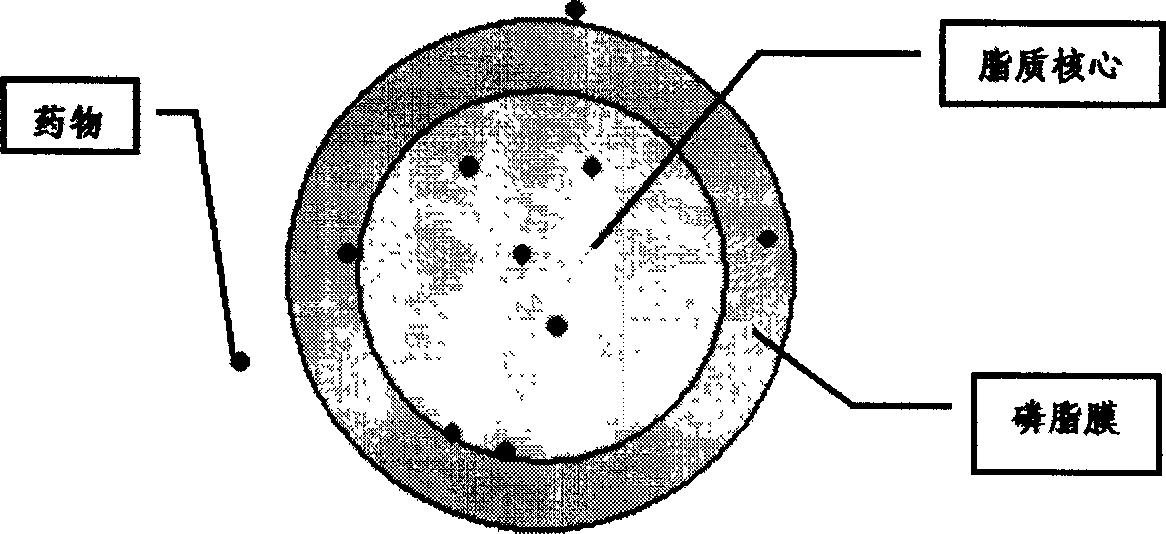
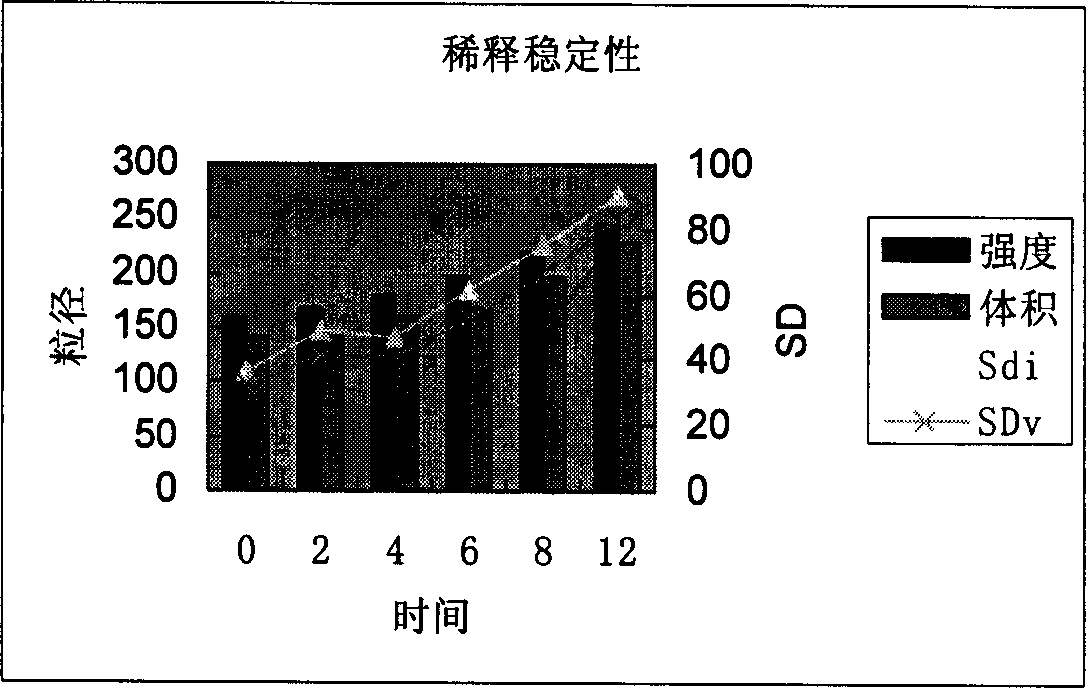
Vinorelbine liposome micro ball injection and its prepn
A technology of vinorelbine and lipid microspheres, which is applied in the field of medicine, can solve the problems of vinorelbine drug instability, inability to achieve vascular stimulating effect, and no provision, so as to reduce systemic toxicity, low irritation, and reduced toxicity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0162] Prescription 4:
[0163] Oil Phase (Soybean Oil / Medium Chain Fatty Acid Triglyceride=2 / 8) 10%
[0164] Vinorelbine 0.1%
[0165] Lecithin 1.2%
[0166] Glycerin 2.5%
[0167] Sodium Oleate 0.1%
[0168] Tween-80 0.2%
[0169] 10-Hydroxy-2-decenoic acid 0.5%
[0170] Span80 0.1%
[0171] Ascorbic acid 0.1%
[0172] Vitamin E 0.05%
[0173] EDTA0.05%
[0174] Add water for injection to 100mL
[0175] Preparation method 4:
[0176] (1) Mix the prescribed amount of lecithin, Tween-80, glycerin, sodium oleate, ascorbic acid, EDTA with an appropriate amount of water for injection preheated at 70-80°C, transfer to a tissue masher, stir for a few minutes, 5 times , until the ingredients are uniformly dispersed to obtain the water phase; at the same time, add the prescribed amount of vitamin E, 10-hydroxy-2-decenoic acid, and Span80 to the mixed oil phase composed of medium-chain fatty acid triglycerides for injection and soybean oil for injection , heated at 70-80°C...
Embodiment 2
[0178] Prescription 5:
[0179] Soybean oil for injection 10%
[0180] Vinorelbine Tartrate 0.1%
[0181] Soy Lecithin 1.2%
[0182] Glycerin 2.5%
[0183] Tween-80 0.2%
[0184] Sodium Oleate 0.1%
[0185] 10-Hydroxy-2-decenoic acid 0.5%
[0186] Span80 0.1%
[0188] Vitamin E 0.05%
[0189] EDTA0.05%
[0190] Add water for injection to 100ml
[0191] Preparation method 5:
[0192] (1) Mix the prescribed amount of soybean lecithin, Tween-80, sodium oleate, glycerin, sodium sulfite, EDTA with an appropriate amount of water for injection preheated at 70-80°C, transfer to a tissue masher, stir for several minutes, 5 Repeat until the ingredients are uniformly dispersed to obtain the water phase; at the same time, add the prescribed amount of vitamin E, 10-hydroxy-2-decenoic acid, and Span80 to the prescribed amount of soybean oil for injection and heat to dissolve at 70-80°C to obtain the oil phase (2) Add the oil phase to the water phase,...
Embodiment 3
[0194] Prescription 6:
[0195] Oil Phase (Soybean Oil / Medium Chain Fatty Acid Triglyceride=2 / 8) 10%
[0196] Vinorelbine Tartrate 0.1%
[0197] Lecithin 1.2%
[0198] Glycerin 2.5%
[0199] Tween-80 0.2%
[0200] Sodium Oleate 0.1%
[0201] 10-Hydroxy-2-decenoic acid 0.5%
[0202] Span80 0.1%
[0203] L-cysteine 0.2%
[0204] Vitamin E 0.05%
[0205] EDTA0.05%
[0206] Add water for injection to 100ml
[0207] Preparation method 6:
[0208] (1) Mix the prescribed amount of lecithin, Tween-80, sodium oleate, glycerin, L-cysteine, EDTA with an appropriate amount of water for injection preheated at 70-80°C, and transfer it into a tissue masher, Stir for several minutes, 5 times, until the ingredients are evenly dispersed to obtain a water phase; at the same time, add the prescribed amount of vitamin E, 10-hydroxy-2-decenoic acid, and Span80 to the prescribed amount of soybean oil for injection and medium-chain fatty acid for injection Heat and dissolve the triglyce...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com