Novel long-circulating liposome composition and preparation method thereof
A long-circulation liposome and composition technology, which is applied in the direction of liposome delivery, drug combination, medical preparations of non-active ingredients, etc., can solve the problem of high production cost, large mass ratio, and increased production of long-circulation liposomes Cost and other issues, to achieve the effect of low charge, stable quality, and obvious long cycle characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
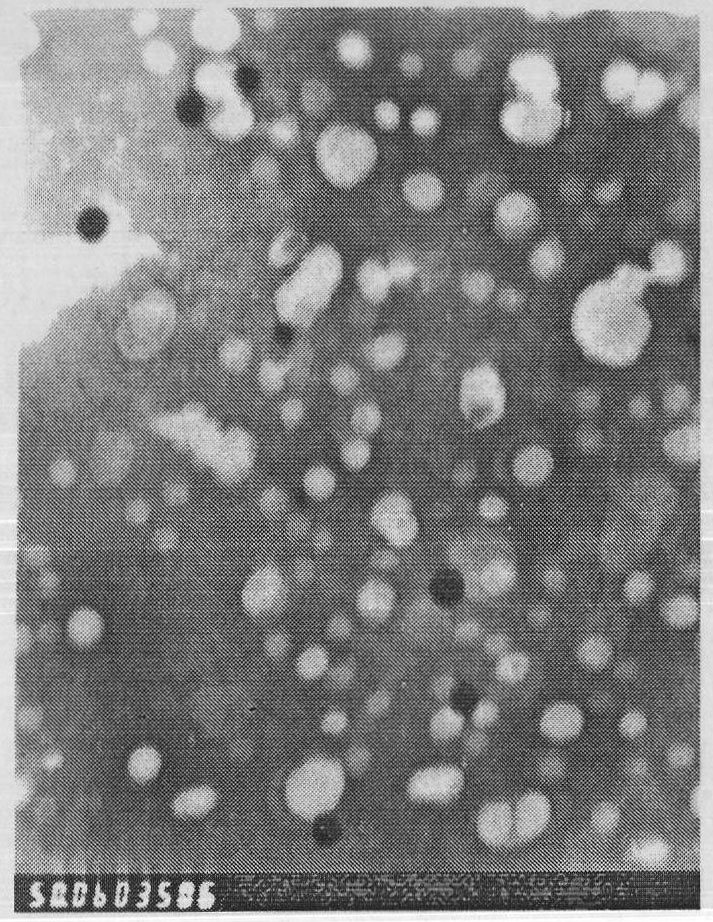
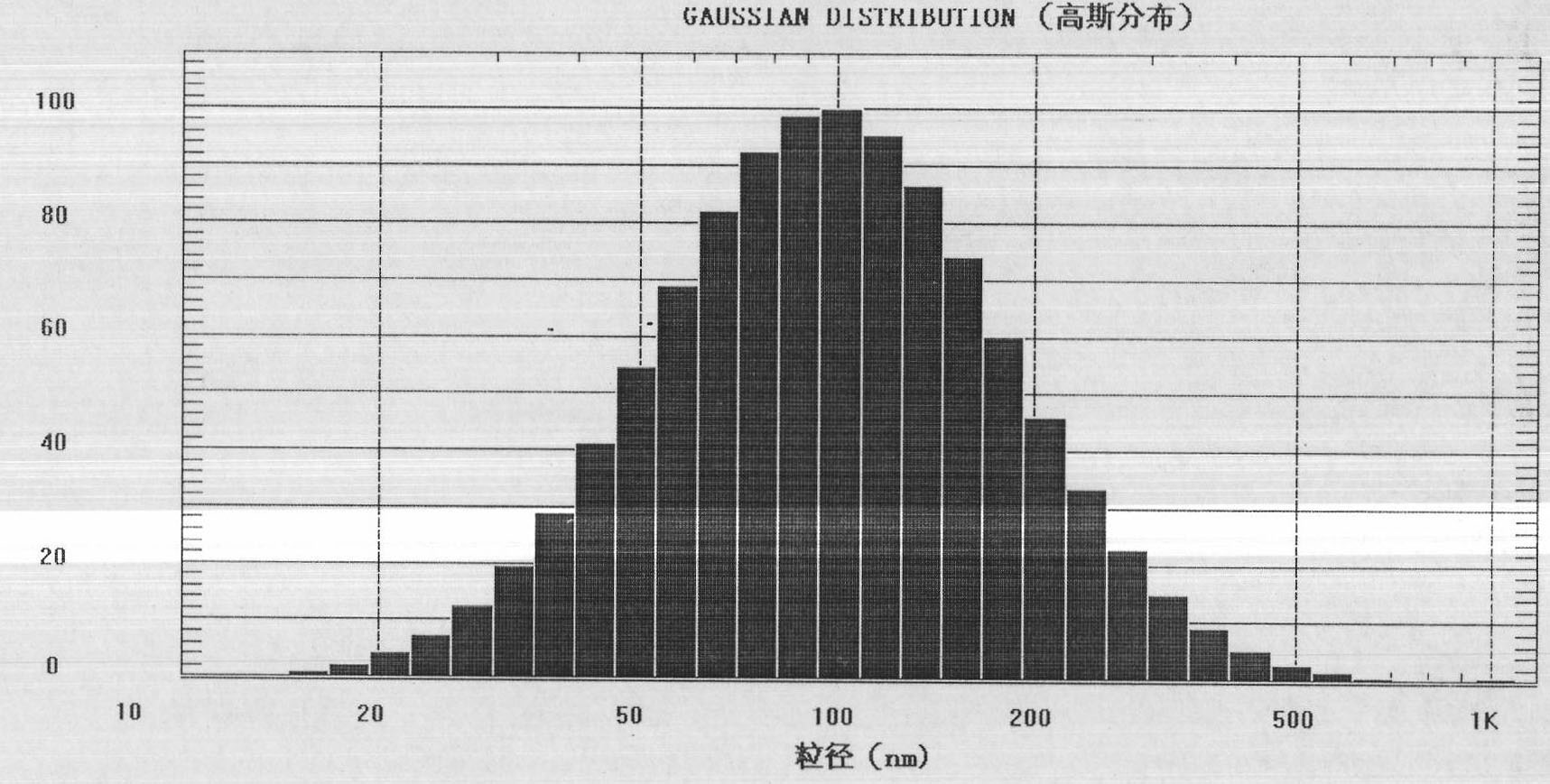
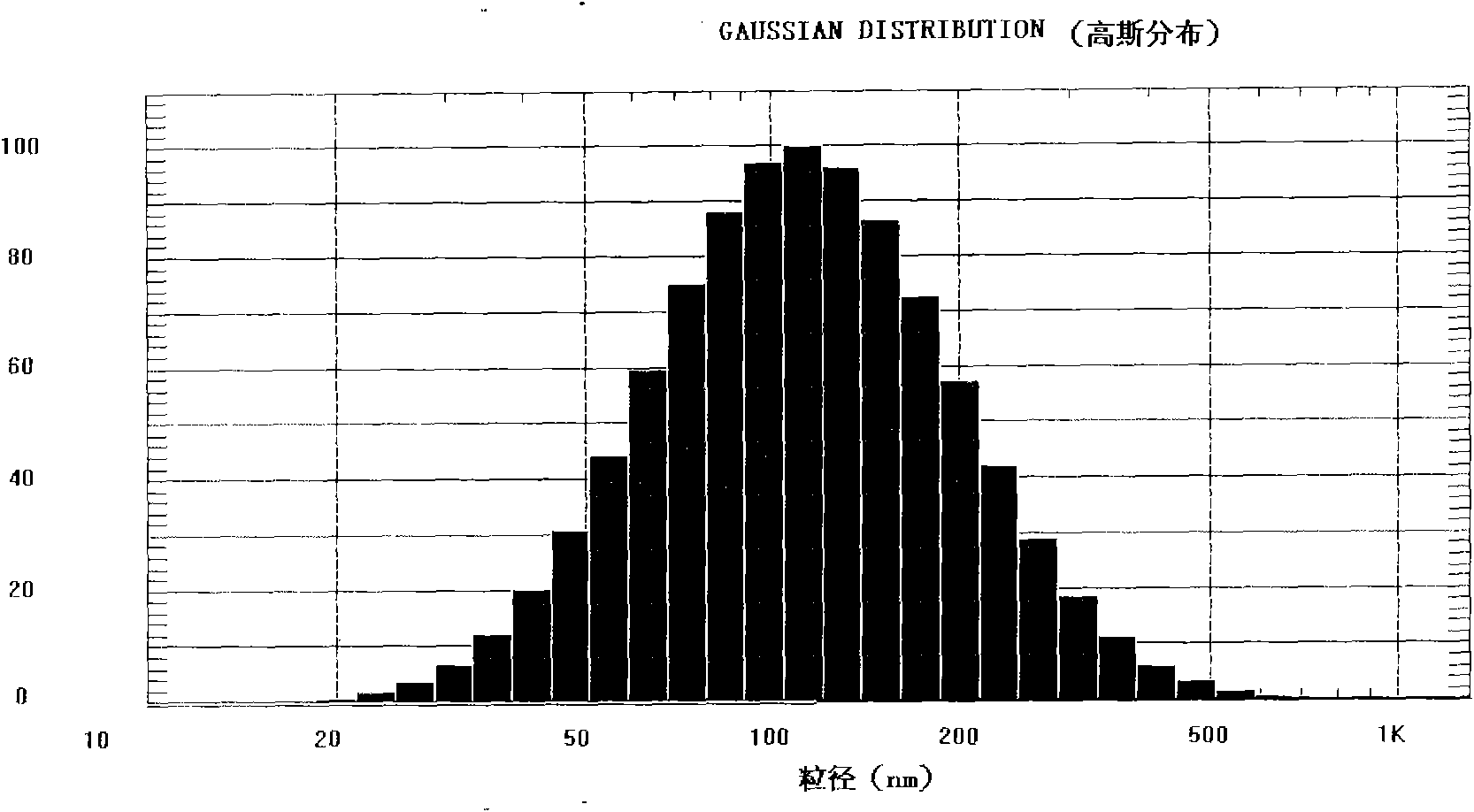
Examples
Embodiment 1
[0080] The preparation of embodiment 1 novel paclitaxel long circulation liposome
[0081] Paclitaxel 0.60g Hydrogenated Soy Lecithin 16g
[0082] Cholesterol 2.5g Vitamin E succinate 0.1g
[0083] Vitamin E polyethylene glycol succinate (TPGS 2000) 2.5g
[0084] Vitamin E 0.05g
[0085] Sucrose 20g
[0086] Water for injection 200ml
[0087] Weigh respectively paclitaxel, hydrogenated soybean lecithin, cholesterol, vitamin E succinate (VES), vitamin E polyethylene glycol succinate (TPGS 2000), vitamin E, add 100ml absolute ethanol, after dissolving, place Rotary evaporator, under the condition of nitrogen flow protection, 50 ℃ water bath constant temperature, drain the solvent, prepare a film, place it in a vacuum desiccator, and fully remove the residual solvent. Add sucrose solution dissolved in water for injection, keep the temperature at 55°C, stir with magnetic force for 30 minutes, disperse evenly in a water bath, homogenize with a high-pressure homogenizer, filter...
Embodiment 2
[0091] Embodiment 2: Preparation of novel paclitaxel long-circulating liposome
[0092] Paclitaxel 0.15g Hydrogenated Soy Lecithin 16g
[0093] Cholesterol 4.5g Vitamin E succinate 0.5g
[0094] Vitamin E Polyethylene Glycol Succinate (TPGS 1000) 2.5g
[0095] Vitamin E 0.08g
[0096] Trehalose 20g
[0097] Water for injection 200ml
[0098] Weigh paclitaxel, hydrogenated soybean lecithin, cholesterol, vitamin E succinate (VES), vitamin E polyethylene glycol succinate (TPGS 1000) and vitamin E respectively, add 100ml absolute ethanol, after dissolving, place Rotary evaporator, under the condition of nitrogen flow protection, 50 ℃ water bath constant temperature, drain the solvent, prepare a film, place it in a vacuum desiccator, and fully remove the residual solvent. Add sucrose solution dissolved in water for injection, keep the temperature at 55°C, stir with magnetic force for 30 minutes, disperse evenly in a water bath ultrasonically, extrude with an extruder with a 10...
Embodiment 3
[0100] Embodiment 3: Preparation of novel paclitaxel long-circulating liposome
[0101] Paclitaxel 0.90g Hydrogenated Soy Lecithin 16g
[0102] Cholesterol 1.25g Vitamin E succinate 1.0g
[0103] Vitamin E Polyethylene Glycol Succinate (TPGS 500) 2.0g
[0104] Vitamin E 0.1g
[0105] Lactose 15g
[0106] Water for injection 200ml
[0107] Weigh the prescribed amount of paclitaxel, hydrogenated soybean lecithin, cholesterol, vitamin E succinate, vitamin E polyethylene glycol succinate (TPGS 2000), and vitamin E, add 100ml of absolute ethanol, dissolve, and place in a rotary evaporator , under the condition of nitrogen flow protection, keep the temperature in a water bath at 50°C, drain the solvent, prepare a film, place it in a vacuum desiccator, and fully remove the residual solvent. Add sucrose solution dissolved in water for injection, keep the temperature at 55°C, stir with magnetic force for 30 minutes, disperse evenly in a water bath, homogenize with a high-pressure ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com