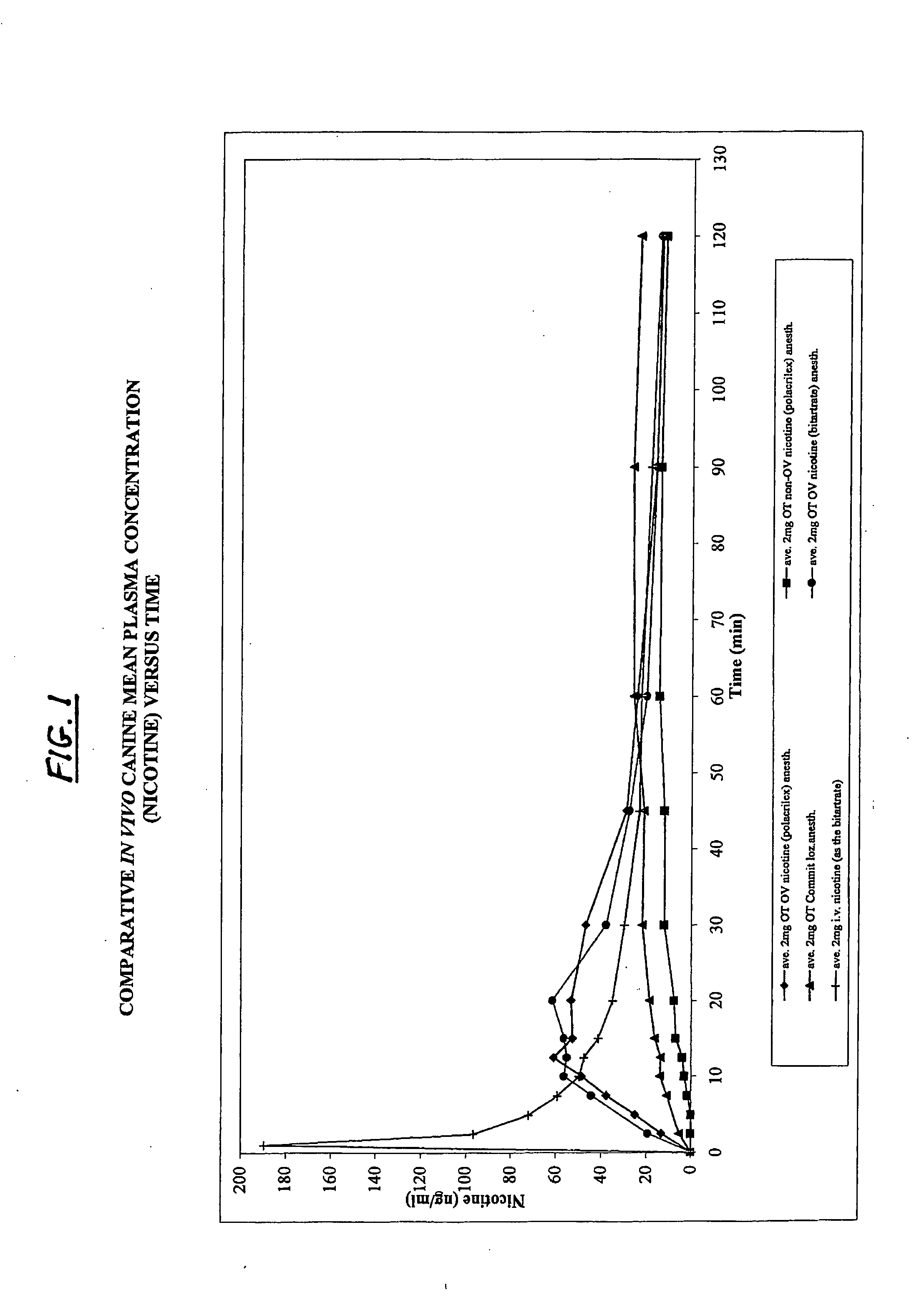
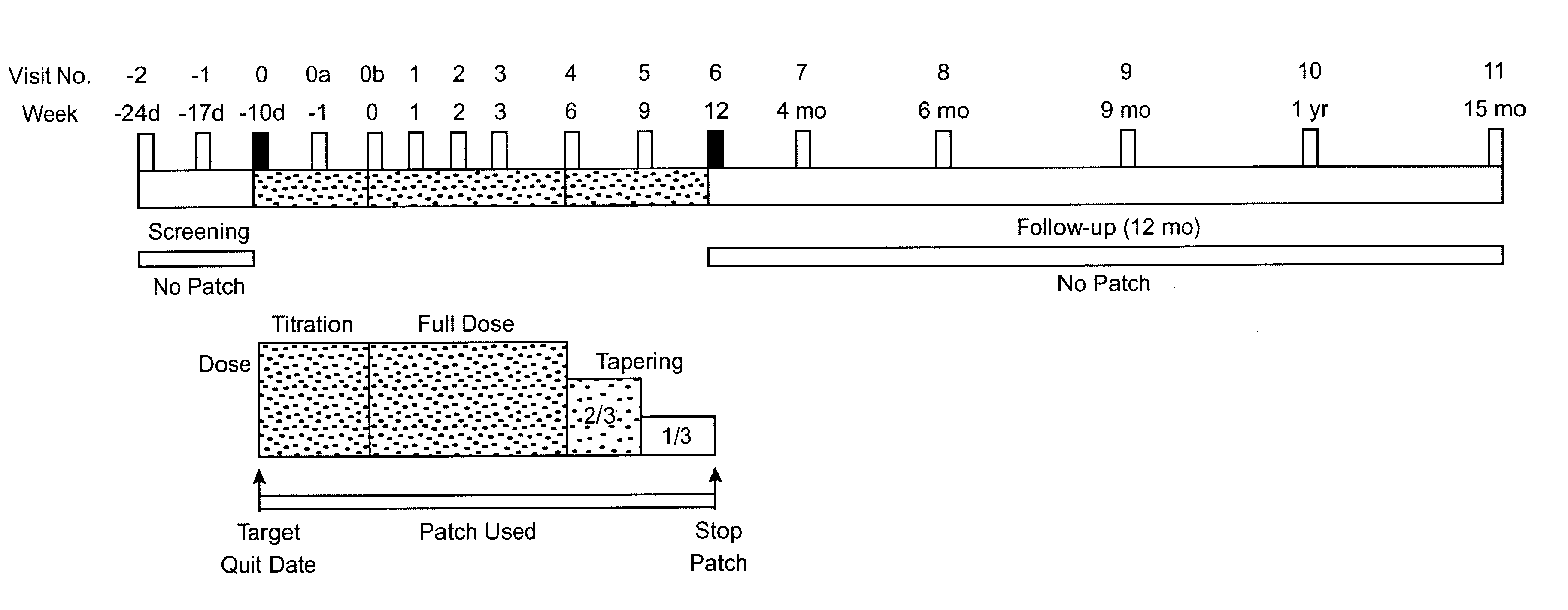
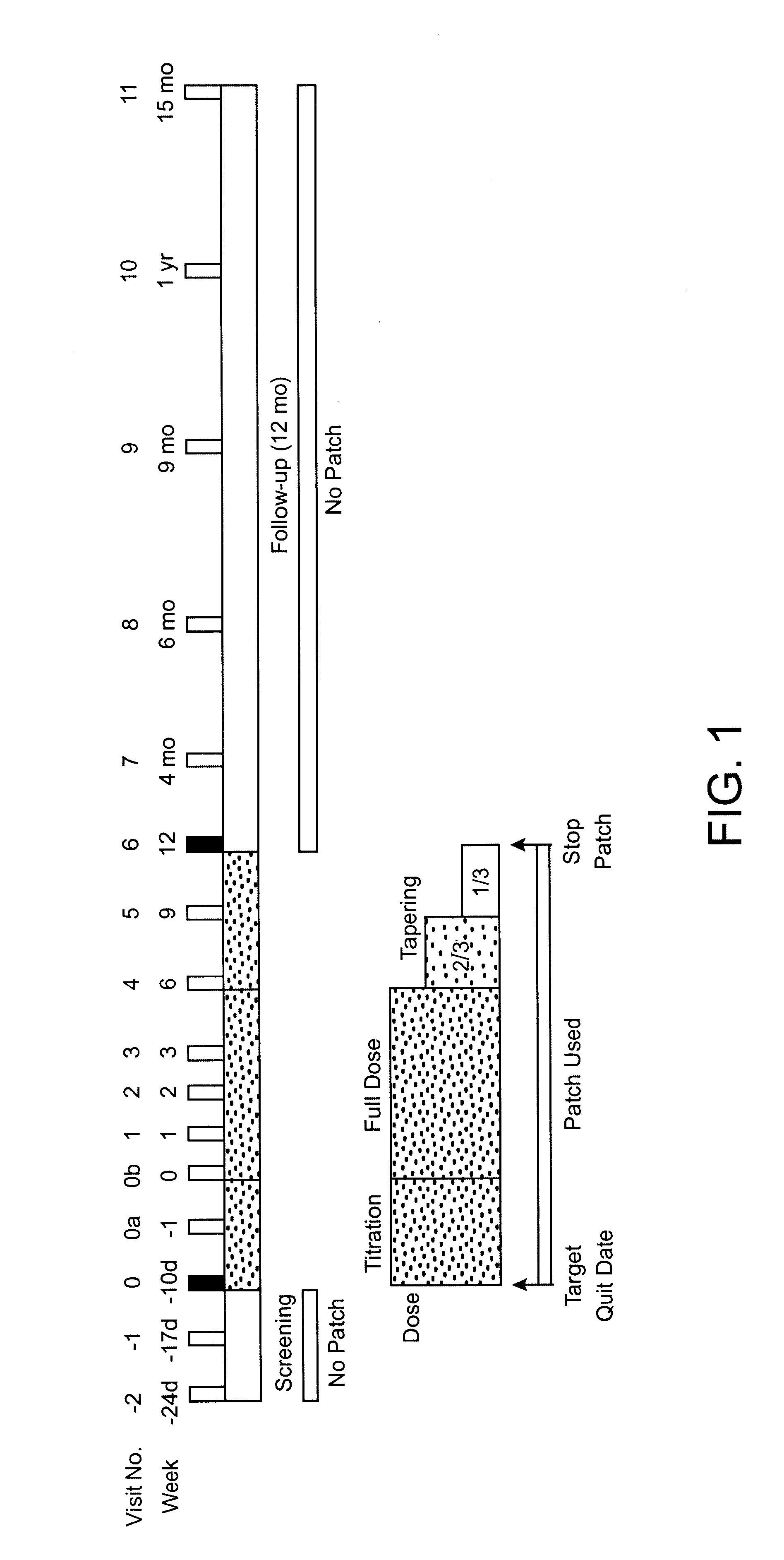
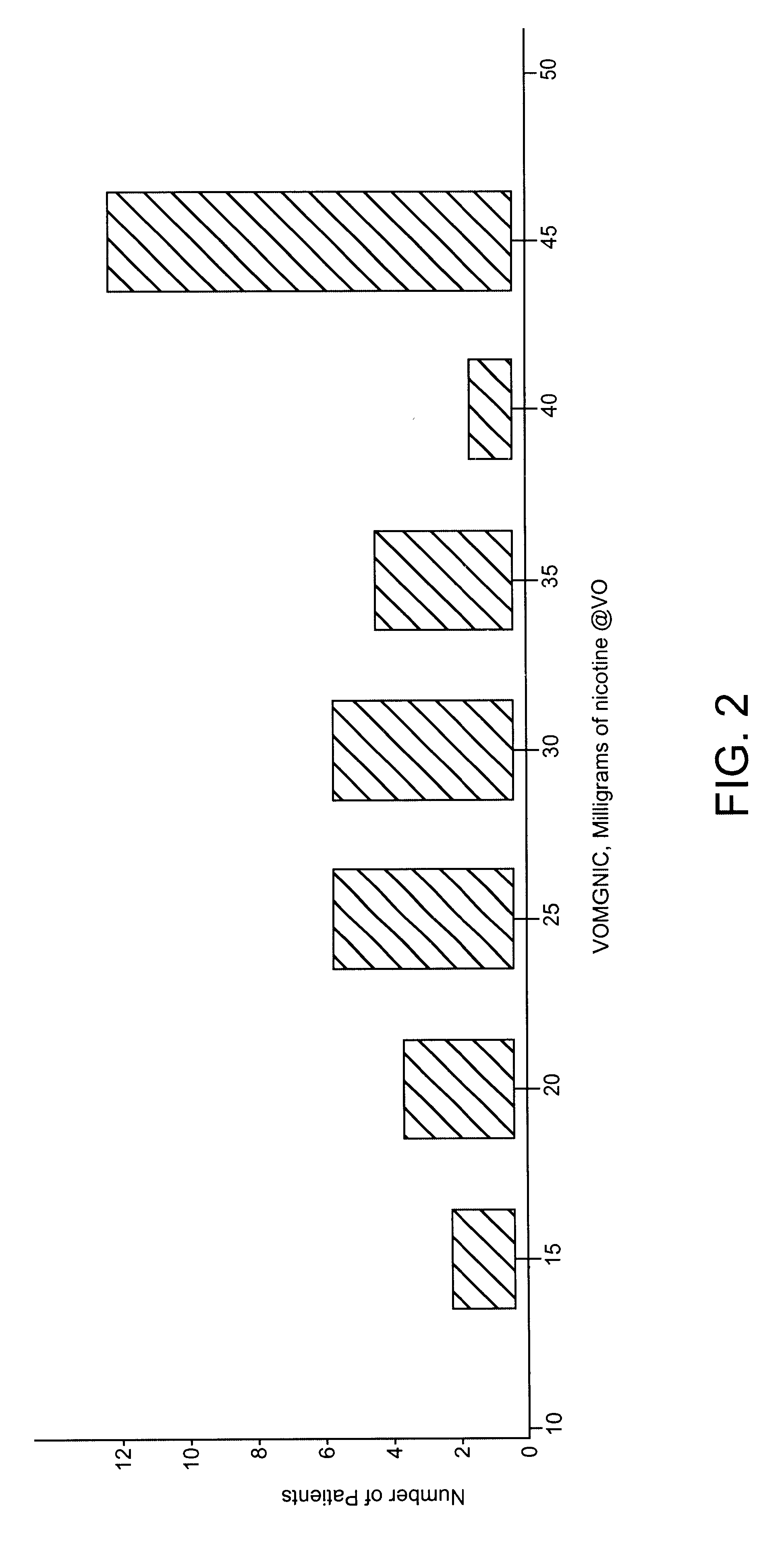
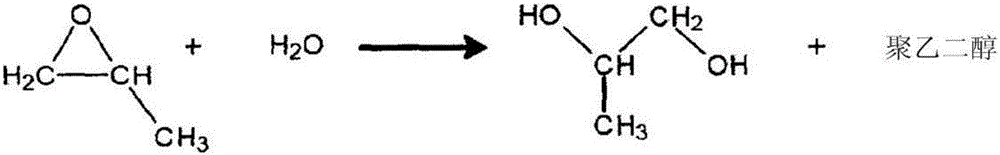
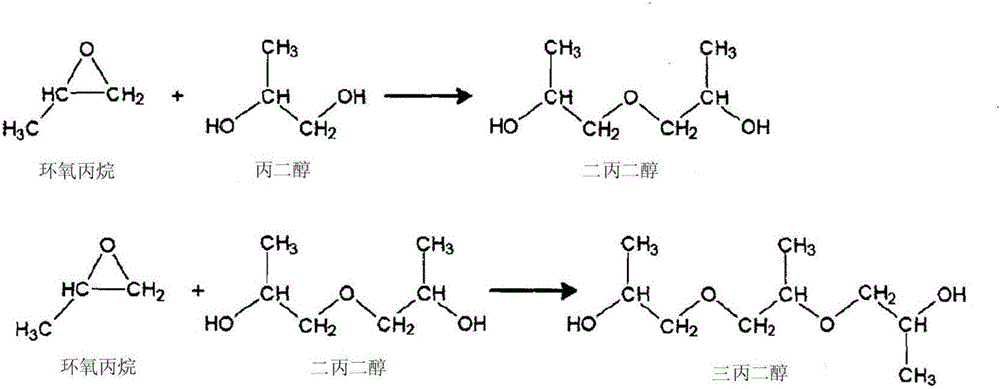
Patents
Literature
32 results about "Nicotine replacement" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
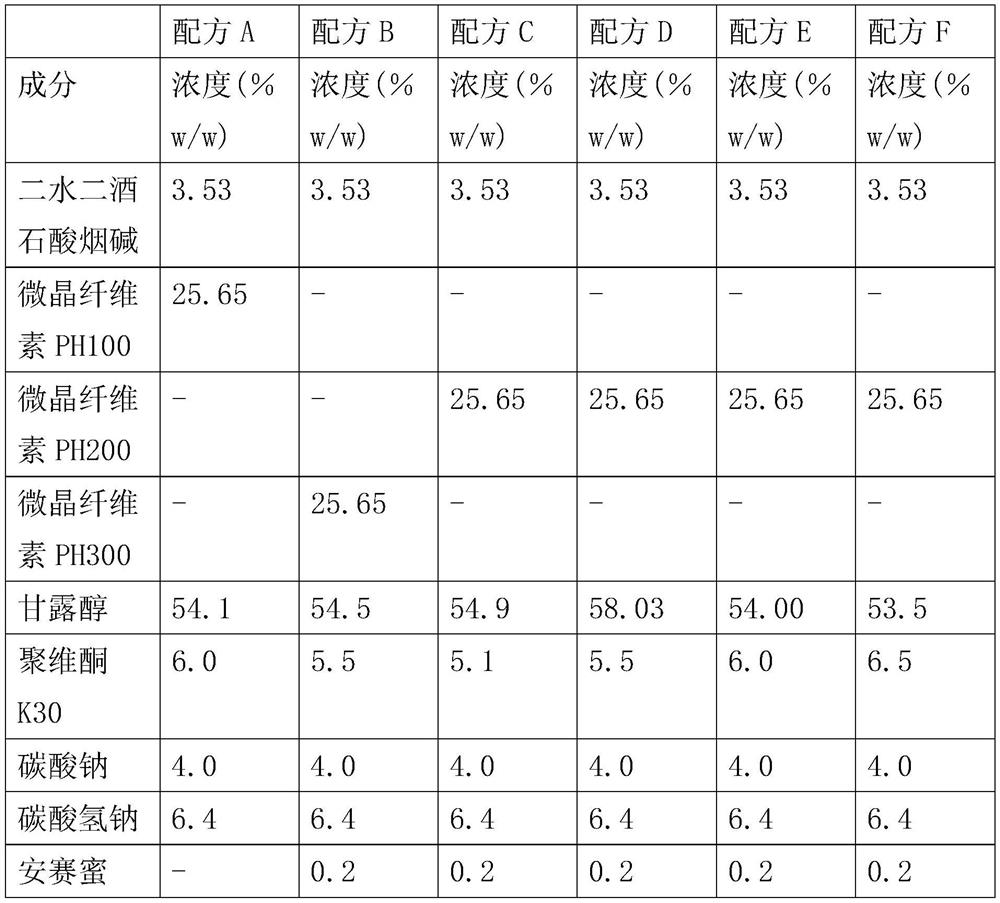
Nicotine replacement therapy (NRT) is the most commonly used family of quit smoking medications. NRT reduces withdrawal feelings by giving you a small controlled amount of nicotine─but none of the other dangerous chemicals found in cigarettes. This small amount of nicotine helps satisfy your craving for nicotine and reduces the urge to smoke.
Nicotine-containing pharmaceutical compositions giving a rapid transmucosal absorption
Formulations of nicotine for use in nicotine replacement therapy. The formulations are intended for application in the oral cavity where upon the uptake of nicotine mainly takes place through the buccal mucosa. The formulations essentially comprise apolar, polar and surface-active components. The formulations may be administered in combination with other nicotine formulations.
Owner:MCNEIL AB
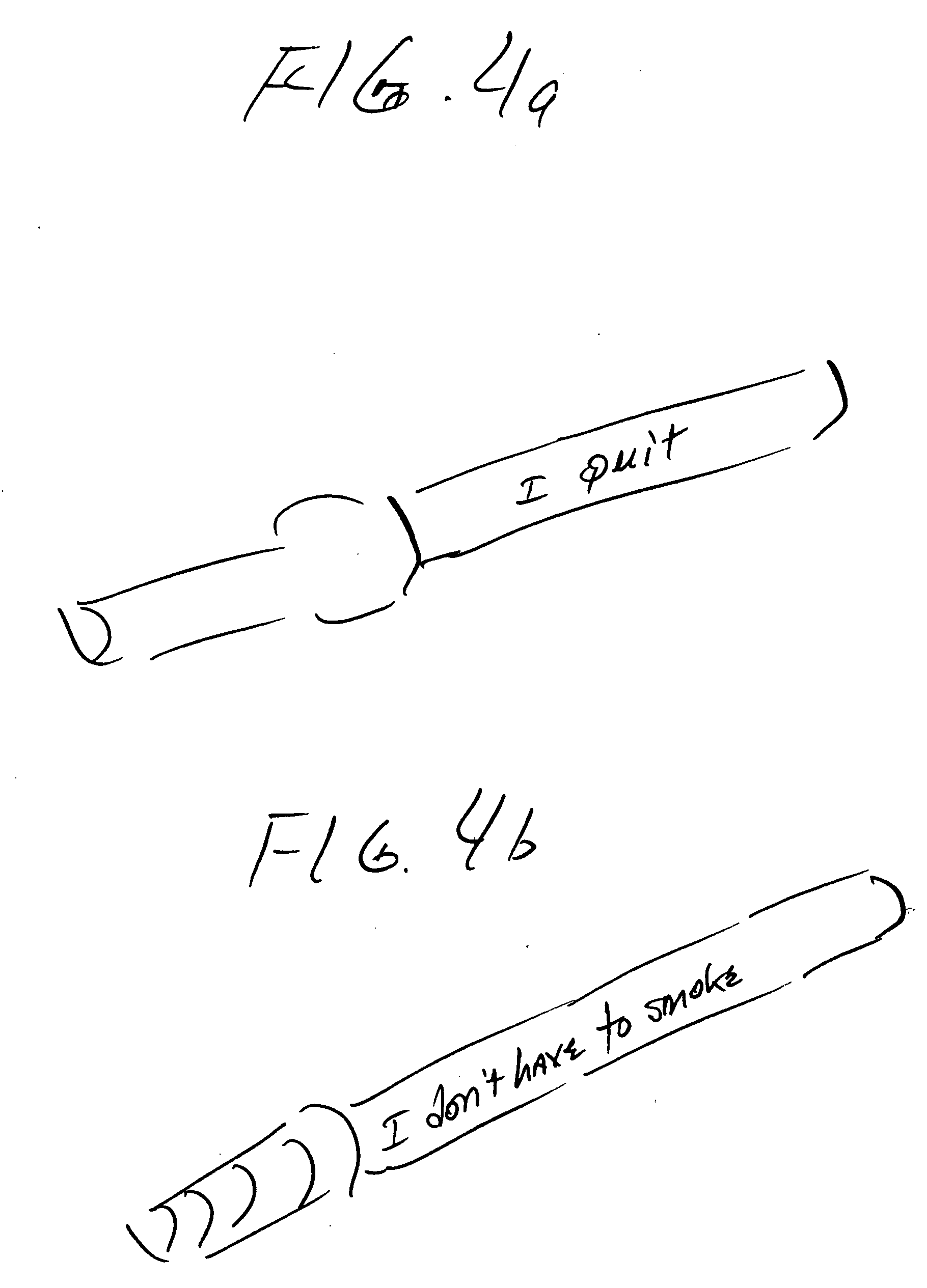
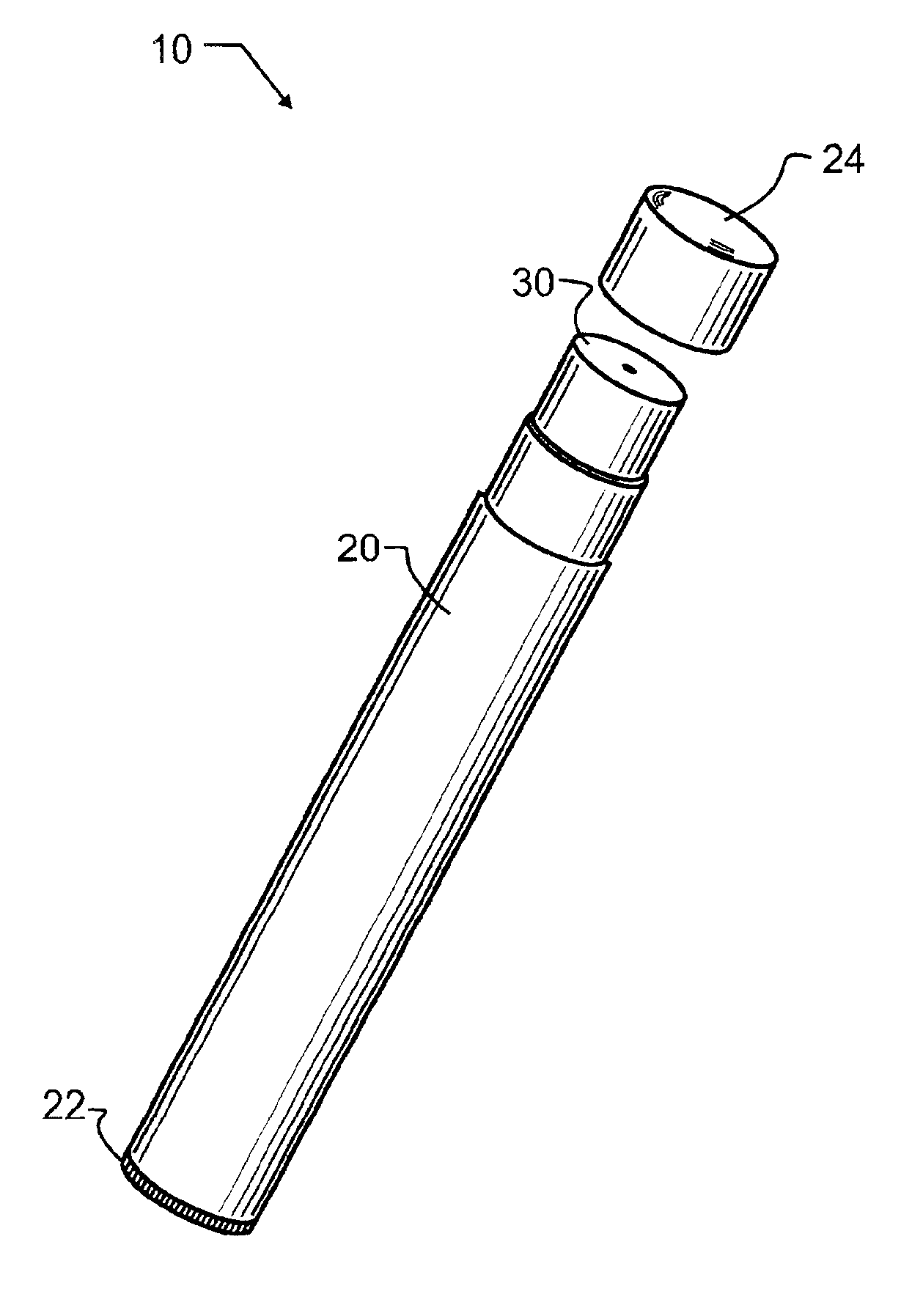
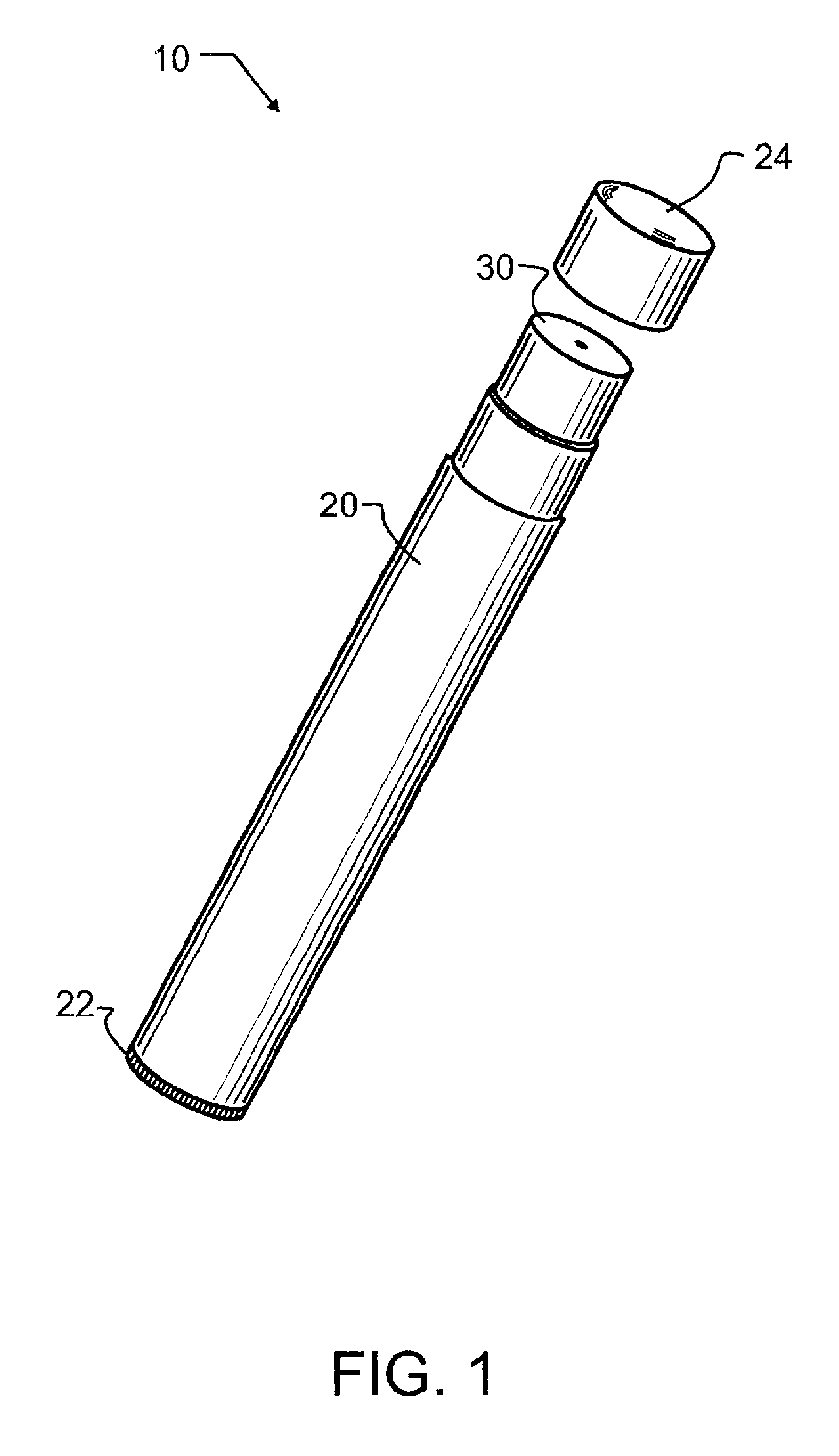
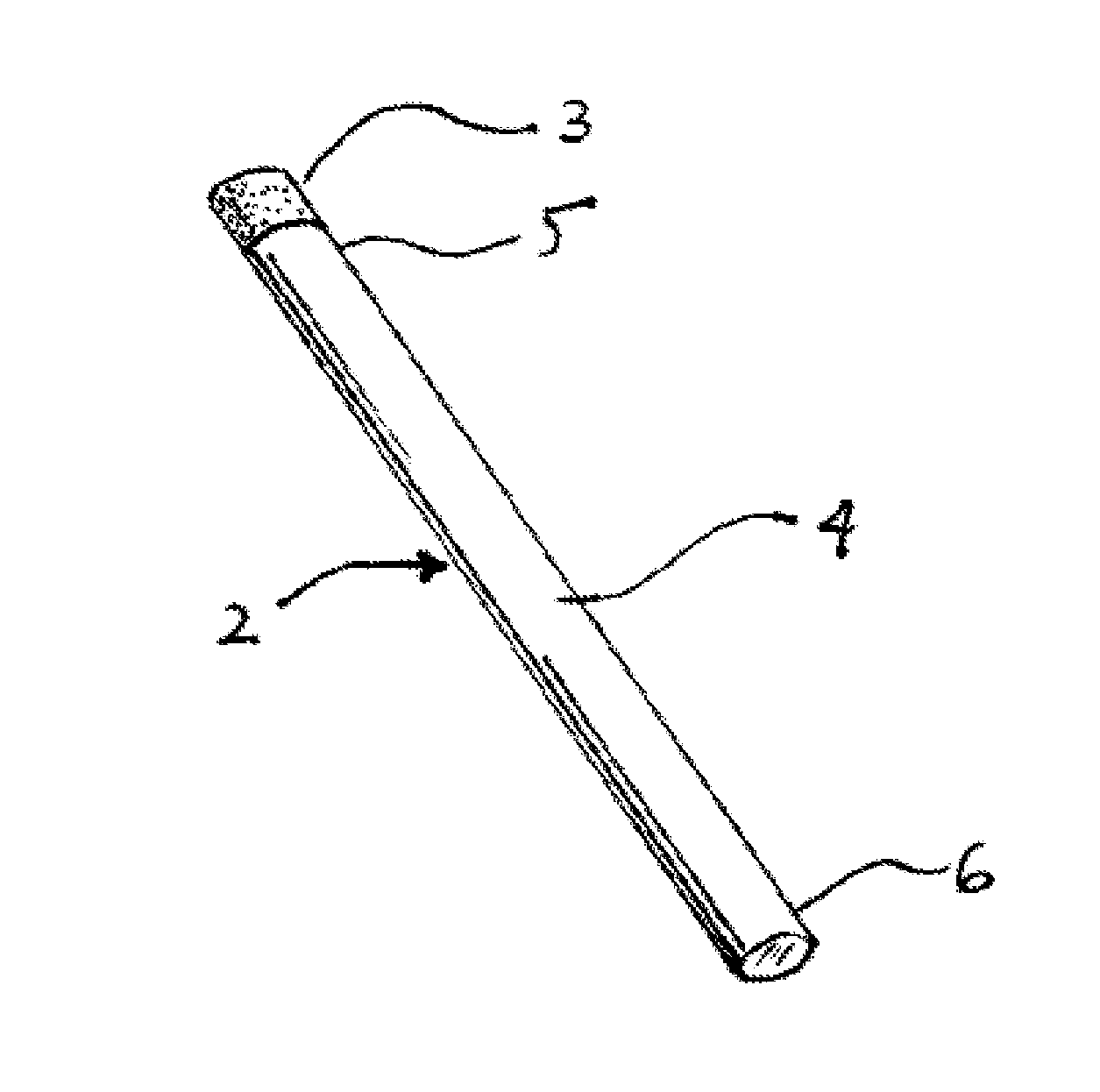
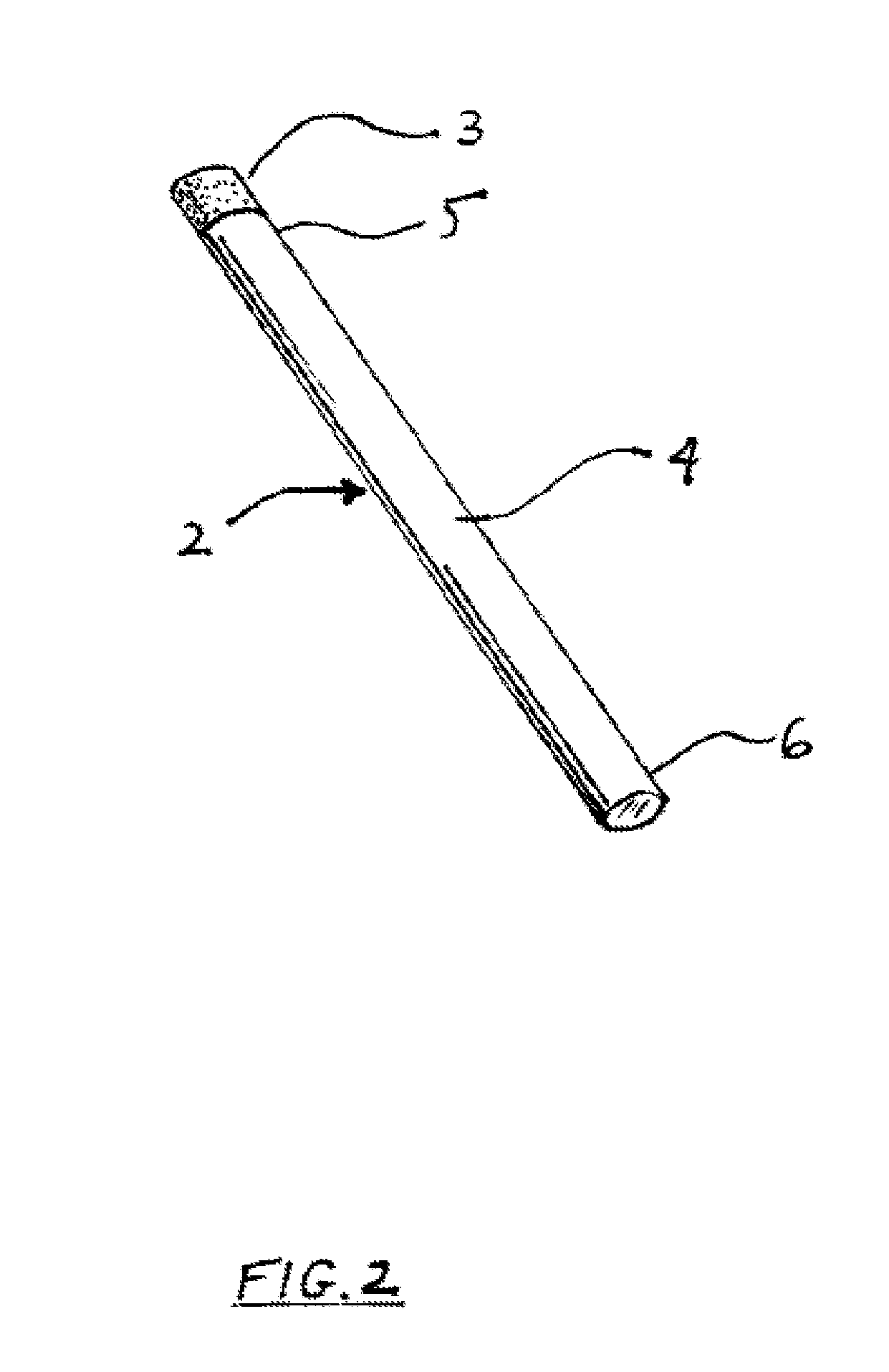
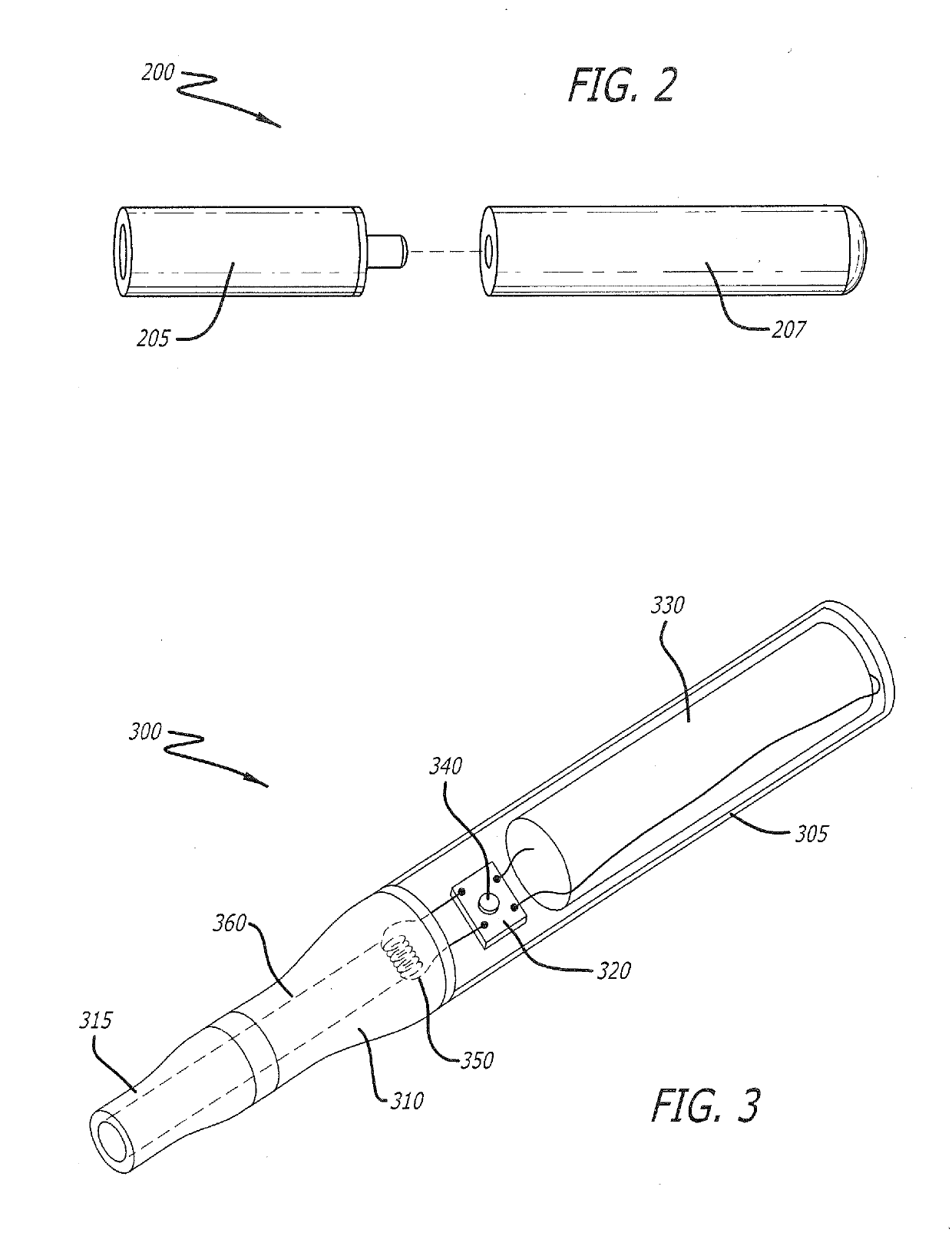
Smoking cessation devices, methods of use and methods of conducting business therewith
InactiveUS20050236006A1Eliminate requirementsCigar manufactureTobacco devicesEnvironmental healthNicotine replacement
The present invention provides several smoking and tobacco use cessation devices and methods which entirely eliminate the requirement of ignition and burning of substances which emit harmful substances and vapors while simultaneously providing therapeutically efficacious amounts of nicotine and / or nicotine substitutes, and optionally tobacco aroma and taste and / or other undesirable habit inhibiting compounds and antagonists and other desirable orally ingestible substances.
Owner:CHROMAGLASS
Nicotine replacement applique
InactiveUS7105173B1Easy to carryWithout adverse cosmetic effectCosmetic preparationsImpression capsNicotine replacementsCosmetic ingredient
Nicotine in base or salt form combined with cosmetic ingredients to form a lip balm or similar stick. The stick is readily dispensed not only onto a person's lips but also may be used on the skin, for example on the wrist. Packaging the nicotine this way enables a smoker to obtain nicotine discretely anywhere in public, without offending others by smoking. Furthermore, the dosage is readily controlled, and the person's hands and mouth are active, similar to the lighting and smoking of a cigarette.
Owner:ROLLING KENNETH J
Nicotine-alternative compositions and methods of producing such compositions
InactiveUS20060204598A1Eliminate needLess-expensive to produceOrganic active ingredientsBiocideNicotine replacementAlkaloid
A method for producing a consumable nicotine-alternative composition includes measuring a quantity of a cigarette nicotine-alternative alkaloid and / or a larger quantity of lobeline. The quantities of cigarette nicotine-alternative alkaloid and / or lobeline are diluted into one or more successive intermediate solutions, a last of which constitutes a final solution. The final solution is apportioned so that each portion contains a precise quantity of cigarette nicotine-alternative alkaloid and / or lobeline appropriate for consumption in a single use by a single person. Each portion is introduced into a separate single-serving dispenser.
Owner:THOMPSON MARSHALL ANLAUF
Modifying taste and sensory irritation of smokeless tobacco and non-tobacco products
ActiveUS20140271946A1Reduce and eliminate sensory irritationLess irritatingTobacco preparationBiocideNicotine replacementsTRPA1 Channel
Tobacco products comprising smokeless tobacco products and active ingredients, including those that antagonize nicotinic acetylcholine receptors, the TRPV1 channel, and / or the TRPA1 channel are disclosed. Nicotine replacement therapies comprising active ingredients, including those that antagonize nicotinic acetylcholine receptors, the TRPV1 channel, and / or the TRPA1 channel. The active ingredient may reduce or eliminate sensory irritation arising due to use of the product. Analgesic compositions comprising active ingredients. Methods of reducing taste and sensory irritation by employing an active ingredient.
Owner:AKRIA CLIENT SERVICES LLC
Tobacco product labeling system
InactiveUS20060237024A1Reducing nicotine contentLess nicotineTobacco preparationTobacco treatmentTobacco Use CessationTobacco-specific nitrosamines
The disclosed invention relates to visual content indicators of a tobacco product. More specifically, the invention includes labeling systems that provide at least one visual indicator of a tobacco product, methods for using the systems, and tobacco products that display one or more visual indicators of content. The present invention generally relates to tobacco products, which have a reduced amount of nicotine and tobacco specific nitrosamines (TSNAs), and contain one or more nicotine substitutes. The use of these tobacco products to reduce the exposure of an individual to nicotine, as well as, facilitating tobacco-use cessation is disclosed.
Owner:VECTOR TOBACCO LLC
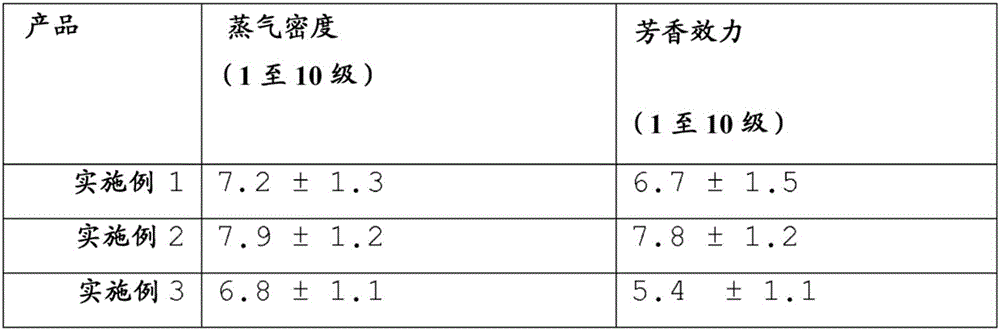
Use of a composition containing 1,3-propanediol as e-liquid
InactiveUS20160262443A1Improve bioavailabilityReinforce aromatic powerTobacco treatmentTobacco devicesElectronic cigarette1,3-Propanediol
A composition containing 1,3-propanediol for use as electronic cigarette liquid. A liquid composition for an electronic cigarette, including 1,3-propanediol, as well as at least one compound selected from nicotine, a nicotine substitute, and an aroma, and an electronic cigarette containing this composition.
Owner:LAB CERES
Tobacco product labeling system
InactiveUS20060243290A1Tobacco preparationTobacco treatmentBiotechnologyTobacco-specific nitrosamines
The disclosed invention relates to visual content indicators of a tobacco product. More specifically, the invention includes labeling systems that provide at least one visual indicator of a tobacco product, methods for using the systems, and tobacco products that display one or more visual indicators of content. The present invention generally relates to tobacco products, which have a reduced amount of nicotine and tobacco specific nitrosamines (TSNAs), and contain one or more nicotine substitutes. The use of these tobacco products to reduce the exposure of an individual to nicotine, as well as, facilitating tobacco-use cessation is disclosed.
Owner:VECTOR TOBACCO LLC
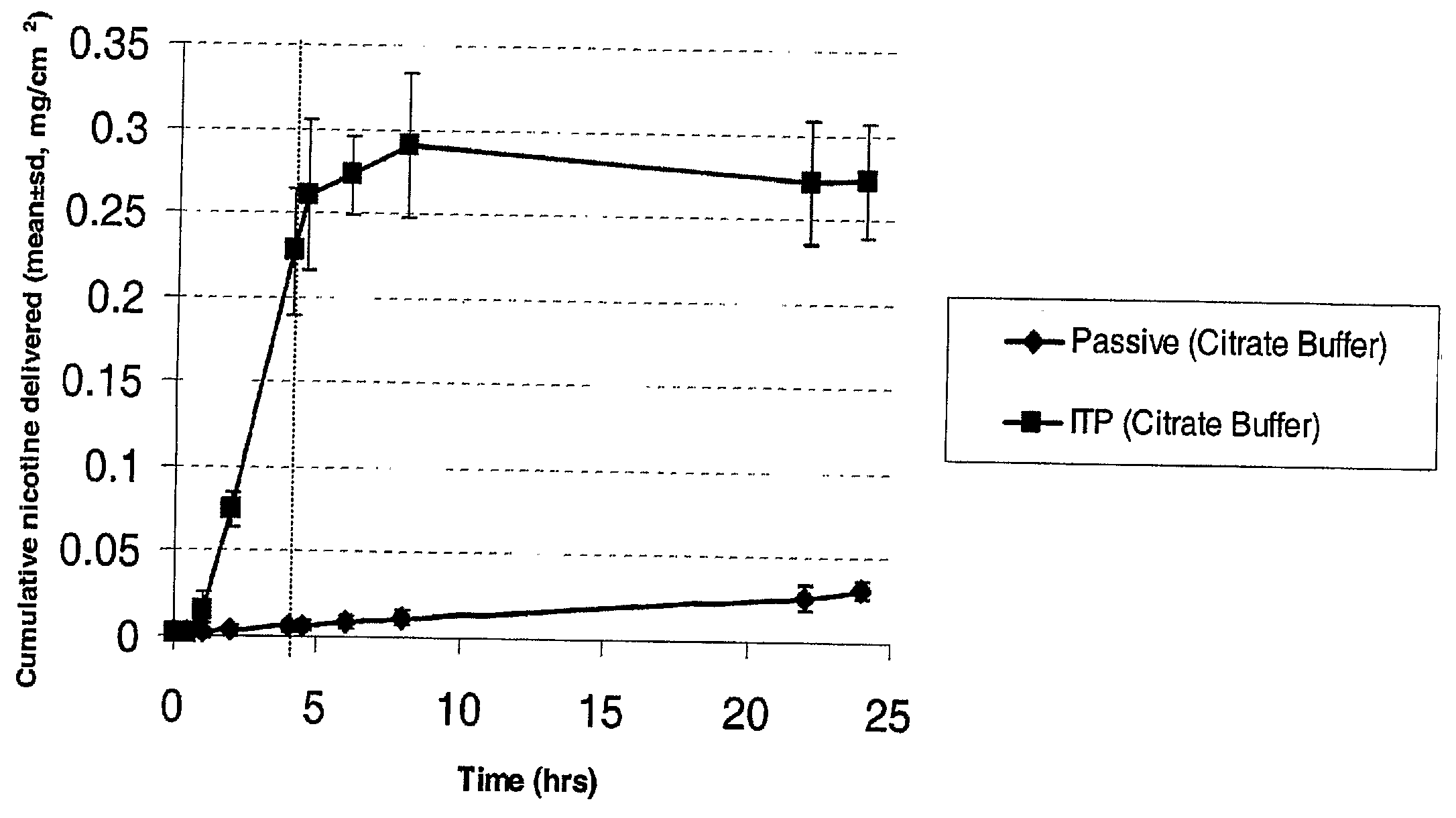
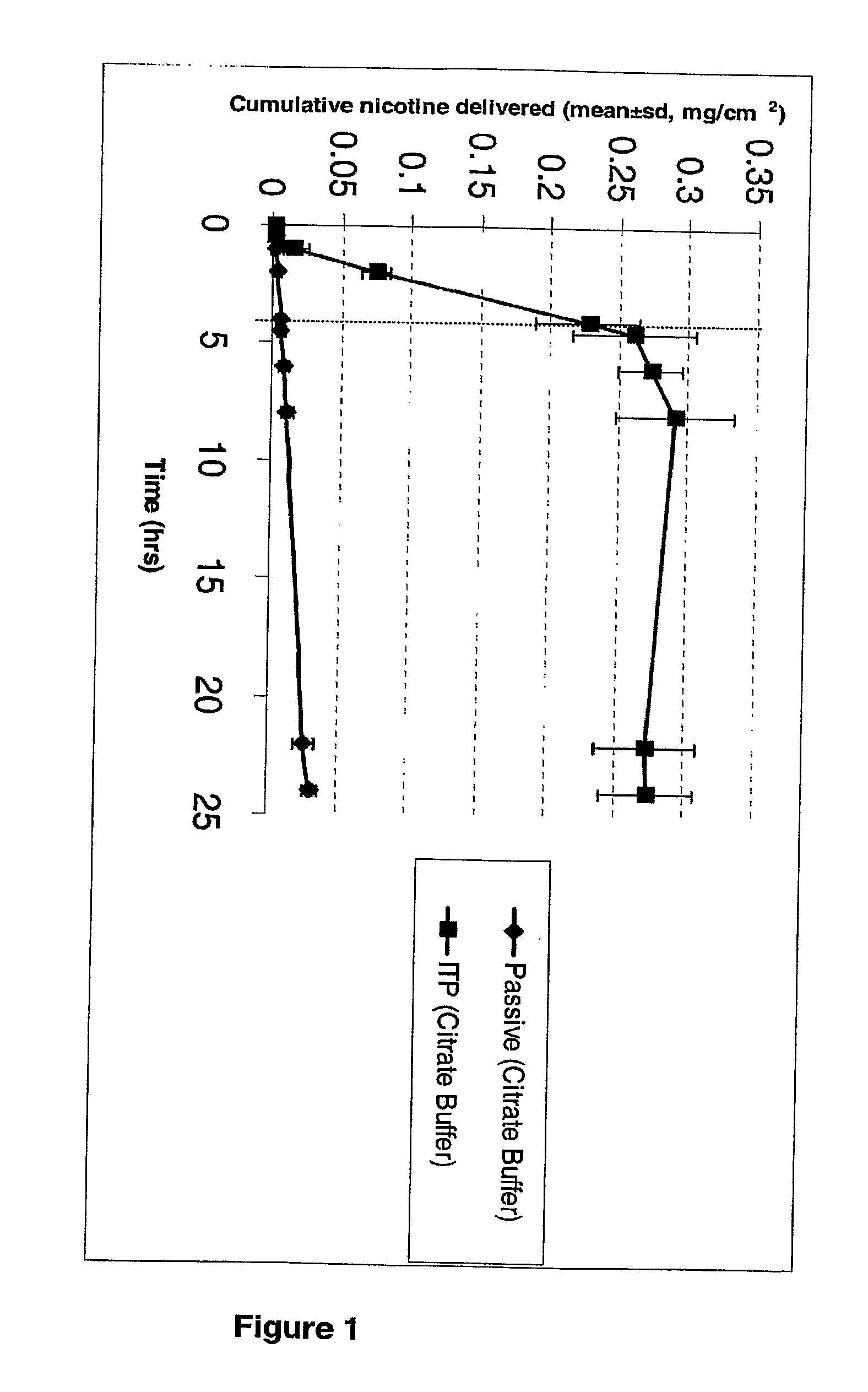
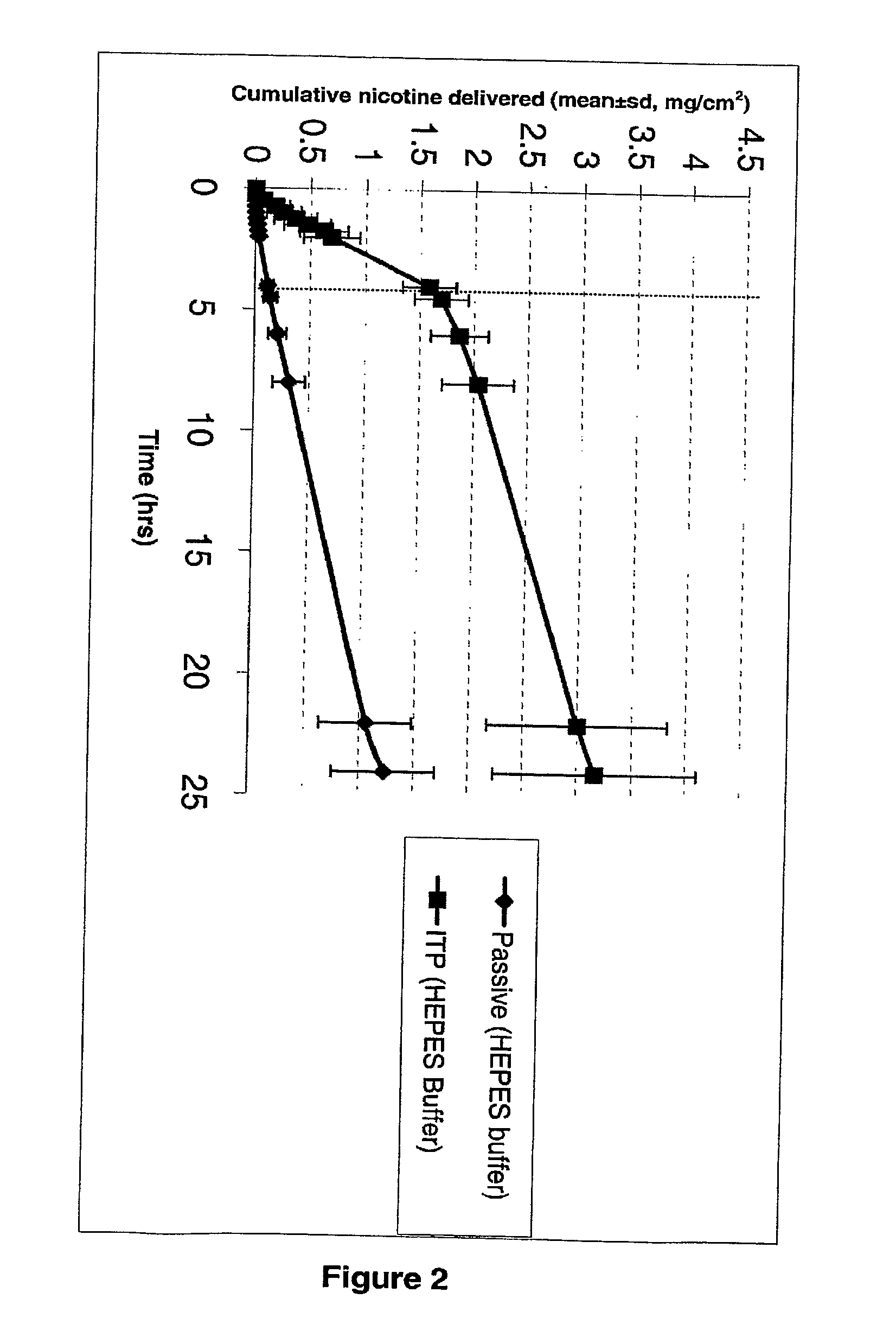
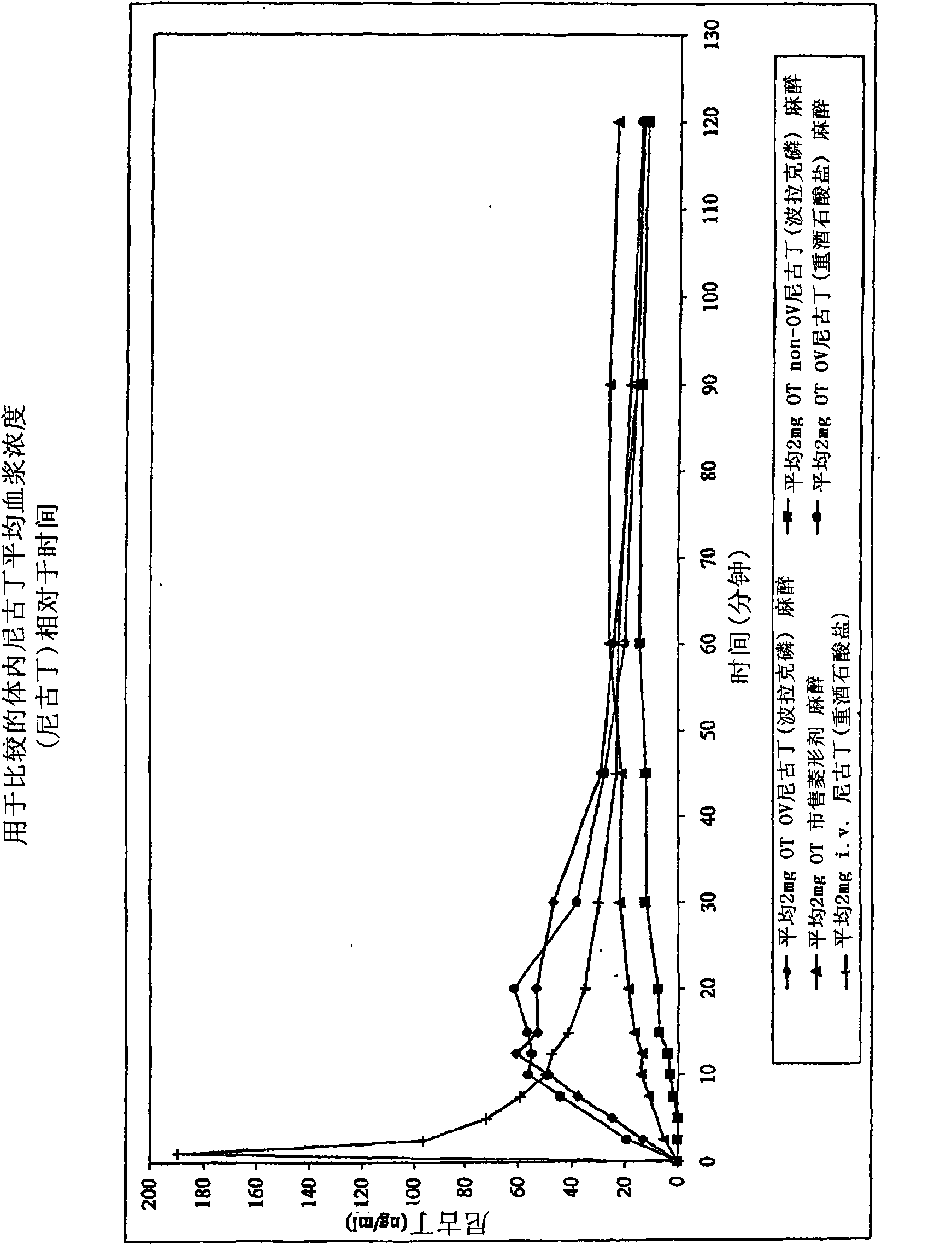
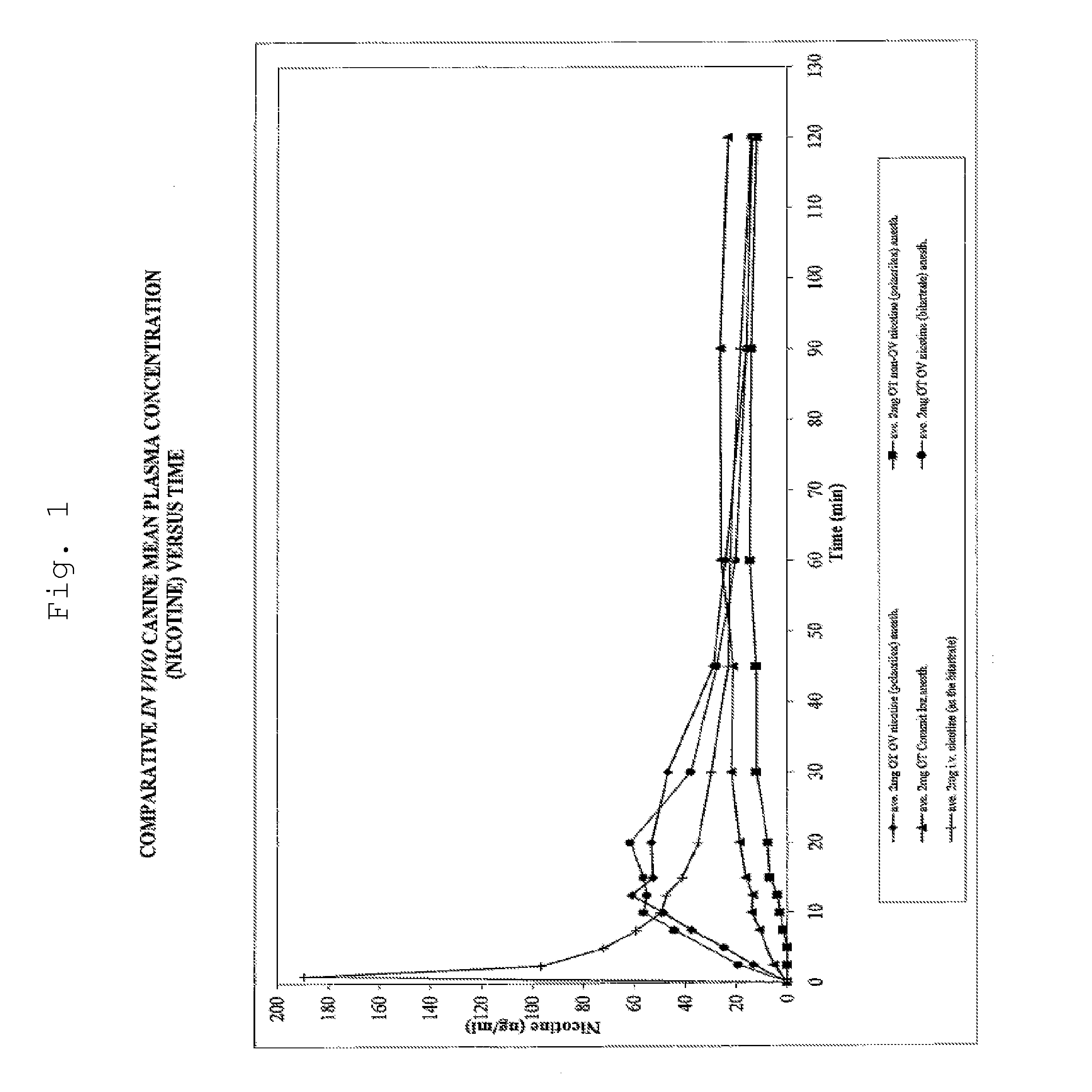
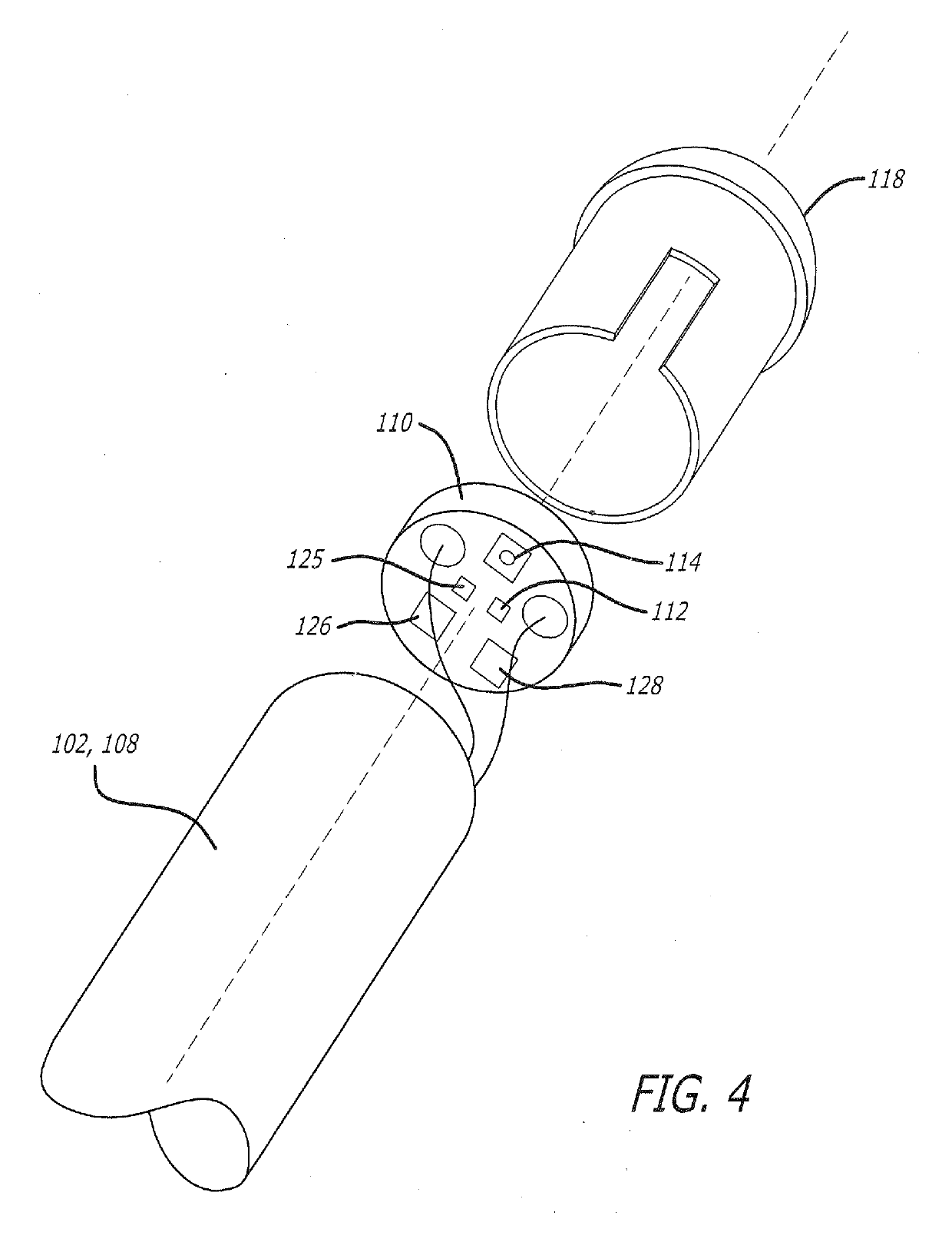
Iontophoretic Transdermal Delivery of Nicotine Salts
The present invention relates to iontophoretic transdermal delivery of nicotine salts useful for nicotine replacement therapy for an individual in need thereof. The present invention further relates to the iontophoretic transdermal delivery of nicotine maleate and nicotine citrate. Methods of reducing skin irritation generally caused by transdermal nicotine delivery by iontophoretic transdermal delivery of nicotine salts are also disclosed.
Owner:SMITHKLINE BECKMAN CORP
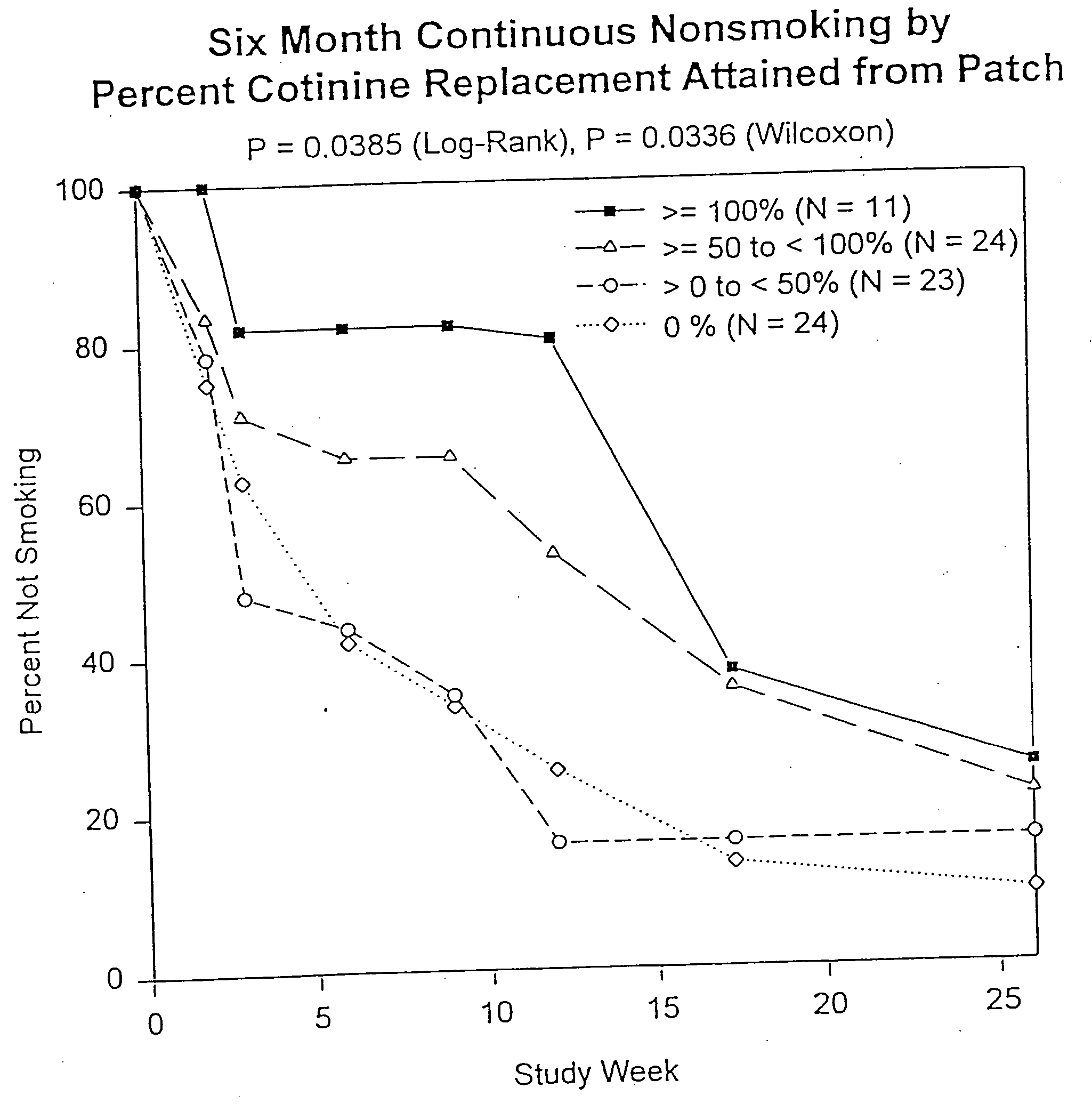
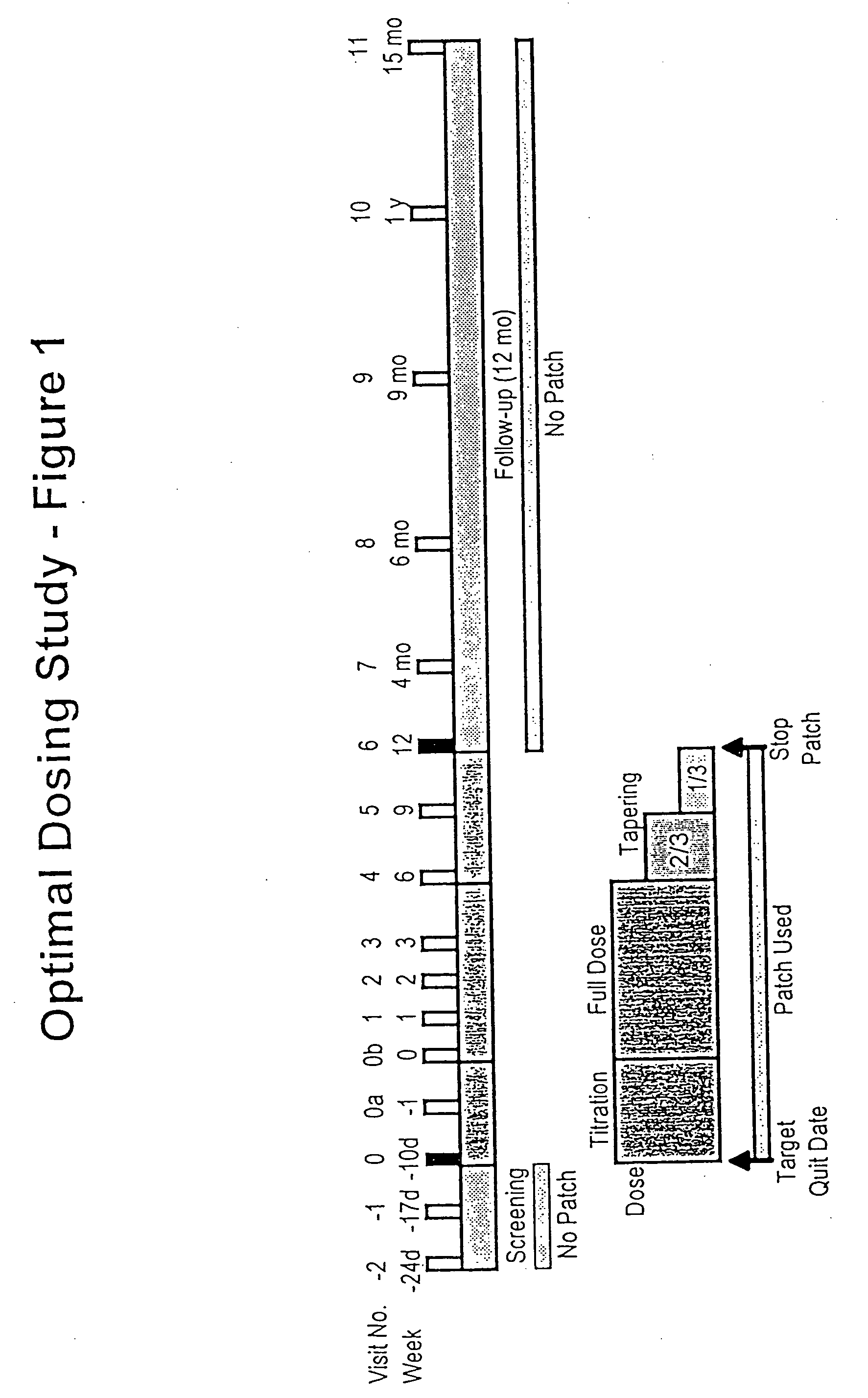
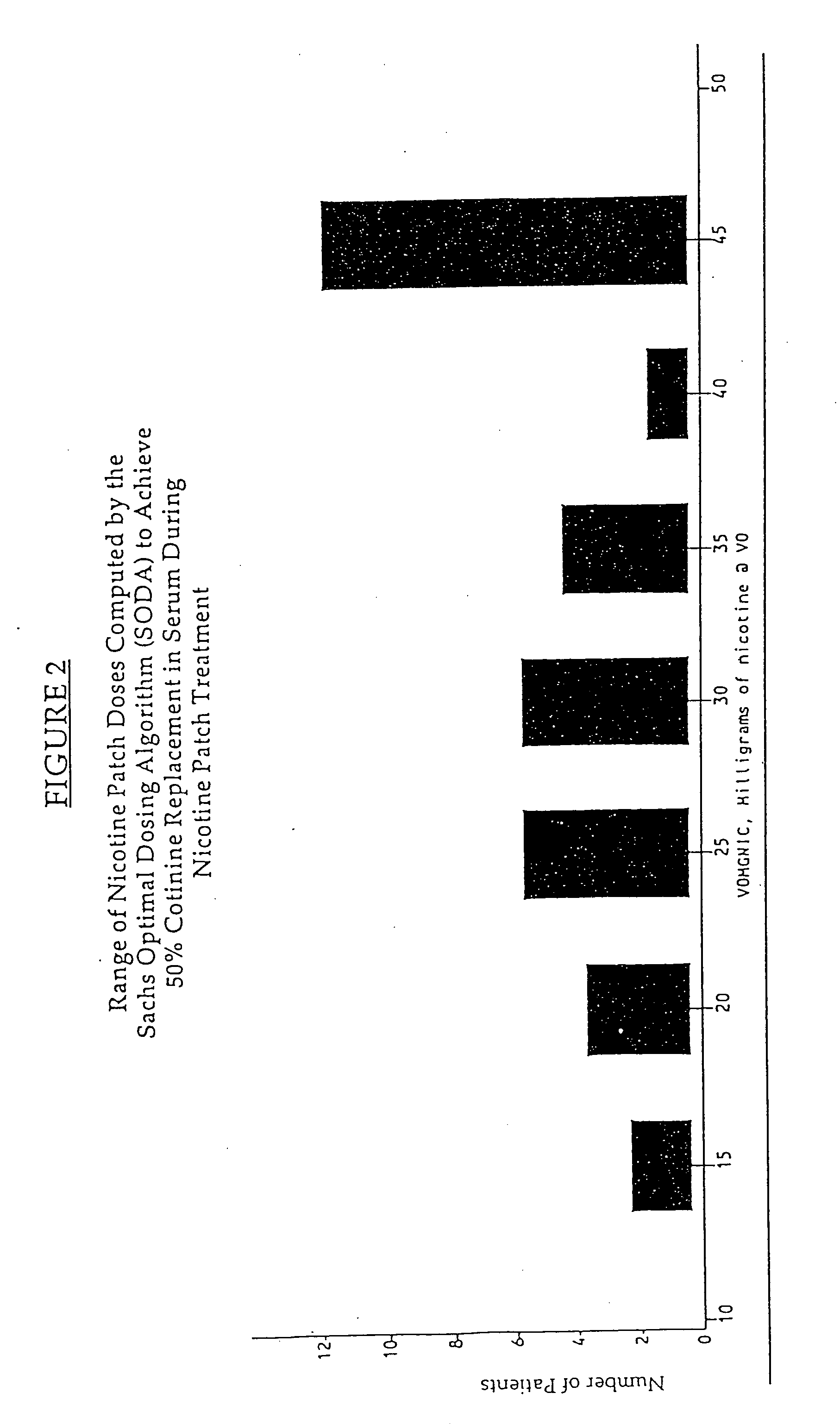
Methods for nicotine replacement dosage determination
InactiveUS20040006113A1Increased riskQuickly reachCompounds screening/testingBiocideNicotine replacementsSerum concentration
Owner:SACHS DAVID P L
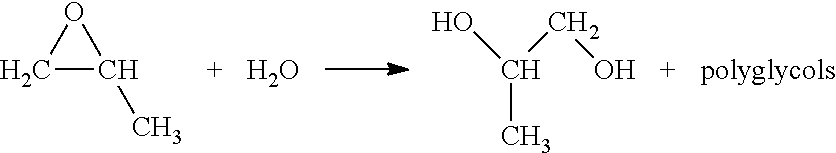
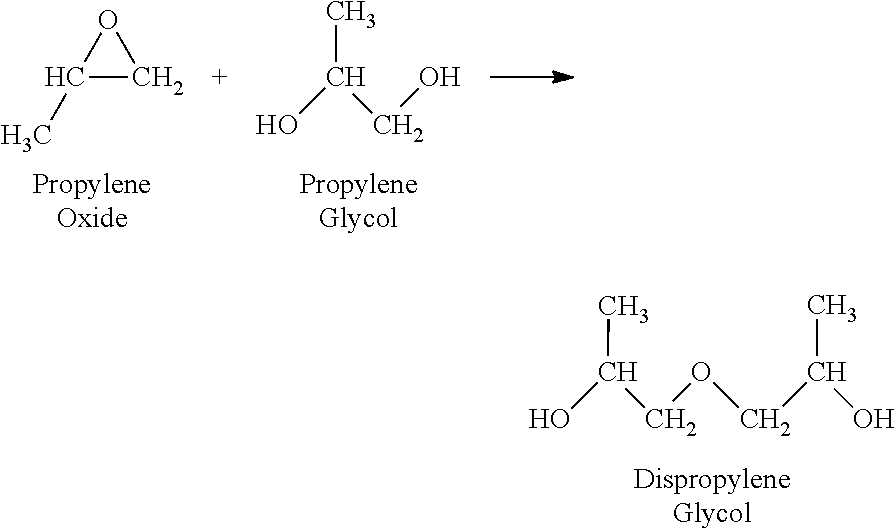
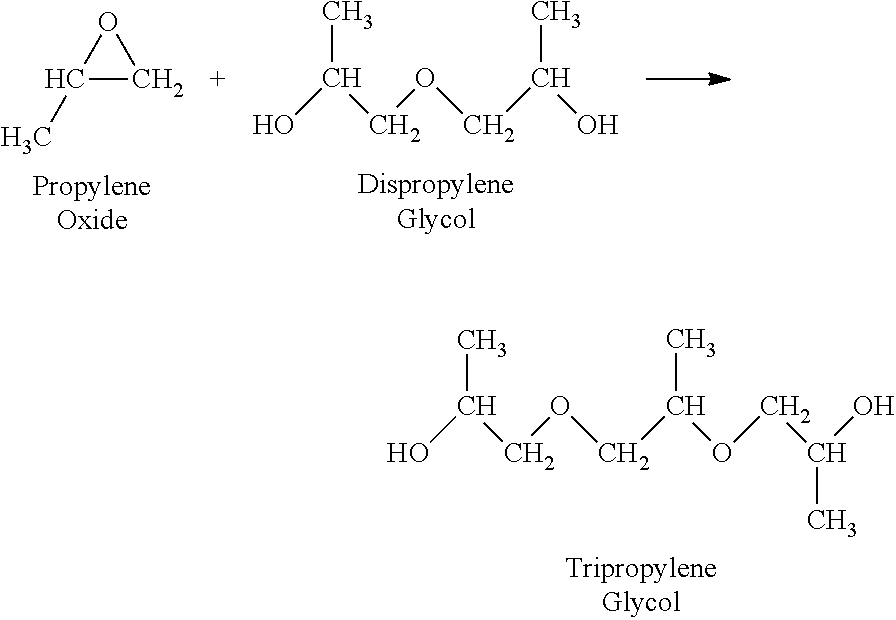
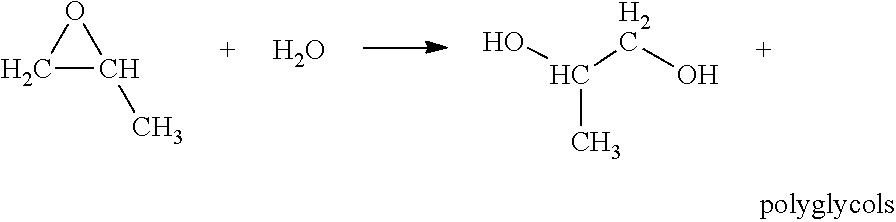
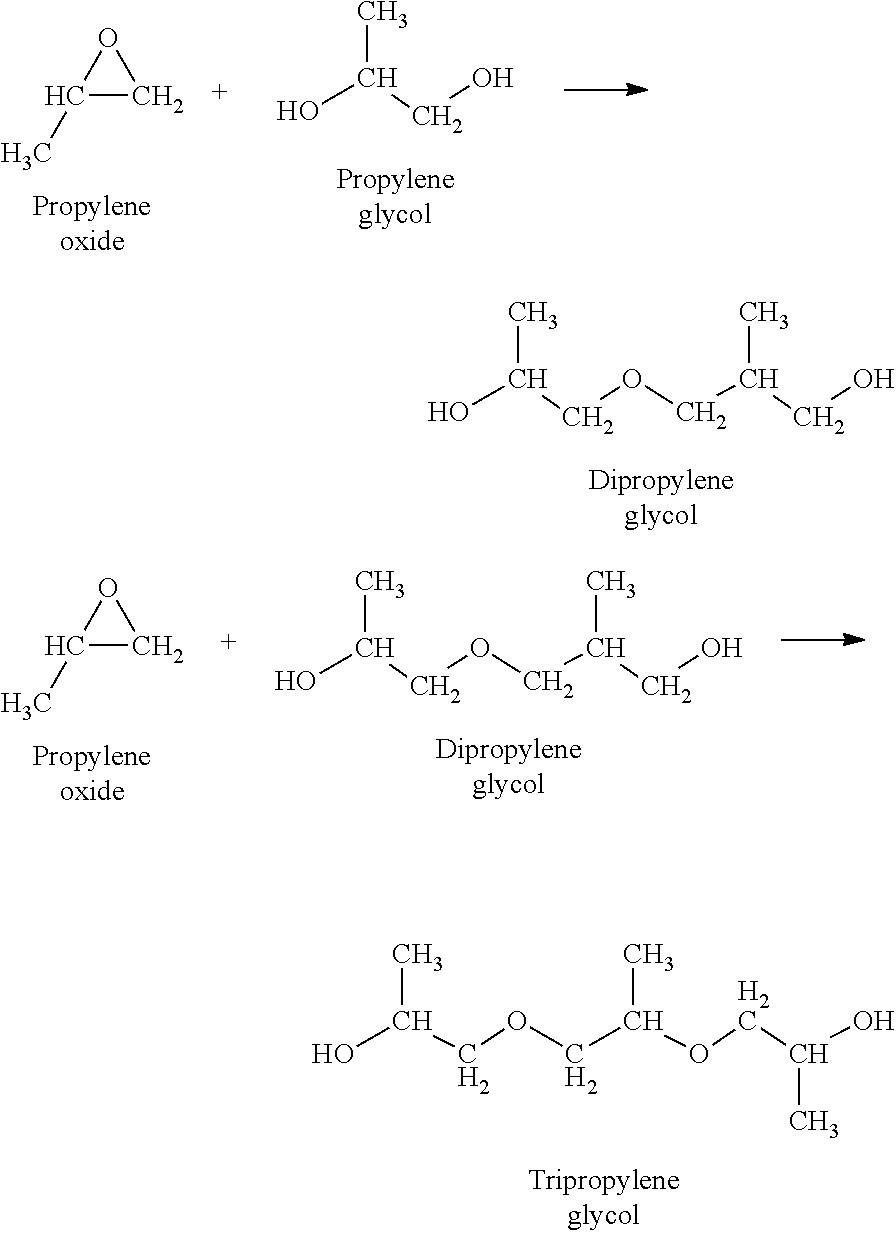
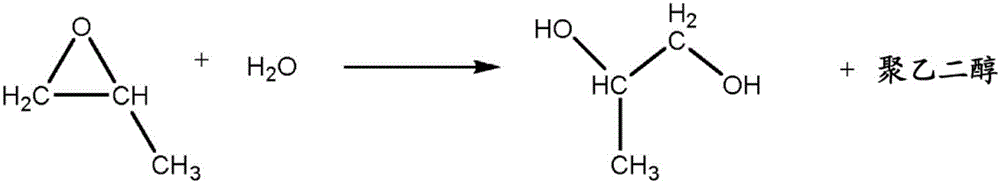
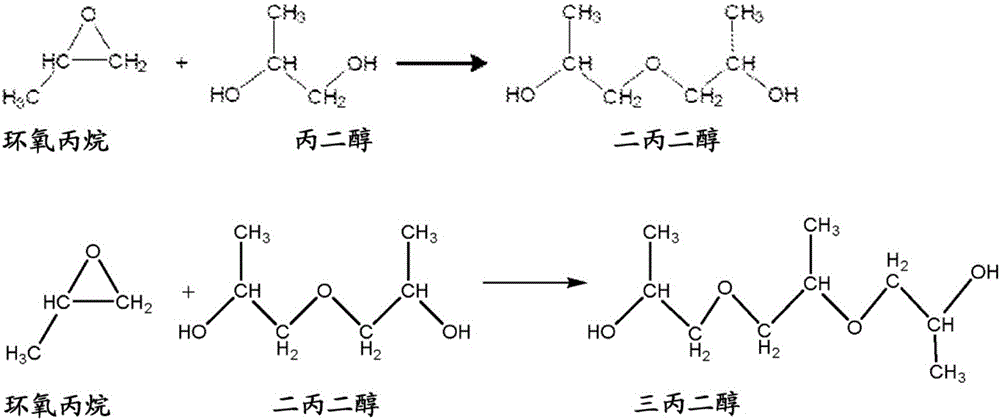
Use of a composition containing a long-chain polyol as a base for e-liquids
InactiveUS20170215470A1Reinforce aromatic potencyLimit and eliminate formationOrganic active ingredientsTobacco treatmentPolyolChemical compound
Provided is a composition containing a polyol that has 4 to 8 carbon atoms and 2 hydroxyl functions that can be used as a liquid for electronic cigarettes. The disclosure further relates to a liquid composition for electronic cigarettes, the composition containing a polyol that has 4 to 8 carbon atoms and 2 hydroxyl functions, as well as at least one compound selected from among nicotine, a nicotine substitute and an aroma. The disclosure also relates to an electronic cigarette containing the composition.
Owner:LAB CERES
Nicotine Containing Soft Gelatin Pastilles
The present invention relates to soft pastilles for nicotine replacement therapy, said pastille comprises about 0.05% to about 1% of nicotine active; about 5% to about 40% of gelling agent; about 30% to about 70% of plasticizer; about 0.05% to about 10% of sweetener; 0.5% to about 30% of releasing agent; about 0.05% to about 2% of preservative; about 0.01% to 5% of flavouring agent; and about 5% to about 20% of water.
Owner:THAKKAR JATIN
Oral transmucosal nicotine dosage form
InactiveUS20080131508A1Effectively and rapidly therapeutically effective amountImprove bioavailabilityBiocideNervous disorderNicotine agentSolid Dose Form
The invention described herein relates to an oral transmucosal solid dosage form useful in treating nicotine addiction or as a nicotine substitute or replacement. By virtue of the formulation in combination with nicotine, the invention transmucosally delivers an effective amount of nicotine to the recipient while permitting the accomplishing of such, and manufacture of such, using a relatively small, convenient and orally comfortable dosage form (e.g., tablet) size.
Owner:CIMA LABS +1
Nicotine-alternative compositions and methods of producing such compositions
InactiveUS20100160376A1Low production costImprove marketabilityBiocideNervous disorderNicotine replacementAlkaloid
A method for producing a consumable nicotine-alternative composition includes measuring a quantity of a cigarette nicotine-alternative alkaloid and / or a larger quantity of lobeline. The quantities of cigarette nicotine-alternative alkaloid and / or lobeline are diluted into one or more successive intermediate solutions, a last of which constitutes a final solution. The final solution is apportioned so that each portion contains a precise quantity of cigarette nicotine-alternative alkaloid and / or lobeline appropriate for consumption in a single use by a single person. Each portion is introduced into a separate single-serving dispenser.
Owner:THOMPSON MARSHALL ANLAUF
Methods for nicotine replacement dosage determination
InactiveUS6602892B1Increased riskQuickly reachCompounds screening/testingBiocideNicotine replacementsSerum concentration
A method for predicting nicotine replacement dosage to achieve a target nicotine serum concentration relies on measuring blood nicotine concentration prior to smoking cessation. At least two values corresponding to other patient characteristics, such as body mass, cumulative smoking, psychological dependence, age, and menopausal status, are also determined and used to predict expected blood nicotine concentrations based on nicotine replacement dosages. Such methods are useful in achieving target blood nicotine concentrations for smoking cessation and therapy.
Owner:SACHS DAVID P L
Use of a composition containing 1,3-propanediol as an e-liquid
The invention relates to the use of a composition containing 1, 3-propanediol as an electronic cigarette liquid. The invention also relates to a liquid composition for electronic cigarettes comprising 1, 3-propanediol, as well as at least one compound selected from nicotine, a nicotine substitute and an aroma. The invention also relates to an electronic cigarette containing said composition.
Owner:LAB CERES
Oral transmucosal nicotine dosage form
InactiveCN101563071AReduced bioavailabilityEarly bioavailabilityOrganic active ingredientsNervous disorderNicotine replacementEndocrinology
The invention described herein relates to an oral transmucosal solid dosage form useful in treating nicotine addiction or as a nicotine substitute or replacement. By virtue of the formulation in combination with nicotine, the invention transmucosally delivers an effective amount of nicotine to the recipient while permitting the accomplishing of such, and manufacture of such, using a relatively small, convenient and orally comfortable dosage form (e.g., tablet) size.
Owner:CEPHALON INC +1
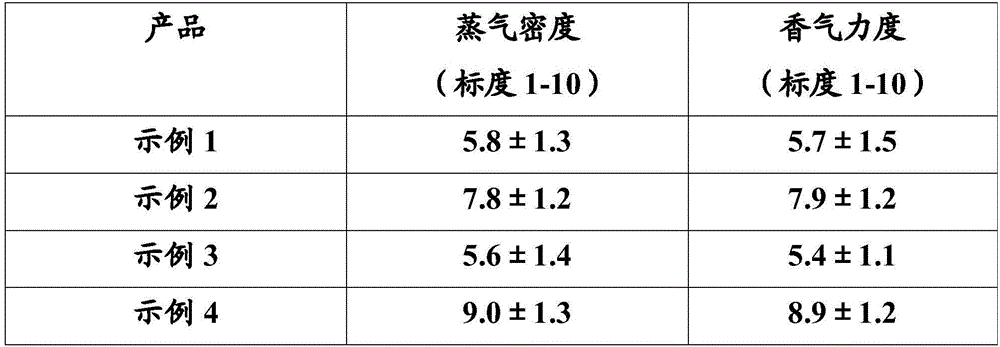
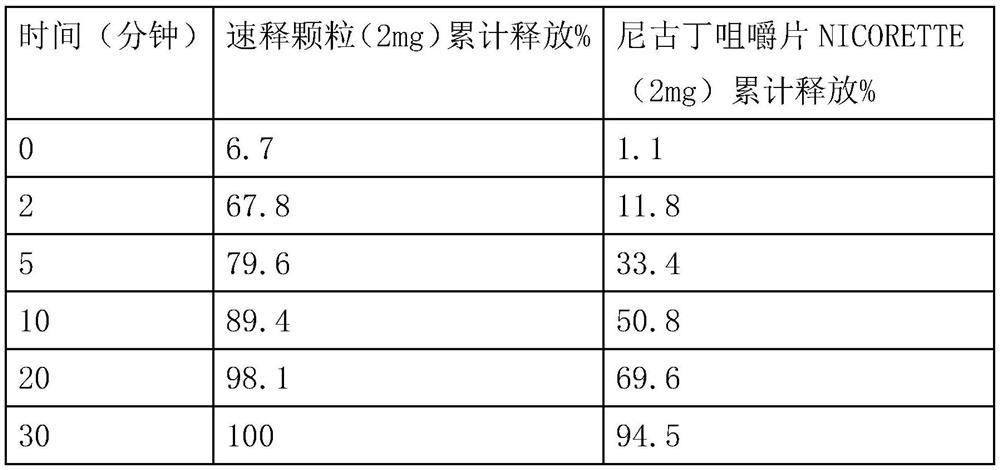
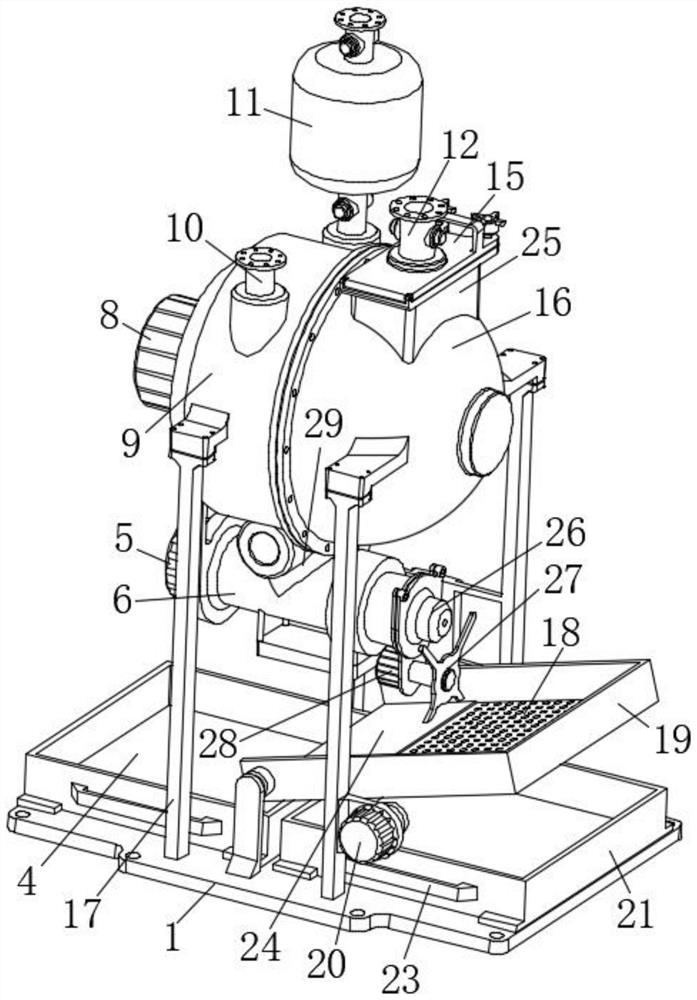
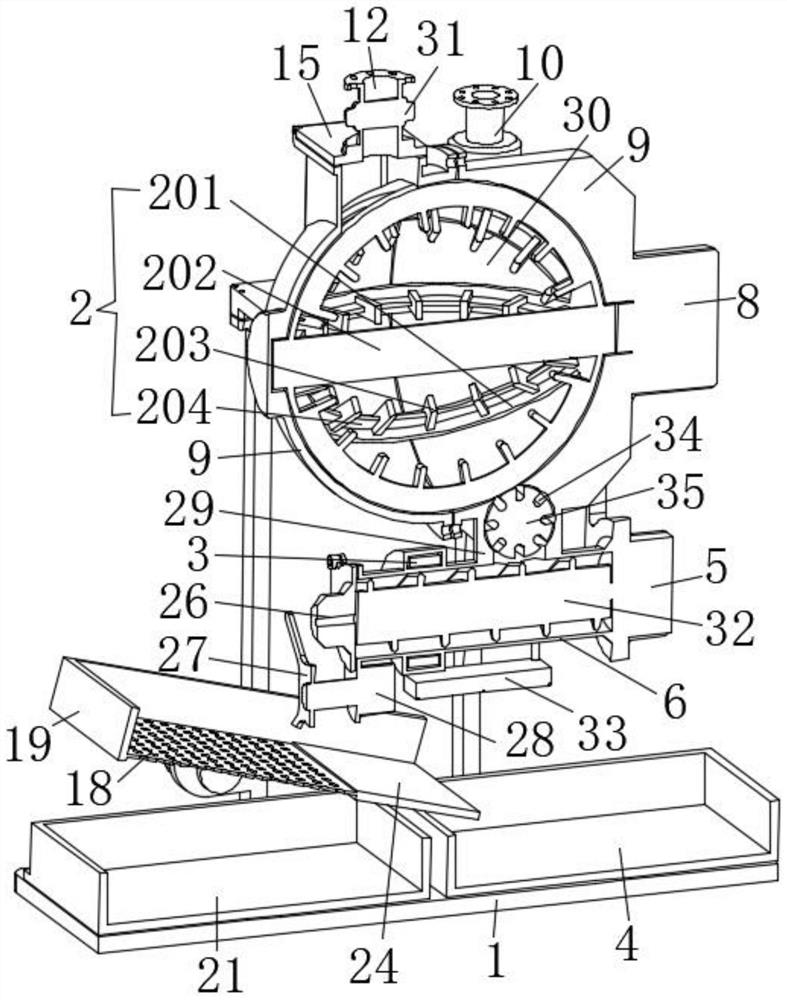
Nicotine granule composition and preparation method thereof
ActiveCN112220756AExtension of timeInhibition-dependentOrganic active ingredientsNervous disorderNicotine replacementsNicotine replacement
The invention relates to the technical field of nicotine replacement therapy products, and discloses a nicotine granule composition, which mainly comprises nicotine quick-release granules and nicotinesustained-release granules, and the nicotine quick-release granules mainly comprise a nicotine raw material, a filler, an adhesive, a buffer agent, a sweetener and an edible essence; the nicotine sustained-release granules mainly comprise the following components: a nicotine raw material, a polymer sustained-release material, an adhesive, a buffer agent, a sweetener and an edible essence. The invention also discloses a method for preparing the nicotine granule mixture and a preparation device thereof. The nicotine granule composition provided by the invention can guarantee the speed of obtaining pleasant sensation of a human body and the duration of nicotine maintaining the concentration with a treatment effect in a human body through the nicotine quick-release granules and the nicotine sustained-release granules, so that smoking addicts can get rid of the dependence on smoking better, and has a better treatment effect than the similar products sold on the market.
Owner:重庆市义力医药科技有限公司
Nicotine containing soft gelatin pastilles
The present invention relates to soft pastilles for nicotine replacement therapy, said pastille comprises about 0.05% to about 1% of nicotine active; about 5% to about 40% of gelling agent; about 30% to about 70% of plasticizer; about 0.05% to about 10% of sweetener; 0.5% to about 30% of releasing agent; about 0.05% to about 2% of preservative; about 0.01% to 5% of flavoring agent; and about 5% to about 20% of water.
Owner:THAKKAR JATIN
Oral transmucosal nicotine dosage form
InactiveUS20110206621A1Effectively delivered transmucosallyEffectively and rapidly therapeutically effective amountBiocideOrganic active ingredientsNicotine replacementDosage form
The invention described herein relates to an oral transmucosal solid dosage form useful in treating nicotine addiction or as a nicotine substitute or replacement. By virtue of the formulation in combination with nicotine, the invention transmucosally delivers an effective amount of nicotine to the recipient while permitting the accomplishing of such, and manufacture of such, using a relatively small, convenient and orally comfortable dosage form (e.g., tablet) size.
Owner:CEPHALON INC +1
Use of a composition containing a long-chain polyol as a base for e-liquids
InactiveCN106572694AImprove bioavailabilityOrganic active ingredientsTobacco treatmentPolyolChemical compound
The invention relates to the use of a composition containing a polyol that comprises 4 to 8 carbon atoms and 2 hydroxyl functions, as a liquid for electronic cigarettes. The invention further relates to a liquid composition for electronic cigarettes, containing a polyol that comprises 4 to 8 carbon atoms and 2 hydroxyl functions, as well as at least one compound selected from among nicotine, a nicotine substitute and an aroma. The invention also relates to an electronic cigarette containing said composition.
Owner:LAB CERES
Nicotine granule composition, preparation method and prepartion device thereof
ActiveCN112220757AExtension of timeInhibition-dependentOrganic active ingredientsNervous disorderNicotine replacementsNicotine replacement
The invention relates to the technical field of nicotine replacement therapy products, and discloses a nicotine granule composition, which mainly comprises nicotine quick-release granules and nicotinesustained-release granules, and the nicotine quick-release granules mainly comprise a nicotine raw material, a filler, an adhesive, a buffer agent, a sweetener and an edible essence; the nicotine sustained-release granules mainly comprise the following components: a nicotine raw material, a polymer sustained-release material, an adhesive, a buffer agent, a sweetener and an edible essence. The invention also discloses a method for preparing the nicotine granule mixture and a preparation device thereof. The nicotine granule composition provided by the invention can guarantee the speed of obtaining pleasant sensation of a human body and the duration of nicotine maintaining the concentration with a treatment effect in a human body through the nicotine quick-release granules and the nicotine sustained-release granules, so that smoking addicts can get rid of the dependence on smoking better, and has a better treatment effect than the similar products sold on the market.
Owner:重庆市义力医药科技有限公司
Systems and Methods for Buffered Aerosol Drug Delivery
A method for delivering a drug to a user including an electronic cigarette wherein the electronic cigarette itself includes a liquid formulation. The liquid formulation can include at least one drug and at least one biologically acceptable carrier. The at least one drug can be formed with an acid or alcohol and has a first vapor pressure at a first temperature, and is heated by a heating element within the electronic cigarette resulting in the generation of an aerosol suitable for inhaling by the user. Often, the drug can include nicotine, but may also be configured to include cannabinol or can be associated with nicotine replacement therapies, or other medication-assisted therapies. The drugs may also have a salt or co-salt added in order to increase the effectiveness of drug delivery while lowering the need for a higher powered heating element.
Owner:TURBI ZAYD ABDULFUHAH
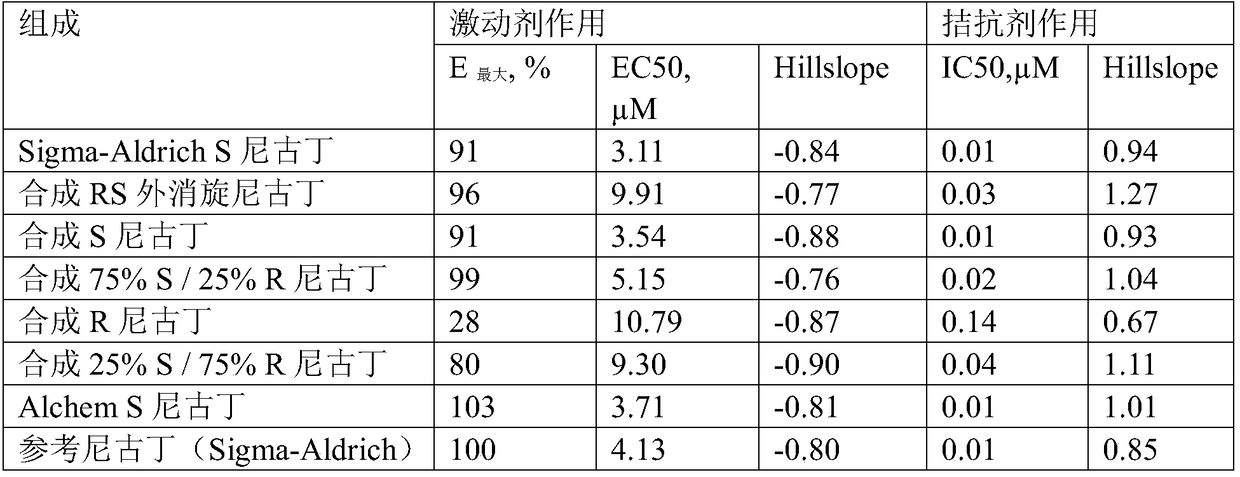
Nicotine replacement therapy products comprising synthetic nicotine
A composition suitable for use in nicotine replacement therapy products includes a nicotine product that includes a synthetic nicotine that is substantially free of one or more contaminants and / or impurities normally associated with tobacco-derived nicotine. For example, the synthetic nicotine is substantially free of one or more of nicotine-1'-N-oxide, nicotyrine, nornicotyrine, 2',3-bipyridyl, cotinine, anabasine, and / or anatabine. The composition further comprises one or more pharmaceutically acceptable excipients, additives and / or carriers. The nicotine replacement therapy products may include any number of such products, including transdermal nicotine delivery patches, nicotine gums, synthetic chewing tobacco, synthetic snuff, and synthetic strips (e.g., dissolvable synthetic tobacco). Additionally, a method of treating nicotine addiction includes administering a nicotine replacement composition, e.g., via a nicotine replacement therapy product, to a user.
Owner:NEXT GENERATION LABS LLC
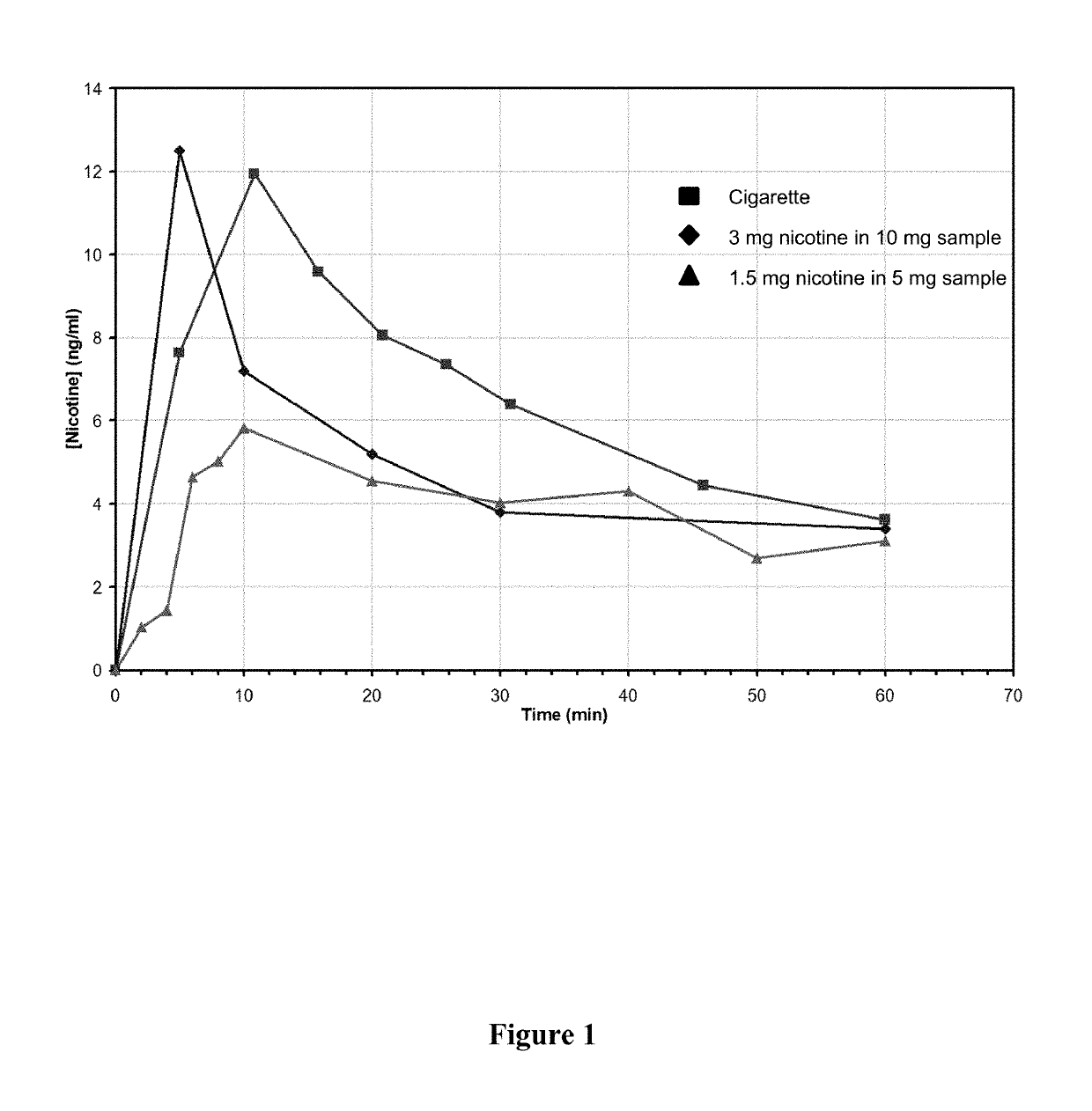
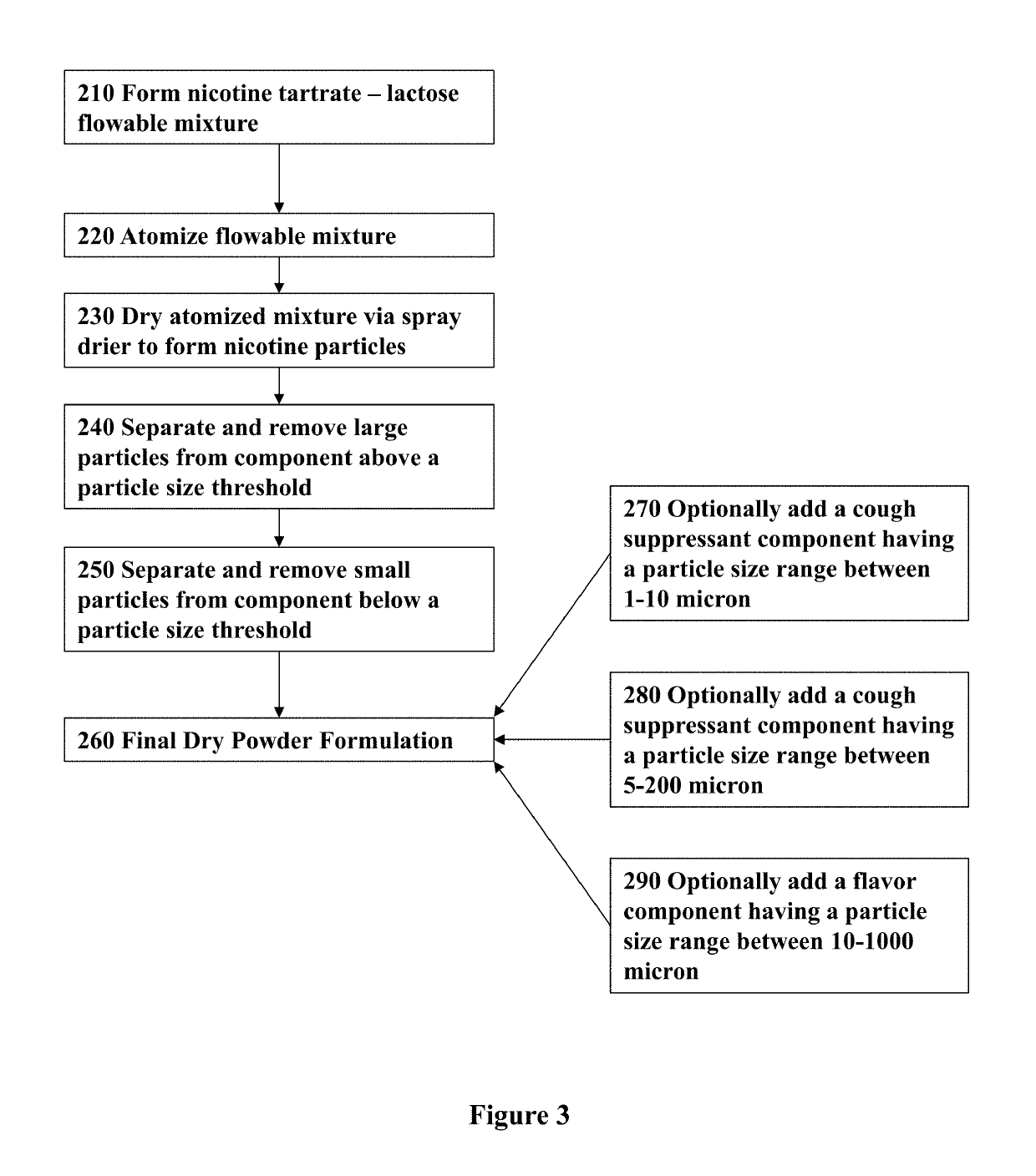
Nicotine Formulations and Methods of Making and Using the Same
ActiveUS20170071929A1Reducing nicotine cravingReduces nicotine cravingPowder deliveryNervous disorderNicotine replacementsInhalation
A method of reducing nicotine cravings is described. The method includes inhalation of a dry powder formulation containing a dose of nicotine by a subject seeking nicotine cravings reduction. The formulation includes amounts and concentrations of nicotine that are significantly lower than cigarettes or nicotine replacement therapies.
Owner:PHILIP MORRIS PROD SA
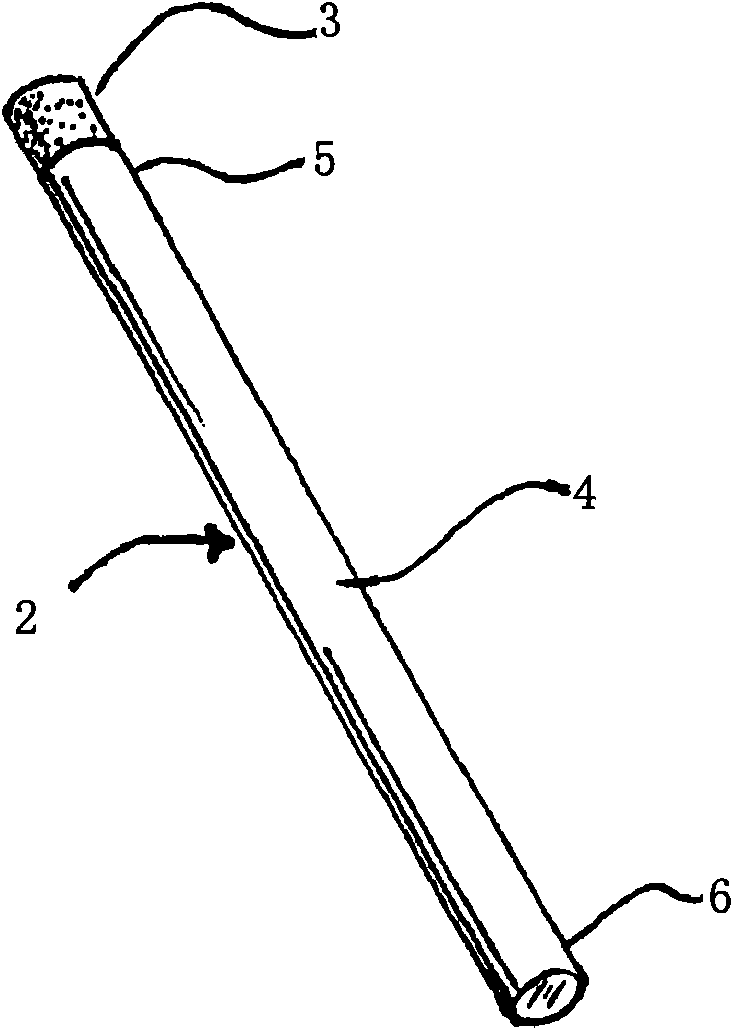
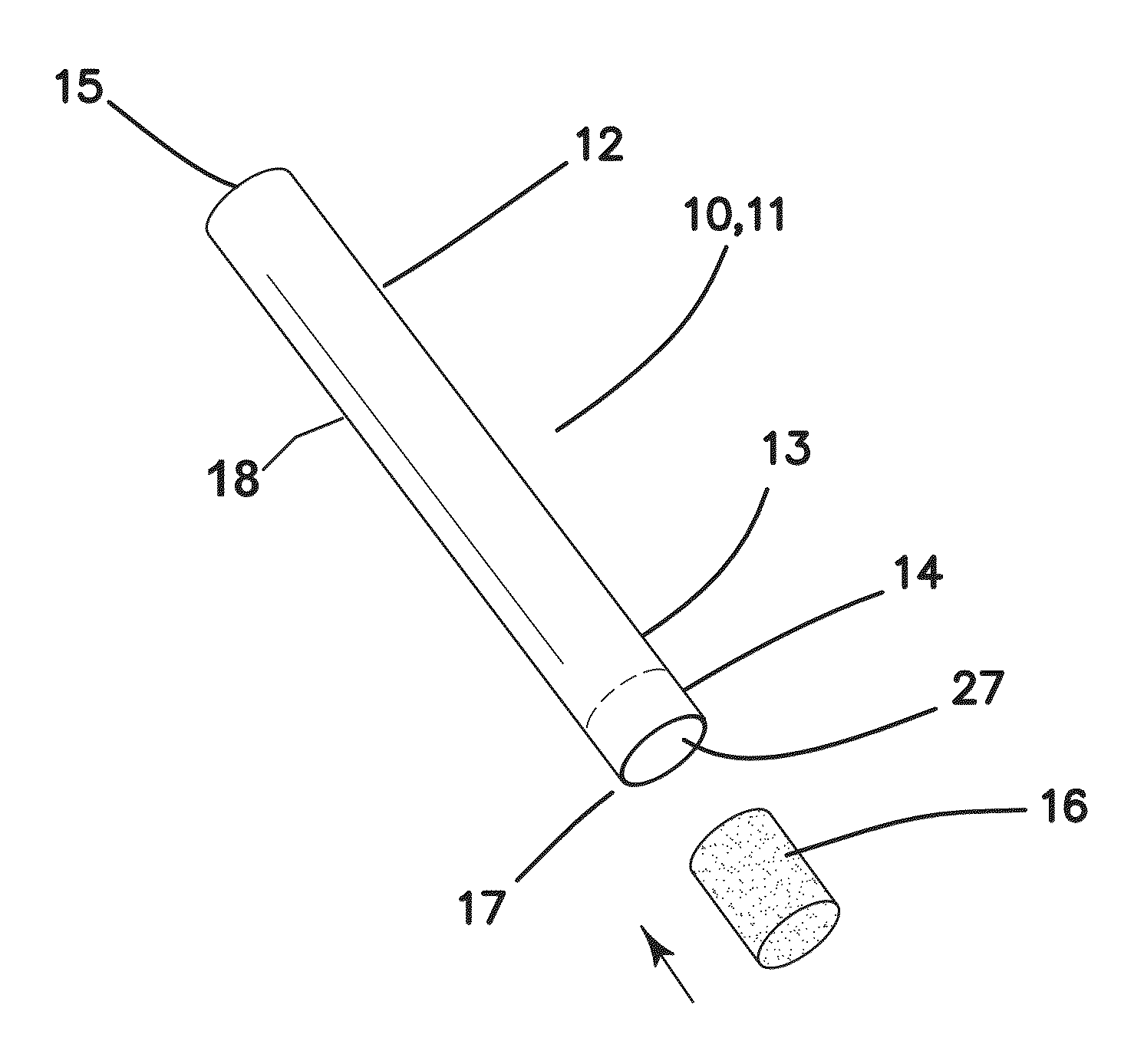
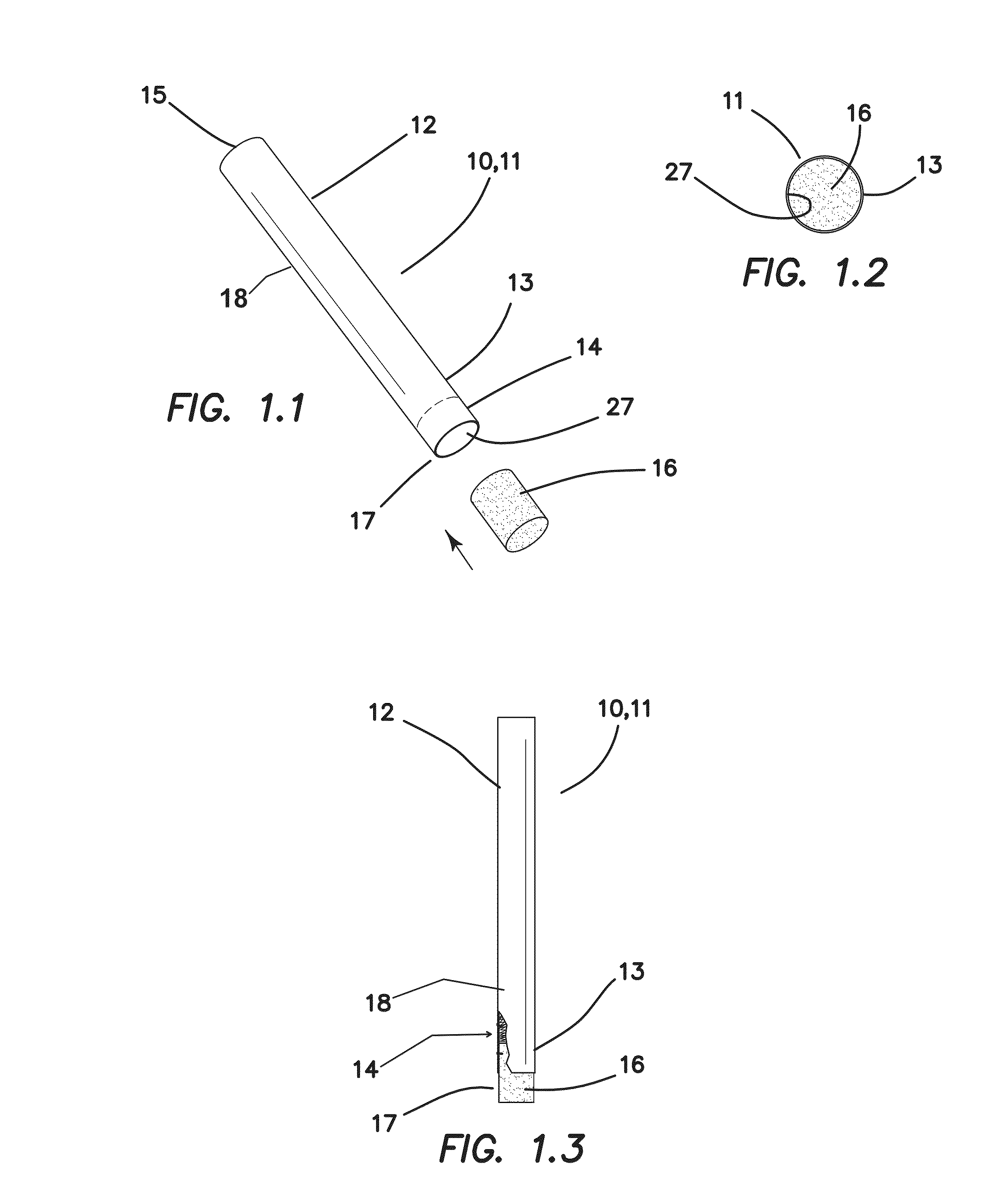
Smoking cessation device
InactiveUS20150201677A1Increase ratingsImprove therapyOrganic active ingredientsPharmaceutical delivery mechanismNicotine replacementsEnvironmental health
A smoking cessation device for use in nicotine replacement therapy includes an imitation smoking implement with a body sized and shaped substantially identical to a conventional smoking implement chosen from the group consisting of a cigarette, a cigarillo, a cigar, and a pipe. The device also includes one or more nicotine replacement modules removably disposed at one end of the body, the one or more nicotine replacement modules removable from the imitation smoking implement by a user to ingest said nicotine replacement modules as part of a nicotine replacement therapy. Optionally, a plurality of said imitation smoking implements can be held in a package sized and shaped to resemble a conventional box of cigarettes, cigarillos or cigars. Said imitation smoking implement can be held by the user in their hands or lips while consuming the one or more nicotine replacement modules to simulate a conventional smoking ritual.
Owner:Q CIGARETTES
A kind of nicotine particle composition and its preparation method and its preparation device
ActiveCN112220757BExtension of timeInhibition-dependentOrganic active ingredientsNervous disorderNicotine replacementsImmediate release
The invention relates to the technical field of products for nicotine replacement therapy, and discloses a nicotine granule composition, which is mainly composed of nicotine immediate-release granules and nicotine sustained-release granules, wherein the components of the nicotine immediate-release granules mainly include: nicotine raw materials, fillers , binders, buffers, sweeteners and food flavors; the components of nicotine sustained-release granules mainly include: nicotine raw materials, polymer slow-release materials, binders, buffers, sweeteners and food flavors. The invention also discloses a method and a preparation device for preparing the nicotine particle mixture. The nicotine granule composition provided by the present invention can ensure the speed at which the human body obtains pleasure and the duration of nicotine in the human body to maintain a therapeutically effective concentration through the nicotine immediate-release granule and the nicotine sustained-release granule, thereby facilitating smokers to better get rid of the habit of smoking The dependence of the drug has better therapeutic effect compared with similar products sold in the existing market.
Owner:重庆市义力医药科技有限公司
Nicotine formulations and methods of making and using the same
Owner:PHILIP MORRIS PROD SA
Nicotine replacement therapy products containing synthetic nicotine
A composition suitable for use in a nicotine replacement therapy product comprising synthetic nicotine substantially free of one or more contaminants and / or impurities normally associated with tobacco-derived nicotine. For example, the synthetic nicotine is substantially free of nicotine-1'-N-oxide, nicotine diene, nicotine nordiene, 2',3-bipyridine, cotinine, anabacin and / or Or one or more of anatapin. The composition further comprises one or more pharmaceutically acceptable excipients, additives and / or carriers. Nicotine replacement therapy products may comprise any number of such products, including transdermal nicotine delivery patches, nicotine gum, synthetic chewing tobacco, synthetic snus, and synthetic tobacco rods (eg, dissolvable synthetic tobacco). Additionally, methods of treating nicotine addiction include administering a nicotine replacement composition, eg, via a nicotine replacement therapy product, to a user.
Owner:NEXT GENERATION LABS LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com