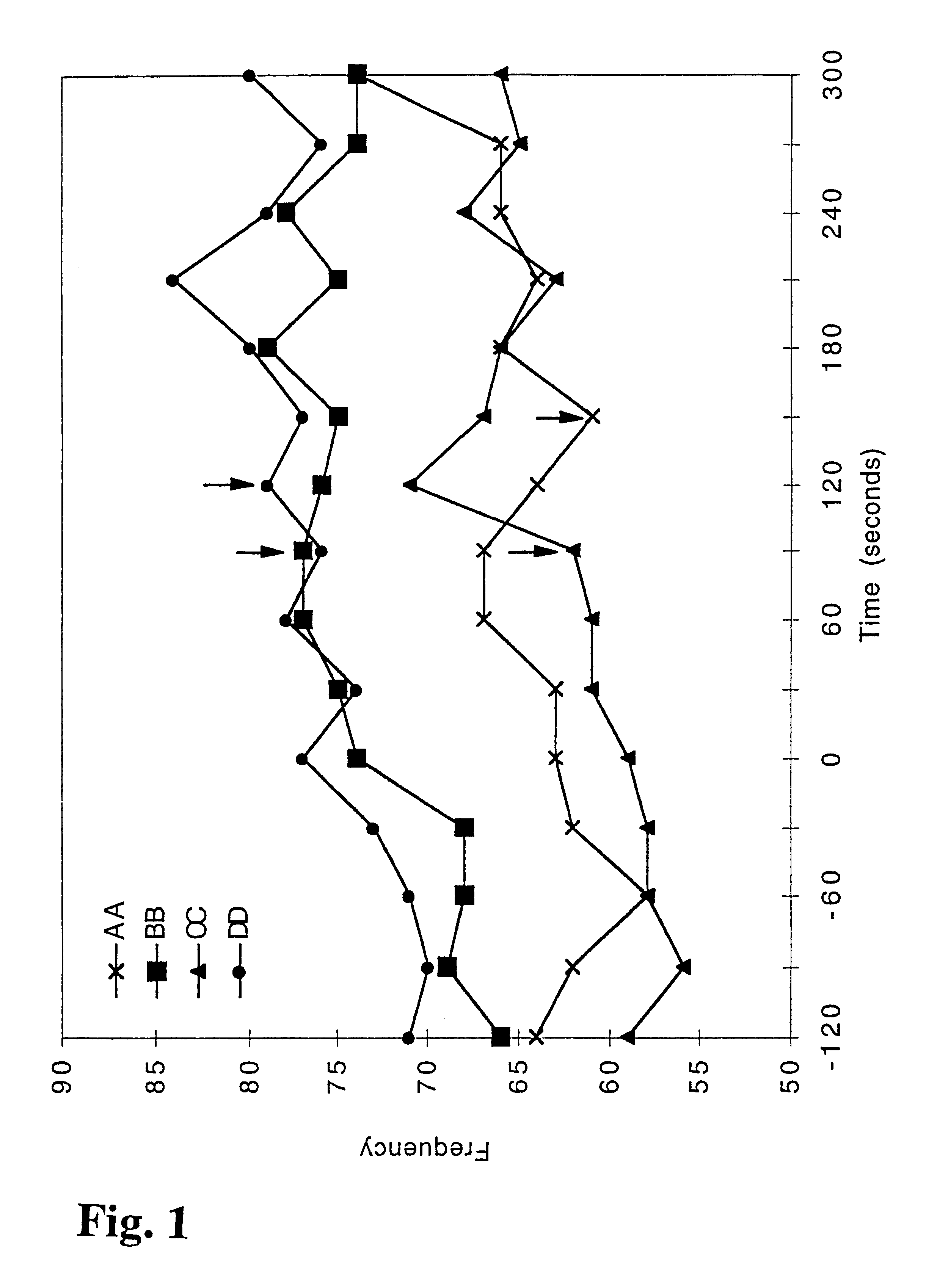
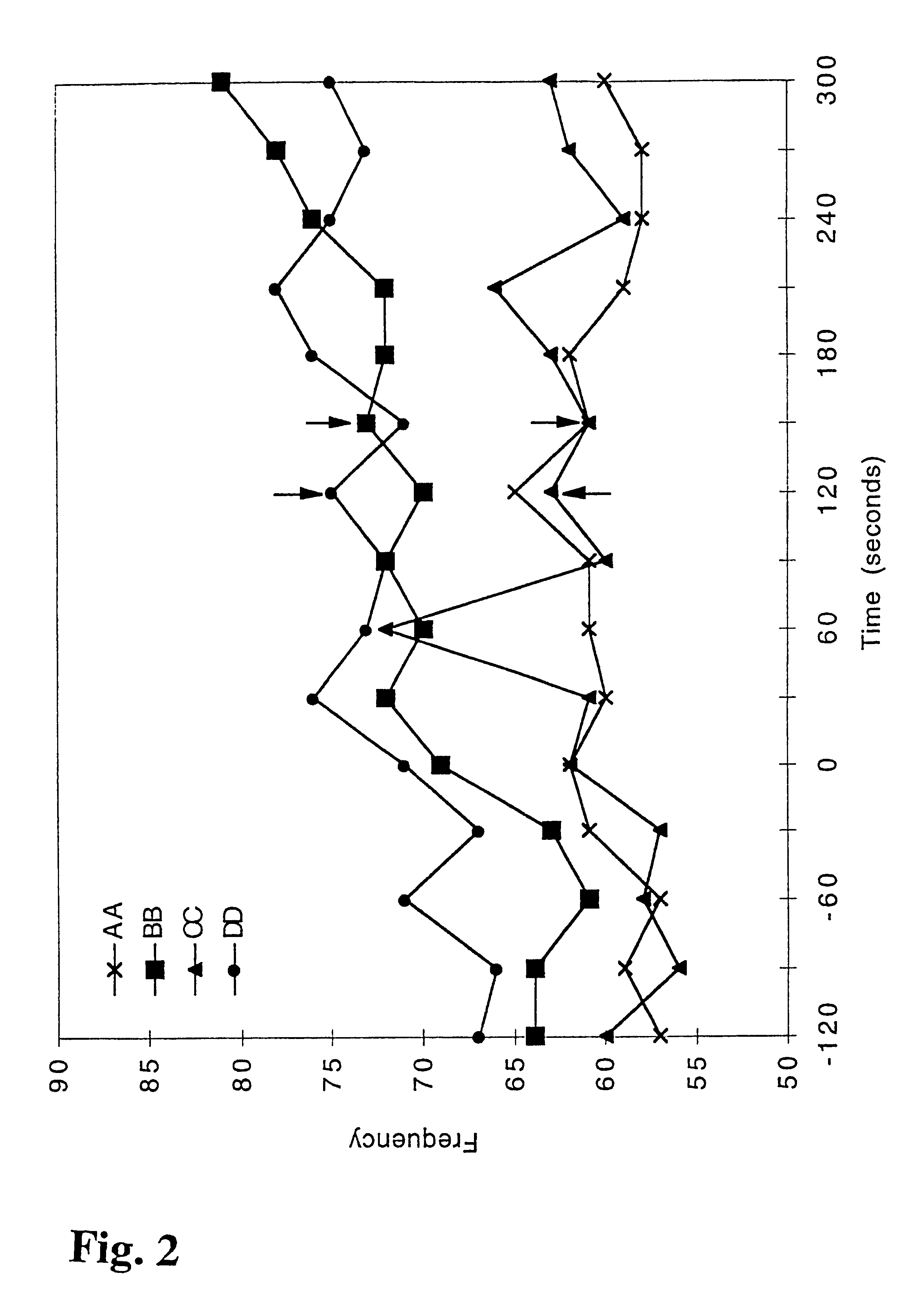
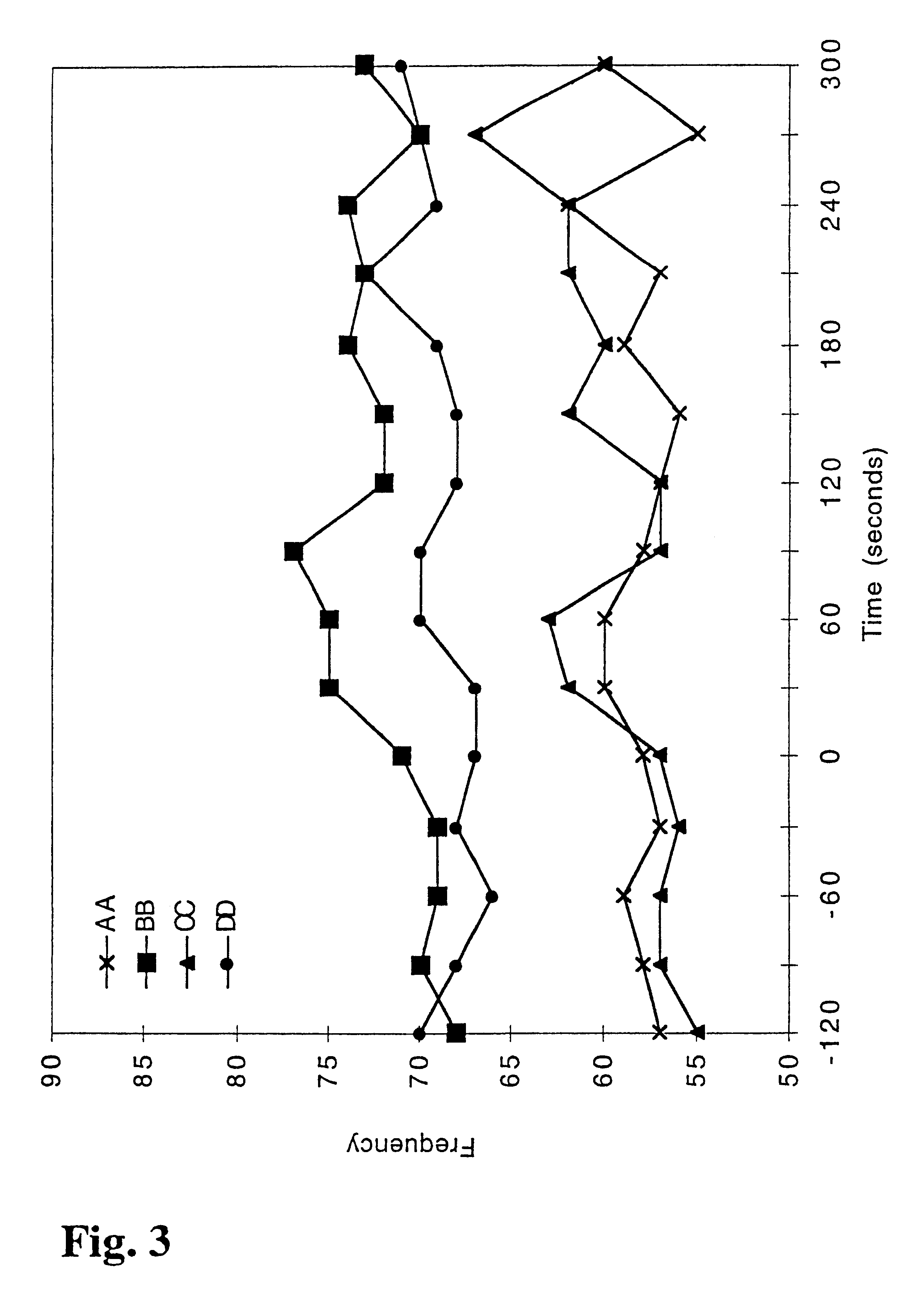
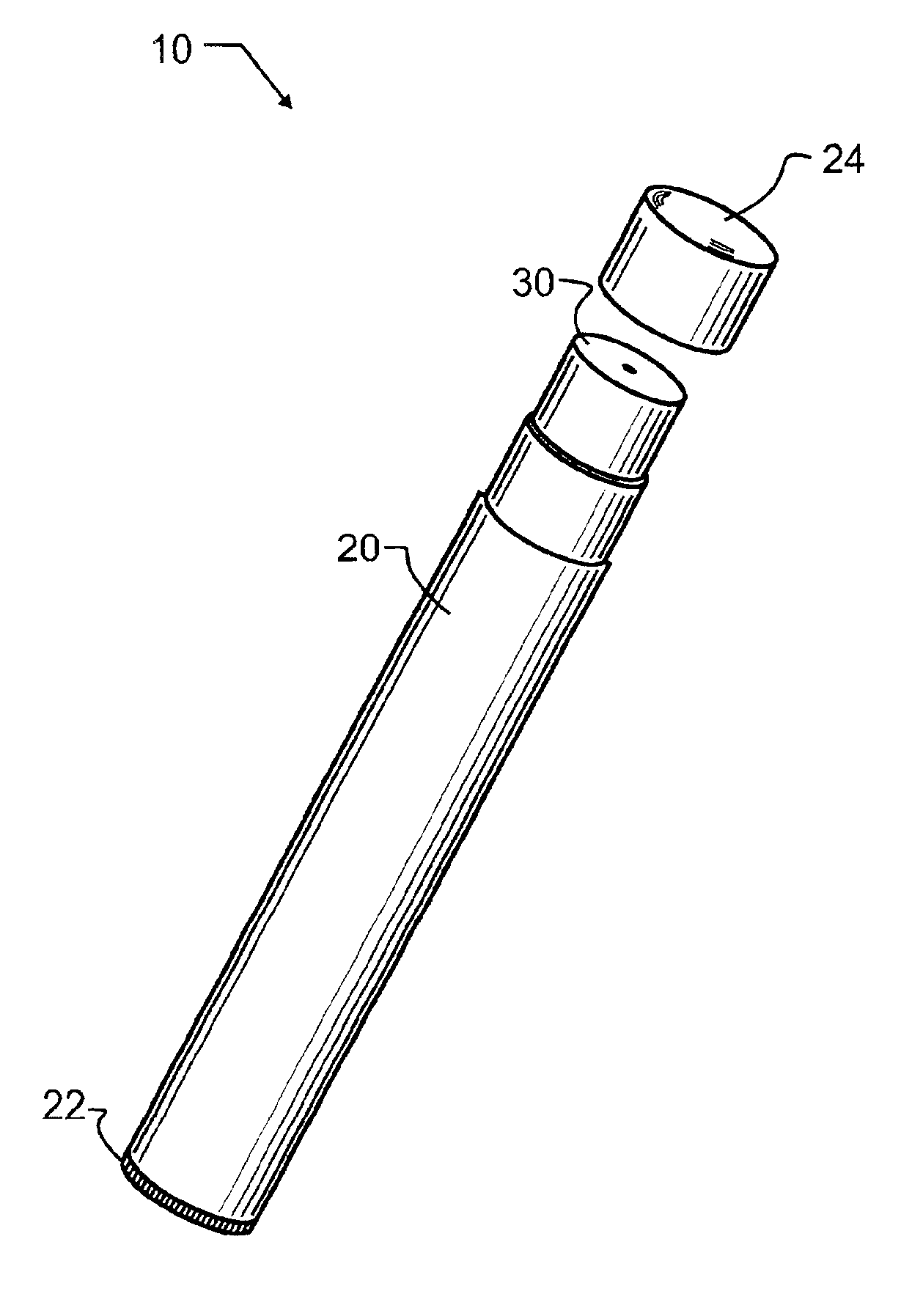
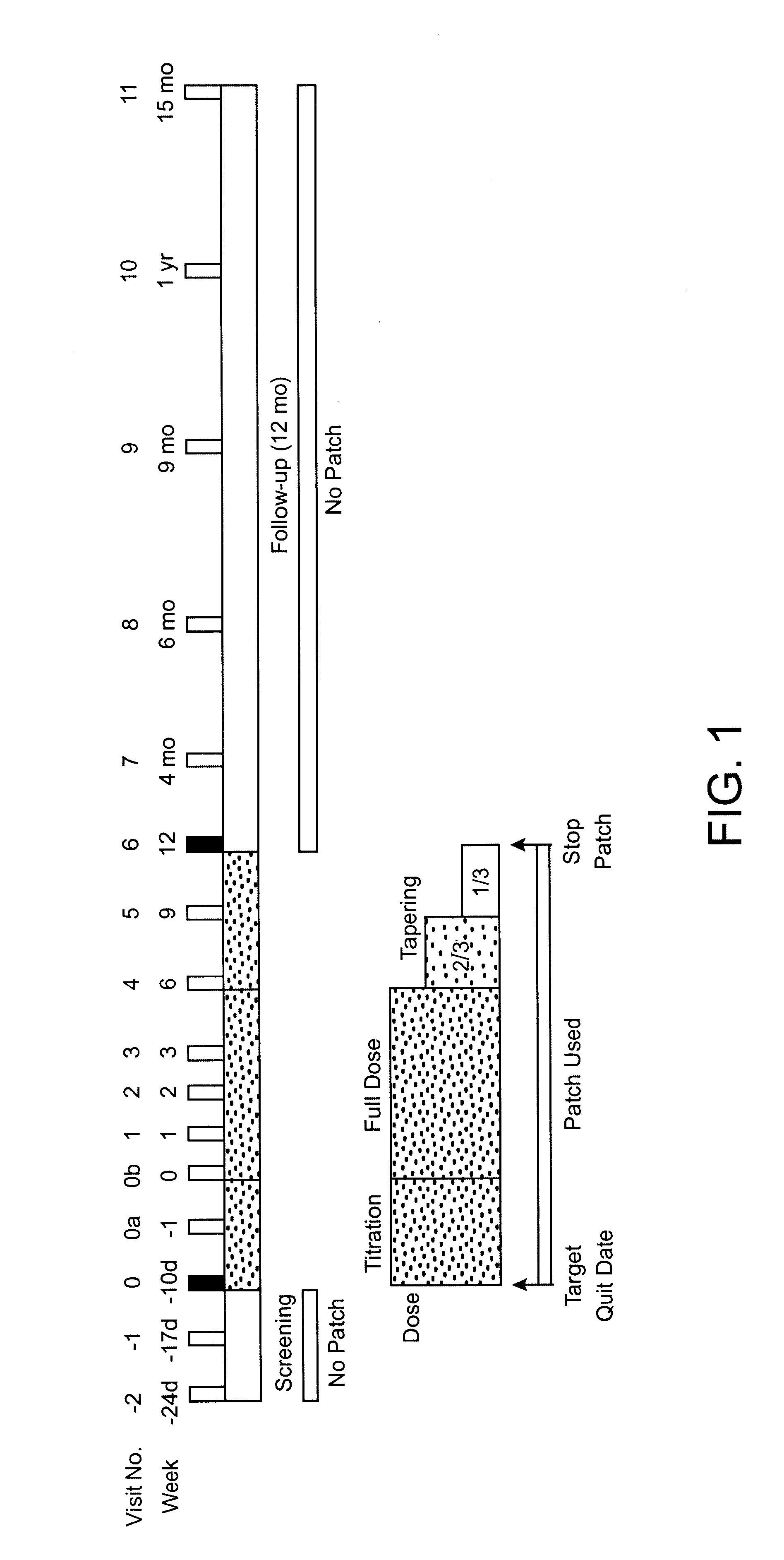
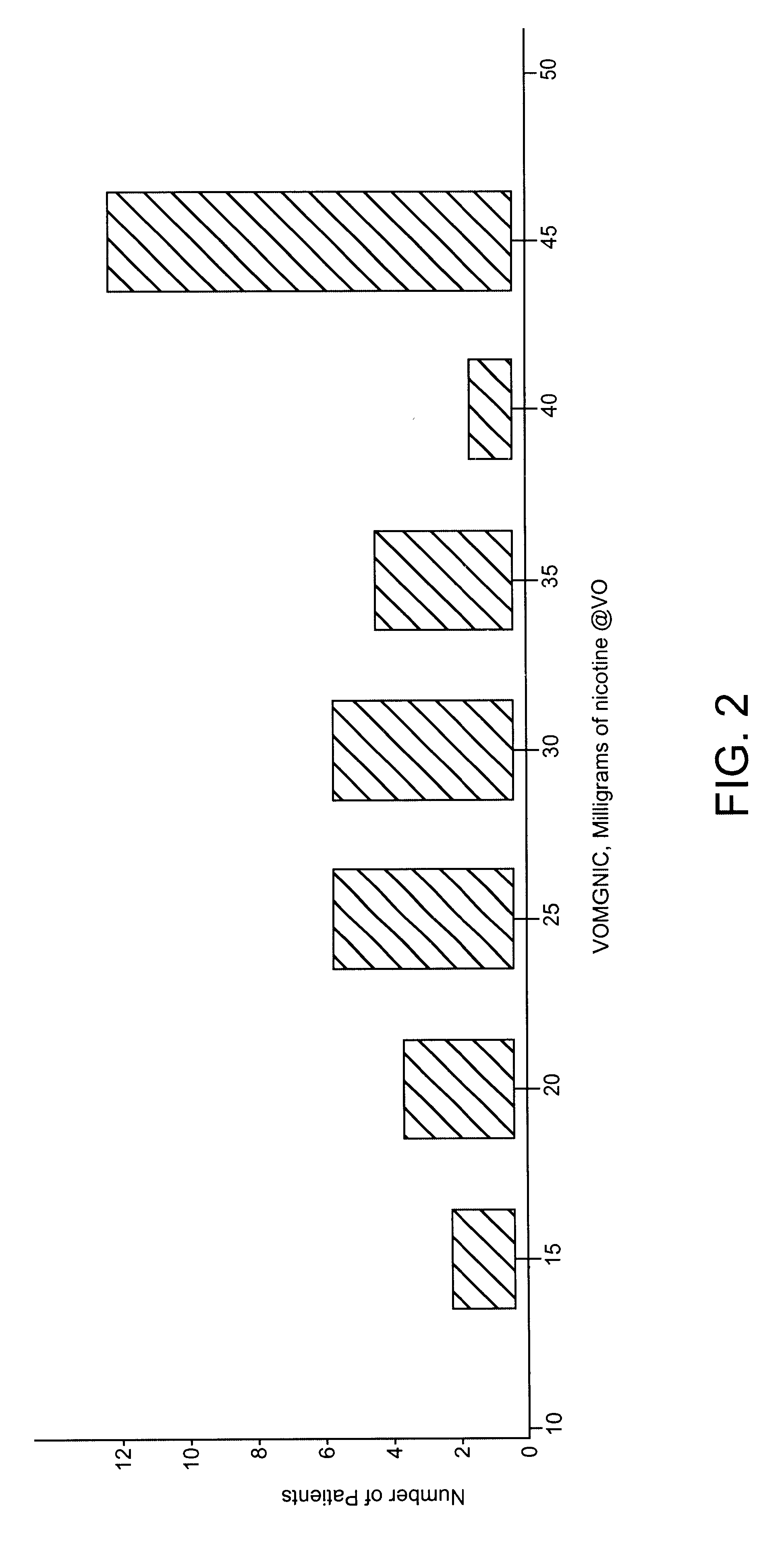
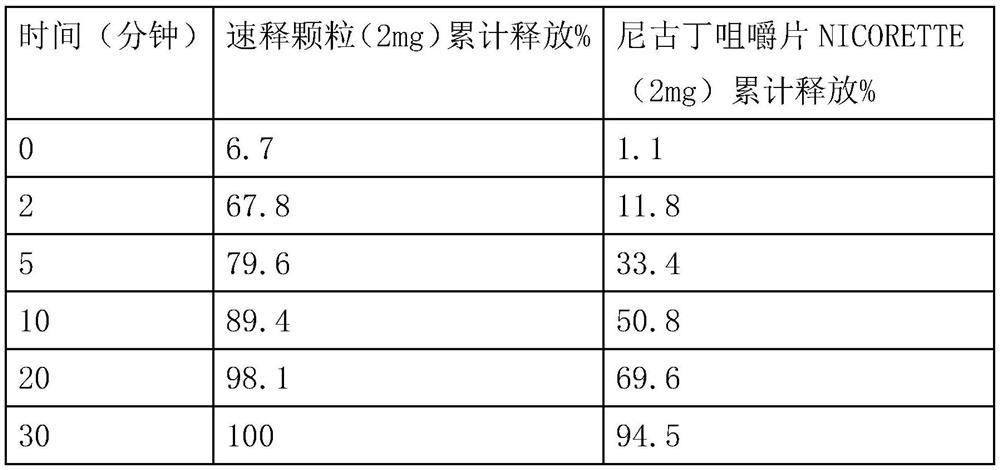
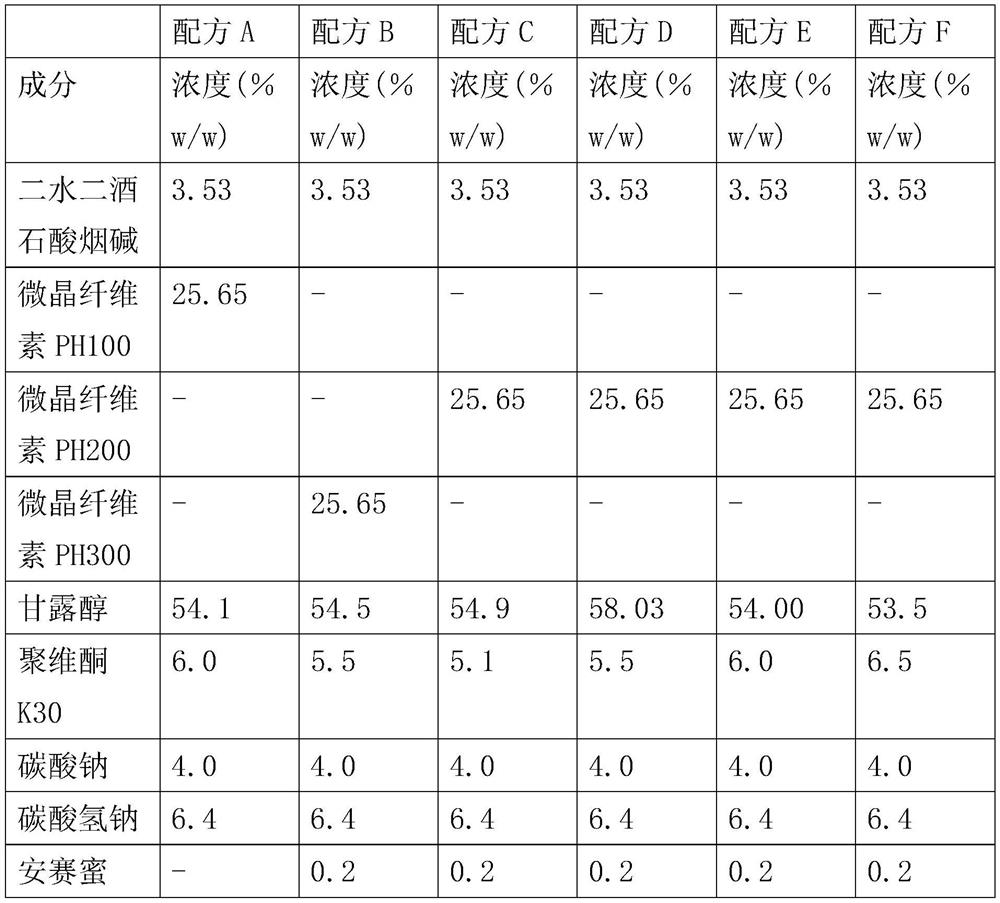
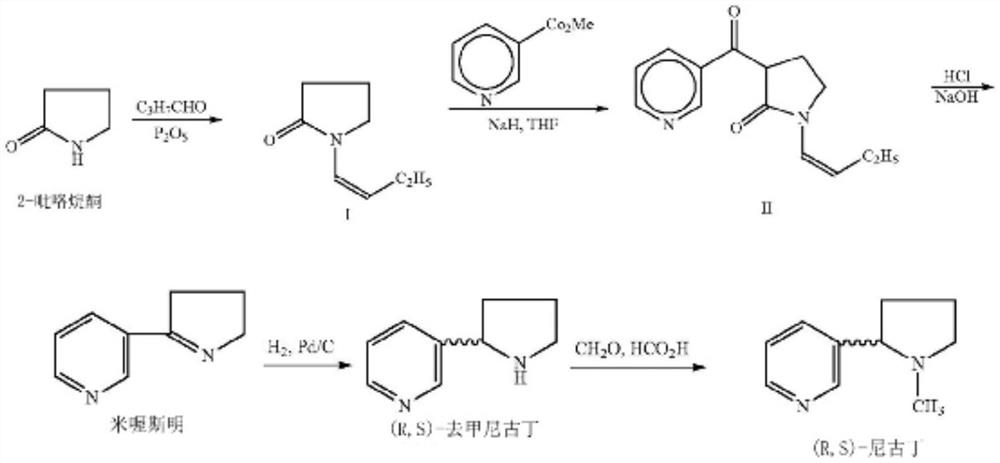
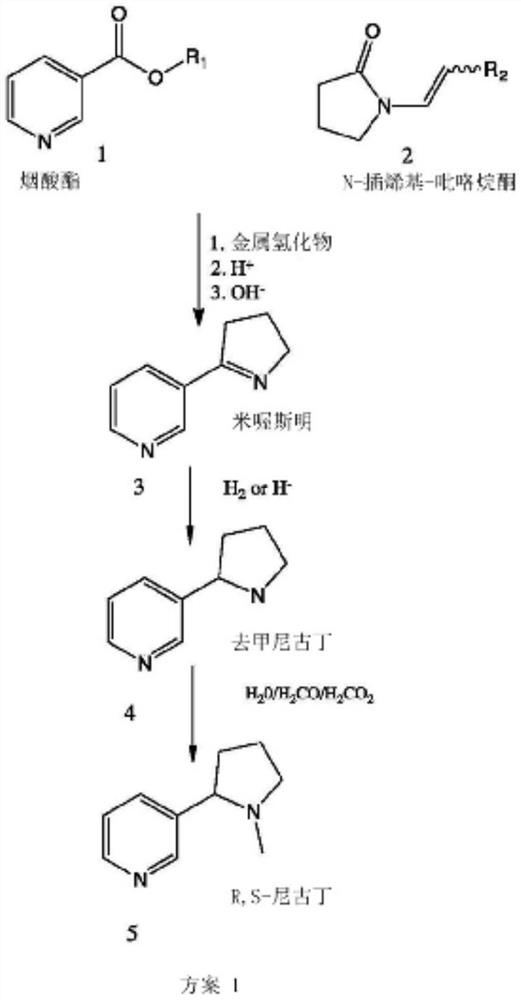
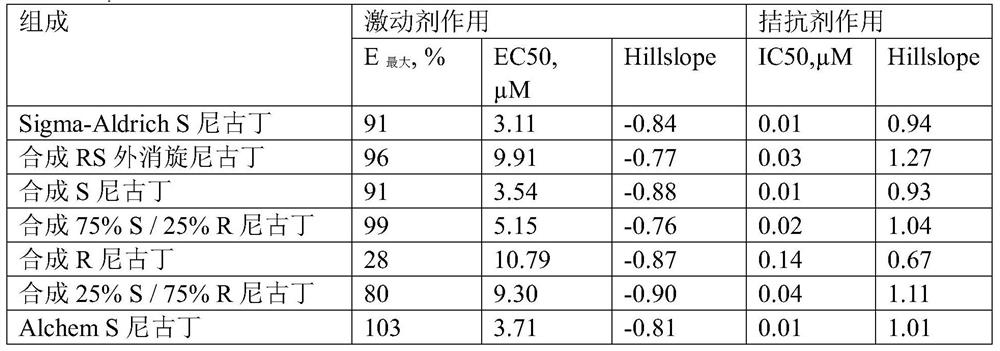
Patents
Literature
30 results about "Nicotine replacements" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Nicotine replacement therapy (NRT) is a medically-approved way to take nicotine by means other than tobacco. It is used to help with quitting smoking or stopping chewing tobacco.
Nicotine-containing pharmaceutical compositions giving a rapid transmucosal absorption
Formulations of nicotine for use in nicotine replacement therapy. The formulations are intended for application in the oral cavity where upon the uptake of nicotine mainly takes place through the buccal mucosa. The formulations essentially comprise apolar, polar and surface-active components. The formulations may be administered in combination with other nicotine formulations.
Owner:MCNEIL AB
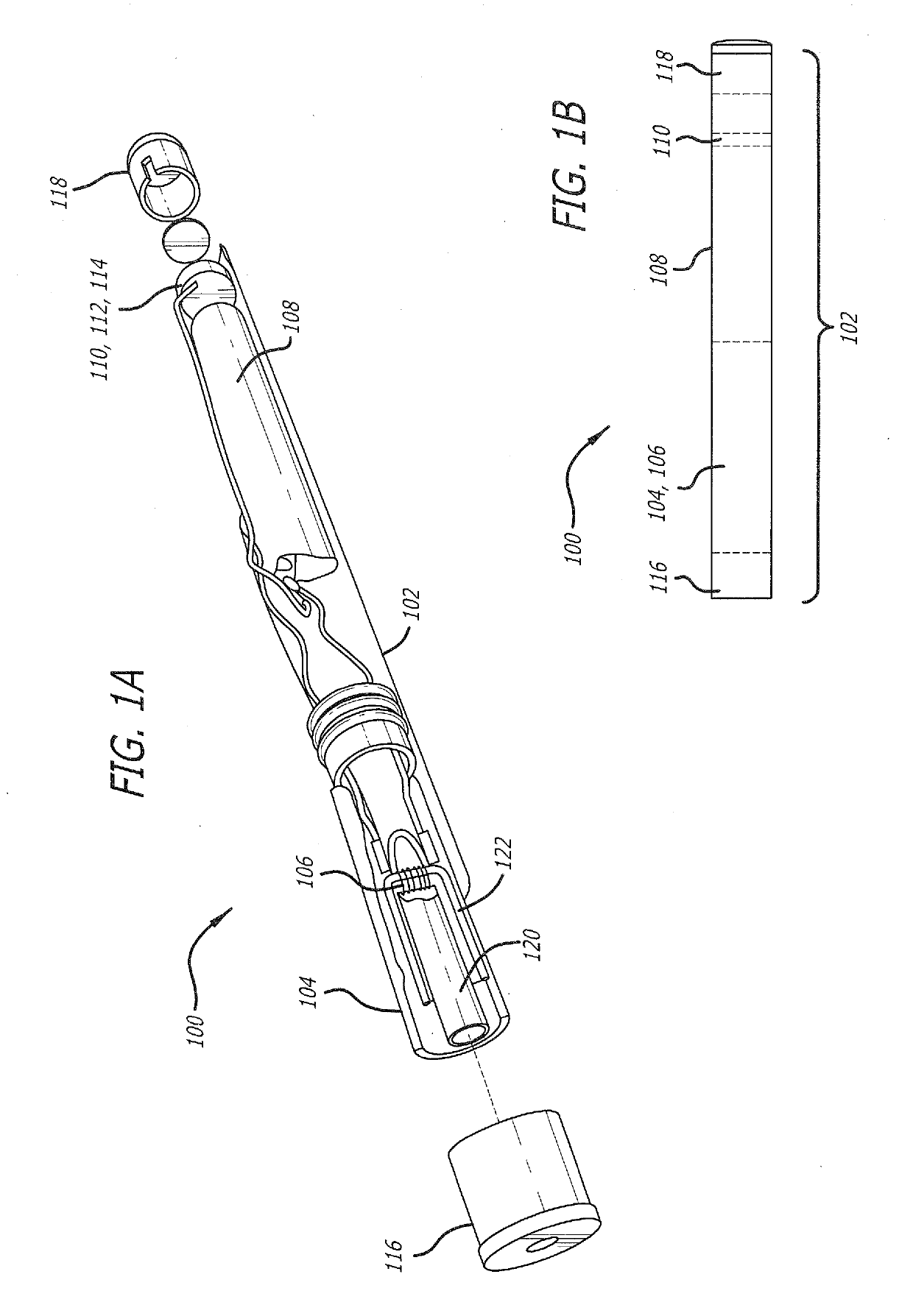
Nicotine replacement applique
InactiveUS7105173B1Easy to carryWithout adverse cosmetic effectCosmetic preparationsImpression capsNicotine replacementsCosmetic ingredient
Nicotine in base or salt form combined with cosmetic ingredients to form a lip balm or similar stick. The stick is readily dispensed not only onto a person's lips but also may be used on the skin, for example on the wrist. Packaging the nicotine this way enables a smoker to obtain nicotine discretely anywhere in public, without offending others by smoking. Furthermore, the dosage is readily controlled, and the person's hands and mouth are active, similar to the lighting and smoking of a cigarette.
Owner:ROLLING KENNETH J
Modifying taste and sensory irritation of smokeless tobacco and non-tobacco products
ActiveUS20140271946A1Reduce and eliminate sensory irritationLess irritatingTobacco preparationBiocideNicotine replacementsTRPA1 Channel
Tobacco products comprising smokeless tobacco products and active ingredients, including those that antagonize nicotinic acetylcholine receptors, the TRPV1 channel, and / or the TRPA1 channel are disclosed. Nicotine replacement therapies comprising active ingredients, including those that antagonize nicotinic acetylcholine receptors, the TRPV1 channel, and / or the TRPA1 channel. The active ingredient may reduce or eliminate sensory irritation arising due to use of the product. Analgesic compositions comprising active ingredients. Methods of reducing taste and sensory irritation by employing an active ingredient.
Owner:AKRIA CLIENT SERVICES LLC
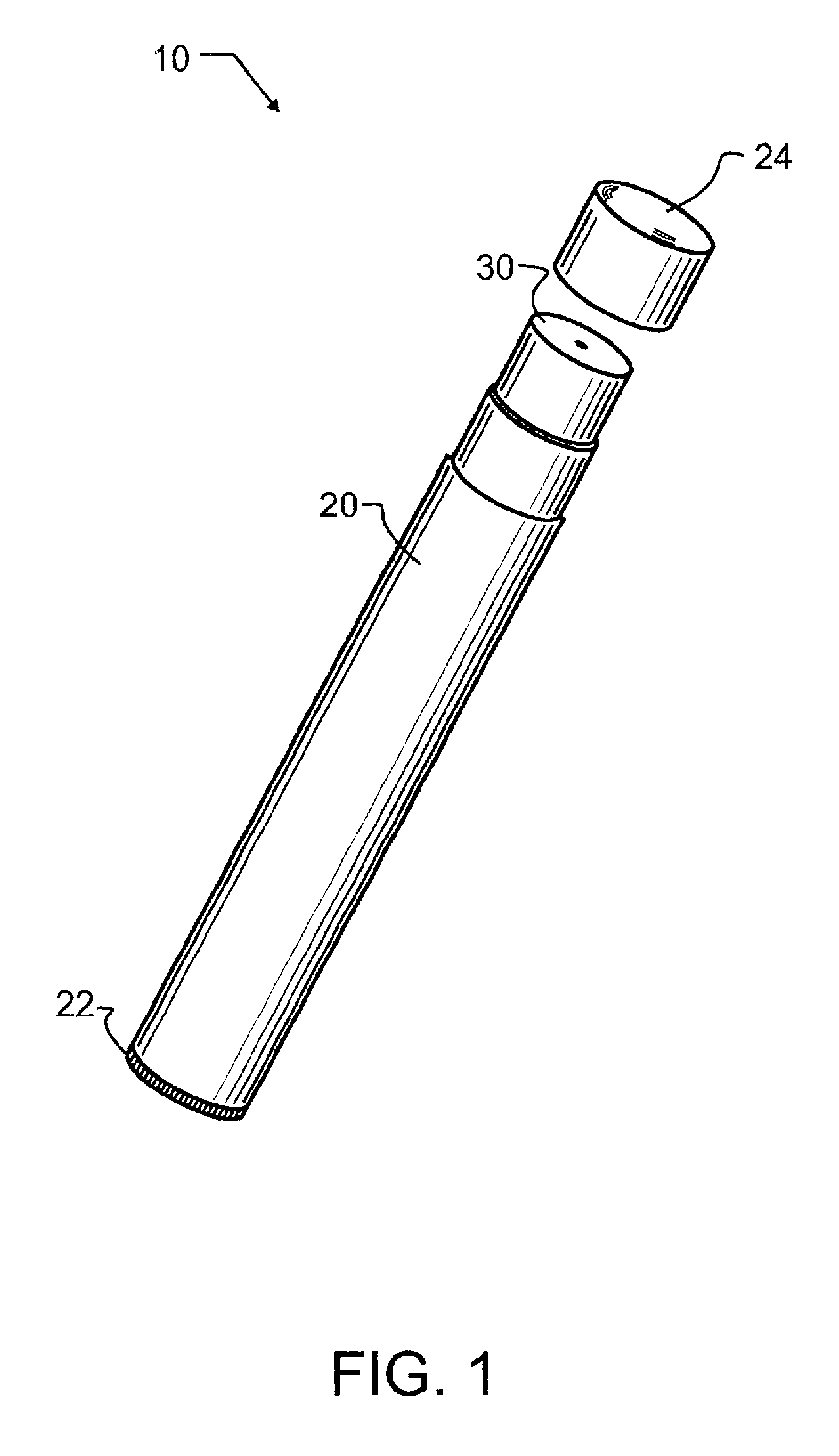
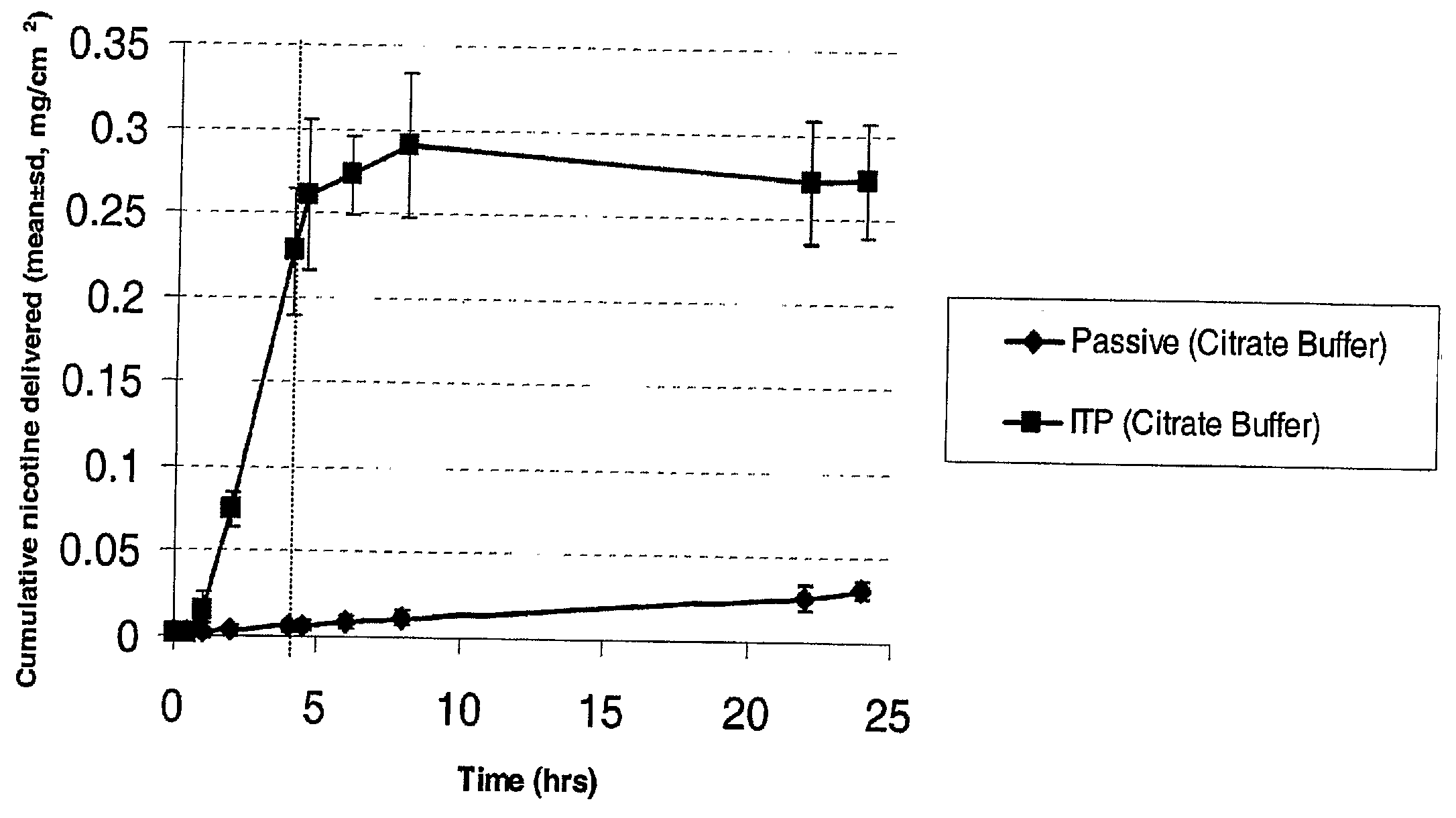
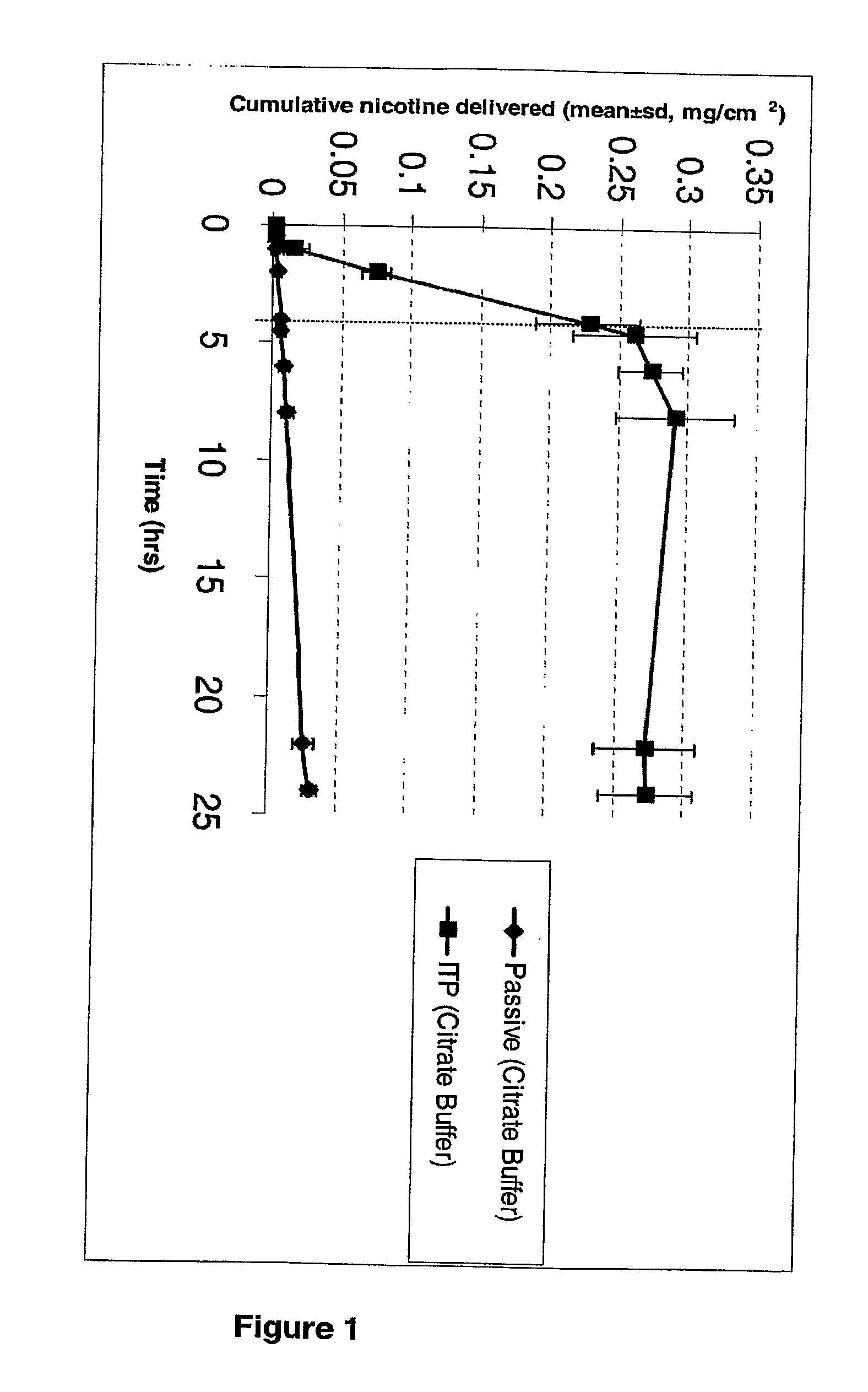
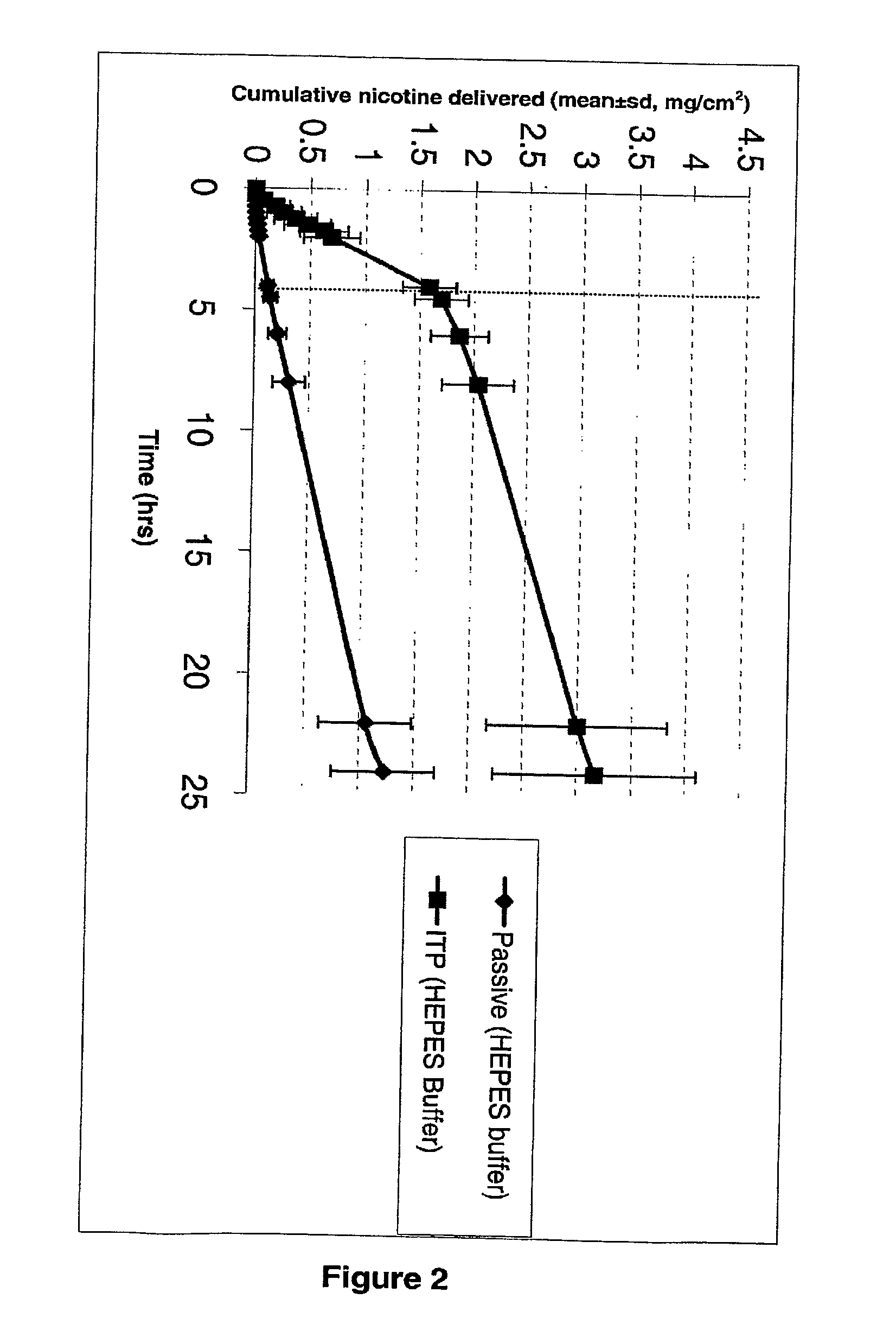
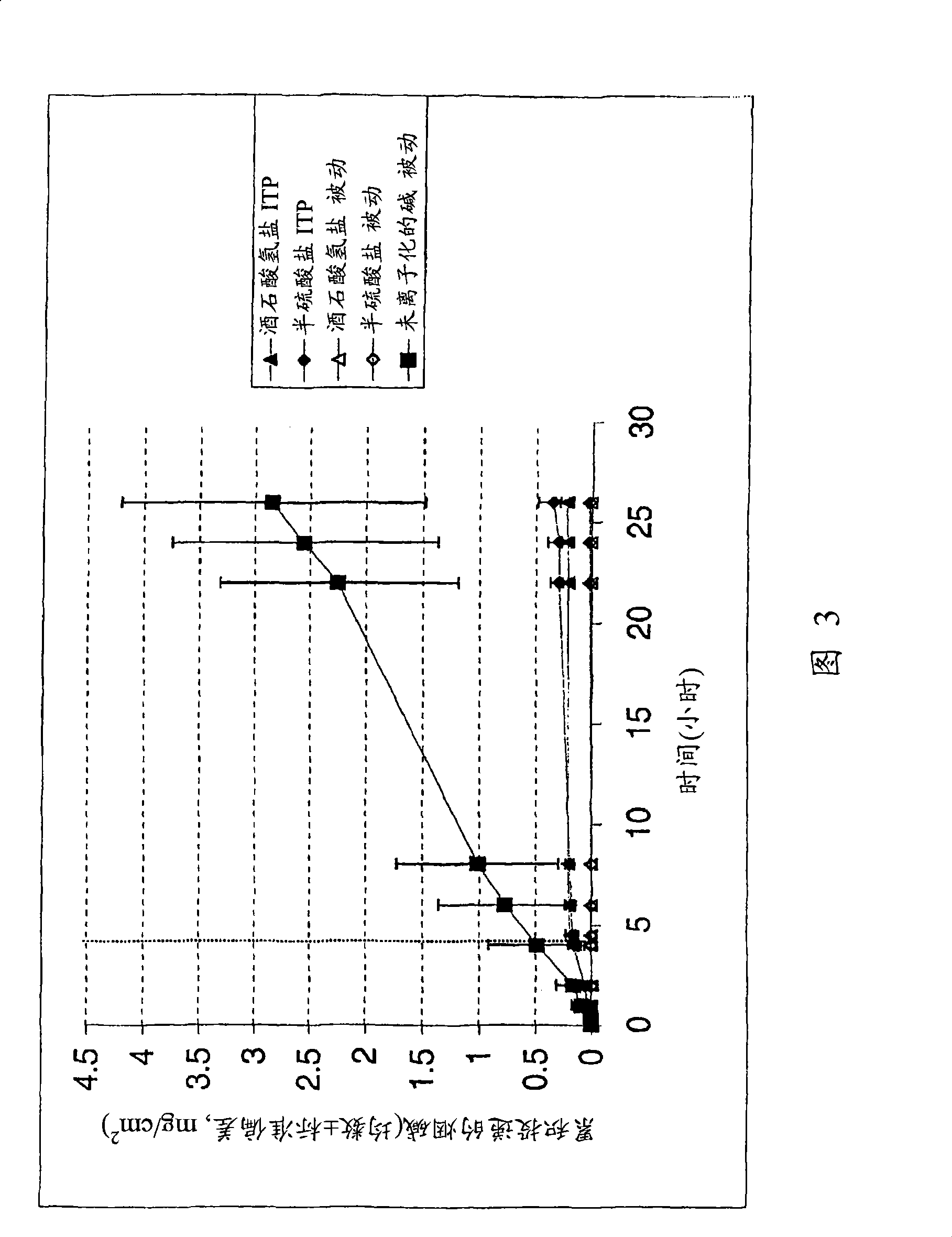
Iontophoretic Transdermal Delivery of Nicotine Salts
The present invention relates to iontophoretic transdermal delivery of nicotine salts useful for nicotine replacement therapy for an individual in need thereof. The present invention further relates to the iontophoretic transdermal delivery of nicotine maleate and nicotine citrate. Methods of reducing skin irritation generally caused by transdermal nicotine delivery by iontophoretic transdermal delivery of nicotine salts are also disclosed.
Owner:SMITHKLINE BECKMAN CORP
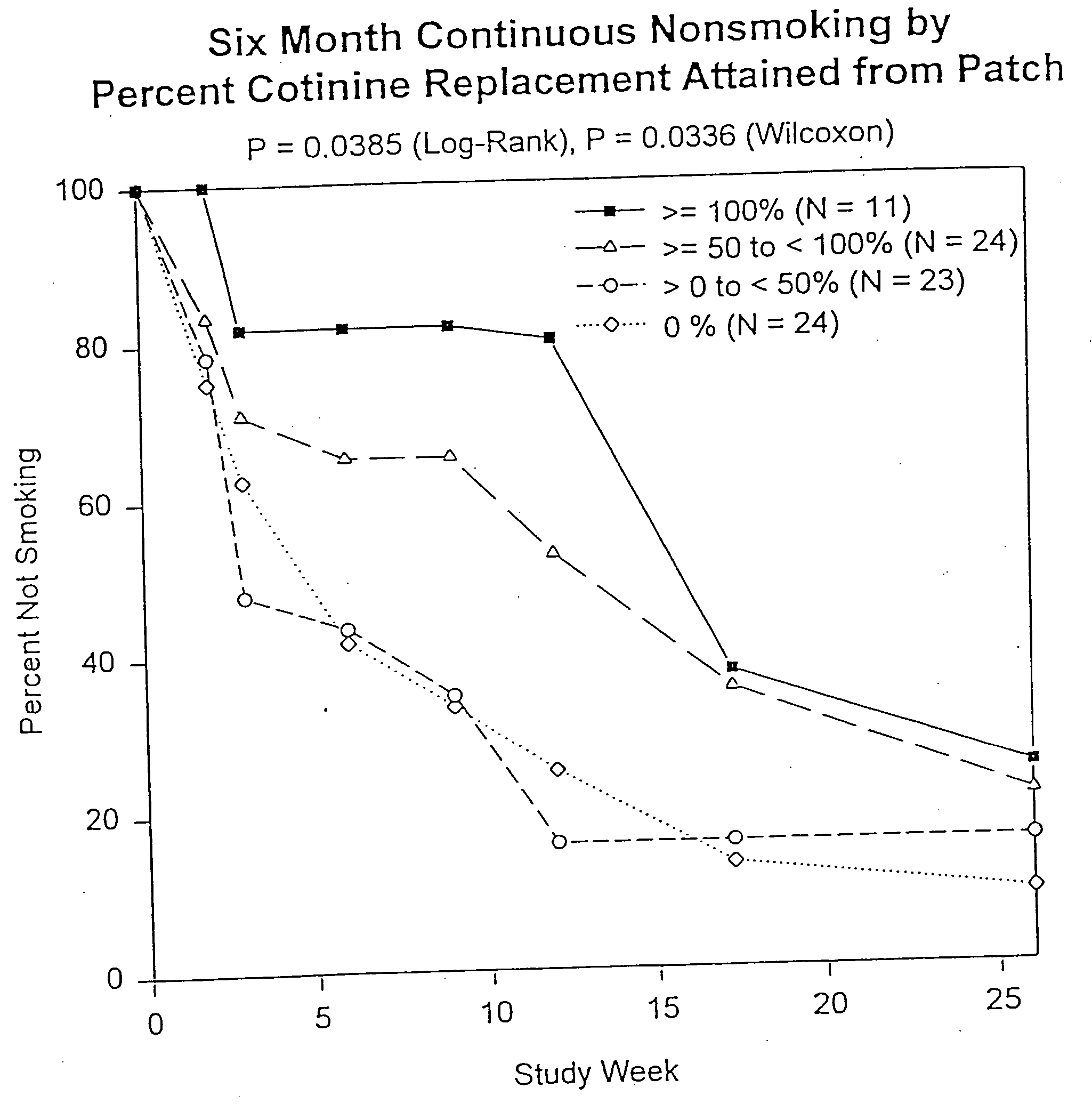
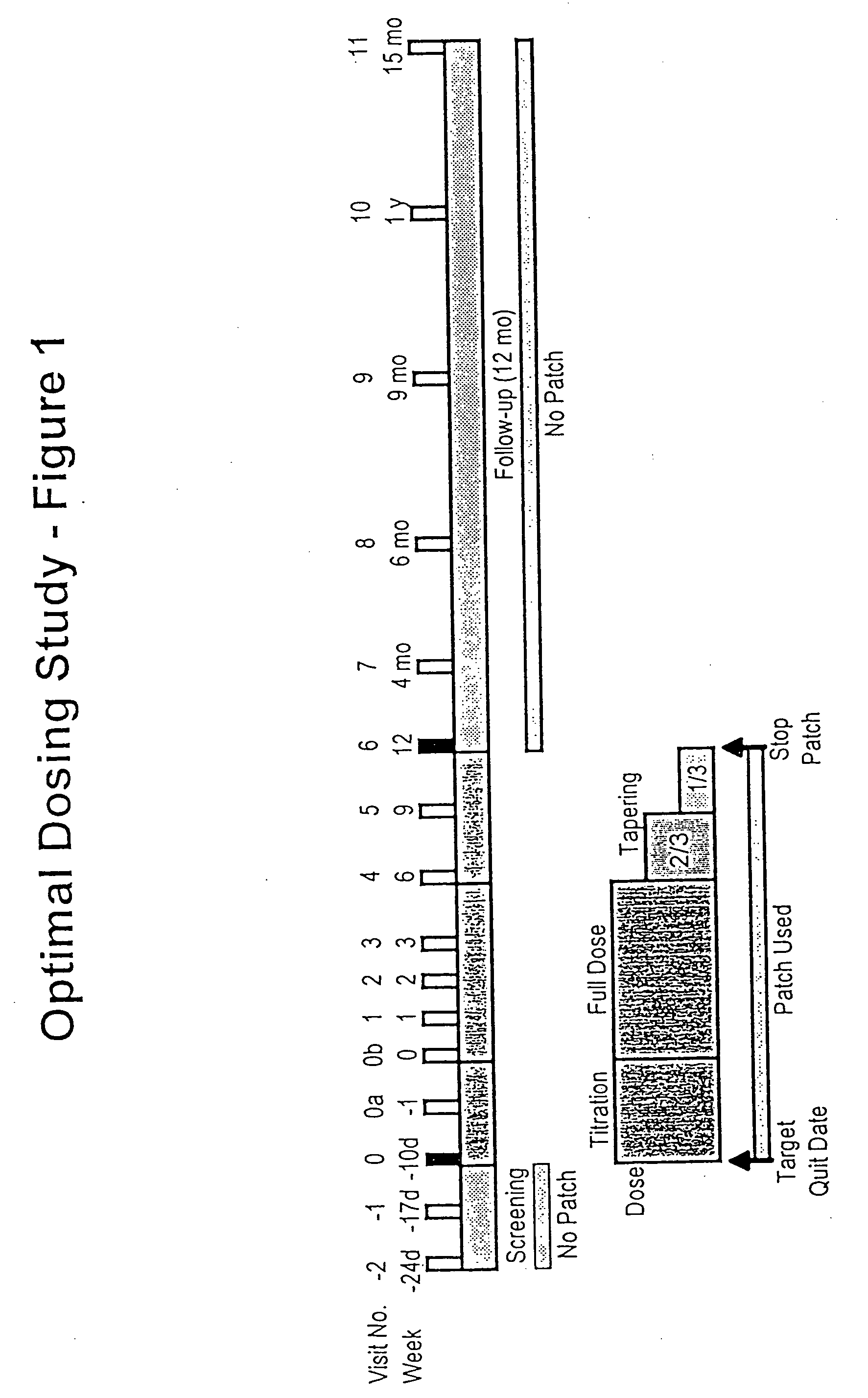
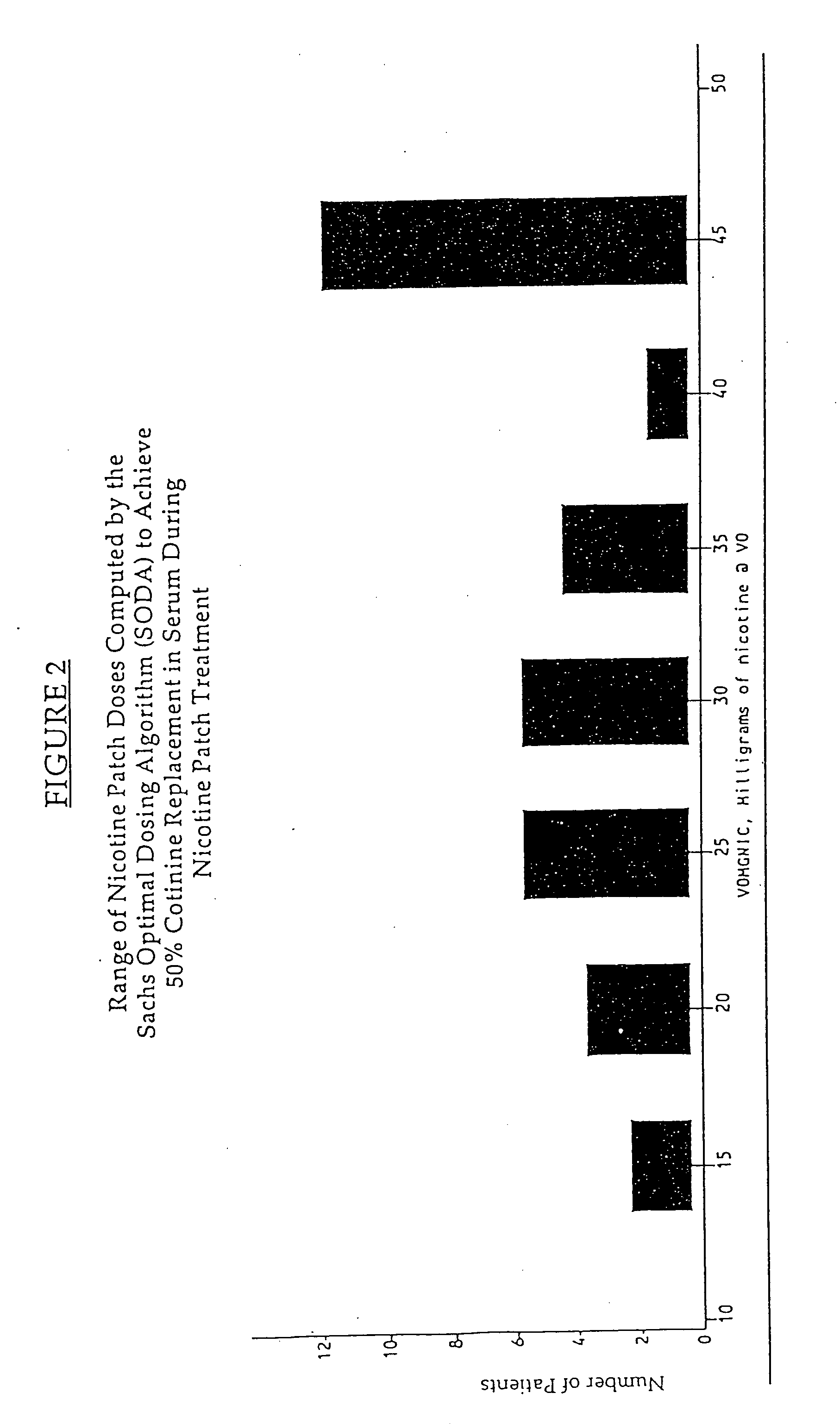
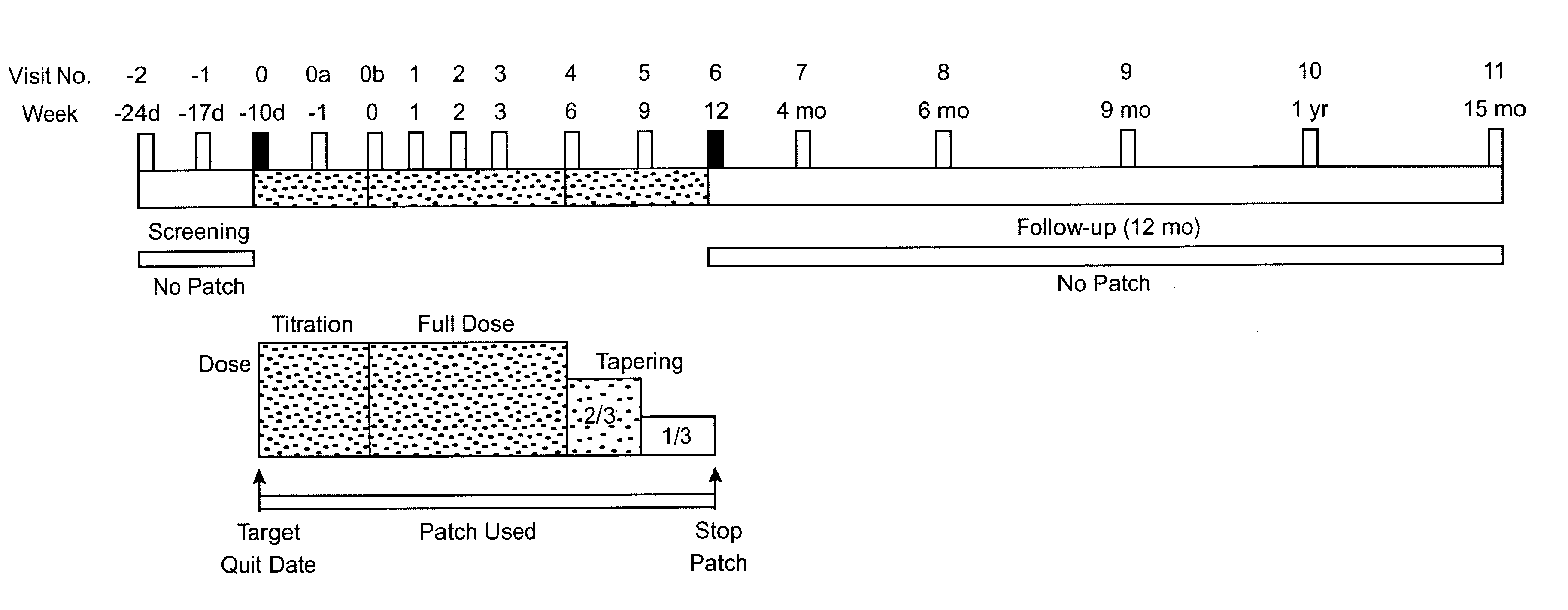
Methods for nicotine replacement dosage determination
InactiveUS20040006113A1Increased riskQuickly reachCompounds screening/testingBiocideNicotine replacementsSerum concentration
Owner:SACHS DAVID P L
Methods for nicotine replacement dosage determination
InactiveUS6602892B1Increased riskQuickly reachCompounds screening/testingBiocideNicotine replacementsSerum concentration
A method for predicting nicotine replacement dosage to achieve a target nicotine serum concentration relies on measuring blood nicotine concentration prior to smoking cessation. At least two values corresponding to other patient characteristics, such as body mass, cumulative smoking, psychological dependence, age, and menopausal status, are also determined and used to predict expected blood nicotine concentrations based on nicotine replacement dosages. Such methods are useful in achieving target blood nicotine concentrations for smoking cessation and therapy.
Owner:SACHS DAVID P L
Composition for improving cognition and memory
ActiveUS20060229340A1Improve cognitive functionEnhance memoryBiocideNervous disorderNicotine replacementsMelatonin agonist
The invention relates to a pharmacologically active combination, having utility in treating insomnia patients, which comprises: (a) at least one first active ingredient selected from melatonin, other melatonergic agents, melatonin agonists and melatonin antagonists; and (b) at least one second active ingredient selected from nicotine and nicotine receptor agonists; to use of a medicament containing component (a) with or without component (b) for alleviation of at least one adverse effect which occurs in a patient in the course of nicotine replacement therapy, or otherwise, selected from impairment of the quality of sleep, impairment of cognition and impairment of memory, as well as to a kit having utility in treating insomnia patients, which comprises components (a) and (b) in unit dosage form.
Owner:NEURIM PHARMA
Nicotine granule composition and preparation method thereof
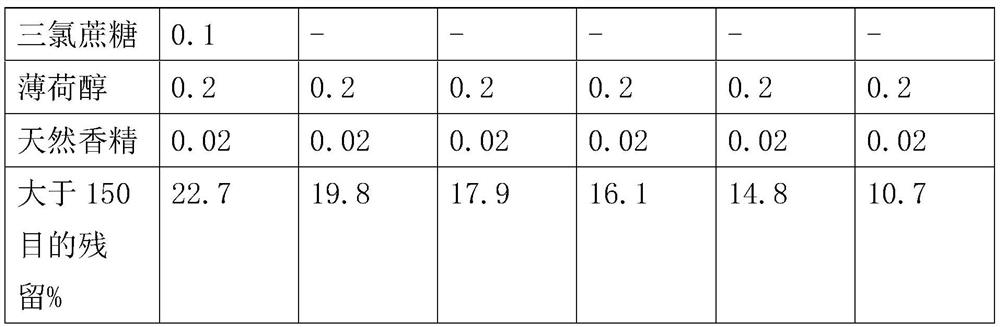
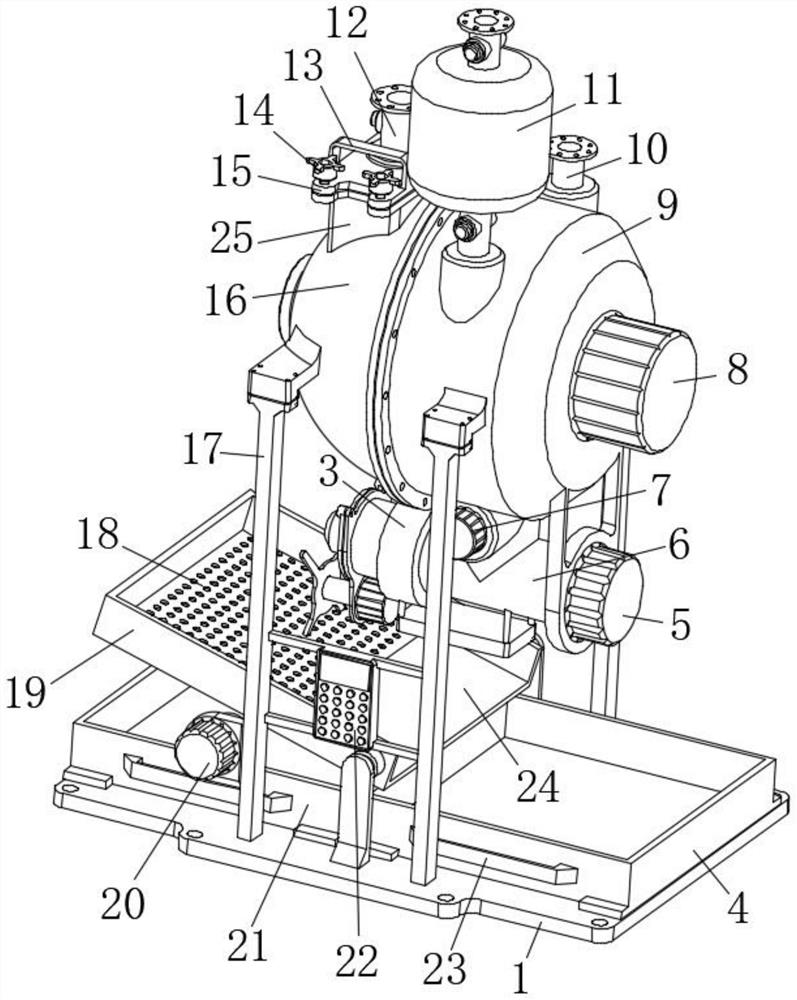
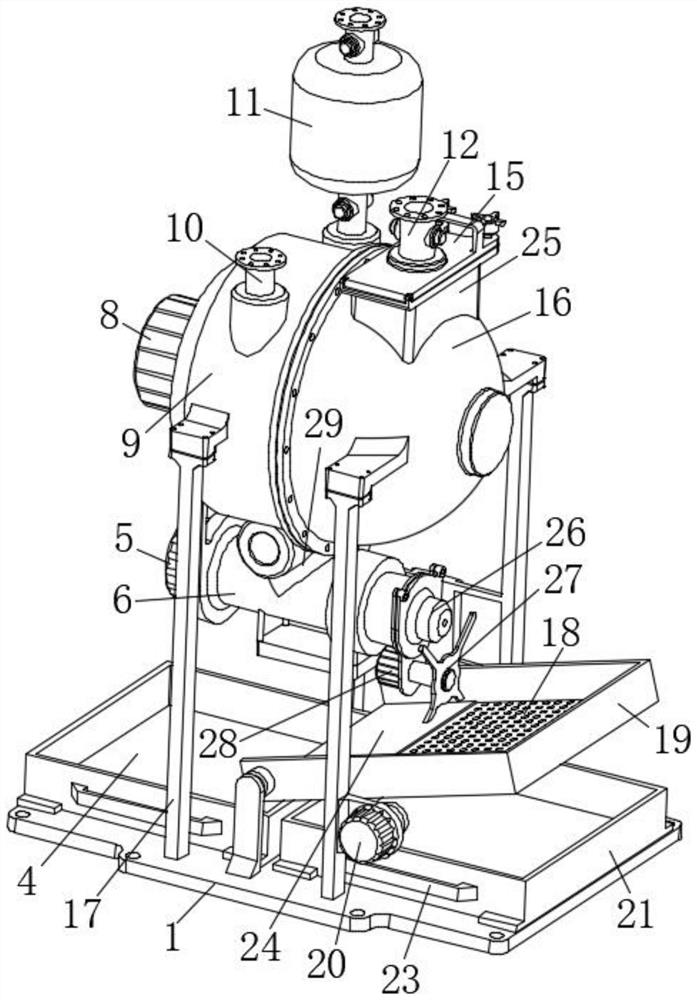
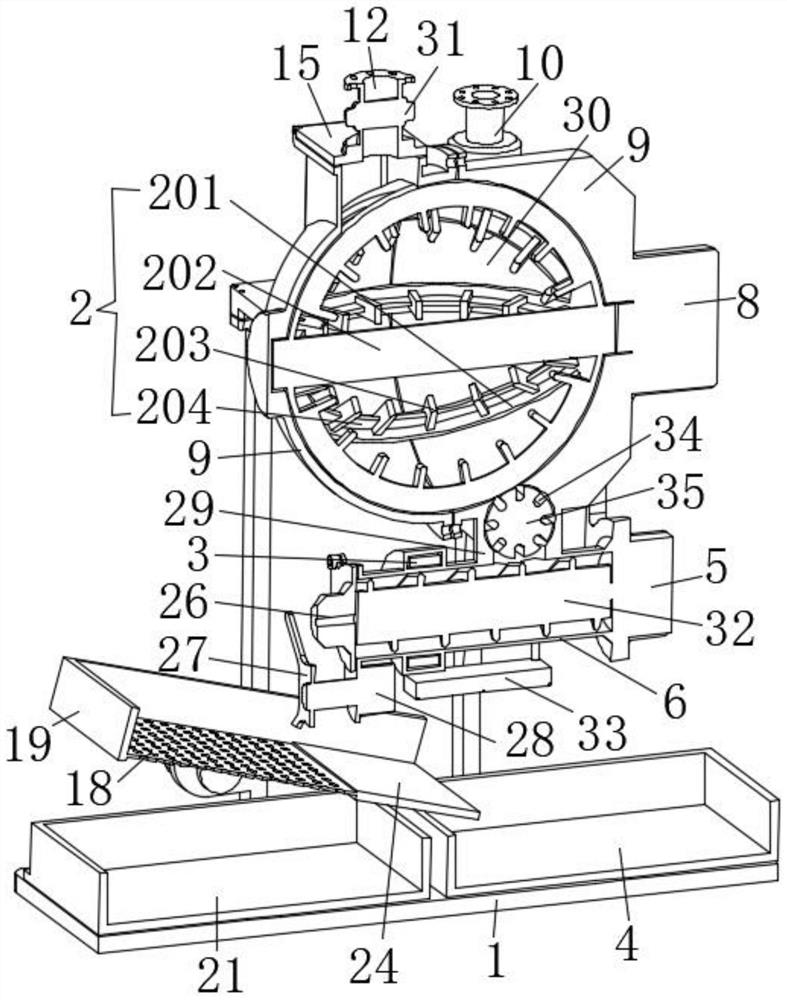
ActiveCN112220756AExtension of timeInhibition-dependentOrganic active ingredientsNervous disorderNicotine replacementsNicotine replacement
The invention relates to the technical field of nicotine replacement therapy products, and discloses a nicotine granule composition, which mainly comprises nicotine quick-release granules and nicotinesustained-release granules, and the nicotine quick-release granules mainly comprise a nicotine raw material, a filler, an adhesive, a buffer agent, a sweetener and an edible essence; the nicotine sustained-release granules mainly comprise the following components: a nicotine raw material, a polymer sustained-release material, an adhesive, a buffer agent, a sweetener and an edible essence. The invention also discloses a method for preparing the nicotine granule mixture and a preparation device thereof. The nicotine granule composition provided by the invention can guarantee the speed of obtaining pleasant sensation of a human body and the duration of nicotine maintaining the concentration with a treatment effect in a human body through the nicotine quick-release granules and the nicotine sustained-release granules, so that smoking addicts can get rid of the dependence on smoking better, and has a better treatment effect than the similar products sold on the market.
Owner:重庆市义力医药科技有限公司
Nicotine containing soft gelatin pastilles
The present invention relates to soft pastilles for nicotine replacement therapy, said pastille comprises about 0.05% to about 1% of nicotine active; about 5% to about 40% of gelling agent; about 30% to about 70% of plasticizer; about 0.05% to about 10% of sweetener; 0.5% to about 30% of releasing agent; about 0.05% to about 2% of preservative; about 0.01% to 5% of flavoring agent; and about 5% to about 20% of water.
Owner:THAKKAR JATIN
Nicotine-containing pharmaceutical compositions
InactiveCN102933199ANervous disorderPharmaceutical delivery mechanismNicotine replacementsPharmaceutical Substances
A composition intended to be employed for therapeutic purposes incorporates a source of nicotine and at least one levulinate moiety. Representative forms of nicotine include free base (e.g., as a mixture of nicotine and microcrystalline cellulose), a nicotine salt (e.g., as nicotine bitartrate) or nicotine polacrilex. The levulinate moiety can have the form of an acid (e.g., levulinic acid), a levulinate salt (e.g., sodium levulinate), or an ester of levulinic acid (e.g., methyl levulinate or ethyl levulinate). The composition can incorporate nicotine and levulinic acid in a salt form (e.g., nicotine levulinate). The composition can be composed of at least two forms of nicotine, and one of the forms of nicotine is in the form of nicotine levulinate. The composition is useful for treatment of central nervous system conditions, diseases, and disorders, and as a nicotine replacement therapy.
Owner:NICONOVUM USA
Nicotine granule composition, preparation method and prepartion device thereof
ActiveCN112220757AExtension of timeInhibition-dependentOrganic active ingredientsNervous disorderNicotine replacementsNicotine replacement
The invention relates to the technical field of nicotine replacement therapy products, and discloses a nicotine granule composition, which mainly comprises nicotine quick-release granules and nicotinesustained-release granules, and the nicotine quick-release granules mainly comprise a nicotine raw material, a filler, an adhesive, a buffer agent, a sweetener and an edible essence; the nicotine sustained-release granules mainly comprise the following components: a nicotine raw material, a polymer sustained-release material, an adhesive, a buffer agent, a sweetener and an edible essence. The invention also discloses a method for preparing the nicotine granule mixture and a preparation device thereof. The nicotine granule composition provided by the invention can guarantee the speed of obtaining pleasant sensation of a human body and the duration of nicotine maintaining the concentration with a treatment effect in a human body through the nicotine quick-release granules and the nicotine sustained-release granules, so that smoking addicts can get rid of the dependence on smoking better, and has a better treatment effect than the similar products sold on the market.
Owner:重庆市义力医药科技有限公司
Systems and Methods for Buffered Aerosol Drug Delivery
A method for delivering a drug to a user including an electronic cigarette wherein the electronic cigarette itself includes a liquid formulation. The liquid formulation can include at least one drug and at least one biologically acceptable carrier. The at least one drug can be formed with an acid or alcohol and has a first vapor pressure at a first temperature, and is heated by a heating element within the electronic cigarette resulting in the generation of an aerosol suitable for inhaling by the user. Often, the drug can include nicotine, but may also be configured to include cannabinol or can be associated with nicotine replacement therapies, or other medication-assisted therapies. The drugs may also have a salt or co-salt added in order to increase the effectiveness of drug delivery while lowering the need for a higher powered heating element.
Owner:TURBI ZAYD ABDULFUHAH
Nicotine replacement therapy products comprising synthetic nicotine
A composition suitable for use in nicotine replacement therapy products includes a nicotine product that includes a synthetic nicotine that is substantially free of one or more contaminants and / or impurities normally associated with tobacco-derived nicotine. For example, the synthetic nicotine is substantially free of one or more of nicotine-1'-N-oxide, nicotyrine, nornicotyrine, 2',3-bipyridyl, cotinine, anabasine, and / or anatabine. The composition further comprises one or more pharmaceutically acceptable excipients, additives and / or carriers. The nicotine replacement therapy products may include any number of such products, including transdermal nicotine delivery patches, nicotine gums, synthetic chewing tobacco, synthetic snuff, and synthetic strips (e.g., dissolvable synthetic tobacco). Additionally, a method of treating nicotine addiction includes administering a nicotine replacement composition, e.g., via a nicotine replacement therapy product, to a user.
Owner:NEXT GENERATION LABS LLC
Nicotine-containing pharmaceutical compositions
A composition intended to be employed for therapeutic purposes incorporates nicotine and at least one other nicotinic compound. Representative forms of nicotine can be as a free base (e.g., as a mixture of nicotine and microcrystalline cellulose), as a form of nicotine salt (e.g., as nicotine bitartrate) or as nicotine polacrilex. The other nicotinic compound is a compound that can be considered to bind selectively to certain nicotinic receptor subtypes, and particularly those of the central nervous system. For example, the other nicotinic compound can be a compound that binds selectively to the nicotinic receptor subtypes a7 or a4ss2. The composition is useful for treatment of central nervous system conditions, diseases and disorders, and as a nicotine replacement therapy.
Owner:NICONOVUM USA
Nicotine-containing chewing gum compositions
InactiveUS20200138706A1Rapid satisfactionOvercomes drawbackOrganic active ingredientsNervous disorderNicotine replacementsOrganic chemistry
The present invention relates to nicotine-containing chewing gum compositions and the method of preparing the same. The present invention provides improved and stable nicotine-containing chewing gum compositions for use in nicotine replacement therapy, which provide rapid nicotine release in the oral cavity and effectively satisfy the craving that most smokers experience.
Owner:ENORAMA PHARMA AB
Iontophoretic transdermal delivery of nicotine salts
The present invention relates to iontophoretic transdermal delivery of nicotine salts useful for nicotine replacement therapy for an individual in need thereof. The present invention further relates to the iontophoretic transdermal delivery of nicotine maleate and nicotine citrate. Methods of reducing skin irritation generally caused by transdermal nicotine delivery by iontophoretic transdermal delivery of nicotine salts are also disclosed.
Owner:SMITHKLINE BECKMAN CORP
Composition for improving cognition and memory
Owner:NEURIM PHARMA
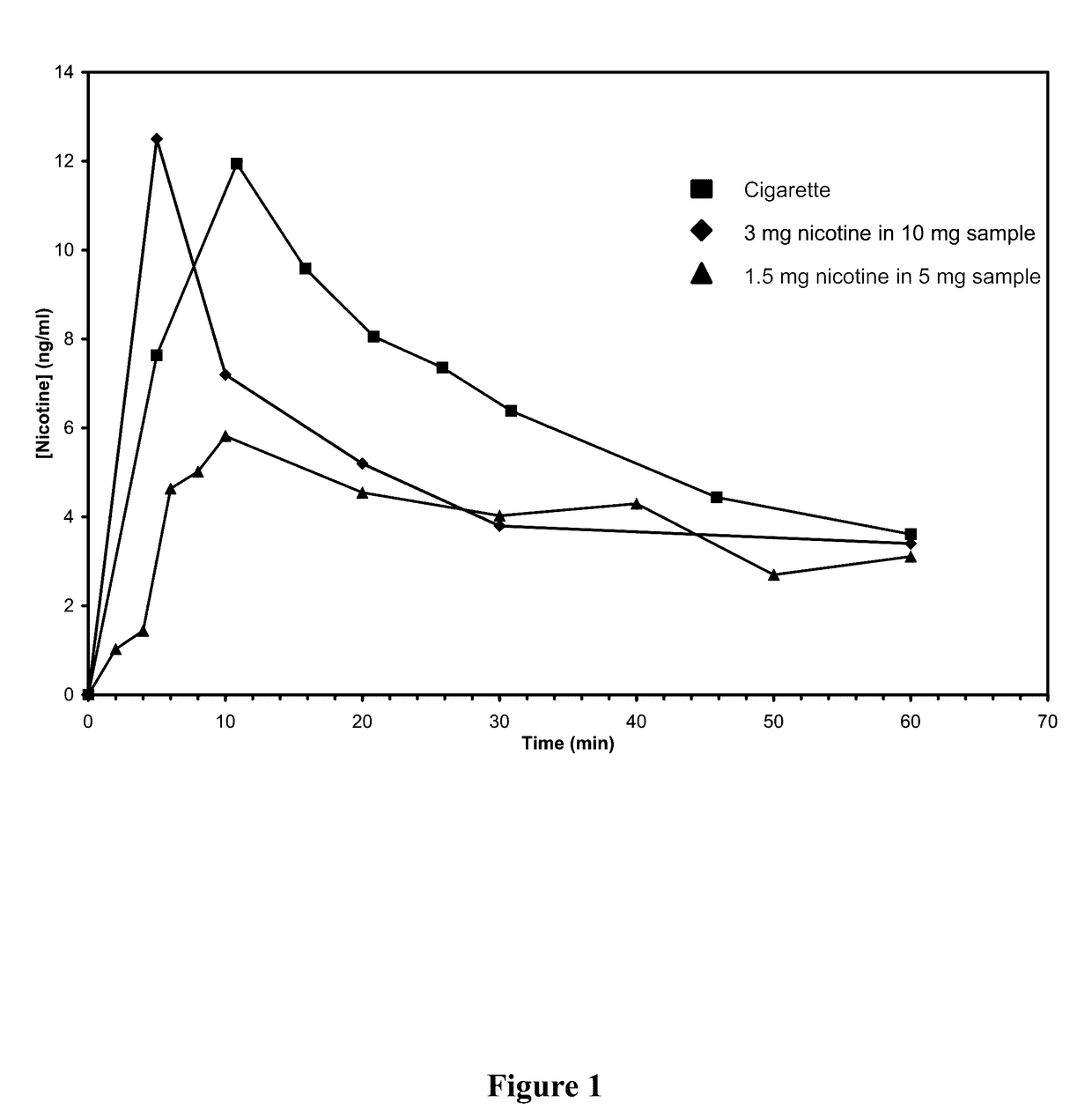
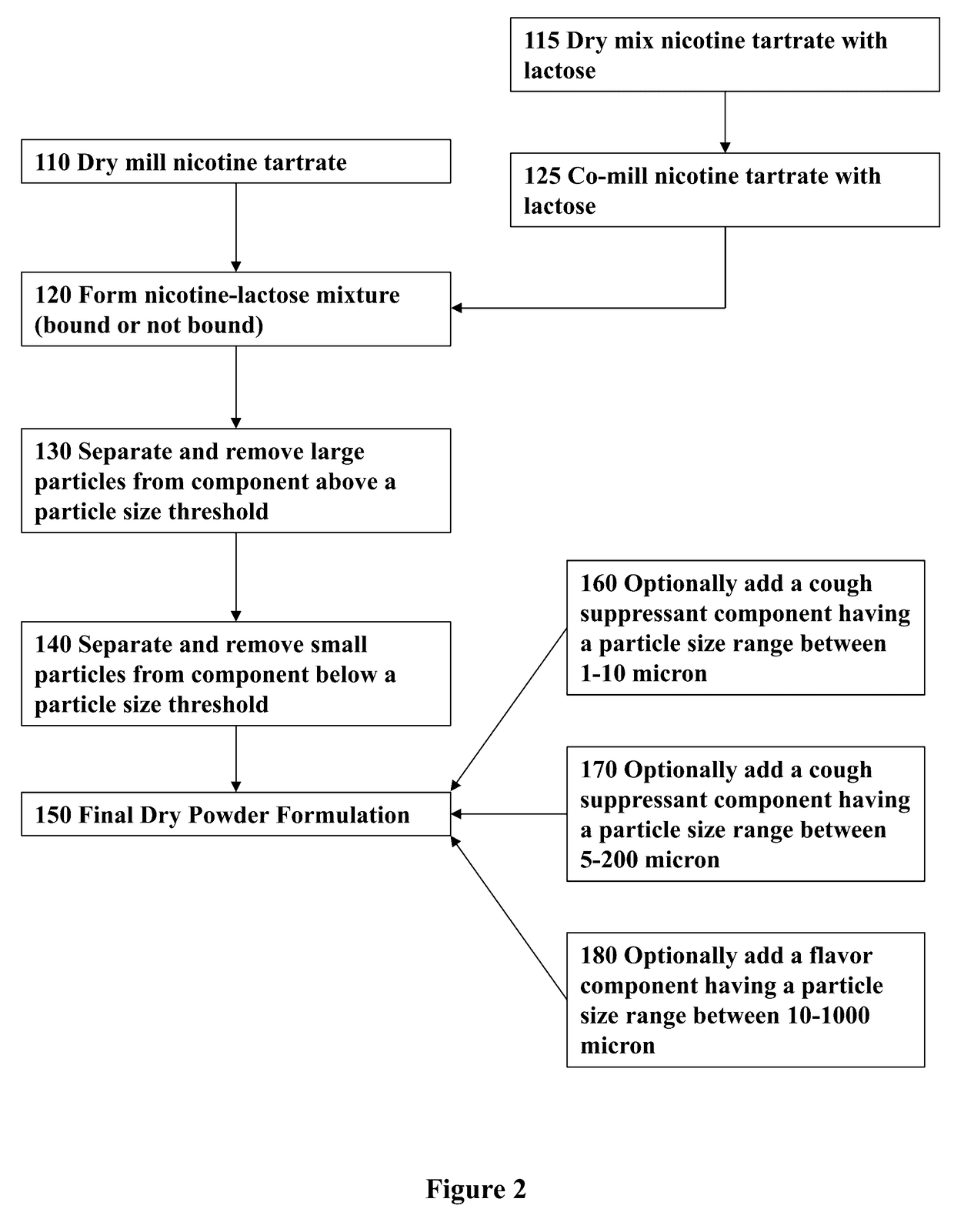
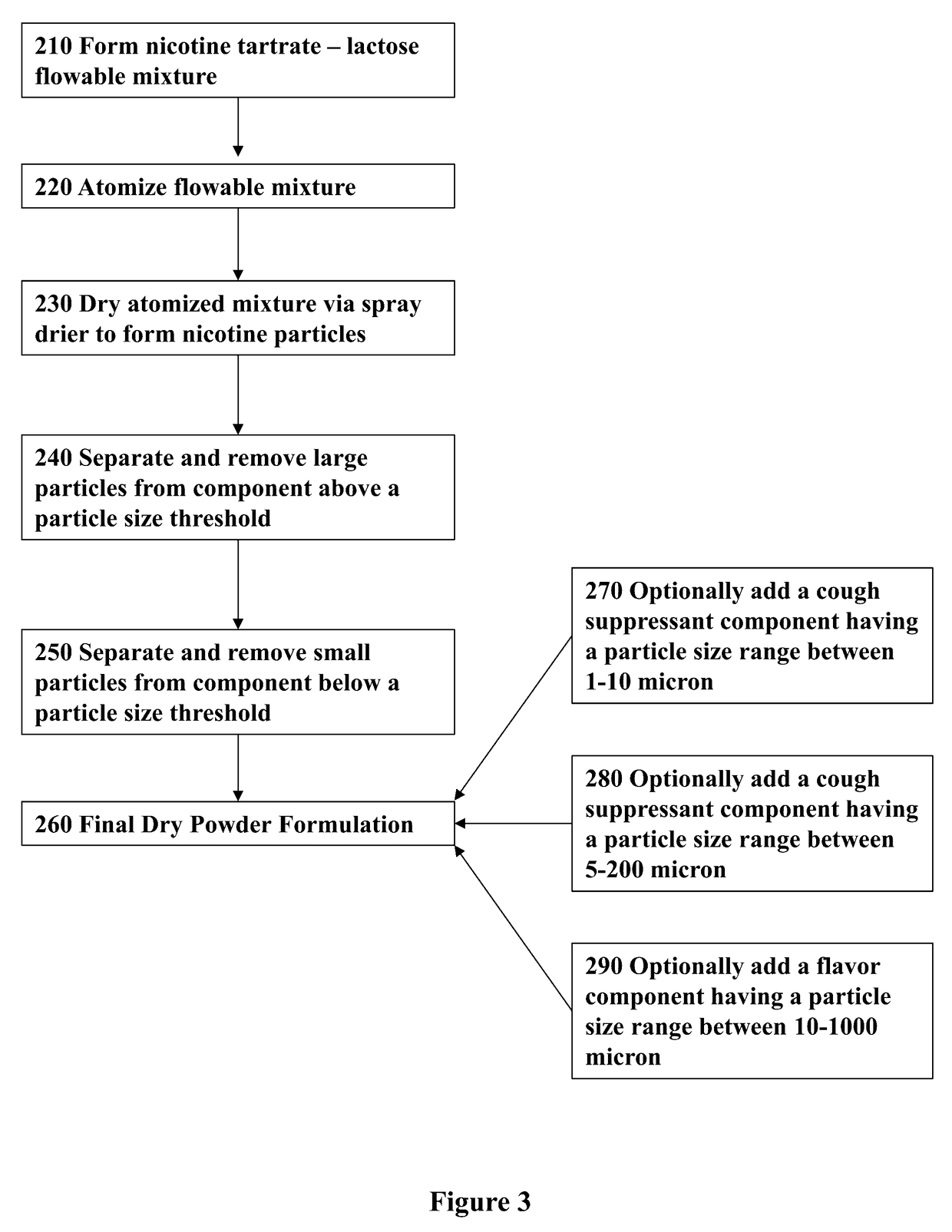
Nicotine Formulations and Methods of Making and Using the Same
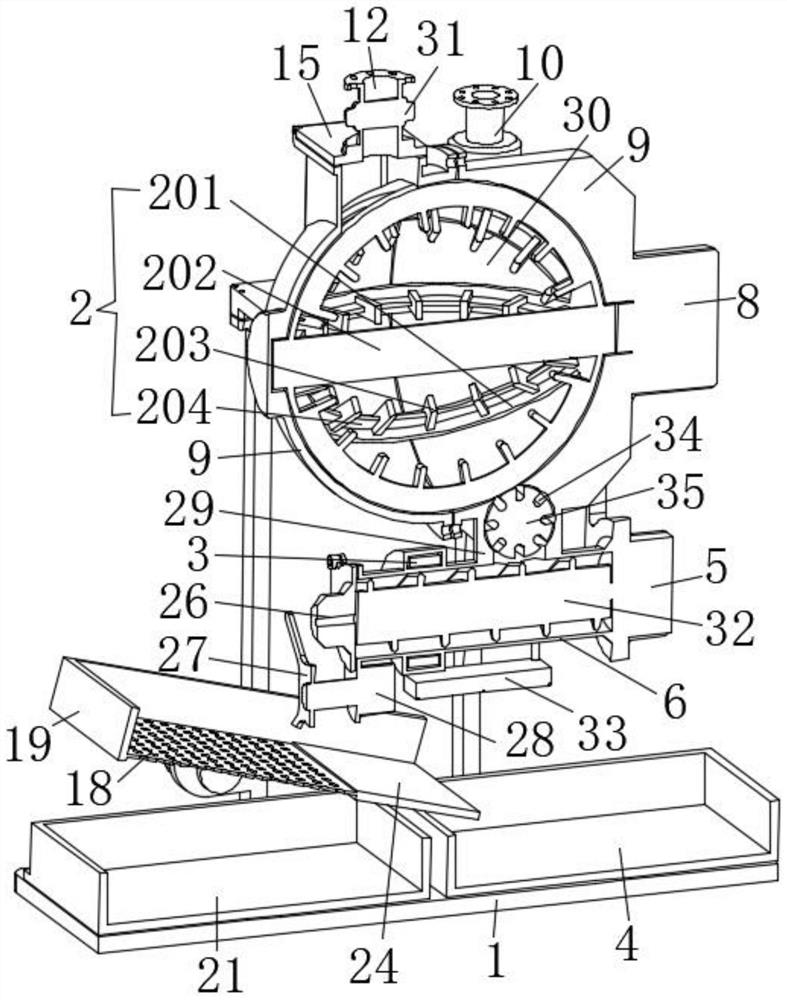
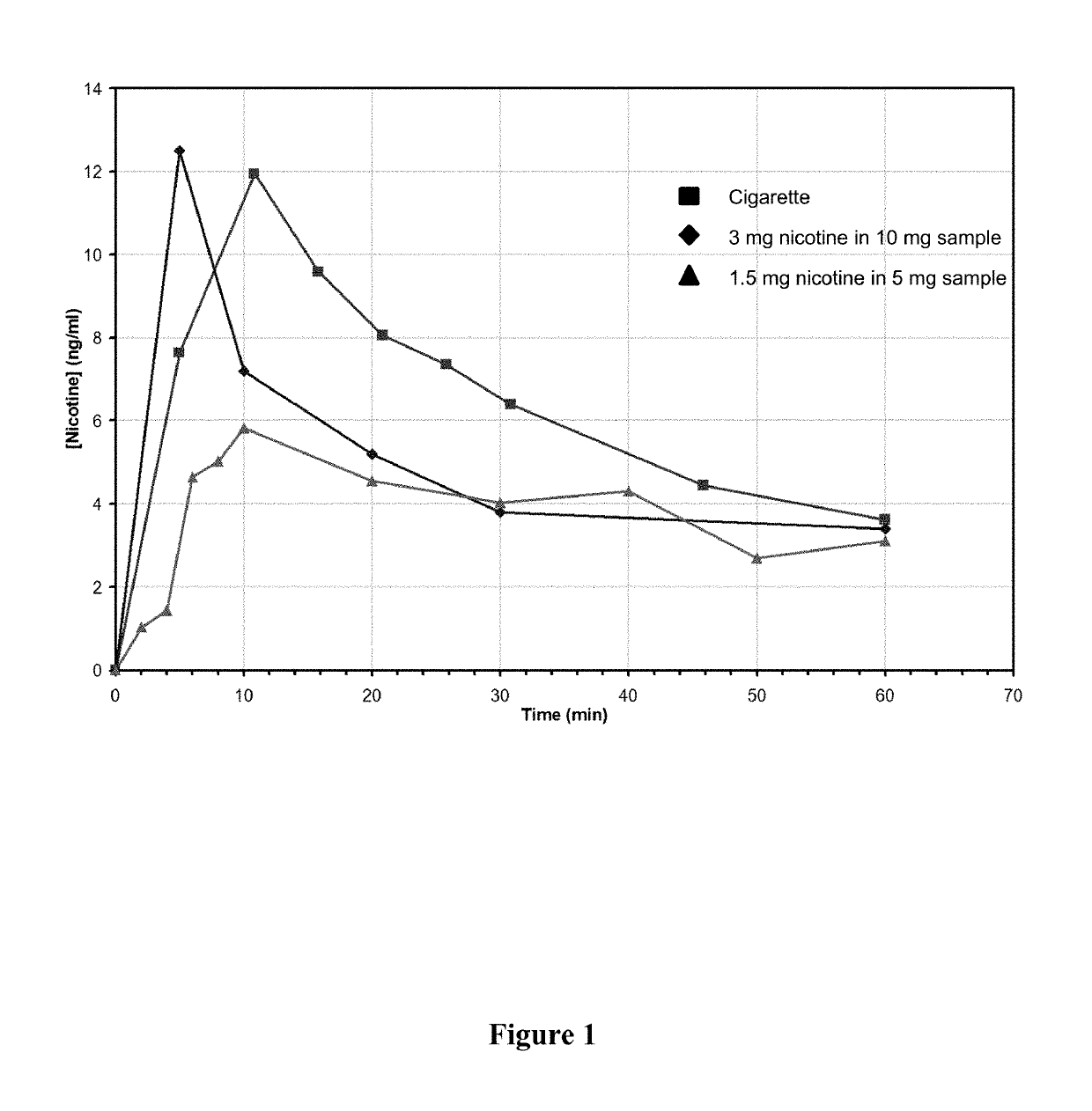
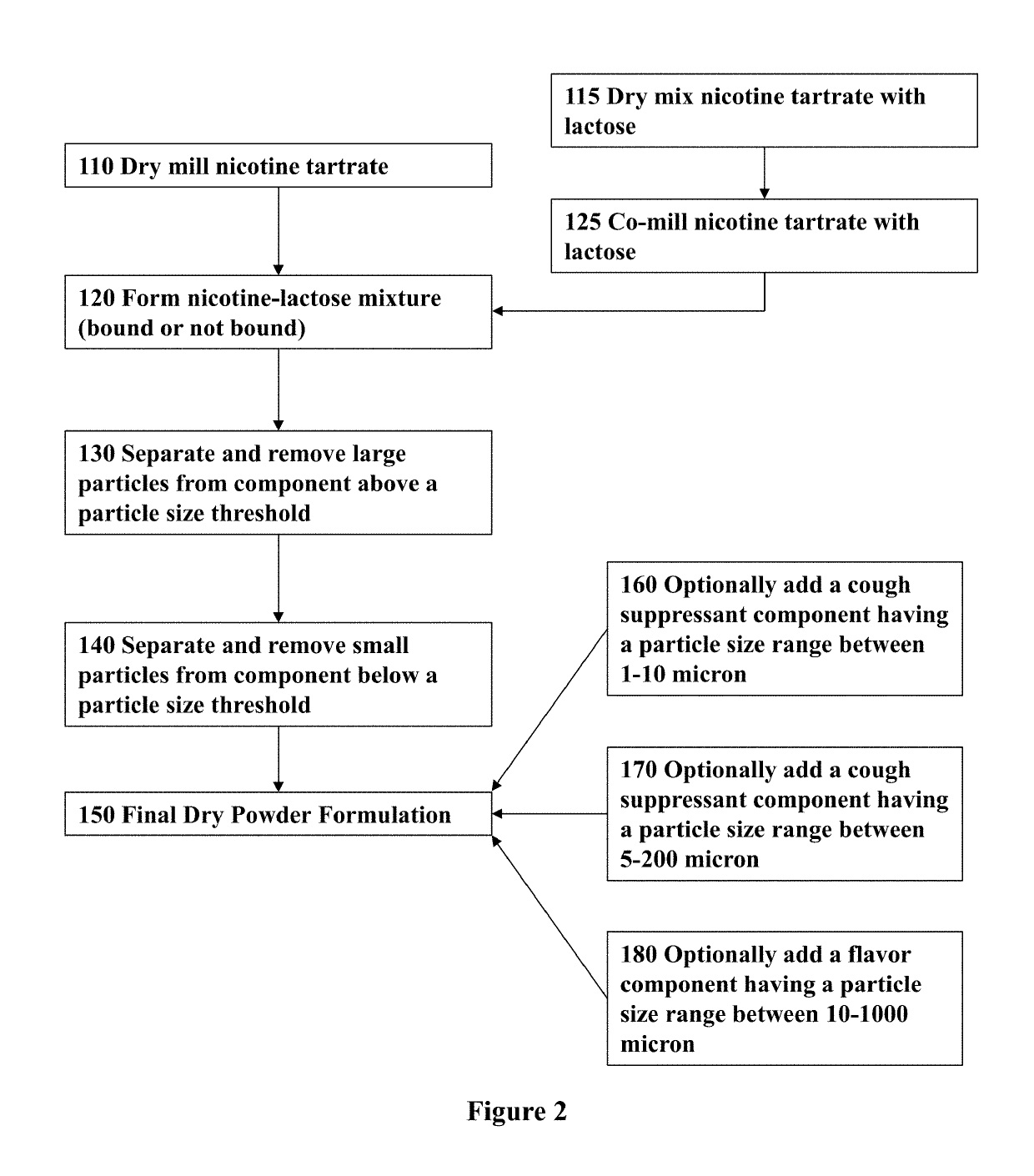
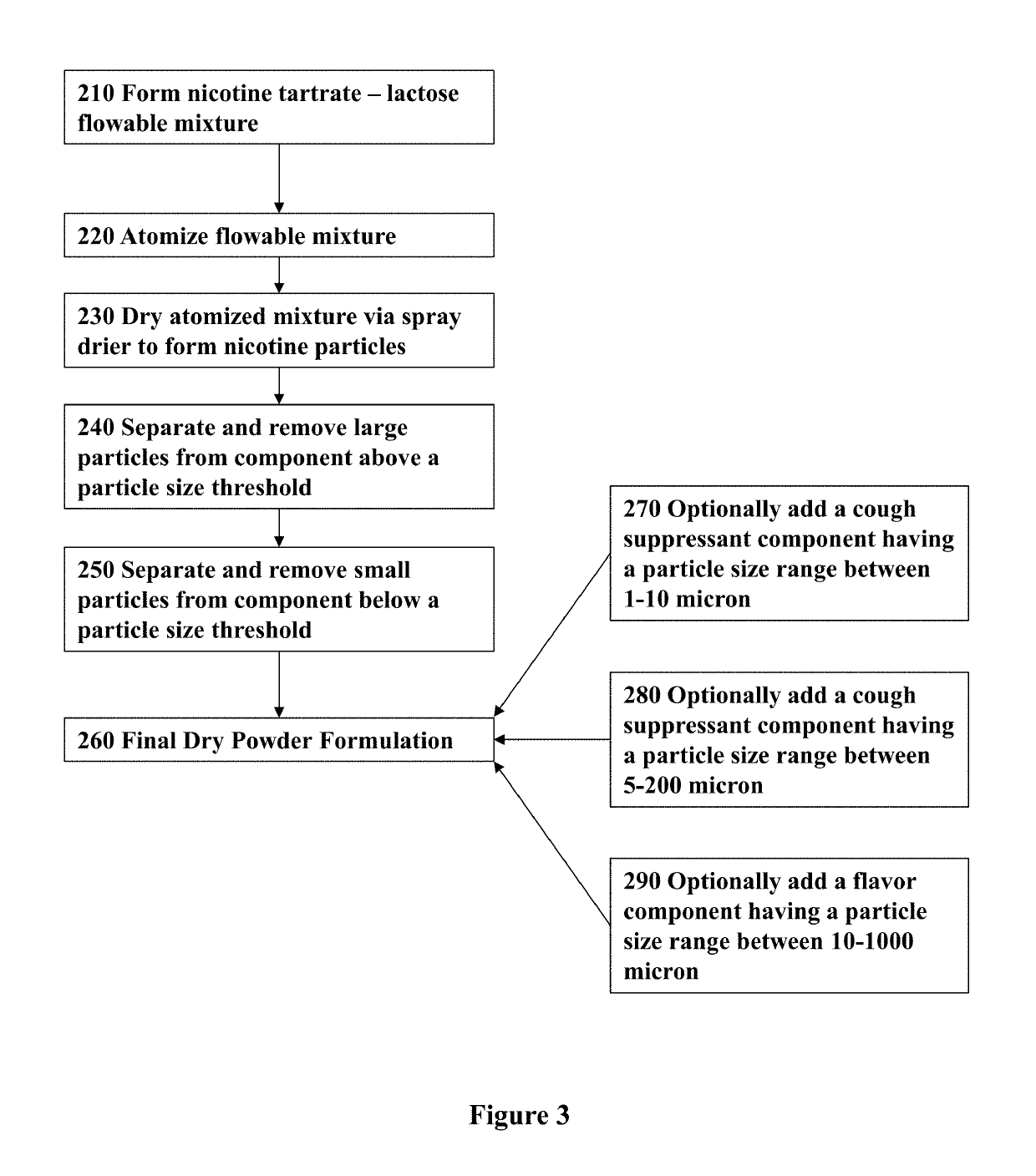
ActiveUS20170071929A1Reducing nicotine cravingReduces nicotine cravingPowder deliveryNervous disorderNicotine replacementsInhalation
A method of reducing nicotine cravings is described. The method includes inhalation of a dry powder formulation containing a dose of nicotine by a subject seeking nicotine cravings reduction. The formulation includes amounts and concentrations of nicotine that are significantly lower than cigarettes or nicotine replacement therapies.
Owner:PHILIP MORRIS PROD SA
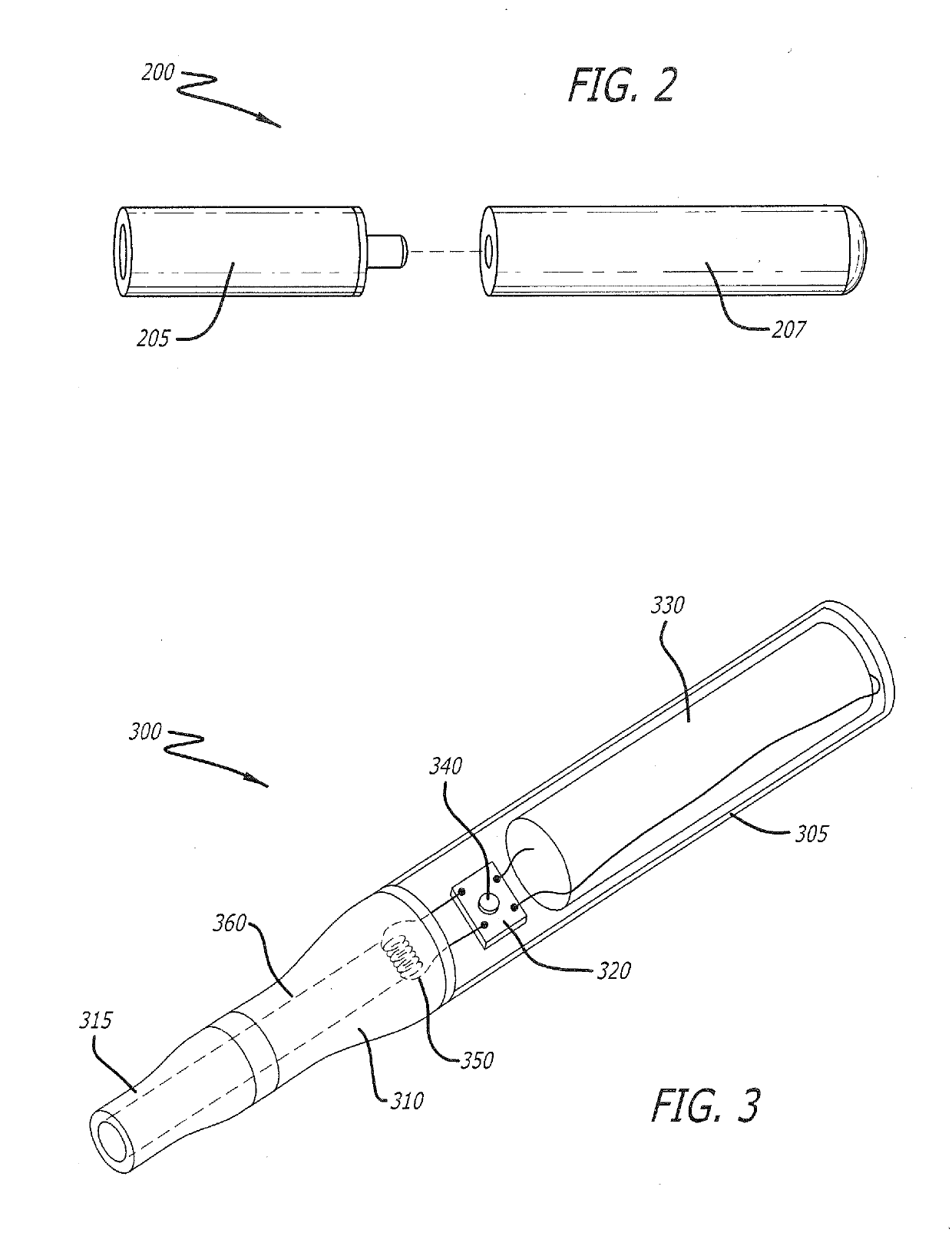
Smoking cessation device
InactiveUS20150090276A1Increase ratingsImprove therapyOrganic active ingredientsPharmaceutical delivery mechanismNicotine replacementsTobacco pipe
A smoking cessation device for use in nicotine replacement therapy includes an imitation smoking implement with a body sized and shaped substantially identical to a conventional smoking implement chosen from the group consisting of a cigarette, a cigarillo, a cigar, and a pipe. The device also includes one or more nicotine replacement modules removably disposed at one end of the body, the one or more nicotine replacement modules removable from the imitation smoking implement by a user to ingest said nicotine replacement modules as part of a nicotine replacement therapy. Optionally, a plurality of said imitation smoking implements can be held in a package sized and shaped to resemble a conventional box of cigarettes, cigarillos or cigars. Said imitation smoking implement can be held by the user in their hands or lips while consuming the one or more nicotine replacement modules to simulate a conventional smoking ritual.
Owner:Q CIGARETTES
Smoking cessation device
InactiveUS20150201677A1Increase ratingsImprove therapyOrganic active ingredientsPharmaceutical delivery mechanismNicotine replacementsEnvironmental health
A smoking cessation device for use in nicotine replacement therapy includes an imitation smoking implement with a body sized and shaped substantially identical to a conventional smoking implement chosen from the group consisting of a cigarette, a cigarillo, a cigar, and a pipe. The device also includes one or more nicotine replacement modules removably disposed at one end of the body, the one or more nicotine replacement modules removable from the imitation smoking implement by a user to ingest said nicotine replacement modules as part of a nicotine replacement therapy. Optionally, a plurality of said imitation smoking implements can be held in a package sized and shaped to resemble a conventional box of cigarettes, cigarillos or cigars. Said imitation smoking implement can be held by the user in their hands or lips while consuming the one or more nicotine replacement modules to simulate a conventional smoking ritual.
Owner:Q CIGARETTES
A kind of nicotine particle composition and its preparation method and its preparation device
ActiveCN112220757BExtension of timeInhibition-dependentOrganic active ingredientsNervous disorderNicotine replacementsImmediate release
The invention relates to the technical field of products for nicotine replacement therapy, and discloses a nicotine granule composition, which is mainly composed of nicotine immediate-release granules and nicotine sustained-release granules, wherein the components of the nicotine immediate-release granules mainly include: nicotine raw materials, fillers , binders, buffers, sweeteners and food flavors; the components of nicotine sustained-release granules mainly include: nicotine raw materials, polymer slow-release materials, binders, buffers, sweeteners and food flavors. The invention also discloses a method and a preparation device for preparing the nicotine particle mixture. The nicotine granule composition provided by the present invention can ensure the speed at which the human body obtains pleasure and the duration of nicotine in the human body to maintain a therapeutically effective concentration through the nicotine immediate-release granule and the nicotine sustained-release granule, thereby facilitating smokers to better get rid of the habit of smoking The dependence of the drug has better therapeutic effect compared with similar products sold in the existing market.
Owner:重庆市义力医药科技有限公司
Nicotine formulations and methods of making and using the same
Owner:PHILIP MORRIS PROD SA
Masticatory pattern cigarette alternative food formula
PendingCN112806561AAvoid pollutionAvoid carcinogensPre-extraction tea treatmentFood shapingBiotechnologyNicotine replacements
The invention provides masticatory pattern cigarette alternative food. The raw materials of the alternative food include barbary wolfberry fruit, peach gum, herba rhodiolae, honey-fried licorice root, rose, peppermint, dried tangerine peel and tobacco leaves. The alternative food can be used as nicotine replacement therapy to relieve the onset of a craving for tobacco and can avoid all the associative negative effects brought by smoking.
Owner:中卫清华园生物科技有限公司
Nicotine replacement therapy products containing synthetic nicotine
A composition suitable for use in a nicotine replacement therapy product comprising synthetic nicotine substantially free of one or more contaminants and / or impurities normally associated with tobacco-derived nicotine. For example, the synthetic nicotine is substantially free of nicotine-1'-N-oxide, nicotine diene, nicotine nordiene, 2',3-bipyridine, cotinine, anabacin and / or Or one or more of anatapin. The composition further comprises one or more pharmaceutically acceptable excipients, additives and / or carriers. Nicotine replacement therapy products may comprise any number of such products, including transdermal nicotine delivery patches, nicotine gum, synthetic chewing tobacco, synthetic snus, and synthetic tobacco rods (eg, dissolvable synthetic tobacco). Additionally, methods of treating nicotine addiction include administering a nicotine replacement composition, eg, via a nicotine replacement therapy product, to a user.
Owner:NEXT GENERATION LABS LLC
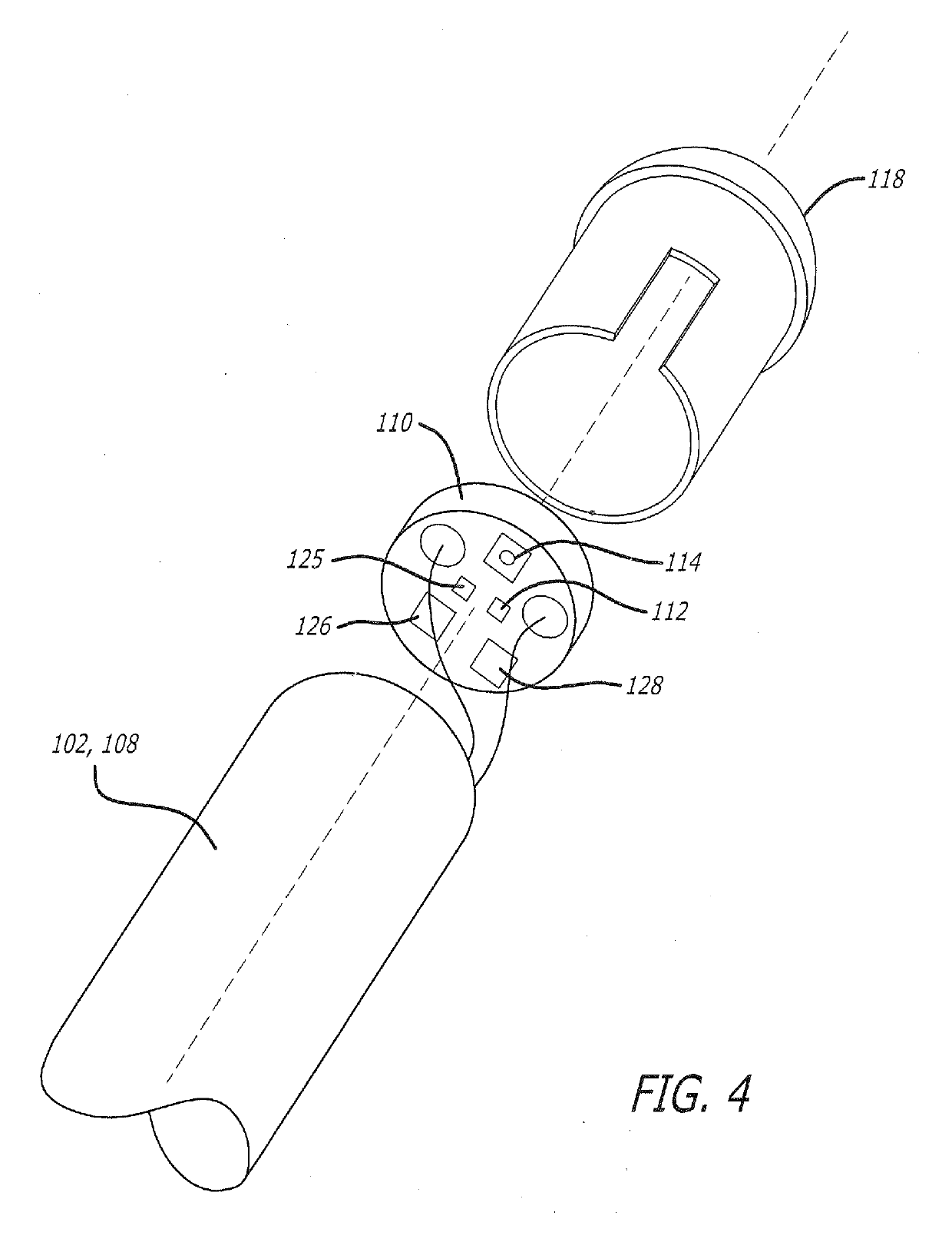
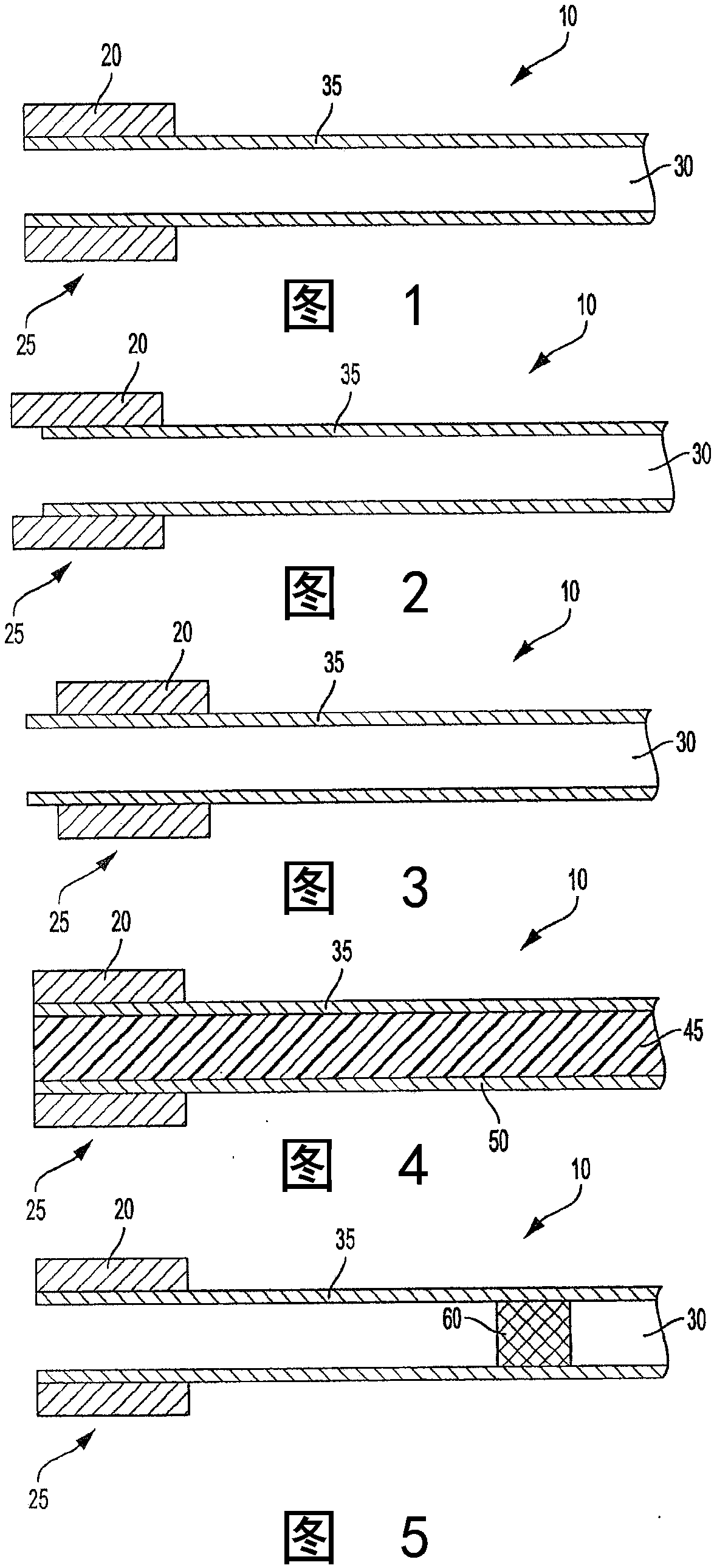
Therapeutic composition and configuration
InactiveCN108024910AOrganic active ingredientsPharmaceutical delivery mechanismNicotine replacementsAlternative treatment
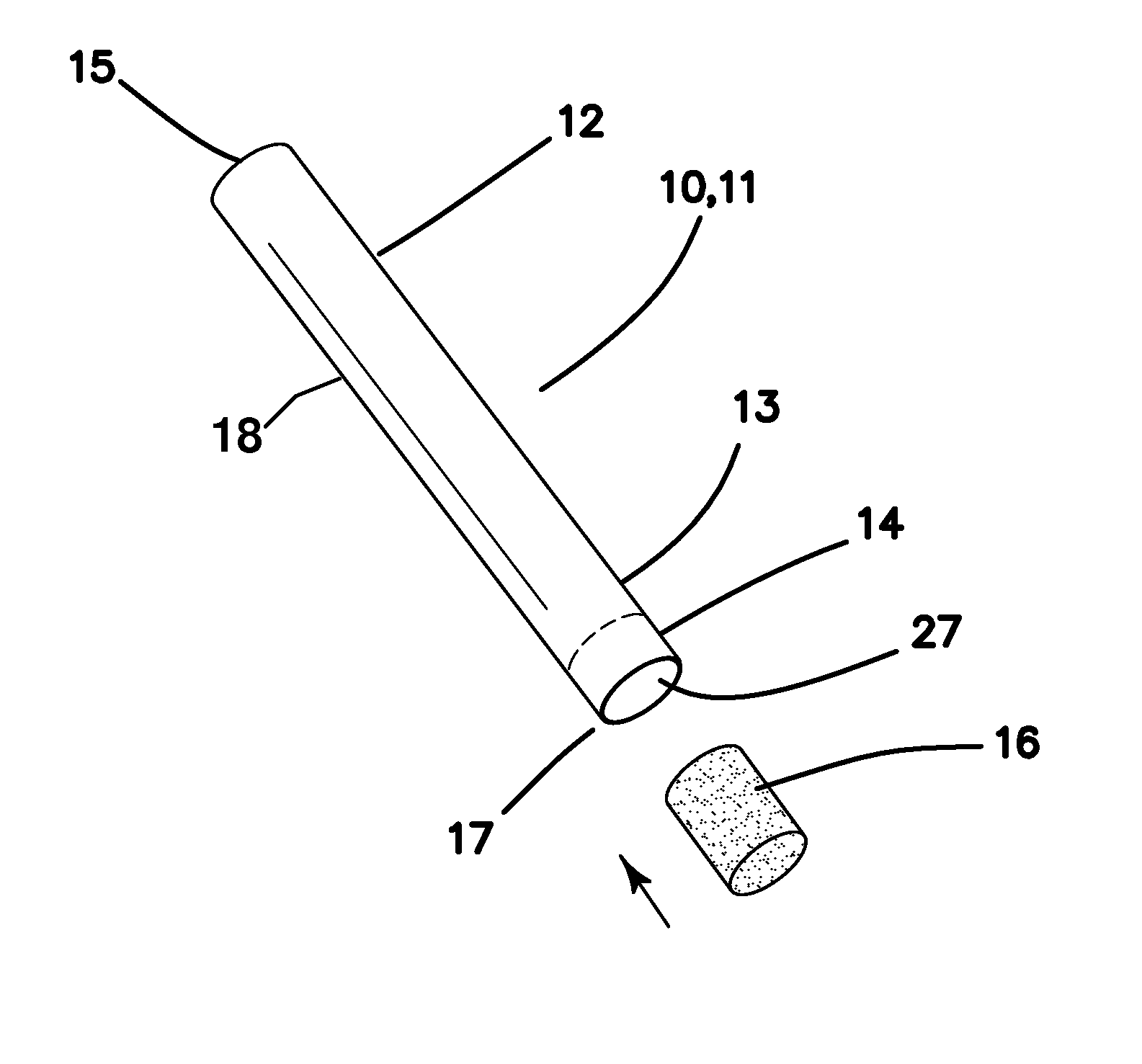
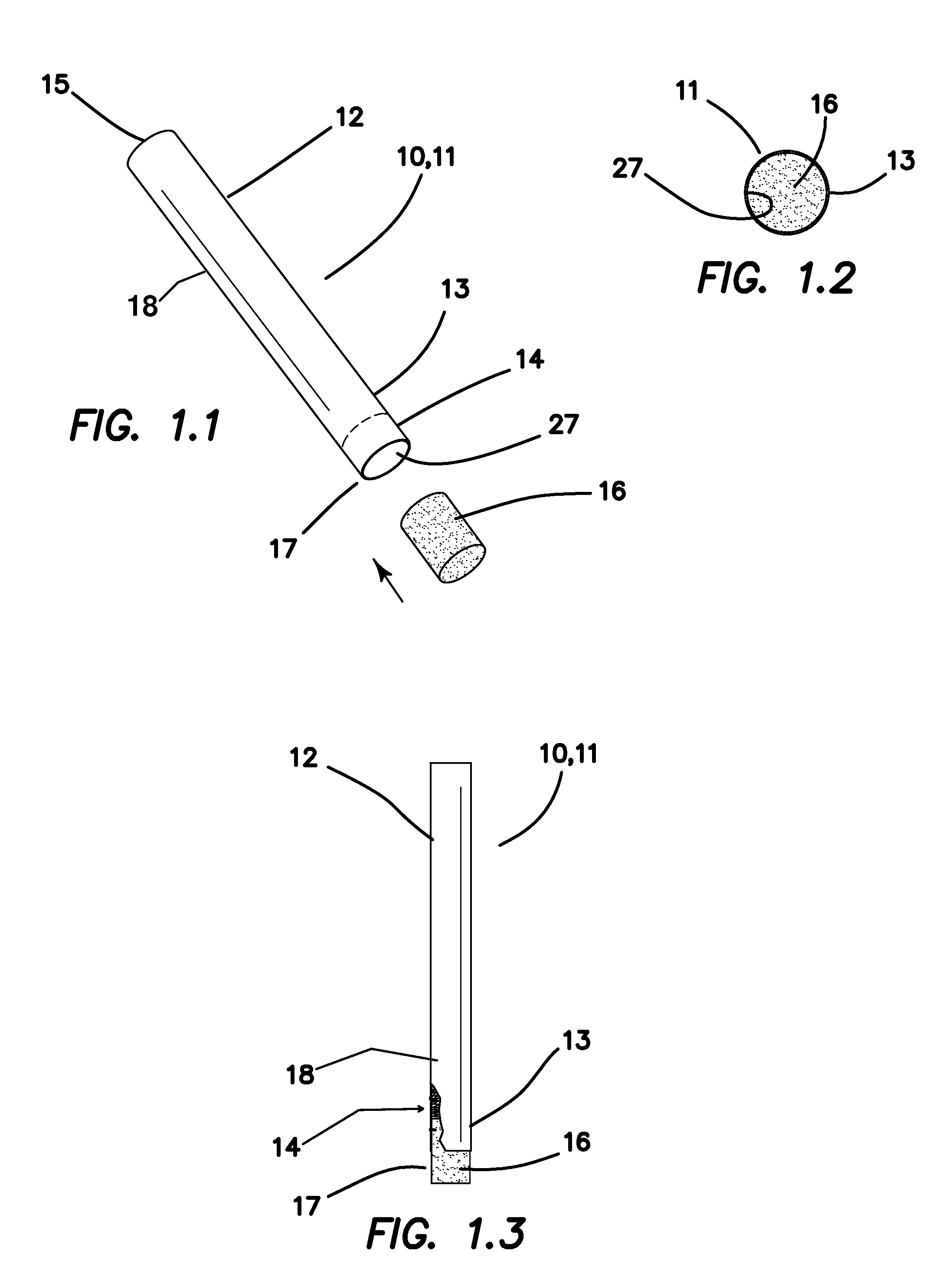
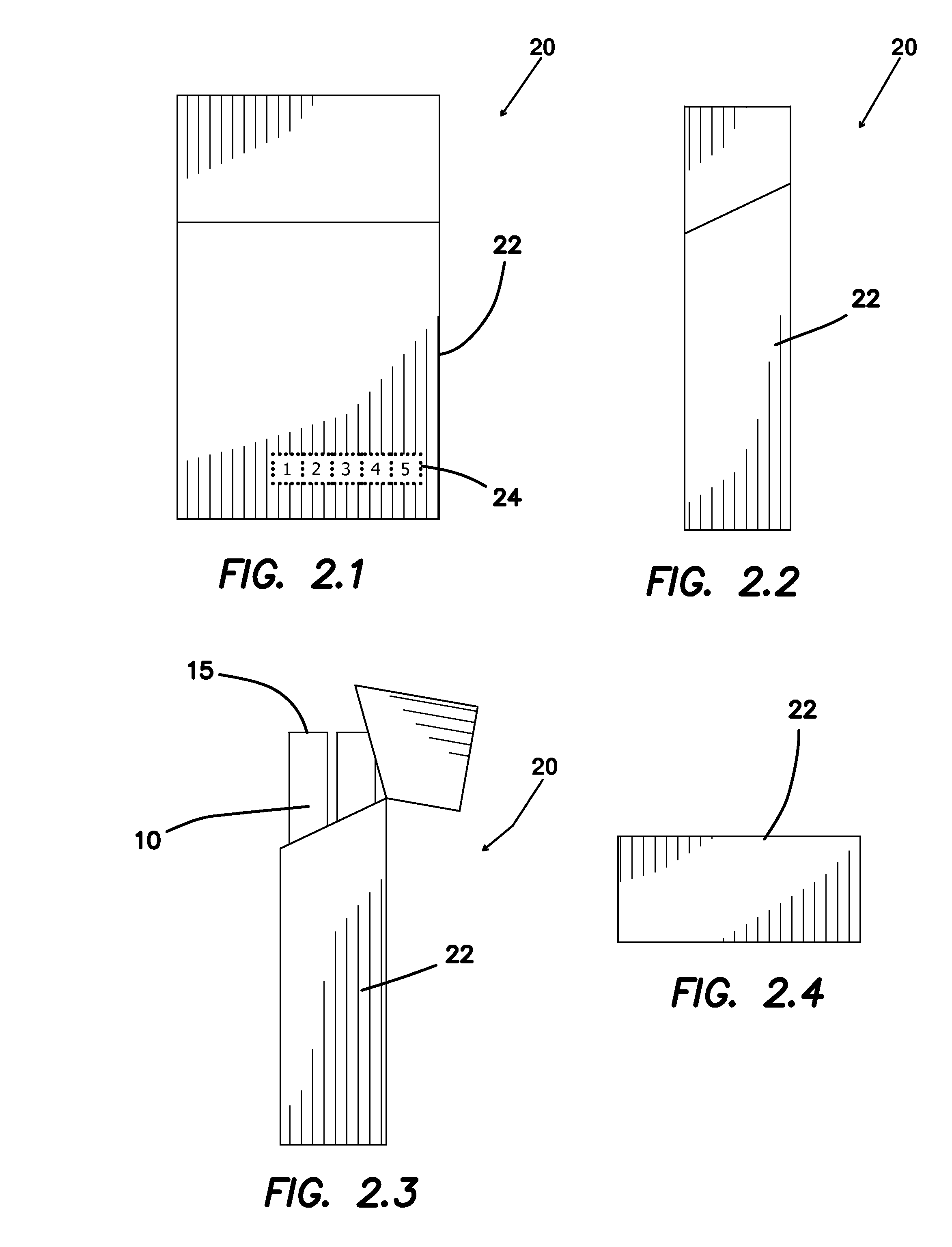
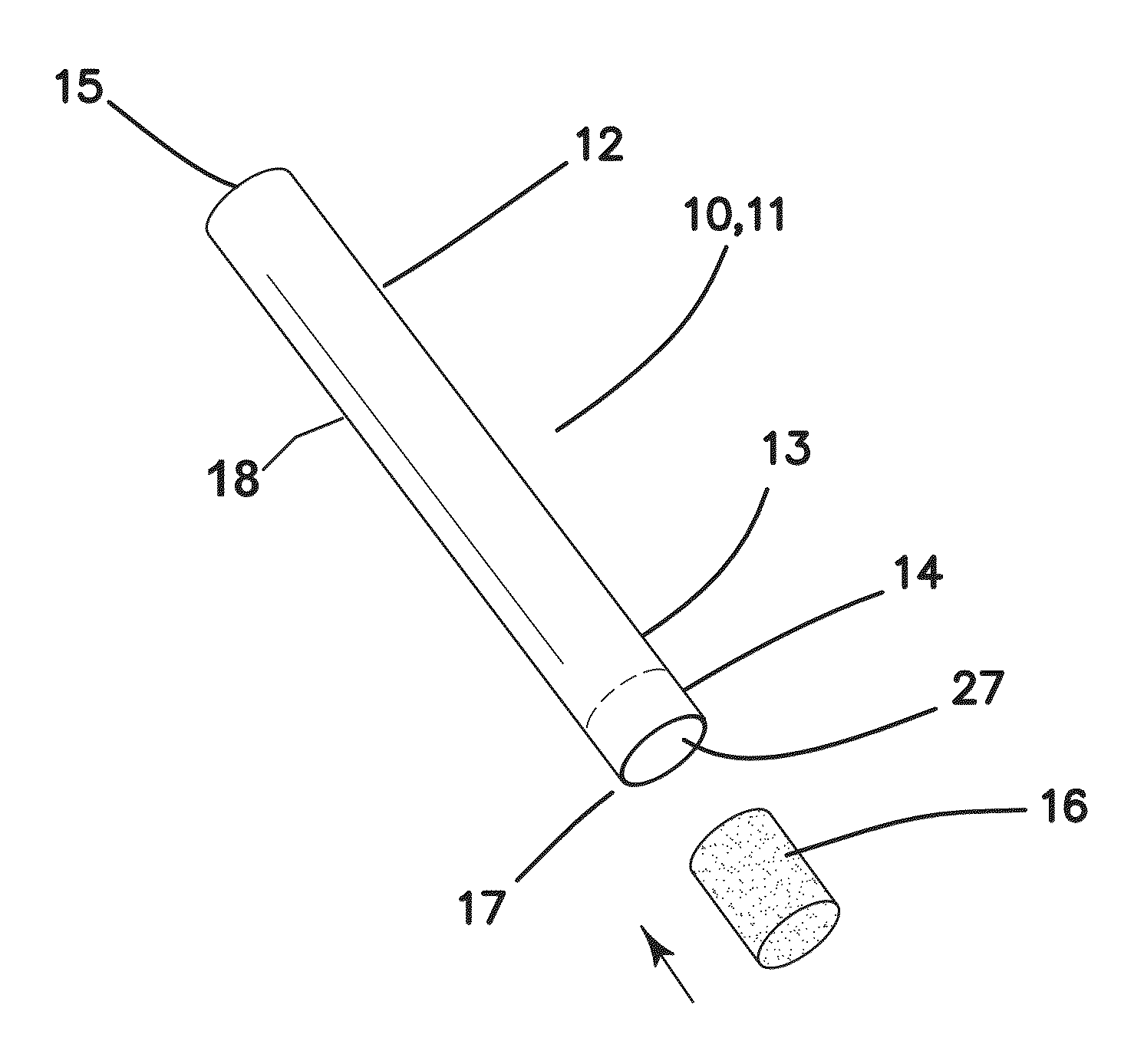
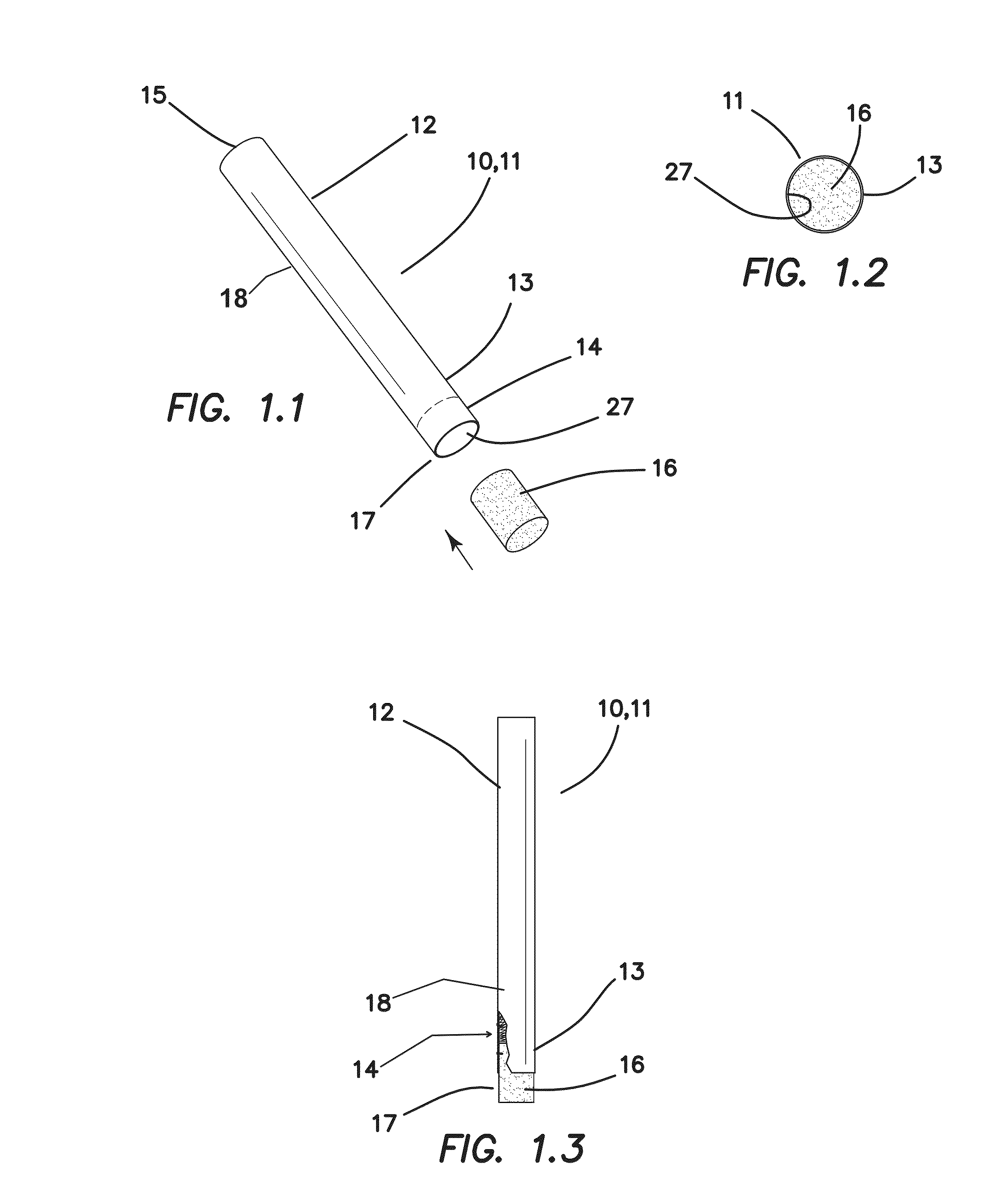
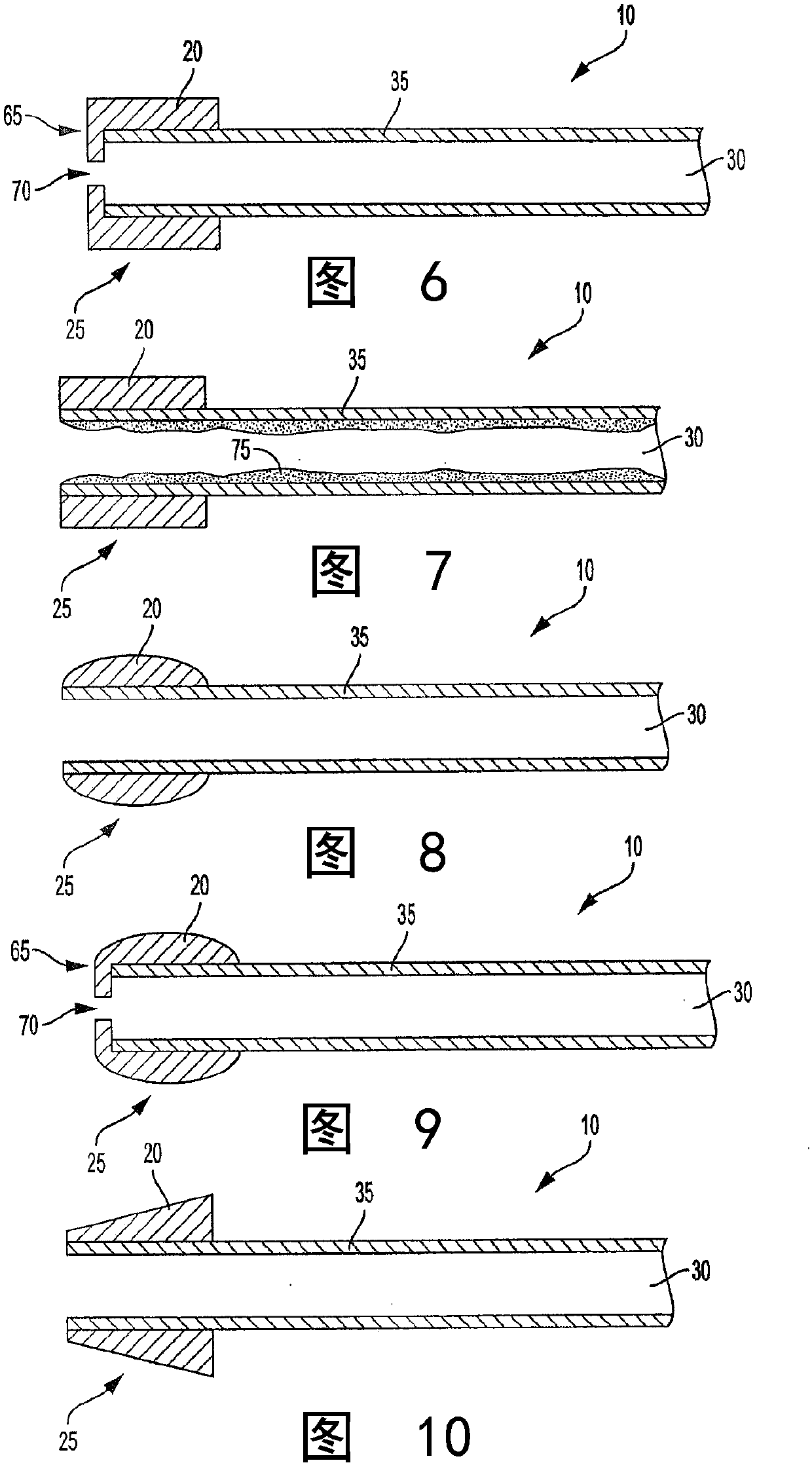
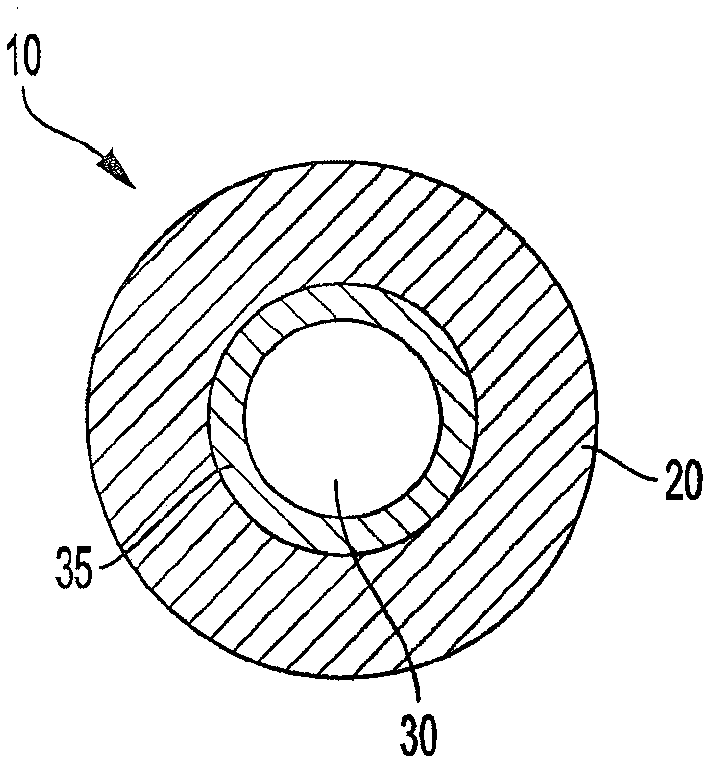
A system intended to be employed for therapeutic purposes incorporates an active ingredient (e.g., a source of nicotine). Representative forms of nicotine include free base (e.g., as a mixture of nicotine and microcrystalline cellulose), a nicotine salt (e.g., as nicotine bitartrate) and nicotine polacrilex. The system preferably comprises a lozenge incorporating the active ingredient, adapted toprovide oral administration of nicotine. The lozenge is in contact with a substrate (e.g., hollow tube) that can be manipulated within the mouth of the user (e.g., the hollow tube can be drawn upon tosimulate the inhalation of cigarette smoke). As such, the active ingredient is administered and the user is able to experience certain other physiological sensations. The composition is useful for treatment of central nervous system conditions, diseases, and disorders, and can be used as a nicotine replacement therapy. The system comprising: a substrate portion (35) having an upstream end and a downstream end, the upstream end allowing for passage of drawn atmospheric air into the substrate and the downstream end adapted for positioning into a user's mouth for draw upon the substrate and inhalation of atmospheric air by the user, a lozenge portion (20) incorporating a source of active ingredient in a pharmaceutically acceptable form, the lozenge portion providing for oral ingestion of theactive ingredient, the lozenge portion and the substrate portion being physically separate from one another but in contact with each other, the lozenge being positioned at the downstream end of the substrate, and the lozenge and substrate portions being positioned so that the lozenge portion and a portion of the substrate portion can be located in the user's mouth during use, to provide for delivery of active ingredient from the lozenge and drawn air through the substrate.
Owner:NICONOVUM USA
Nicotine-containing chewing gum compositions
PendingCN110621307ASatisfy the desireOrganic active ingredientsNervous disorderNicotine replacementsChewing gum
The present invention relates to nicotine-containing chewing gum compositions and the method of preparing the same. The present invention provides improved and stable nicotine-containing chewing gum compositions for use in nicotine replacement therapy, which provide rapid nicotine release in the oral cavity and effectively satisfy the craving that most smokers experience.
Owner:ENORAMA PHARMA AB
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com