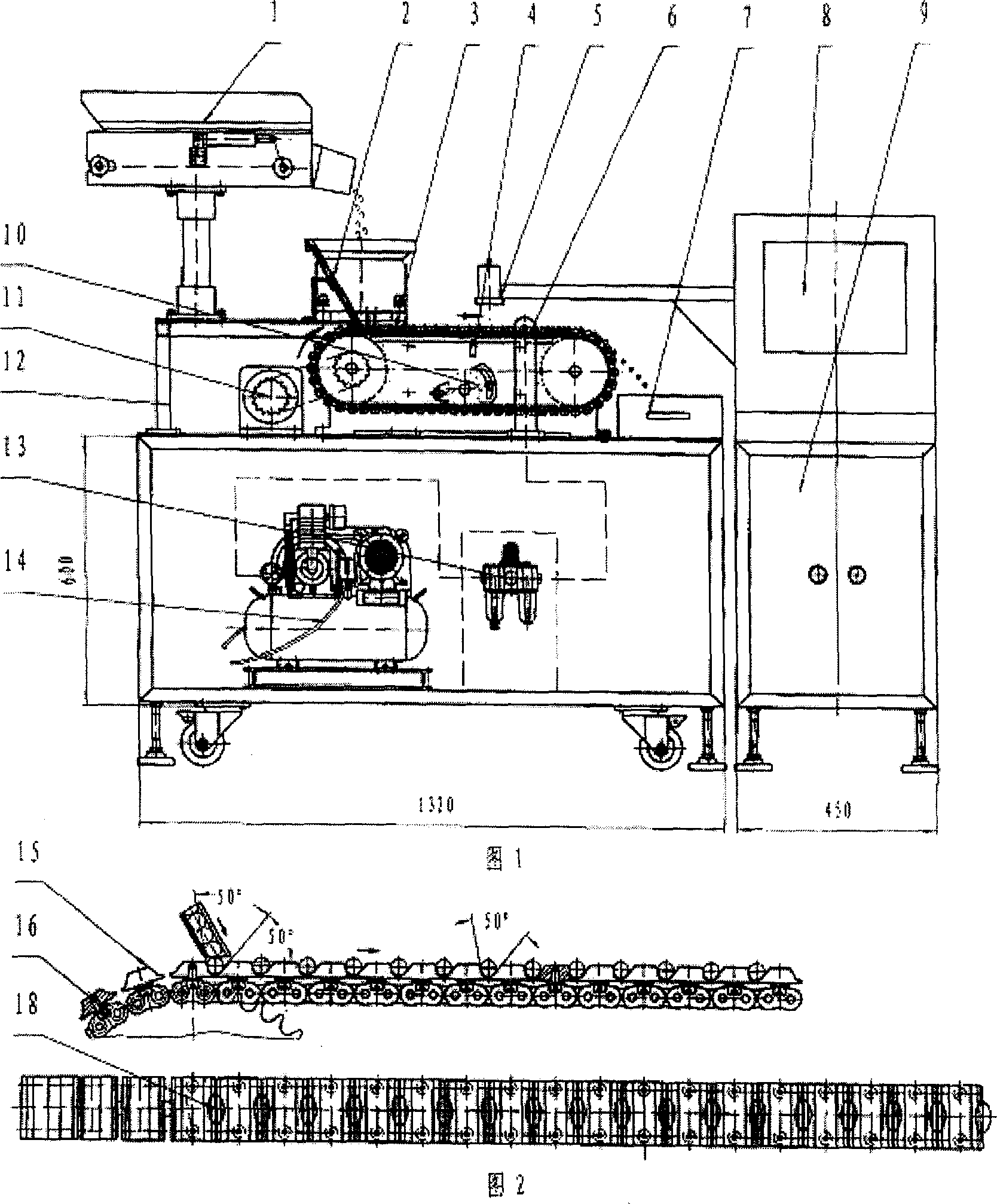
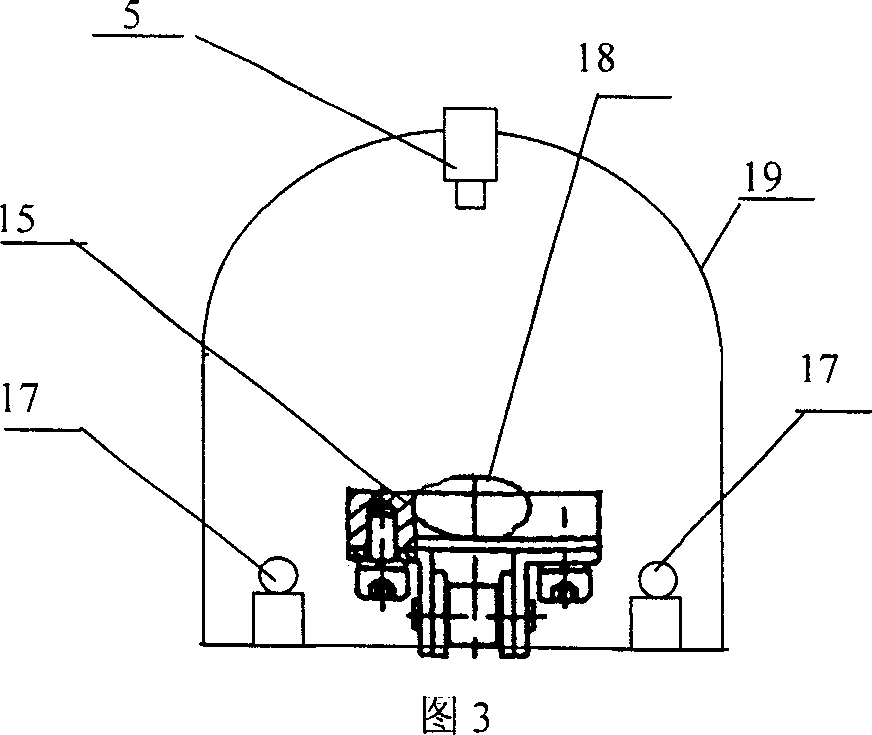
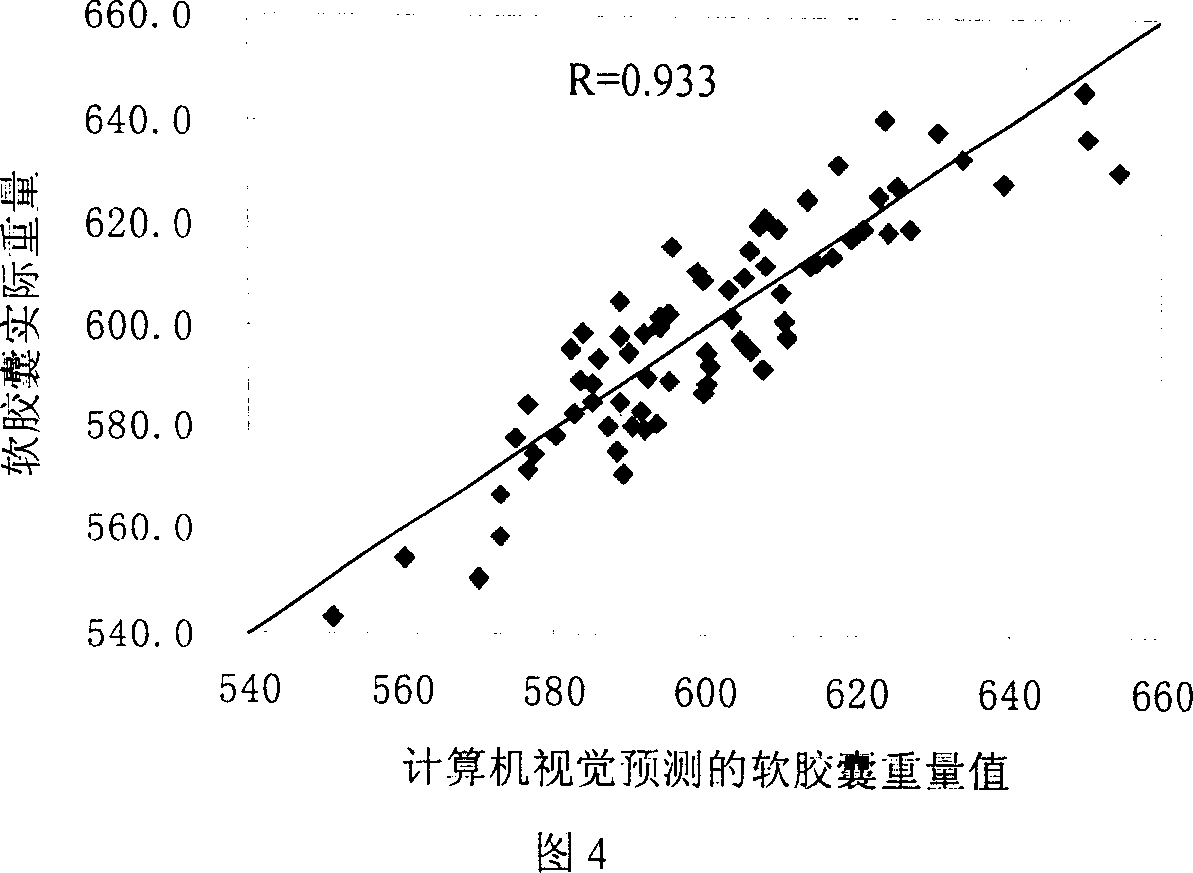
Online detecting device and method based on computer vision for soft capsule quality
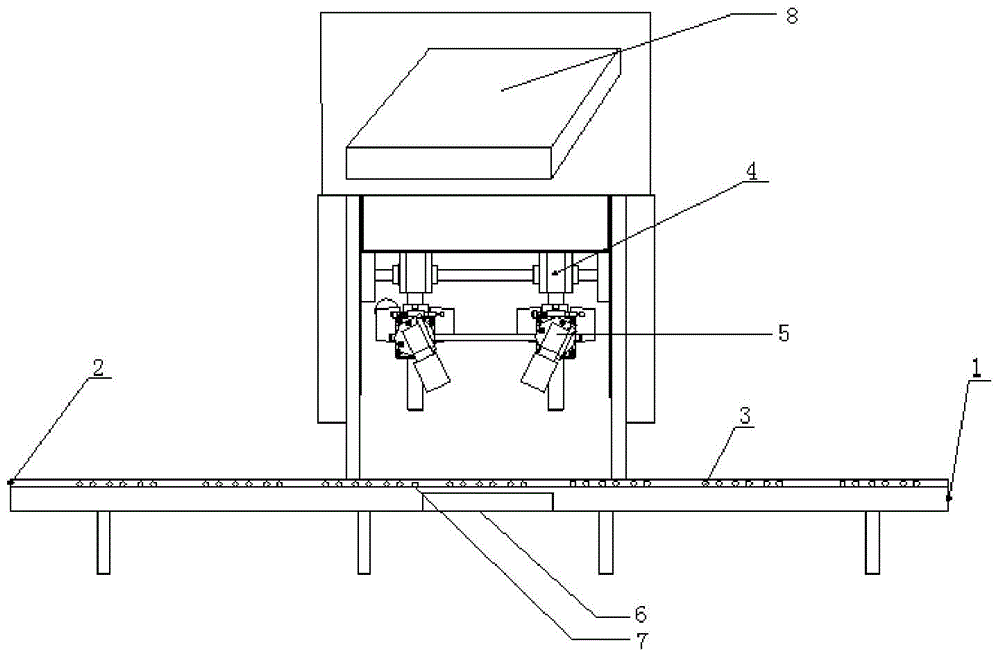
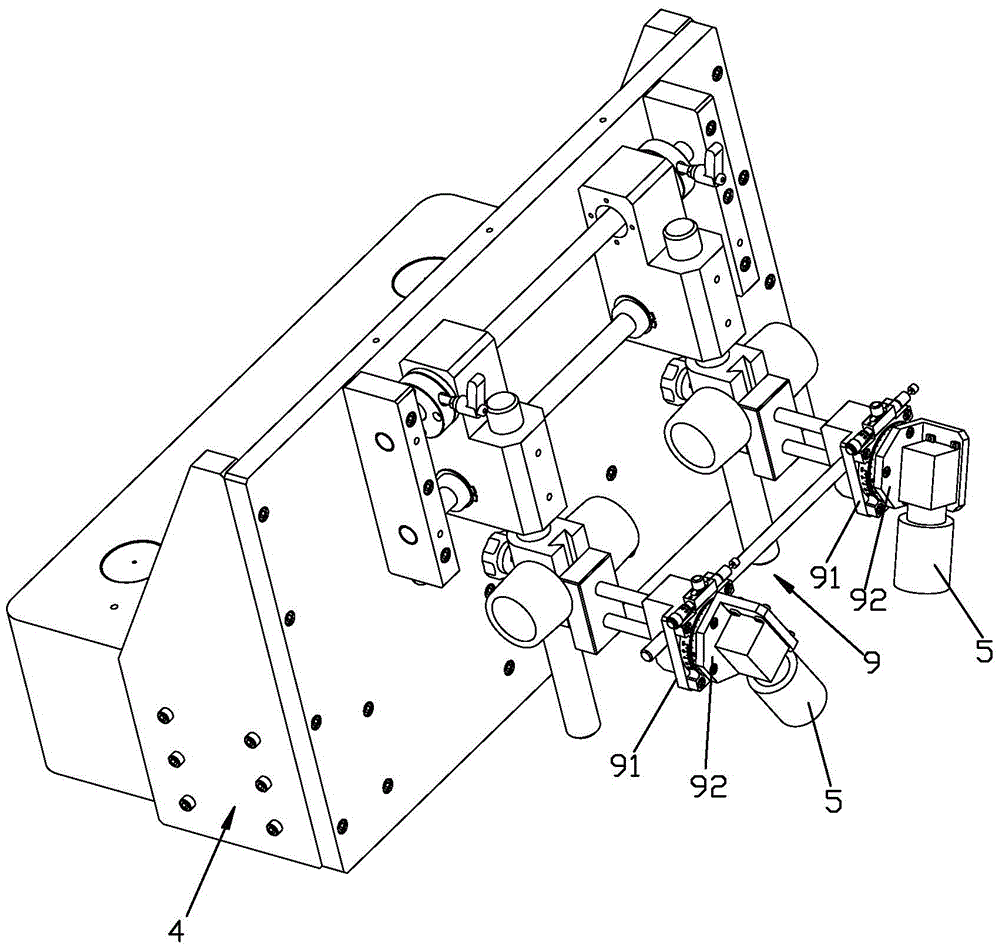
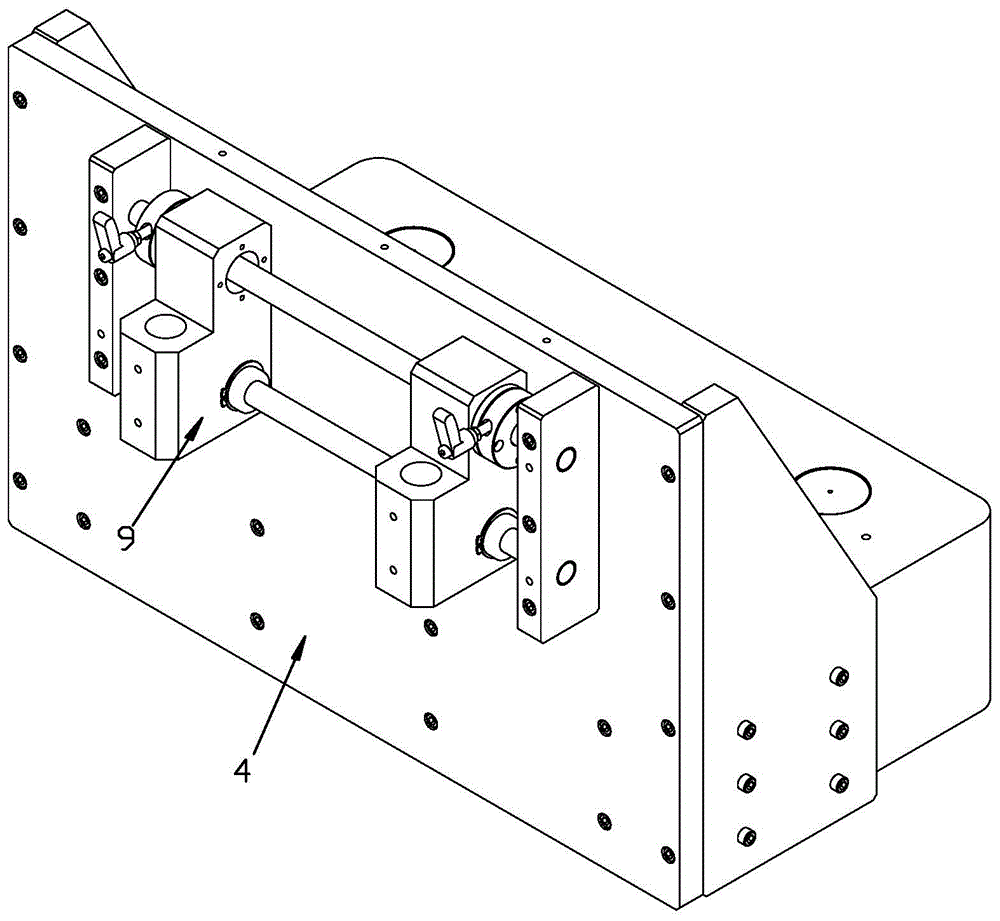
InactiveCN1943886AGuaranteed accuracyGuaranteed detection speedInvestigating moving fluids/granular solidsCharacter and pattern recognitionComputer hardwareMachine vision
The present invention is computer vision detecting device and method for real-time on-line detection based on the characteristics of article. The device consists of one capsule arranging and conveying unit, one computer vision recognizing unit and one capsule eliminating unit; and features the computer vision recognizing unit with CCD camera mounted over the capsule arranging and conveying unit and the capsule eliminating unit mounted beside the conveying chain of the capsule arranging and conveying unit. The capsule arranging and conveying unit includes one vibrating feeder and one conveying chain mainly; the capsule eliminating unit eliminating soft capsules in unqualified weight and shape consists of air source, solenoid valve and nozzle connected successively; and the computer vision recognizing unit consists of video camera system, lighting box, photoelectronic sensor, control module and corresponding software.
Owner:JIANGSU UNIV
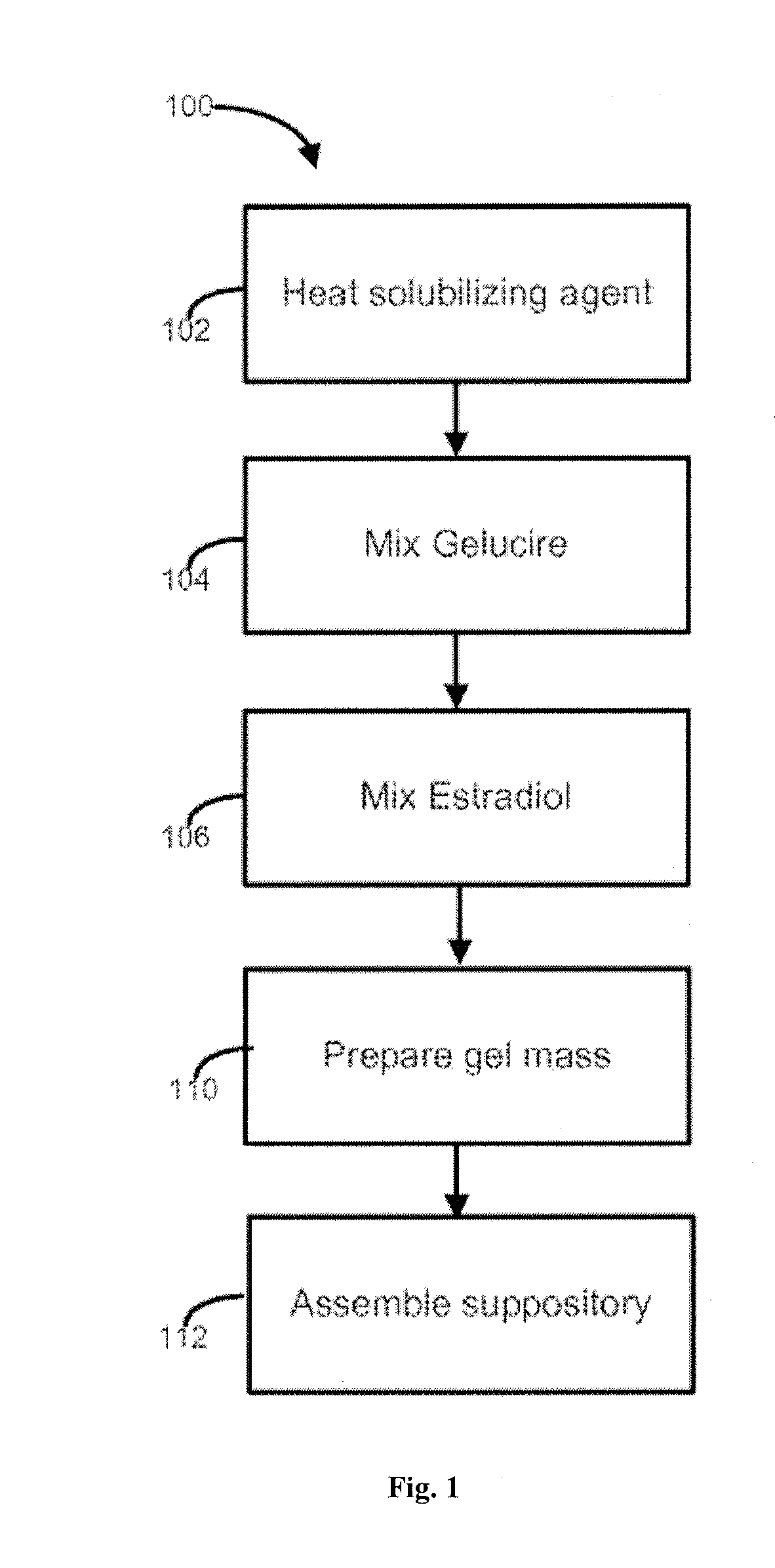
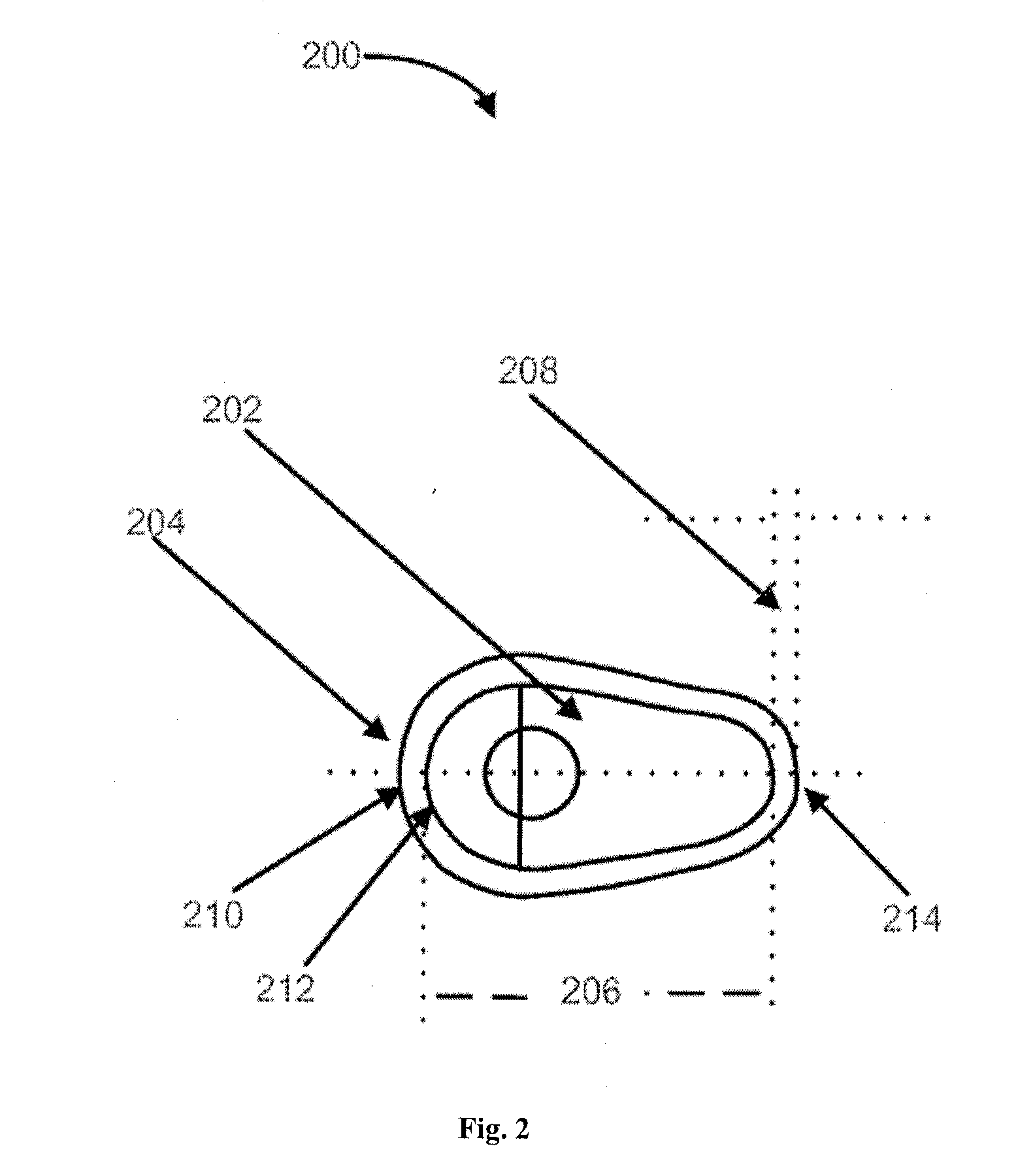
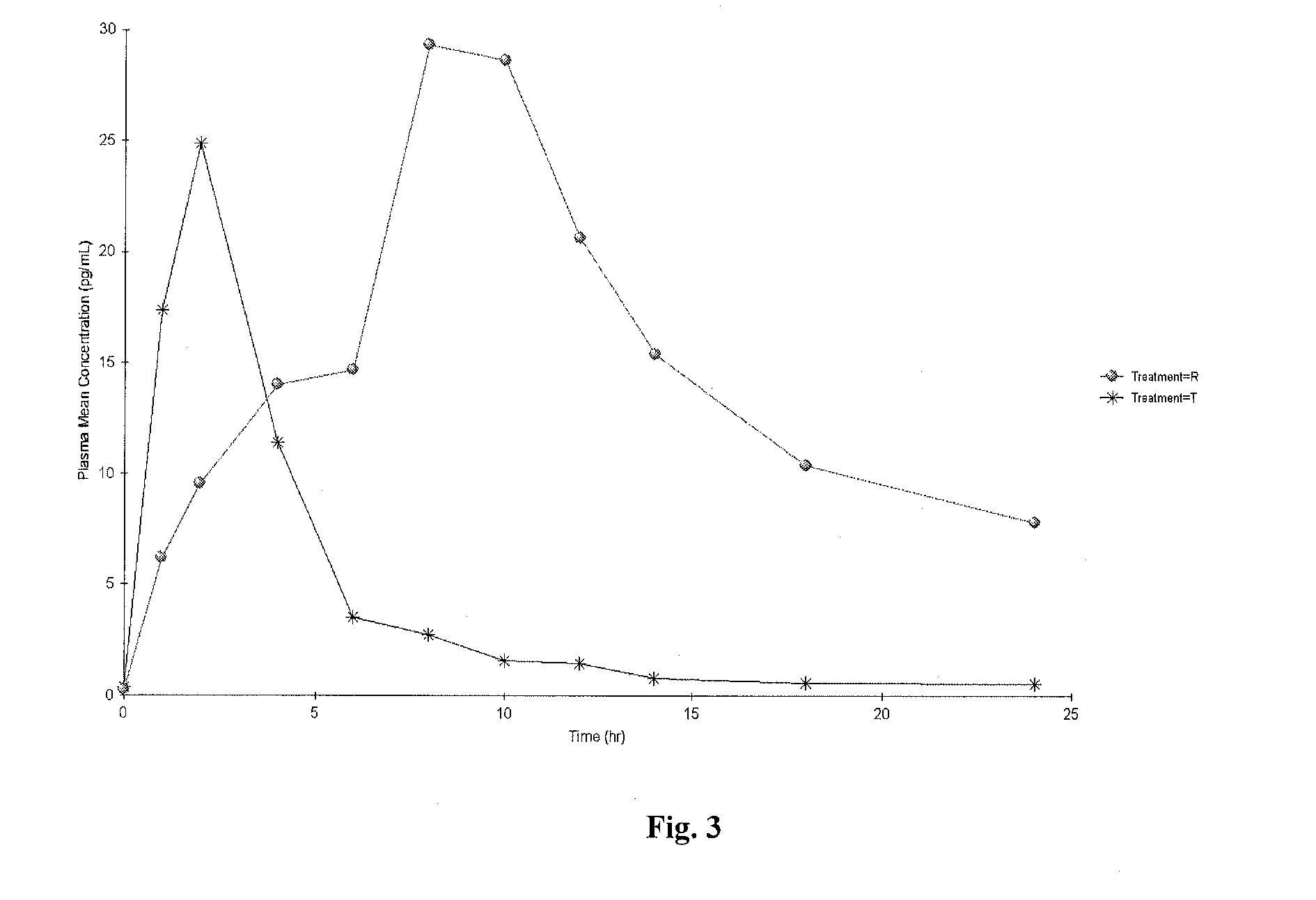
Vaginal inserted estradiol pharmaceutical compositons and methods
ActiveUS20150045335A1Reduce in quantityIncrease the number ofOrganic active ingredientsOintment deliveryVaginal atrophyVagina
According to various embodiments of this disclosure, pharmaceutical compositions comprising solubilized estradiol are provided. In various embodiments, such compositions are encapsulated in soft capsules which may be vaginally inserted for the treatment of vulvovaginal atrophy.
Owner:THERAPEUTICSMD
Ubiquinol and alpha lipoic acid compositions
ActiveUS20080226710A1Reduce the amount of solutionReduced form requirementsPeptide/protein ingredientsOrganic compound preparationAlpha-Lipoic AcidFatty acid
The present invention is directed to compositions and methods of delivery of CoQ that is reduced in the presence of lipoic acid and, optionally a fatty acid and / or optionally in a monoterpene. The compositions that include the reduced CoQ can be formulated in soft gel capsules.
Owner:SOFT GEL TECHNOLGIES
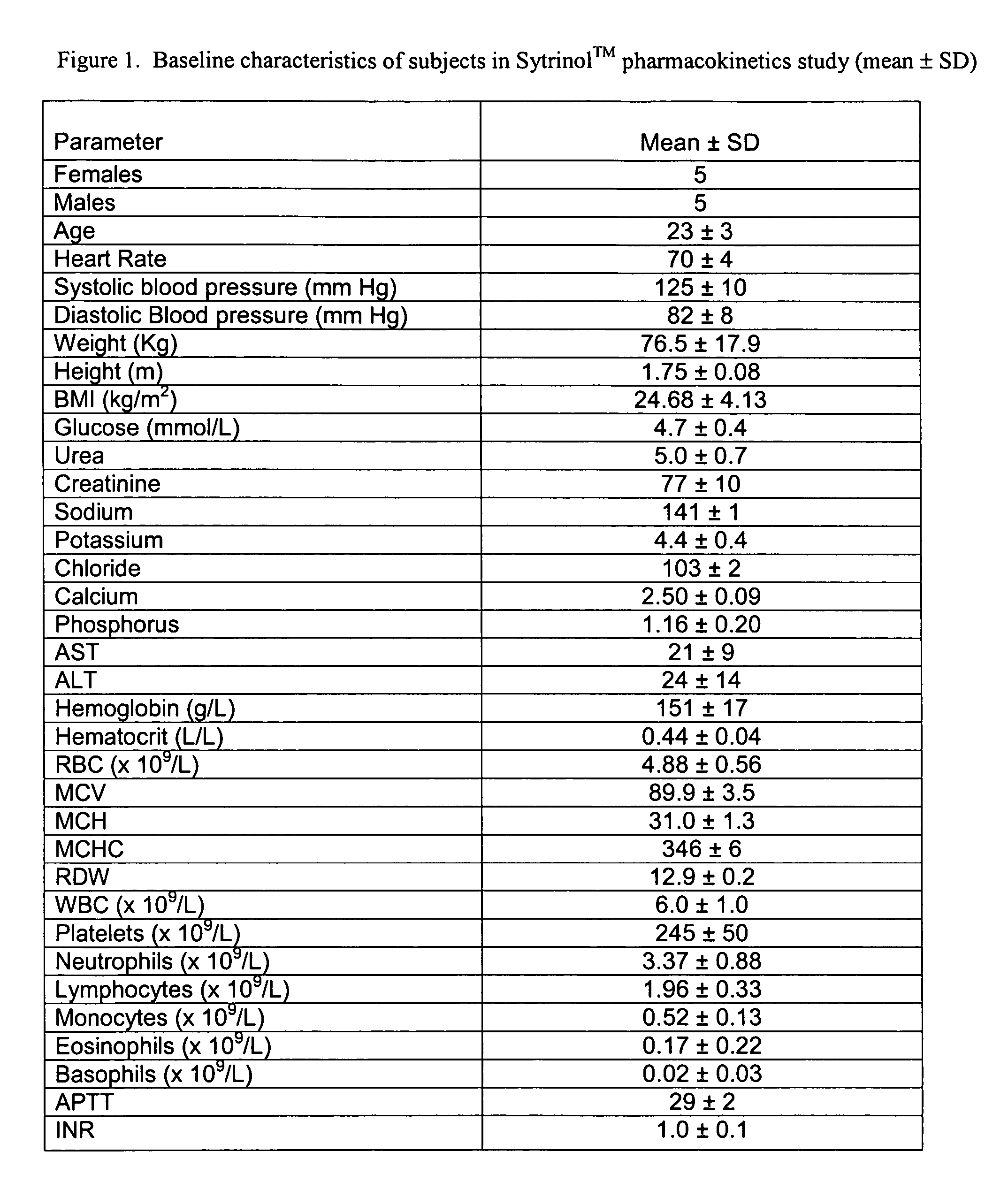
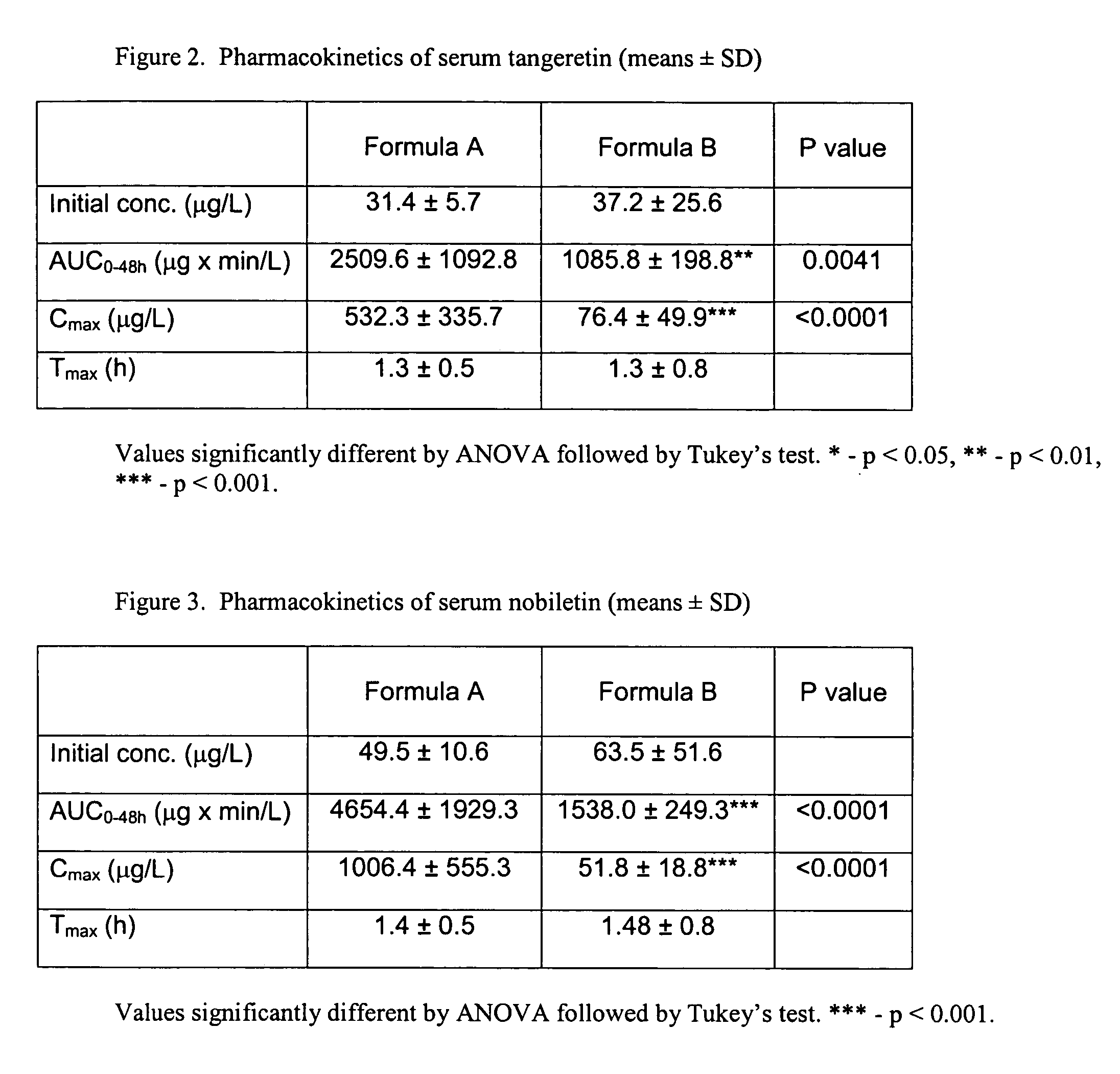
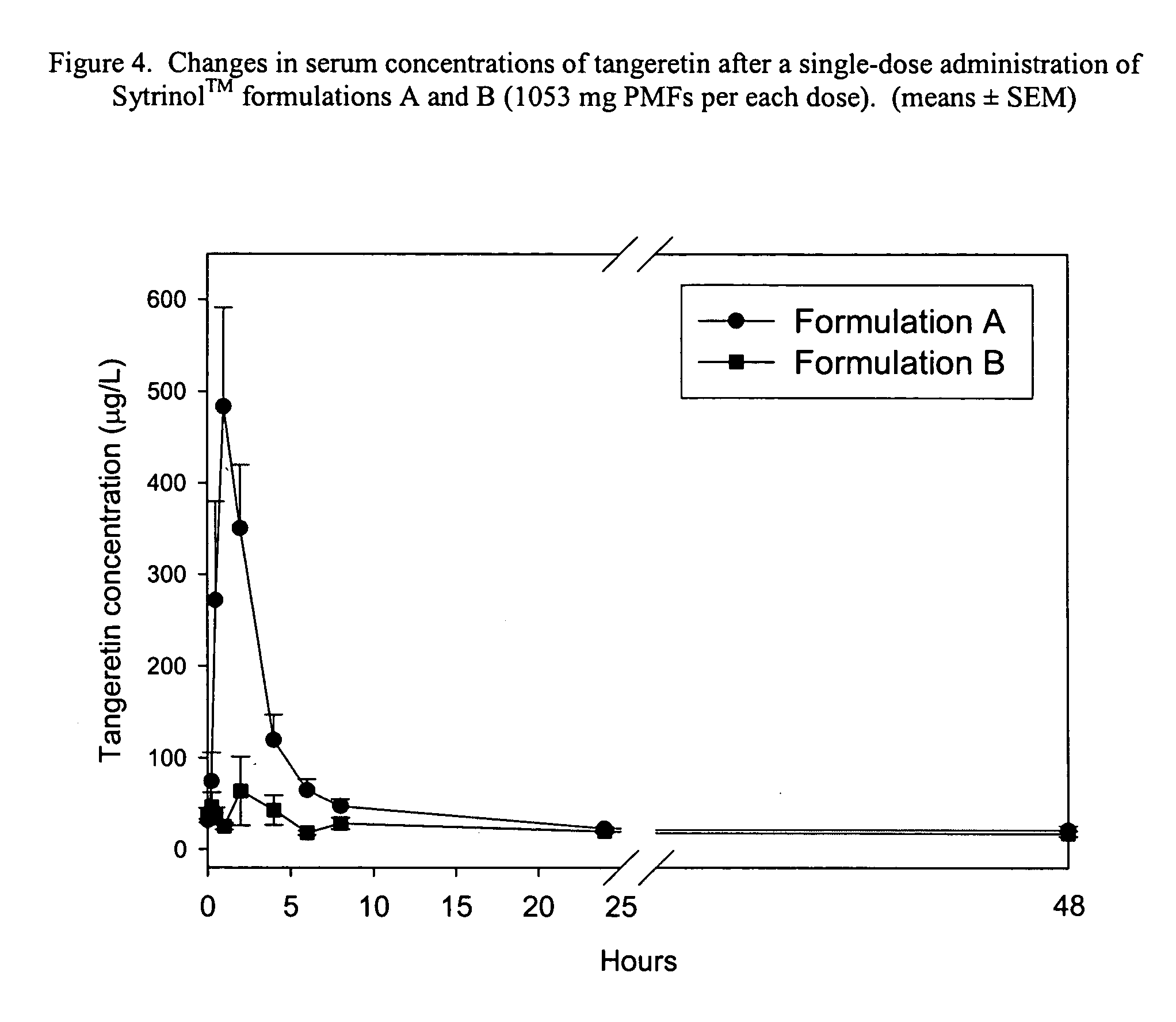
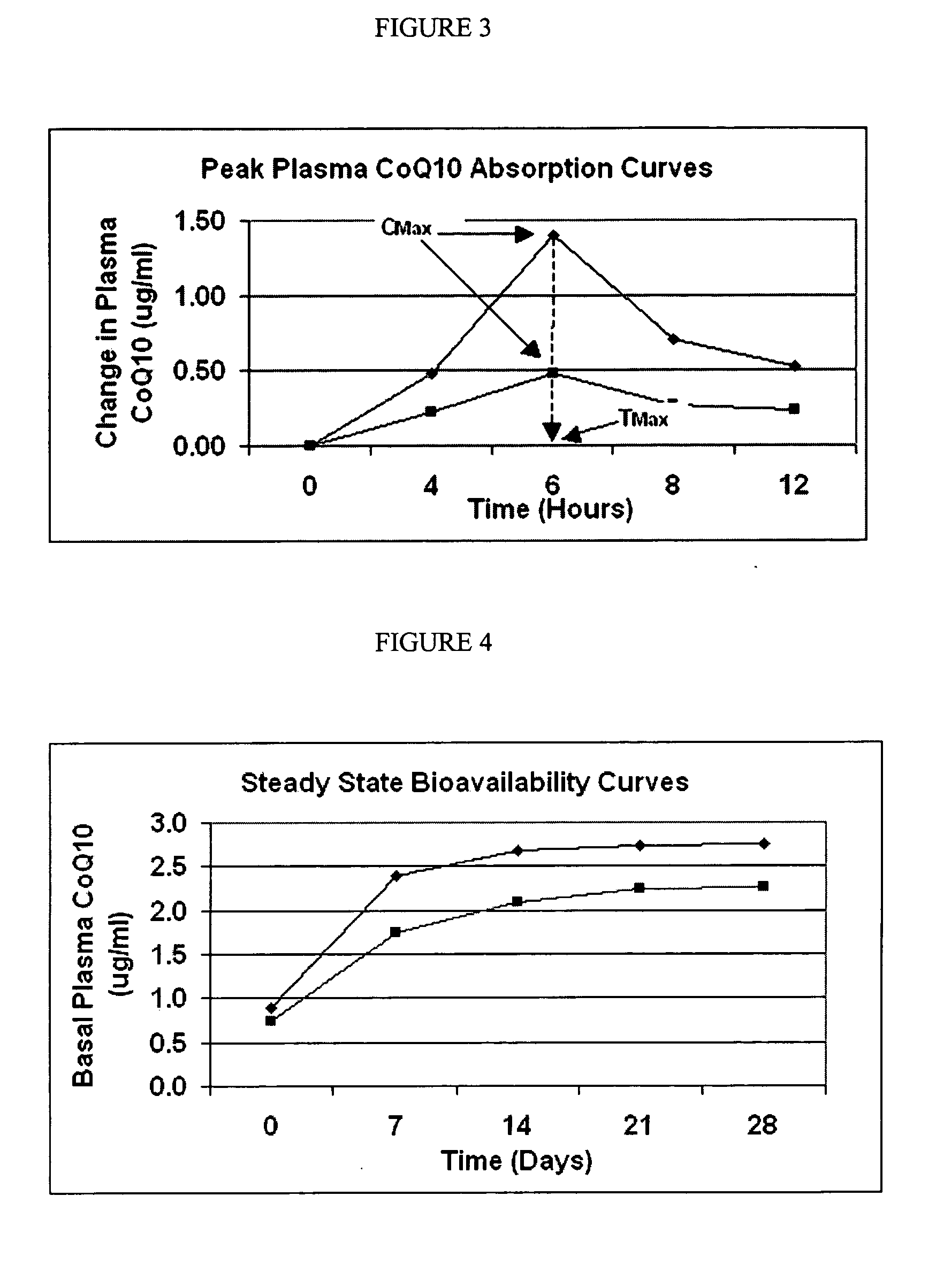
Formulation and manufacturing process for coenzyme Q10 soft gel capsules
InactiveUS6855733B2Improve solubility and stabilityPromote absorptionBiocidePeptide/protein ingredientsSoft gel capsuleCo enzyme q10
Owner:SOFT GEL TECHNOLGIES
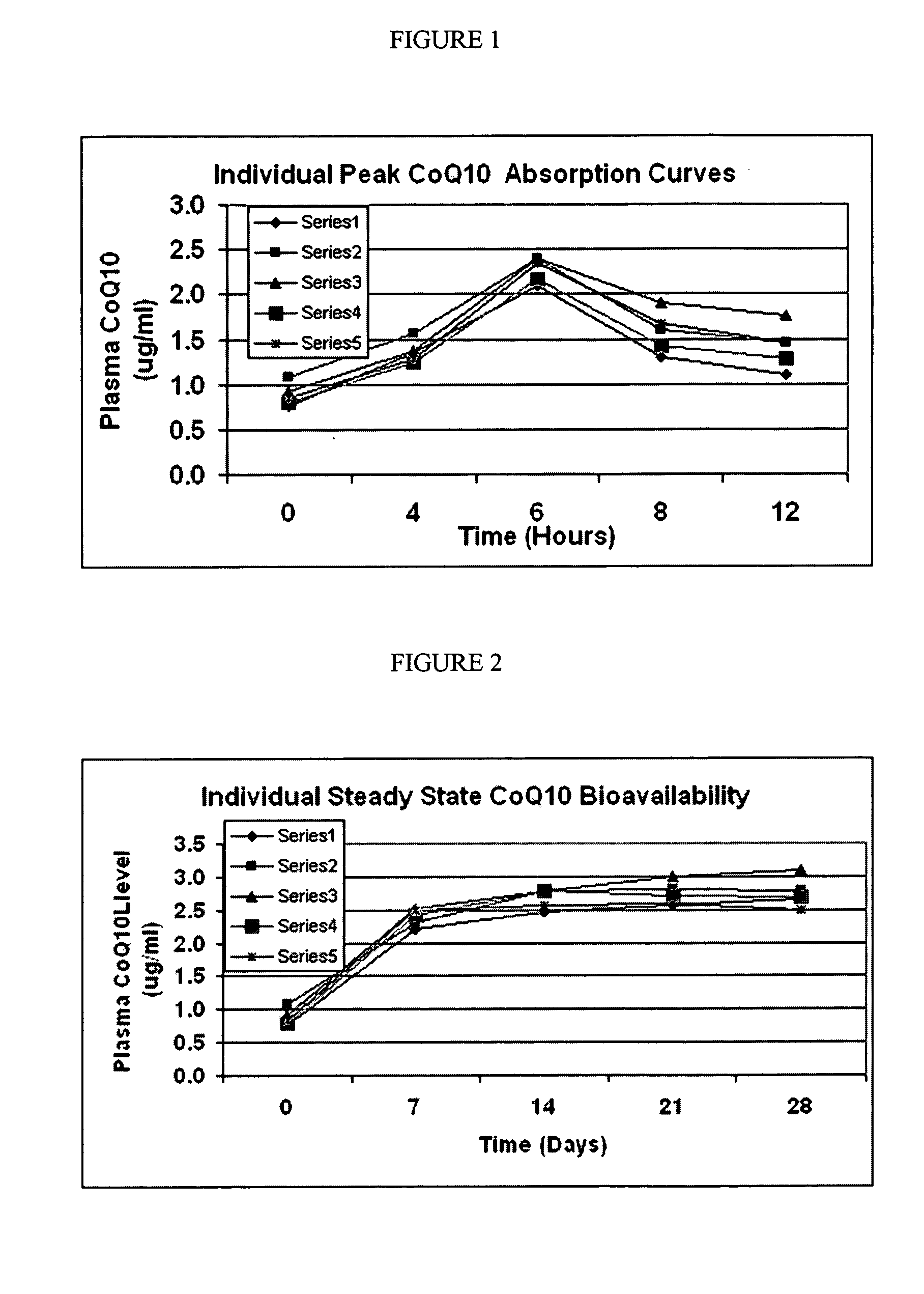
Super absorption Coenzyme Q10
An improved soft gelatin formulation and process methodology that increases single Coenzyme Q10 molecules presented to the absorption channels of the small intestines by providing medium chain triglycerides, Vitamin E and natural beta carotene to Coenzyme Q10 in a soft gel capsule to increase the absorption thereof.
Owner:SOFT GEL TECHNOLGIES
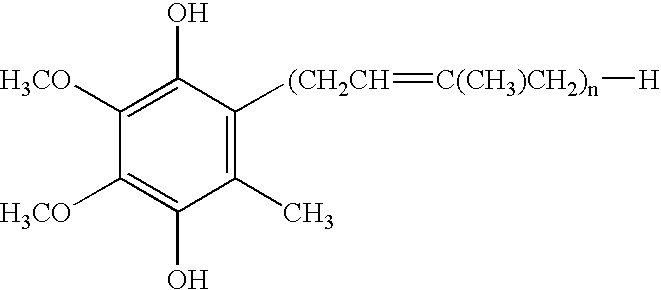
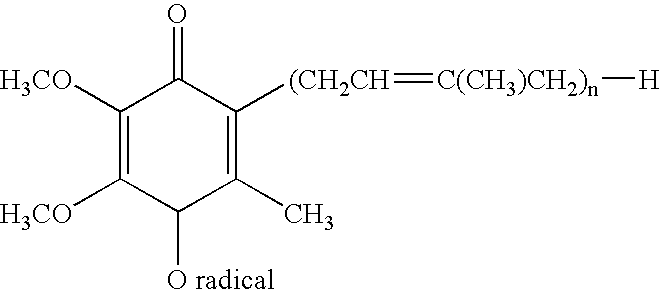
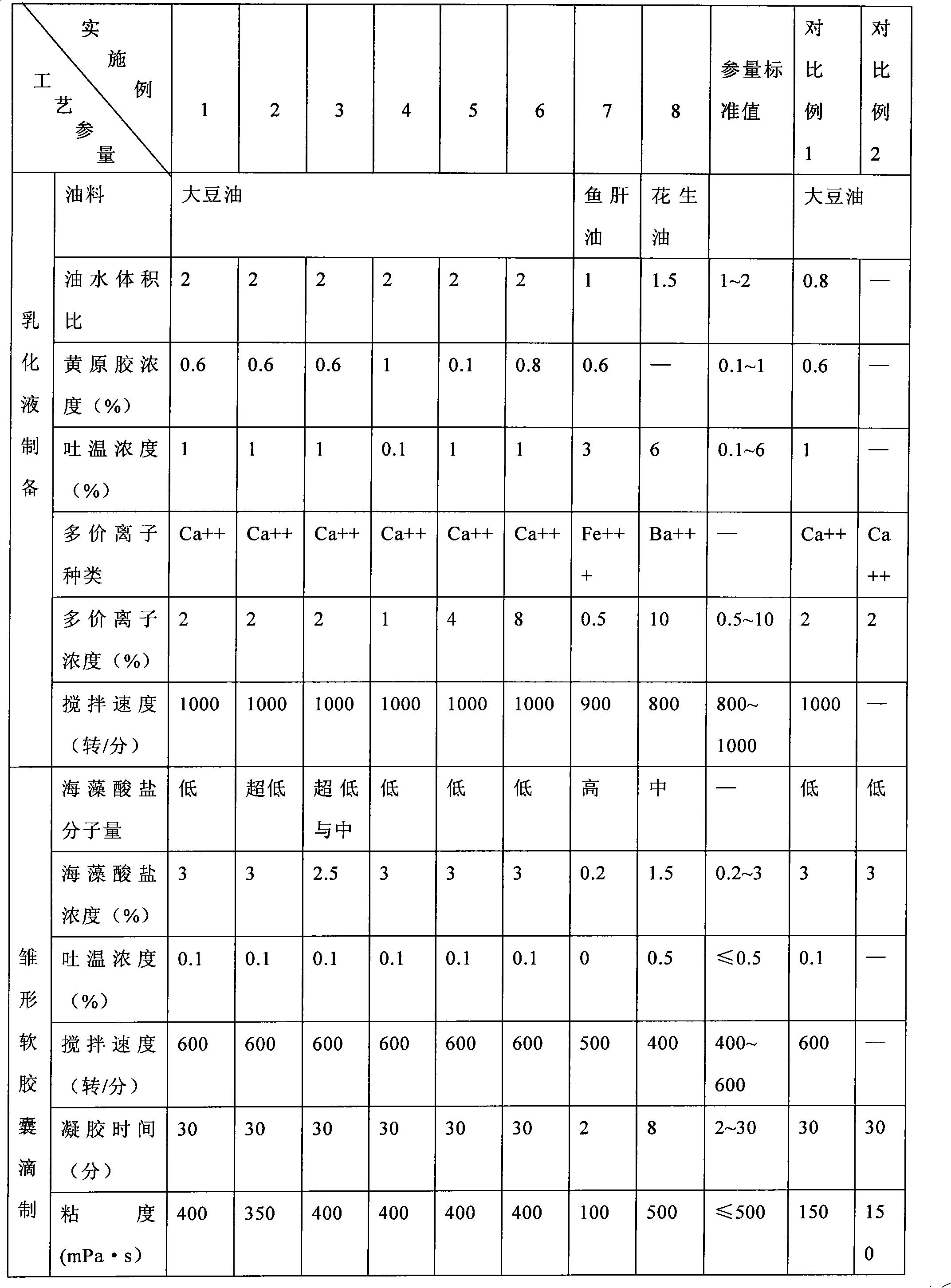
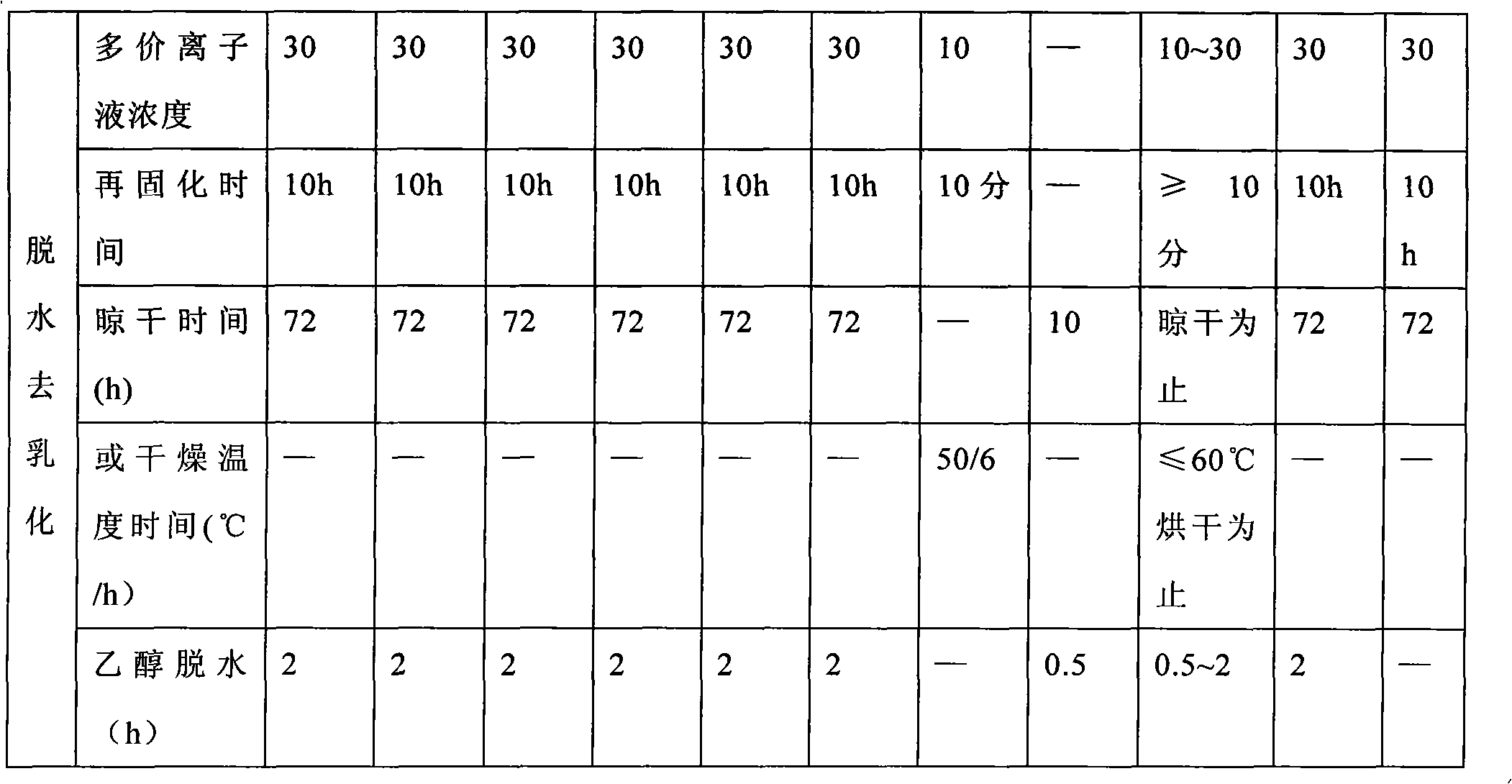
Method for preparing alginate soft capsule
ActiveCN101564667AAvoid eccentricityShrinkage is smallPharmaceutical non-active ingredientsCapsule deliveryLiquid stateOil water
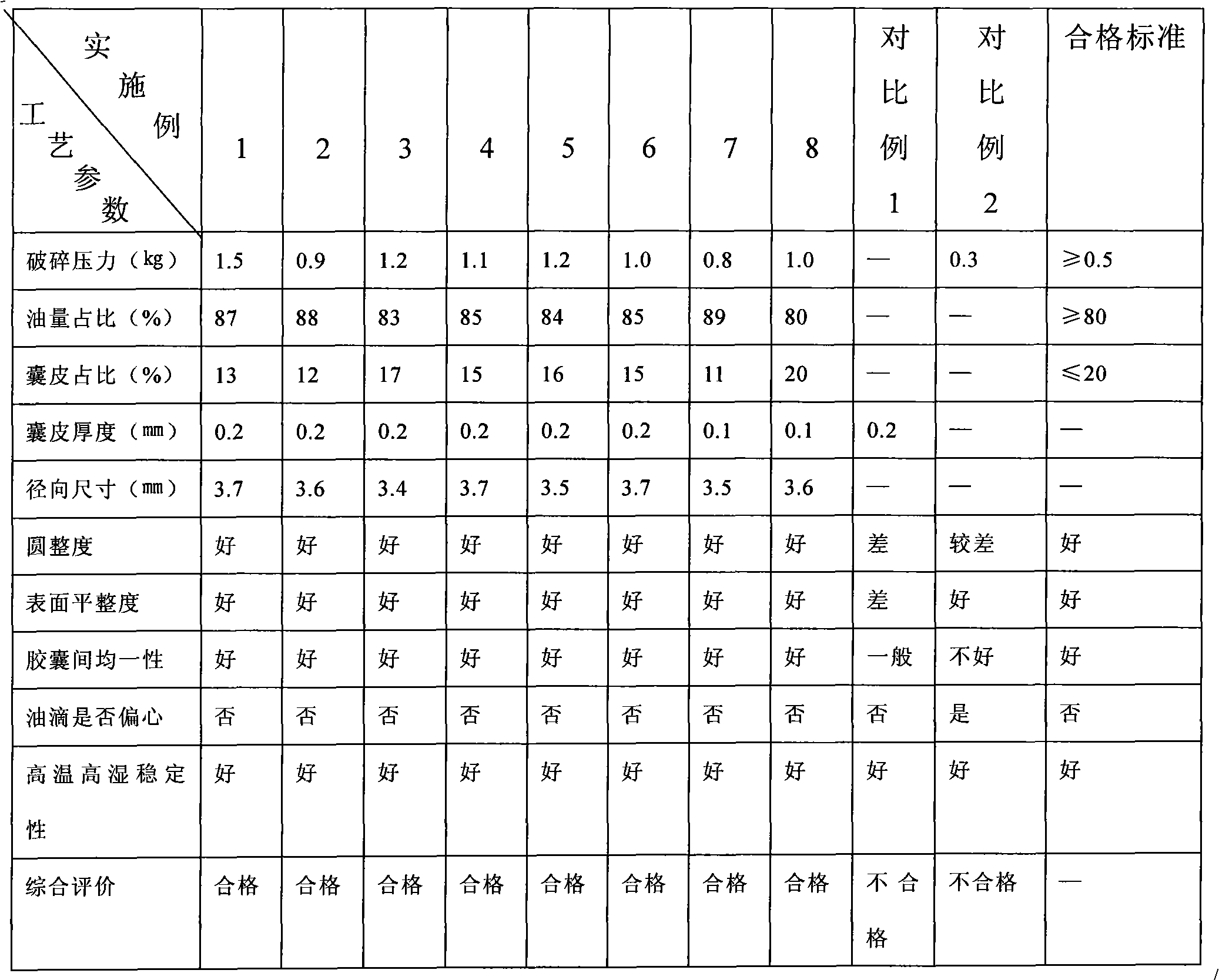
The invention relates to a method for preparing alginate soft capsule. By adopting a mode of emulsifying / de-emulsifying and emulsion dropping, oil-in-water emulsion containing polyvalent metal ions with high oil-water ratio is dropped into univalent soluble alginate solution, and then the polyvalent metal ions react with the univalent alginate solution to form insoluble alginate gel so as to obtain a primary soft capsule form of which outer layer is wrapped with the alginate gel; then, the primary soft capsule form is subjected to steps of drying and dehydrating so that the emulsion inside the soft capsule is de-emulsified; and finally, a circular soft capsule is obtained, wherein the interior of the circular soft capsule has oily liquid substances, the exterior of the circular soft capsule has an alginate gel wrapping layer, the radial size of the circular soft capsule is in millimeter scale, and the circular soft capsule can be directly taken orally. The soft capsule obtained by the method has the advantages of good mechanical property, even and round appearance, non-eccentric oil drops and high oil content; and the preparation method is simple and has low cost. Under the condition that the internal oily liquid substances are the same, the soft capsule which is prepared by the method and can be directly taken orally can be used for replacing gelatin soft capsules which are directly taken orally.
Owner:南京健辉生物科技有限公司
Formulation and manufacturing process for Coenzyme Q10 soft gel capsules
InactiveUS20050037066A1Improve solubility and stabilityPromote absorptionBiocidePeptide/protein ingredientsManufacturing technologyMedicine
A soft gelatine capsule formulation improved manufacturing of Coenzyme Q10, comprising Coenzyme Q10 in a thixotropic gelatine carrier capable of admixing without heating with Coenzyme Q10, and capable of keeping Coenzyme Q10 in suspension at ambient temperature.
Owner:SOFT GEL TECHNOLGIES
Solubilized CoQ-10 and carnitine
The present invention is directed to compositions and methods of delivery of CoQ-10 and an amino acid solubilized in monoterpenes. Use of monoterpenes as dissolving agents, greatly effects the ability to incorporate greater amounts of bioactive CoQ-10 and the amino acid, such as carnitine, i.e., carnitine tartrate, in formulations, such as soft gel capsules.
Owner:SOFT GEL TECHNOLGIES
Method for extracting squalene by using camellia oleosa seeds as raw materials and tea oil and squalene soft capsules
InactiveCN102146014AWide variety of sourcesPromote regenerationHydrocarbon active ingredientsAntipyreticChromatographic separationCamellia oleifera
The invention discloses a method for extracting squalene by using camellia oleosa seeds as raw materials and tea oil and squalene soft capsules and relates to the technical field of biomedicine. The extraction method comprises the following process flows of material preparation, grinding, leaching, concentration, methyl esterification, complexant-borax reaction, macroporous resin chromatographic separation and purification, concentration, extraction, washing, concentration, preparation of squalene crude oil and supercritical carbon dioxide extraction, so that squalene essential oil with the squalene content of more than or equal to 80 percent is obtained. The tea oil and squalene soft capsules are prepared from the following raw materials in percentage by weight: 87 to 90 percent of squalene essential oil with the squalene content of more than or equal to 80 percent, 3 to 5 percent of polyethylene glycol 400, 2 to 3 percent of beewax and 2 to 5 percent of phospholipid. The raw material resources are wide. The prepared tea oil and squalene soft capsules not only can be used for medicine, but also can be used for food therapy, are particularly suitable for administration of treatment and food therapy of patients suffering from hepatitis, fatty liver, acute and chronic inflammation, tumor, chemotherapy, diabetes mellitus and cardio-cerebrovascular disease and have no toxic or side effects.
Owner:李文东
Maniod ebish flower extract, extraction and analysis method and extract preparation and use thereof
InactiveCN101385748AGood treatment effectNotable mechanism of actionPill deliveryCapsule deliveryDiseaseAdditive ingredient
An extract from Sunset Abelmoschus, containing 50-75wt% of total flavone counted by hyperin, wherein, the contents of hyperin, isoquercitrin and quercetin-3'-glucoside are 10.0-15.0wt%, 7.0-12.0wt% and 8.0-12.0wt% respectively, and a total flavone finger-print is provided. The extract is obtained by the following steps: a concentrated solution of a Sunset Abelmoschus ethanol extract undergoes chromatography through an HPD400 or HPD700 macroporous absorbent resin column to derive a crude extract or is extracted by normal butanol to derive the crude extract; a refined extract is obtained by refluxing extraction with ethyl acetate-ethanol azeotropic mixture or ethyl acetate-methanol mixture; the refined extract goes through pressure reducing exsolution and drying. Corresponding contents and a method for detecting finger-print are also provided. The extract preparation is a dripping pill, tablet, capsule or soft capsule obtained through processing with the extract as an active ingredient and pharmaceutical excipients. The extract can be used for preparing a medicine preparation for treating cardiac anencephalohemia diseases.
Owner:周春晓 +1
Cod liver oil soft capsule appearance defect visual detection method and device
ActiveCN105784711AAccurate detectionImprove accuracyOptically investigating flaws/contaminationCod liver oilImaging Feature
The invention discloses a cod liver oil soft capsule appearance defect visual detection method and a detection device thereof. The method consists of: shooting horizontally transferred bubble caps line by line with a camera tilting downward to obtain shot pictures; calling bubble cap templates matching the shot pictures to obtain the inner slot positions of the bubble caps; acquiring the regional images of the inner slot positions corresponding to the shot pictures, extracting a soft capsule outer contour from the regional images by gray threshold segmentation, and judging whether particle deficiency exists accordingly, and if so, sending out appearance defect information; matching the regional images with the cod liver oil soft capsule shape to separate and extract a fish body regional image and a fish tail regional image; and respectively calculating the image features of the fish body regional image and the fish tail regional image, and judging whether abnormity or empty shell exists accordingly, and if so, sending out appearance defect information. The detection method provided by the invention not only can rapidly and accurately detect the defects of multiple particles and particle deficiency in cod liver oil soft capsules within bubble caps, but also can detect a variety of other appearance defects in cod liver oil soft capsules.
Owner:厦门威芯泰科技有限公司
Formulation and manufacturing process for Coenzyme Q10 soft gel capsules
InactiveUS20050031681A1Improve solubility and stabilityPromote absorptionBiocidePeptide/protein ingredientsManufacturing technologyMedicine
A soft gelatine capsule formulation improved manufacturing of Coenzyme Q10, comprising Coenzyme Q10 in a thixotropic gelatine carrier capable of admixing without heating with Coenzyme Q10, and capable of keeping Coenzyme Q10 in suspension at ambient temperature.
Owner:SOFT GEL TECHNOLGIES
Grifola frondosa strain, culture method and application thereof
The invention discloses a drug and edible two-use fungus grey ramalic GF-932[Grifola frondosa (Dichs.ex Fr.) S.F.Gray] and producing method of grey ramalic beta-dextran, which is characterized by the following: applying in the tablet, soft capsule and hard capsule, liquid drugs injection and freeze dried; reserving in the China biological bacterial reserving management committee common microbe center with CGMCC No.1709 as reserving number.
Owner:CHAMBROAD CHEM IND RES INST CO LTD
A method for preparing hirudin by making leech bloodsucker as raw material and application thereof
ActiveCN1883510AFix extraction issuesStrong targetingMetabolism disorderCardiovascular disorderMedicineCurative effect
The invention discloses a process for preparing hirudin by using blood- suction leeches, as well as its use, the preparing process consists of pulping leeches, formulating suspension and drying. The substance can be prepared into capsules, soft capsules, granules and tablets. The obtained medicament can be used for treating cardiovascular and cerebrovascular diseases including cerebral apoplexy, hypertension, hyperlipemia and coronary disease.
Owner:周维官
Solubilized CoQ-10 and carnitine
The present invention is directed to compositions and methods of delivery of CoQ-10 and an amino acid solubilized in monoterpenes. Use of monoterpenes as dissolving agents, greatly effects the ability to incorporate greater amounts of bioactive CoQ-10 and the amino acid, such as carnitine, i.e., carnitine tartrate, in formulations, such as soft gel capsules.
Owner:SOFT GEL TECHNOLGIES
Light-stabilized soft capsule formulations
ActiveUS20100021535A1Effective lightingSufficient lightingBiocideOrganic active ingredientsWater solubleBULK ACTIVE INGREDIENT
An object of the present invention is to provide a small-sized light-stabilized soft capsule formulation, which has a shell that ensures effective light shielding of an active ingredient encapsulated thereby.The present invention provides a light-stabilized soft capsule formulation comprising a shell containing a non-water-soluble light-shielding agent and having an average thickness of 200 μm or less, and a medicament encapsulated by the shell.
Owner:CHUGAI PHARMA CO LTD
System and method for dusting soft capsules
InactiveUS20080166477A1Prevent surfaceConfectioneryVacuum evaporation coatingParticulatesSystems design
A capsule dusting system is designed to expose capsules to a dusting agent in a controlled manner. The system incorporates a tumbling basket positioned within an enclosure. The tumbling basket is loaded with capsules and is rotatably connected to a drive shaft. A dust injection system meters the dusting agent into the dust injection system. The dust injection system may include a dusting injector that translates between two positions. At one position, the dusting injector is loaded with dusting agent by a powder supply system. At the other position, the dusting injector is positioned to inject the dusting agent into the tumbling basket. A gas is fluidly connected to the dusting injector to cause the dusting agent to disperse into the tumbling basket. The enclosure contains the dusting agent within the system to reduce the environmental, health, and safety hazards associated with airborne particulates.
Owner:R P SCHERER TECH INC
Weight-losing soft capsules and preparation method thereof
ActiveCN102188565AImmune Boosting PropertiesReduce absorptionOrganic active ingredientsMetabolism disorderBlueberry extractMedicine
The invention relates to weight-losing soft capsules, which comprise soft capsule bodies and a capsule content, and are characterized by being prepared by the following steps: weighing the content including 60 to 80 weight percent of conjugated linoleic acid, 5 to 15 percent of L-carnitine, 5 to 15 percent of green tea extract, 1 to 10 percent of konjaku flour, 1 to 10 percent of blueberry extract, uniformly stirring, grinding, and degassing; weighing gelatin, water and glycerol according to a ratio of 100:100:40, heating to dissolve the material, and degassing to obtain capsule body filtrate; and delivering the capsule body filtrate and the content to a pelleting press to make pellets, sizing, drying, washing pellets, sorting and packaging to obtain the finished product. The konjaku flour, blueberry extract, conjugated linoleic acid, L-carnitine and green tea extract, which are selected in the invention, act together to achieve an optimal weight-losing effect. The dose is scientific, and the weight-losing soft capsules have health-care effects; and the preparation method is reasonable in process and high in operability and makes large-scale production easy.
Owner:WEIHAI BAIHE BIOTECH
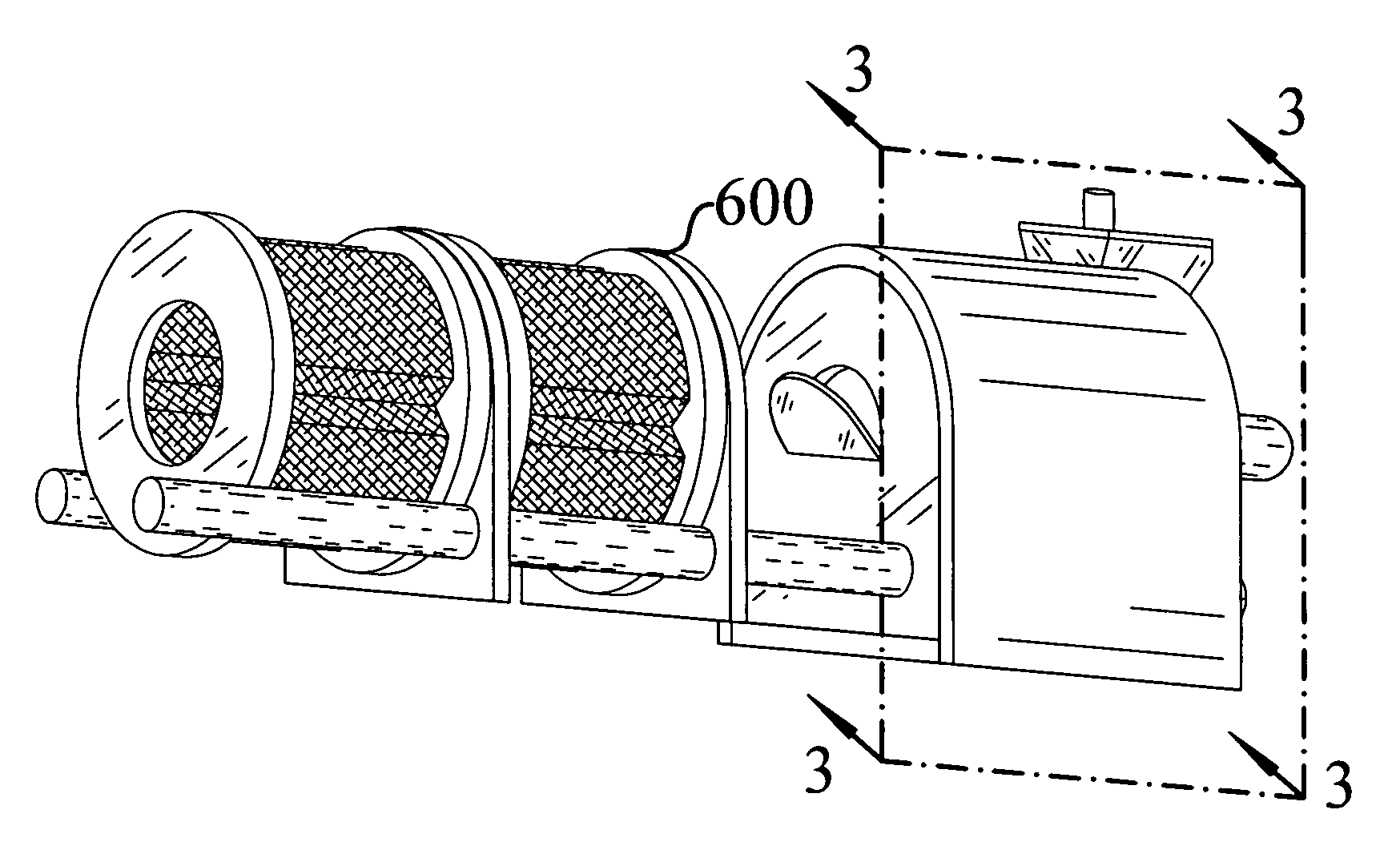
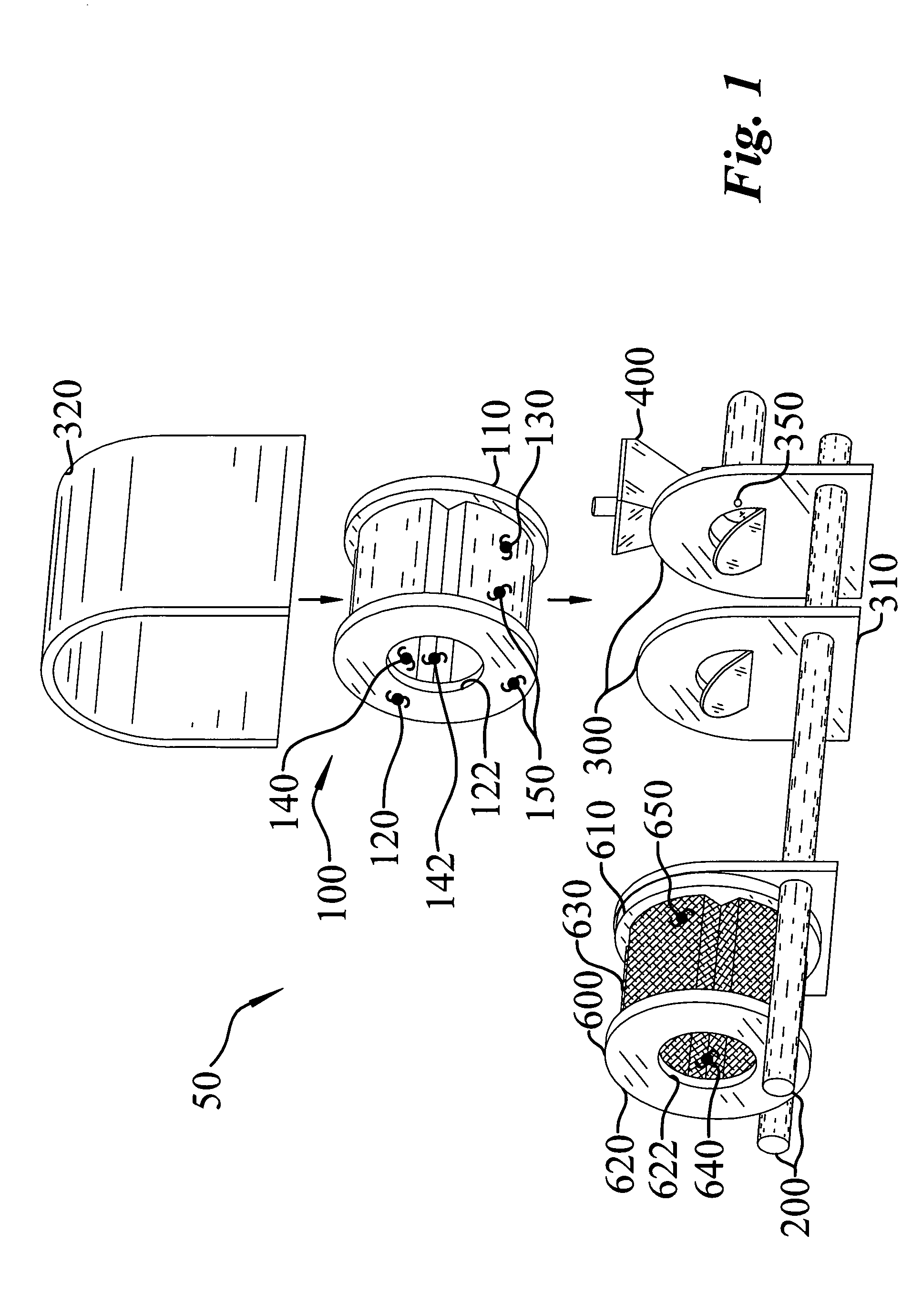
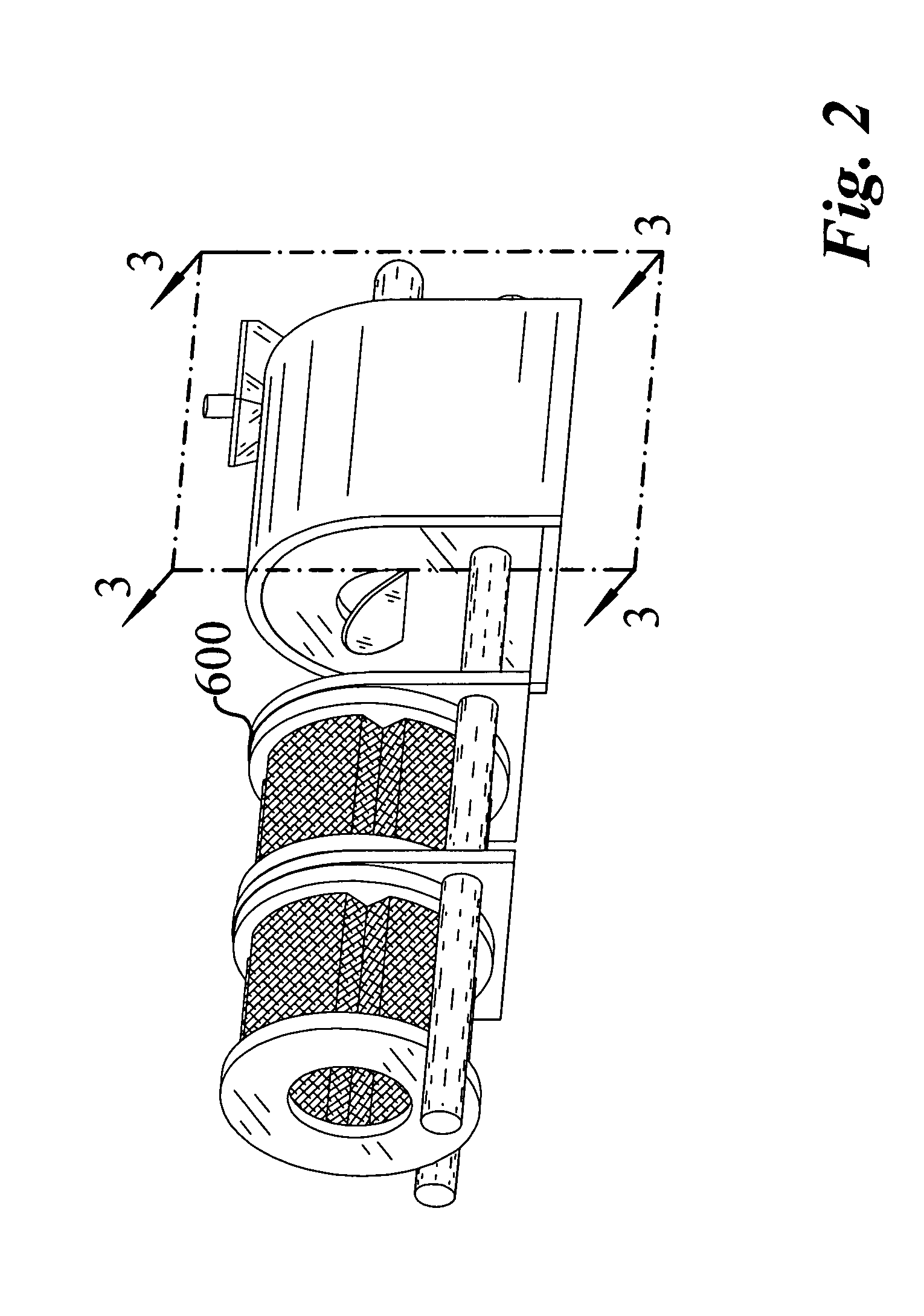
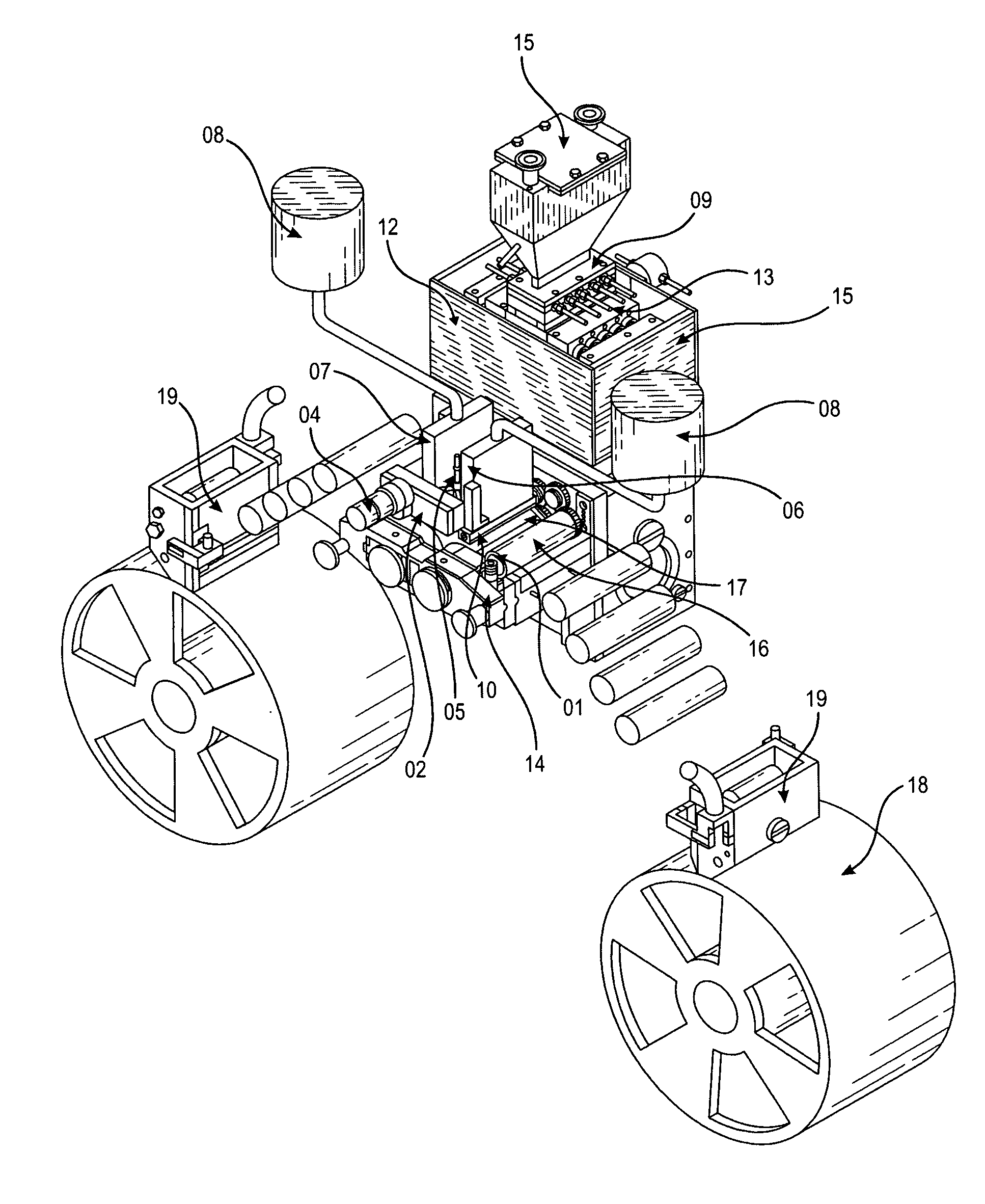
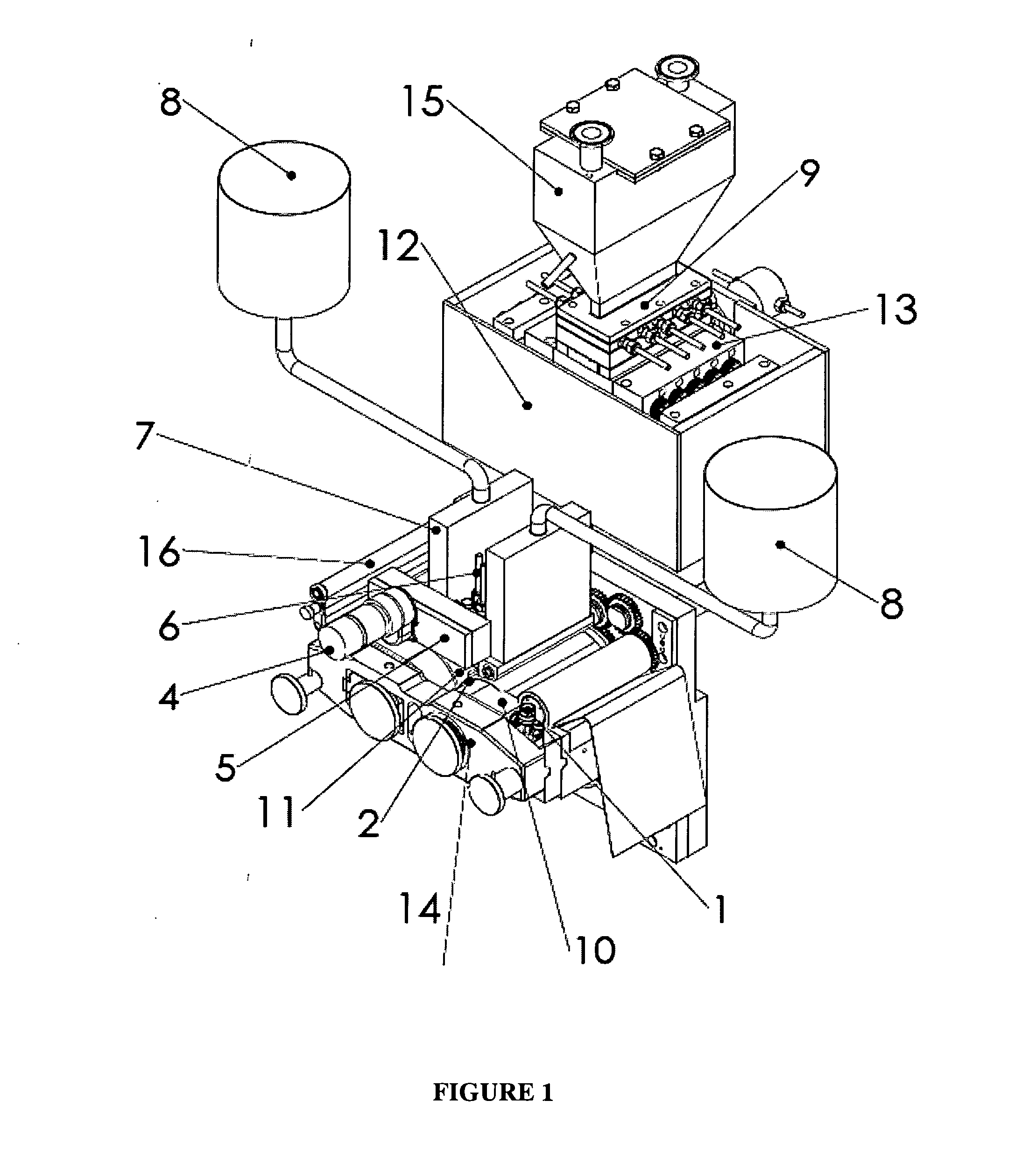
Apparatus and process for encapsulating microparticles with liquid in soft gel capsules
ActiveUS20120058179A1Pharmaceutical product form changeCeramic shaping apparatusNanoparticleEngineering
The invention provides an apparatus for producing soft gel capsules having encapsulated therein microparticles, nanoparticles and fluids, said apparatus comprising: (a) two spreader boxes; (b) two casting drums; (c) a pair of rotary dies; (d) a liquid fill system (medicine pump system); (e) a wedge for heating gelatine ribbons and feeding said fill; and (f) one or more microgranule or nanogranule feeders located on each side of the rotary dies, said feeders being synchronized to rotate at the same tangential speed as the rotary dies.
Owner:PROCAPS
Homogeneous, thermoreversible low viscosity polymannan gum films and soft capsules made therefrom
Abstract of the Disclosure The present invention is directed to a homogeneous, thermoreversible gel film comprising a film forming amount of low viscosity polymannan gum, e.g., low viscosity guar gum, and optionally at least one of a plasticizer, a second film former, a bulking agent, and a pH controlling agent; and processes for the preparation thereof. The present invention is also directed to soft capsules and solid forms containing the gel film, as well as processes for the preparation thereof.
Owner:FMC CORP
Traditional Chinese medicine for preventing and treating Alzheimer disease and preparation method thereof
ActiveCN102078460AApplicable treatmentNo side effectsNervous disorderPlant ingredientsDiseaseChemical synthesis
The invention discloses a traditional Chinese medicine for preventing and treating Alzheimer disease and a preparation method thereof. The traditional Chinese medicine is prepared from the following raw materials in parts by weight: 12-16 parts of prepared fleece flower root, 8-12 parts of ginseng, 9-13 parts of rhizoma acori graminei, 5-9 parts of coptis and 6-10 parts of chuanxiong rhizome. The traditional Chinese medicine can be prepared into granules, capsules, soft capsules, dissolved medicines, dripping pills and soft extracts, has the advantages of simple manufacturing process and low cost, is completely prepared from natural plants, does not contain hormones, has no addition of pigments and other chemical synthetics, and does not have toxic side effects on human bodies after being continuously used. The traditional Chinese medicine contains various effective components such as flavones, saponins, phenolic acids, alkaloids, polysaccharides and the like capable of being easily absorbed by human bodies, can be effectively used for nourishing the kidney and invigorating the brain, replenishing qi and dissolving turbidity and promoting blood circulation and eliminating toxicity, and is suitable for treating deficiency of the kidney, mild cognitive impairment caused by obstruction of the orifices by blood stasis, Alzheimer disease and the like.
Owner:XIYUAN HOSPITAL OF CHINA ACAD OF CHINESE MEDICAL SCI
Propolis-lycopene composition and preparation method thereof
ActiveCN102824371AAnthropod material medical ingredientsFungi medical ingredientsBiotechnologyLycopersene
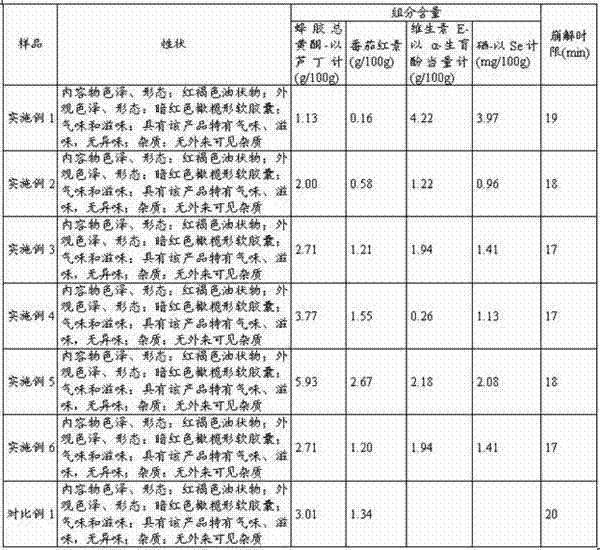
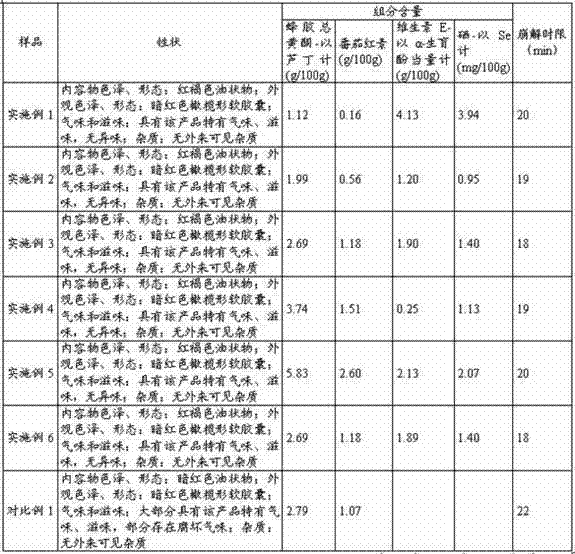
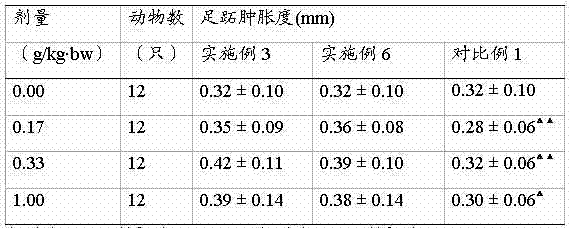
The invention relates to a propolis-lycopene composition. The propolis-lycopene composition comprises: by weight, 2 to 6 parts of propolis, 0.8 to 7.5 parts of a lycopene raw material, 0.07 to 2.2 parts of vitamin E, and 0.075 to 0.5 parts of a selenium nutrient. The invention also relates to a propolis-lycopene composition preparation and especially relates to a propolis-lycopene composition soft capsule and a preparation method thereof. The propolis-lycopene composition is prepared from natural raw materials and has a scientific formula and controllable quality. The propolis-lycopene composition soft capsule has good stability and good disintegration effects. An animal experiment proves that the propolis-lycopene composition soft capsule has an effect of improving immunity and also has good safety.
Owner:石药集团中诺药业(泰州)有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com