Compositions including triciribine and bortezomib and derivatives thereof and methods of use thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
8.1. Example 1
In Vitro Screening
[0229]Cell Lines and NCI Diversity Set. All cell lines can be purchased from ATCC or described previously (Cheng et al., 1997, Oncogene 14: 2793-2801; West et al., 2002, Drug Resist Updat 5: 234-248; Satyamoorthy et al., 2001, Cancer Res 61: 7318-7324). The NCI Structural Diversity Set is a library of 1,992 compounds selected from the approximately 140,000-compound NCI drug depository. In-depth data on the selection, structures, and activities of these diversity set compounds can be found on the NCI Developmental Therapeutics Program web site.
[0230]Screening for Inhibition of Akt-transformed Cell Growth. AKT2 transformed NIH3T3 cells or LXSN vector-transfected NIH3T3 control cells (Cheng et al., 1997, Oncogene 14: 2793-2801) are plated into 96-well tissue culture plate. Following treatment with 5 μM of NCI Diversity Set compound, cell growth can be detected with CellTier 96 One Solution Cell Proliferation kit (Promega). Compounds that inhibit growth i...
example 2
8.2 Example 2
Antitumor Activity in the Nude Mouse Tumor Xenograft Model
[0237]Tumor cells can be harvested, suspended in PBS, and can be injected s.c. into the right and left flanks (2×106 cells / flank) of 8-week-old female nude mice as reported previously (Sun et al., 1999, Cancer Res 59: 4919-4926). When tumors reach about 100-150 mm3, animals are randomized and dosed i.p. with 0.2 ml vehicle of the triciribine compound and / or one or more platinum compounds daily. Control animals receive DMSO (20%) vehicle, whereas treated animals can be injected with API-2 (1 mg / kg / day) in 20% DMSO.
[0238]API-2 Inhibits the Growth of Tumors in Nude Mice that Overexpress Akt. Frequent overexpression / activation and / or amplification of AKT1 and AKT2 in human ovarian and pancreatic cancer was shown (Cheng et al., AKT signal transduction pathway in oncogenesis, in Schwab D (ed.) Encyclopedic Reference of Cancer, Springer, pp 35-7). Inhibition of Akt pathway by inhibitors of PI3K, HSP70, Src and farnesylt...
example 3
8.3 Example 3
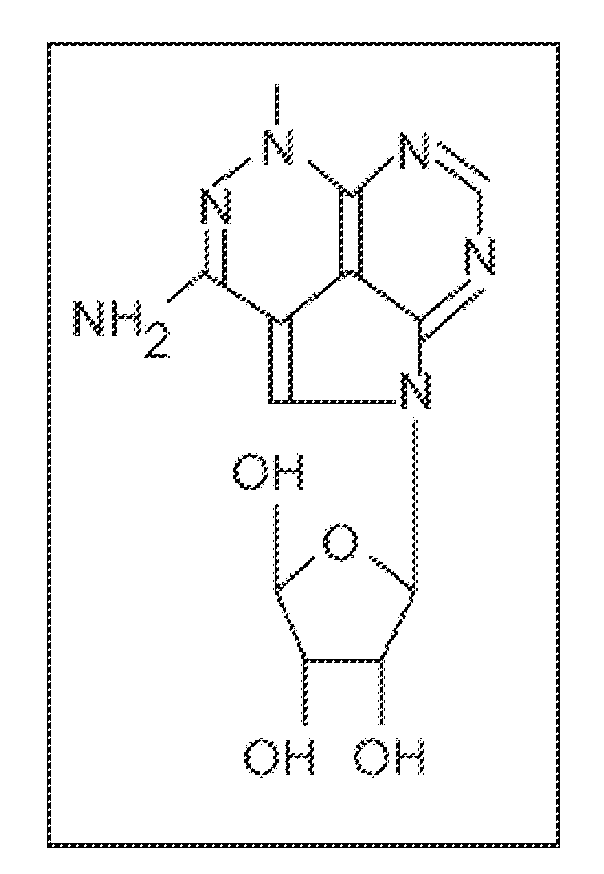
TCN Directly Inhibits Wild Type Akt Kinase Activity
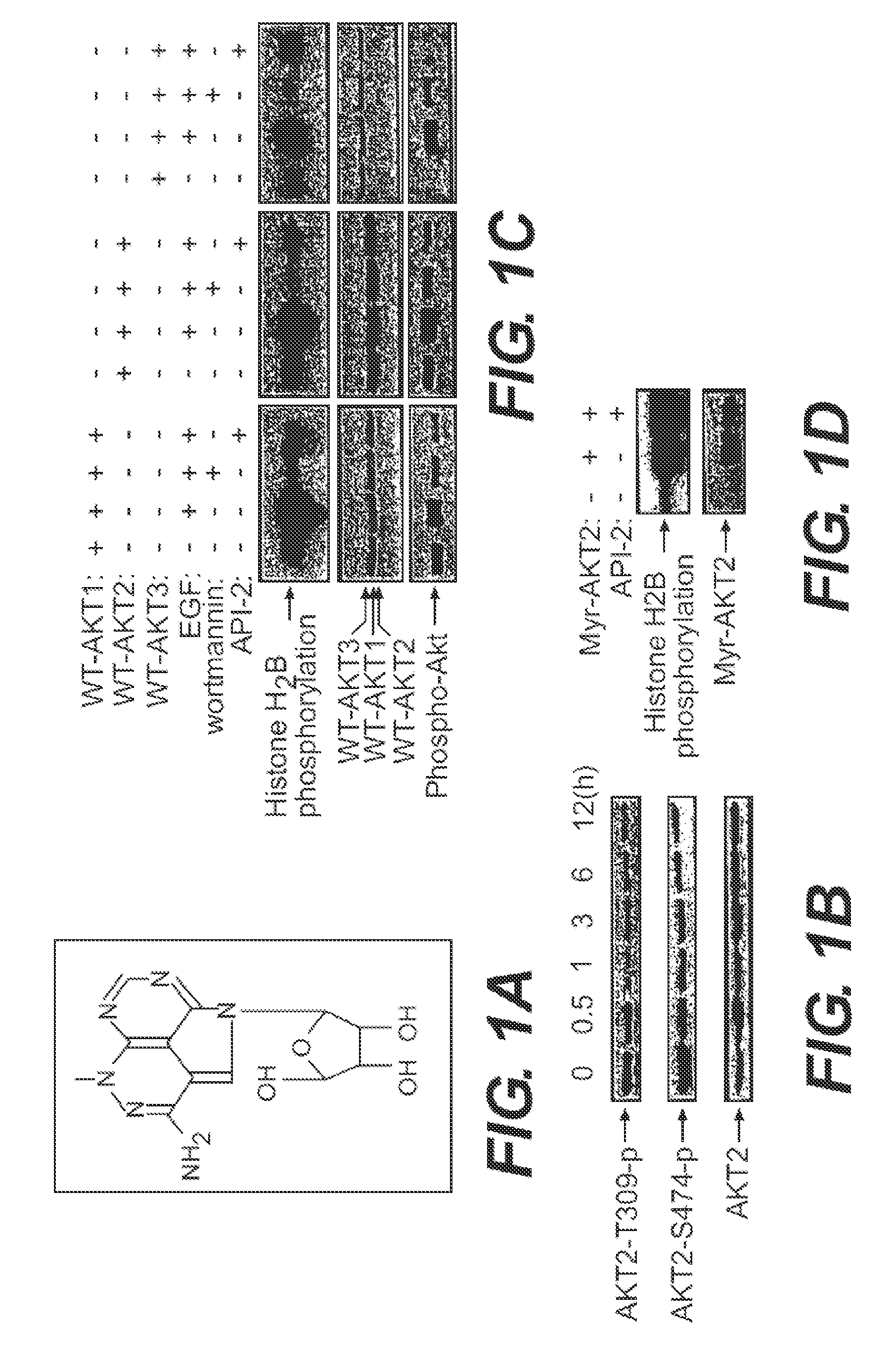
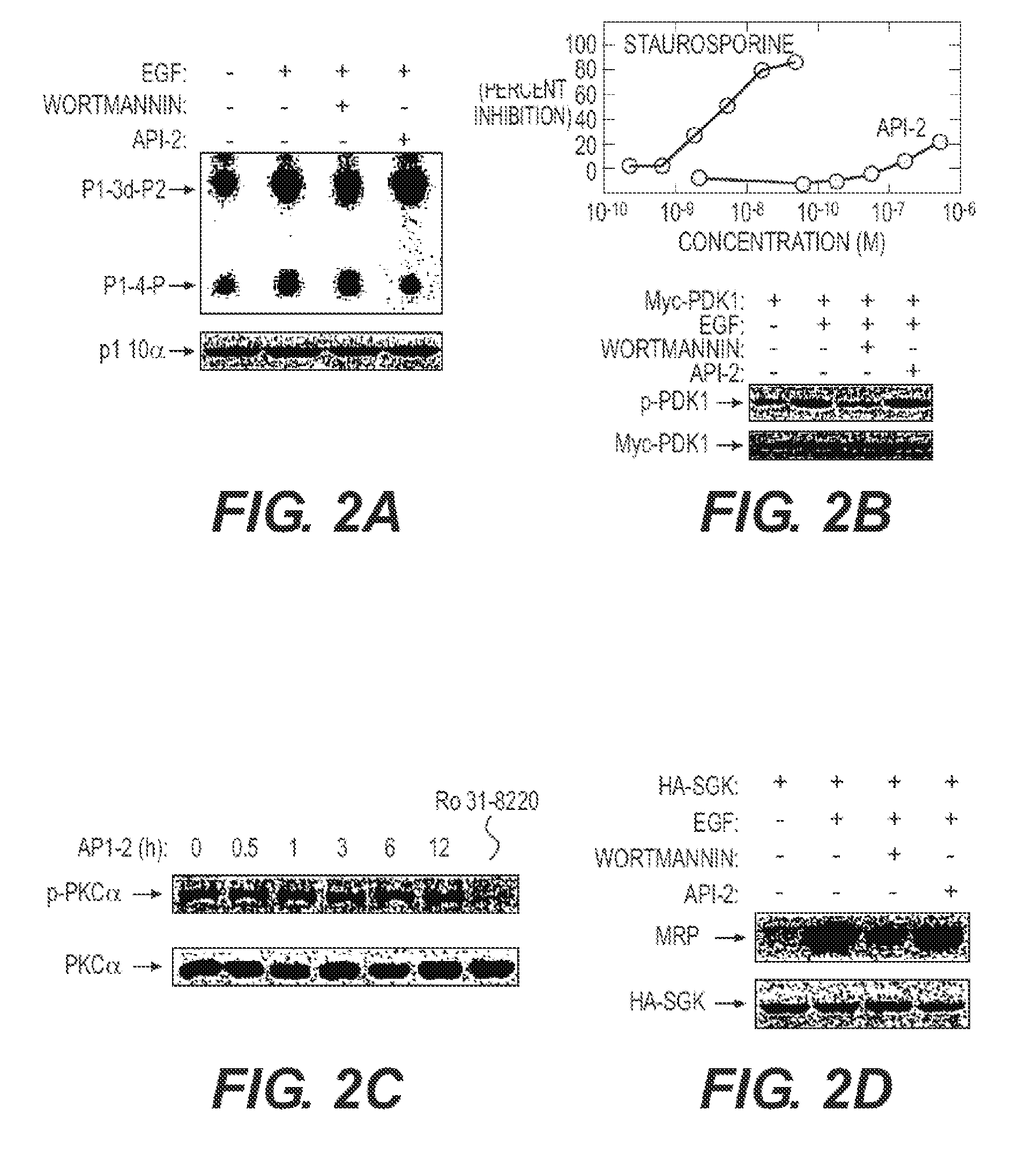
[0239]API-2 (TCN) can directly inhibit wild type Akt kinase activity induced by PDK1 in vitro (FIG. 1). This result supports that API-2 is a direct Akt inhibitor and that the underlying mechanism may be API-2 binding to PH domain and / or threonine-308 of Akt. An In vitro kinase assay is performed with recombinant of PDK1 and Akt in a kinase buffer containing phosphatidylinositol-3,4,5-P3 (PIP3). API-2 and histone H2B as substrate. After incubation of 30 min, the reactions were separated by SDS-PAGE and exposed in a film.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com