Methods for nicotine replacement dosage determination
a nicotine replacement and dosage technology, applied in the field of smoking cessation assistance, can solve the problems of patient relapse, inability to accurately predict the nicotine replacement level of blood, and inability to adapt the nicotine transdermal replacement dosage to previous daily cigarette consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
Materials and Methods
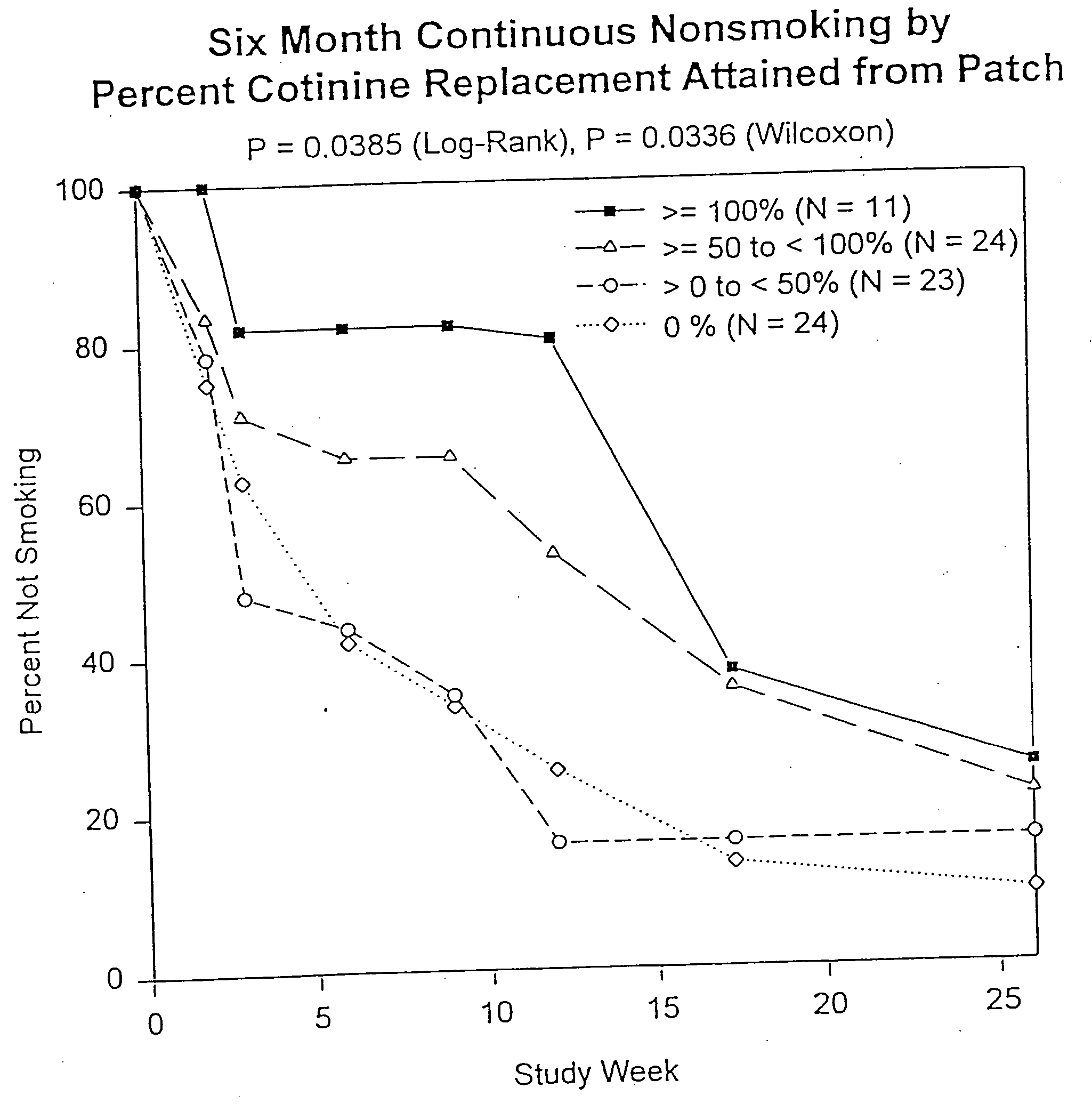
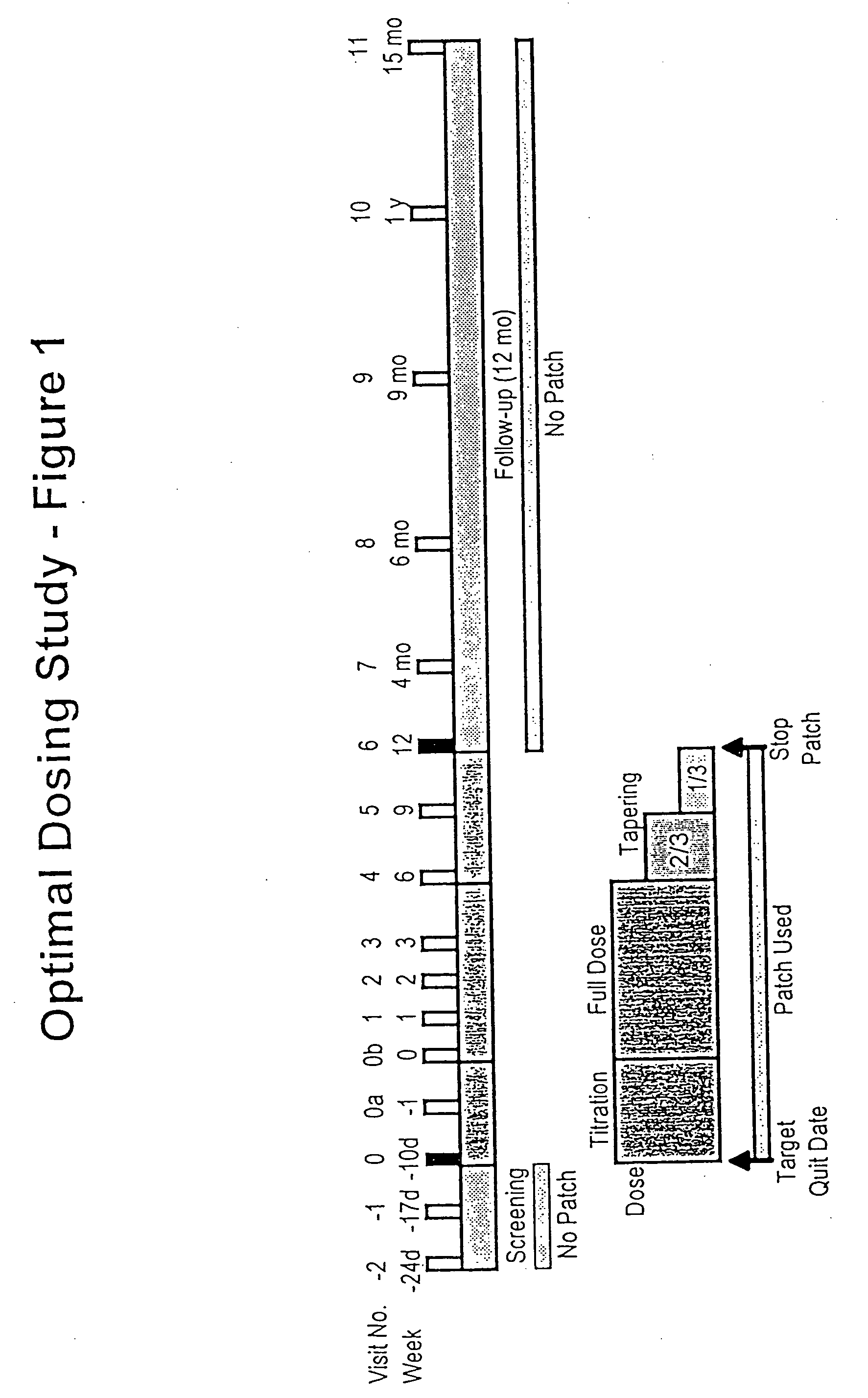
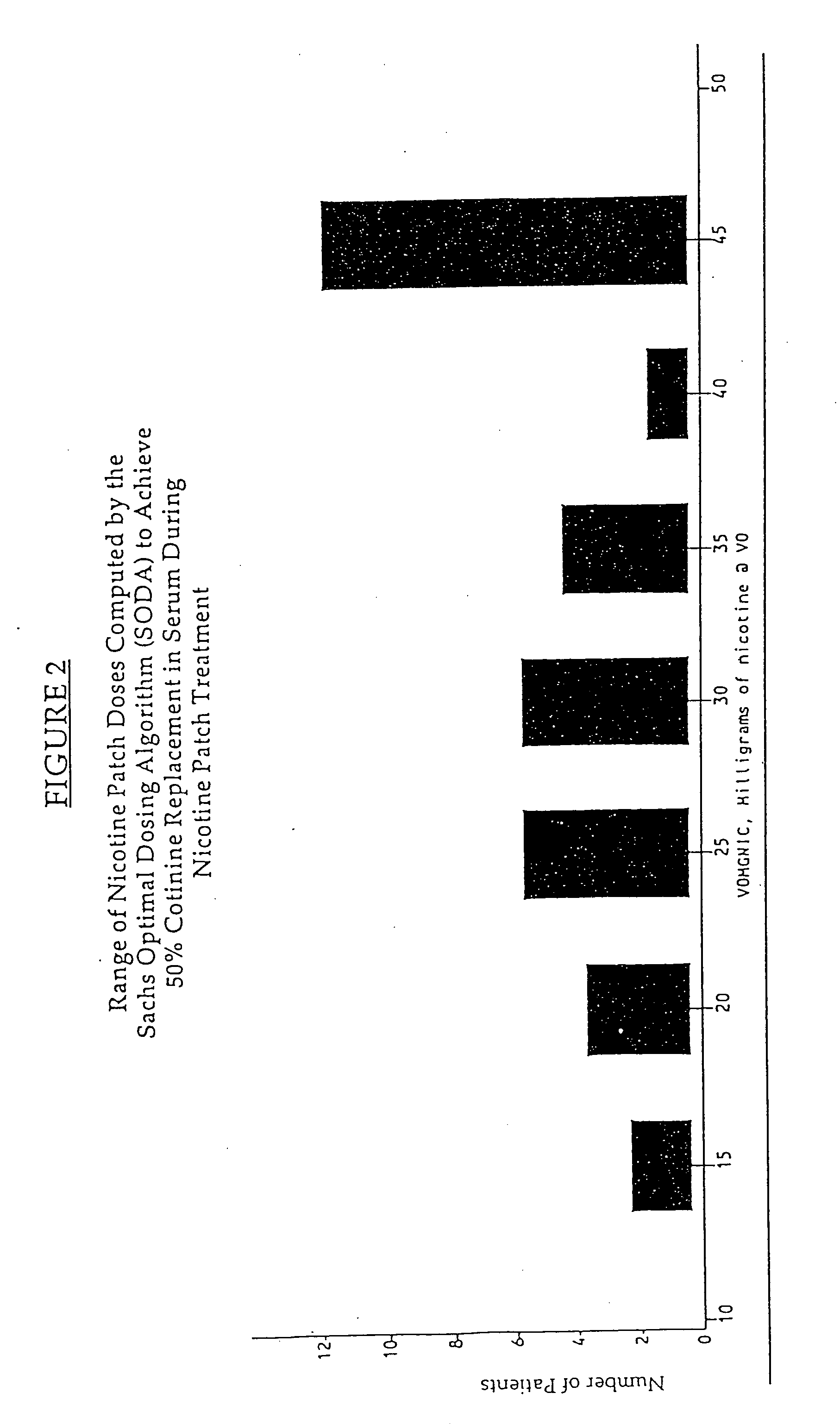
[0029] 1. Study Design
[0030] Subject characteristics. Subjects were smokers, aged 18 years or more, were eligible if they had smoked at least 10 cigarettes per day for a minimum of 3 years, were motivated to stop smoking completely, and were basically in good health. Exclusion criteria were severe or symptomatic cardiovascular disease, pregnancy or breast feeding, current regular use of psychotropic medications, current or past alcohol or other drug abuse, current use of smokeless tobacco, or chronic dermatological disorders such as psoriasis, urticaria, or chronic dermatitis.
[0031] Allocation to Treatment. Subjects were sequentially and randomly assigned to receive one of three treatments based on a target percent cotinine replacement (% Cot Repl): 0% (placebo), 50%, or 100% cotinine replacement. In addition, subject enrollment was stratified by sex and nicotine dependency according to Table 1. Ninety-one subjects were assigned into the matrix shown in Table 1 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| half life | aaaaa | aaaaa |
| half life | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com