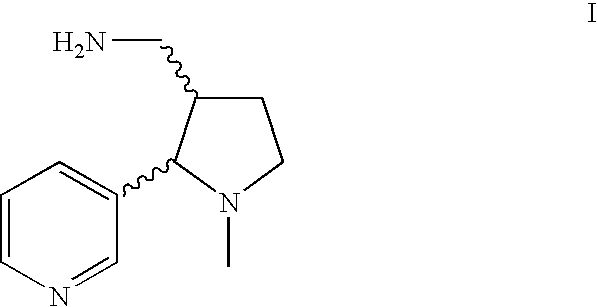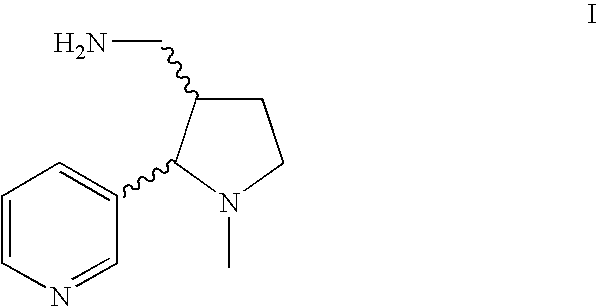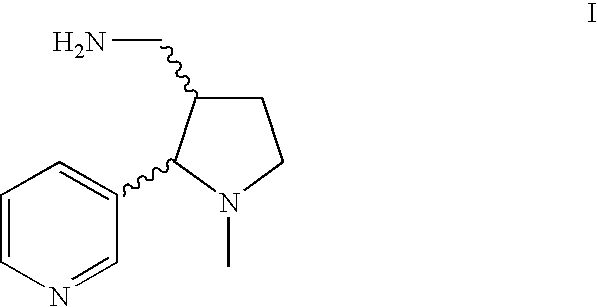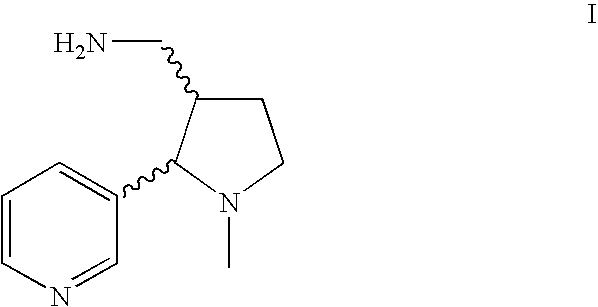Patents
Literature
106 results about "Nicotine Addiction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
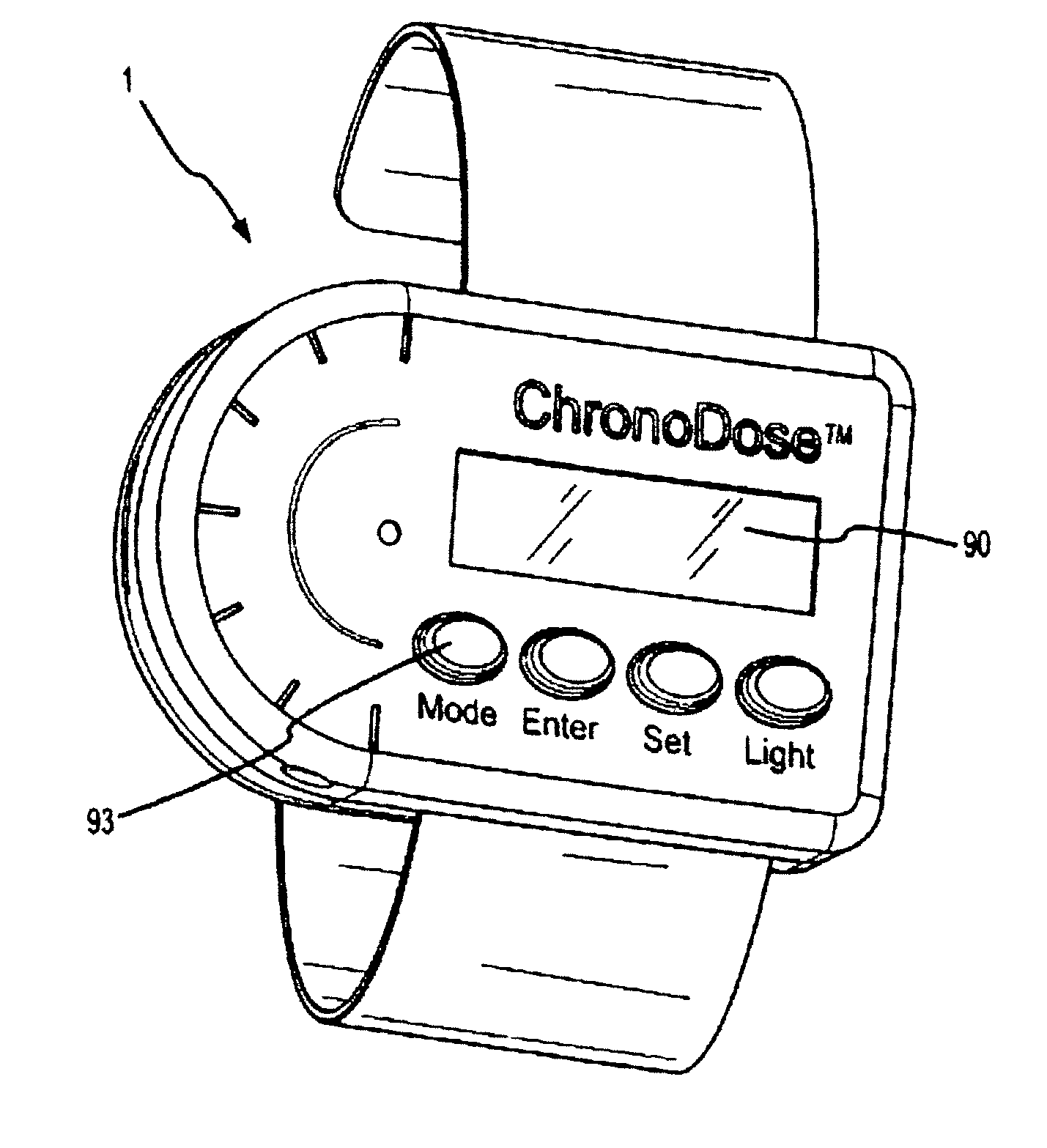
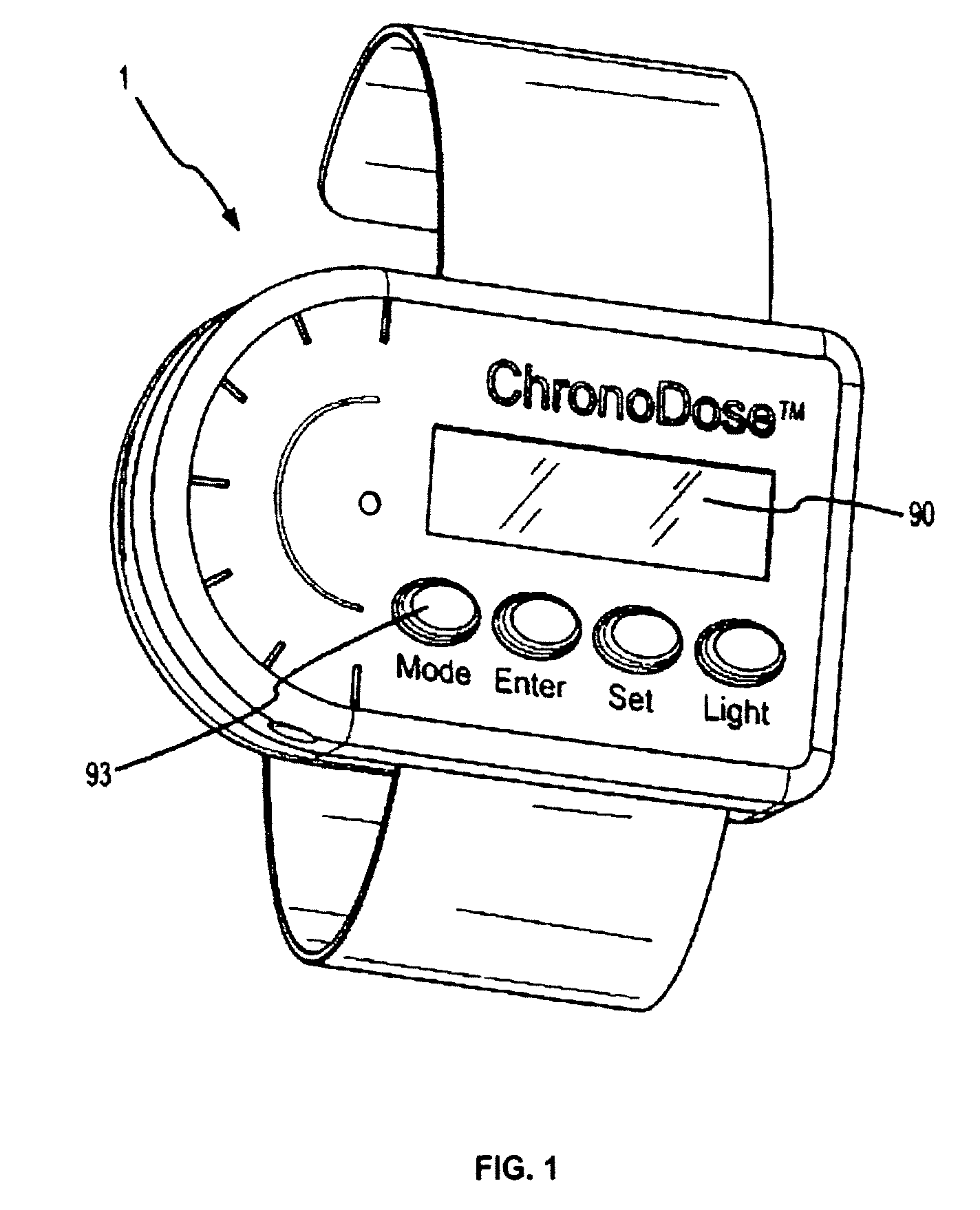
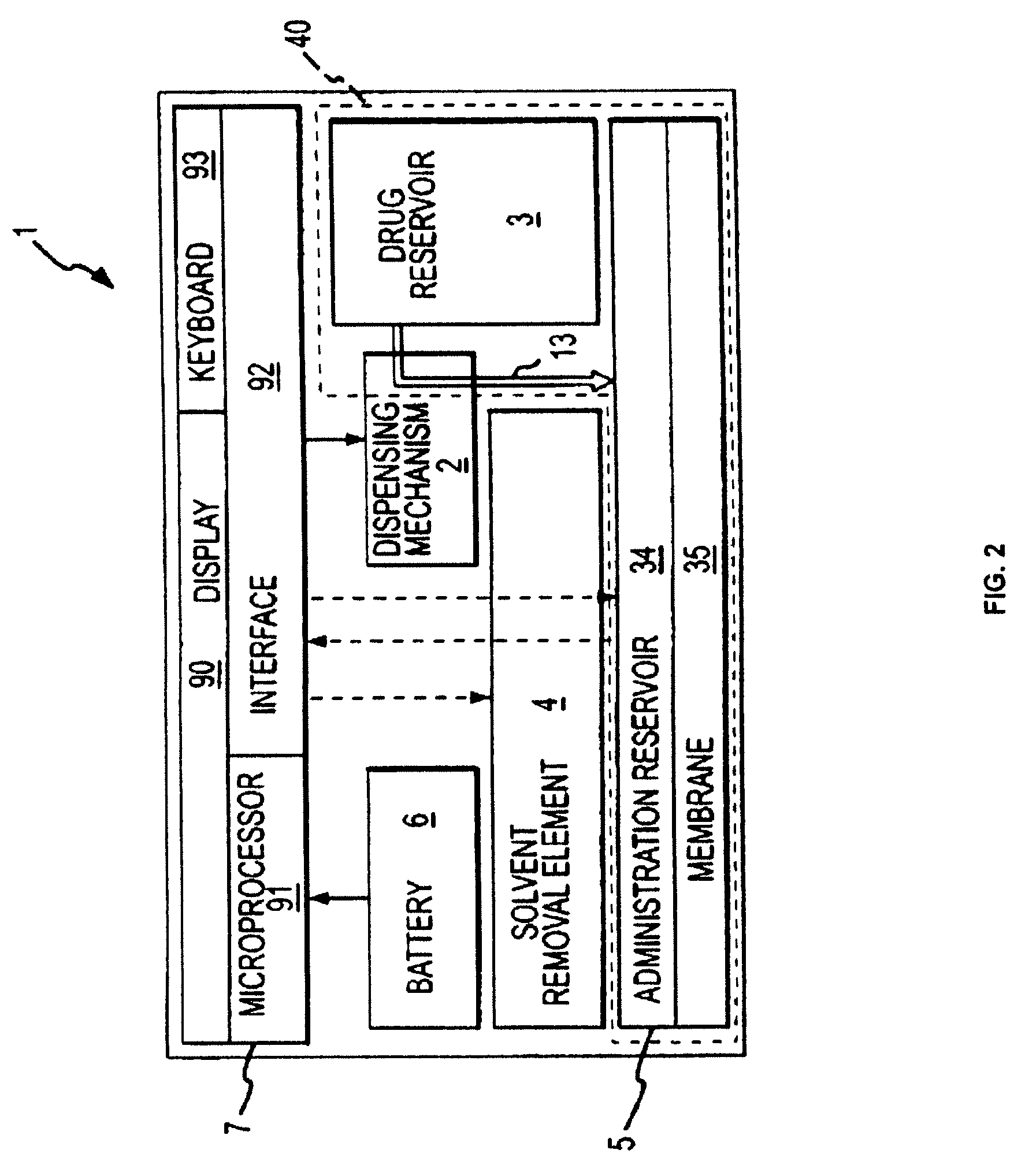
Biosynchronous transdermal drug delivery for longevity, anti-aging, fatigue management, obesity, weight loss, weight management, delivery of nutraceuticals, and the treatment of hyperglycemia, alzheimer's disease, sleep disorders, parkinson's disease, aids, epilepsy, attention deficit disorder, nicotine addiction, cancer, headache and pain control, asthma, angina, hypertension, depression, cold, flu and the like
ActiveUS20080220092A1Improve performanceReduce the amount requiredHeavy metal active ingredientsBiocidePhytochemicalAntioxidant
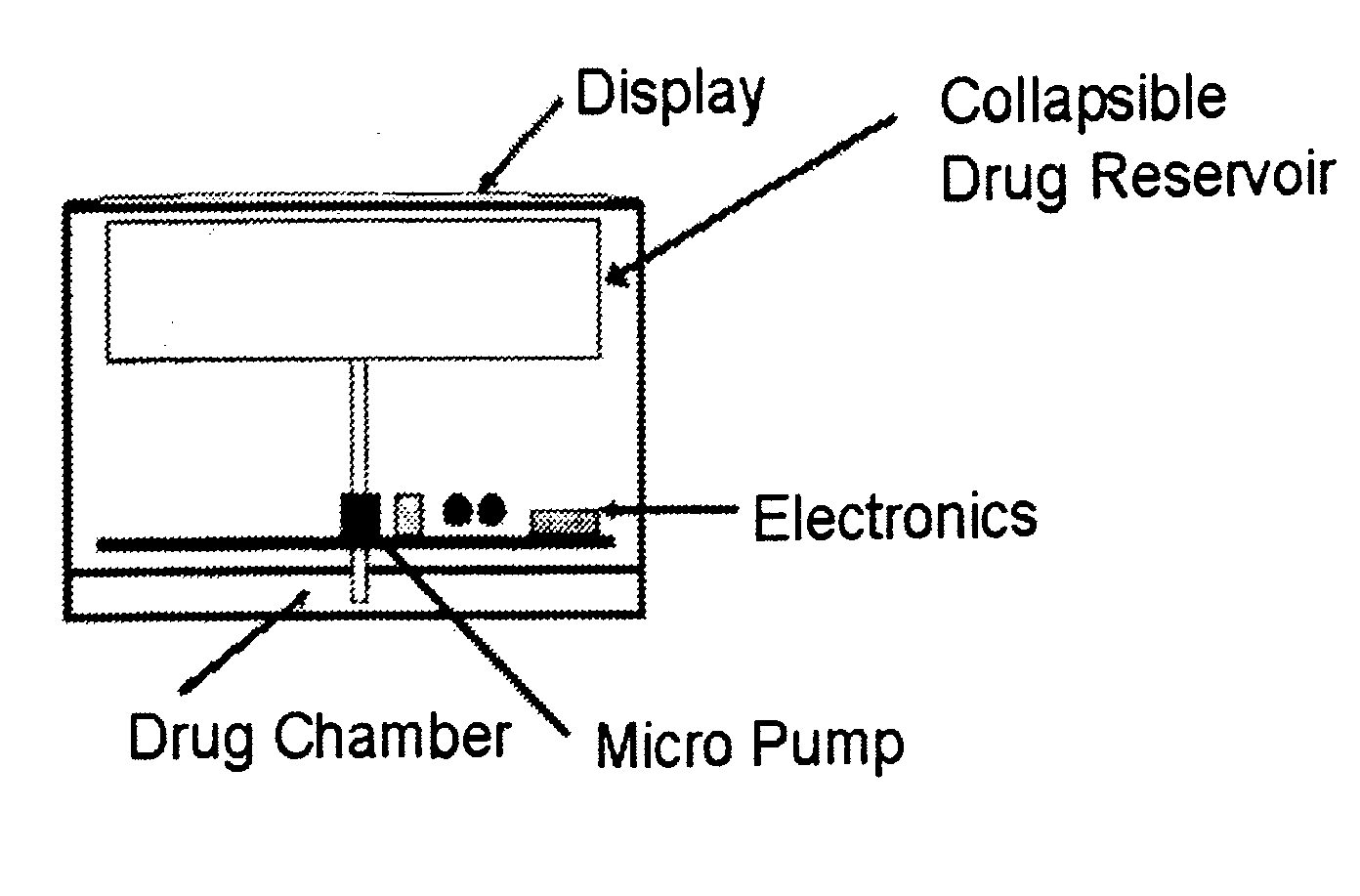
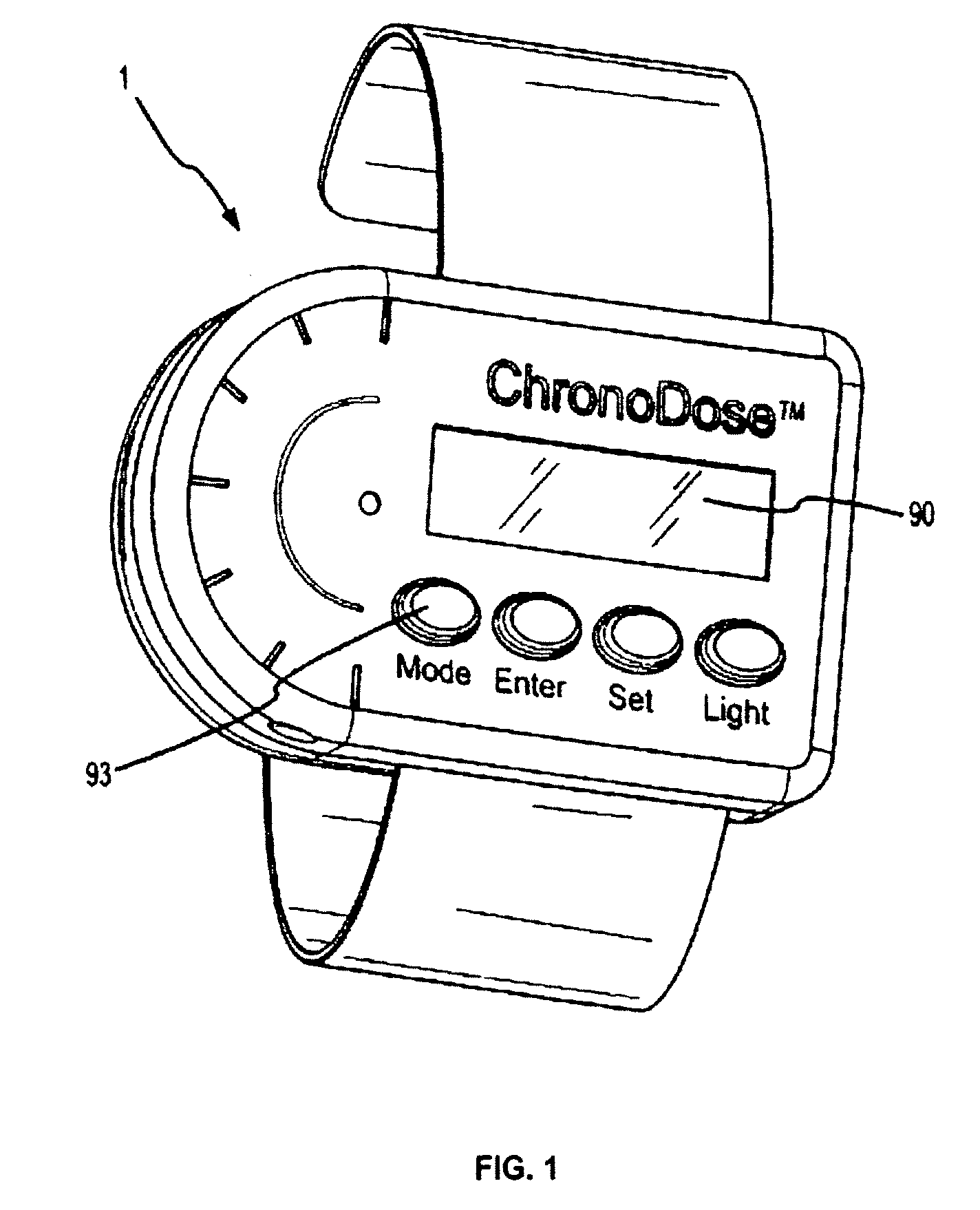
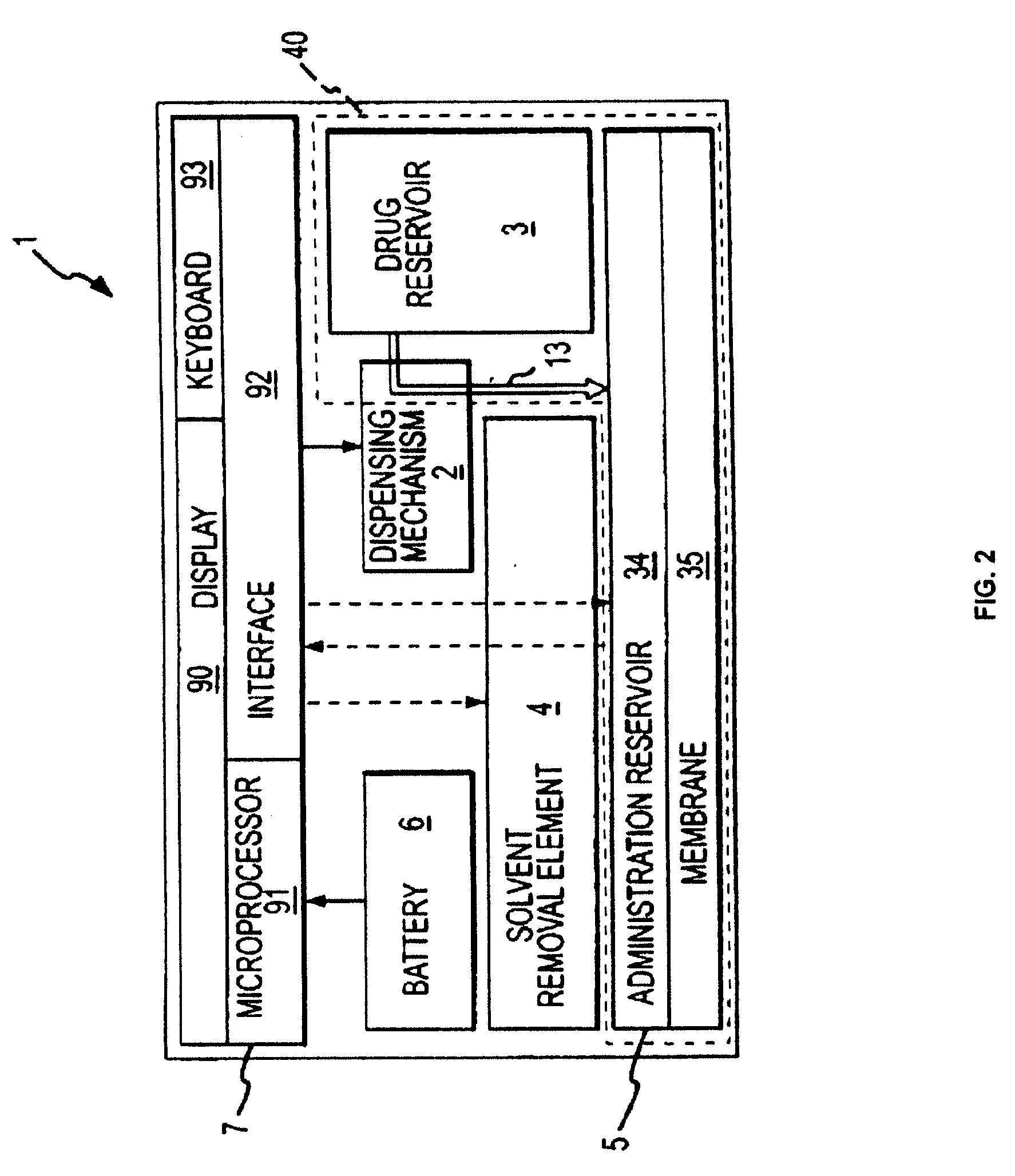
Systems and methods for longevity, anti-aging, fatigue management, obesity, weight loss, weight management, delivery of nutraceuticals, and treating hyperglycemia, Alzheimer's disease, sleep disorders, Parkinson's disease, Attention Deficit Disorder and nicotine addiction involve synchronizing and tailoring the administration of nutraceuticals, medications and other substances (for example, stimulants) in accordance with the body's natural circadian rhythms, meal times and other factors. Improved control of blood glucose levels, extended alertness, and weight control, and counteracting of disease symptoms when they are at their worst are possible. An automated, pre-programmable transdermal administration system is used to provide pulsed doses of medications, pharmaceuticals, hormones, neuropeptides, anorexigens, pro-drugs, stimulants, plant extracts, botanicals, nutraceuticals, cosmeceuticals, phytochemicals, phytonutrients, enzymes, antioxidants, essential oils, fatty acids, minerals, vitamins, amino acids, coenzymes, or other physiological active ingredient or precursor. The system can utilize a pump, pressurized reservoir, a system for removing depleted carrier solution, or other modulated dispensing actuator, in conjunction with porous membranes or micro-fabricated structures.
Owner:MORNINGSIDE VENTURE INVESTMENTS
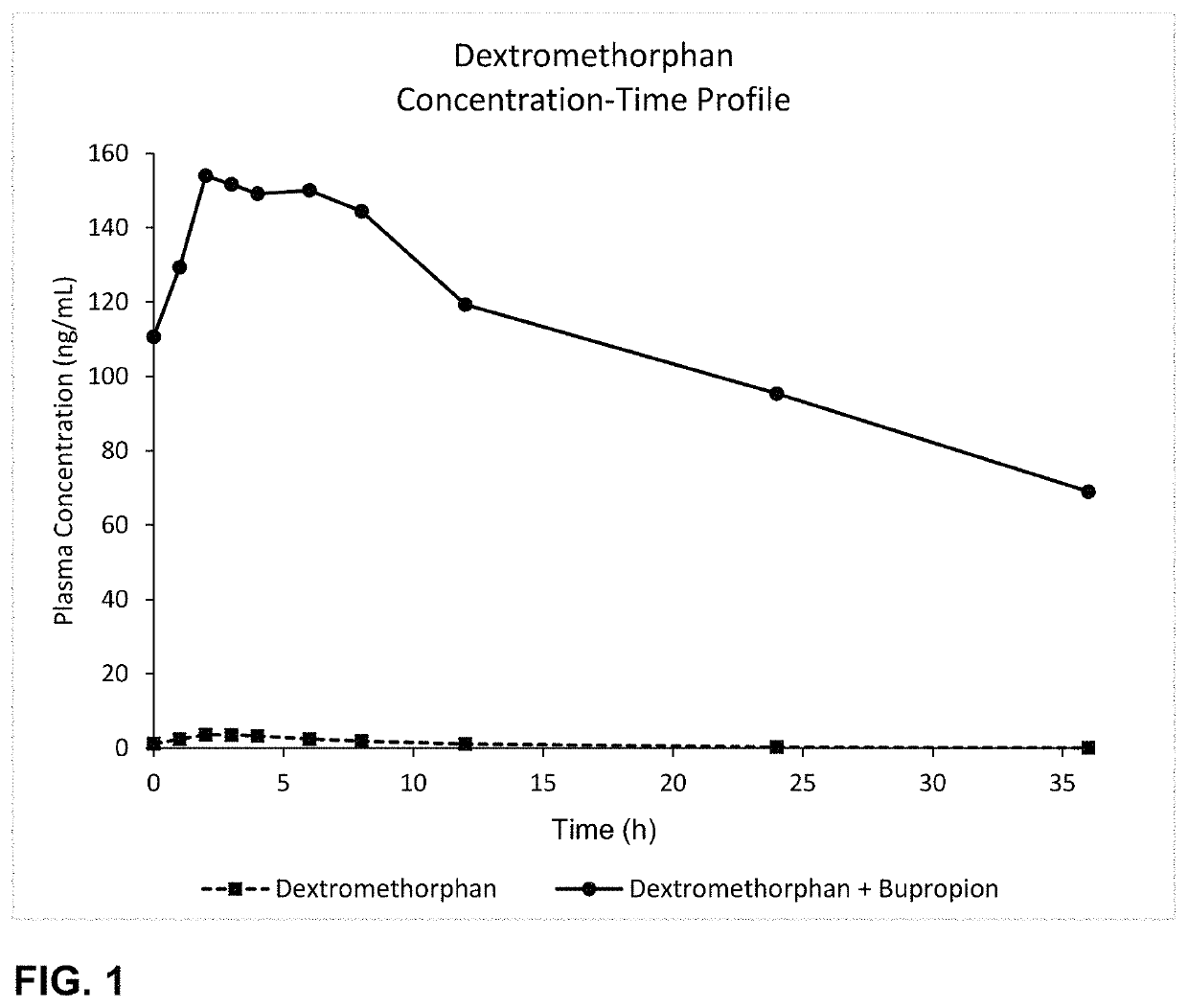
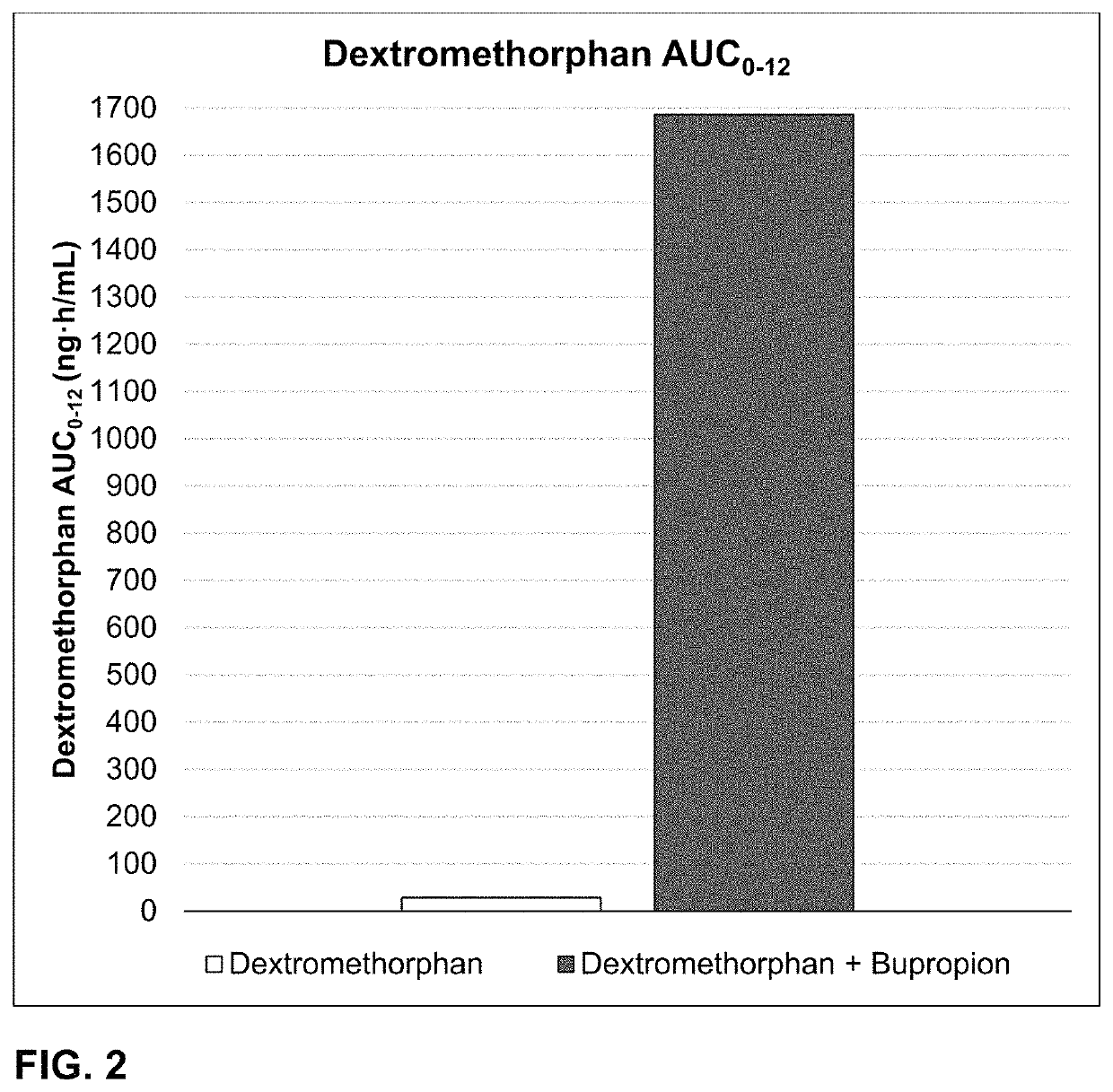
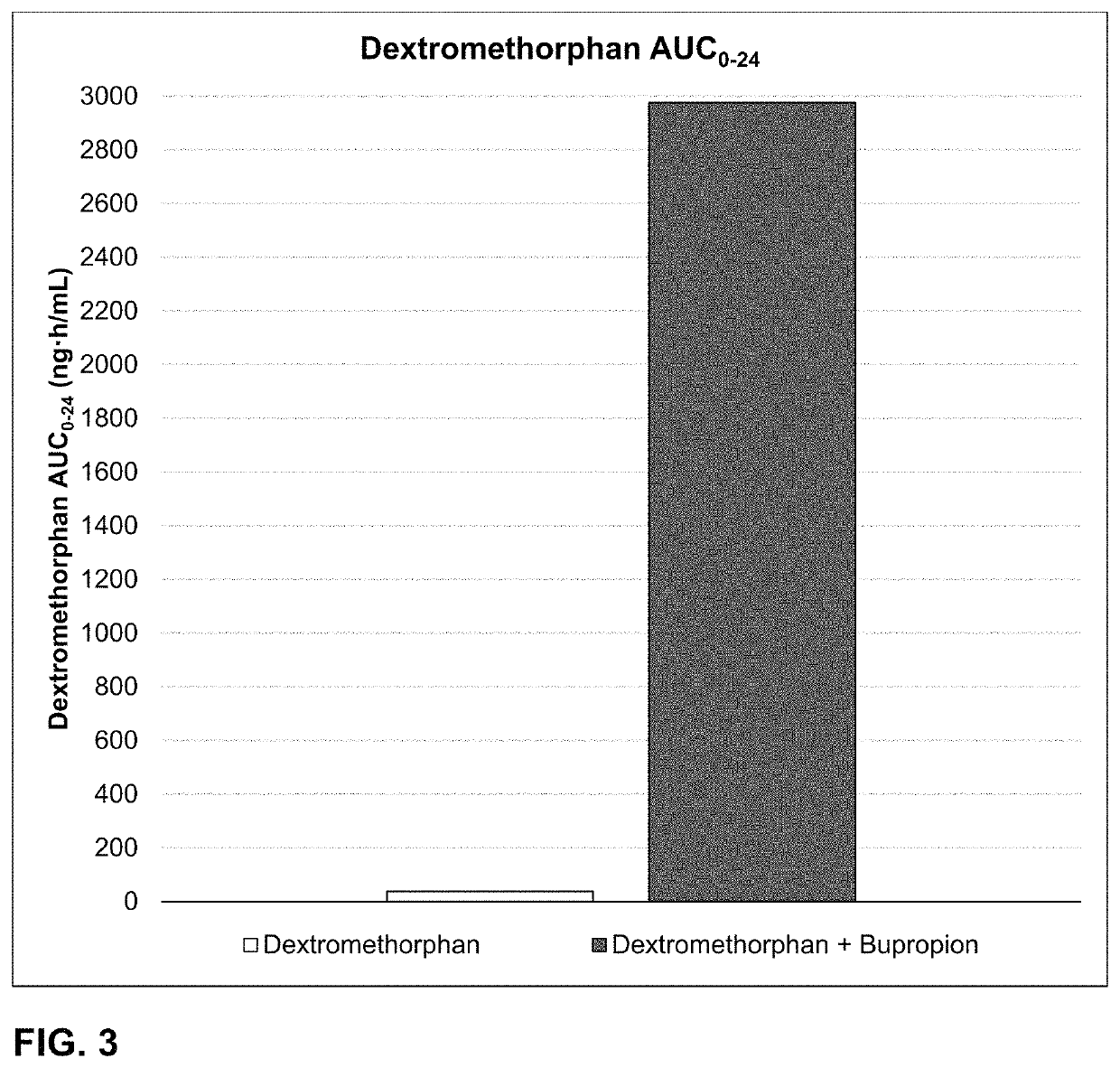
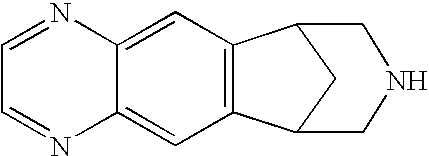
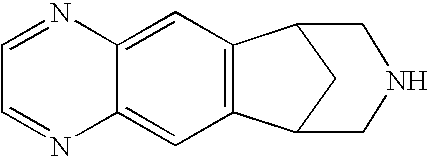
Bupropion and dextromethorphan for treating nicotine addiction
ActiveUS10688066B2Reduce in quantityOrganic active ingredientsNervous disorderPharmaceutical drugTherapeutic effect
Dosage forms, drug delivery systems, and methods related to sustained release of dextromethorphan or improved therapeutic effects are disclosed. Typically, bupropion or a related compound is orally administered to a human being to be treated with, or being treated with, dextromethorphan.
Owner:ANTECIP BIOVENTURES II
Compositions and methods for intranasal, buccal, sublingual and pulmonary delivery of varenicline
InactiveUS20060084656A1Promote nasal absorptionReducing nicotine addictionBiocidePowder deliveryNasal cavityBuccal use
A composition for nasal administration comprising varenicline or its pharmaceutically acceptable salt and at least one excipient. The invention also provides a composition for buccal administration comprising varenicline or its pharmaceutically acceptable salt and at least one excipient to form a solid dosage form, wherein the solid dosage form disintegrates in an oral cavity at body temperature and may adhere to body tissue of the oral cavity; a composition for pulmonary administration comprising varenicline or its pharmaceutically acceptable salt and at least one excipient; and, a method for reducing nicotine addiction, aiding in the cessation of, or lessening of tobacco use in a subject.
Owner:PFIZER INC
Tobacco Raw Material
InactiveUS20170112183A1Enhanced smell and tasteTobacco preparationTobacco treatmentBiotechnologySulfite
Bleached tobacco raw material comprising less than about 4 weight-% fermentable carbohydrates, calculated on the dry total weight of the bleached tobacco raw material. Smoking tobacco composition comprising the bleached tobacco raw material. Smokeless tobacco composition comprising the bleached tobacco raw material. Nasal snuff comprising the smokeless tobacco composition. Oral smokeless tobacco product comprising the smokeless tobacco composition. A process for production of bleached tobacco raw material comprising: (a) treating tobacco raw material at acidic pH at about 70° C. to about 180° C. with sulfite ion; (b) defibrating the tobacco raw; and (c) treating the defibrated material with a bleaching agent at about 60° C. to about 90° C. Bleached tobacco raw material for use in the treatment of nicotine addiction.
Owner:WINNINGTON AB
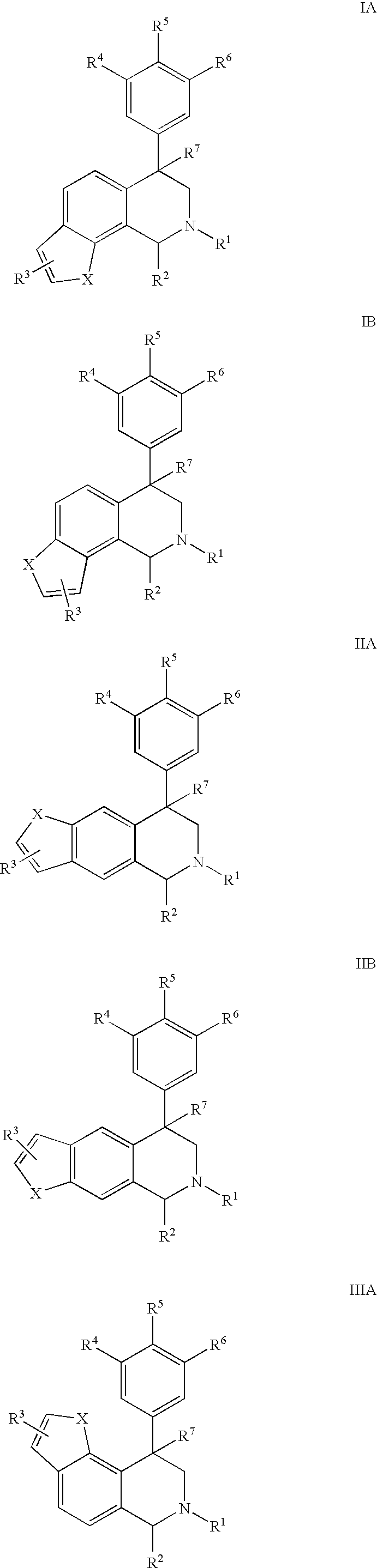
Novel 4-phenyl substituted tetrahydroisoquinolines and therapeutic use thereof
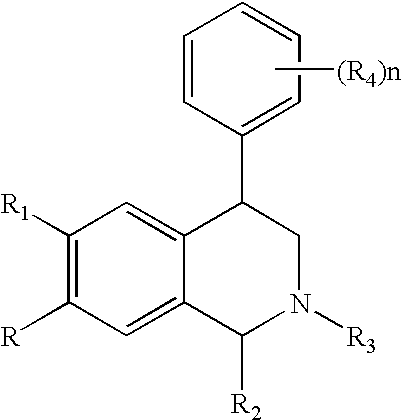
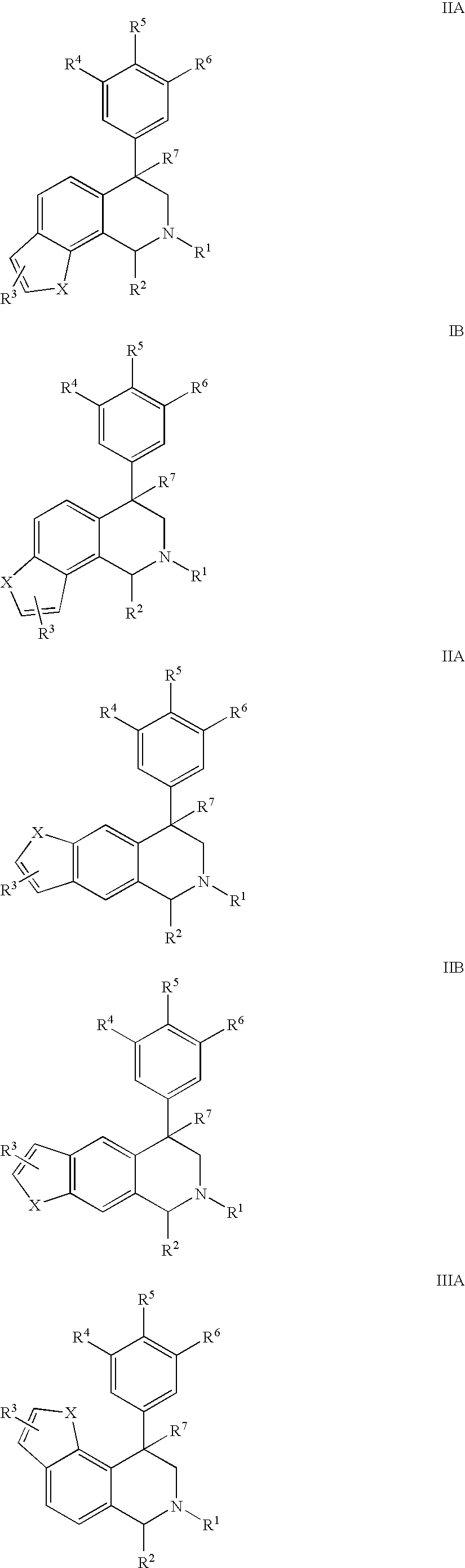
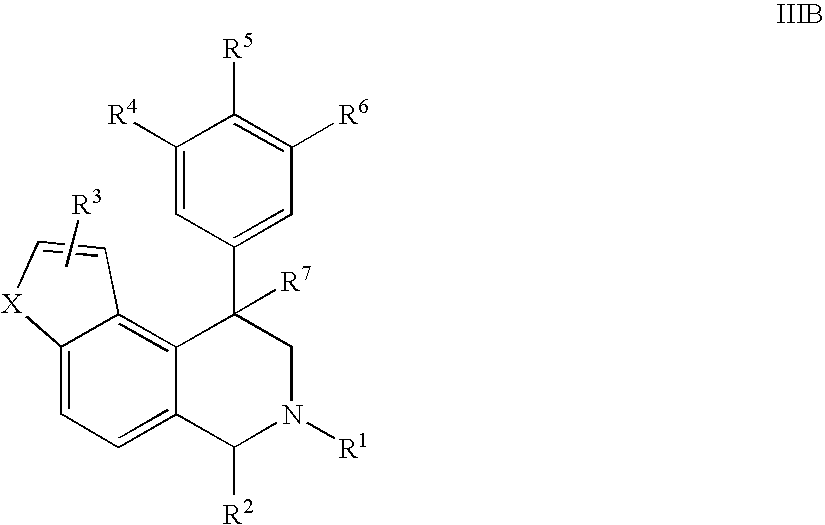
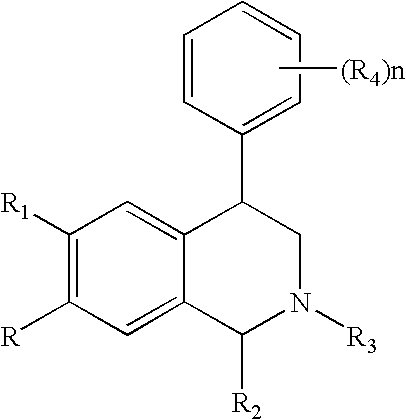
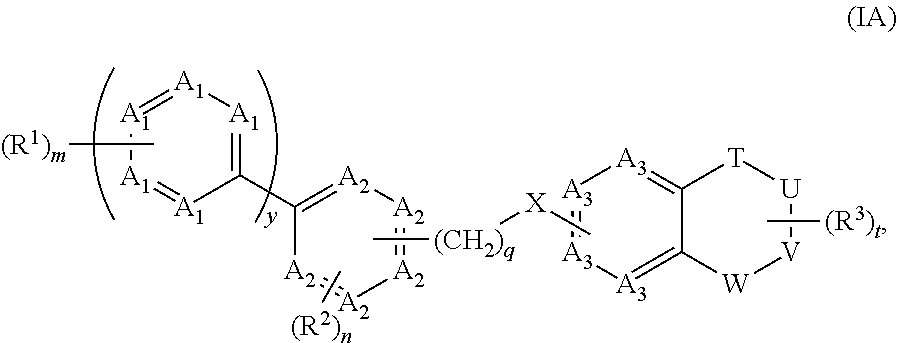
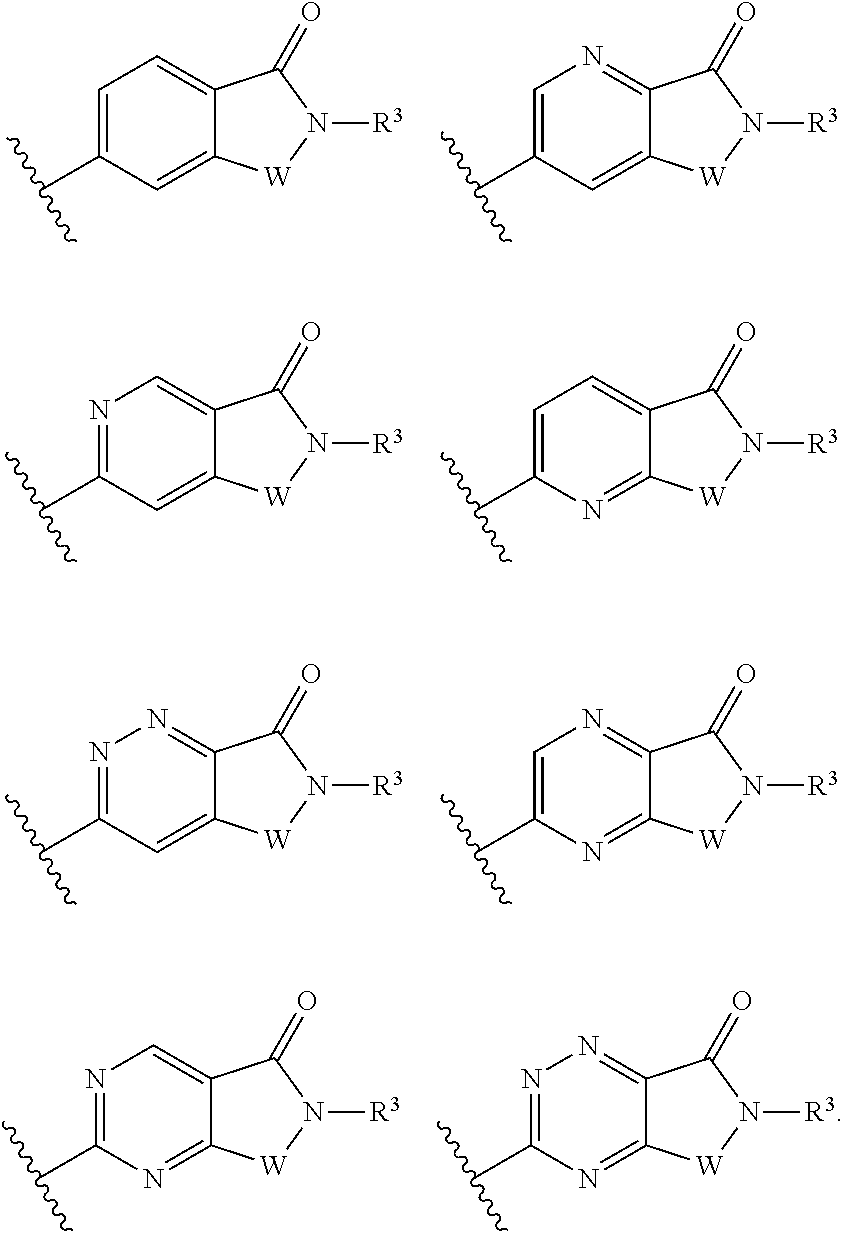
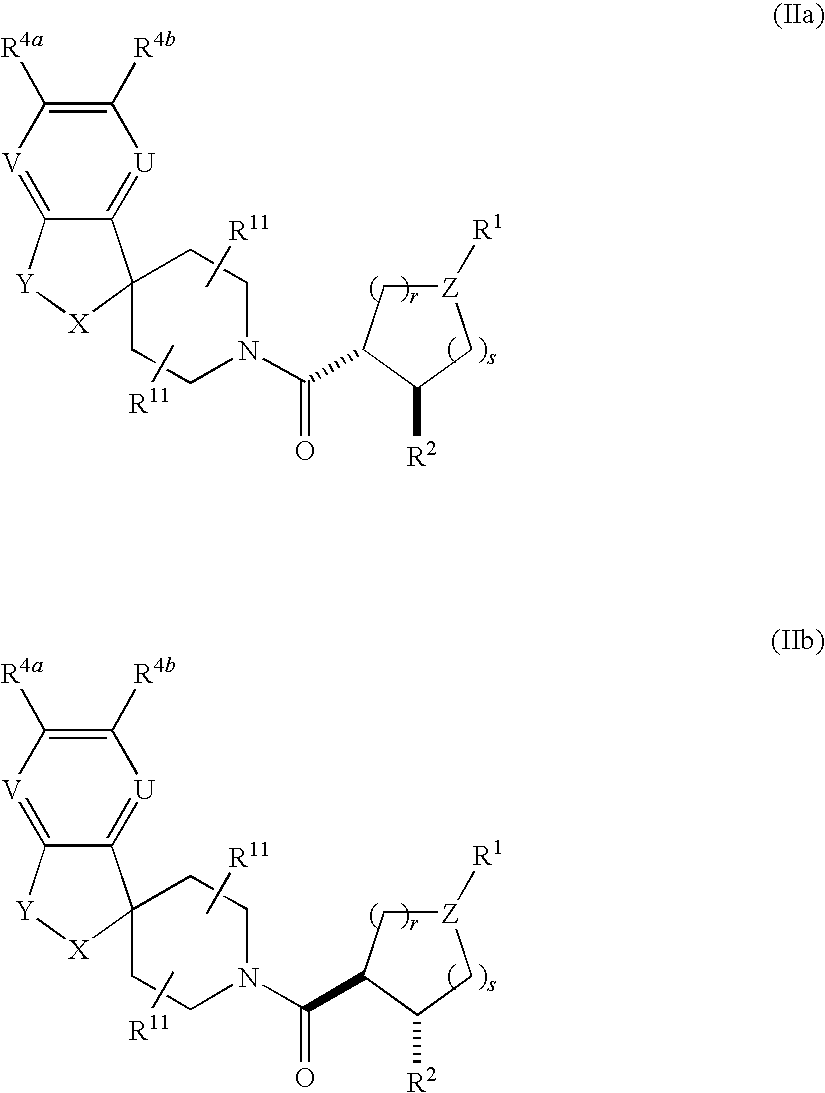
The present invention relates to a method of treating disorders including cognition impairment, generalized anxiety disorder, acute stress disorder, social phobia, simple phobias, pre-menstrual dysphoric disorder, social anxiety disorder, major depressive disorder, eating disorders, obesity, anorexia nervosa, bulimia nervosa, binge eating disorder, substance abuse disorders, chemical dependencies, nicotine addiction, cocaine addiction, alcohol addiction, amphetamine addiction, Lesch-Nyhan syndrome, neurodegenerative diseases, late luteal phase syndrome, narcolepsy, psychiatric symptoms anger, rejection sensitivity, movement disorders, extrapyramidal syndrome, Tic disorder, restless leg syndrome, tardive dyskinesia, sleep related eating disorder, night eating syndrome, stress urinary incontinence, migraine, neuropathic pain, diabetic neuropathy, fibromyalgia syndrome, chronic fatigue syndrome, sexual dysfunction, premature ejaculation, and male impotence. This method involves administering to a patient in need of such treatment a therapeutically effective amount of a disclosed compound. Such compounds are 4-phenyl substituted tetrahydroisoquinolines having the Formula IA, IB, IIA, IIB, IIIA or IIIC as set forth herein.
Owner:ALBANY MOLECULAR RESEARCH INC
Novel 4-phenyl substituted tetrahydroisoquinolines and therapeutic use thereof
The present invention relates to a method of treating disorders including cognition impairment, generalized anxiety disorder, acute stress disorder, social phobia, simple phobias, pre-menstrual dysphoric disorder, social anxiety disorder, major depressive disorder, eating disorders, obesity, anorexia nervosa, bulimia nervosa, binge eating disorder, substance abuse disorders, chemical dependencies, nicotine addiction, cocaine addiction, alcohol addiction, amphetamine addiction, Lesch-Nyhan syndrome, neurodegenerative diseases, late luteal phase syndrome, narcolepsy, psychiatric symptoms anger, rejection sensitivity, movement disorders, extrapyramidal syndrome, Tic disorder, restless leg syndrome, tardive dyskinesia, sleep related eating disorder, night eating syndrome, stress urinary incontinence, migraine, neuropathic pain, diabetic neuropathy, fibromyalgia syndrome, chronic fatigue syndrome, sexual dysfunction, premature ejaculation, and male impotence. This method involves administering to a patient in need of such treatment a therapeutically effective amount of a disclosed compound. Such compounds are 4-phenyl substituted tetrahydroisoquinolines having the Formula IA, IB, IIA, IIB, IIIA or IIIC as set forth herein.
Owner:ALBANY MOLECULAR RESEARCH INC
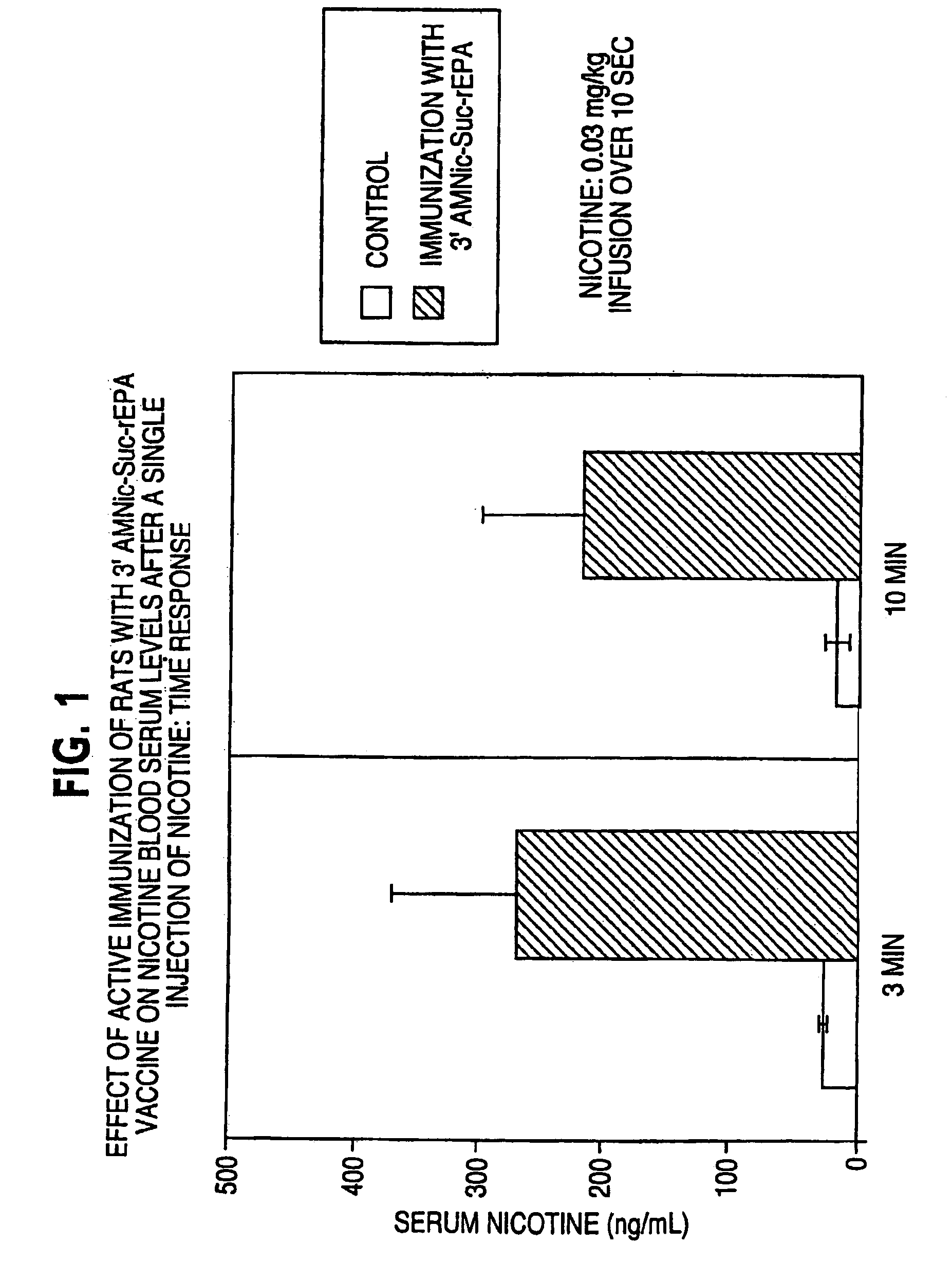
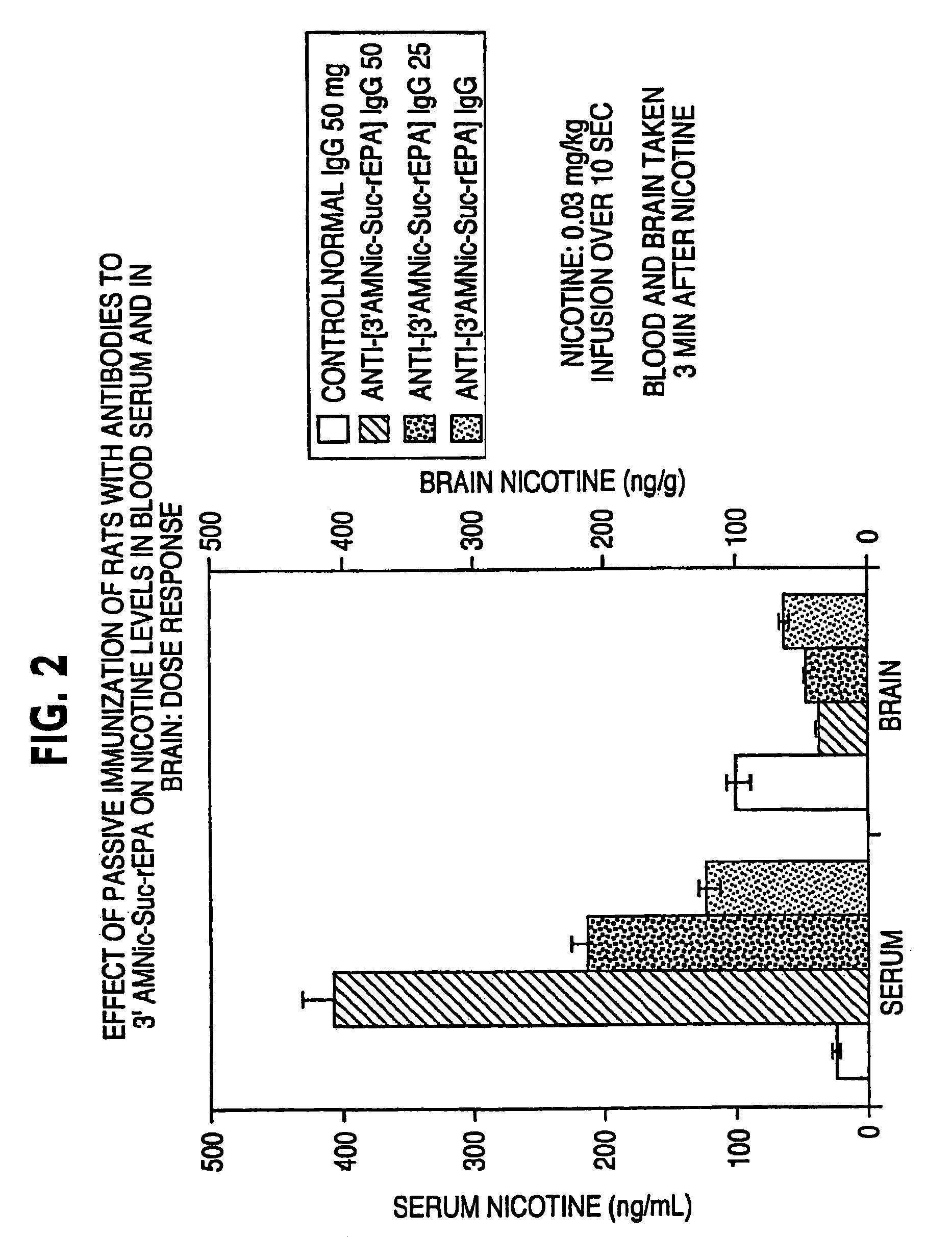
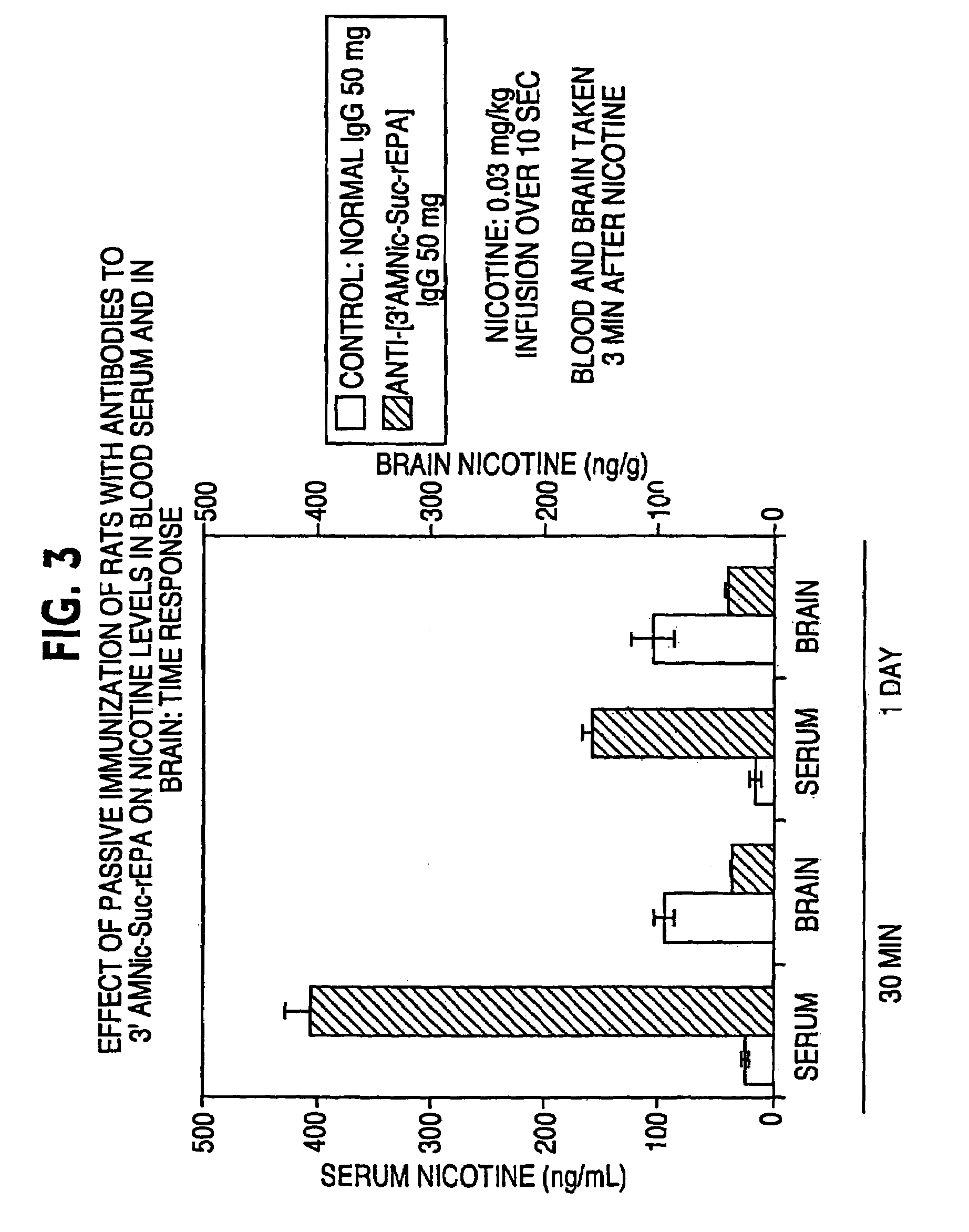
Hapten-carrier conjugates for treating and preventing nicotine addiction
InactiveUS7247502B2Easy to produceImprove the level ofBiocideNervous disorderPassive ImmunizationsCarrier protein
Owner:NABI BIOPHARMLS
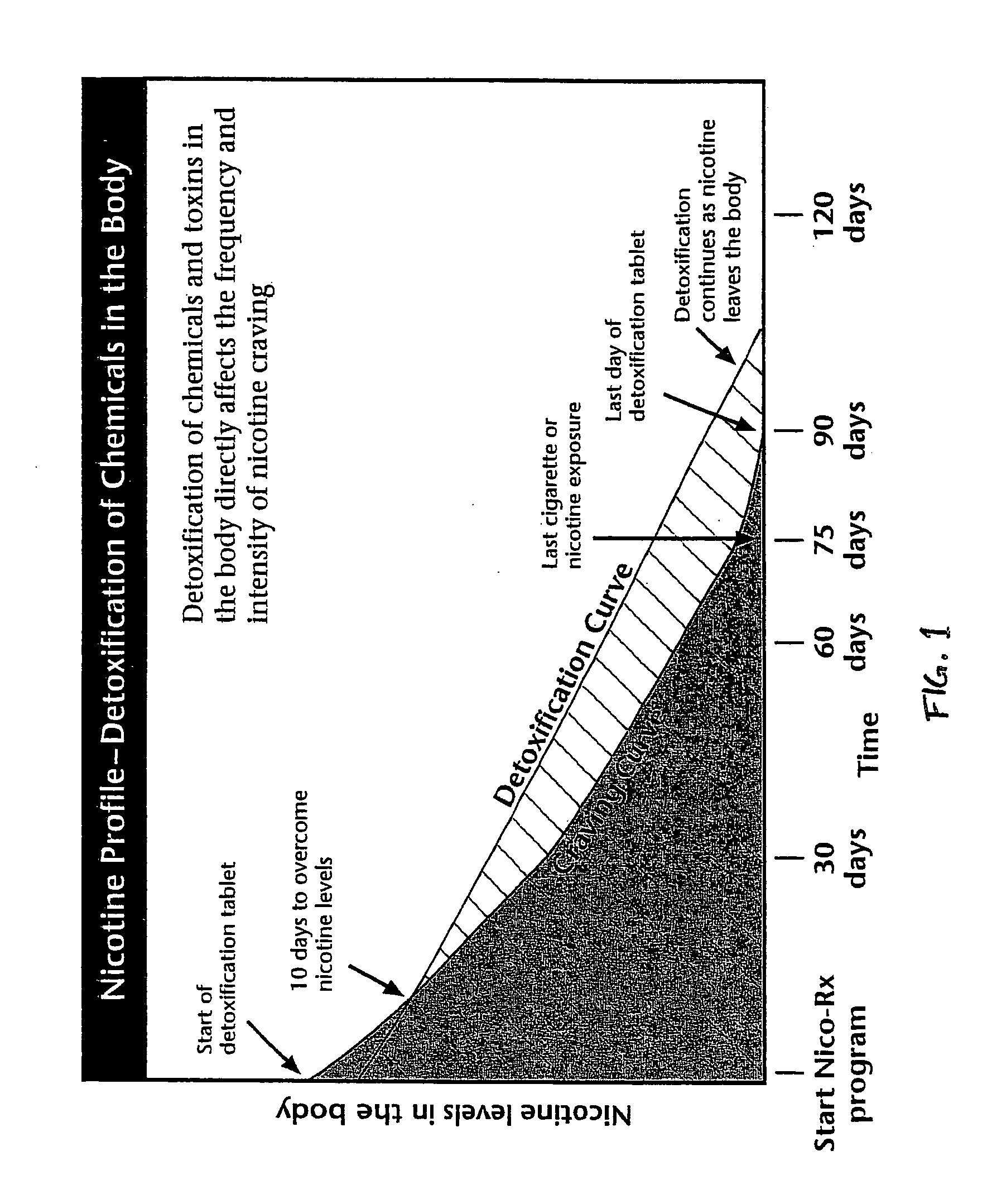
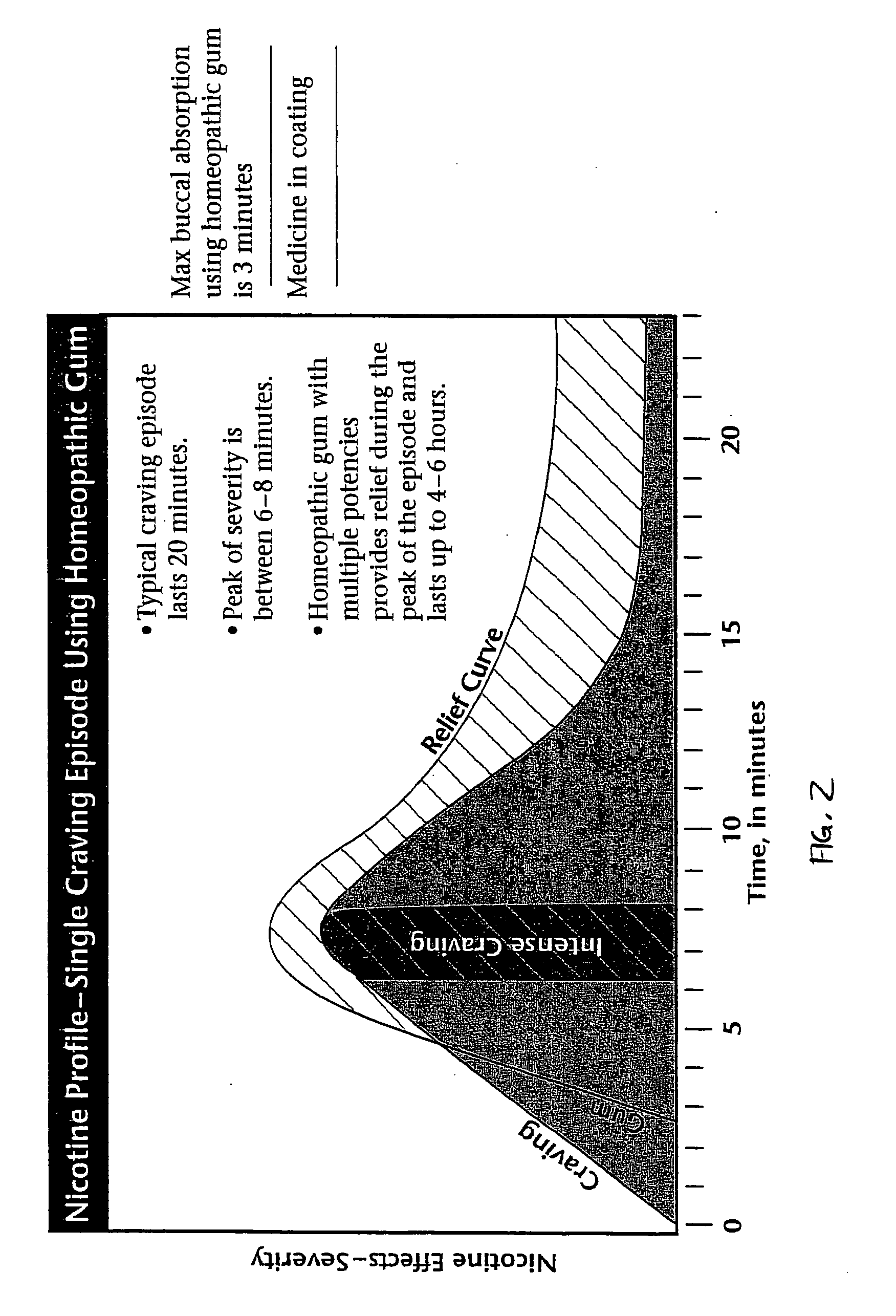
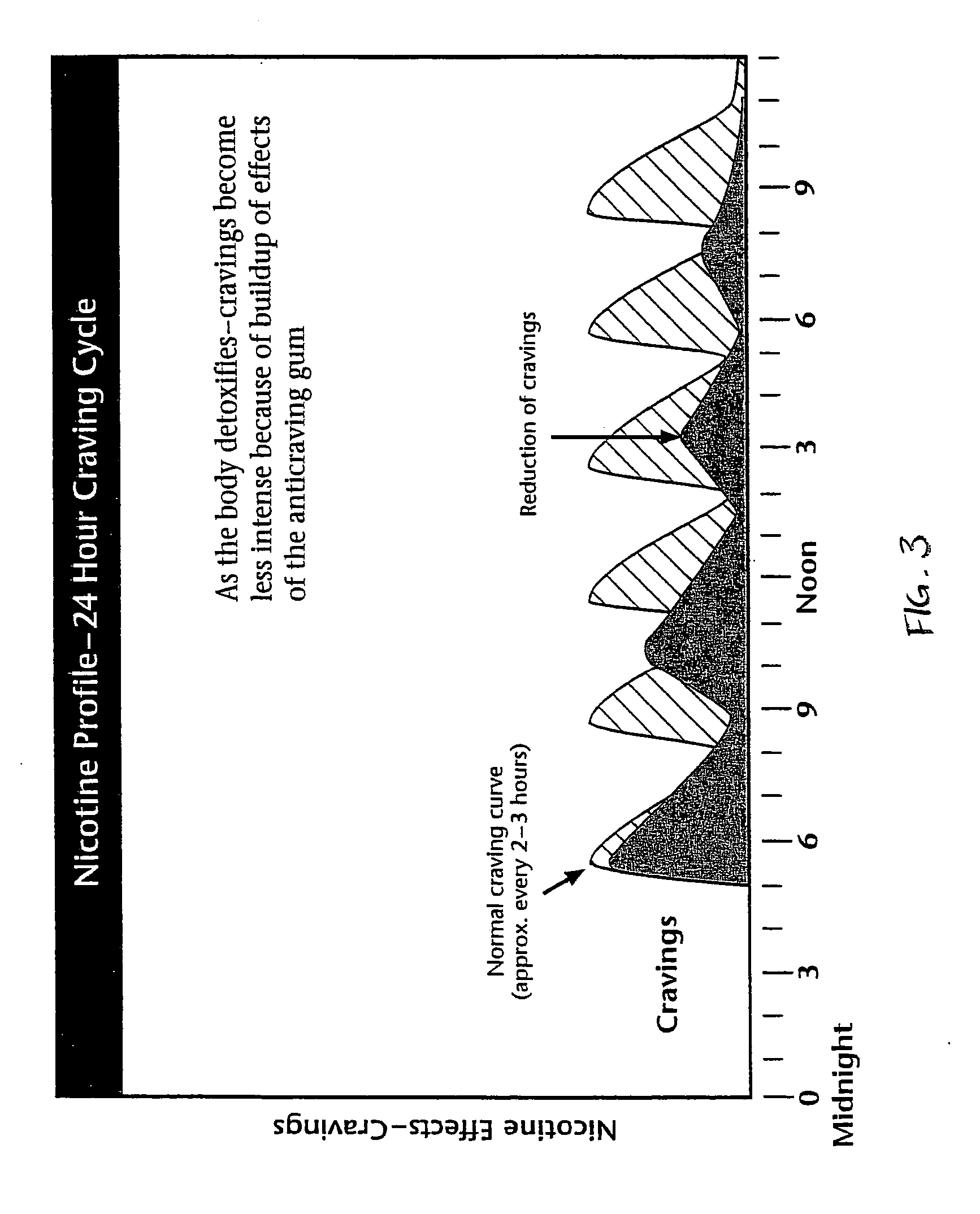
Homeopathic method and system for treating nicotine addiction
The present invention includes a method for aiding an individual in the cessation of nicotine use. The method has the steps of administering a first homeopathic composition to the individual. The first homeopathic composition is formulated to reduce nicotine craving by the individual. A second homeopathic composition is contemporaneously administered in conjunction with the first homeopathic composition. The second homeopathic composition formulated to detoxify the individual of residual nicotine and nicotine byproducts.
Owner:WATKINS MARY BETH +1
Biosynchronous transdermal drug delivery for longevity, anti-aging, fatigue management, obesity, weight loss, weight management, delivery of nutraceuticals, and the treatment of hyperglycemia, alzheimer's disease, sleep disorders, parkinson's disease, aids, epilepsy, attention deficit disorder, nicotine addiction, cancer, headache and pain control, asthma, angina, hypertension, depression, cold, flu and the like
ActiveUS8252321B2Improve performanceReduce the amount requiredHeavy metal active ingredientsBiocideDiseasePhytochemical
Owner:MORNINGSIDE VENTURE INVESTMENTS
Pharmaceutical compositions and methods for relieving pain and treating central nervous system disorders
InactiveUS20050282823A1Prevent and suppress symptomsIncrease the number ofBiocideNervous disorderS syndromeRacemic mixture
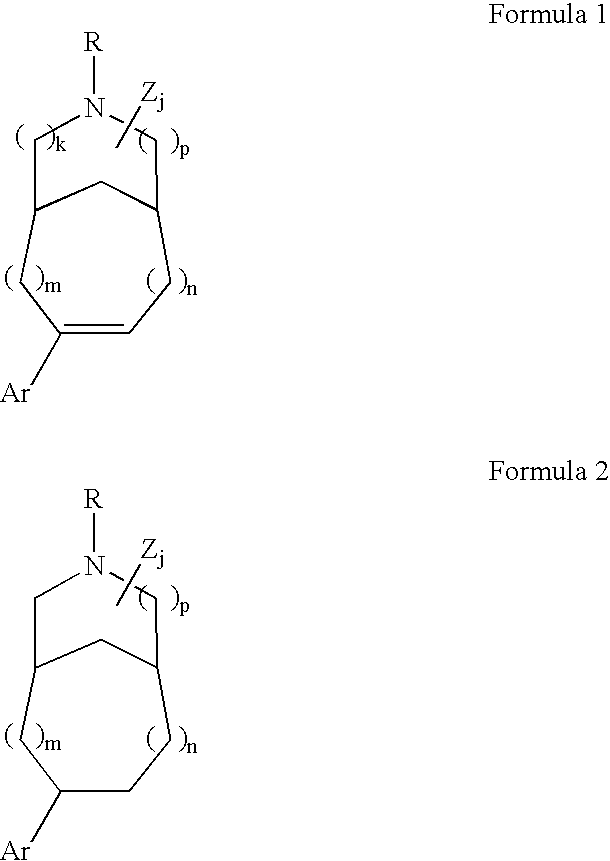
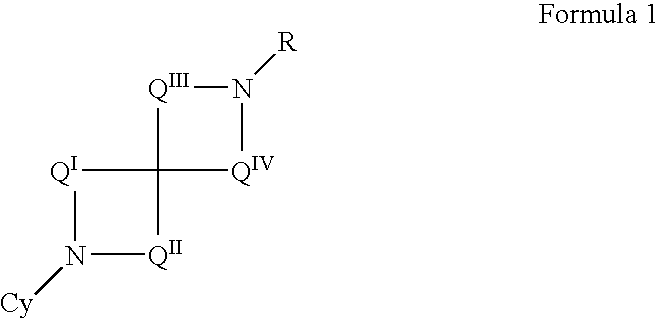
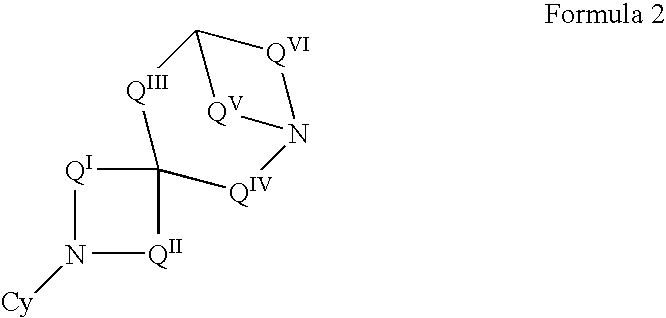
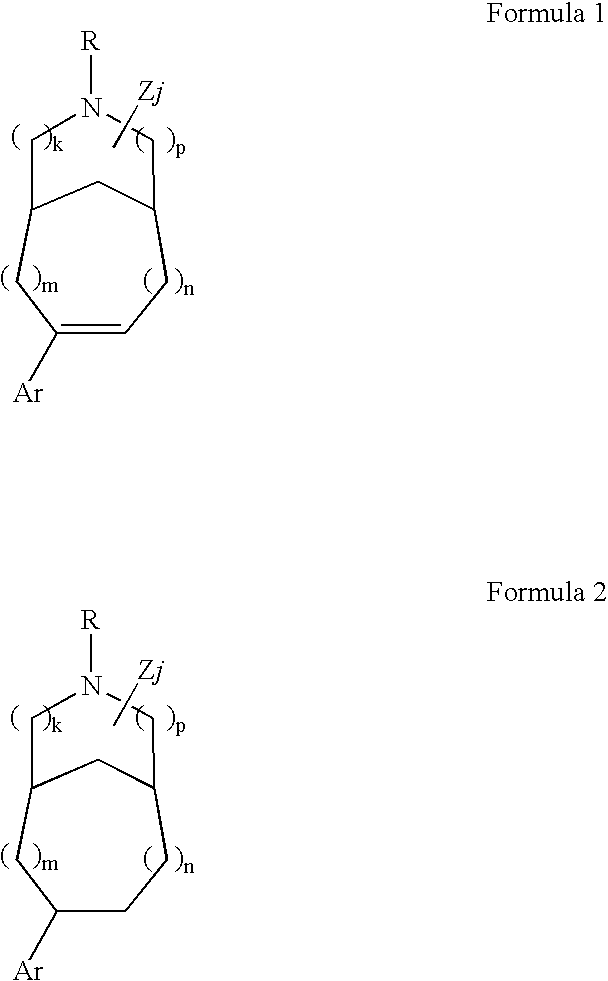
Patients susceptible to or suffering from disorders, such as central nervous system disorders, which are characterized by an alteration in normal neurotransmitter release, such as dopamine release (e.g., Parkinsonism, Parkinson's Disease, Tourette's Syndrome, attention deficient disorder, or schizophrenia), are treated by administering a compound of Formulas 1 or 2, as described herein. The compounds of Formulas 1 and 2 are also useful for treating pain, and treating drug addiction, nicotine addiction, and / or obesity. The compounds can exist as individual stereoisomers, racemic mixtures, diastereomers and the like.
Owner:TARGACEPT INC
Oral transmucosal nicotine dosage form
InactiveUS20080131508A1Effectively and rapidly therapeutically effective amountImprove bioavailabilityBiocideNervous disorderNicotine agentSolid Dose Form
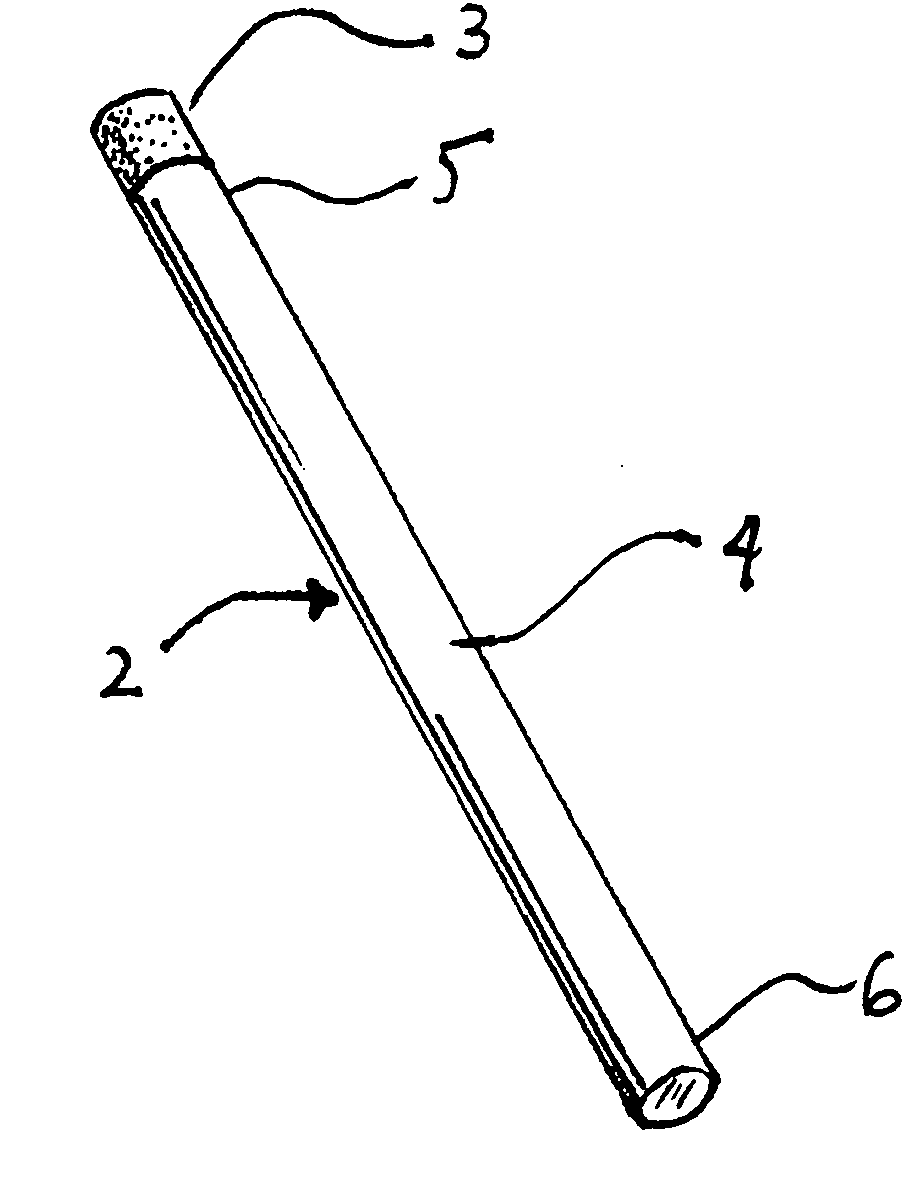
The invention described herein relates to an oral transmucosal solid dosage form useful in treating nicotine addiction or as a nicotine substitute or replacement. By virtue of the formulation in combination with nicotine, the invention transmucosally delivers an effective amount of nicotine to the recipient while permitting the accomplishing of such, and manufacture of such, using a relatively small, convenient and orally comfortable dosage form (e.g., tablet) size.
Owner:CIMA LABS +1
Compositions and compounds for use as molecular adjuvant for a nicotine vaccine
Owner:BOARD OF RGT UNIV OF NEBRASKA
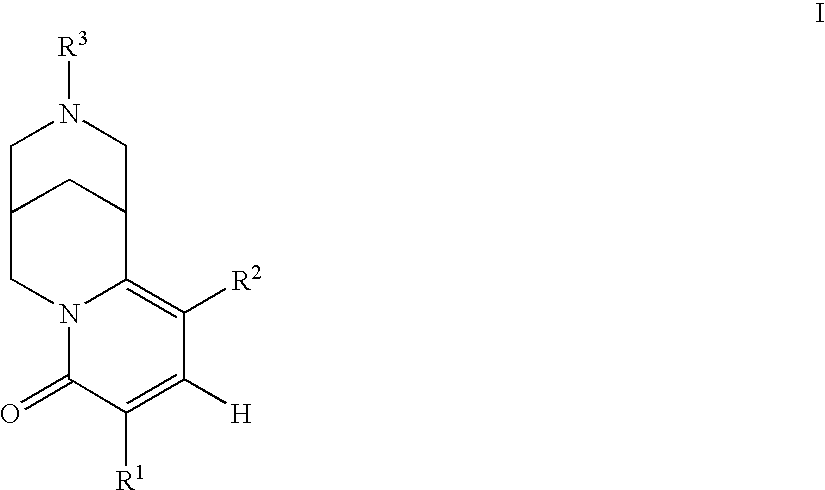
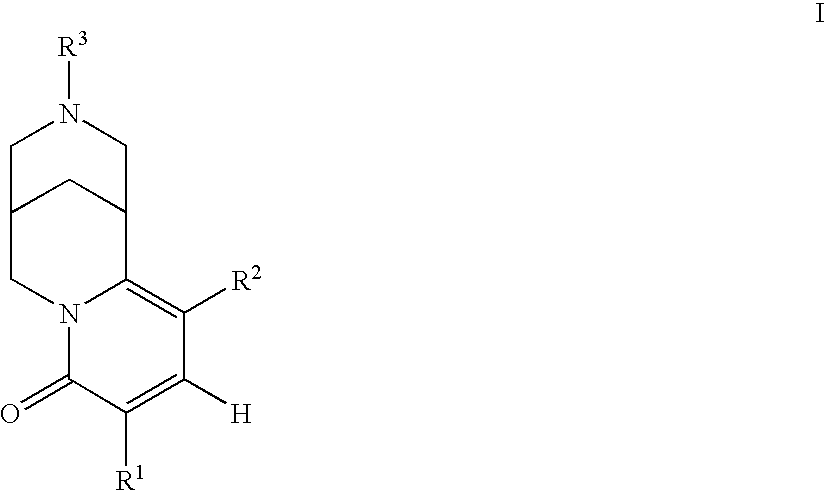
Pyridone-fused azabicyclic- or cytisine derivatives, their preparation and their use in addiction therapy
InactiveUS6630467B2Reduce addictionLessing of tobacco useBiocideNervous disorderUlcerative colitisAzabicyclo Compounds
Owner:PFIZER INC
Positive allosteric modulators of group ii mglurs
The disclosure provides compounds and compositions, and methods of using these compounds and compositions, as positive allosteric modulators of the metabotropic glutamate subtype 2 (mGlu2) receptor, and for treating CNS disorders associated with the mGlu2 receptor including schizophrenia, anxiety, addiction, e.g. cocaine addiction, nicotine addiction, and the like.
Owner:SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INST +1
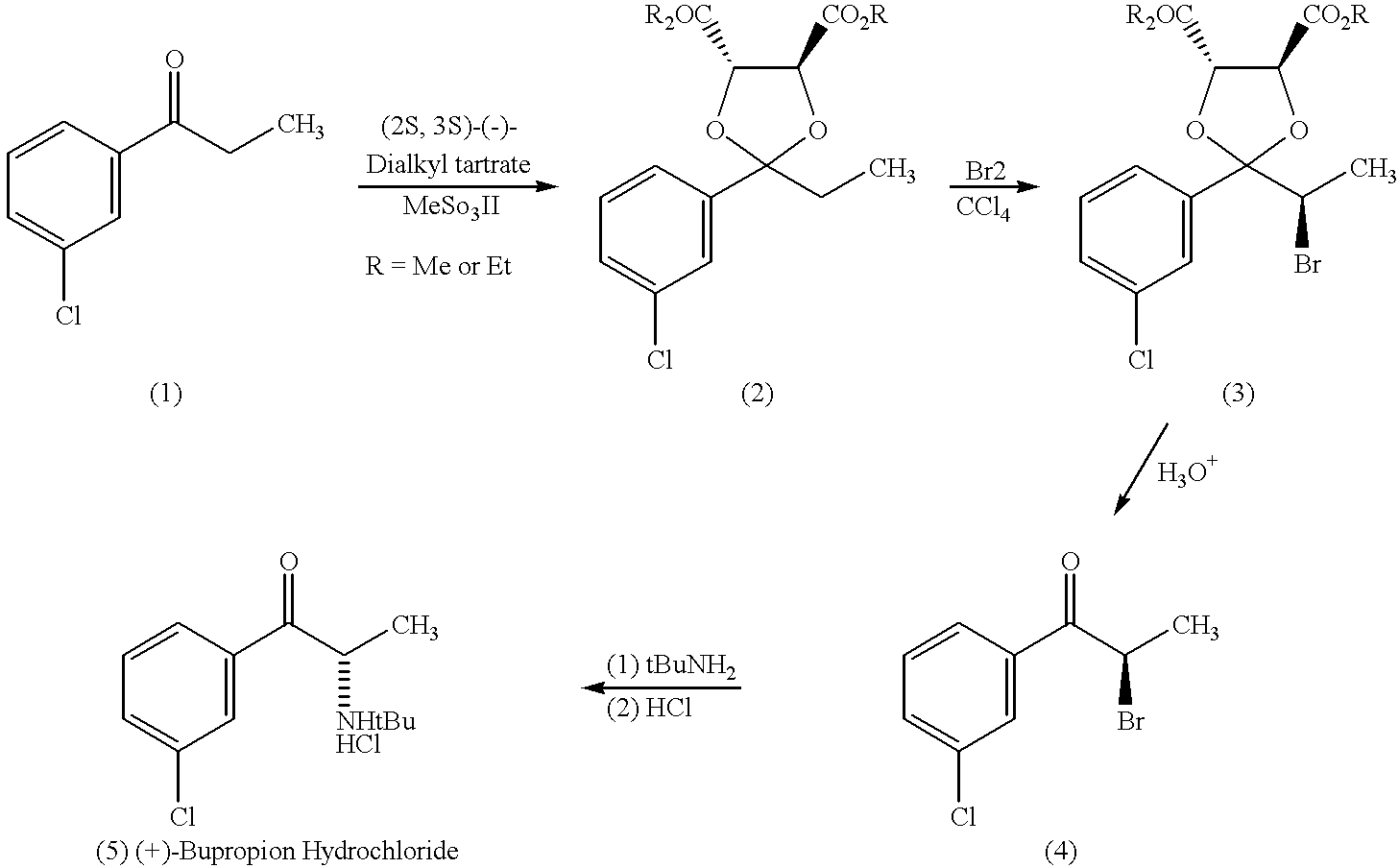
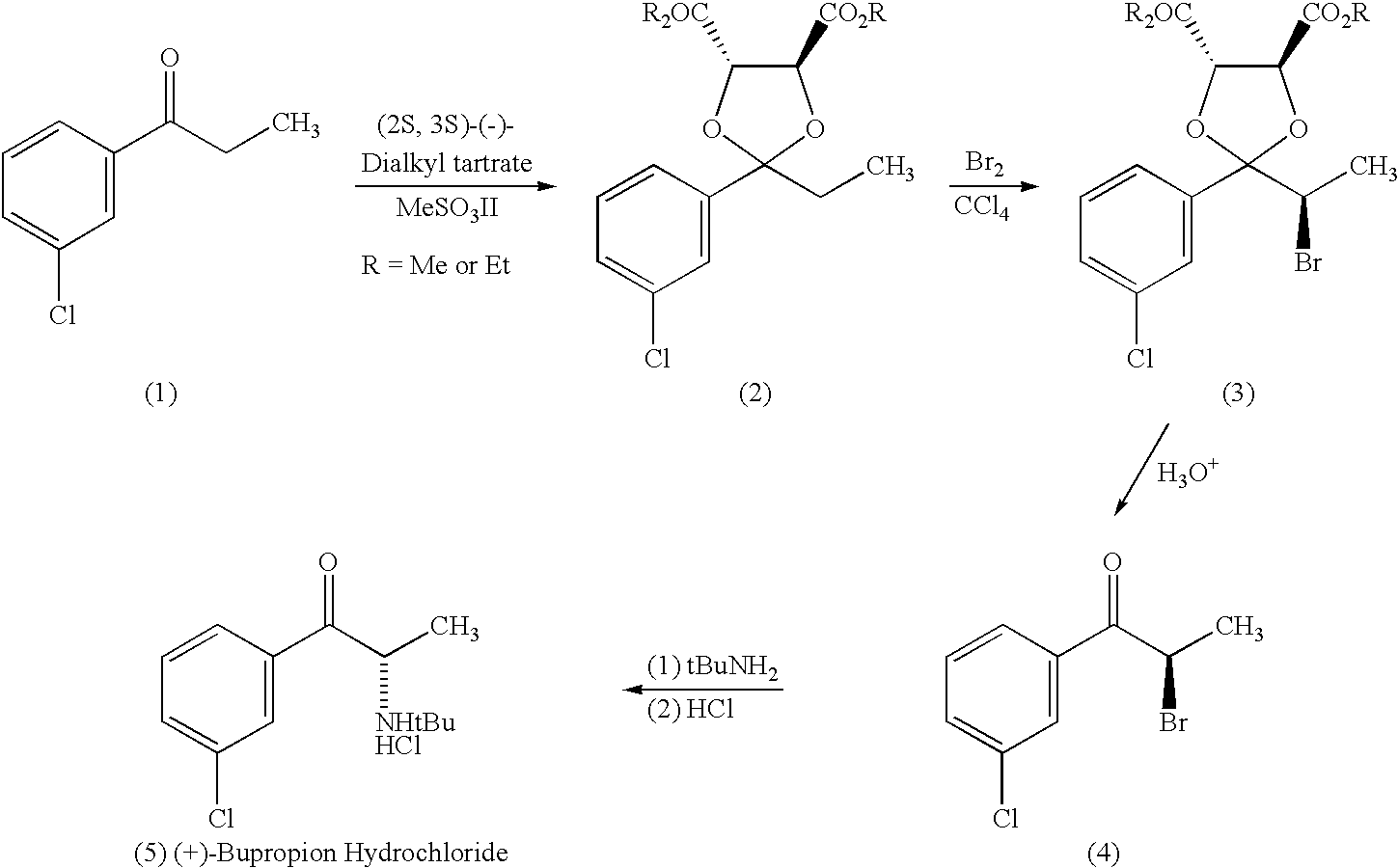
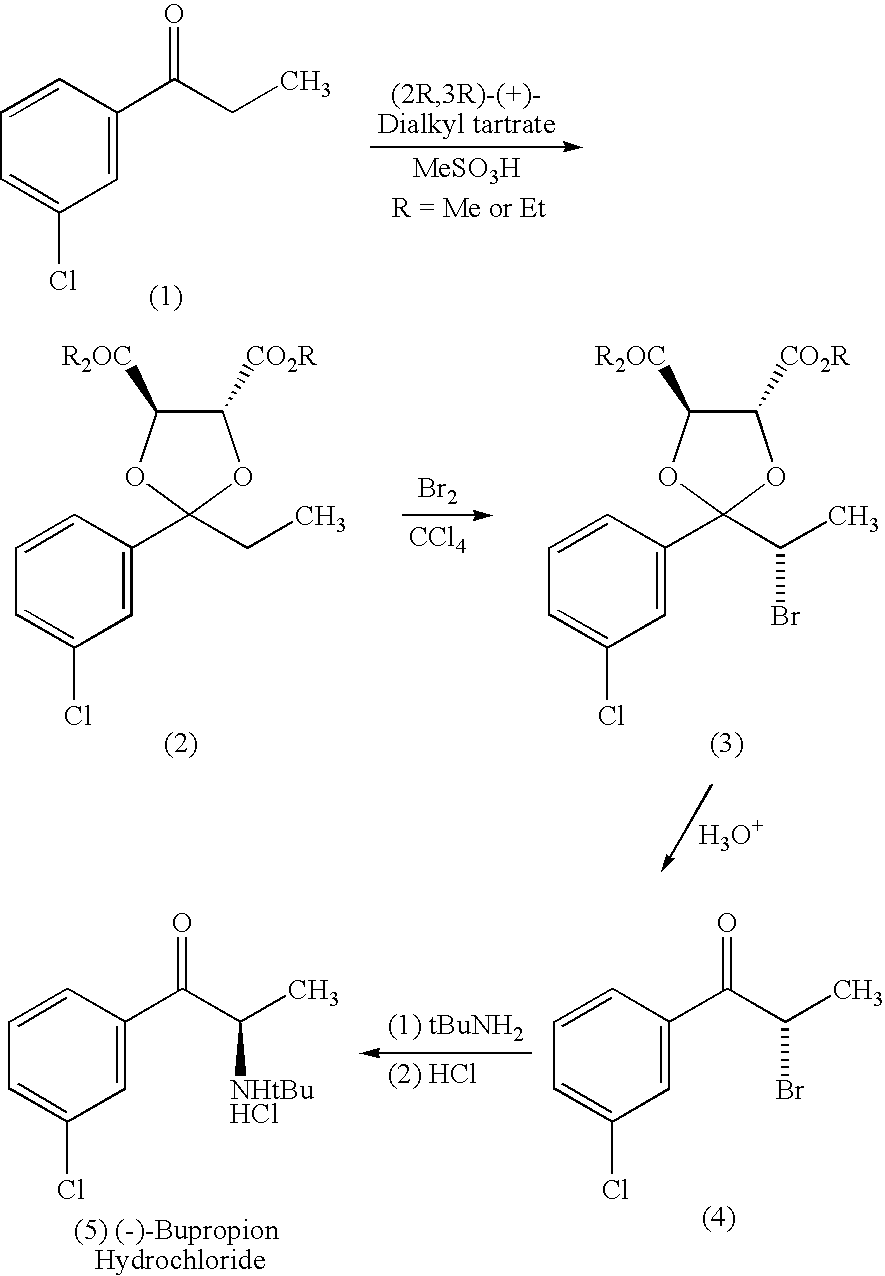
Methods and compositions for aiding in smoking cessation and for treating pain and other disorders using optically pure (+)-bupropion
InactiveUS20010011103A1Reduce adverse effectsEffective treatmentOrganic active ingredientsBiocideReflexSeasonal Affective Disorders
Methods and compositions are disclosed utilizing the optically pure (+)-isomer of bupropion to assist in smoking cessation, for treating smoking and nicotine addiction, and for treating pain, including, but not limited to, chronic pain, neuropathetic pain and reflex sympathetic dystrophy, and other disorders such as narcolepsy, chronic fatigue syndrome, fibromyalgia, seasonal affective disorder and premenstrual syndrome, while avoiding adverse affects associated with racemic bupropion.
Owner:SEPACOR INC
Use of N-aryl diazaspiracyclic compounds in the treatment of addiction
InactiveUS20060058328A1Reduces dopamine releaseReduce releaseBiocideNervous disorderMetaboliteSide effect
Compounds, compositions and methods for treating drug addiction, nicotine addiction, and / or obesity are disclosed. The compounds are N-aryl diazaspirocyclic compounds, bridged analogs of N-heteroaryl diazaspirocyclic compounds, or prodrugs or metabolites of these compounds. The aryl group can be a five- or six-membered heterocyclic ring (heteroaryl). The compounds are effective at inhibiting dopamine production and / or secretion, and accordingly are effective at inhibiting the physiological “reward” process that is associated with ingestion of nicotine and / or illicit drugs. The compounds and compositions can be administered in effective amounts to inhibit dopamine release, without resulting in appreciable adverse side effects (e.g., side effects such as significant increases in blood pressure and heart rate, significant negative effects upon the gastro-intestinal tract, and significant effects upon skeletal muscle).
Owner:BHATTI BALWINDER S +2
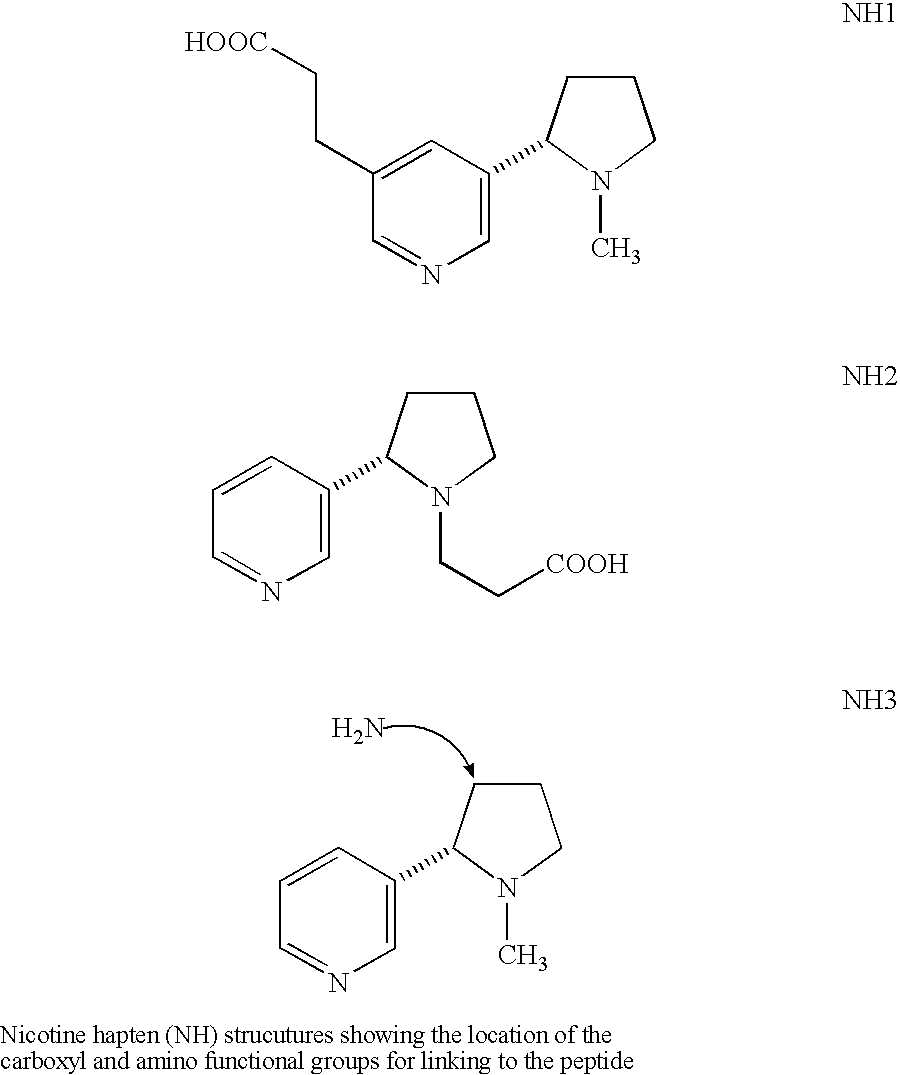
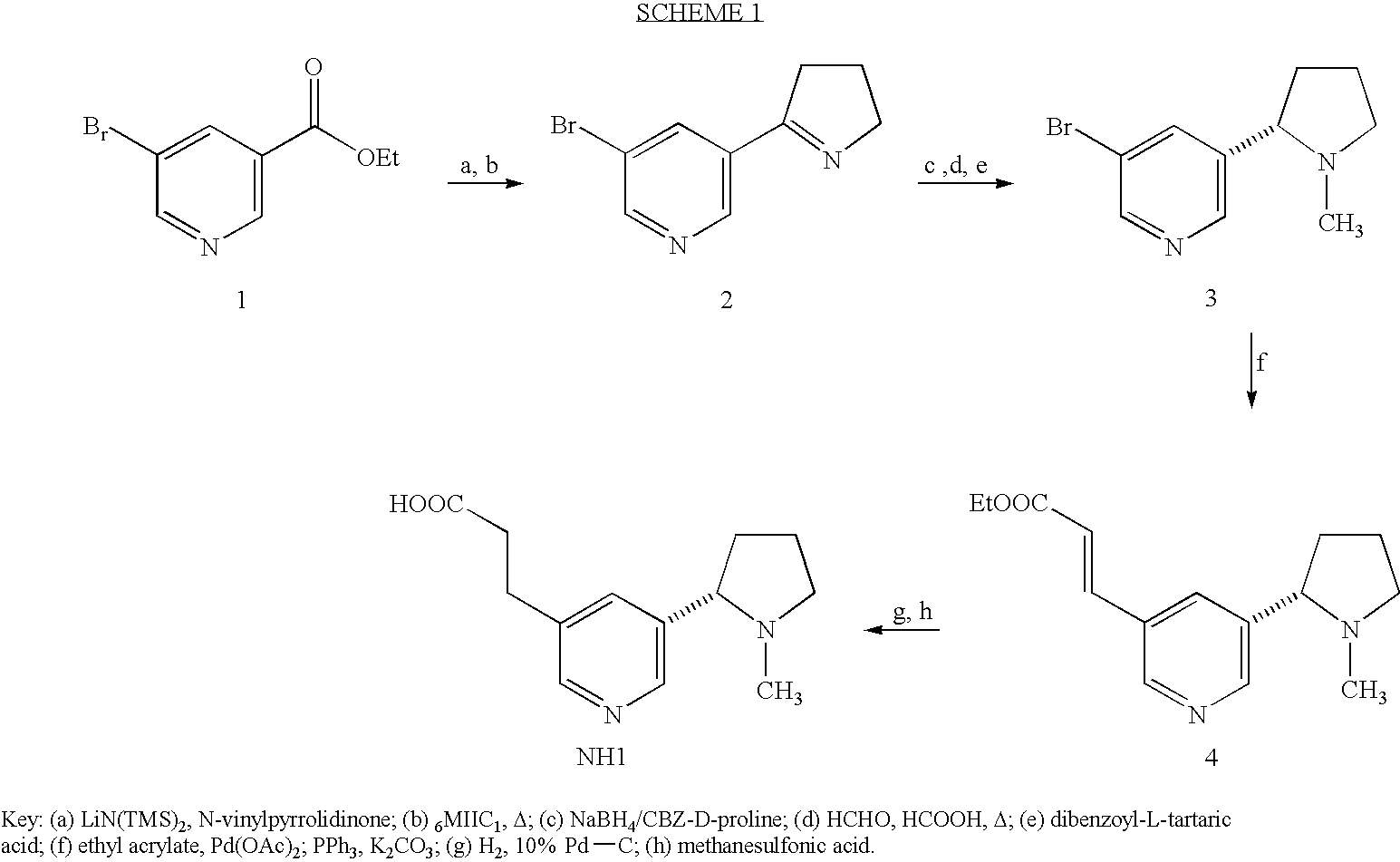
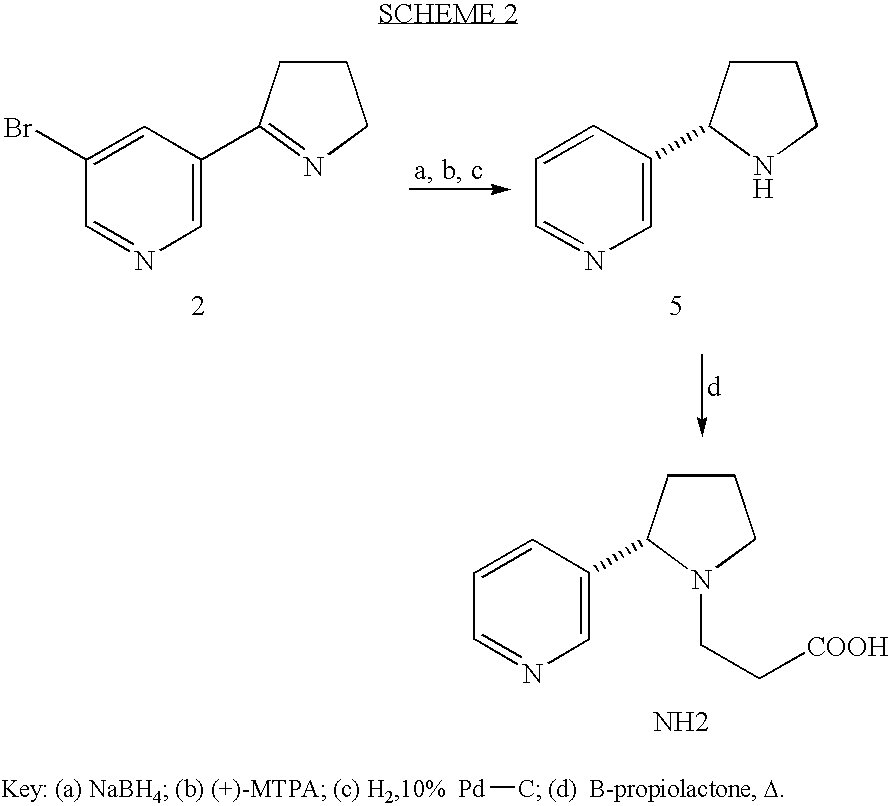
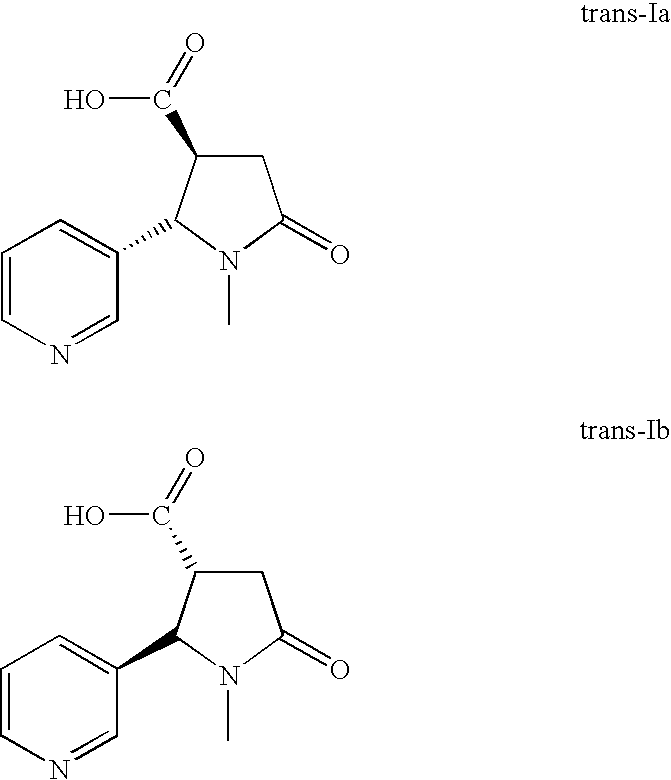
Method for making nicotine hapten
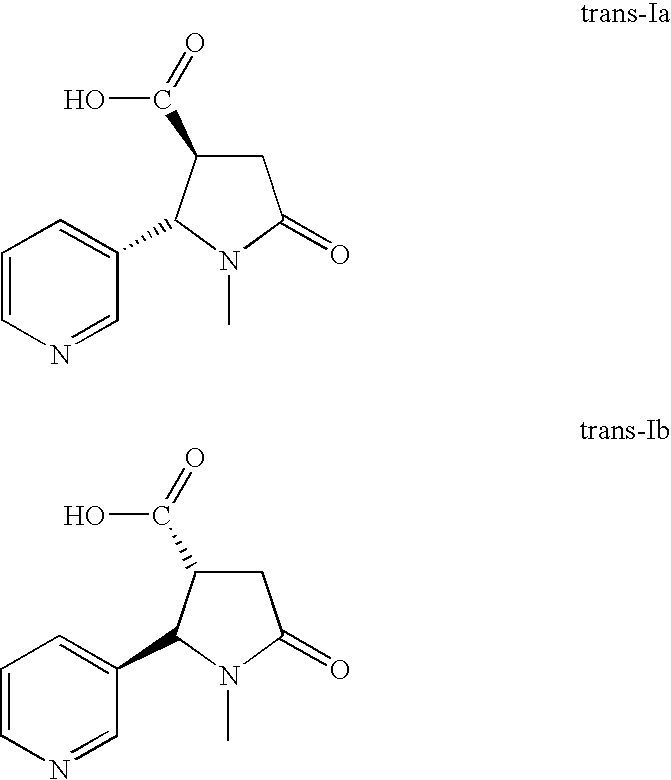
A high yielding and short process for preparing the 3′-aminomethylnicotine hapten of general formula I permits the isolation of the hapten in very pure quantities. The process can be adapted for the synthesis of a single stereoisomer or mixtures of stereoisomers, such as the stereoisomers trans-Ia and trans-Ib: Compounds of general formula I are useful for the preparation of hapten-carrier conjugates in the treatment of nicotine addiction.
Owner:NABI BIOPHARMLS
Low dose noribogaine for treating nicotine addiction and preventing relapse of nicotine use
ActiveUS20150238503A1Reduce smokeIncrease volumeBiocidePharmaceutical delivery mechanismPharmacologyNoribogaine
This invention provides methods and compositions for treating nicotine addiction or treating or preventing nicotine cravings in a subject. The method comprises administering to the patient in need thereof a therapeutically effective amount of noribogaine, noribogaine derivative, or a pharmaceutically acceptable salt thereof.
Owner:DEMERX
Method for making nicotine hapten
A high yielding and short process for preparing the 3′-aminomethylnicotine hapten of general formula Ipermits the isolation of the hapten in very pure quantities. The process can be adapted for the synthesis of a single stereoisomer or mixtures of stereoisomers, such as the stereoisomers trans-Ia and trans-Ib:Compounds of general formula I are useful for the preparation of hapten-carrier conjugates in the treatment of nicotine addiction.
Owner:NABI BIOPHARMLS
Methods and compositions for aiding in smoking cessation and for treating pain and other disorders using optically pure (+)-bupropion
InactiveUS6495605B2Reduce adverse effectsEffective treatmentBiocideOrganic active ingredientsReflexSeasonal Affective Disorders
Methods and compositions are disclosed utilizing the optically pure (+)-isomer of bupropion to assist in smoking cessation, for treating smoking and nicotine addiction, and for treating pain, including, but not limited to, chronic pain, neuropathetic pain and reflex sympathetic dystrophy, and other disorders such as narcolepsy, chronic fatigue syndrome, fibromyalgia, seasonal affective disorder and premenstrual syndrome, while avoiding adverse affects associated with racemic bupropion.
Owner:SEPACOR INC
Treatment of addiction and addiction-related behavior
InactiveUS6057368AAmeliorating addiction and addictive behaviorInhibits and eliminates cravingBiocidePeptide/protein ingredientsPharmacologySubstance addiction
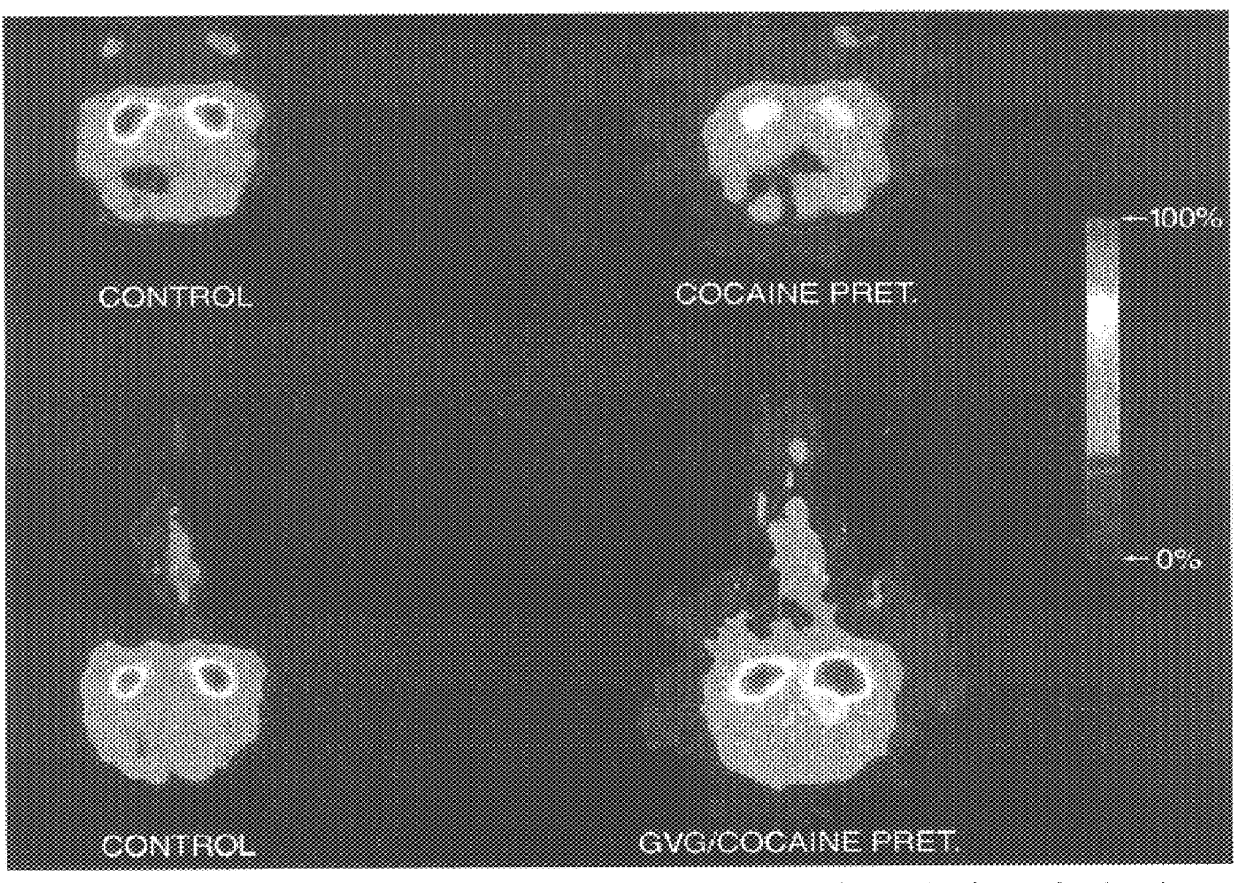
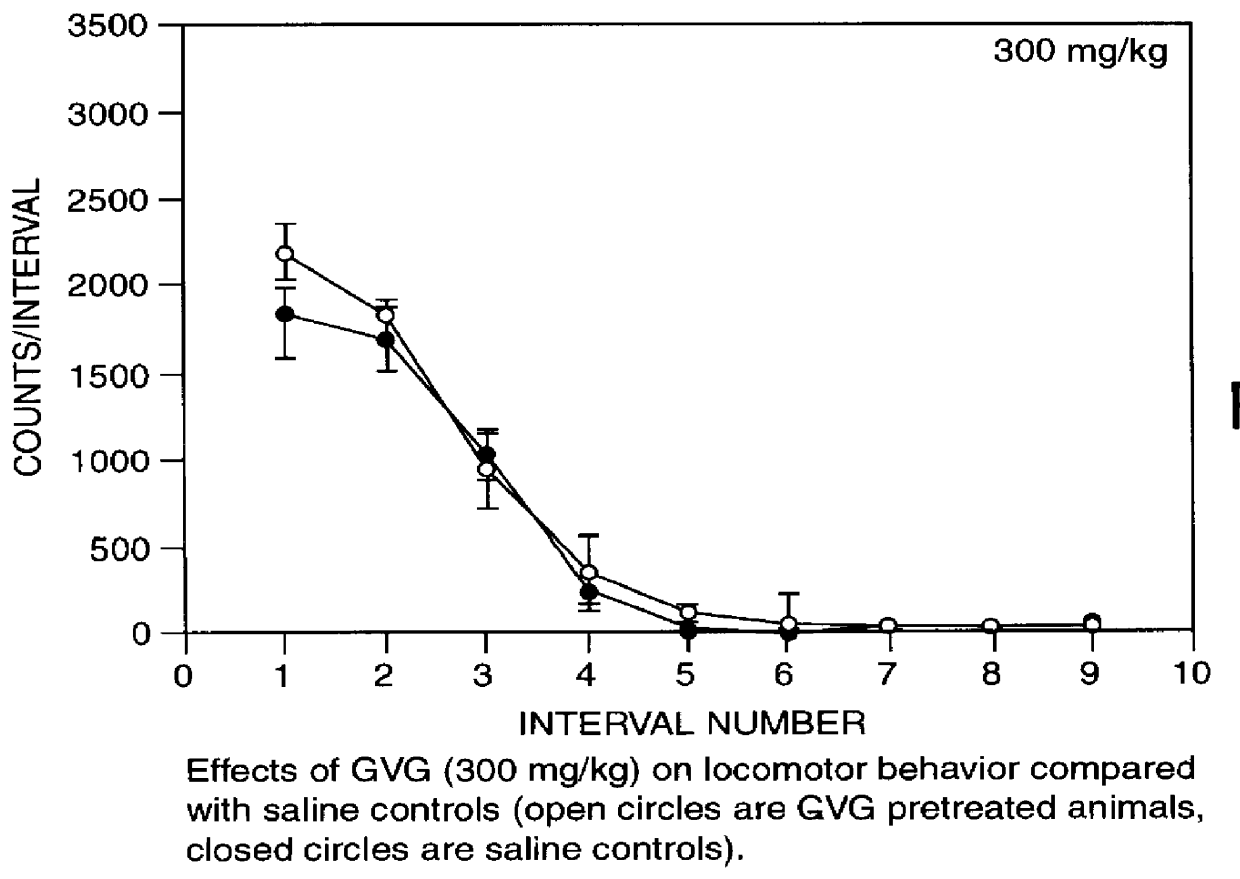
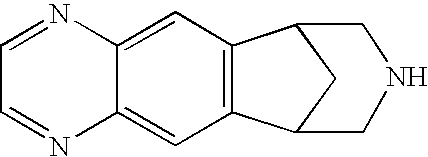
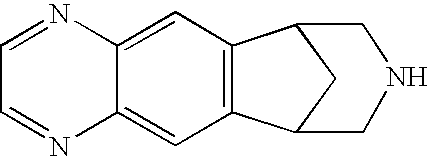
The present invention provides a highly efficient method for treating substance addiction and for changing addiction-related behavior of a primate suffering from substance addiction. The method includes administering to a primate an effective amount of a pharmaceutical composition including gamma vinylGABA. The present invention also provides a method of treatment of nicotine addiction by treating a patient with an effective amount of a composition including gamma vinylGABA.
Owner:BROOKHAVEN SCI ASSOCS
Chewing Gum Compositions of Varenicline
InactiveUS20080181933A1Reducing nicotine addictionCessation of lesseningBiocideNervous disorderAdditive ingredientWater insoluble
A chewing gum composition including a water insoluble base portion; a water soluble portion; and a therapeutically effective amount of varenicline or its pharmaceutical acceptable salt thereof. A method for reducing nicotine addiction and aiding in the cessation or lessening of tobacco use in an individual by administering to an oral cavity of an individual a chewing gum composition including an effective amount of varenicline or its pharmaceutical acceptable salt thereof; and chewing the gum composition to cause the varenicline or its pharmaceutical acceptable salt thereof to be released from the chewing gum composition into the oral cavity of the individual. A method of manufacturing a chewing gum composition.
Owner:PFIZER INC
Smoking Cessation Treatment By Reducing Nicotine Cravings, Apetite Suppression, And Altering The Perceived Taste Of Tobacco Smoke
InactiveUS20100021570A1Alteration in taste perceptionReduce cravingsBiocideNervous disorderDrug withdrawal symptomsSide effect
A multi-component compound for the simultaneous treatment of nicotine addiction and the side effects of nicotine withdrawal, such as excessive appetite. The first component is a bivalent negative sulfur compound in an amount effective to control nicotine craving or the withdrawal symptoms resulting from nicotine withdrawal. The bivalent negative sulfur is selected from a group that includes, but is not limited to, alkyl sulfides, colloidal sulfur, hydropersulfides, organic thio compounds or their salts. The second component is a serotonin precursor, such as tryptophan or its derivative 5-HTP, which is used to assist the body in producing more serotonin which in turn suppresses appetite. The appetite suppressant(s) are combined with the bivalent negative sulfur compound(s) to provide a single compound that reduces nicotine craving and simultaneously suppresses increased appetite resulting from nicotine withdrawal. The compound alters the perceived taste of tobacco smoke such that it is no longer enjoyable.
Owner:BIELEY HARLAN CLAYTON
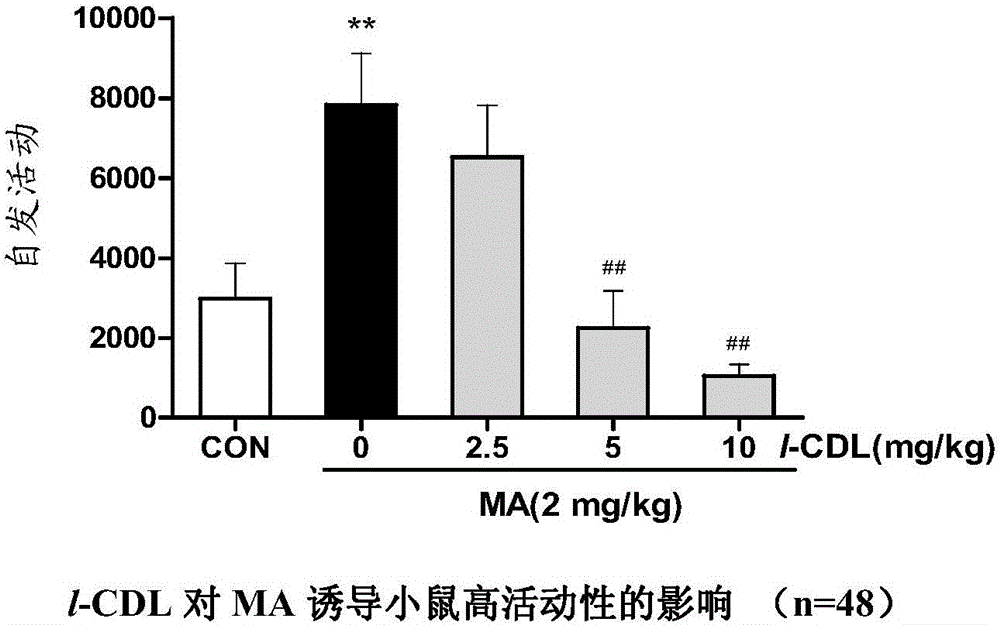
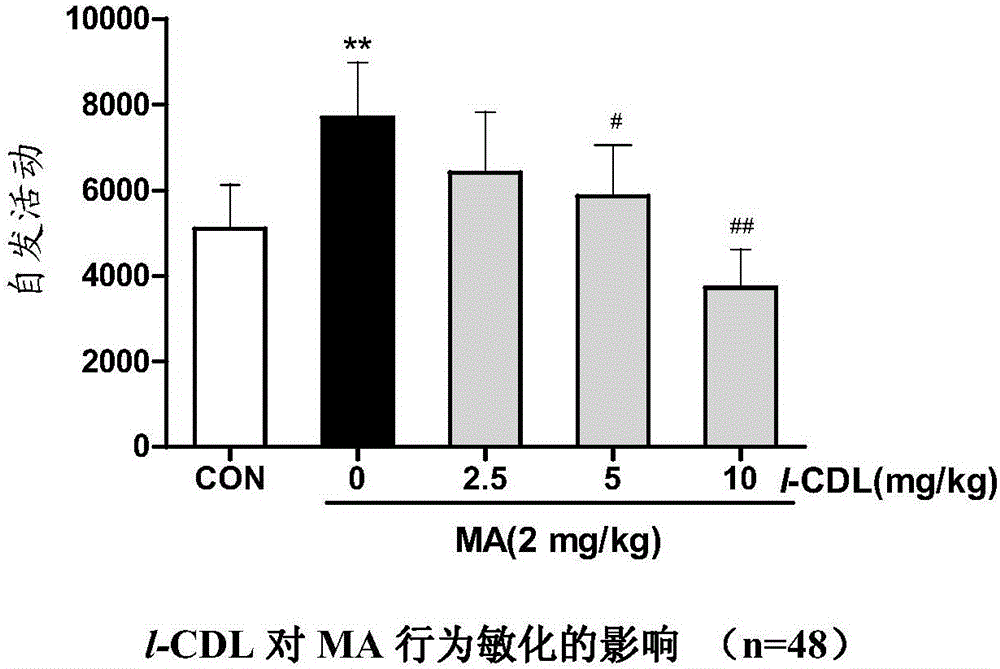
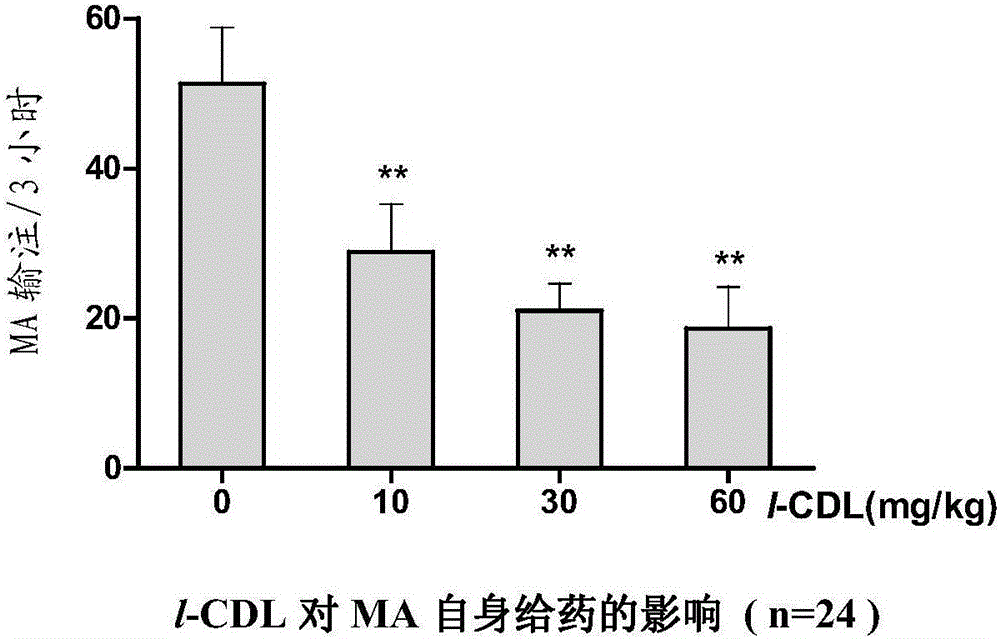
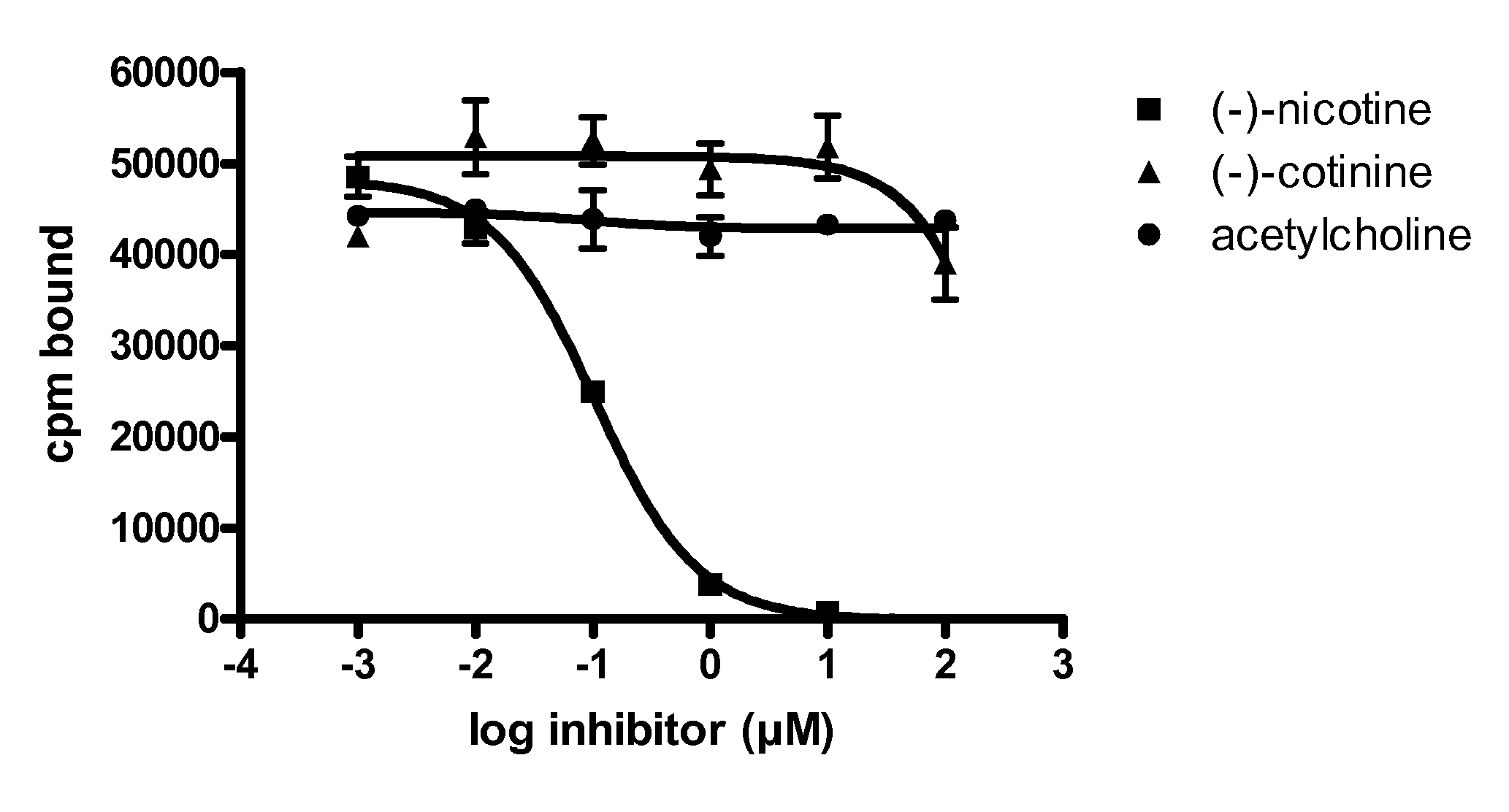
Anti-addiction medical application of L-corydalmine (L-CDL)
ActiveCN106176740AReduced reinforcementIncrease mobilityOrganic active ingredientsNervous disorderDiseaseIsoquinoline
The invention relates to an application of a lead compound namely L-corydalmine (L-CDL) of isoquinoline compounds on treating diseases related with addiction. The diseases comprise drug addiction (heroin, opium, methyl amphetamine, morphine, marihuana, cocaine, and the like), nicotine addiction, and alcohol addiction, and also comprise novel drug addiction (stimulant type novel drugs) and brain damage caused by novel drugs.
Owner:杨征
Methods and compositions for aiding in smoking cessation and for treating pain and other disorders using optically pure (-) -bupropion
InactiveUS20040225020A1Reduce adverse effectsEffective treatmentBiocideOrganic active ingredientsReflexSeasonal Affective Disorders
Methods and compositions are disclosed utilizing the optically pure (-)-isomer of bupropion to assist in smoking cessation, for treating smoking and nicotine addiction, and for treating pain, including, but not limited to, chronic pain, neuropathetic pain and reflex sympathetic dystrophy, and other disorders such as narcolepsy, chronic fatigue syndrome, fibromyalgia, seasonal affective disorder and premenstrual syndrome, while avoiding adverse affects associated with racemic bupropion.
Owner:SEPACOR INC
Smoking Cessation Treatment with Appetite Suppression
InactiveUS20080103111A1Suppress appetiteSuppress cravingBiocidePeptide/protein ingredientsSide effectThio-
A multi-component compound for the simultaneous treatment of nicotine addiction and the side effects of nicotine withdrawal, such as excessive appetite. The first component is a bivalent negative sulfur compound in an amount effective to control nicotine craving or the withdrawal symptoms resulting from nicotine withdrawal. The bivalent negative sulfur is selected from a group that includes, but is not limited to, alkyl sulfides, colloidal sulfur, hydropersulfides, organic thio compounds or their salts. The second component is a serotonin precursor, such as tryptophan or its derivative 5-HTP, which is used to assist the body in producing more serotonin which in turn suppresses appetite. The appetite suppressant(s) are combined with the bivalent negative sulfur compound(s) to provide a single compound that reduces nicotine craving and simultaneously suppresses increased appetite resulting from nicotine withdrawal.
Owner:BIELEY HARLAN CLAYTON
Acylated spiropiperidine derivatives as melanocortin-4 receptor modulators
Certain novel N-acylated spiropiperidine derivatives are ligands of the human melanocortin receptor(s) and, in particular, are selective ligands of the human melanocortin-4 receptor (MC-4R). They are therefore useful for the treatment, control, or prevention of diseases and disorders responsive to the modulation of MC-4R, such as obesity, diabetes, nicotine addiction, alcoholism, sexual dysfunction, including erectile dysfunction and female sexual dysfunction.
Owner:MERCK SHARP & DOHME LLC
Pharmaceutical compositions and methods for relieving pain and treating central nervous system disorders
InactiveUS7402592B2Increase the number ofPreventing and/orBiocideNervous disorderAttention deficitsS syndrome
Patients susceptible to or suffering from disorders, such as central nervous system disorders, which are characterized by an alteration in normal neurotransmitter release, such as dopamine release (e.g., Parkinsonism, Parkinson's Disease, Tourette's Syndrome, attention deficient disorder, or schizophrenia), are treated by administering a compound of Formulas 1 or 2, as described herein. The compounds of Formulas 1 and 2 are also useful for treating pain, and treating drug addiction, nicotine addiction, and / or obesity. The compounds can exist as individual stereoisomers, racemic mixtures, diastereomers and the like.
Owner:TARGACEPT INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com