KGM (Konjac Glucomannan)-g (grafted)-AH (Alicyclic Amine) drug loaded nano micelle and preparation method
A technology of konjac glucomannan and glucomannan, which can be used in pharmaceutical formulations, anti-tumor drugs, drug combinations, etc., can solve the problem of less pH-sensitive micelles, and achieve reduced toxic and side effects, good biocompatibility, and better biocompatibility. Biodegradability, the effect of improving drug efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
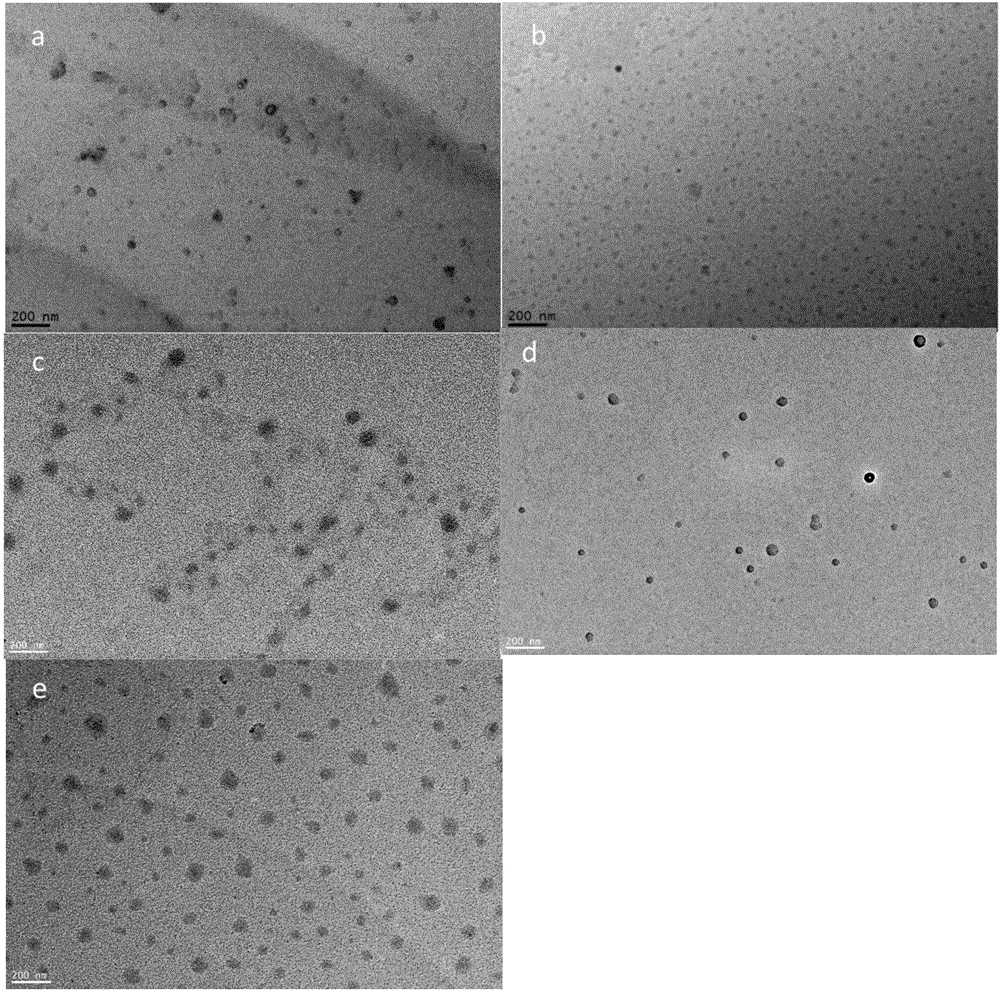
Examples
Embodiment 1
[0033] Example 1: Preparation of Dialdehyde Konjac Glucomannan (DAK)
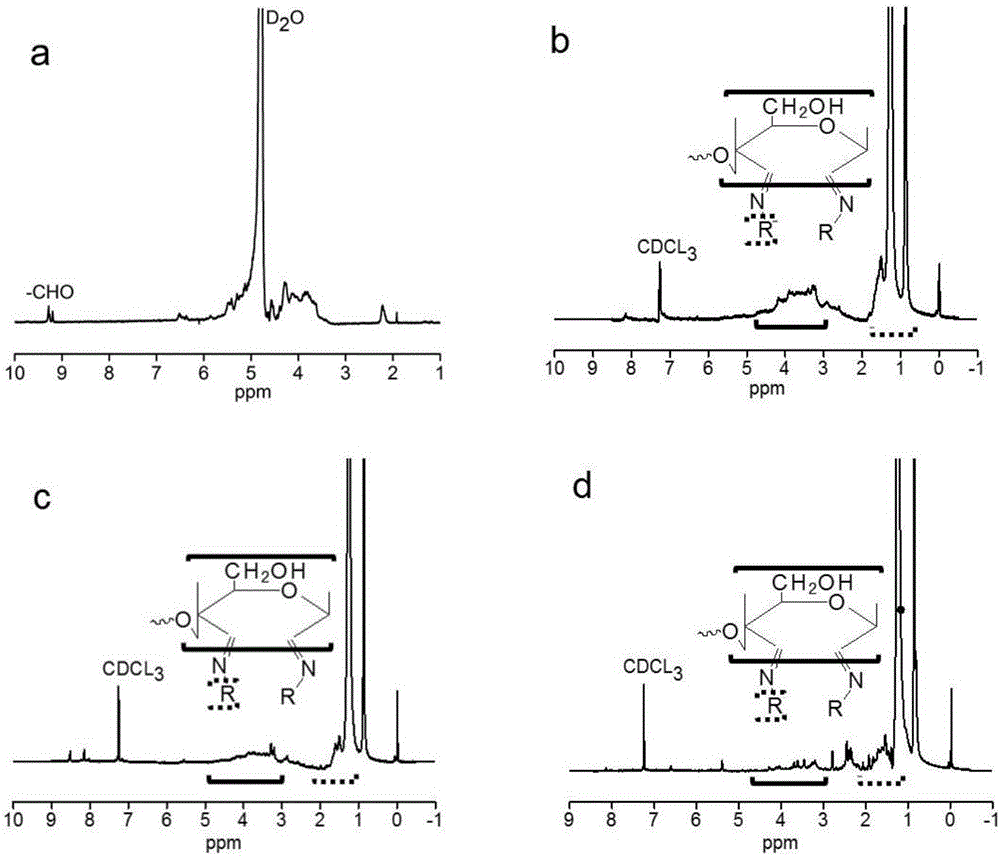
[0034] Weigh 3.00 g of KGM, disperse in 500 mL of double-distilled water under mechanical stirring, and then swell at room temperature for 12 h to obtain a KGM dispersion; weigh 2.40 g of sodium periodate and dissolve in 100 mL of double-distilled water, drop Add it to the KGM dispersion, react for 24 h at room temperature in the dark, remove the reaction bottle, concentrate under reduced pressure at 55 °C to about 300 mL, transfer it to a dialysis bag with a molecular weight cut-off of 3500, After dialysis for 7 days, the dialysate was freeze-dried to obtain white dialdehyde konjac glucomannan (DAK).
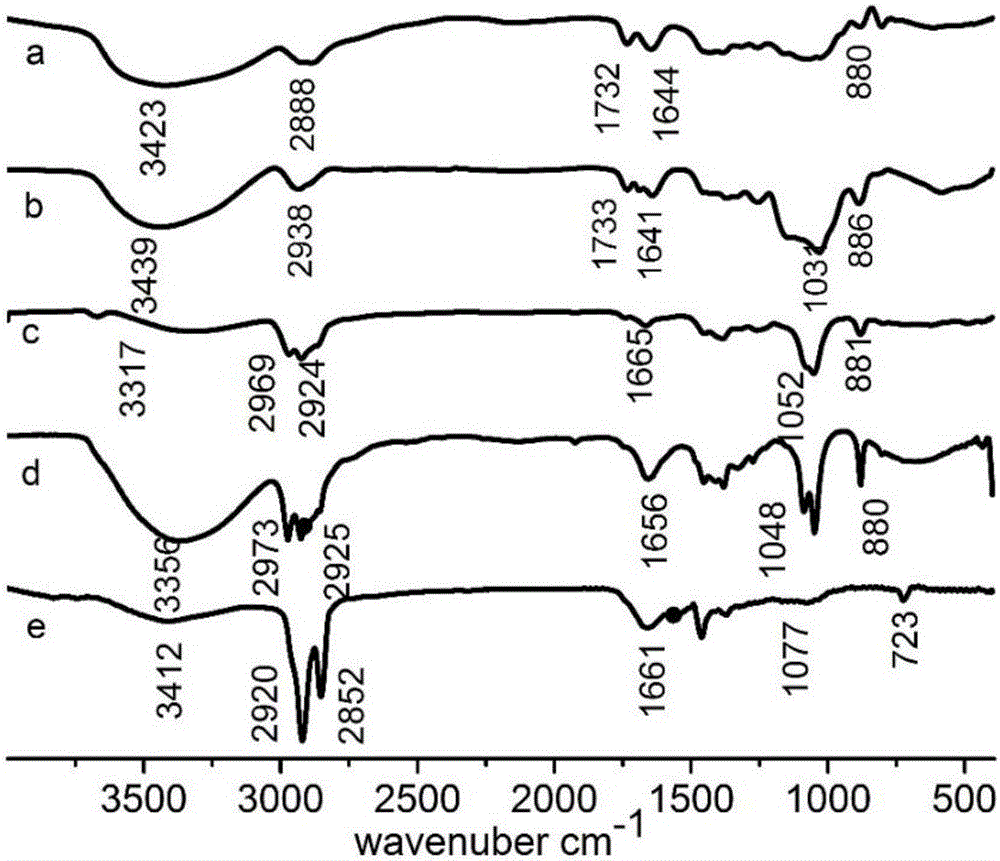
[0035] Such as figure 1 As shown in the infrared spectrum b, at 3429 cm -1 There is a -OH stretching vibration peak at 2938 cm -1 There is a C-H stretching vibration peak at 1733 cm -1 There is a carbonyl stretching vibration peak at , indicating that C=O is produced after KGM is oxidized by sodium peri...
Embodiment 2
[0036] Embodiment 2: the preparation of octylamine grafted KGM (KGM- g -AH 8 )
[0037] Weigh 0.3 g of DAK in 30 mL of double-distilled water and dissolve at 50 °C for 10 min; weigh 0.19 mL of octylamine and dissolve it in 100 mL of ethanol, mix the ethanol solution of octylamine with DAK aqueous solution, and dissolve in the presence of ethanol Reflux at the boiling point for 8 h, remove the reaction bottle, remove ethanol under reduced pressure at 30 °C, wash with ethyl acetate or chloroform three times, take the organic phase, remove the organic reagent under reduced pressure at about 30 °C, and finally dissolve it with 2 mL of ethanol, Transfer to a dialysis bag, dialyze in double distilled water for 2 days, change the water every 4 h, take out the dialyzed fluid, and freeze-dry to obtain brown grafted konjac glucomannan KGM- g -AH 8 .
[0038] Such as figure 1 As shown in the infrared spectrum of c, at 3317 cm -1 There is a -OH stretching vibration peak at 2969 cm ...
Embodiment 3
[0040] Embodiment 3: the preparation of dodecylamine grafted KGM (KGM- g -AH 12 )
[0041] KGM-g-AH 12 Preparation process: Weigh 0.3 g of DAK in 30 mL of double distilled water and dissolve at 50 °C for 10 min; weigh 0.22 g of dodecylamine and dissolve it in 100 mL of ethanol, mix the ethanol solution of dodecylamine with the DAK aqueous solution , reflux at the boiling point of ethanol for 10 h, remove the reaction bottle, remove ethanol under reduced pressure at 30 °C, wash with ethyl acetate three times, take the organic phase, remove the organic reagent under reduced pressure at about 30 °C, and finally, use 2 mL of Dissolved in ethanol, transferred to a dialysis bag, dialyzed in double distilled water for 2 days, changed the water every 4 h, took out the dialyzed fluid, and freeze-dried to obtain brown grafted konjac glucomannan KGM- g -AH 12 .
[0042] Such as figure 1 As shown in the infrared spectrum of d, at 3356 cm -1 There is a -OH stretching vibration pea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com