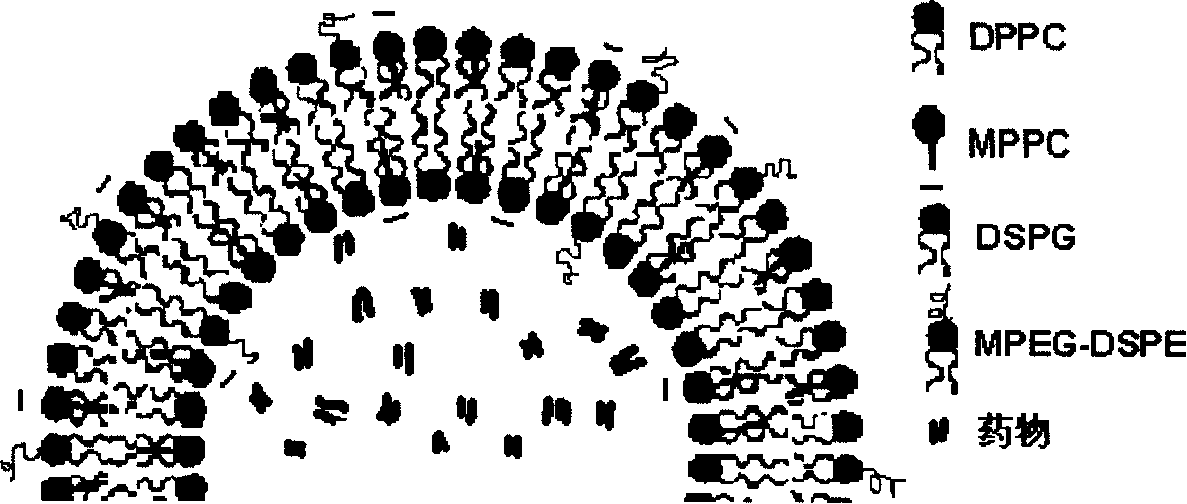
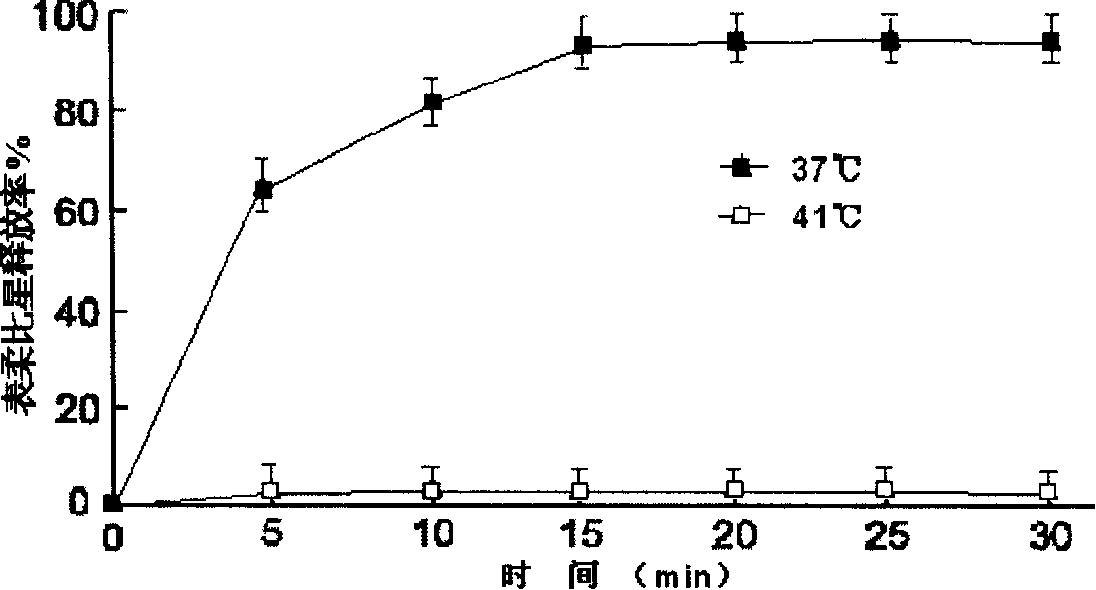
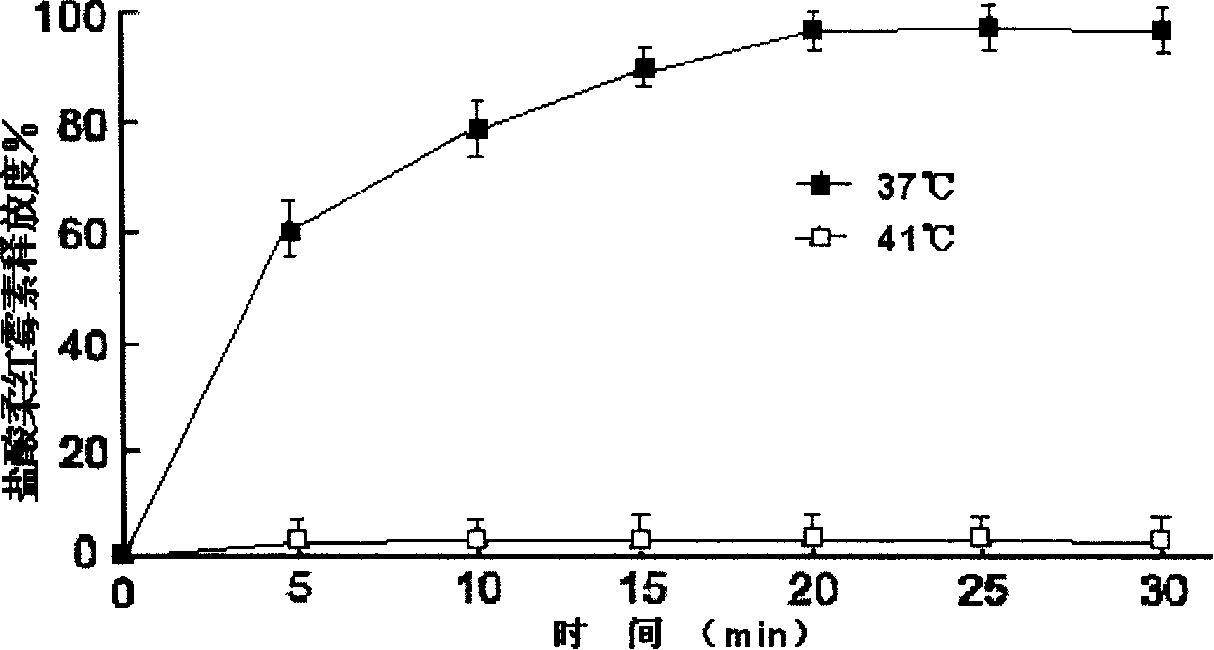
Anthracene nucleus medicinal liposome injection and preparation method
A technology of liposomes and anthracyclines, which is applied in drug combinations, pharmaceutical formulations, liquid delivery, etc., can solve problems such as ineffective effects, low concentration of free drugs, and short shelf life, so as to avoid excessively long circulation time and increased Large storage stability, avoid the effect of hand-foot effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Preparation prescription (50ml capacity)
[0067] Epirubicin Hydrochloride 100mg Citric Acid 365mg
[0068] DPPC 1700mg Sodium Citrate 380mg
[0069] MPPC 100mg Sodium Carbonate 340mg
[0070] DSPG 100mg Lactose 2500mg
[0071] PEG-DSPE 100mg Water for Injection Dilute to 50ml
[0072] Preparation Process:
[0073] Add phospholipids into a round-bottomed flask according to the prescription ratio, add chloroform-methanol (3:1) mixed solvent to dissolve, and remove the solvent by rotary evaporation under reduced pressure at 45°C. The phospholipid forms a film on the inner wall of the round bottom flask, and the prepared film is dried under vacuum for 20 hours to ensure that the organic solvent is completely removed. The citric acid-sodium citrate buffer solution (pH4.0) added to the prepared film, containing 5% lactose, was hydrated at 45-60°C for 1 hour to obtain multilamellar liposomes (MLVs). Under high-pressure conditions at 45°C, pass the prepared MLVs through ...
Embodiment 2
[0075] Preparation prescription (50ml capacity)
[0076] Epirubicin Hydrochloride 100mg Sodium Citrate 380mg
[0077] DPPC 1600mg Sodium Carbonate 340mg
[0078] DSPG 200mg Trehalose 5000mg
[0079] PEG-DSPE 200mg PVP 500mg
[0080] Citric acid 365mg
[0081] Preparation Process:
[0082]Add phospholipids into a round-bottomed flask according to the prescription ratio, add chloroform-methanol (3:1) mixed solvent to dissolve, and remove the solvent by rotary evaporation under reduced pressure at 45°C. The phospholipid forms a film on the inner wall of the round bottom flask, and the prepared film is dried under vacuum for 20 hours to ensure that the organic solvent is completely removed. Add citric acid-sodium citrate buffer (pH4.0) to the prepared film, containing 10% trehalose, and hydrate at 45-60°C for 1 hour to obtain multilamellar liposomes (MLVs). Under high-pressure conditions at 45°C, pass the prepared MLVs through polycarbonate membranes with pore diameters of 2...
Embodiment 3
[0084] Preparation prescription (50ml capacity)
[0085] Epirubicin Hydrochloride 150mg Citric Acid 730mg
[0086] DPPC 1600mg Sodium Citrate 760mg
[0087] MSPC 100mg Sodium Carbonate 680mg
[0088] DSPG 100mg Trehalose 7500mg
[0089] PEG-DSPE 200mg PVP 1000mg
[0090] Preparation Process:
[0091] Add phospholipids into a round-bottomed flask according to the prescription ratio, add a small amount of ethanol to dissolve it, inject it into a preheated buffer solution containing 15% trehalose at 60°C, ultrasonicate for 15 minutes, and dialyze with hydration buffer solution to remove the outer phase Organic solvent, hydration at 60°C for 1 hour to obtain multilamellar liposomes (MLVs), pass the prepared MLVs through polycarbonate membranes with pore diameters of 200nm, 100nm, and 80nm in sequence at a temperature greater than 45°C under high pressure conditions, and obtain particle sizes For large unilamellar liposomes (LUVs) of about 50-250nm, cool the prepared blank lip...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com