Patents
Literature
103 results about "Ethanol Injection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
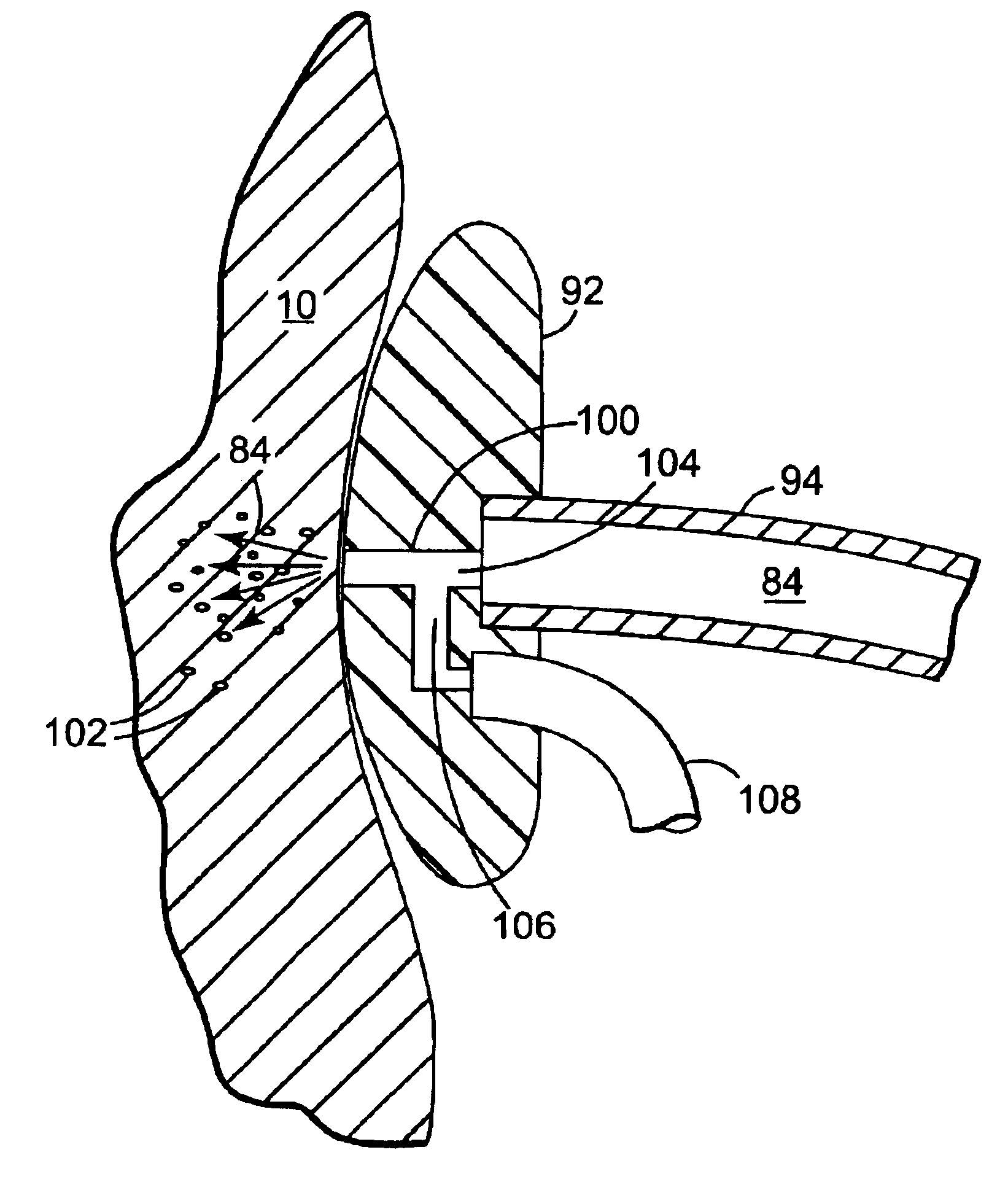
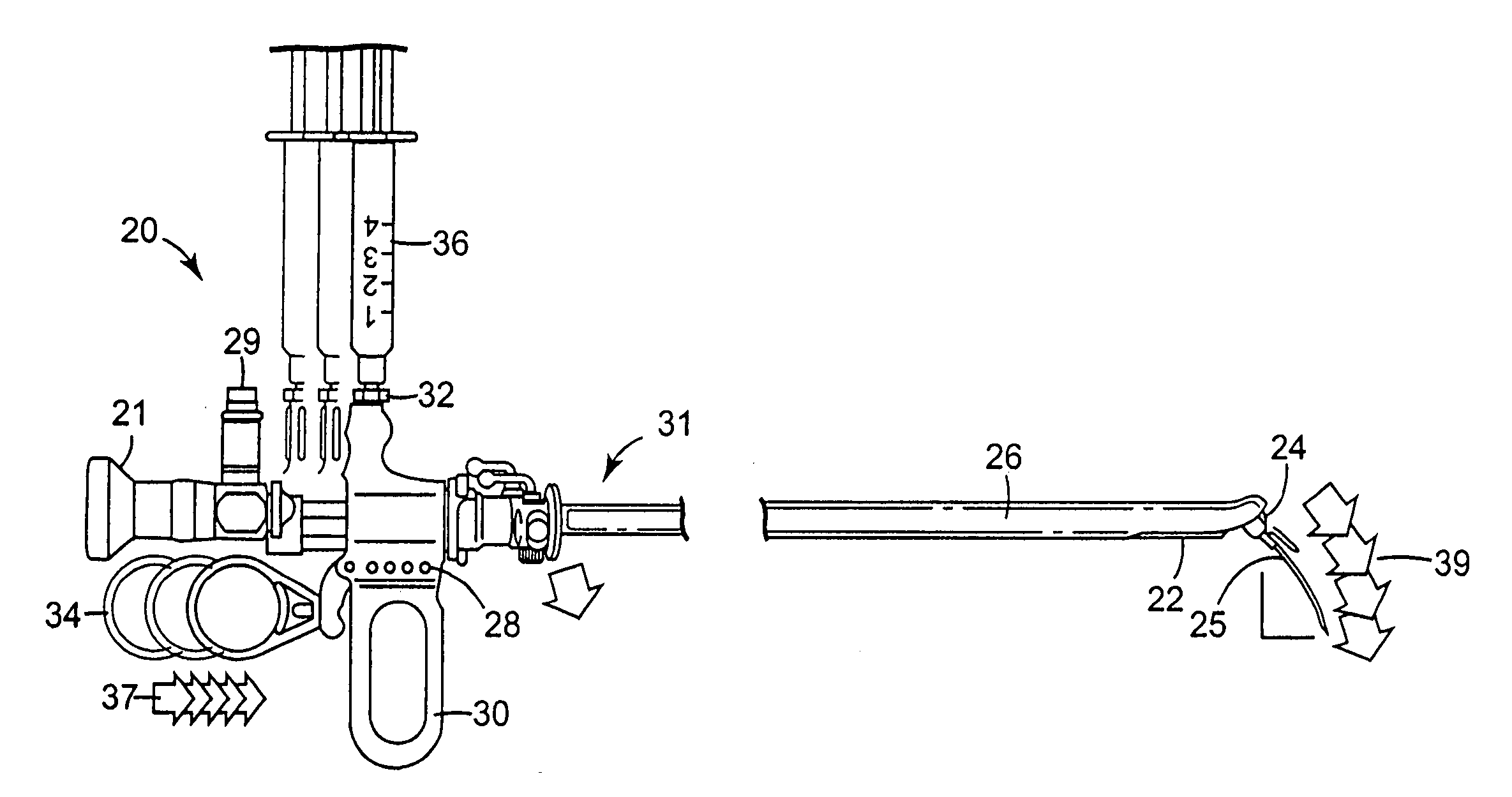
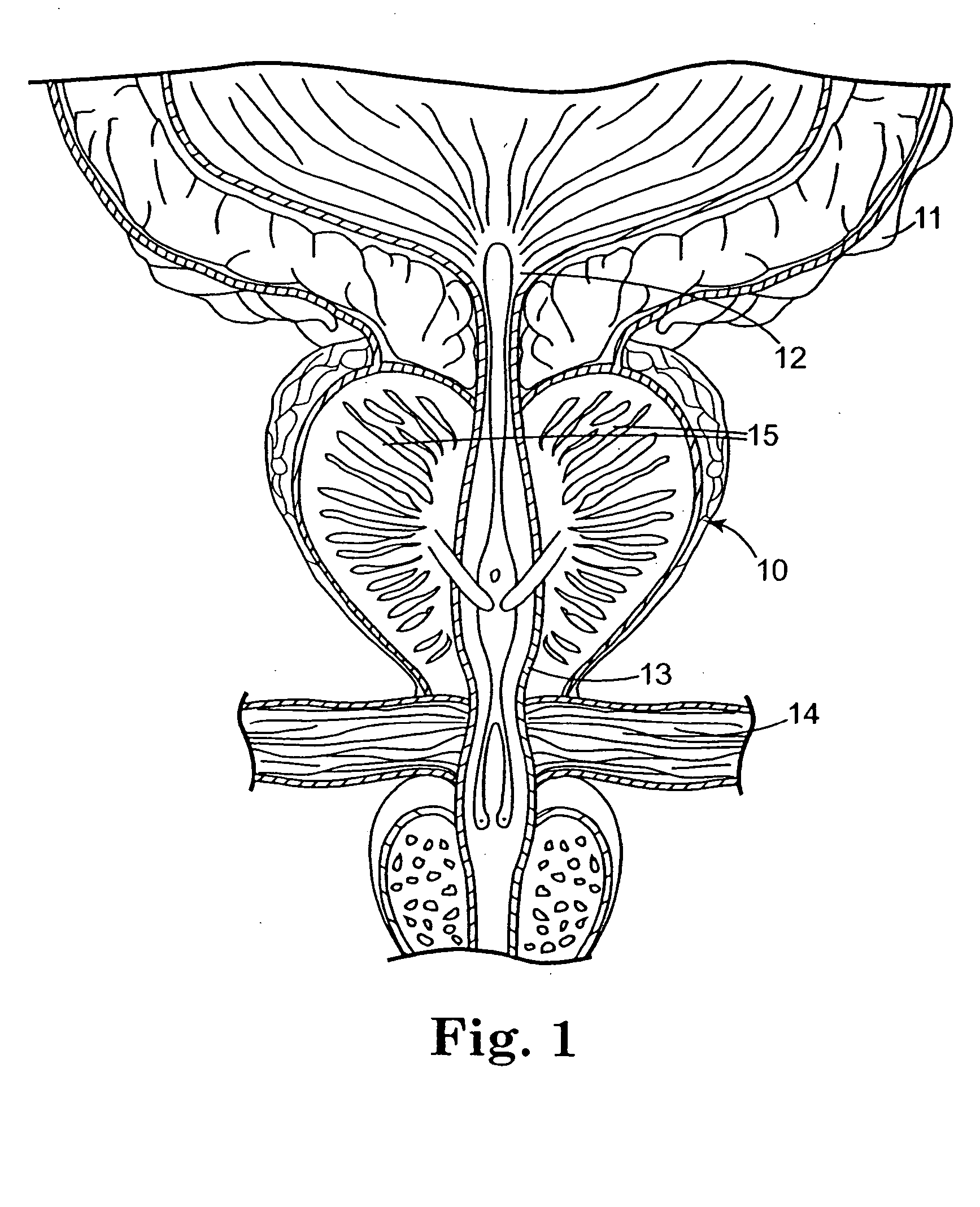
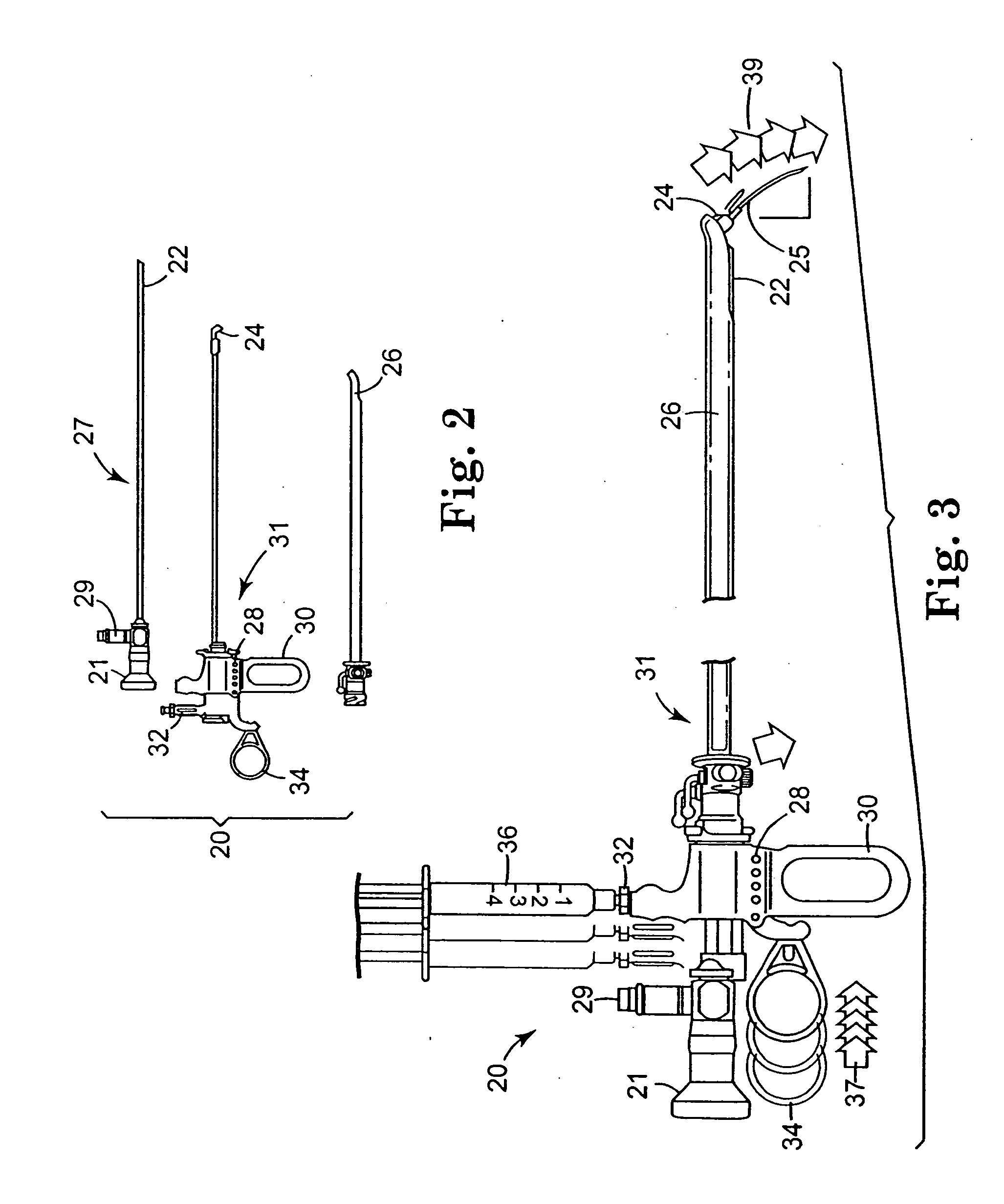
Method of injecting a drug and echogenic bubbles into prostate tissue
InactiveUS6905475B2Ultrasonic/sonic/infrasonic diagnosticsJet injection syringesNeedle Free InjectionEthanol Injection
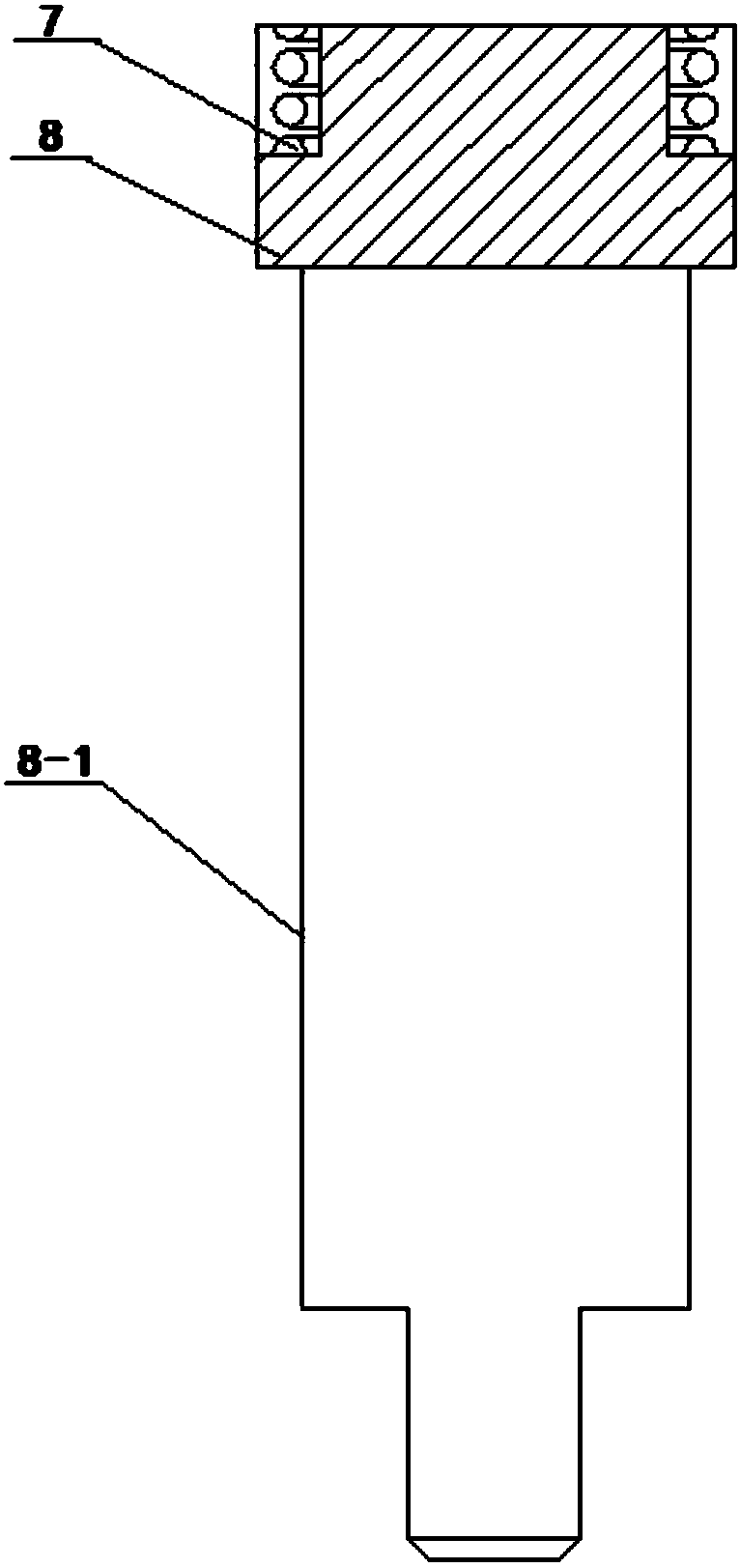
Method and surgical instrument for treating prostate tissue including a surgical instrument having a main body, a needle deployment port, a needle, first and second handles and a lockout release mechanism to limit needle extension. Additionally, a kit includes the surgical instrument, together with a cystoscope, and optionally a syringe and reservoir of ethanol. The method includes needle-less injection and visualizing the ethanol injection by delivering both an echogenic agent and ethanol either by needle or needle-less injection or by providing an ultrasonically visible marker near the tip of the ethanol delivery cannula. The method also includes extending the needle transversely of the instrument housing using a link assembly.
Owner:BOSTON SCI SCIMED INC
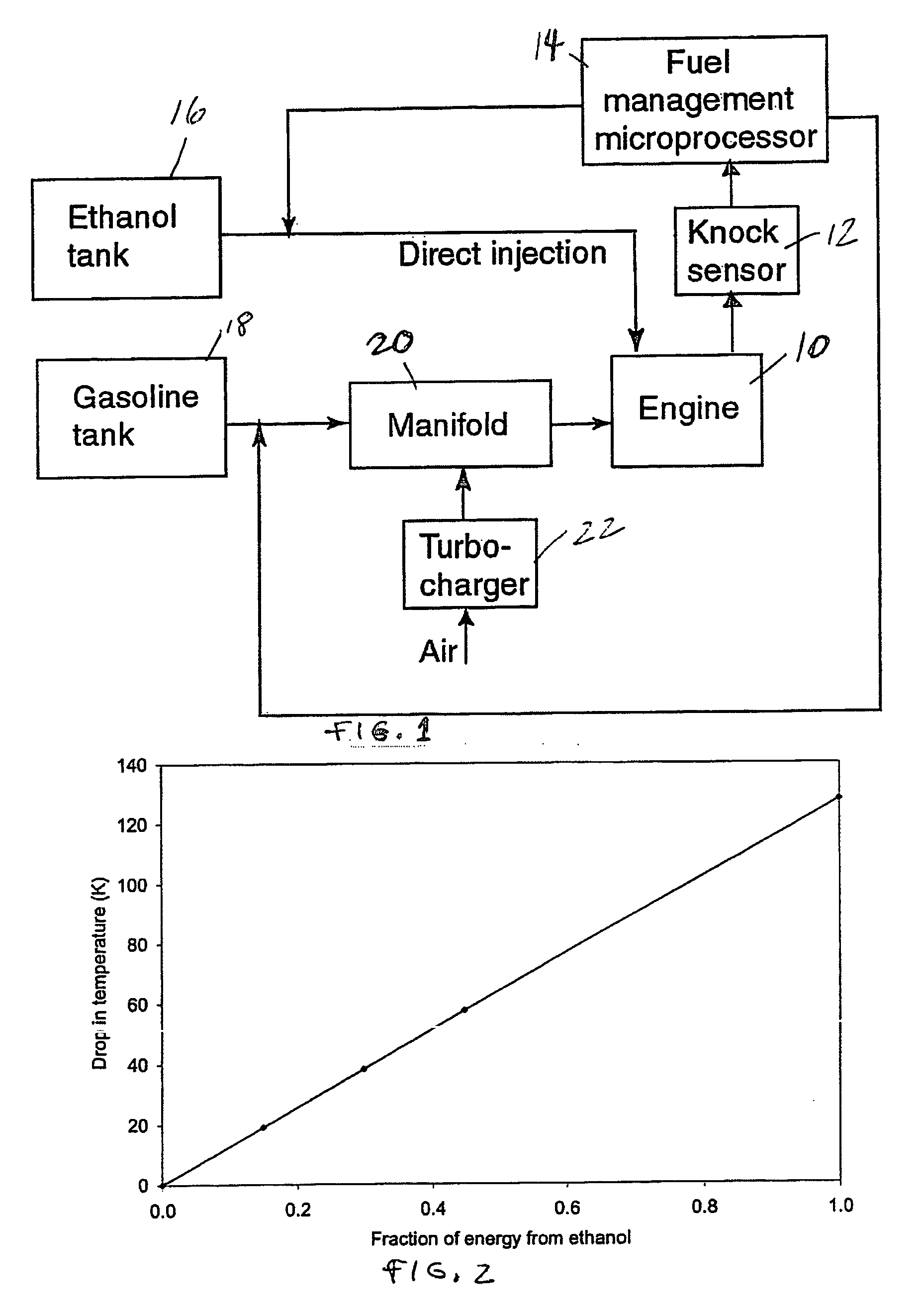
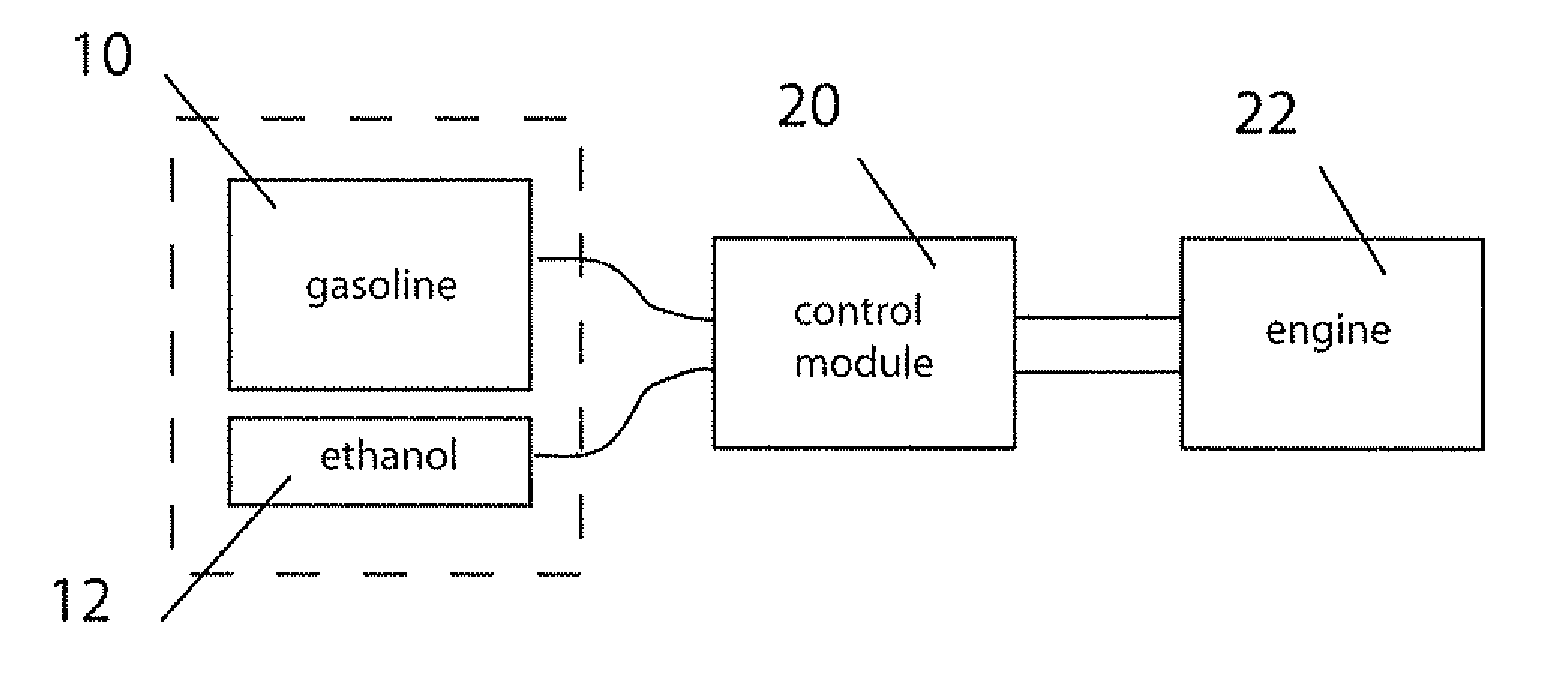
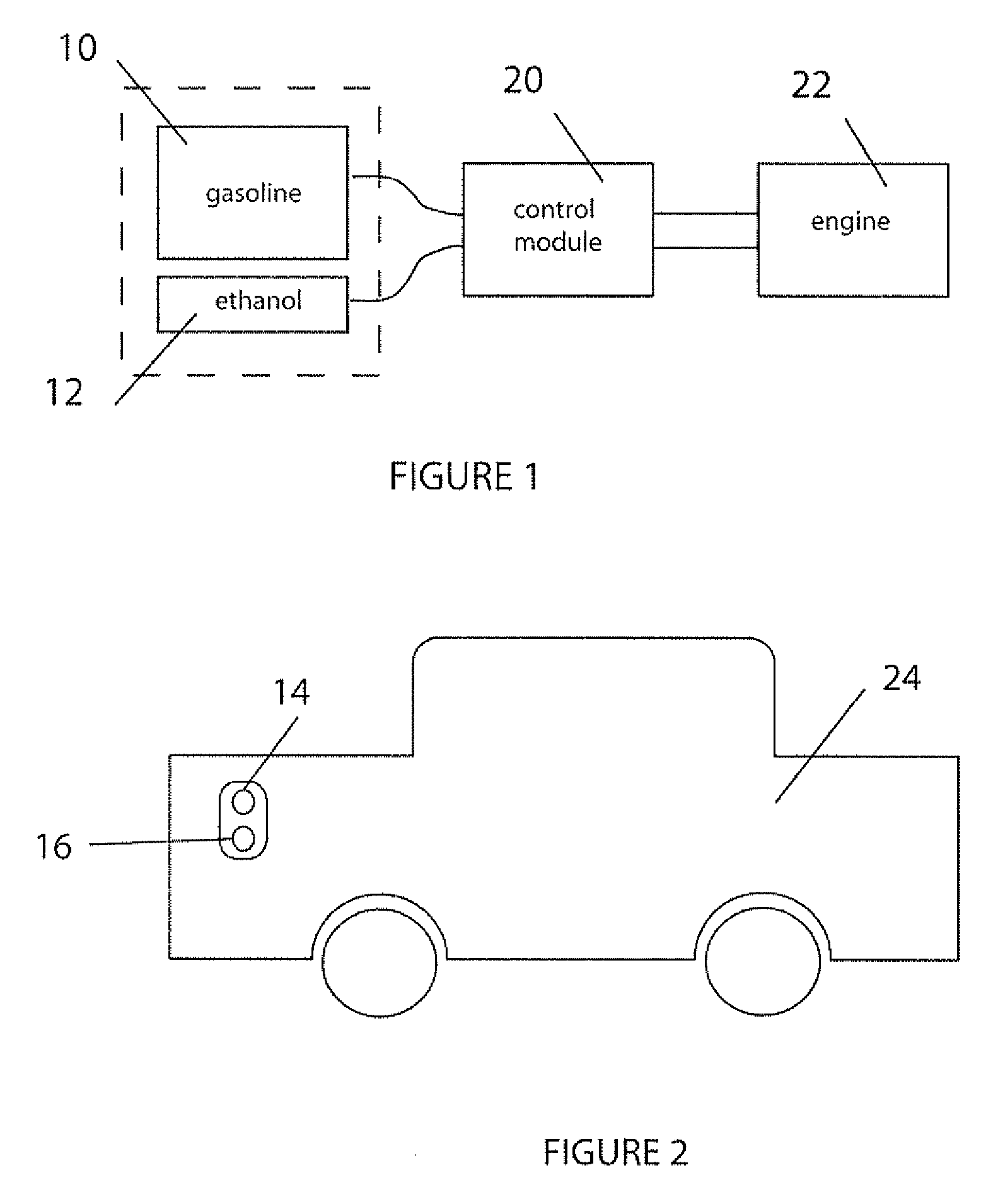
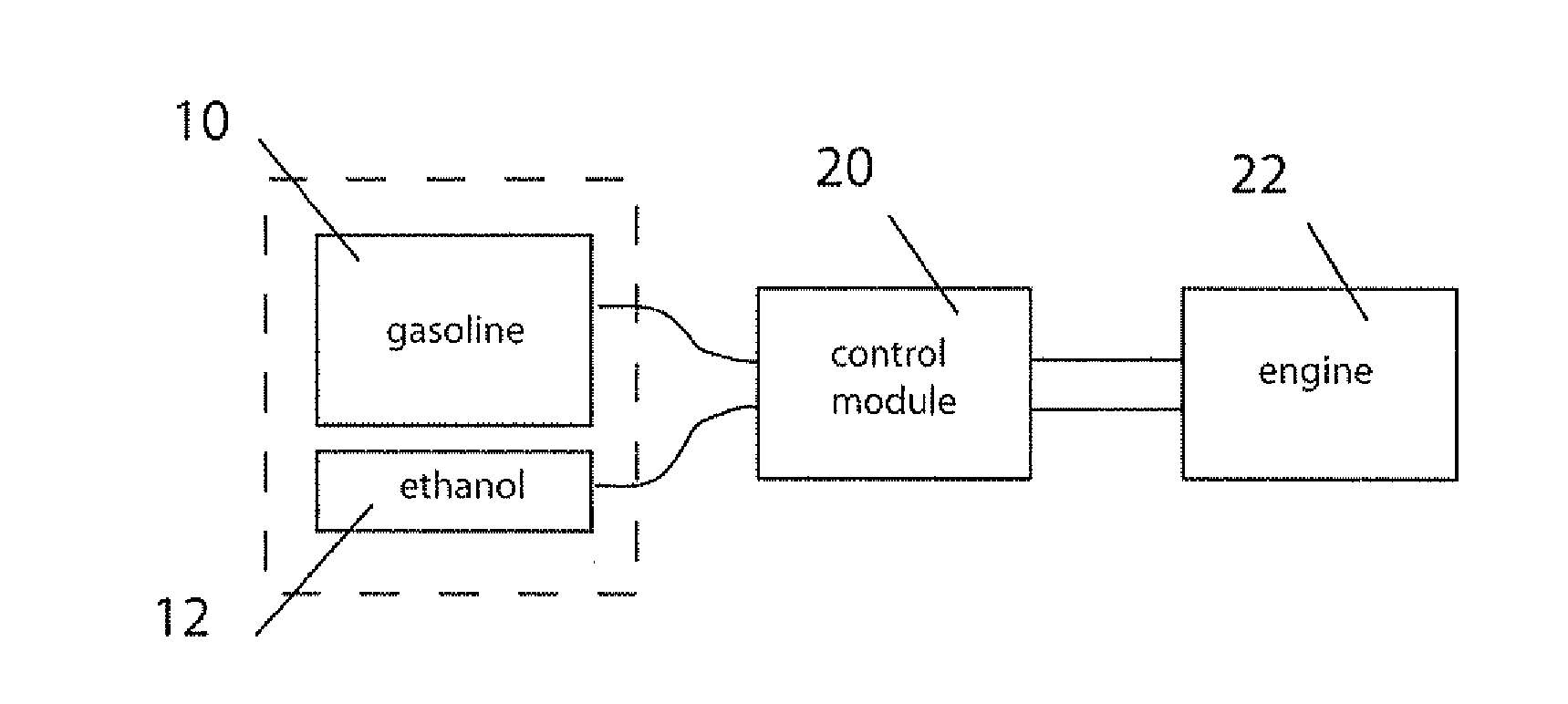
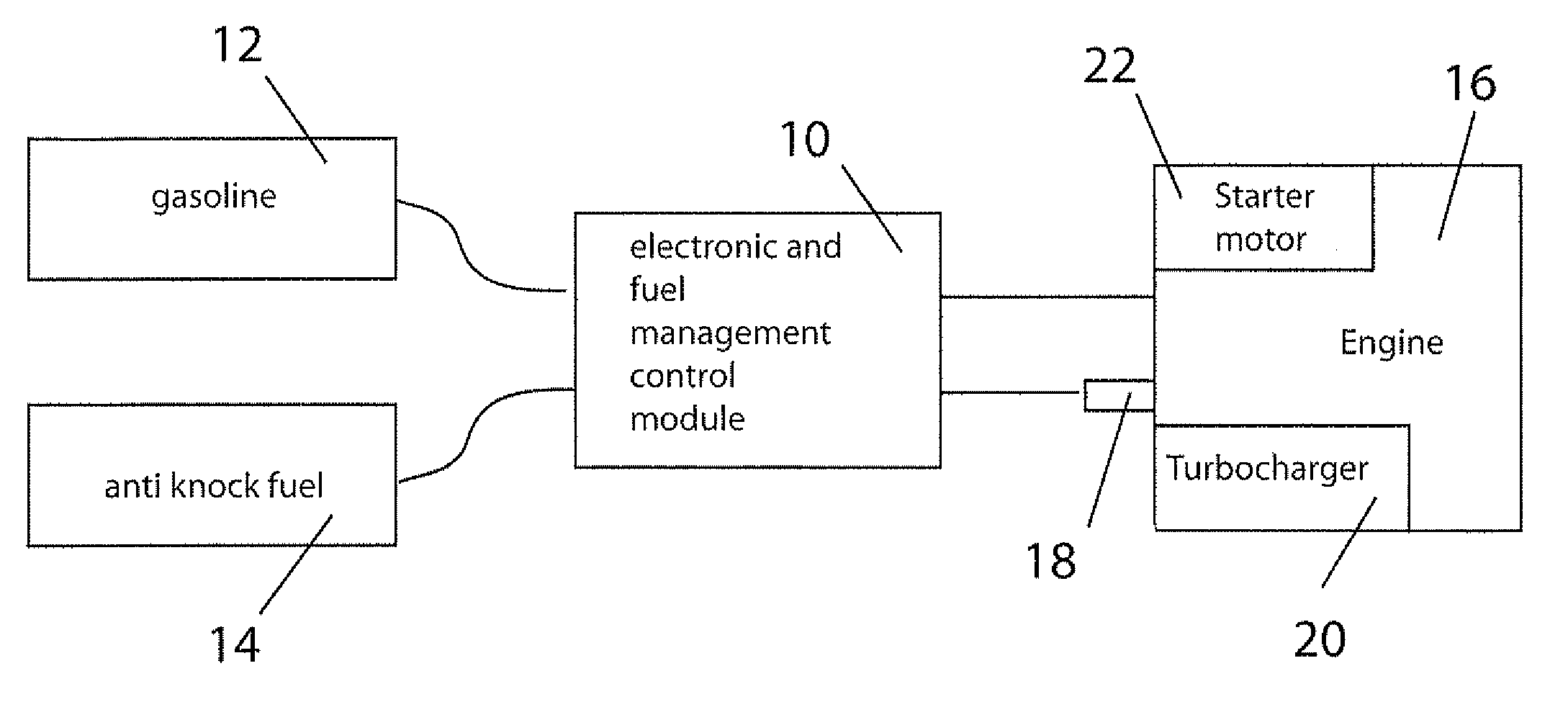
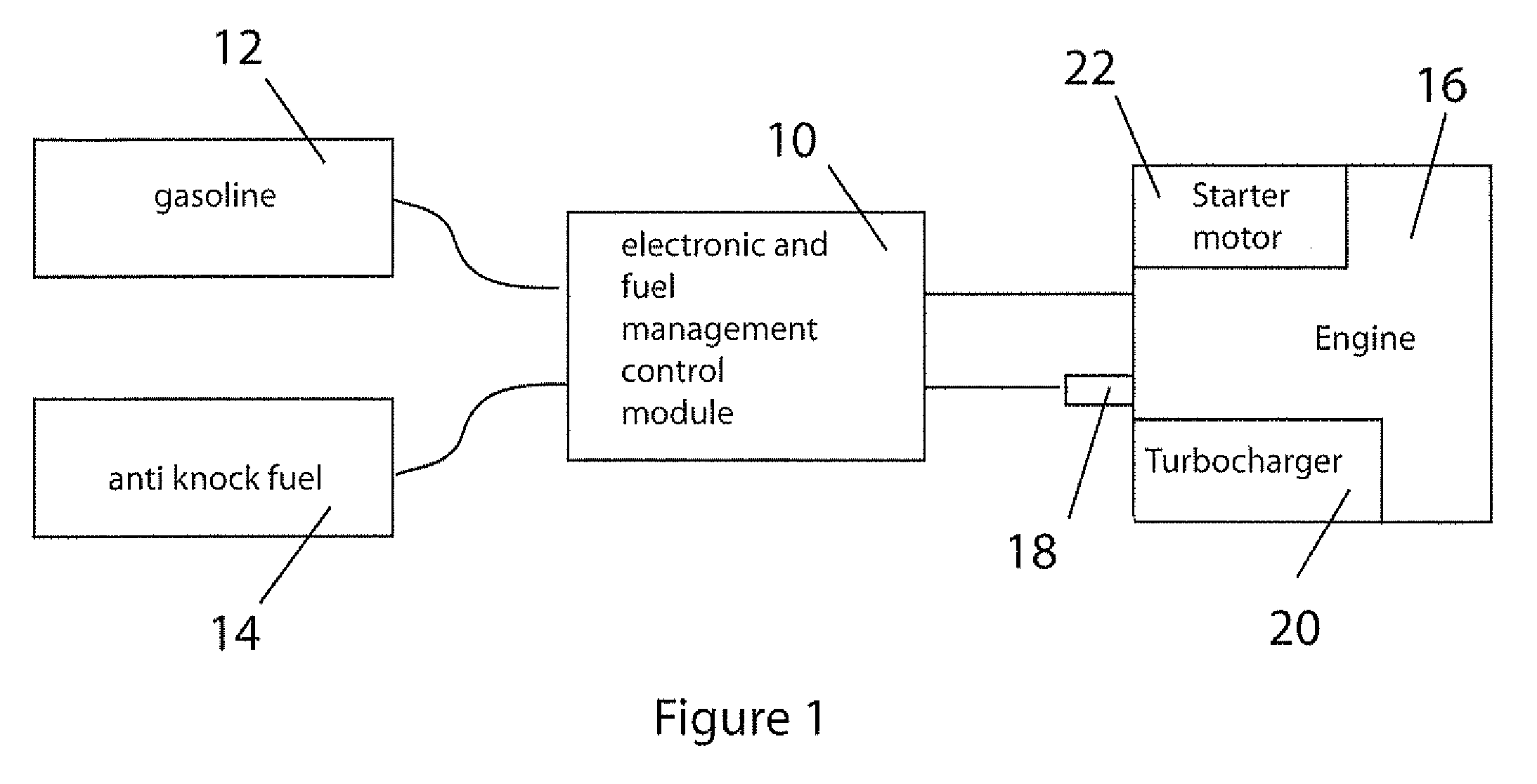
Fuel management system for variable ethanol octane enhancehment of gasoline engines
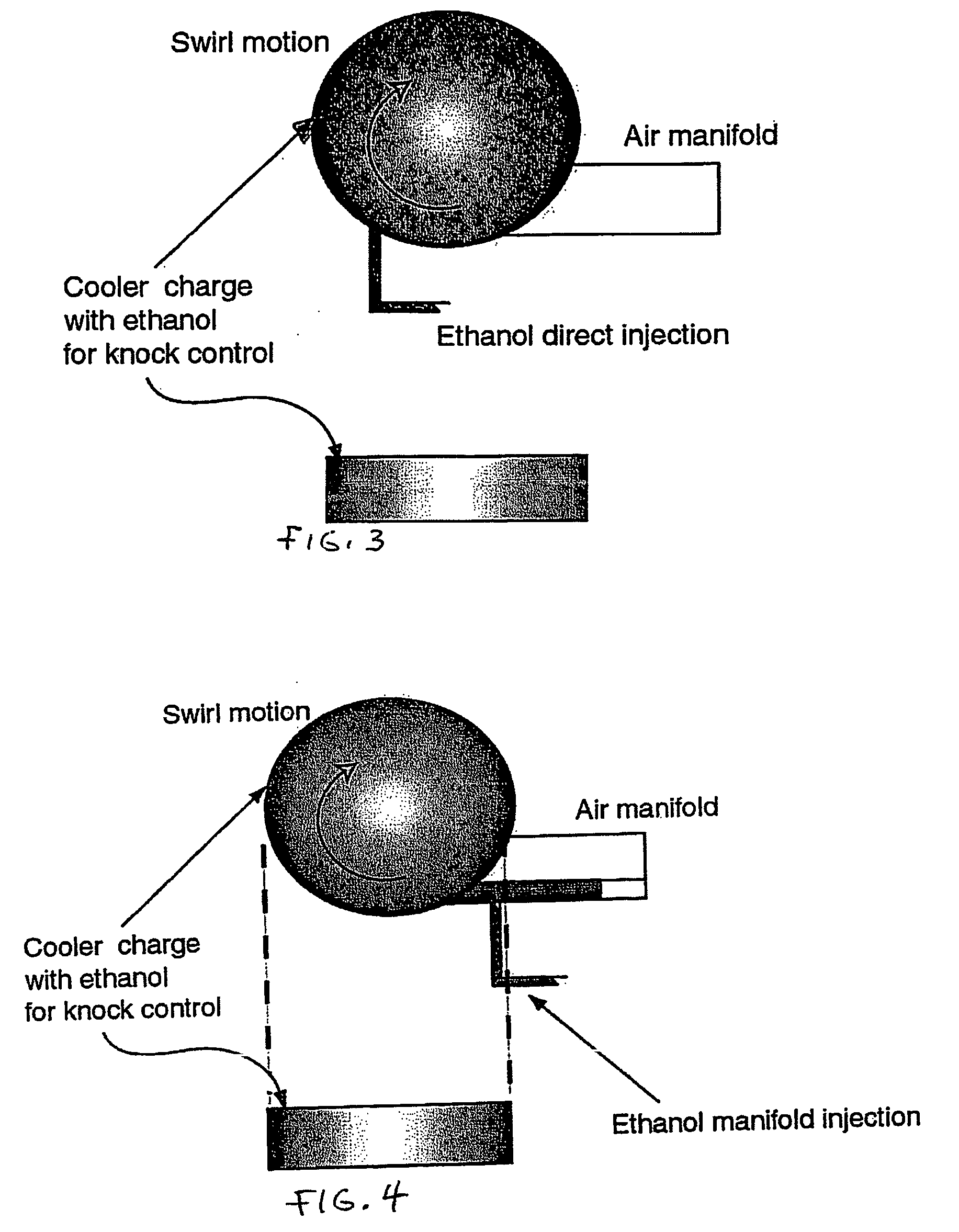
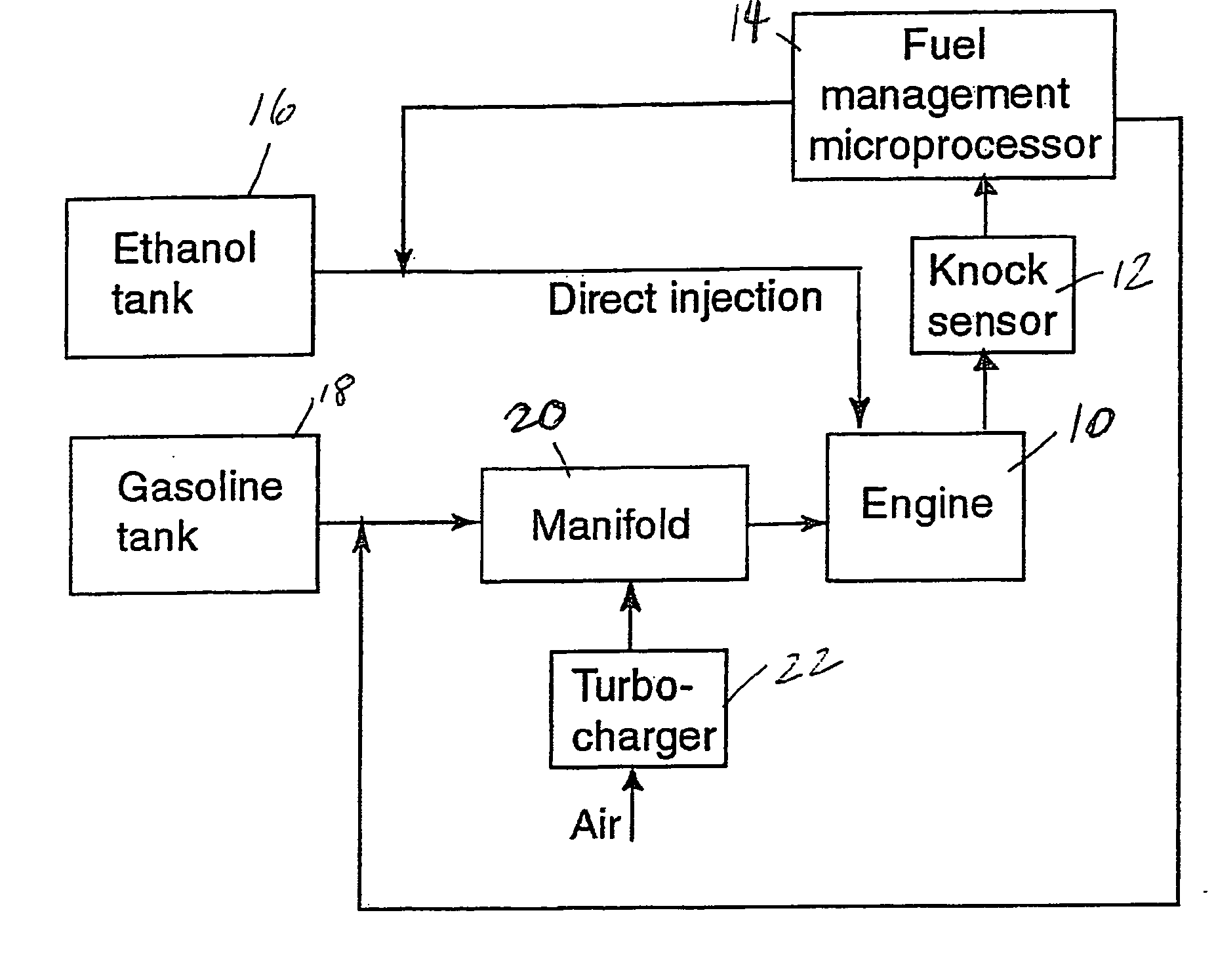
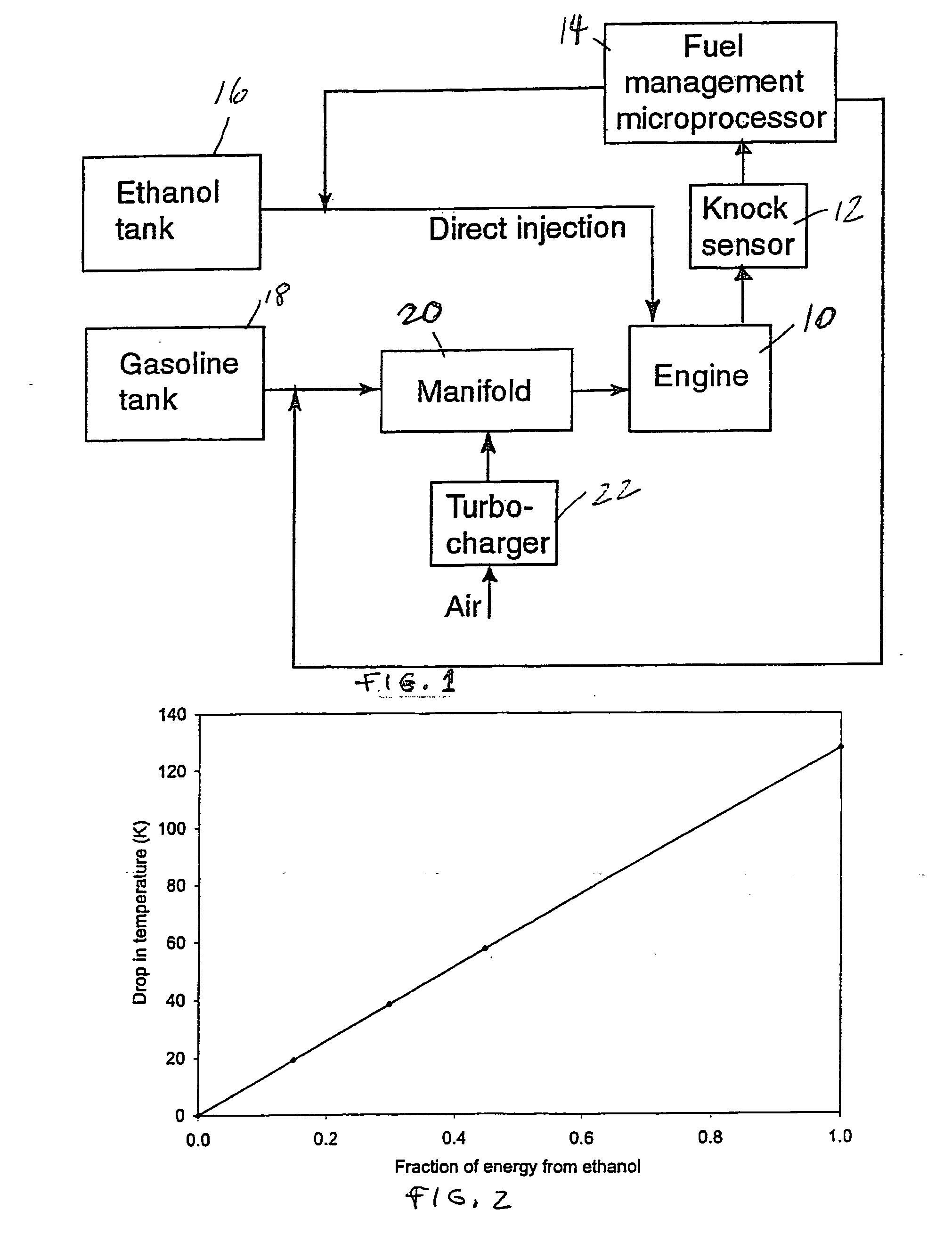
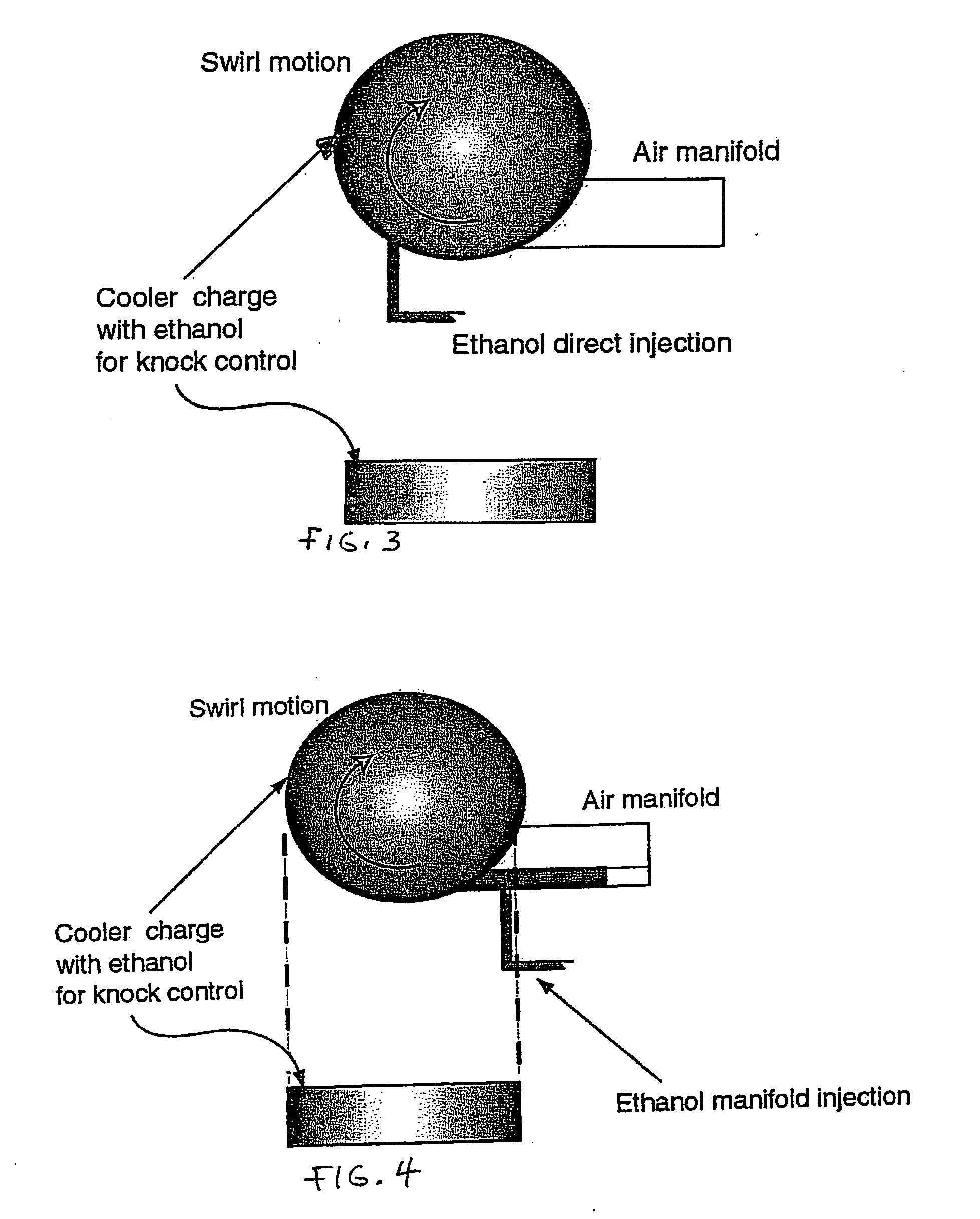
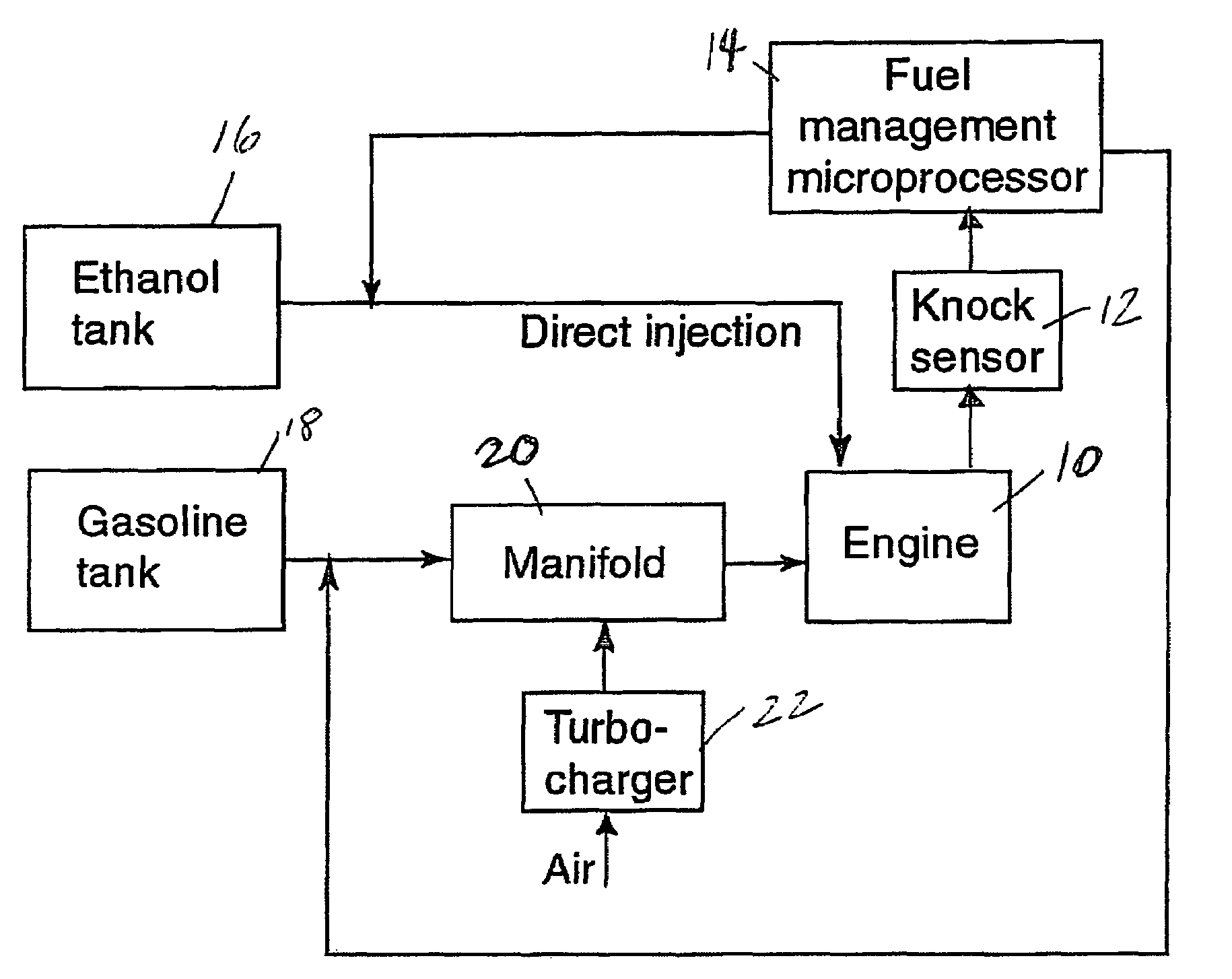
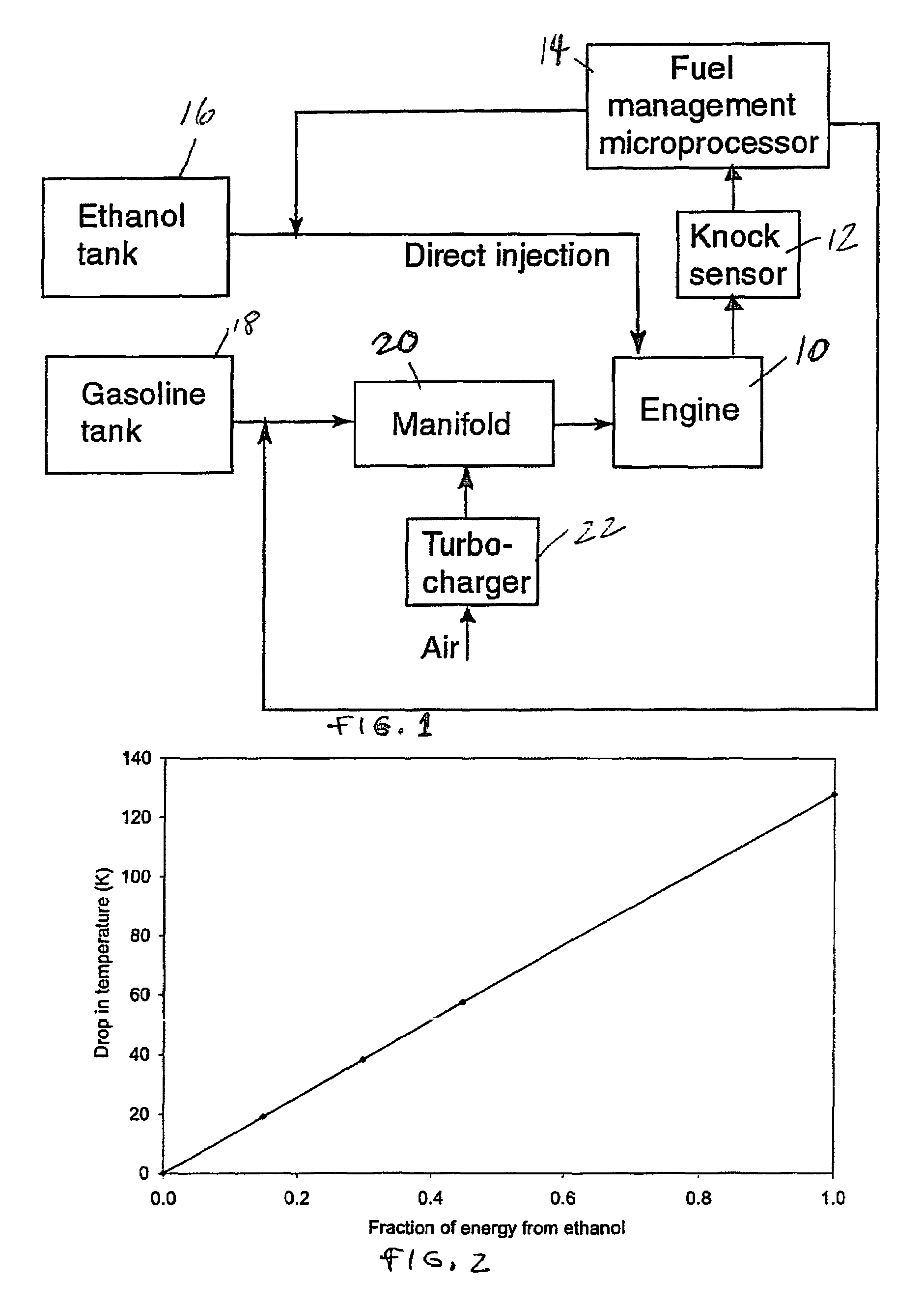
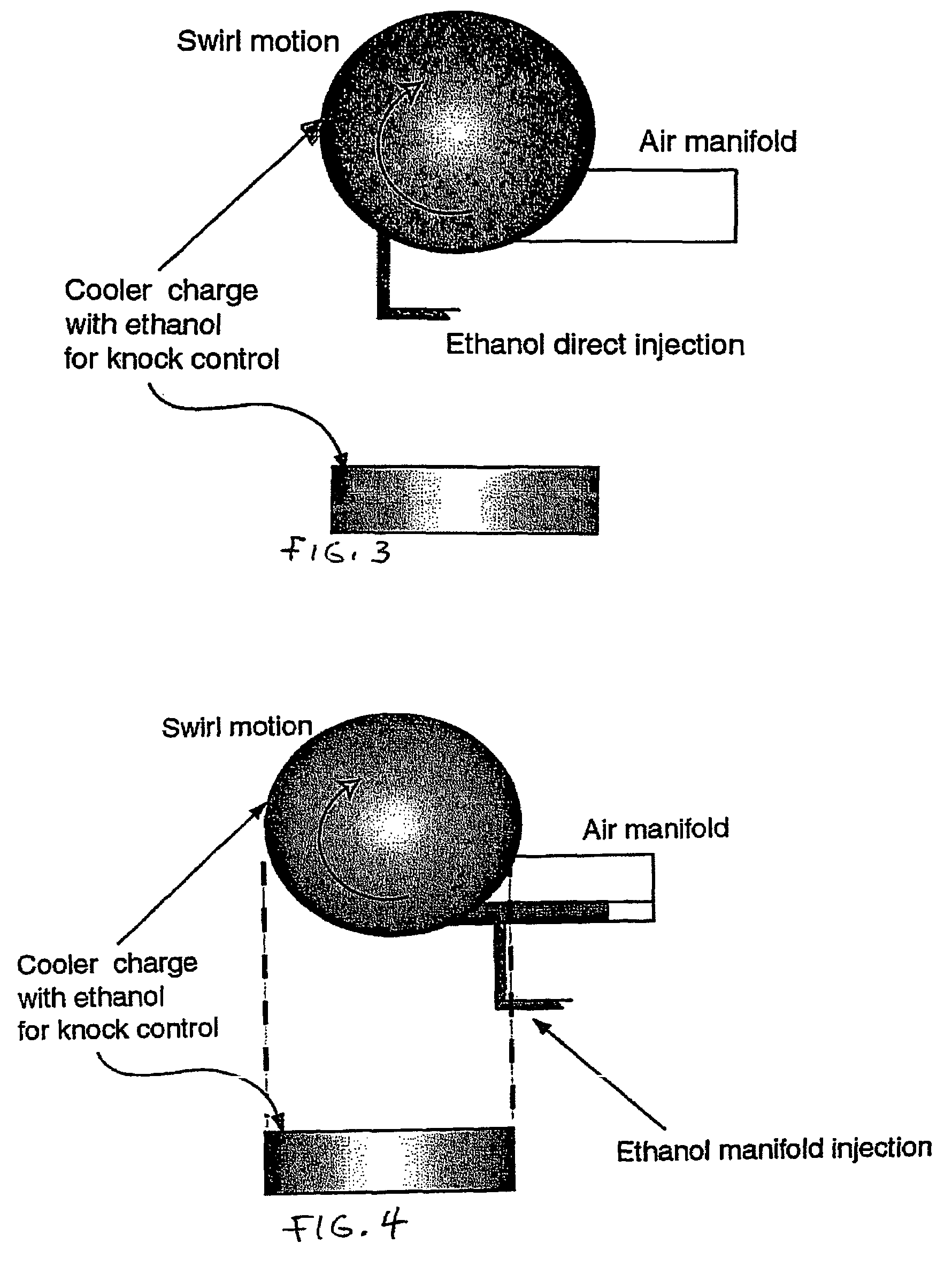
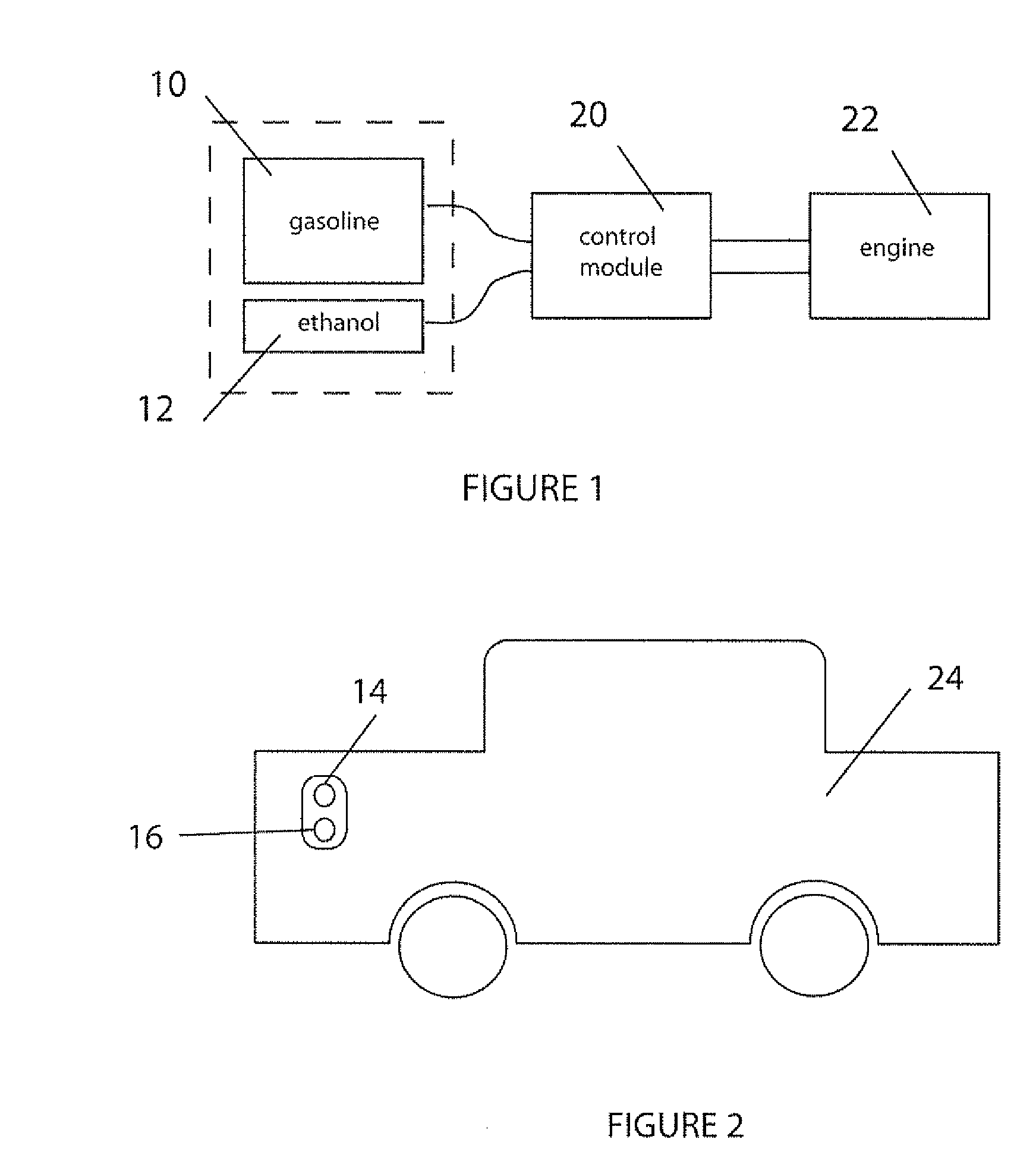
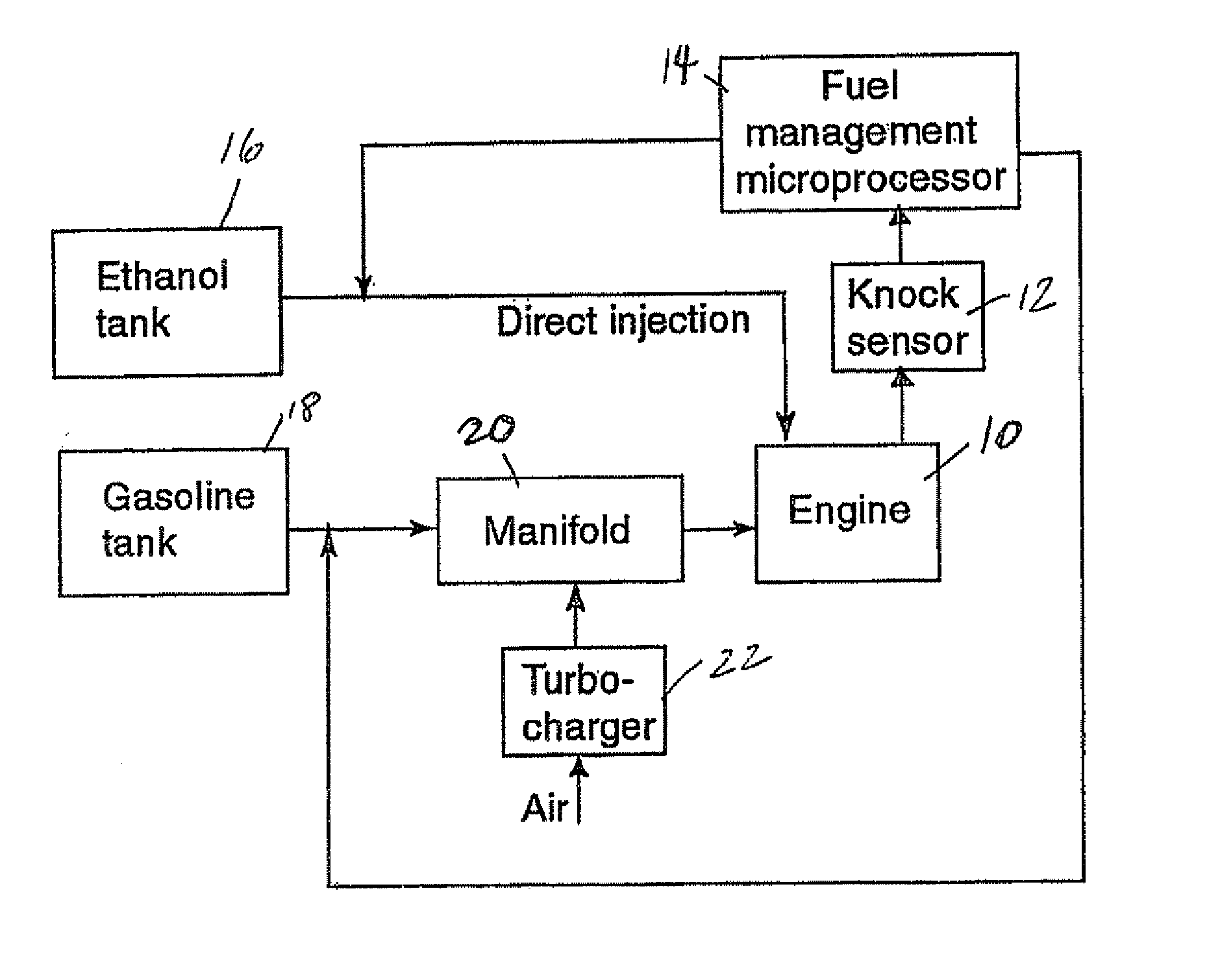
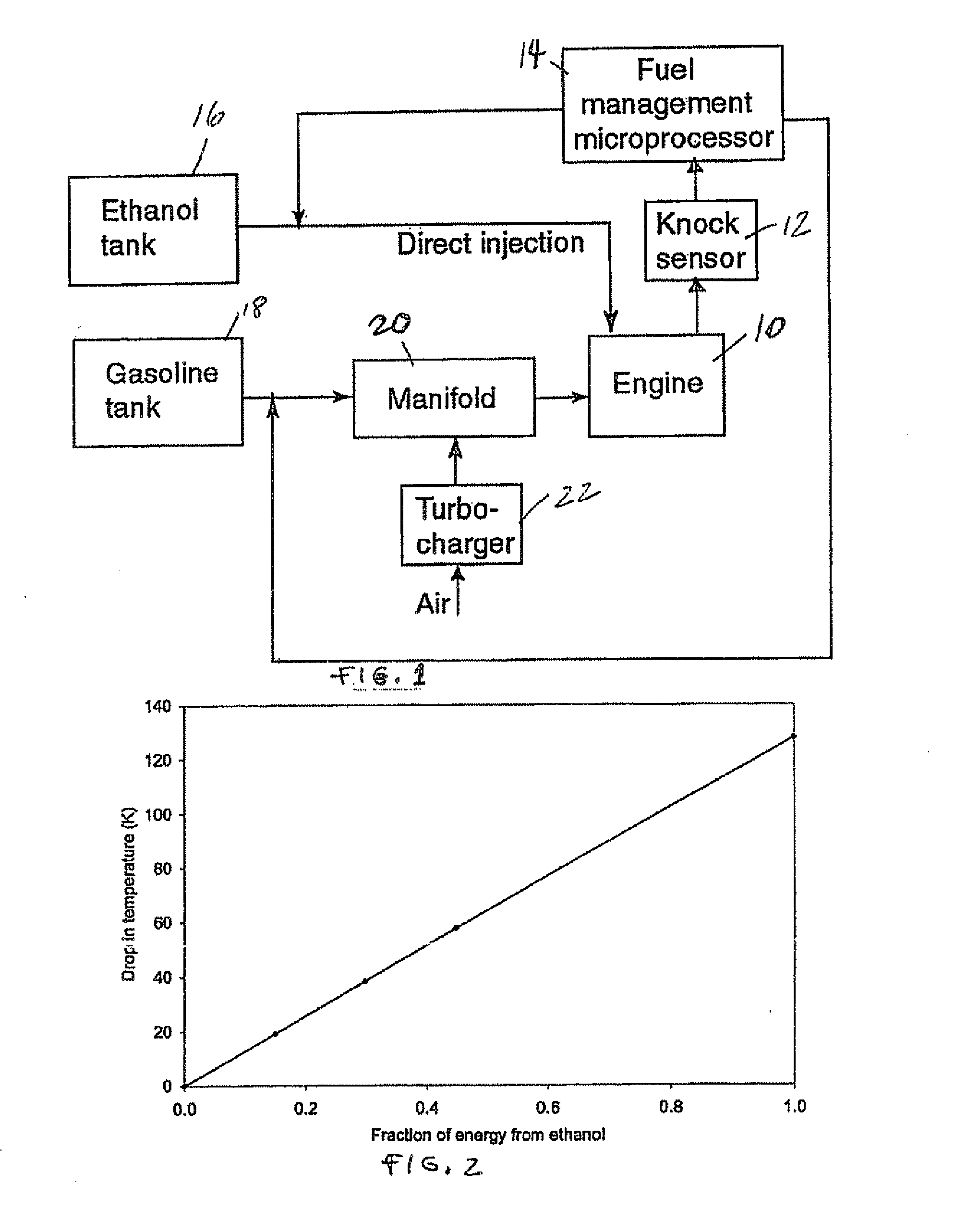
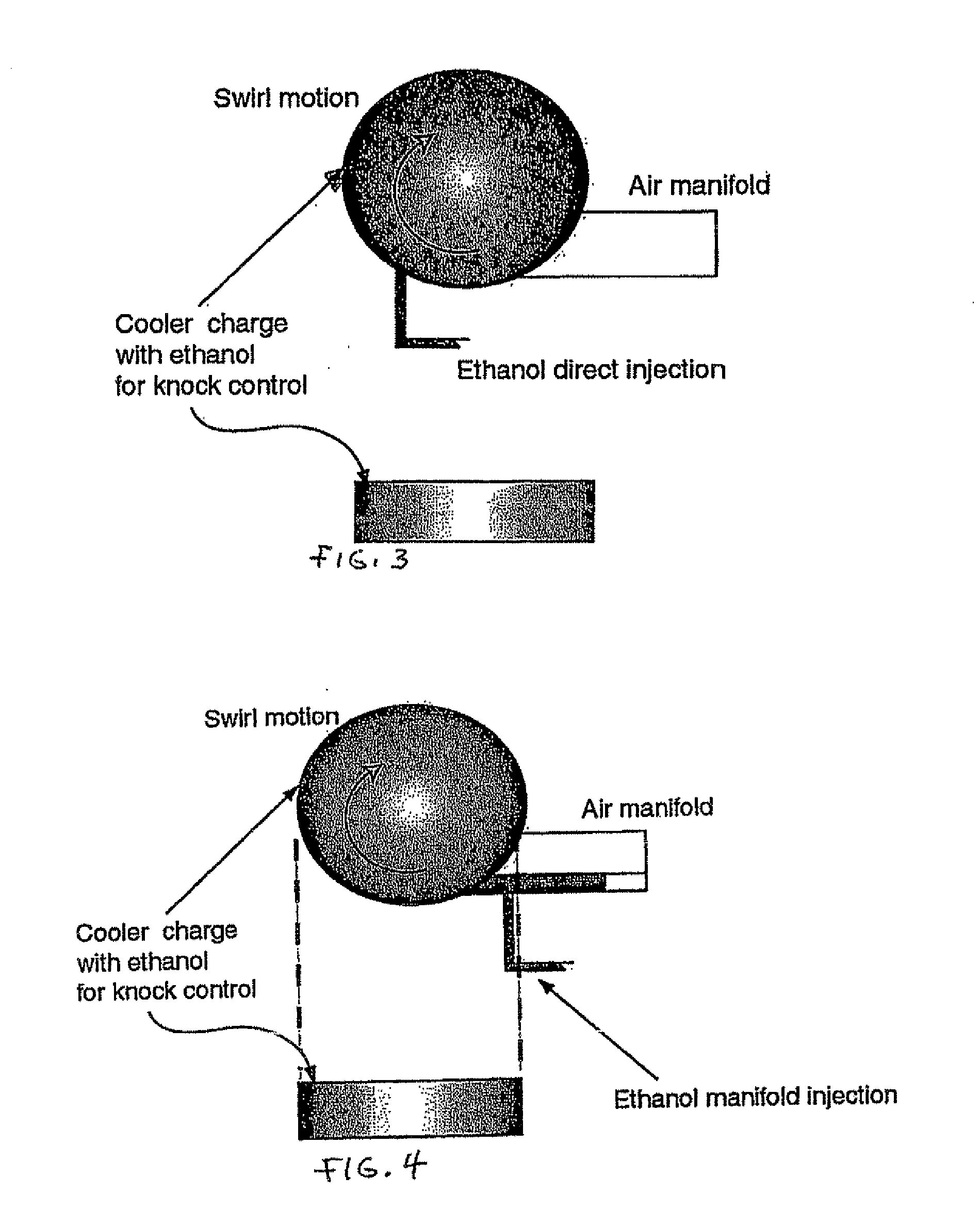
InactiveUS20060102145A1Increase heatMeet cutting requirementsElectrical controlInternal combustion piston enginesEthanol InjectionEngineering
Fuel management system for efficient operation of a spark ignition gasoline engine. Injectors inject an anti-knock agent such as ethanol directly into a cylinder of the engine. A fuel management microprocessor system controls injection of the anti-knock agent so as to control knock and minimize that amount of the anti-knock agent that is used in a drive cycle. It is preferred that the anti-knock agent is ethanol. The use of ethanol can be further minimized by injection in a non-uniform manner within a cylinder. The ethanol injection suppresses knock so that higher compression ratio and / or engine downsizing from increased turbocharging or supercharging can be used to increase the efficiency of the engine.
Owner:MASSACHUSETTS INST OF TECH
Fuel management system for variable anti-knock agent octane enhancement of gasoline engines
InactiveUS20060102146A1Increase heatReduces octane requirementElectrical controlNon-fuel substance addition to fuelEthanol InjectionEngineering
Fuel management system for efficient operation of a spark ignition gasoline engine. Injectors inject an anti-knock agent such as ethanol directly into a cylinder of the engine. A fuel management microprocessor system controls injection of the anti-knock agent so as to control knock and minimize that amount of the anti-knock agent that is used in a drive cycle. It is preferred that the anti-knock agent is ethanol. The use of ethanol can be further minimized by injection in a non-uniform manner within a cylinder. The ethanol injection suppresses knock so that higher compression ratio and / or engine downsizing from increased turbocharging or supercharging can be used to increase the efficiency of the engine.
Owner:MASSACHUSETTS INST OF TECH
Fuel management system for variable ethanol octane enhancement of gasoline engines
InactiveUS7314033B2Increase heatMeet cutting requirementsElectrical controlNon-fuel substance addition to fuelEngineeringAntiknock agent
Fuel management system for efficient operation of a spark ignition gasoline engine. Injectors inject an anti-knock agent such as ethanol directly into a cylinder of the engine. A fuel management microprocessor system controls injection of the anti-knock agent so as to control knock and minimize that amount of the anti-knock agent that is used in a drive cycle. It is preferred that the anti-knock agent is ethanol. The use of ethanol can be further minimized by injection in a non-uniform manner within a cylinder. The ethanol injection suppresses knock so that higher compression ratio and / or engine downsizing from increased turbocharging or supercharging can be used to increase the efficiency of the engine.
Owner:MASSACHUSETTS INST OF TECH
Method of injecting a drug and echogenic bubbles into prostate tissue
Method and surgical instrument for treating prostate tissue including a surgical instrument having a main body, a needle deployment port, a needle, first and second handles and a lockout release mechanism to limit needle extension. Additionally, a kit includes the surgical instrument, together with a cystoscope, and optionally a syringe and reservoir of ethanol. The method includes needle-less injection and visualizing the ethanol injection by delivering both an echogenic agent and ethanol either by needle or needle-less injection or by providing an ultrasonically visible marker near the tip of the ethanol delivery cannula. The method also includes extending the needle transversely of the instrument housing using a link assembly.
Owner:BOSTON SCI SCIMED INC
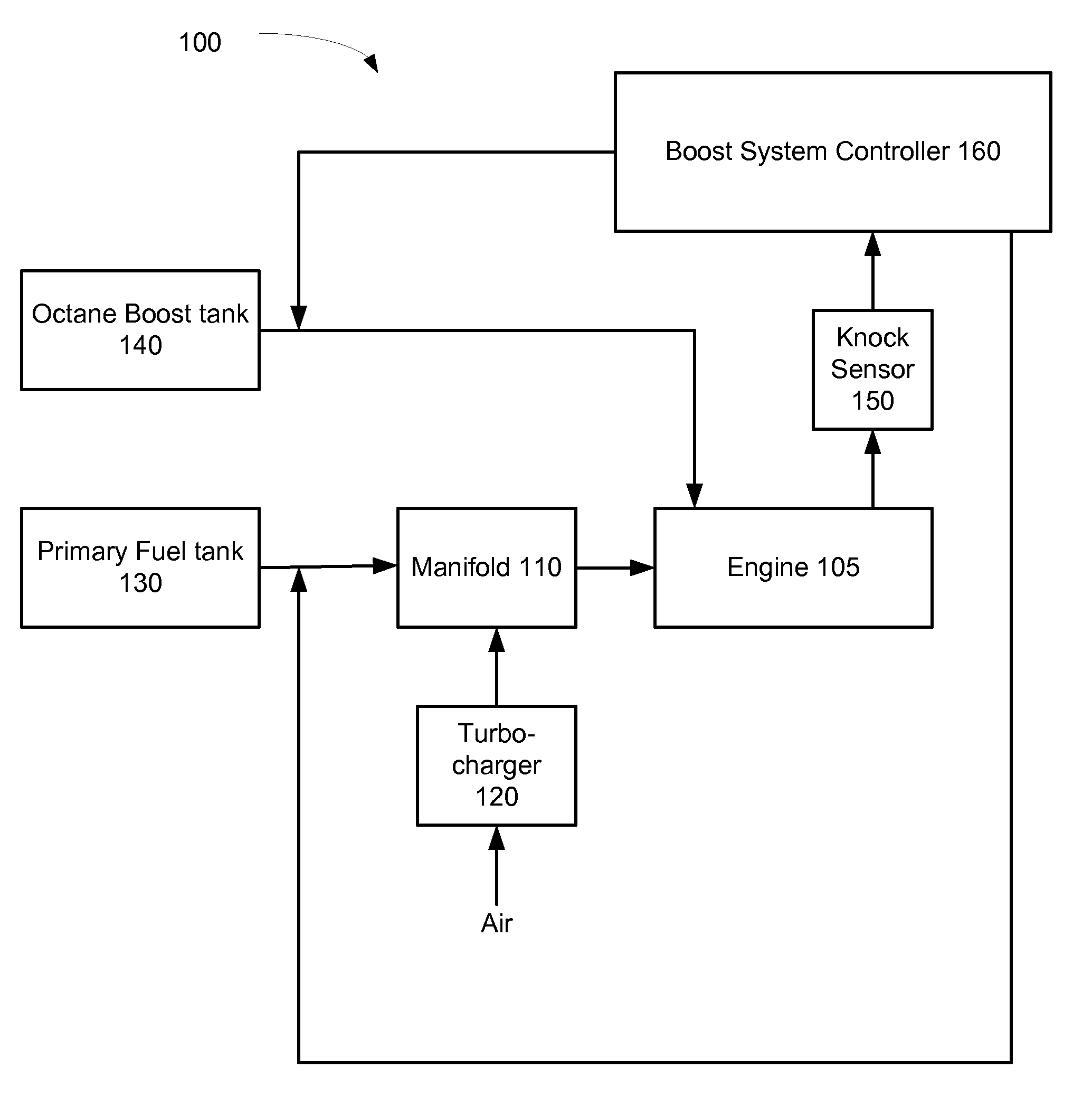
Fuel tank system for direct ethanol injection octane boosted gasoline engine
InactiveUS7726265B2Reduce consumptionFacilitate pressureNon-fuel substance addition to fuelInternal combustion piston enginesEthanol InjectionE85
Fuel tank system for a direct ethanol injection octane boosted gasoline engine. The system includes a gasoline engine and a main fuel tank that contains a mix of gasoline and gasoline E85. A smaller secondary tank is provided to contain ethanol or E85. An injector directly injects in a separately controlled fashion ethanol or E85 into a cylinder of the engine to boost octane. A control module controls the relative amounts of gasoline and ethanol used and structure is provided for fueling the main and secondary fuel tanks.
Owner:ETHANOL BOOSTING SYST LLC
Biogastrone acid prosome liposome with long circulation function and preparation method thereof
InactiveCN101366698AAchieve long cycleSmall particle sizeOrganic active ingredientsMetabolism disorderFreeze-dryingCholesterol
The invention belongs to the new technical field of a drug preparation and in particular relates to a precursor liposome containing a glycyrrhetinic acid and a method for preparing the same. The precursor liposome containing the glycyrrhetinic acid consists of glycyrrhetinic acid, phospholipids, cholesterol, a surfactant and a water-soluble material; a suspension solution of the glycyrrhetinic acid liposome is prepared by an ethanol injection method or a thin-film dispersion method; and solid liposome powder is prepared by a freeze drying method or a spray drying method. The precursor liposome has simple process and good repeatability, is suitable for industrialized production; through drug administration by intravenous injection, the precursor liposome has long-circulating function, can remarkably reduce the toxicity of drug and achieve the function of prolonging the drug effect; and through oral administration, a solid preparation of the liposome can increase absorption and improve bioavailability by three to five times.
Owner:CHINA PHARM UNIV
Fuel Tank System for Direct Ethanol Injection Octane Boosted Gasoline Engine
InactiveUS20080053399A1Facilitate pressureReduce consumptionInternal combustion piston enginesNon-fuel substance addition to fuelEthanol InjectionFuel tank
Fuel tank system for a direct ethanol injection octane boosted gasoline engine. The system includes a gasoline engine and a main fuel tank that contains a mix of gasoline and gasoline E85. A smaller secondary tank is provided to contain ethanol or E85. An injector directly injects in a separately controlled fashion ethanol or E85 into a cylinder of the engine to boost octane. A control module controls the relative amounts of gasoline and ethanol used and structure is provided for fueling the main and secondary fuel tanks.
Owner:ETHANOL BOOSTING SYST
Fuel Management System for Variable Ethanol Octane Enhancement of Gasoline Engines
InactiveUS20080060612A1Increase heatMeet cutting requirementsElectrical controlNon-fuel substance addition to fuelEthanol InjectionEngineering
Fuel management system for efficient operation of a spark ignition gasoline engine. Injectors inject an anti-knock agent such as ethanol directly into a cylinder of the engine. A fuel management microprocessor system controls injection of the anti-knock agent so as to control knock and minimize that amount of the anti-knock agent that is used in a drive cycle. It is preferred that the anti-knock agent is ethanol. The use of ethanol can be further minimized by injection in a non-uniform manner within a cylinder. The ethanol injection suppresses knock so that higher compression ratio and / or engine downsizing from increased turbocharging or supercharging can be used to increase the efficiency of the engine.
Owner:MASSACHUSETTS INST OF TECH
Method for preparing vitamin E nano liposome
InactiveCN101502326AAchieve protectionAchieve sustained releaseOrganic active ingredientsAntinoxious agentsWater basedUltra high pressure
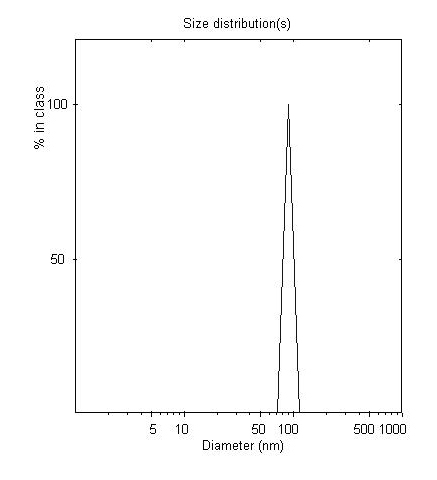
The invention provides a preparation method of a vitamin E nano liposome, belonging to the functional health food technology field. The vitamin E is used as core material and lecithin, cholesterol and Tween 80 are used as wall material and vitamin E common liposome is prepared using an ethanol injection method and then subjected to ultra-high pressure homogeneity, thus transparent or semi-transparent vitamin E nano liposome with average diameter of les than 100nm and encapsulation rate of higher than 90% is obtained. The preparation method is suitable for continuous production and solves problem of saled vitamin E in aspects of stability, water dispersibility and oral absorbency and is widely applied on preparation method of functional water based food.
Owner:JIANGNAN UNIV
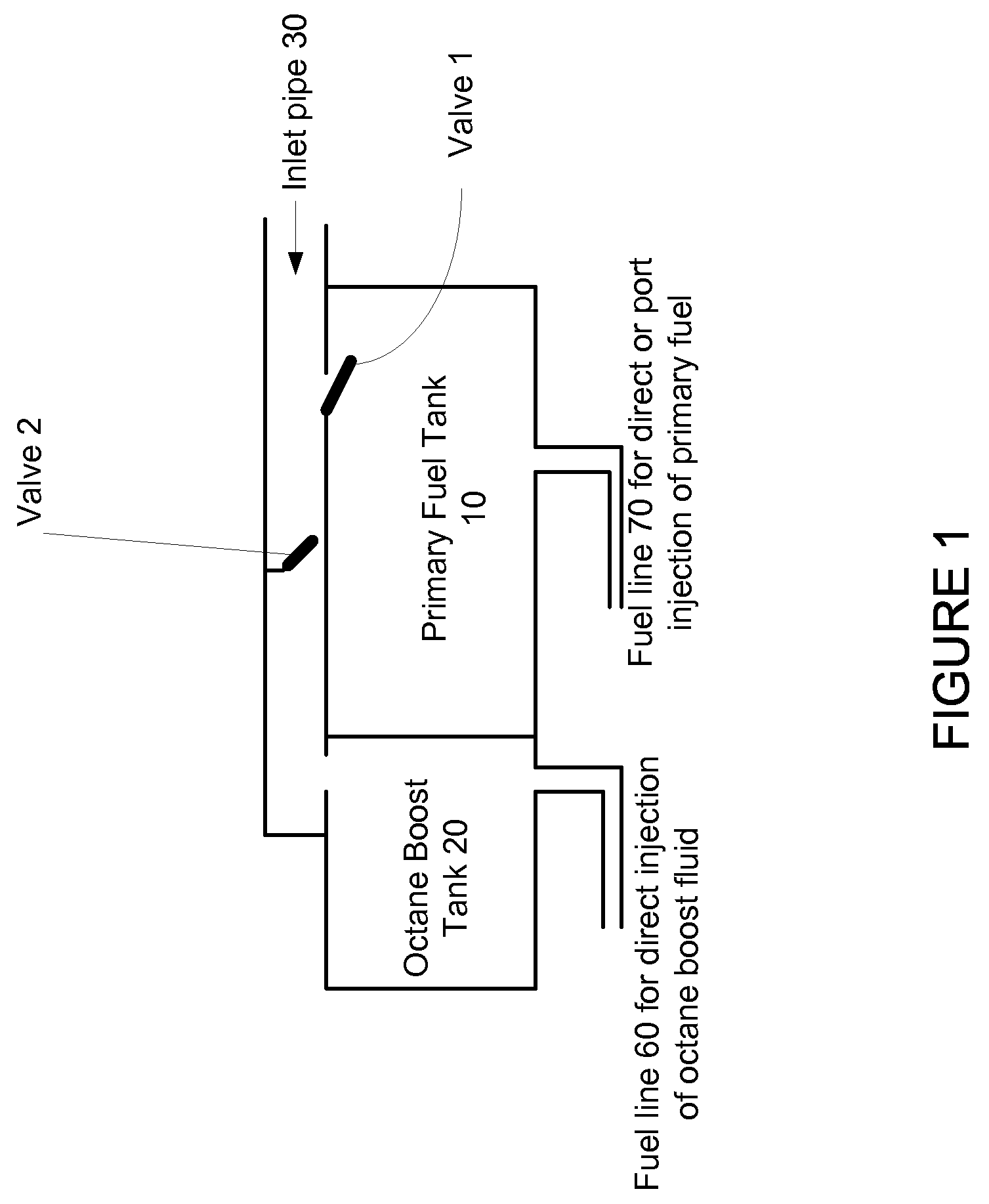
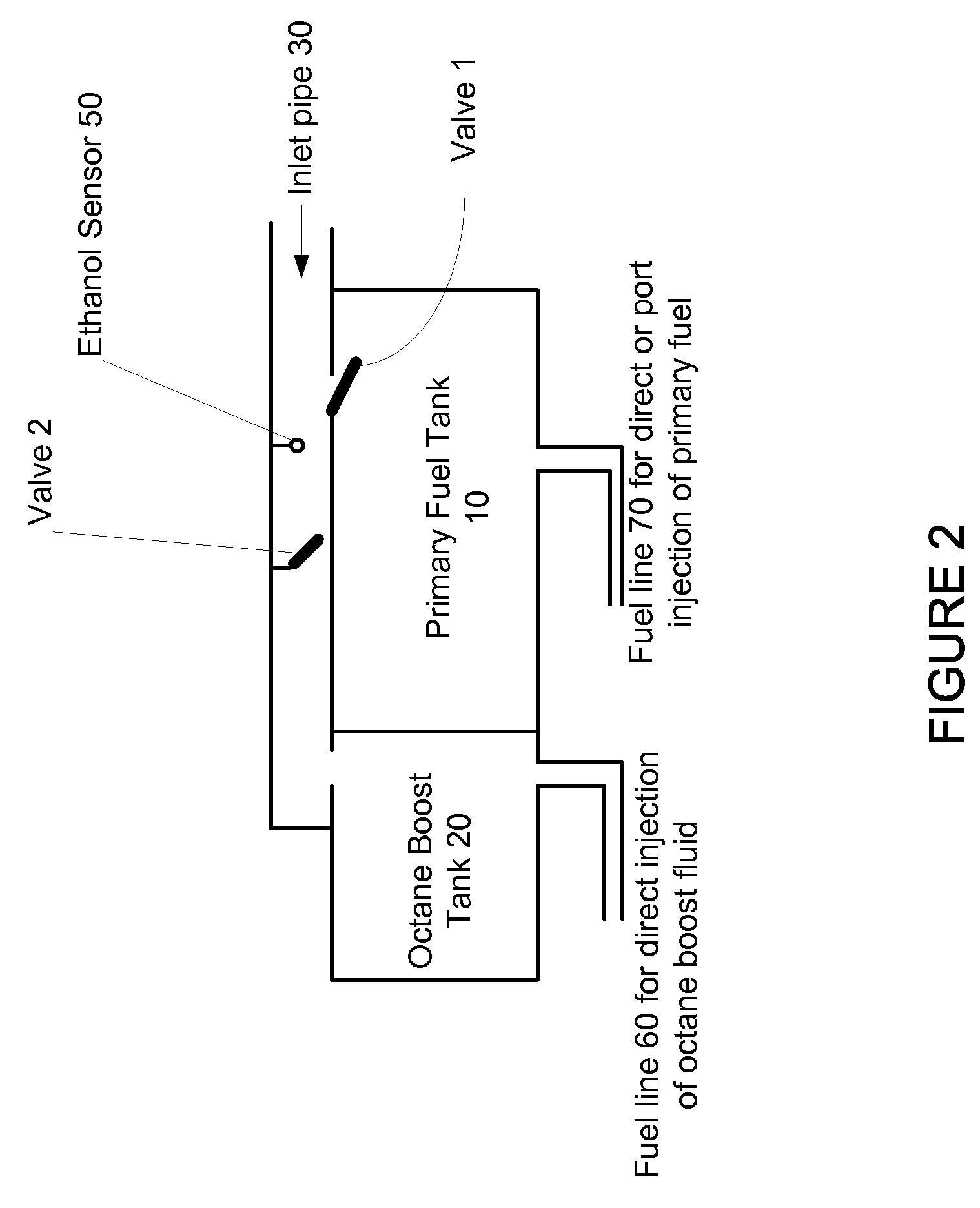
Fuel Tank System For Gasoline And Flexible Ethanol Powered Vehicles Using On-Demand Direct Ethanol Injection Octane Boost
InactiveUS20110120569A1Large on-demand octane boostConvenient and quick and flexible and minimal costNon-fuel substance addition to fuelInternal combustion piston enginesEthanol InjectionFuel tank
A fuel tank system for gasoline or flexible gasoline / ethanol powered vehicles that use independently controlled direct ethanol injection to provide a large on-demand octane boost is disclosed. The on-demand octane boost is used when needed to prevent knock. The ethanol can be in the form of 100% ethanol or E85 (a 85% ethanol, 15% gasoline mixture) and is stored in a second tank that is separate from the tank that which contains the primary fuel. The primary fuel can be gasoline, E85, ethanol or a mix of these fuels. The fuel tank system enables convenient, quick, flexible and minimal cost refueling of the separate fuel tank. A range of fueling options is available to provide the driver with the maximum freedom to choose fuels depending upon price and availability. Valves may be utilized to direct the flow in fuel to the various tanks.
Owner:ETHANOL BOOSTING SYST LLC
Hydroxypropyl-beta-cyclodextrin inclusion liposome of zedoary turmeric oil and preparation method thereof
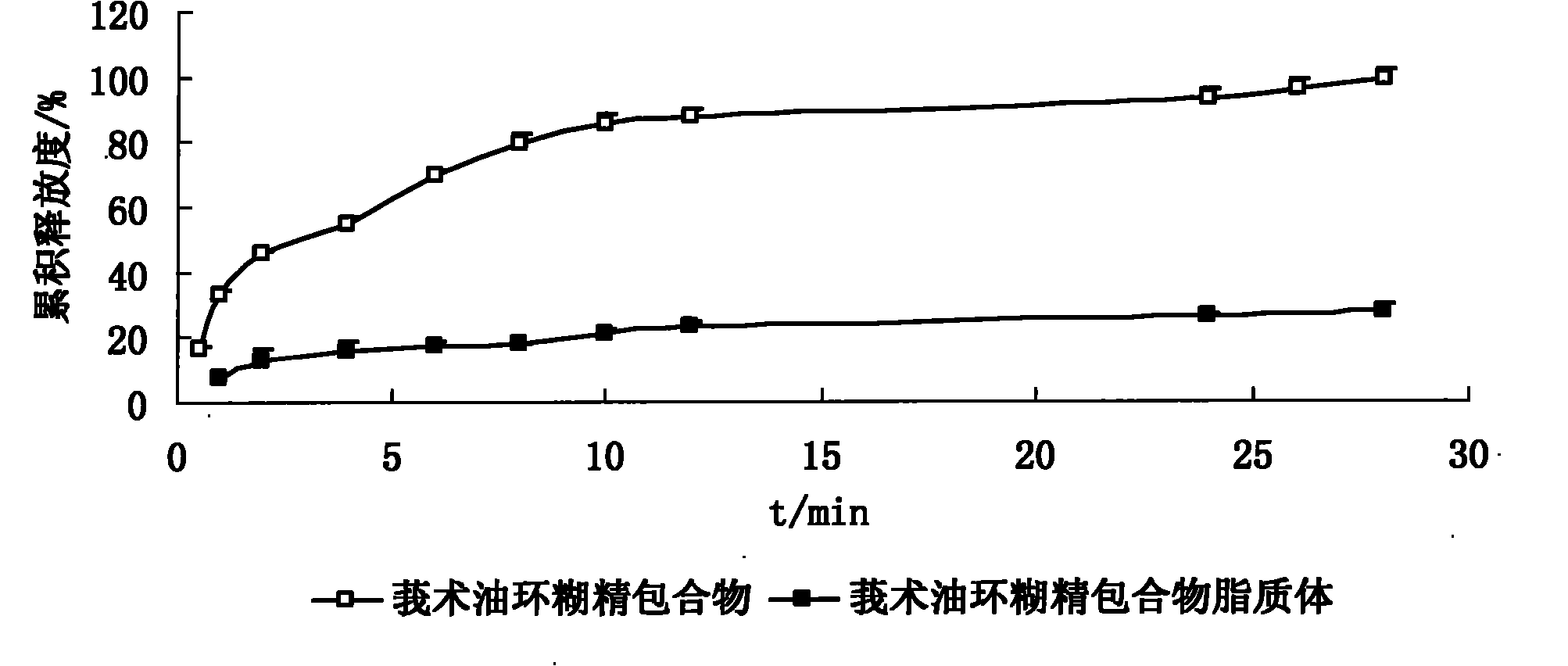
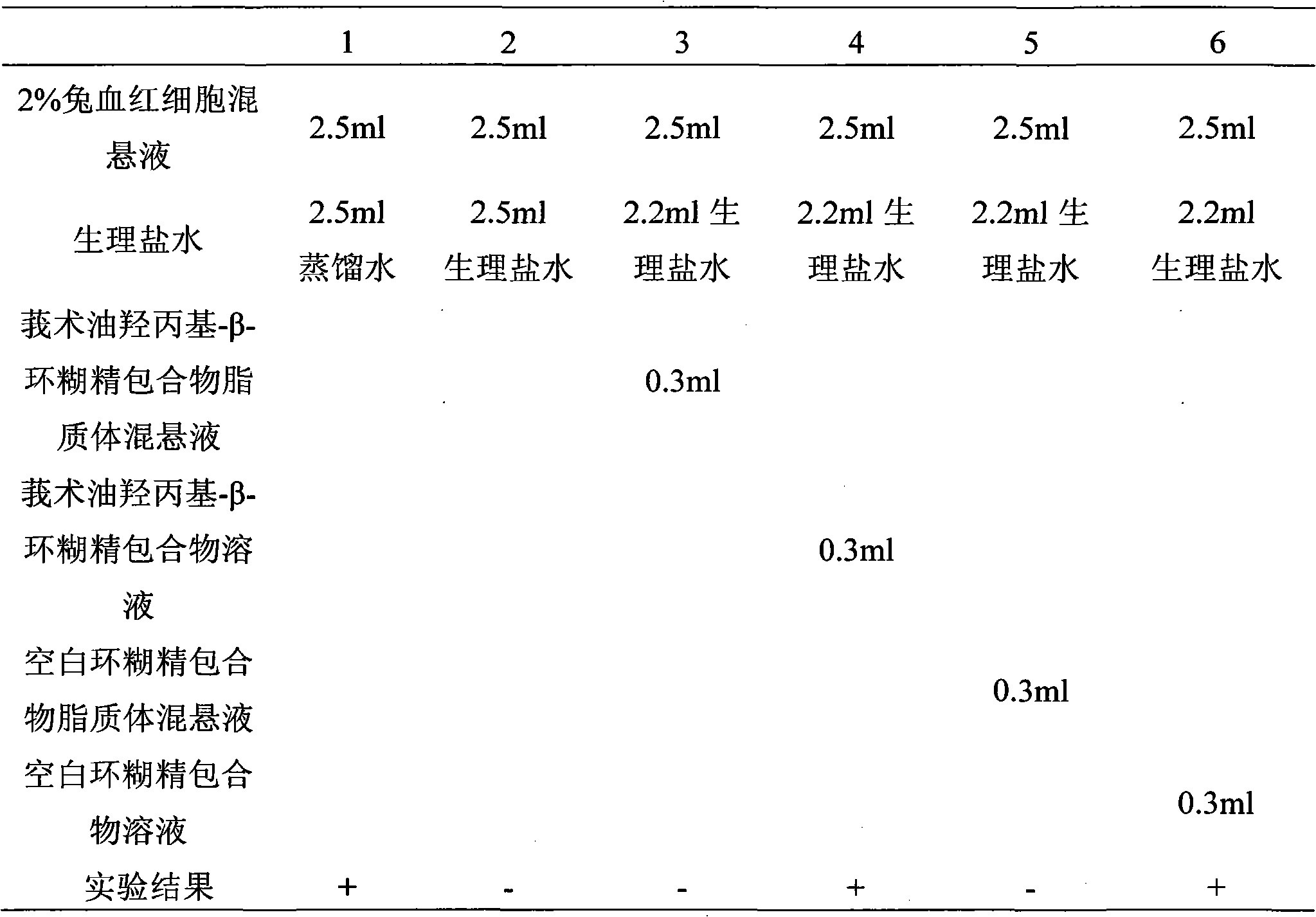
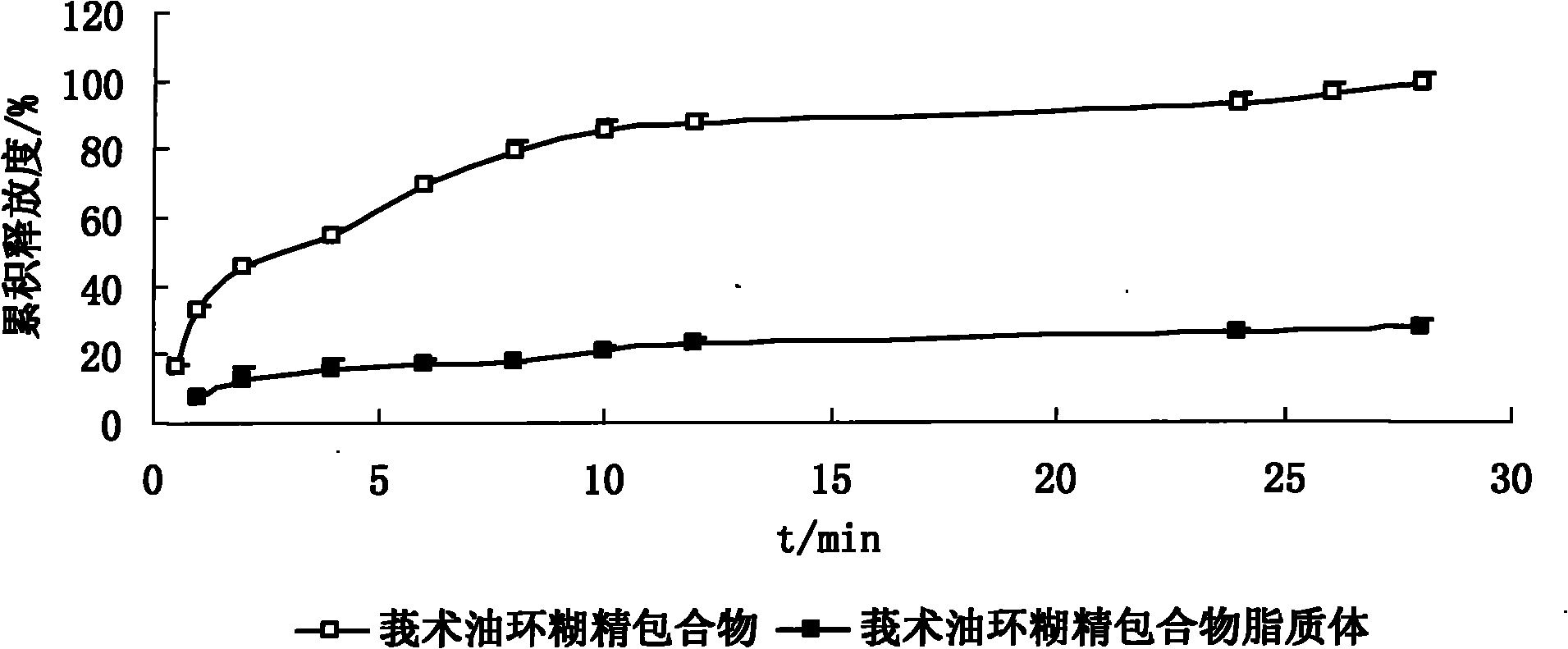
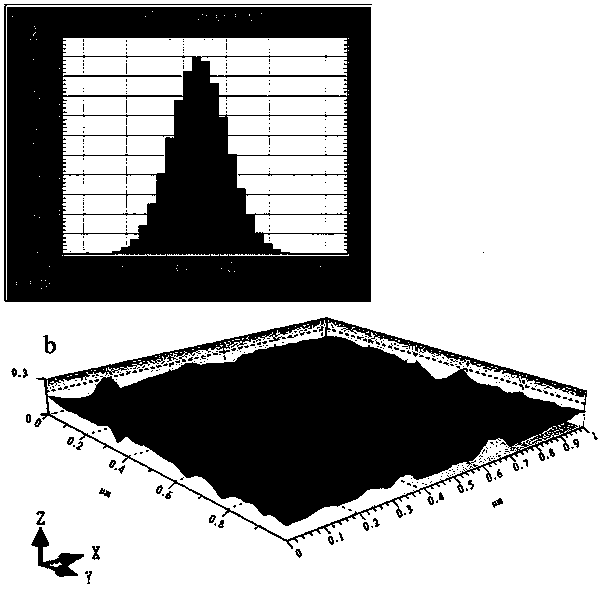
InactiveCN101926962ANo hemolytic toxicityObvious slow-release and long-actingPharmaceutical non-active ingredientsAntineoplastic agentsHemolysisEthanol Injection
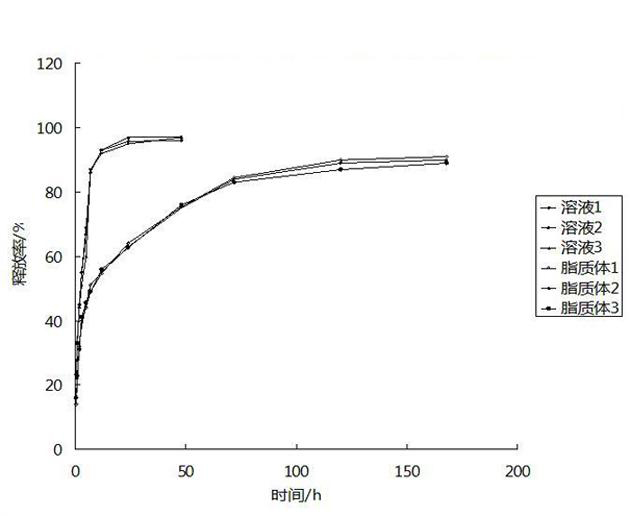
The invention discloses a hydroxypropyl-beta-cyclodextrin inclusion liposome of zedoary turmeric oil and a preparation method thereof. The liposome is prepared by the following steps of: preparing a hydroxypropyl-beta-cyclodextrin inclusion of the zedoary turmeric oil from the zedoary turmeric oil and hydroxypropyl-beta-cyclodextrin through an inclusion process; and preparing the hydroxypropyl-beta-cyclodextrin inclusion liposome of the zedoary turmeric oil from the hydroxypropyl-beta-cyclodextrin inclusion, phospholipid and cholesterol through an ethanol injection method. Experimental results show that: the hydroxypropyl-beta-cyclodextrin inclusion liposome of the zedoary turmeric oil has the advantages of good sustained release and long action, high loading rate, particularly no untoward effect such as hemolysis, and higher safety.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Preparation method of tea polyphenol nano-liposomes by ethanol injection-dynamic high-pressure microfluidization-enzymolysis
InactiveCN103637989AHigh encapsulation efficiencyEvenly distributedCosmetic preparationsOrganic active ingredientsZeta potentialEthanol Injection
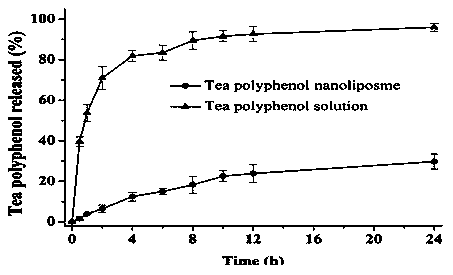
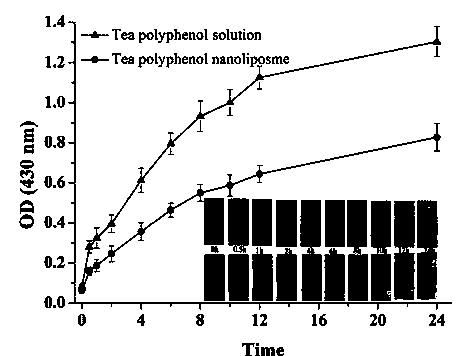
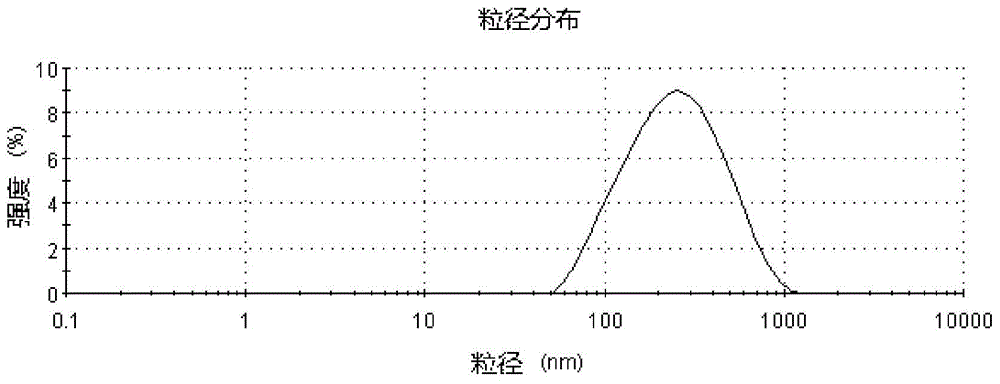
The invention discloses a preparation method of tea polyphenol nano-liposomes by combination of an ethanol injection method and dynamic high-pressure microfluidization-enzymolysis. Tea polyphenol, lecithin, tween-80 and a phosphate buffer solution are adopted as raw materials. The tea polyphenol nano-liposomes are prepared by utilization of an ethanol injection-dynamic high-pressure microfluidization-enzymolysis method. According to the prepared tea polyphenol nano-liposomes, the average particle size is 67.4 nm plus or minus 3.0 nm, the zeta potential is -6.07 mV plus or minus 0.59 mV, the distribution coefficient is 0.220 plus or minus 0.010, and the encapsulation efficiency is 79.7% plus or minus 5.4%. The prepared tea polyphenol nano-liposomes show good slow-release property. Only 30.3% plus or minus 2.9% of the tea polyphenol is released after 24 h. In a small-intestine simulation environment where pH is 7.4, the tea polyphenol nano-liposomes show good stability.
Owner:NANCHANG UNIV
Lavender essential oil liposome and preparation method thereof
InactiveCN104800119AImprove stabilityWith sustained releaseCosmetic preparationsToilet preparationsSolubilityAdditive ingredient
The invention provides a lavender essential oil nanometer liposome which is prepared from the following components in percentage by weight: 1-30% of lavender essential oil, 30-75% of lecithin, 3-20% of cholesterol, 5-40% of Tween 80 and the balance of an anti-oxidant. In the lavender essential oil nanometer liposome, the content of the lavender essential oil accounts for 1-30% of solid substances, the average particle diameter is smaller than 200 nm, and the encapsulation rate can reach 90-98%. The invention further provides a preparation method of the lavender essential oil nanometer liposome. The lavender essential oil nanometer liposome is prepared according to an ethanol injection-filtration film extrusion technology, so that the stability of the lavender essential oil is enhanced, the solubility of the lavender essential oil nanometer liposome is improved, bioactive ingredients in the lavender essential oil are effectively protected, the absorption effect and bioavailability of the lavender essential oil are improved, the quality of the lavender essential oil is improved, and the application rage of the lavender essential oil nanometer liposome is enlarged.
Owner:YANGTZE UNIVERSITY
Gasoline engine system using variable direct ethanol injection and engine shutdown
InactiveUS7637250B2Emission minimizationMinimize timeElectrical controlNon-fuel substance addition to fuelEthanol InjectionControl system
Owner:ETHANOL BOOSTING SYST LLC
Irinotecan liposome and preparation method thereof
InactiveCN102485213AOrganic active ingredientsPharmaceutical non-active ingredientsAlcoholEthanol Injection
The invention belongs to the technical field of medicine and relates to irinotecan liposome and a preparation method thereof. The method can control ethanol content in liposome during a preparation process and ensure no substantial influence of residual ethanol on liposome property in the preparation process. The invention employs improved ethanol injection method to prepare irinotecan liposome, and a membrane material comprises phospholipid or / and other components. The method comprises steps of: preparing a hydrating medium at 45-75 DEG C and insulating for standby; dissolving lipid phase with ethanol, including absolute ethyl alcohol, in a volume less than 10% of a final volume of a preparation at 45-75 DEG C; adding the hydrating medium into the lipid phase at a certain speed; stirring and dispersing to reduce grain size and obtain a blank liposome; and loading according to a gradient method. The invention can effectively control residual ethanol amount during the preparation process, and physical and chemical properties of the liposome have no significant change.
Owner:SHENYANG PHARMA UNIVERSITY
Irinotecan nano circulating liposome and preparation method thereof
InactiveCN101953792ASolve uneven particle size distributionImprove in vivo and in vitro stabilityOrganic active ingredientsPharmaceutical non-active ingredientsEthanol InjectionPolyethylene glycol
The invention provides a novel Irinotecan carrier which is circulating nano liposome and a novel preparation method thereof which is an ethanol injection-ammonium sulfate active medicament-loading method. The preparation process comprises: a, dissolving a lipid material for forming the liposome in ethanol to prepare solution; b, dissolving a polyethylene glycol compound in solution of ammonium sulfate to prepare a hydration medium; c, injecting solution obtained by the step a into the hydration medium obtained by the step b with stirring in a water bath, stirring at a constant temperature for a certain period under a condition of introducing N2 to form circulating blank liposome; d, dialyzing the blank liposome obtained by the step c in physiological saline; and e, adding solution of Irinotecan into the blank liposome, regulating the pH value of an external phase, and performing incubation and medicament loading at a certain temperature to obtain the Irinotecan circulating nano liposome. The process is simple, the particle size is 80 to 150 nanometers, the coating rate is over 95 percent, the sterile preparation can be obtained easily, and an industrialization requirement is met.
Owner:中华人民共和国卫生部肝胆肠外科研究中心 +1
Application of phospholipid-Vitamin E tocopherol acid polyethylene glycol succinate micelle
InactiveCN103142479AGood curative effectImprove retentionCosmetic preparationsPowder deliveryCuticlePolyethylene glycol
The invention discloses an application of a transdermal enhancer with phospholipid and Vitamin E tocopherol acid polyethylene glycol succinate (TPGS) micelle as medicines or functional compositions of cosmetics. A cuticle of a skin epidermis has a multi-layer compact film structure and consists of cuticle cells and lipid compositions among the cuticle cells, the cuticle cells and the lipid compositions together form a compact barrier to prevent influences from the external factors such as the medicines, and therefore, transdermal absorption of the cosmetic compositions needs to overcome the barrier function of the cuticle. According to the application disclosed by the invention, an ethanol injection method is adopted on the phospholipid and the TPGS with a certain proportion to prepare a water soluble micelle, and the water soluble micelle is frozen and dried to prepare a phospholipid-TPGS water soluble powder; the phospholipid-TPGS water soluble powder is self assembled into nano-micelle with the average grain diameter being about 25nm after being dissolving into water and can enter the internals of skin cuticle cells to mutually react with keratin, so that the compact degree of the cuticle cells is lowered, and meanwhile, the phospholipid carried by the nano-micelle can form a lipid channel; and the stability of the micelle is very good, and the micelle has better transdermal promotion effect on various functional compositions of the cosmetics.
Owner:CHINA PHARM UNIV
Sunitinib malate liposome and preparation method thereof
InactiveCN102485212AOrganic active ingredientsPharmaceutical non-active ingredientsSunitinib malateEthanol Injection
The invention discloses sunitinib malate liposome and a preparation method thereof, belonging to the technical field of medicines. A prescription for the sunitinib malate liposome in the invention is as follows: drugs and lipid substances used for constructing a bilayer membrane of the liposome are prepared by using an improved ethanol injection method. In the invention, influence of the content of ethanol in the preparation prescription on an entrapment rate, particle size distribution, stability and an antineoplastic effect of the liposome is also investigated. According to results of investigation, liposome which is obtained through drug loaded preparation by using a gradient method has an entrapment rate greater than 90%; when the content of ethanol is controlled to be no more than 10%, related parameters of the liposome hardly change, thereby providing reference for control of the content of ethanol in preparation and industrial production of the sunitinib liposome.
Owner:SHENYANG PHARMA UNIVERSITY
Verapamil liposome and preparing method thereof
InactiveCN101199505AIncreased corneal retentionIncrease corneal penetrationPowder deliverySenses disorderLipid formationCholesterol
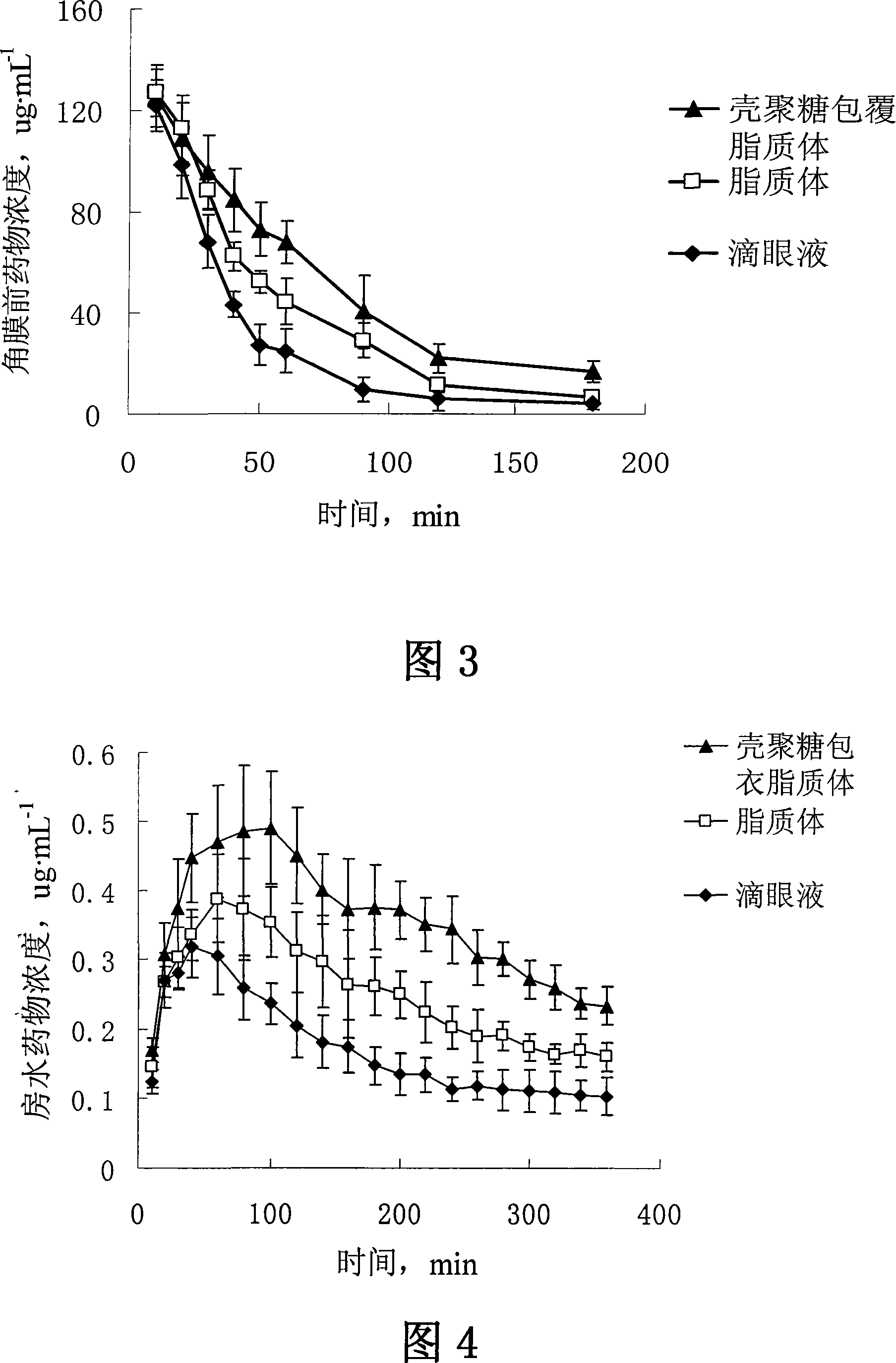
The invention discloses verapamil liposome and a preparation method of the verapamil liposome, belonging to the technical field of medicine. The verapamil liposome is composed of verapamil, phospholipid, cholesterol, chitosan, cationic lipid and antioxidant, etc., all of which are in certain weight percentage. The verapamil liposome is prepared by adopting ammonium sulphate gradients method, film hydration method, ethanol injection method and reverse phase evaporation method. The invention adopts chitosan or derivatives of the chitosan to modify the surface of the liposome, and applies the chitosan to ophthalmic preparation, which can further increase corneal retention of the liposome and can remarkably improve the corneal penetration amount of the drug, with obvious advantages compared with ordinary liposome. With high entrapment efficiency, good stability, good biocompatibility, biological adhesiveness and biodegradability, the prepared liposome can realize sustained release and long-acting administration, particularly suitable for administration on ocular region, and can better play the ocular effects of anti-glaucoma and anti-cataract of verapamil.
Owner:SHENYANG PHARMA UNIVERSITY
TPGS modified docetaxel liposome nano-drug delivery system and preparation method thereof, and application thereof
InactiveCN109224084AReduce systemic toxicityImprove solubilityOrganic active ingredientsPharmaceutical non-active ingredientsZeta potentialCholesterol
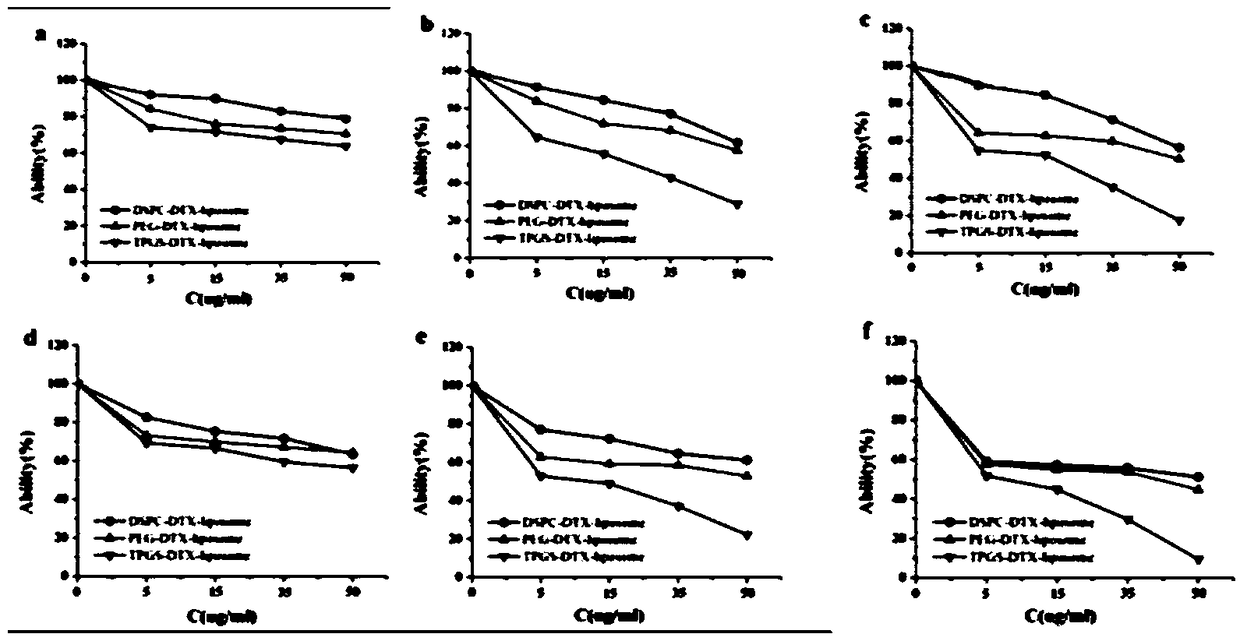
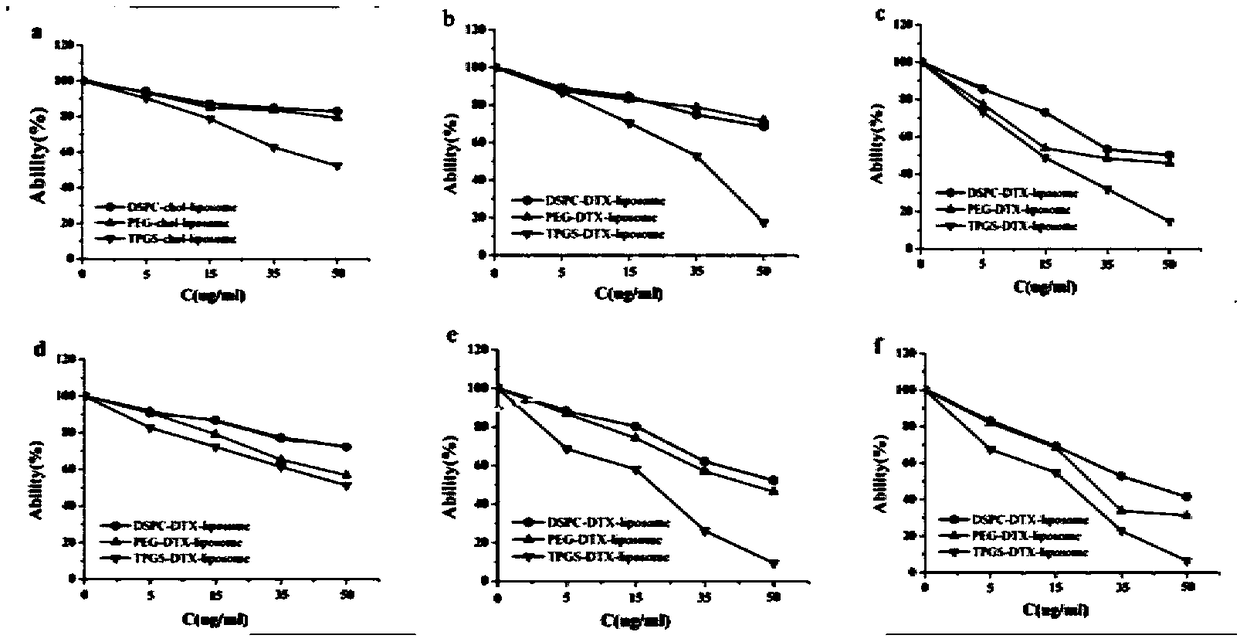
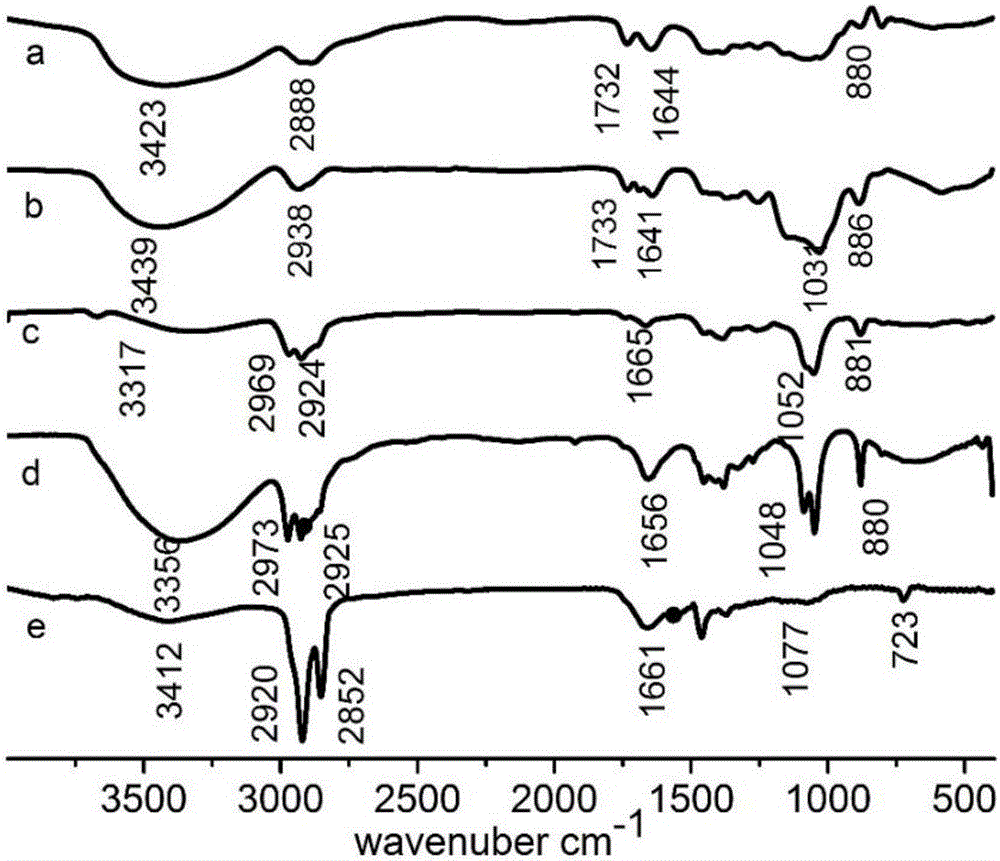
The invention relates to a TPGS modified docetaxel liposome nano-drug delivery system, a preparation method and an application thereof. The TPGS modified docetaxel liposome nano-drug delivery system uses phospholipid and cholesterol as membrane materials, Surfactant TPGS modified liposomes and docetaxel (DTX) as anticancer drug were prepared by membrane dispersion ethanol injection ultrasonic dispersion reverse evaporation and freeze drying. The particle size of nano-doxetaxel liposomes modified by TPGS is 80-200 nm, the zeta potential is 0+30 mV or 0-30 mV, the encapsulation efficiency is 75-99%, with a drug loading of 3-15%. The in vitro cell experiment and in vivo pharmacodynamic experiment of the TPGS modified docetaxel liposome nano-drug delivery system prepared by the invention showthat the nano-drug delivery system can inhibit P. Overexpression of gp, decrease of drug efflux, increase of drug enrichment in tumor cells, increase of cytotoxicity and apoptosis rate, increase of tumor therapeutic effect and reversal of multidrug resistance.
Owner:NINGXIA MEDICAL UNIV
Cordyceps sinensis polysaccharide liposome medicament and preparation thereof
InactiveCN101401789APromote absorptionOrganic active ingredientsMetabolism disorderCholesterolAdditive ingredient
The invention relates to liposome of extract of cordyceps and a method for preparing the same. The main composition of the extract is polysaccharide. The liposome of the cordyceps is prepared by using phospholipid and cholesterol as membrane materials, and the prepared liposome of the cordyceps can be further prepared into dosage forms of tablet, injection, oral agent, capsule, granule, freeze-dried powder and the like. The method for preparing the liposome of the cordyceps can adopt a reverse evaporation method, a film ultrasound method and an ethanol injection method, the encapsulation rate of the methods can reach more than 40 percent, and the in vitro burst rate is low. The preparing process improves the stability of the drug. After the polysaccharide of the cordyceps is coated with the liposome, the polysaccharide of the cordyceps can be absorbed and used better in human body; and through the slow release effect of the liposome, the effective blood drug level of the drug can be maintained for a longer time.
Owner:JILIN UNIV
KGM (Konjac Glucomannan)-g (grafted)-AH (Alicyclic Amine) drug loaded nano micelle and preparation method
InactiveCN106511271AIncrease package loadHigh solubilizing powerKetone active ingredientsPharmaceutical non-active ingredientsCytotoxicityBiocompatibility Testing
The invention relates to synthesis of a KGM (Konjac Glucomannan)-g(grafted)-AH (Alicyclic Amine) material and preparation of drug loaded nano micelle thereof, and belongs to the technical field of biological materials and slow release. The synthesis of the KGM-g-AH material comprises the following steps: firstly carrying out oxidization ring opening on KGM by using periodate, thus obtaining DAK (Dialdehyde KGM); then enabling the DAK to react with alicyclic amine, thus obtaining amphipathic KGM-g-AH. A grafted copolymer can be assembled to form nano micelle through an ethanol injection method; the nano micelle has pH (Potential of Hydrogen) sensitivity; in a neutral environment of normal tissues and blood, the nano micelle is relatively stable; in a weak acid environment of tumor tissues, imine bonds of molecules of the nano micelle are in reversible breakage, loaded drugs can be rapidly released, and the loaded drugs can be gathered in tumor cells, so that site-specific delivery of the drugs on the tumor tissues is realized. The prepared KGM-g-AH drug-loaded nano micelle is stable in structure, simple to synthesise, high in drug loading capacity, good in biocompatibility and low in cytotoxicity, has pH sensitivity and has a wide application prospect in the field of drug controlled release.
Owner:HUBEI UNIV OF TECH
Compound low-molecular heparin sodium liposome gel and preparation method and application thereof
InactiveCN103356693AIncrease drug concentrationImprove permeabilityOrganic active ingredientsAerosol deliveryEthanol InjectionSkin Injury
The invention belongs to the technical field of medicines and relates to a compound low-molecular heparin sodium liposome gel and a preparation method and application thereof. The compound low-molecular heparin sodium liposome gel comprises low-molecular heparin sodium, asiaticoside, lipid components, a membrane softening agent and gel matrix components, wherein the low-molecular heparin sodium and asiaticoside are pharmacological active ingredients, and based on the total gel of 100g, the content of the low-molecular heparin sodium is 5000-35000IU, and the content of the asiaticoside is 0.1-2.5g. The preparation method comprises the following steps of: (1) preparing a compound low-molecular heparin sodium liposome by employing an ethanol injection-ultrasonic method or ethanol injection-high pressure homogenizer method; (2) preparing a blank gel; (3) adding the compound low-molecular heparin sodium liposome into the blank gel, uniformly stirring, and obtaining the compound low-molecular heparin sodium liposome gel. The gel is mainly used for treating skin injury and scar.
Owner:SHENZHEN HEPALINK PHARMA GRP CO LTD
Artemether nanoliposome, and preparation method and application thereof
ActiveCN106474064ANature of controlControl characteristicsOrganic active ingredientsAntiviralsYolkEthanol Injection
The invention discloses an artemether nanoliposome, and a preparation method and an application thereof. The artemether nanoliposome is prepared from the following raw materials in parts by weight: 1 part of artemether, 2 parts of cholesterol and 8-10 parts of egg yolk lecithin. The preparation method comprises a film dispersion-sonication-film filtering method and an ethanol injection-sonication method. The artemether nanoliposome is applied to the preparation of artemether targeting preparation drugs. The encapsulation rate of the artemether nanoliposome is 61.33-63.86%, the average particle size is 161.65-162.73nm, and the poly dispersition index (PDI) is 0.2678-0.4463. The artemether nanoliposome has good stability when being stored at 4 DEG C.
Owner:KPC PHARM INC +1
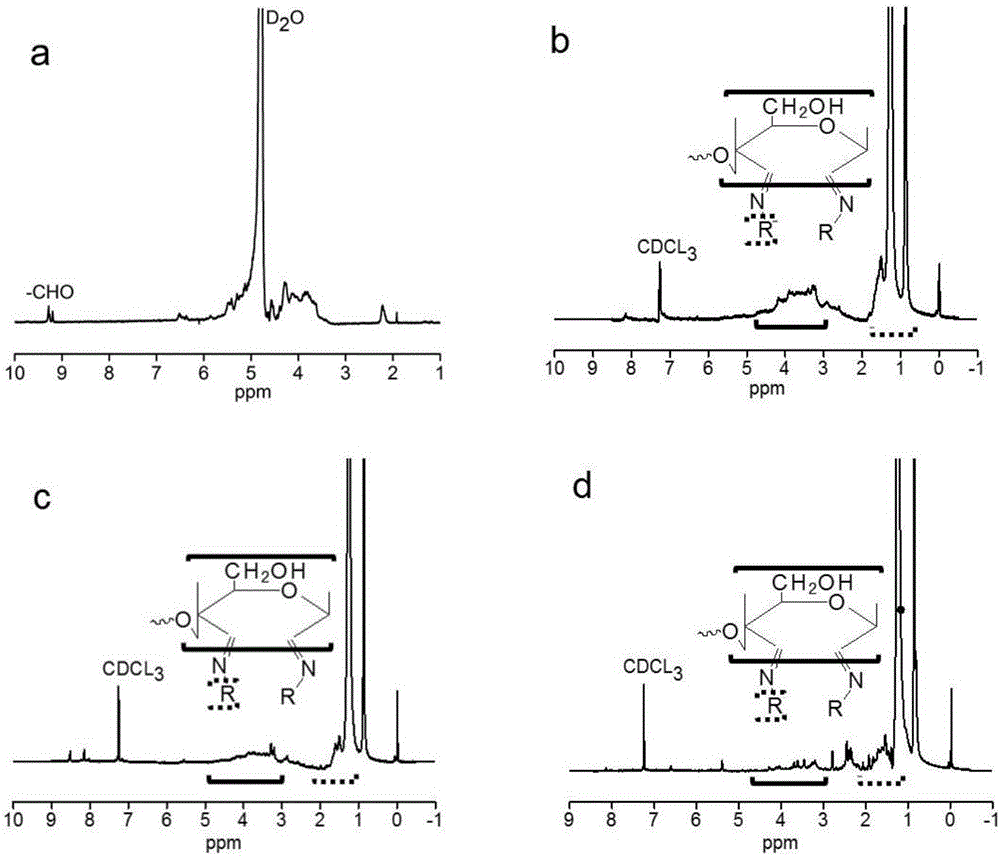
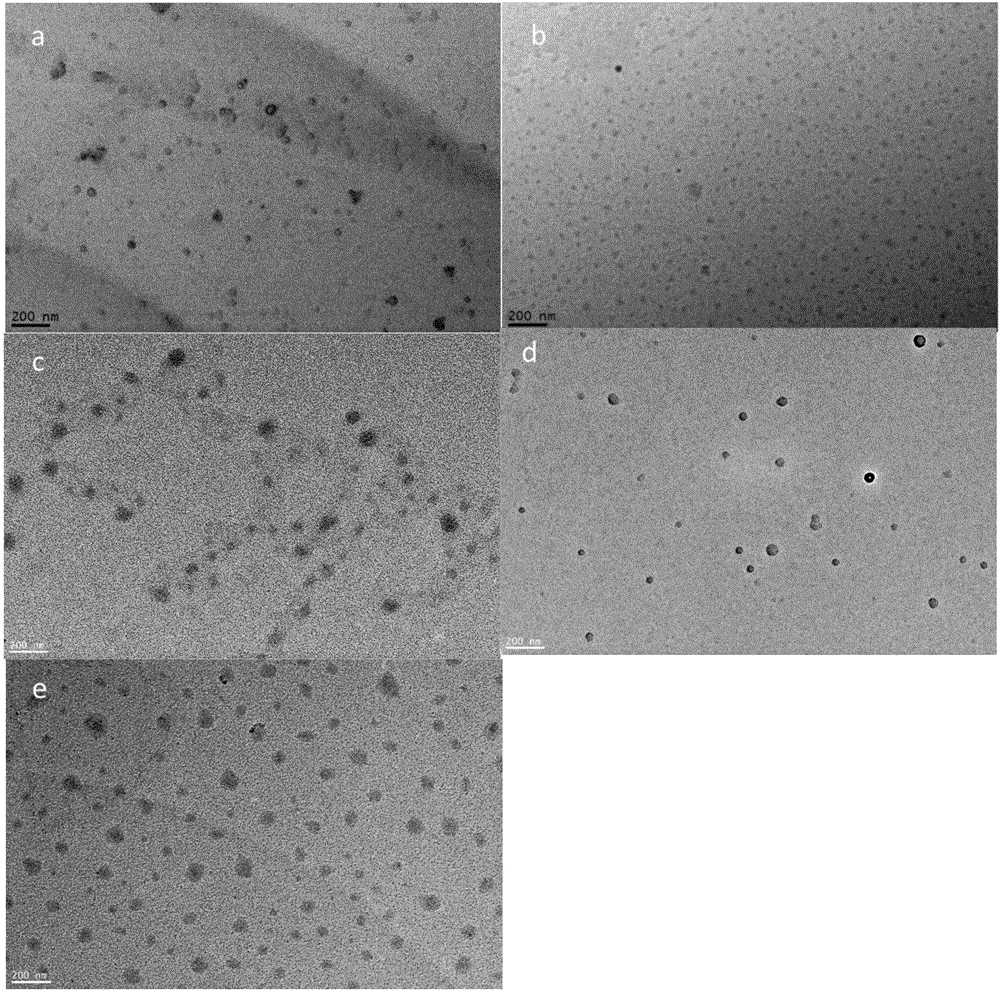
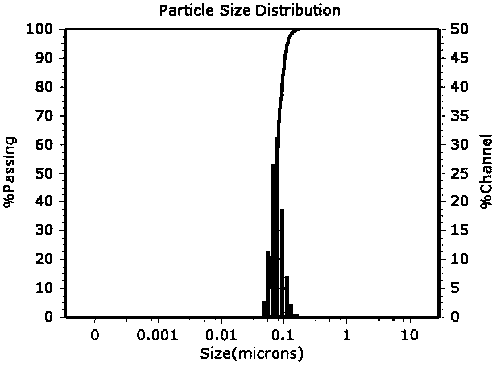
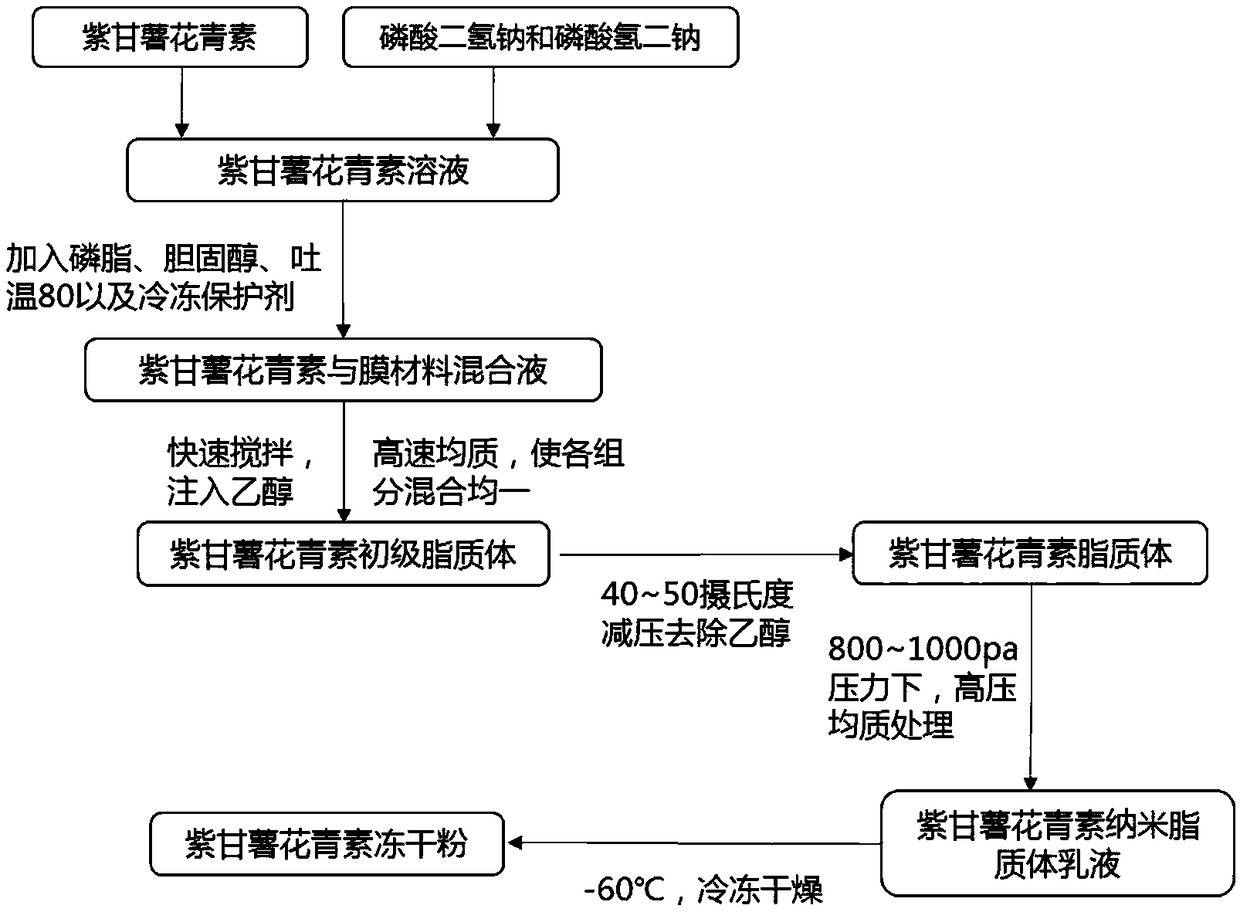
Purple sweet potato anthocyanin nano-liposome and preparation method thereof
InactiveCN108851072AReduce the impactSmall particle sizeFood ingredientsFood shapingEthanol InjectionFreeze-drying
The invention discloses a preparation method of purple sweet potato anthocyanin nano-liposome. According to the preparation method, homogenization treatment is performed by combining an ethanol injection method and a dynamic high-pressure microjet method to prepare the purple sweet potato anthocyanin nano-liposome, so that purple sweet potato anthocyanin is successfully entrapped in the nano-liposome, and the stability of the purple sweet potato anthocyanin in the storage and application fields is improved; a freeze-drying protective additive is added for freeze drying to obtain freeze-dried powder of the purple sweet potato anthocyanin liposome. The prepared purple sweet potato anthocyanin nano-liposome has the advantages of high entrapment rate, small particle size, high thermal stability and high light stability. Moreover, the preparation process is simple, is low in cost, and is easy to realize industrial production. The entrapment rate of the anthocyanin liposome prepared by the method is up to 70 percent, the nano-liposome is 60 to 80 nm in particle size, and the prepared purple sweet potato anthocyanin nano-liposome is small in particle size, is easy for human body to absorb, and has higher bioavailability.
Owner:XUZHOU NORMAL UNIVERSITY
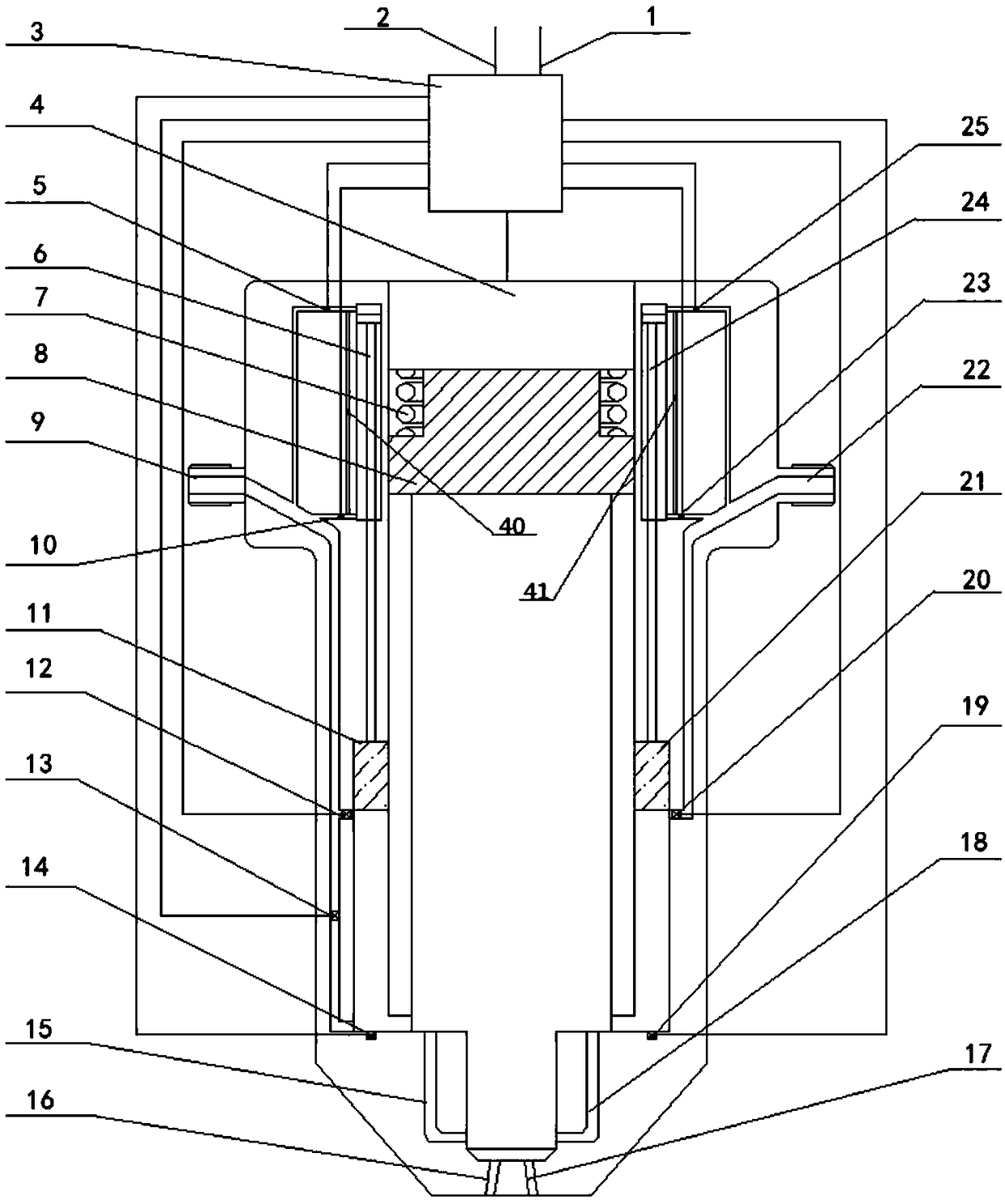
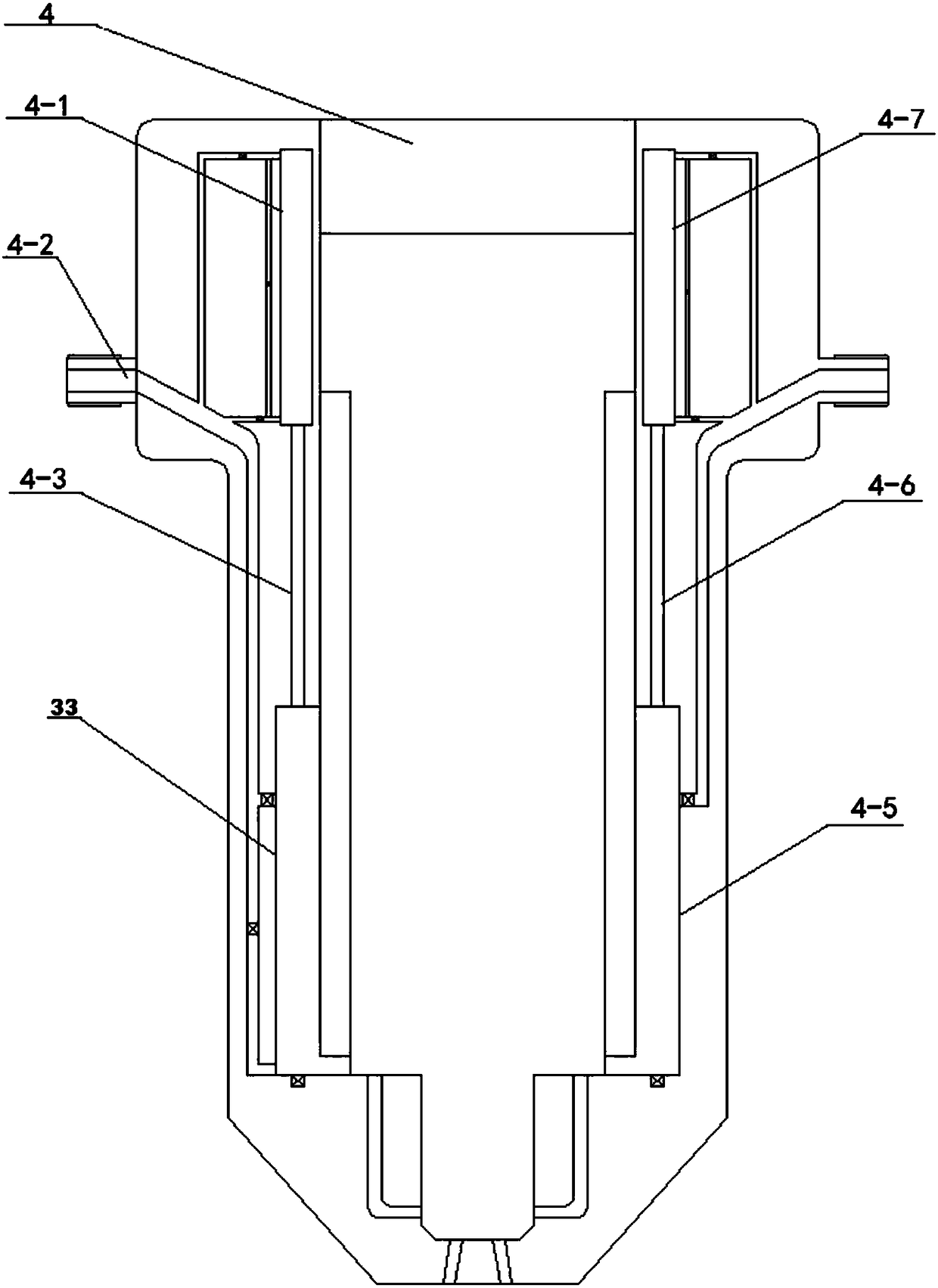
Adjusting method of ethanol-gasoline dual fuel injector with adjustable ratio
InactiveCN108131207AReduce the temperatureIncrease the charge factorElectrical controlFuel injection apparatusEthanol InjectionGasoline
The invention relates to an adjusting method of an ethanol-gasoline dual fuel injector with adjustable ratio. The method specifically comprises the steps that step 1, the operating condition of an engine is determined according to an electronic accelerograph signal of the engine and a rotation speed signal of the engine, and the ethanol volume needed is determined; step 2, according to the determined working condition of the engine and the ethanol volume needed in step 1, moving direction of an ethanol injection piston (11) is controlled, and the ethanol injection piston moves in the up-and-down direction along a push rod chamber of the ethanol injection piston (4-1); step 3, after the moving direction of the ethanol injection piston (11) is determined according to step 2, the precise position of the ethanol injection piston (11) in an ethanol injection piston chamber (33) is controlled, so that target ethanol volume is achieved. According to the method, accurate injection and accuratecontrol of ethanol-gasoline ratio of the dual fuel injector are achieved.
Owner:HUNAN CITY UNIV
Composition with repairing and anti-aging effects, and preparation method and application thereof
InactiveCN109602648ASignificant repairSignificant anti-aging effectCosmetic preparationsToilet preparationsCholesterolLiposome
The present invention relates to a composition with repair and anti-aging effects, and a preparation method and application thereof. The composition comprises the following components in percentage byweight: humectant 10-50%, thickener 0.1-1%, squalane 0.1-10%, ubiquinone 0.01-3%, lecithin 1-20%, cholesterol 1-15%, ceramide 0.1-5%, cypress essential oil 0.01-0.3%, carnosine 0.1-3%, sodium hyaluronate 0.01-0.5%, and deionized water as the balance. The preparation method comprises the steps of: dissolving fat-soluble components of the composition in ethanol, preparing a liposome by using an ethanol injection method, and mixing water-soluble components of the composition with the liposome to obtain the composition with repairing and anti-aging effects. The composition is easily absorbed by skin and has significant repairing and anti-aging benefits.
Owner:广州那比昂生物科技有限公司
Breast cancer treatment triptolide liposome preparation and preparation method thereof
InactiveCN103462898AGood inhibitory effectEnhanced inhibitory effectOrganic active ingredientsAntineoplastic agentsIn vivoOncology
The present invention discloses a breast cancer treatment triptolide liposome preparation and a preparation method thereof, wherein the breast cancer treatment triptolide liposome preparation is a liposome preparation prepared from a breast cancer treatment traditional Chinese medicine active effective component triptolide. The preparation method comprises: adopting an ethanol injection method to prepare liposomes to obtain the breast cancer (breast cancer and triple-negative breast cancer) treatment triptolide liposome preparation. According to the breast cancer treatment triptolide liposome preparation, in vivo animal anti-cancer pharmacological experiment results show that: significant growth inhibition effects are provided for breast cancer and triple-negative breast cancer, and anti-cancer activity of the breast cancer treatment triptolide liposome preparation is respectively and significantly higher than anti-cancer activities of chemotherapy drugs such as paclitaxel and cisplatin. In addition, the triptolide liposome preparation provides a higher anti-cancer effect than the triptolide non-liposome preparation.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
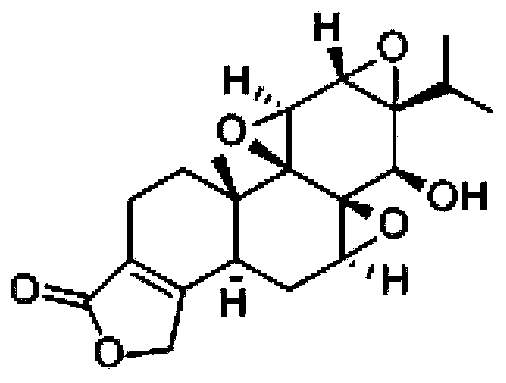
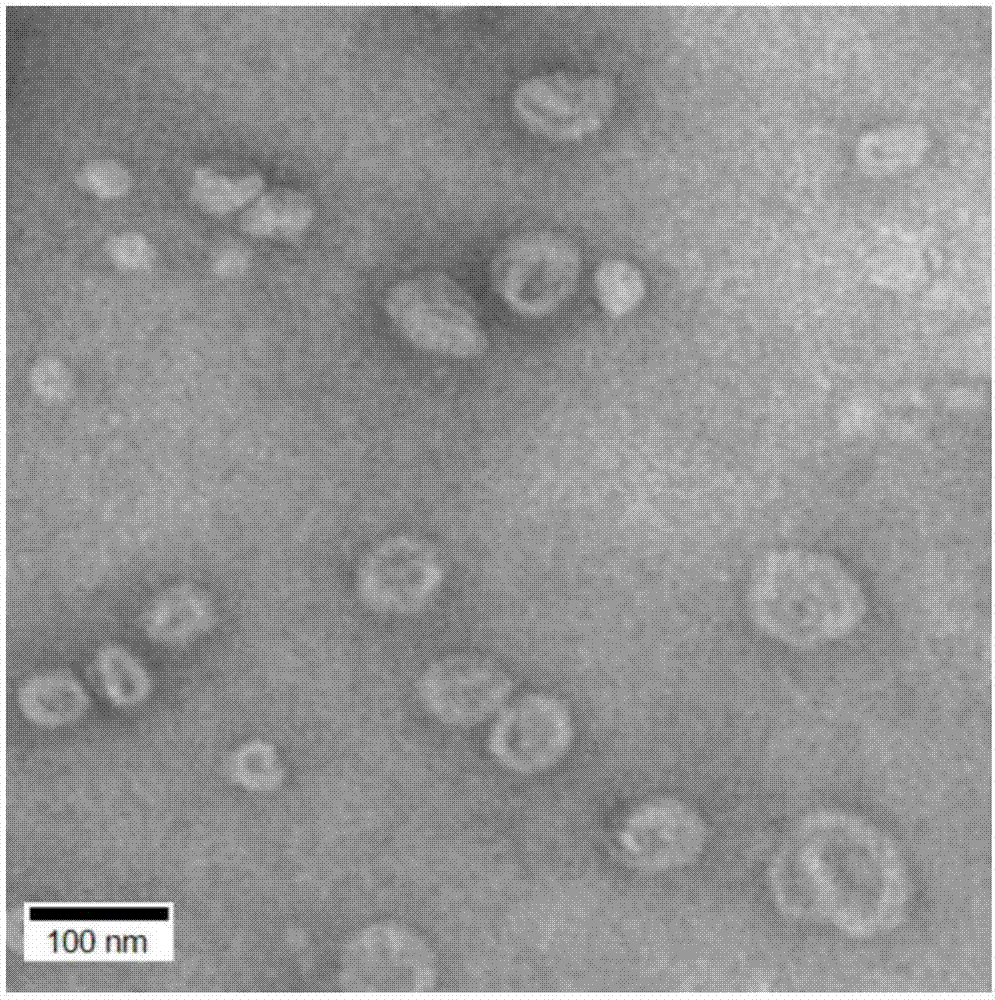
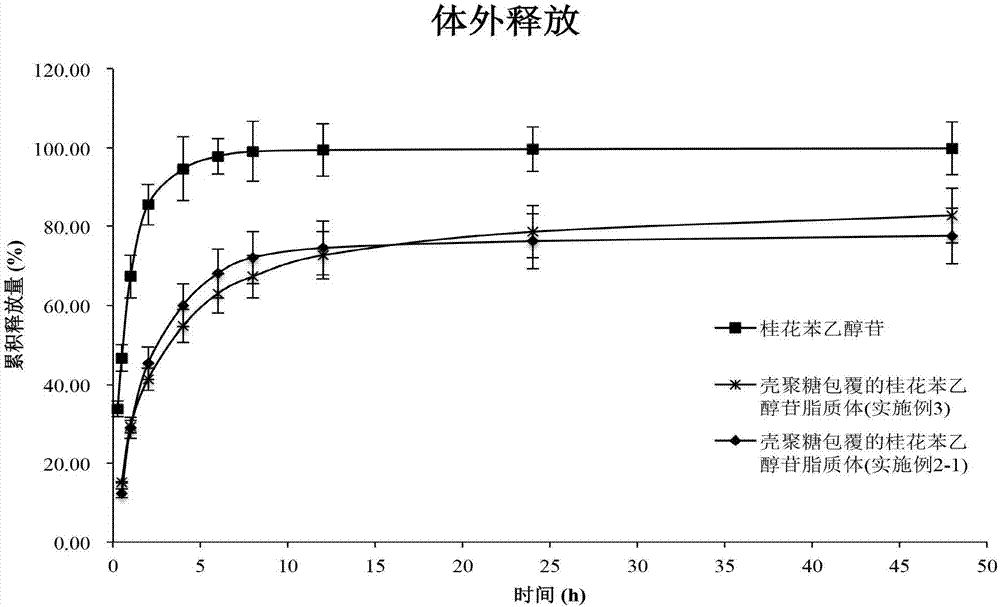
Chitosan-coating sweet-scented osmanthus phenylethanoid glycosides lipidosome and preparation method of chitosan-coating sweet-scented osmanthus phenylethanoid glycosides lipidosome
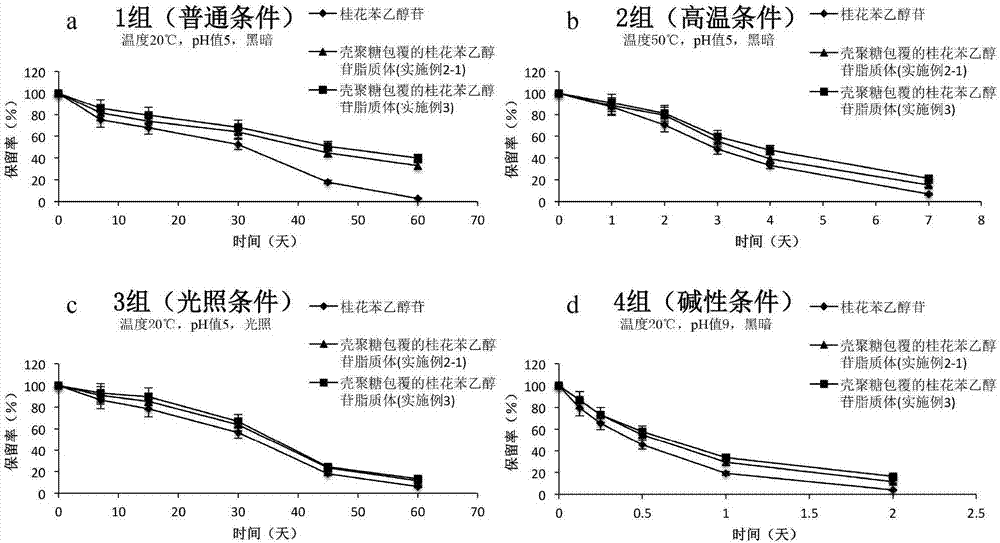
ActiveCN107998183AGood sustained release effectImprove stabilityPharmaceutical non-active ingredientsFood shapingEthanol InjectionChitosan coating
The invention relates to the technical field of healthcare food, and particularly discloses a chitosan-coating sweet-scented osmanthus phenylethanoid glycosides lipidosome and a preparation method ofthe chitosan-coating sweet-scented osmanthus phenylethanoid glycosides lipidosome. The preparation method adopts soybean lecithin and cholesterol as film materials, adopts an ethanol injection methodto prepare the osmanthus phenylethanoid glycosides lipidosome, and utilizes the chitosan to modify the lipidosome, thereby obtaining the chitosan-coating sweet-scented osmanthus phenylethanoid glycosides lipidosome. The embedding rate of the chitosan-coating sweet-scented osmanthus phenylethanoid glycosides lipidosome prepared by the invention is 35.04 to 88.10 percent, and a particle size is 74.14 to 116.47 nm. The stability of the sweet-scented osmanthus phenylethanoid glycosides is improved, and the sweet-scented osmanthus phenylethanoid glycosides has a sustained release effect.
Owner:ZHEJIANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com