Drug/Drug Delivery Systems for the Prevention and Treatment of Vascular Disease
a technology of vascular disease and drug delivery system, which is applied in the direction of drugs, cardiovascular disorders, prosthesis, etc., can solve the problems of ischemic injury, stroke or myocardial infarction, and sudden closure of the vessel, so as to reduce the systemic toxicity, reduce the dose, and facilitate administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
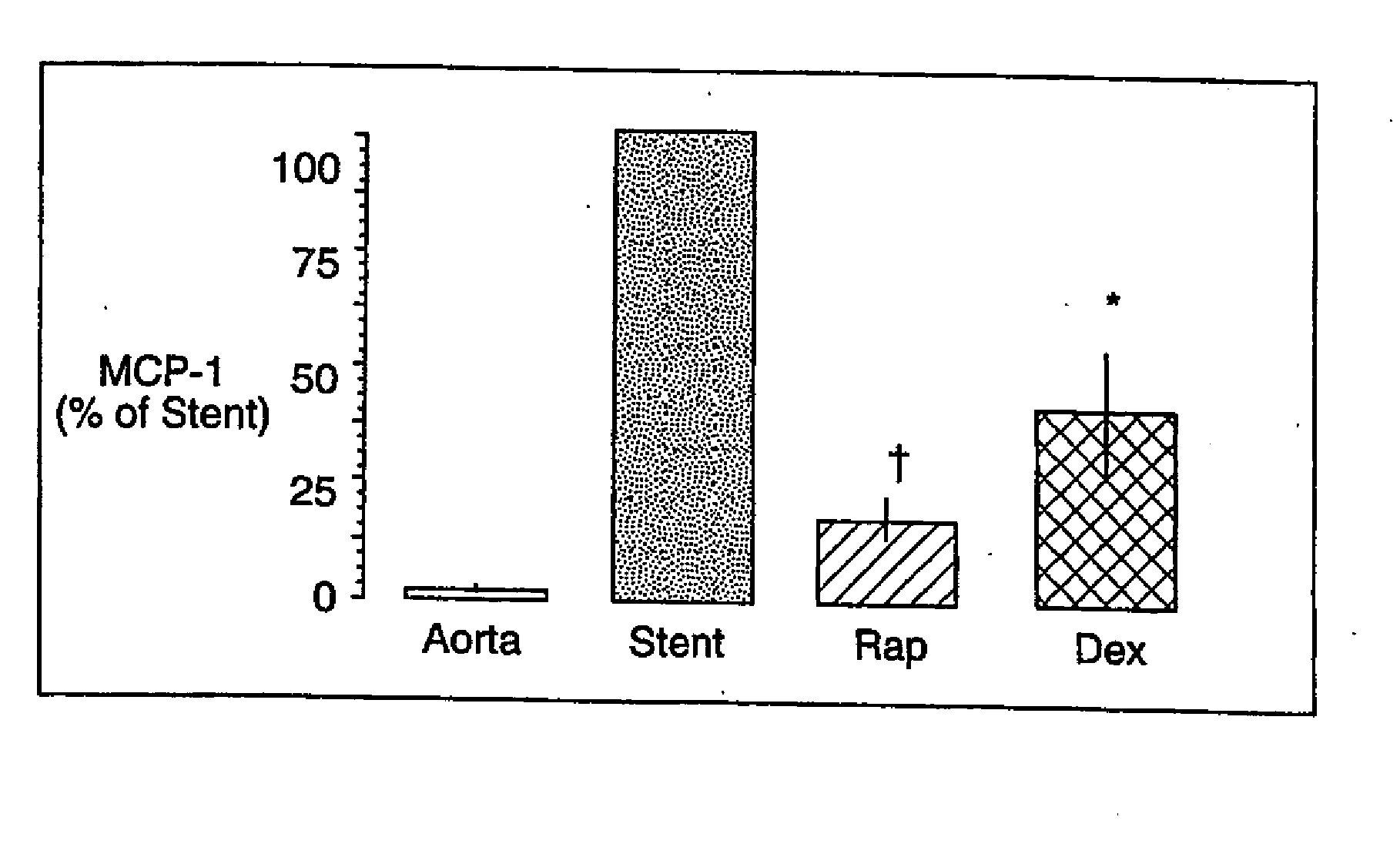
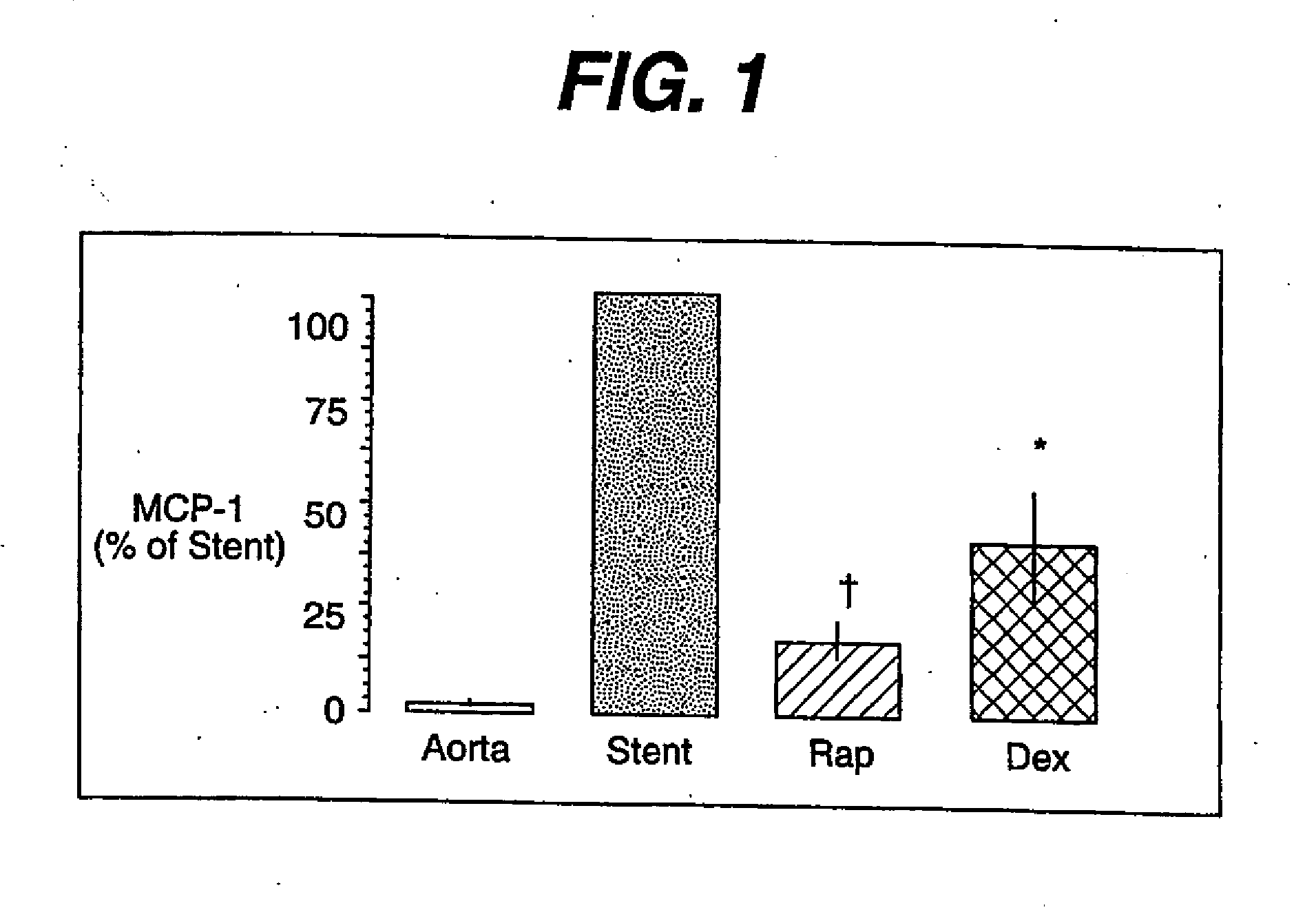
[0027] As stated above, the proliferation of vascular smooth muscle cells in response to mitogenic stimuli that are released during balloon angioplasty and stent implantation is the primary cause of neointimal hyperplasia. Excessive neointimal hyperplasia can often lead to impairment of blood flow, cardiac ischemia and the need for a repeat intervention in selected patients in high risk treatment groups. Yet repeat revascularization incurs risk of patient morbidity and mortality while adding significantly to the cost of health care. Given the widespread use of stents in interventional practice, there is a clear need for safe and effective inhibitors of neointimal hyperplasia.
[0028] Rapamycin is a macroyclic triene antibiotic produced by streptomyces hygroscopicus as disclosed in U.S. Pat. No. 3,929,992. It has been found that rapamycin inhibits the proliferation of vascular smooth muscle cells in vivo. Accordingly, rapamycin may be utilized in treating intimal smooth muscle cell hy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| total mass | aaaaa | aaaaa |
| total mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com