Patents
Literature
175results about How to "Reduce systemic toxicity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
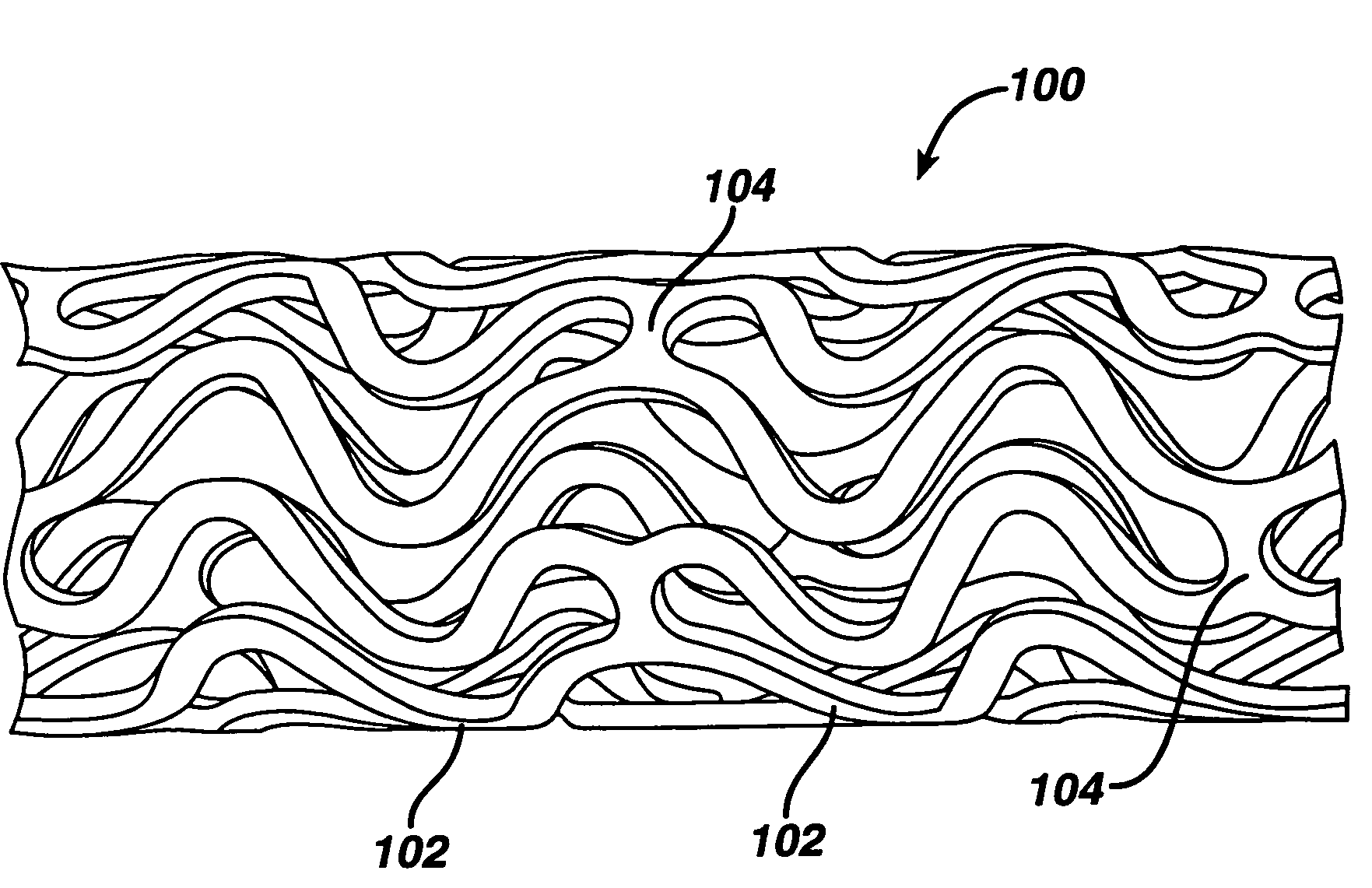
Drug/drug delivery systems for the prevention and treatment of vascular disease
InactiveUS20020007215A1Prevent proliferationGood effectOrganic active ingredientsStentsVascular diseaseWhole body
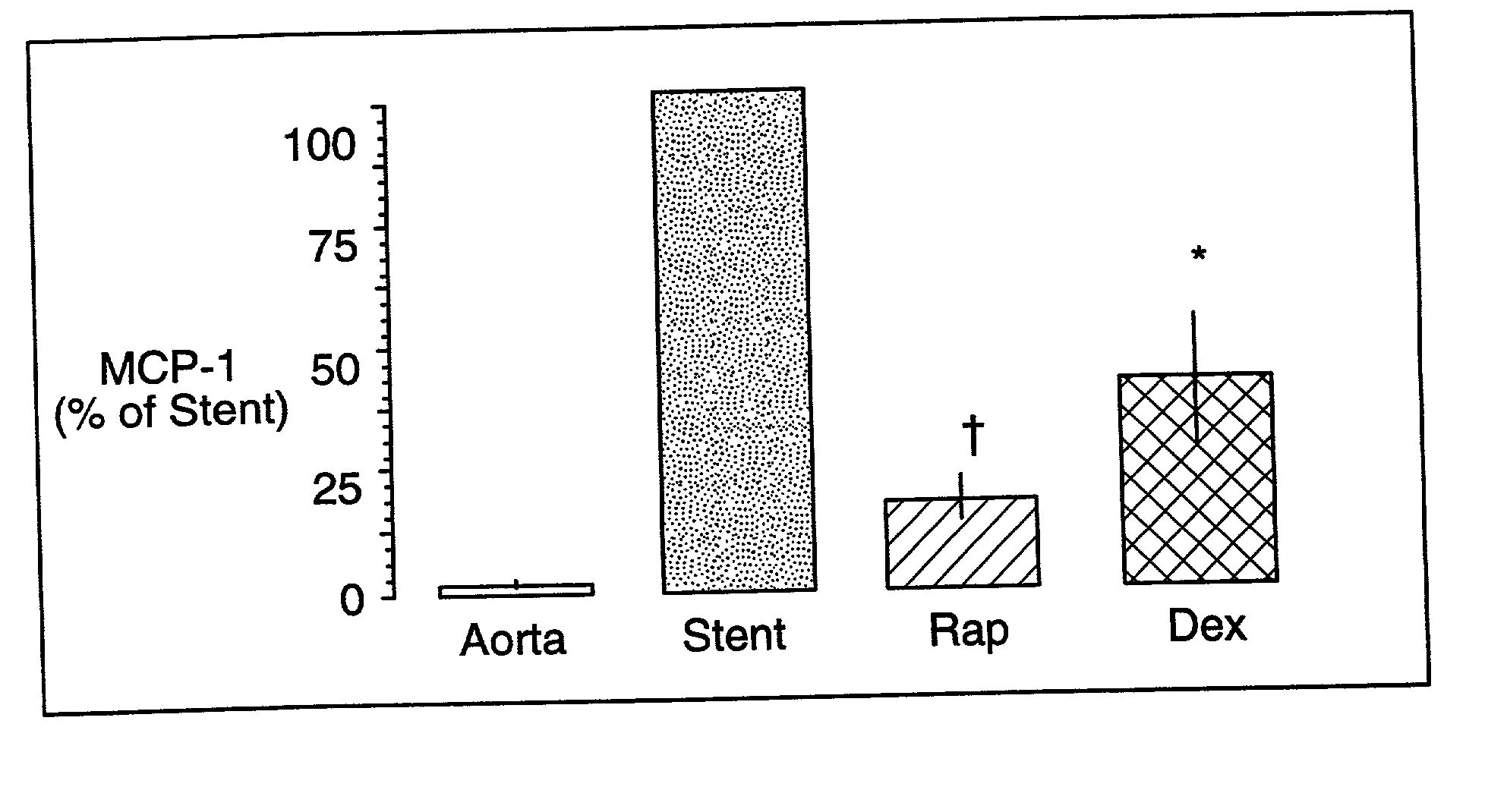
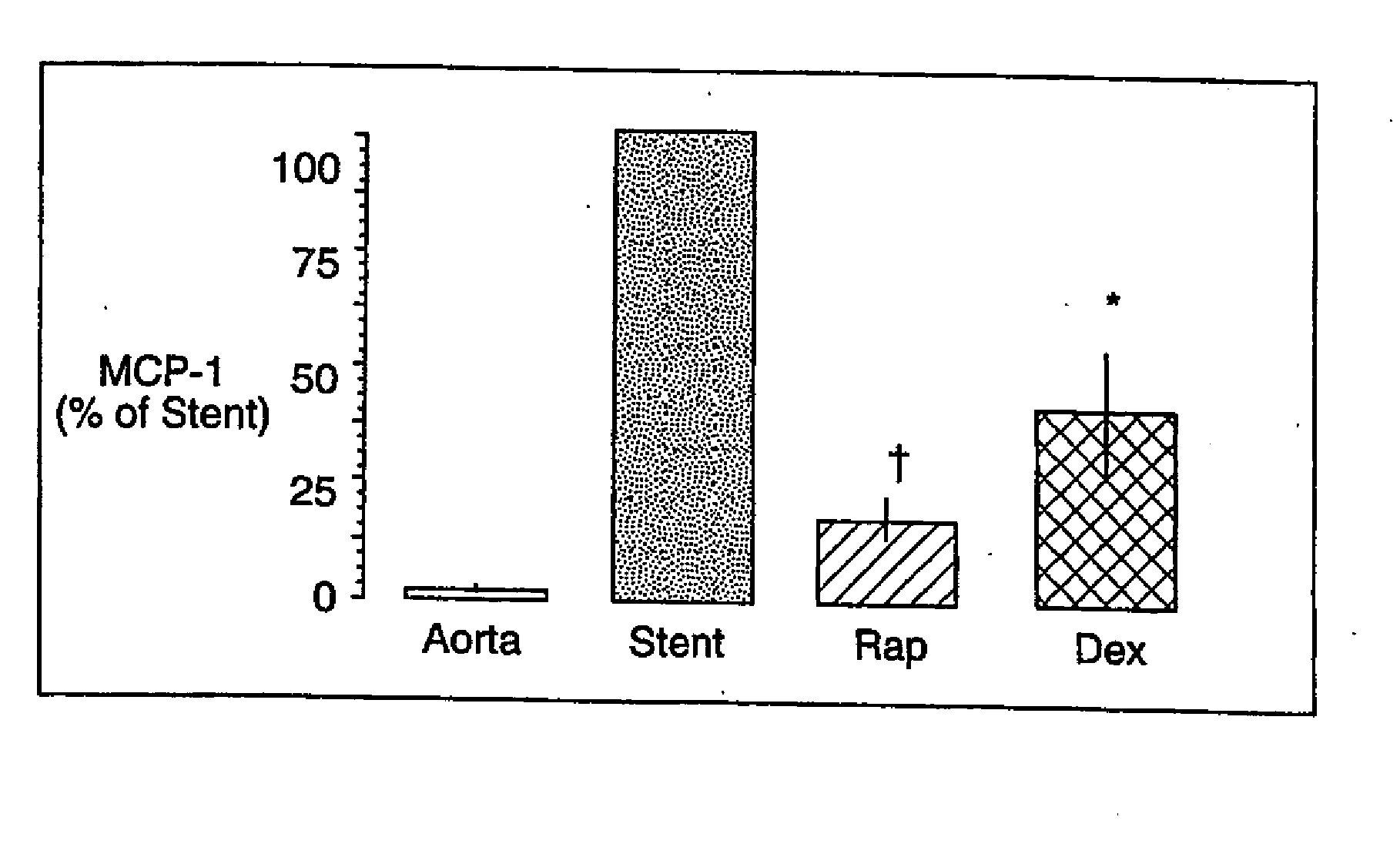
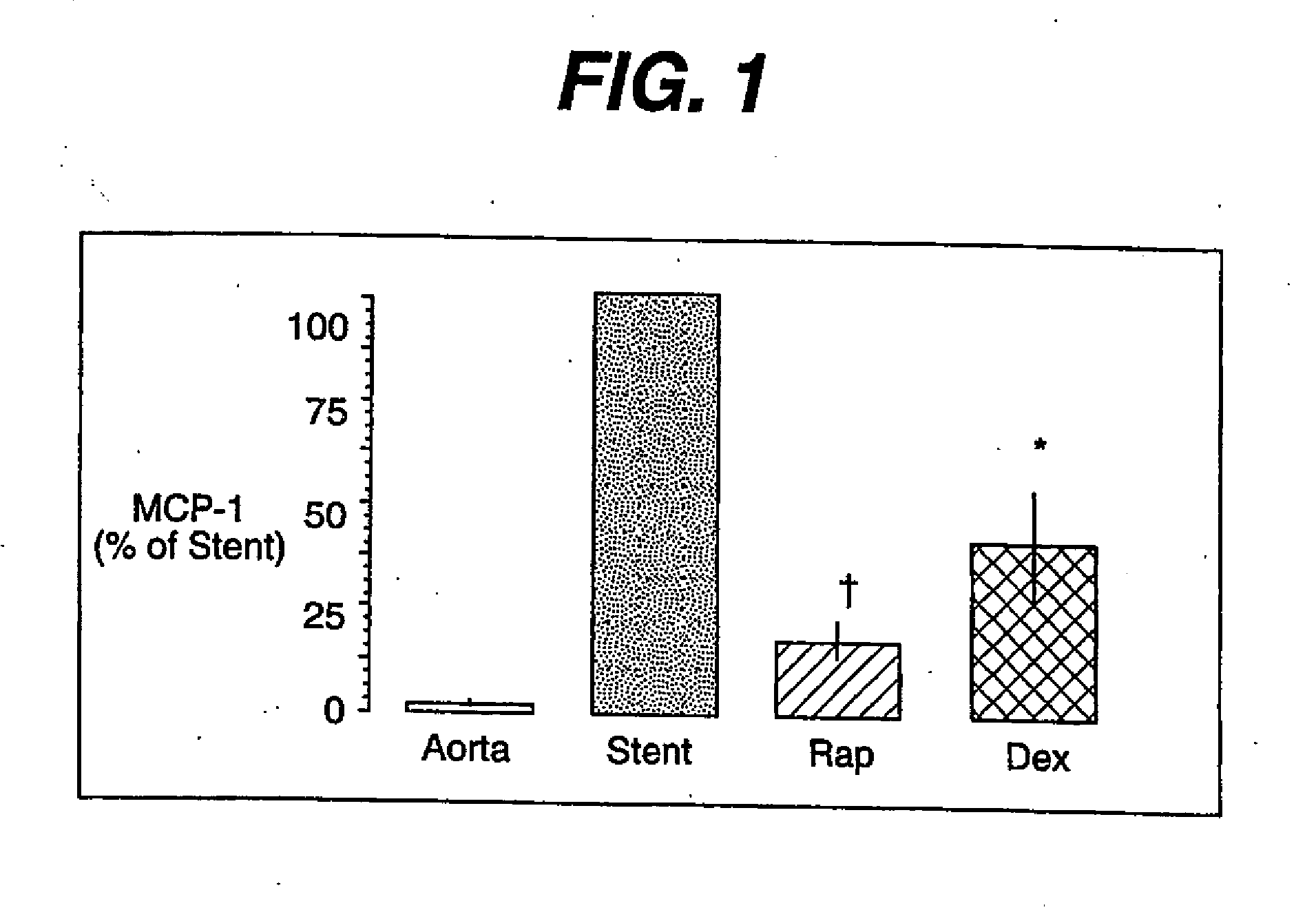
A drug and drug delivery system may be utilized in the treatment of vascular disease. A local delivery system is coated with rapamycin or other suitable drug, agent or compound and delivered intraluminally for the treatment and prevention of neointimal hyperplasia following percutaneous transluminal coronary angiography. The local delivery of the drugs or agents provides for increased effectiveness and lower systemic toxicity.
Owner:WYETH LLC
Disease therapy with chimeric antigen receptor (CAR) constructs and t cells (car-t) or nk cells (car-nk) expressing car constructs
InactiveUS20160361360A1Maintain self-toleranceModulate durationAntibacterial agentsPeptide/protein ingredientsAutoimmune conditionDebulking Procedure
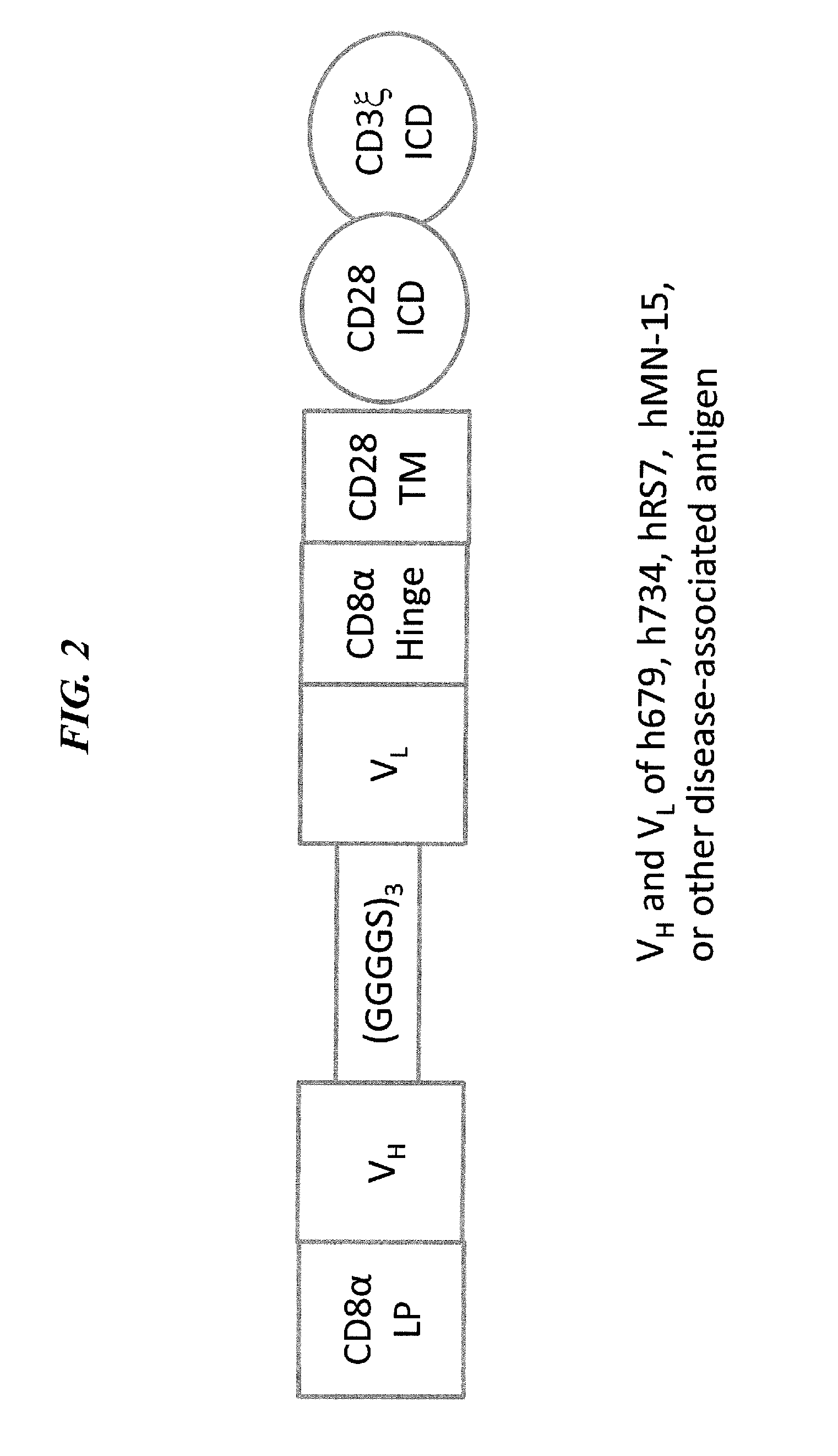
The present invention concerns CAR, CAR-T and CAR-NK constructs, preferably comprising a scFv antibody fragment against a disease-associated antigen or a hapten. More preferably, the antigen is a TAA, such as Trop-2. The constructs may be administered to a subject with a disease, such as cancer, autoimmune disease, or immune dysfunction disease, to induce an immune response against disease-associated cells. Where the constructs bind to a hapten, the subject is first treated with a hapten-conjugated antibody that binds to a disease associated antigen. Therapy may be supplemented by other treatments, such as debulking procedures (e.g., surgery, chemotherapy, radiation therapy) or coadministration of other agents. More preferably, administration of the construct is preceded by predosing with an unconjugated antibody that binds to the same disease-associated antigen. Most preferably, an antibody against CD74 or HLA-DR is administered to reduce systemic immunotoxicity induced by the constructs.
Owner:IMMUNOMEDICS INC
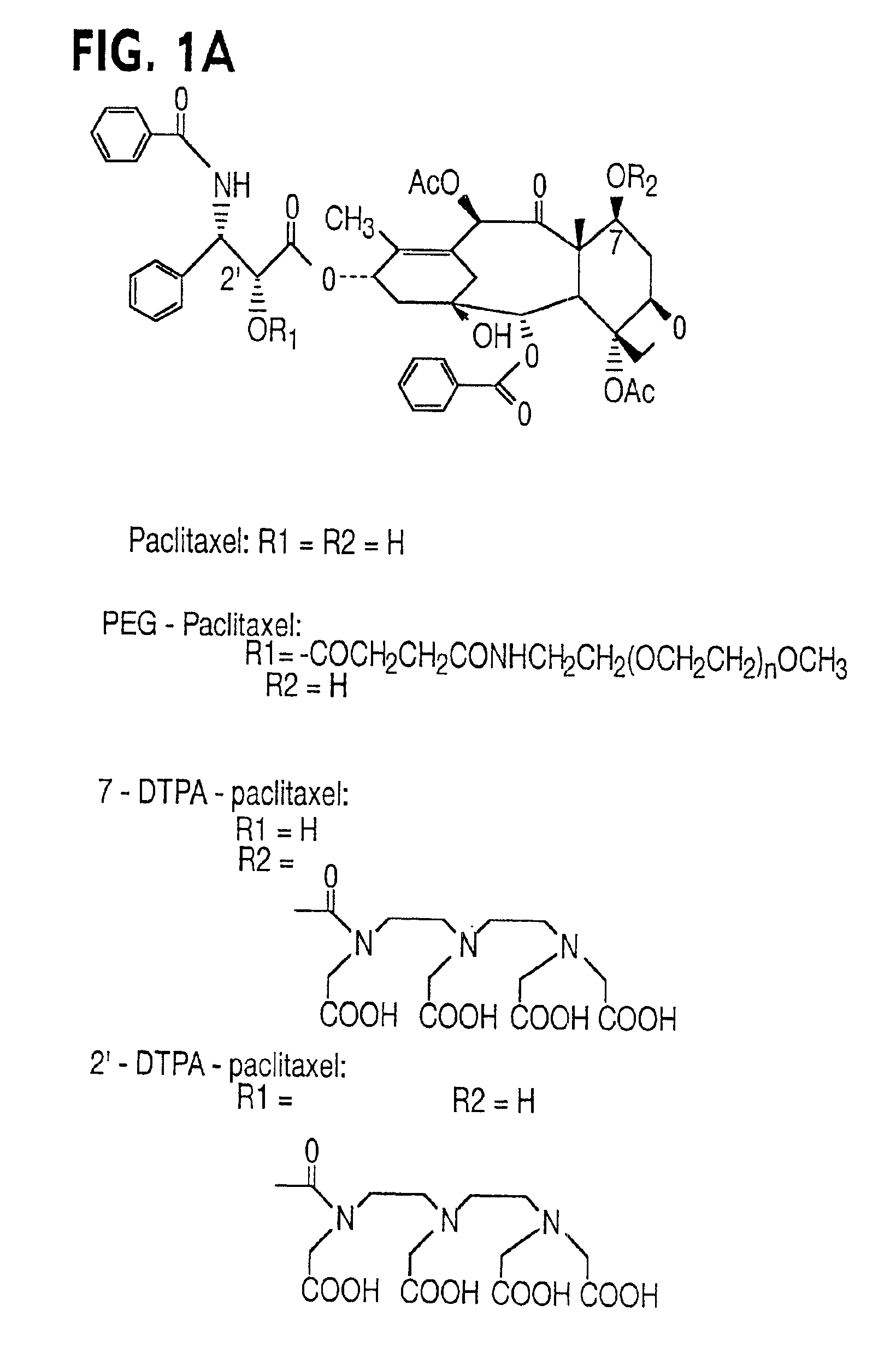
Water soluble paclitaxel derivatives
InactiveUS6884817B2Surprising antitumor activityImprove efficacyHeavy metal active ingredientsBiocideDocetaxel-PNPDocetaxel
Disclosed are water soluble compositions of paclitaxel and docetaxel formed by conjugating the paclitaxel or docetaxel to a water soluble polymer such as poly-glutamic acid, poly-aspartic acid or poly-lysine. Also disclosed are methods of using the compositions for treatment of tumors, auto-immune disorders such as rheumatoid arthritis. Other embodiments include the coating of implantable stents for prevention of restenosis.
Owner:PG TXL COMPANY
Synthetic peptides for use as inhibitors of neurotransmitter secretion and as inducers of cellular relaxation
InactiveUS8318898B2Extended shelf lifeImprove stabilityCosmetic preparationsBiocideMuscle relaxationMuscle contraction
Owner:UNIVERSITY OF LAUSANNE
Polysaccharide containing botulinum toxin pharmaceutical compositions and uses thereof
InactiveUS7758873B2High potencyImprove stabilityCosmetic preparationsOrganic active ingredientsToxinPolysaccharide
This invention relates to the use of a composition comprising a polysaccharide and a botulinum toxin for reducing a skin wrinkle. In some embodiments, the polysaccharide comprises disaccharides. In some embodiments, the average molecular weight of a disaccharide unit of the polysaccharide is between about 345 D and about 1,000 D.
Owner:ALLERGAN INC
Drug/drug delivery systems for the prevention and treatment of vascular disease
InactiveUS7300662B2Prevent proliferationGood effectOrganic active ingredientsOrganic chemistryVascular diseaseWhole body
Owner:WYETH LLC
Water soluble paclitaxel derivatives
InactiveUS7060724B2Surprising antitumor activityImprove efficacyBiocideOrganic active ingredientsDocetaxel-PNPDocetaxel
Disclosed are water soluble compositions of paclitaxel and docetaxel formed by conjugating the paclitaxel or docetaxel to a water soluble polymer such as poly-glutamic acid, poly-aspartic acid or poly-lysine. Also disclosed are methods of using the compositions for treatment of tumors, auto-immune disorders such as rheumatoid arthritis. Other embodiments include the coating of implantable stents for prevention of restenosis.
Owner:PG TXL COMPANY
Coating of devices with effector compounds
InactiveUS20100215708A1Reduce systemic toxicityPrevention and diminishment and reduction in incidenceBiocideGenetic material ingredientsDiseaseSolvent
This invention is directed to substrates, materials and devices coated with a gel, foam, film, particle or composition comprising a polymei solvent and effector compounds attached thereto, processes of producing the same, and methods of use thereof, of in, inter-alia, biological applications, including preventing infection and the treatment of various diseases.
Owner:ZUMBUEHL ANDREAS +4
Targeted mutant alpha-helical bundle cytokines
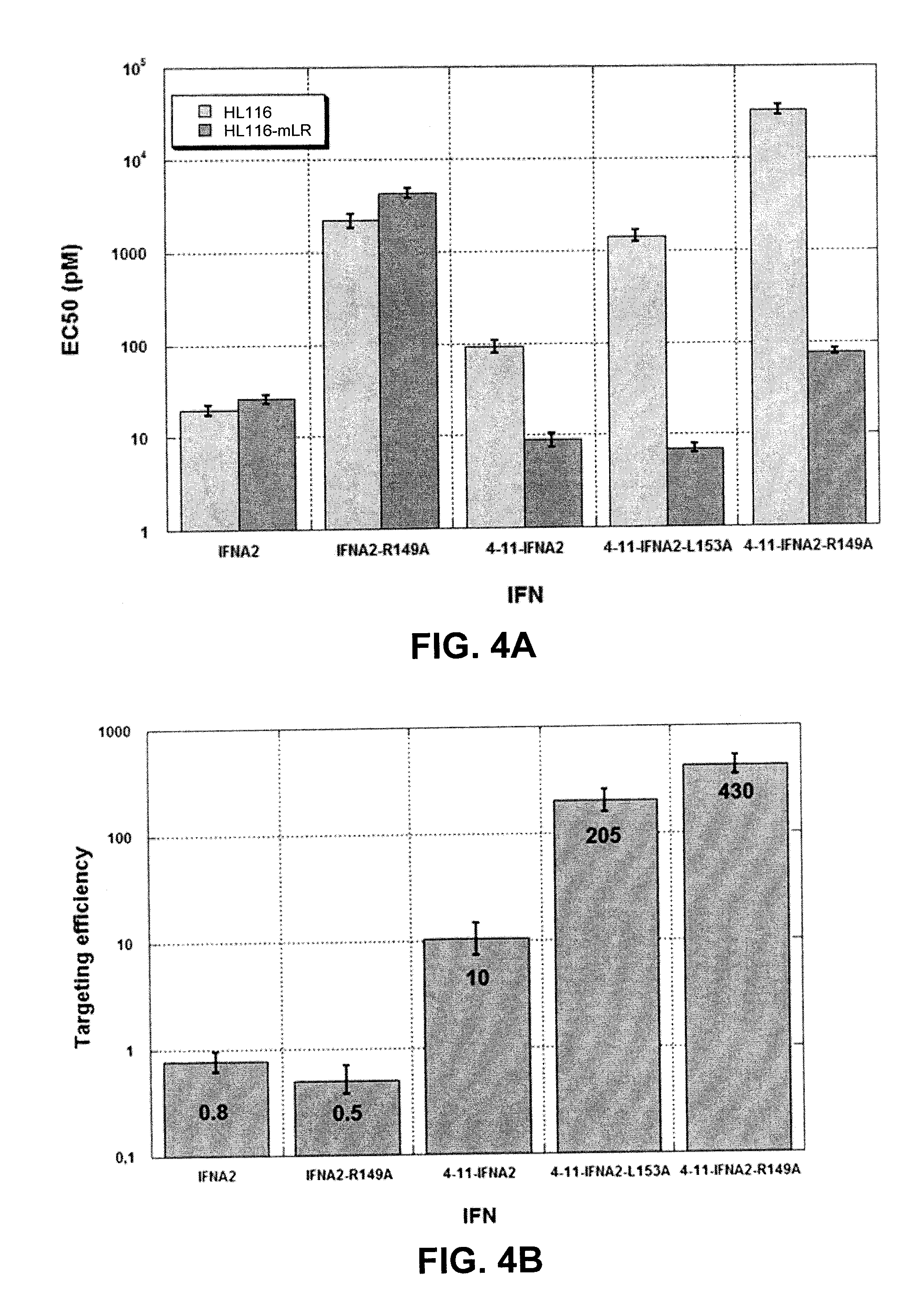
ActiveUS20140348789A1Decrease specific bioactivityLess side effectPeptide/protein ingredientsAntibody mimetics/scaffoldsNeoplasmAntibody
This disclosure relates to a modified α-helical bundle cytokine, with reduced activity via an α-helical bundle cytokine receptor, wherein the α-helical bundle cytokine is specifically delivered to target cells. Preferably, the α-helical bundle cytokine is a mutant, more preferably it is a mutant interferon, with low affinity to the interferon receptor, wherein the mutant interferon is specifically delivered to target cells. The targeting is realized by fusion of the modified α-helical bundle cytokine to a targeting moiety, preferably an antibody. This disclosure relates further to the use of such targeted modified α-helical bundle cytokine to treat diseases. A preferred embodiment is the use of a targeted mutant interferon, to treat diseases, preferably viral diseases and tumors.
Owner:VLAAMS INTERUNIVERSITAIR INST VOOR BIOTECHNOLOGIE VZW +4
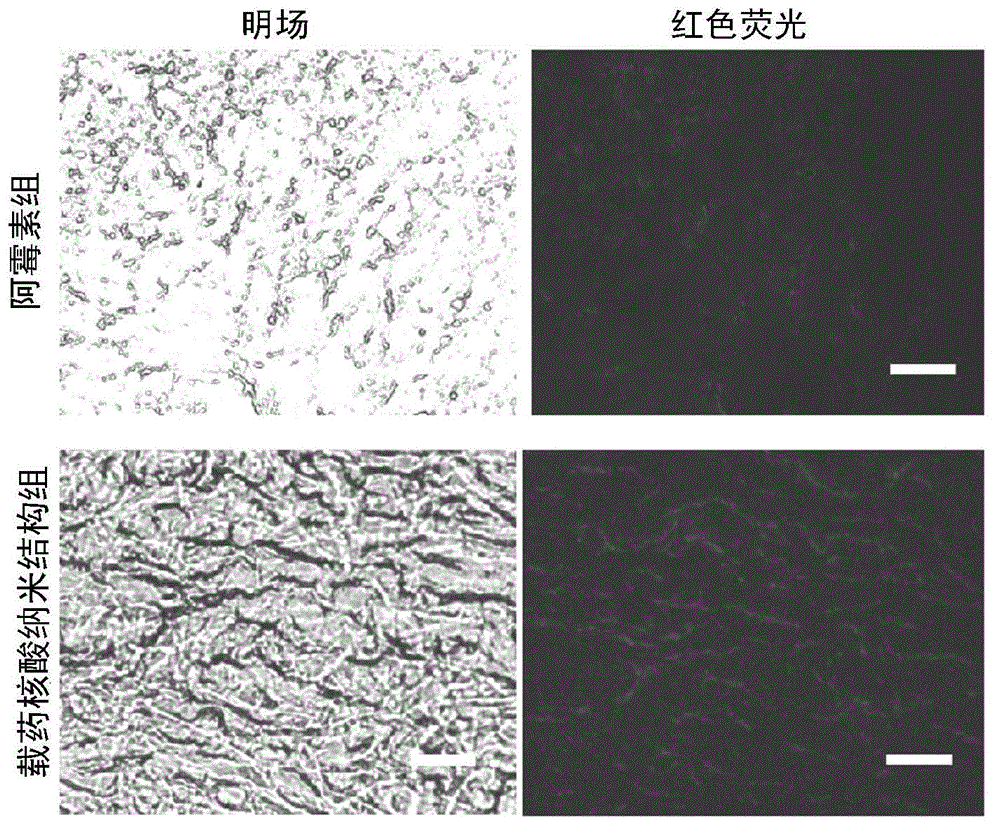
Nucleic acid nano structure for carrying antitumor drugs, preparation method and applications thereof
InactiveCN104368004AGuaranteed antitumor activityImprove targetingNanomedicinePharmaceutical non-active ingredientsNano structuringWhole body
The invention discloses a nucleic acid nano structure for carrying antitumor drugs, a preparation method and applications thereof. The nucleic acid nano structure is a random two-dimensional and / or three-dimensional nano structure constructed through a DNA origami technology. The nucleic acid nano structure is obtained through self-assembly between a scaffold chain and a staple chain for auxiliary folding, wherein the scaffold chain and the stable chain are hybridized according to the base paring principle. The provided nucleic acid nano structure taken as the carrier of antitumor drugs can guarantee the antitumor activity of the carried antitumor drugs, and further improves the targeting property of the antitumor drugs. Thus the antitumor drugs can be enriched in the tumor tissues, and the overall toxicity due to the non-specificity of the antitumor drugs is greatly reduced. Furthermore, the preparation method has the advantages of simple technology, low cost, convenience, and easy application.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA +1
Drug/Drug Delivery Systems for the Prevention and Treatment of Vascular Disease
InactiveUS20070026036A1Reduce systemic toxicityEasy to manageBiocideOrganic chemistryVascular diseaseWhole body
A drug and drug delivery system may be utilized in the treatment of vascular disease. A local delivery system is coated with rapamycin or other suitable drug, agent or compound and delivered intraluminally for the treatment and prevention of neointimal hyperplasia following percutaneous transluminal coronary angiography. The local delivery of the drugs or agents provides for increased effectiveness and lower systemic toxicity.
Owner:WYETH LLC
Compositions including triciribine and bortezomib and derivatives thereof and methods of use thereof
InactiveUS20100009929A1Reduce systemic toxicityMinimize ToxicityBiocideDipeptide ingredientsTriciribineBortezomib
This application relates to combination therapies including triciribine and related compounds and bortezomib and derivatives thereof analogs and compositions with reduced toxicity for the treatment and prevention of tumors, cancer, and other disorders associated with abnormal cell proliferation.
Owner:UNIV OF SOUTH FLORIDA
Conjugated biological molecules and their preparation
ActiveUS7939630B2Favourably alter pharmacokineticsExtended circulation timePeptide/protein ingredientsPeptide preparation methodsHydrogen atomMedicinal chemistry
Novel biologically active compounds of the general formula (I) in which one of X and X′ represents a polymer, and the other represents a hydrogen atom; each Q independently represents a linking group; W represents an electron-withdrawing moiety or a moiety preparable by reduction of an electron-withdrawing moiety; or, if X′ represents a polymer, X-Q-W— together may represent an electron withdrawing group; and in addition, if X represents a polymer, X′ and electron withdrawing group W together with the interjacent atoms may form a ring; each of Z1 and Z2 independently represents a group derived from a biological molecule, each of which is linked to A and B via a nucleophilic moiety; or Z1 and Z2 together represent a single group derived from a biological molecule which is linked to A and B via two nucleophilic moieties; A is a C1-5 alkylene or alkenylene chain; and B is a bond or a C1-4 alkylene or alkenylene chain; are formed by conjugating a suitable polymer to a suitable biologically active molecule via nucleophilic groups in said molecule, preferably via a disulphide bridge.
Owner:ABZENA UK LTD
Engineered TAA antibody-TNFSF member ligand fusion molecules
InactiveUS9534056B2Reduce systemic toxicityImprove efficacyPeptide/protein ingredientsAntibody mimetics/scaffoldsWhole bodyApoptosis
Owner:IMMUNGENE
VEGFR-3 inhibitor materials and methods
InactiveUS7611711B2Extended shelf lifeImprove stabilityImmunoglobulinsCyclic peptide ingredientsDiseaseLymphatic vessel
The present invention relates to the diagnosis, evaluation, and therapeutic intervention of disorders mediated by the activity of cell surface receptor VEGFR-3, which activity often is stimulated by VEGFR-3 ligands VEGF-C and VEGF-D. More particularly, the present invention identifies novel methods and compositions for the inhibition of VEGF-C / D binding to VEGFR-3. The compositions of the present invention will be useful in the inhibition of angiogenesis and lymphangiogenesis.
Owner:VEGENICS PTY LTD
Pharmaceutical compositions containing botulinum toxin
InactiveUS8216591B2Low toxicityLow immunogenicityPowder deliveryOrganic active ingredientsMedicineToxin
This invention relates to the use of a composition comprising a polysaccharide and a botulinum toxin for reducing a skin wrinkle. In some embodiments, the polysaccharide comprises disaccharides. In some embodiments, the average molecular weight of a disaccharide unit of the polysaccharide is between about 345 D and about 1,000 D.
Owner:ALLERGAN INC
Vinorelbine liposome micro ball injection and its prepn
InactiveCN1771954ALess irritatingLow toxicityOrganic active ingredientsGranular deliveryMedicineIrritation
The present invention relates to one kind of vinorelbine liposome micro ball injection and its preparation process. The present invention has vinorelbine medicine in 90-98 % coated inside the oil phase and oil-water interface of liposome micro ball, reduces the clinical toxicity and irritation of vinorelbine greatly and raises its antitumor effect. The preparation of the present invention as one kind of antitumor injection has low irritation, low toxicity and high curative effect.
Owner:沈阳东星医药科技有限公司
Hydrophobic polyamine amides as potent lipopolysaccharide sequestrants
InactiveUS20060122279A1Extended time-window of protectionProlonged temporal window of protectionAntibacterial agentsBiocideMedicinal chemistryGram negative sepsis
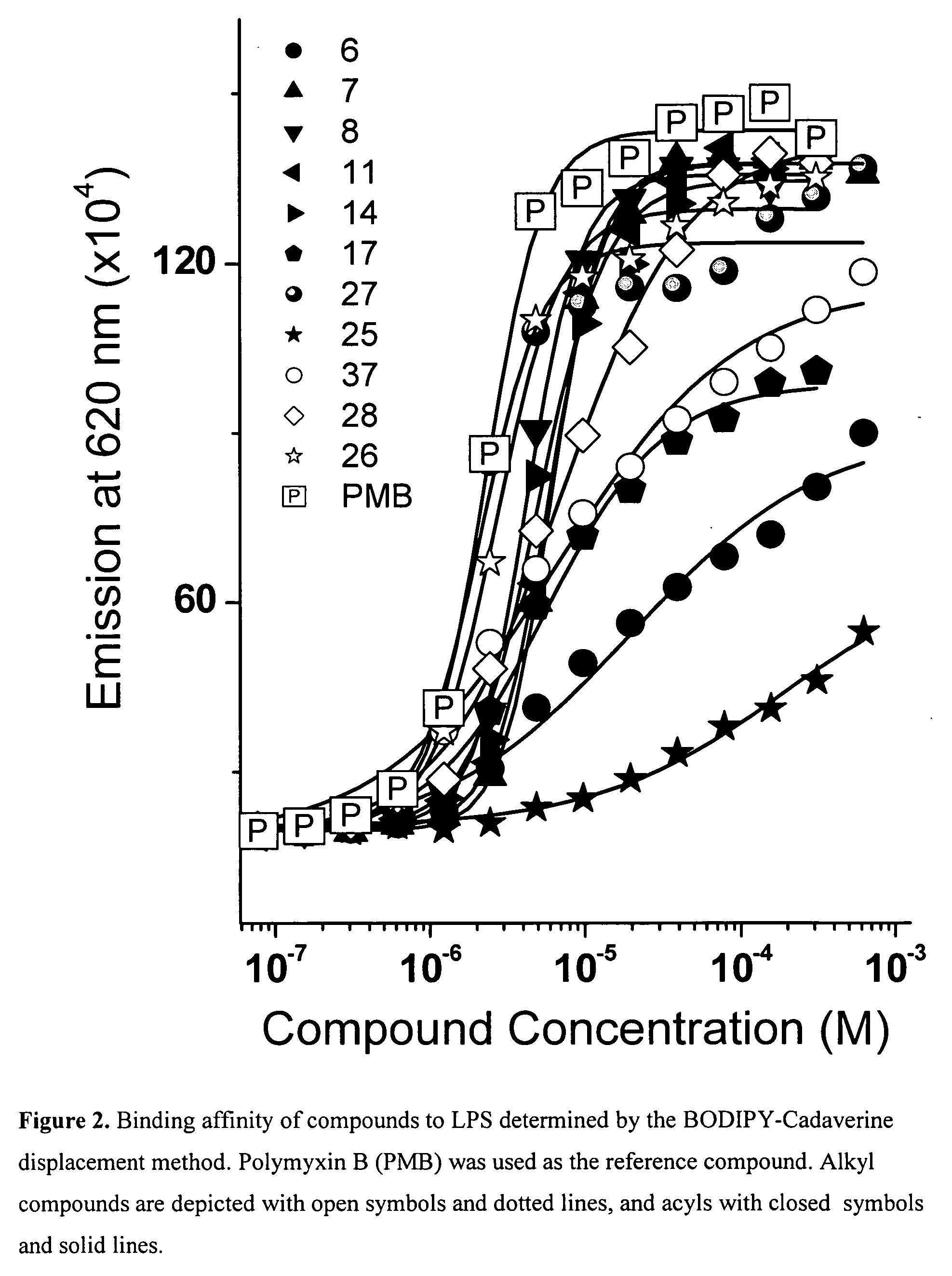
Lysine-spermine conjugates with a long-chain aliphatic (C12-C20) substituent at R1 bind and neutralize bacterial lipopolysaccharides. These compounds reduce lethality in a murine model of lipopolysaccharide-induced shock, and may serve as novel leads for developing novel anti-lipopolysaccharide agents for the therapy of Gram-negative sepsis. These compounds are represented by the formula: wherein X is O or H, H; R is a hydrophobic C12-C20 chain and Y is -NH2 or -H; and pharmaceutically acceptable salts thereof and prodrugs thereof.
Owner:MEDIQUEST THERAPEUTICS INC +1
Novel long-circulating liposome composition and preparation method thereof
ActiveCN101889982ALow chargeReduce chargeOrganic active ingredientsPharmaceutical non-active ingredientsCholesterolDocetaxel
The invention relates to a novel long-circulating liposome composition and an application method thereof. The long-circulating liposome composition is mainly prepared from phospholipid, cholesterol, an electronegative material and a long-circulating material serving as main raw materials, wherein tocopheryl acetate succinate or cholesterol succinate is used as the electronegative material particularly, and tocopheryl acetate polyethylene glycol succinate (TPGS) is used as the long-circulating material particularly, so that the stability of combining the long-circulating liposome composition and medicaments is improved; the long-circulating liposome composition can be used as a carrier of anti-tumor medicaments such as paclitaxel, docetaxel and 10-hydroxycamptothecine, and simultaneously, the tolerance is improved; and when the long-circulating liposome composition is used as the medicinal carrier, the circulating time of the carried medicaments in vivo is longer than that of various carriers sold on the market obviously, and the long-circulating liposome composition has the advantages of low cost and suitability for wide popularization and application.
Owner:LUNAN PHARMA GROUP CORPORATION
Microencapsulated compounds and method of preparing same
InactiveUS6555110B1Inhibit cytokine synthesisReduce systemic toxicityImmunoglobulins against cytokines/lymphokines/interferonsAlbumin peptidesDiseaseMicrosphere
Compositions useful in treating immune modulated disease comprising an anticytokine antibody or immune active drug capable of modifying cytokine activity or modulating the immune system microencapsulated with a biodegradable nonantigenic material, such as albumin or PLGA. When the composition is introduced into a subject, it is phagocytosed by the target organ, the target organ digests the microsphere, releasing the drug or an active form or fragment thereof intracellularly. The drug then modifies the target organ function, thereby modulating it's activity. A method is disclosed for preparation of the microencapsulated composition.
Owner:OF MERCER UNIV THE
Prodrugs for use as ophthalmic agents
ActiveUS20040171596A1Reduced lipid solubilityImproved transcorneal penetrationOrganic active ingredientsSteroidsSolubilityDisease
The subject invention provides a mechanism by which steroidal quinol compounds confer beneficial ophthalmic effects. The subject compounds possess a lipophilic-hydrophilic balance for transcorneal penetration and are readily reduced into parent phenolic A-ring steroid compounds to provide protection or treatment against various ocular symptoms and disorders. The compounds according to the subject invention appear to be highly advantageous as prodrugs to provide protection and / or treatment against ocular disorders. These prodrugs confer lipid solubility optimal for transocorneal penetration and are readily converted to endogenous reducing agents into active phenolic A-ring steroid compounds. To the extent that these prodrugs have reduced feminizing effects and systemic toxicity, they would be expected to be quite advantageous for protecting or treating the eye against ocular disorders such as cataract or glaucoma without undesired (systemic) side effects).
Owner:UNIV OF FLORIDA RES FOUNDATION INC +2
Topical anesthetic formulation
InactiveUS7273887B1Improve permeabilityReduce systemic toxicityBiocidePharmaceutical non-active ingredientsWhole bodyAdditive ingredient
The topical anesthetic formulation of the present invention is typically a solution that preferably includes lidocaine, USP as the active anesthetic ingredient with benzyl alcohol and isopropyl alcohol. This invention deals with problems commonly associated with topical application of local anesthetics such as: slow onset of action; need for occlusion; messiness of creams, ointments or gels; and rapid loss of effect due to rapid systemic dispersion. The invention permits enhanced penetration of the anesthetic and thereby allows for a lesser total dosage of pharmaceutically active ingredient. The use of a lesser total dosage also decreases systemic toxicity.
Owner:TRANSDERMATECH
HER cancer protocols
InactiveUS7309486B1Reduce systemic toxicityAvoid asynchronicityOrganic active ingredientsAntibody ingredientsDual actionIntracrine
Owner:ZAMOYSKI MARK
Anthracene nucleus medicinal liposome injection and preparation method
InactiveCN101190188AGood storage stabilityIncreased drug release rateOrganic active ingredientsPowder deliverySide effectFreeze-drying
The invention discloses an anthracycline liposome injection, including the injection and a frozen powder injection and a preparation process of the injection. The frozen liposome consists of the anthracycline or an anthracycline hydrochloride, compound neutral phospholipids, surfactant, negative-charge phospholipids, buffers, PH regulators and freezing protection agent. The preparation process includes the following steps: preparation of a hollow liposome, a homogenized liposome, an anthracycline lopsome and an anthracycline lopsome suspension; adding the freezing protection agent; constant volume; sterilization; sub-package; freezing and drying; storage, etc. The liposome injection can be preserved for 12 months under the room temperature with better stability. And the encapsulation rate can be above 95 percent and a granule diameter is between 30 and 300mm. The side effect is low and the technique is simple, thus being convenient for industrial production.
Owner:北京天衡药物研究院有限公司
Methods of administering microencapsulated materials for immune modulated diseases
InactiveUS7105158B1Reduce doseInhibit cytokine synthesisOrganic active ingredientsAntibody ingredientsDiseaseMicrosphere
Compositions useful in treating immune modulated disease comprising an anticytokine antibody or immune active drug capable of modifying cytokine activity or modulating the immune system microencapsulated with a biodegradable nonantigenic material, such as albumin or PLGA. When the composition is introduced into a subject, it is phagocytosed by the target organ, the target organ digests the microsphere, releasing the drug or an active form or fragment thereof intracellularly. The drug then modifies the target organ function, thereby modulating it's activity. A method is disclosed for preparation of the microencapsulated composition.
Owner:OF MERCER UNIV THE
Synthetic peptides for use as inhibitors of neurotransmitter secretion and as inducers of cellular relaxation
InactiveUS20090226387A1Reduce depthIncrease in wrinkle sizeCosmetic preparationsNervous disorderMuscle relaxationMuscle contraction
Owner:UNIVERSITY OF LAUSANNE
Composition and methods for targeted delivery of a therapeutic compound to the brain or spinal cord of a subject for treatment of neurodegenerative diseases
ActiveUS9259432B1Good curative effectReduce systemic toxicityPolypeptide with localisation/targeting motifOrganic active ingredientsMedicineSpinal cord
Compositions for targeting to a desired region of the brain or spinal cord include a therapeutic compound useful for the treatment of a neurodegenerative disease; a cell penetrating peptide (CPP); and a thermal targeting polypeptide (TTP).
Owner:UNIV OF MISSISSIPPI MEDICAL CENT
Pharmaceutical compositions containing botulinum neurotoxin
InactiveUS8501196B2Low toxicityLow immunogenicityCosmetic preparationsOrganic active ingredientsPolysaccharideDisaccharide
Owner:ALLERGAN INC
Thermally induced hydrogel containing selenium or tellurium, and preparation method and applications thereof
ActiveCN105287362APossesses thermally induced gelation propertiesGood injectabilityHeavy metal active ingredientsAerosol deliveryWhole bodyTe element
The invention belongs to the field of biomedical polymer materials, and specifically relates to a thermally induced hydrogel containing selenium or tellurium, and a preparation method and applications thereof. The thermally induced hydrogel is composed of an amphiphilic block copolymer containing selenium or tellurium and a solvent; thermally induced gelatinization phase transformation of a water system of the amphiphilic block copolymer and the solvent can be realized with temperature increasing so as to realize spontaneous formation of a physical hydrogel, wherein the amphiphilic block copolymer containing selenium or tellurium is obtained via covalent bonding of small molecules containing selenium or tellurium with an amphiphilic block copolymer. Long-acting platinum drug gel sustained-release preparations can be obtained via coordination of the thermally induced hydrogel with platinum drugs. The long-acting platinum drug gel sustained-release preparations are capable of prolonging and adjusting release behavior of the loaded platinum antitumor drugs. Drug administration of the long-acting platinum drug gel sustained-release preparations in tumor, around tumor, or in tumor cavity can be realized via injection; in situ formation of gel is realized at body temperature, and the platinum drugs loaded via coordination bonds can be released from the gel slowly, so that administration frequency is reduced, and drug whole body toxicity is reduced. The long-acting platinum drug gel sustained-release preparations can be applied to tumor treatment at different periods.
Owner:FUDAN UNIV
Targeted capsules for the delivery of skin whitening agents in the skin
InactiveUS20160250128A1Good whitening effectHigh activityCosmetic preparationsToilet preparationsPeptideDark spot
The present invention relates to targeted microcapsules or nanocapsules comprising a skin whitening compound to target melanocytes with a peptide, which is a MC1 receptor agonist, wherein said peptide is coupled to the outer surface of the microcapsule or nanocapsule. It also relates to the use of said microcapsules or nanocapsules for the cosmetic prevention and / or cosmetic treatment of cutaneous dark spots, and to the cosmetic compositions comprising said microcapsules or nanocapsules. The invention also relates to the MC1 receptor agonist peptide coupled to the microcapsule or nanocapsule. The invention also relates to a peptide for use as skin whitening compound.
Owner:INFINITEC ACTIVOS SL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com