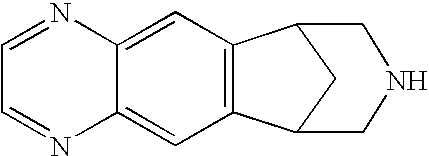
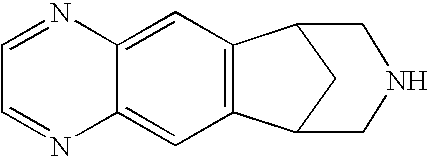
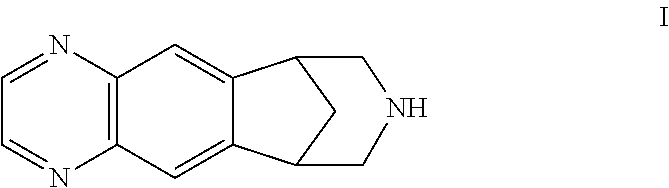
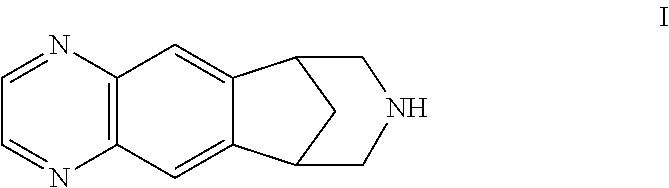
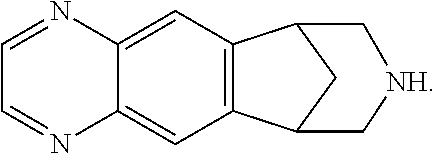
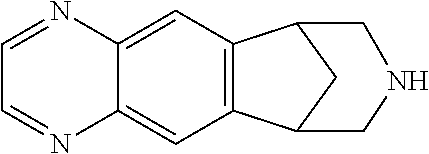
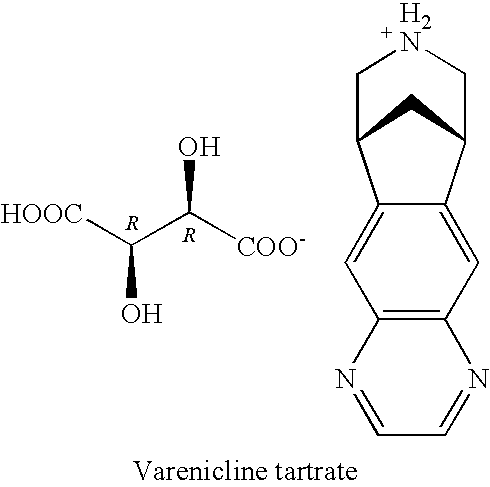
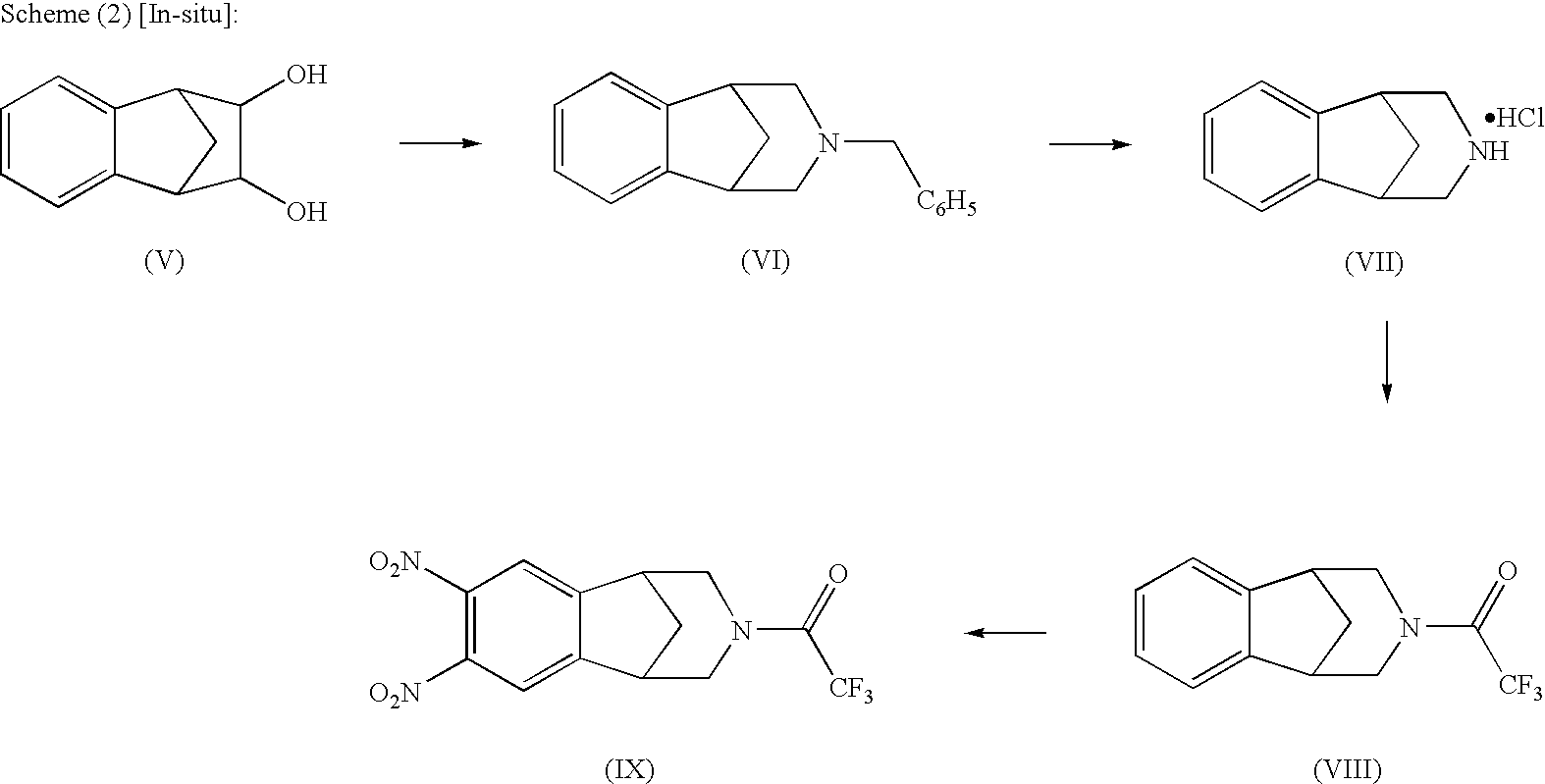
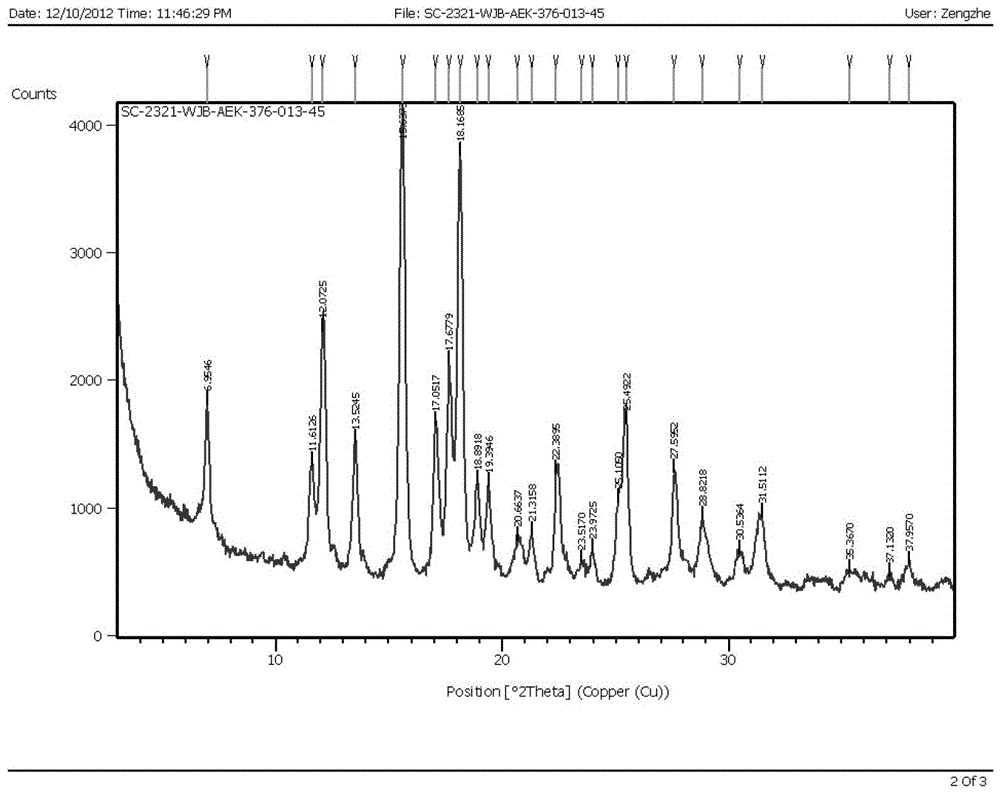
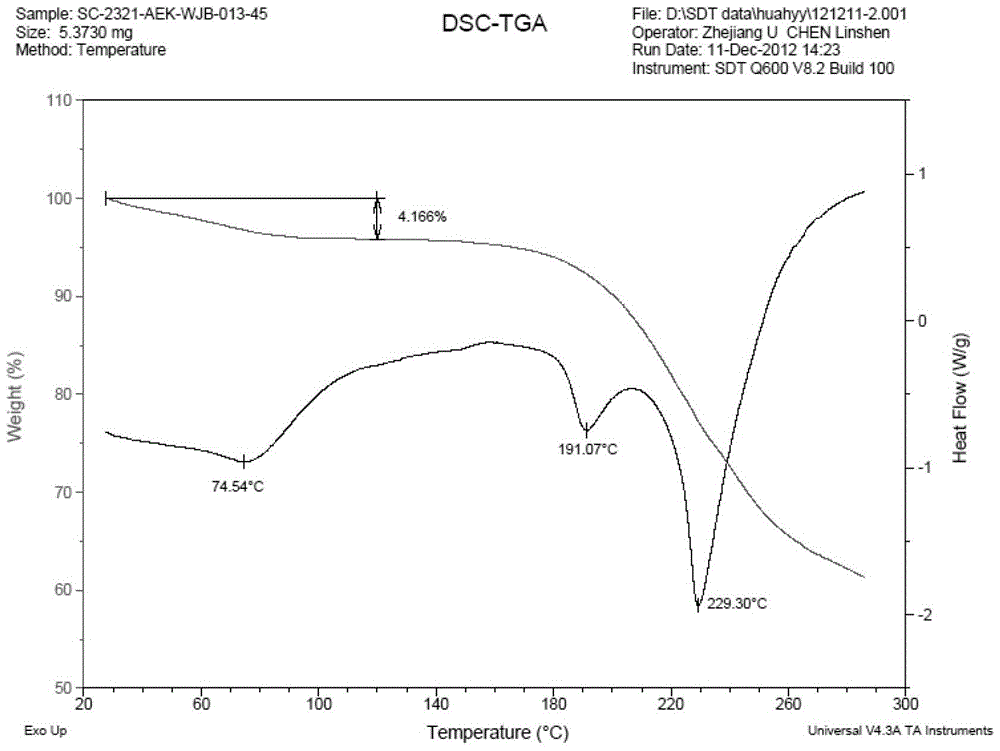
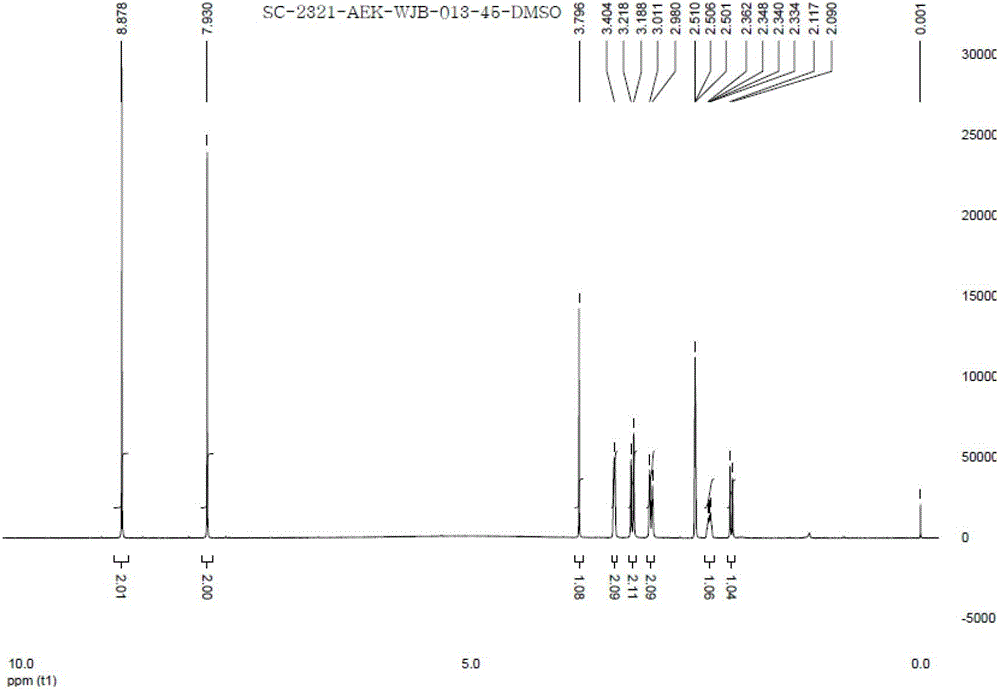
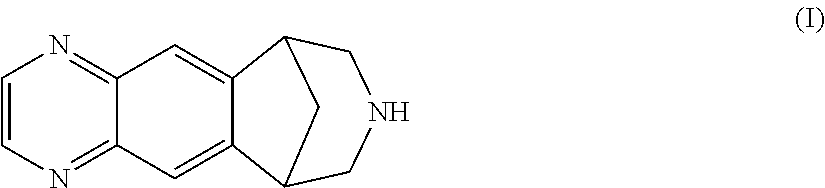
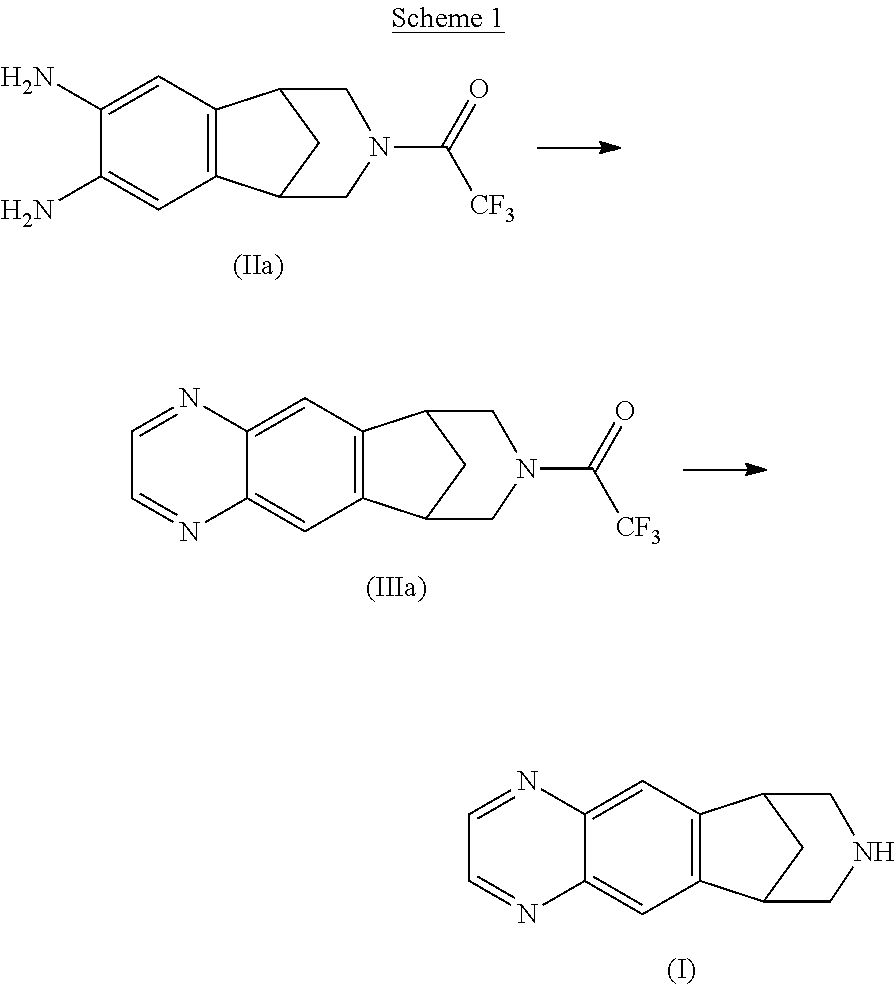
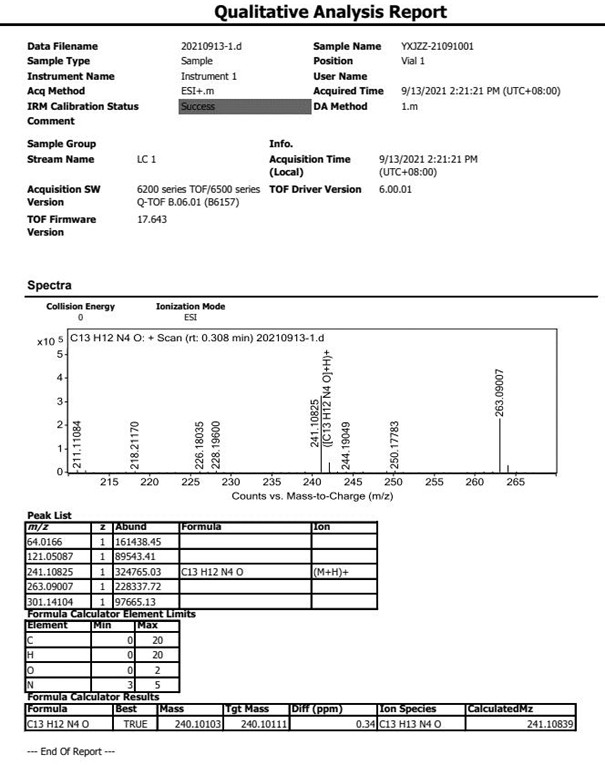
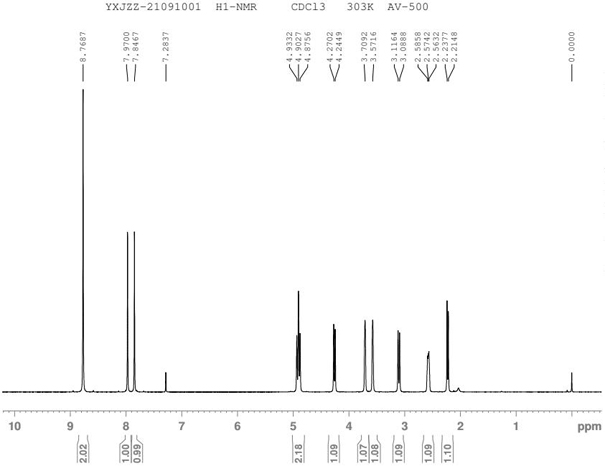
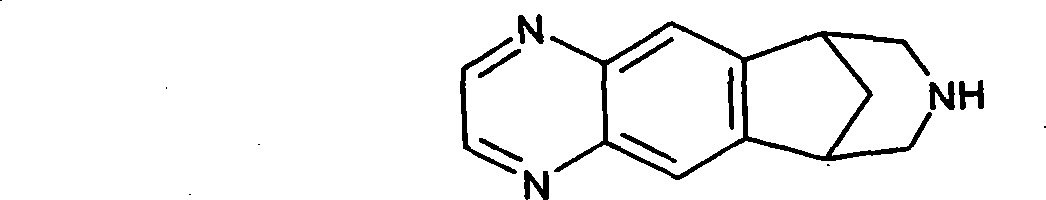
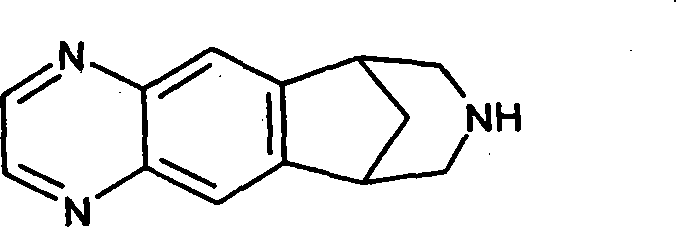
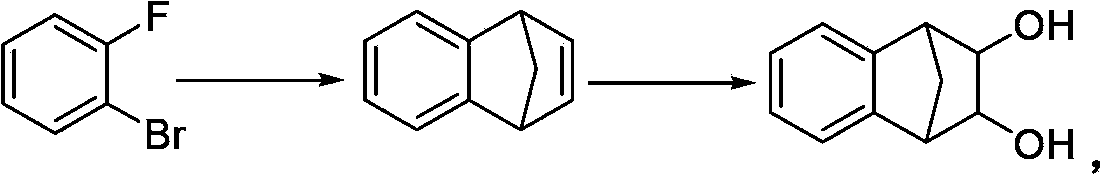
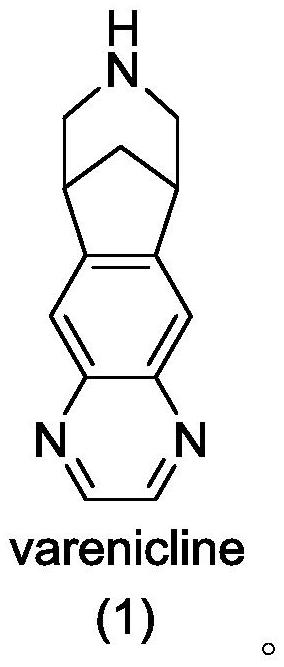
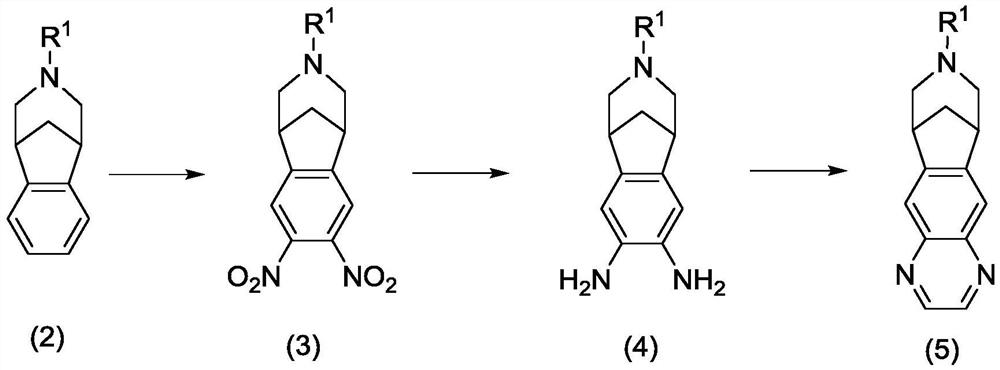
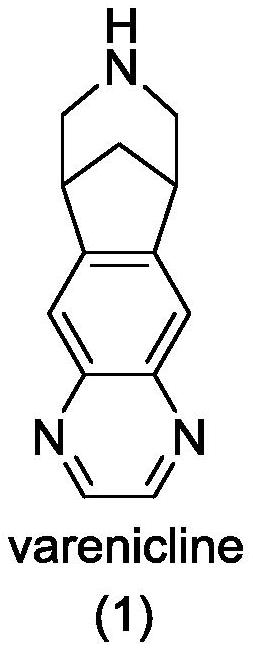
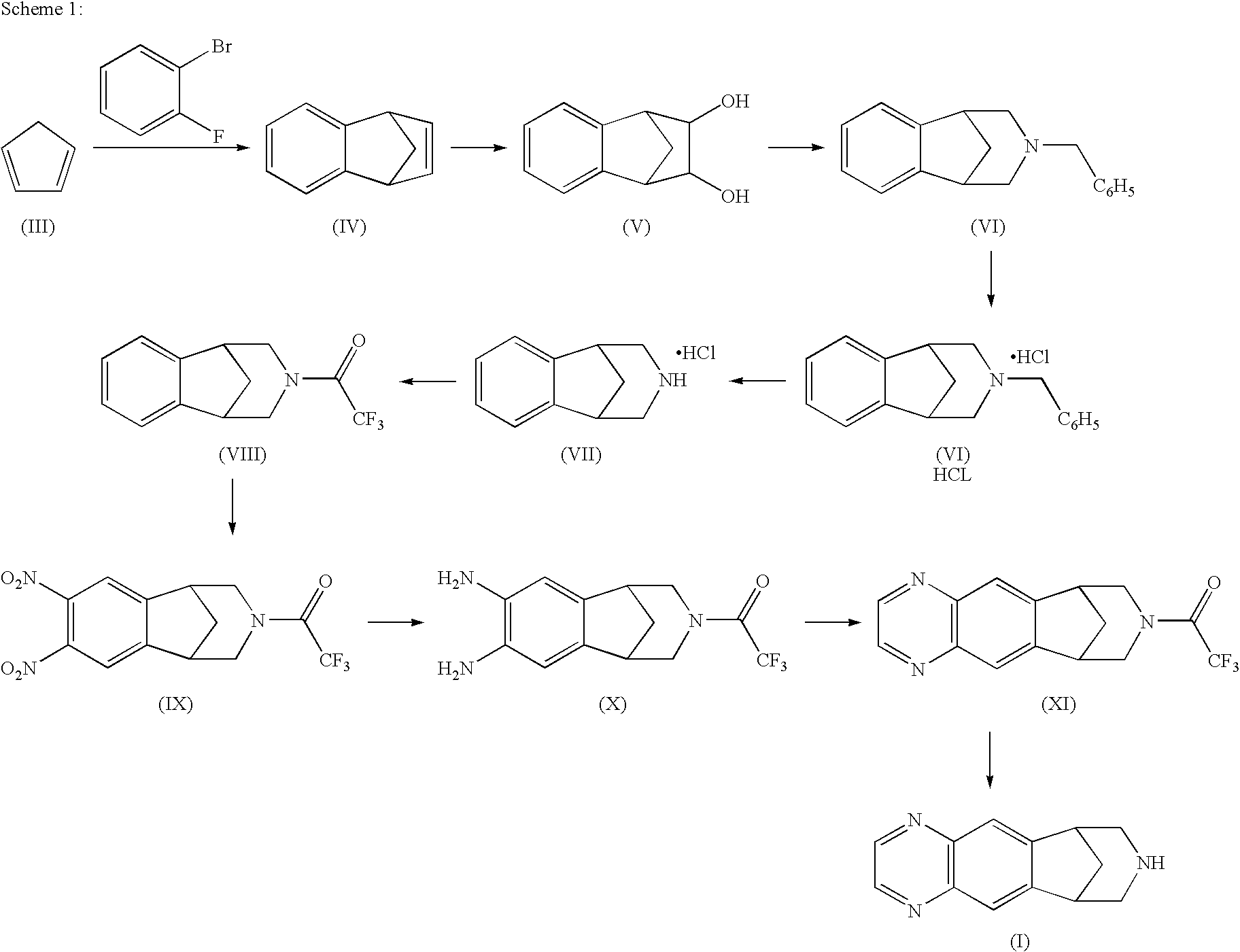Patents
Literature
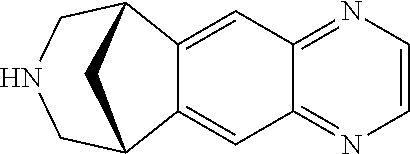
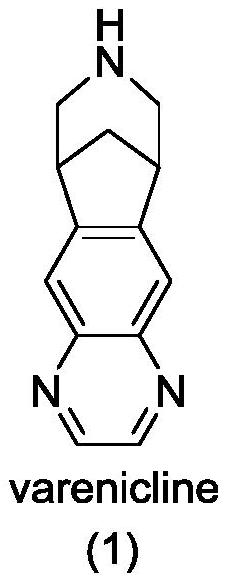
34 results about "Varenicline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
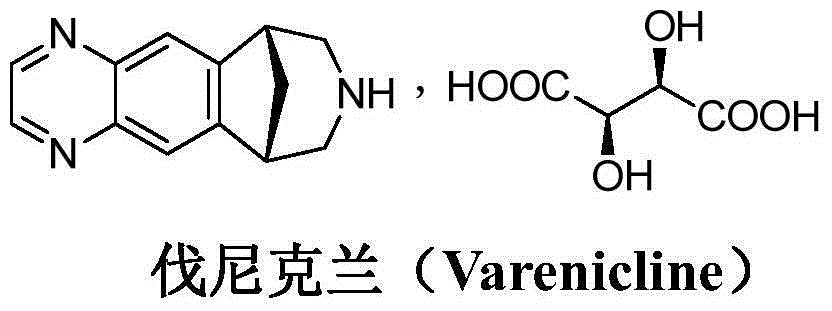
Varenicline helps you stop smoking. To increase your chance of success, use this medication with a stop-smoking program that includes education, support, and counseling. Quitting smoking lowers your risk of heart and lung disease, as well as cancer.
Compositions and methods for intranasal, buccal, sublingual and pulmonary delivery of varenicline
InactiveUS20060084656A1Promote nasal absorptionReducing nicotine addictionBiocidePowder deliveryNasal cavityBuccal use
A composition for nasal administration comprising varenicline or its pharmaceutically acceptable salt and at least one excipient. The invention also provides a composition for buccal administration comprising varenicline or its pharmaceutically acceptable salt and at least one excipient to form a solid dosage form, wherein the solid dosage form disintegrates in an oral cavity at body temperature and may adhere to body tissue of the oral cavity; a composition for pulmonary administration comprising varenicline or its pharmaceutically acceptable salt and at least one excipient; and, a method for reducing nicotine addiction, aiding in the cessation of, or lessening of tobacco use in a subject.
Owner:PFIZER INC
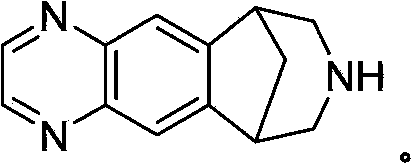
Process for preparing varenicline, varenicline intermediates, pharmaceutically acceptable salts thereof
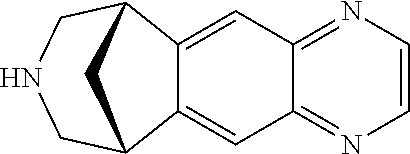
Provided herein is an improved, convenient, commercially viable and environmentally friendly process for the preparation of varenicline or a pharmaceutically acceptable salt thereof comprising reacting 1-(4,5-diamino-10-aza-tricyclo[6.3.1.02 7]dodeca-2(7),3,5-trien-10-yl)-2,2,2-trifluoro-ethanone with chloroacetaldehyde in the presence of an oxygen source. Provided further herein is an improved and industrially advantageous process for the preparation of 1-(4,5-diamino-10-aza-tricyclo[6.3.1.02 7]dodeca-2(7),3,5-trien-10-yl)-2,2,2-trifluoro-ethanone.
Owner:ACTAVIS GRP PTC EHF
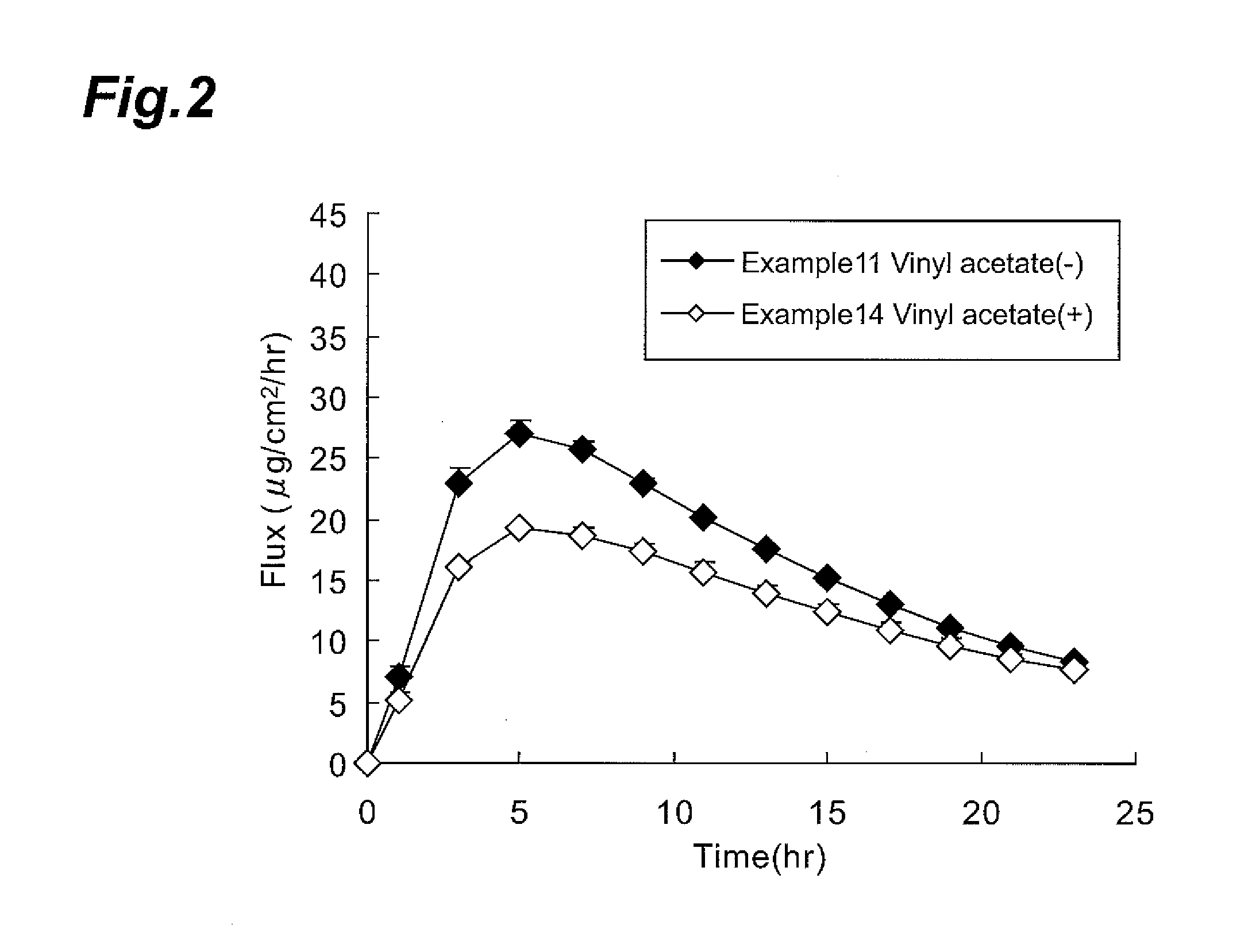
Transdermal system for varenicline
The invention provides transdermal compositions comprising varenicline or its pharmaceutically acceptable salt or prodrug form.
Owner:PFIZER INC
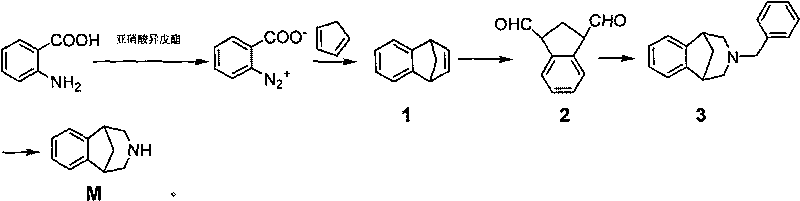
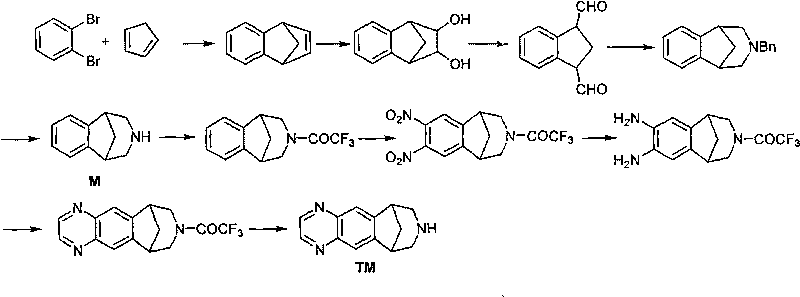
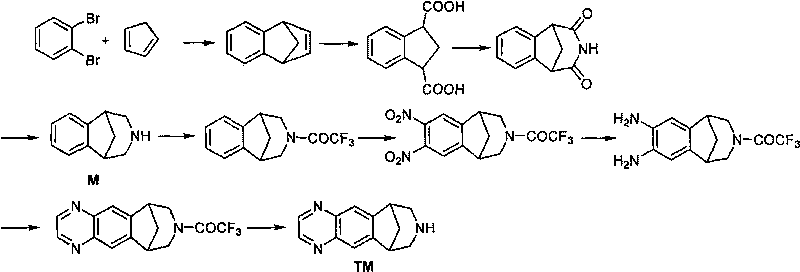
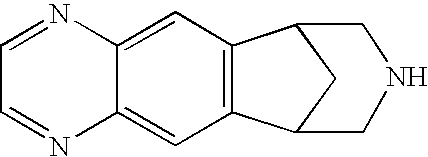
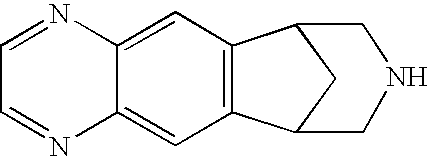
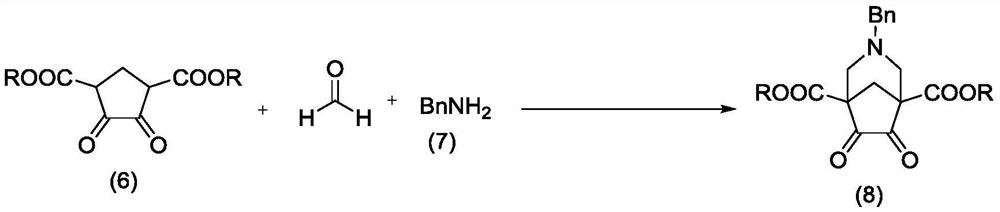
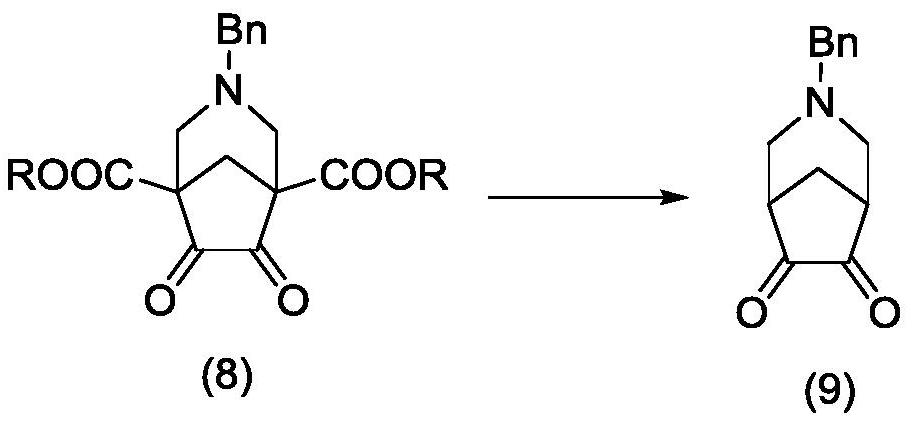
Method for synthesizing Varenicline intermediate 2, 3, 4, 5-tetralin-1, 5-methylene-hydrogen-benzoazepine
The invention relates to a method for synthesizing Varenicline intermediate 2, 3, 4, 5-tetralin-1, 5-methylene-hydrogen-benzoazepine. The method includes the following steps: under the action of catalyst, mixing amyl nitrite with o-aminobenzoic acid solution to generate diazonium salt, then mixing the diazonium salt with cyclopentadiene, and heating to react to generate compound 1; feeding ozone into the solution of compound 1, after complete conversion, adding reducing agent to generate compound 2, then dripping the compound 2 to the mixed solution of triacetoxy sodium borohydride and benzylamine to generate compound 3 by loop closing; and hydrogenating the compound 3 under the action of palladium and carbon for debenzylation and reduction to obtain M intermediate 2, 3, 4, 5-tetralin-1, 5-methylene-hydrogen-benzoazepine. The method has the advantages of greatly simplifying the method for preparing Varenicline intermediate, being simple in production process and safe in operation, well ensuring no harm to the environment and control on production cost, increasing yield and being capable of becoming a process in great industrial production.
Owner:上海立科化学科技有限公司
Medicated patch
Provided is a medicated patch containing a medicinal agent and an adhesive base material and having an acid value of no greater than 28, where the medicinal agent is varenicline or a pharmaceutically acceptable salt of varenicline.
Owner:HISAMITSU PHARM CO INC
Chewing Gum Compositions of Varenicline
InactiveUS20080181933A1Reducing nicotine addictionCessation of lesseningBiocideNervous disorderAdditive ingredientWater insoluble
A chewing gum composition including a water insoluble base portion; a water soluble portion; and a therapeutically effective amount of varenicline or its pharmaceutical acceptable salt thereof. A method for reducing nicotine addiction and aiding in the cessation or lessening of tobacco use in an individual by administering to an oral cavity of an individual a chewing gum composition including an effective amount of varenicline or its pharmaceutical acceptable salt thereof; and chewing the gum composition to cause the varenicline or its pharmaceutical acceptable salt thereof to be released from the chewing gum composition into the oral cavity of the individual. A method of manufacturing a chewing gum composition.
Owner:PFIZER INC
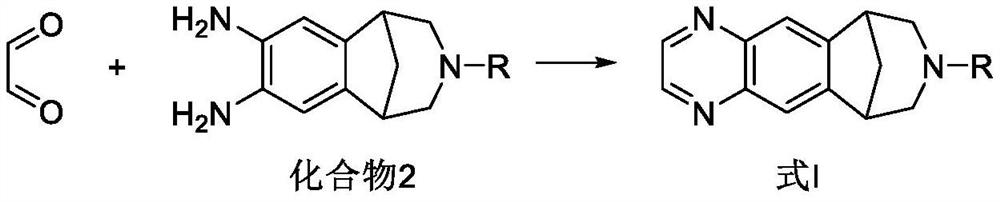
Preparation methods of varenicline intermediate, varenicline and its salt
PendingCN113956255AShort reaction timeIncrease productivityOrganic compound preparationCarboxylic acid salt preparationSolventPharmaceutical Substances
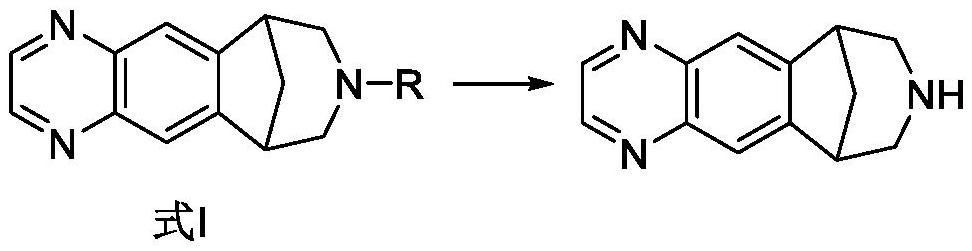
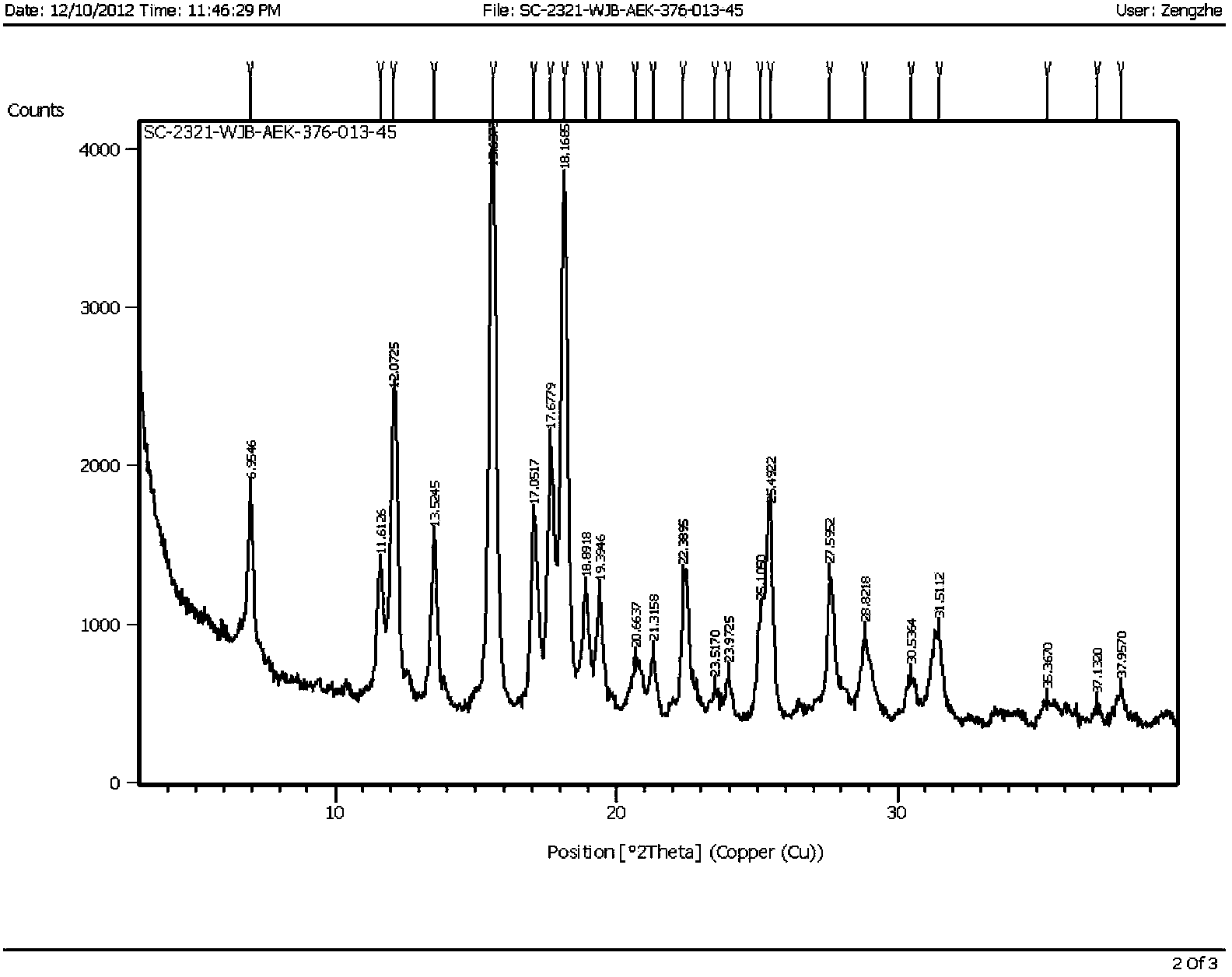
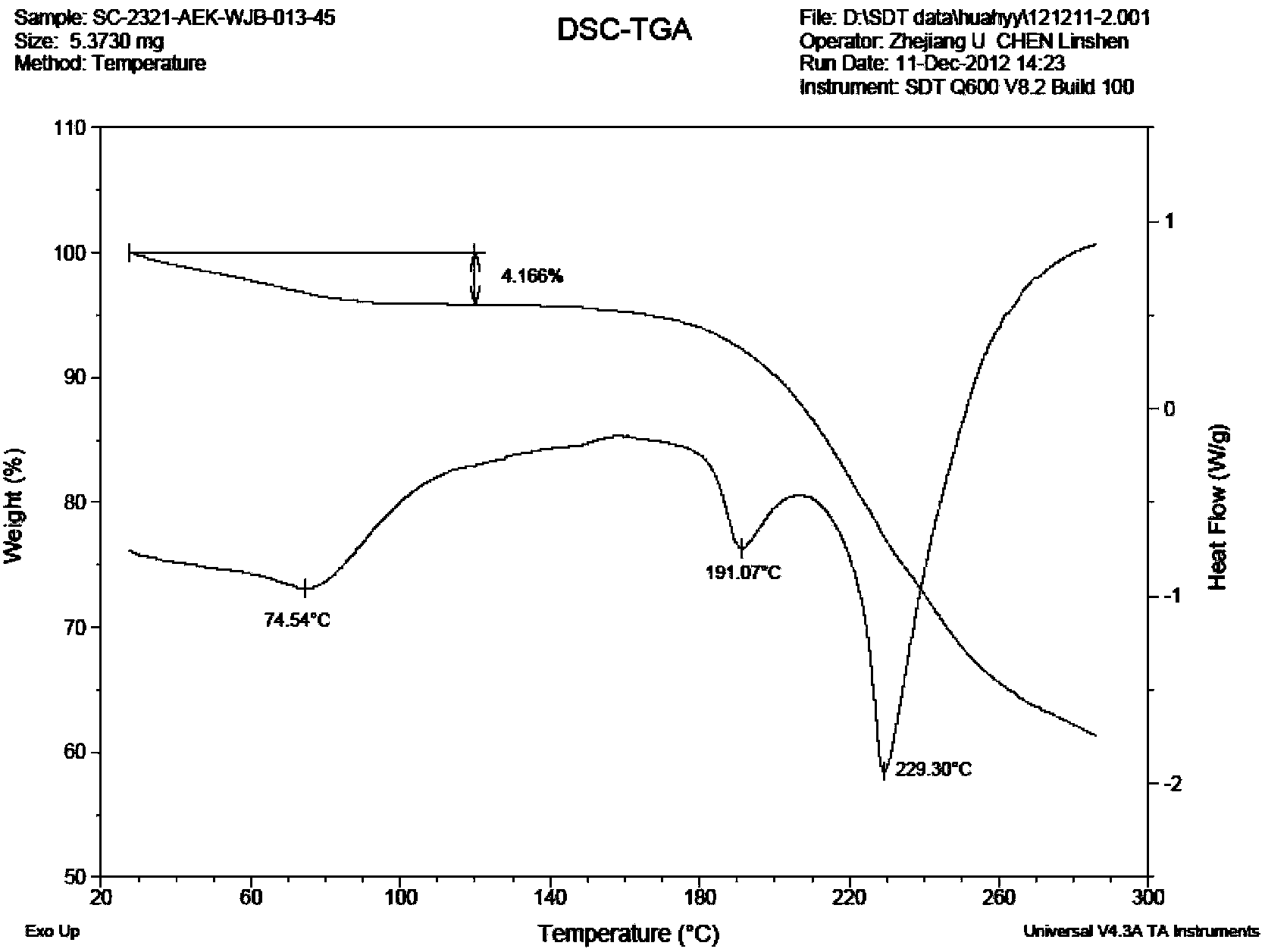
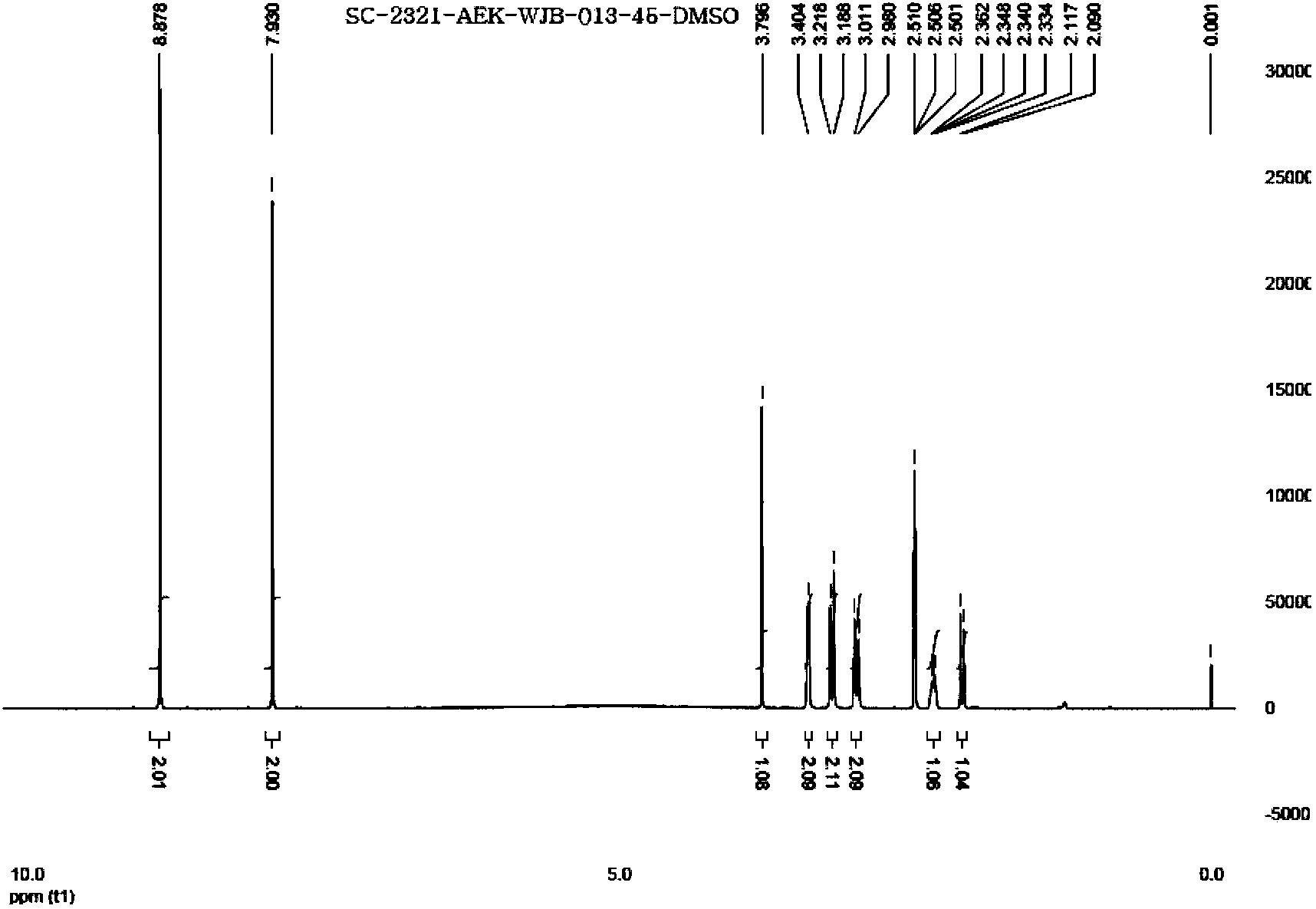
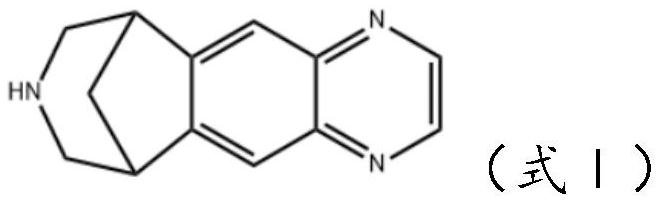
The invention discloses preparation methods of a varenicline intermediate, varenicline and its salt. The invention provides a preparation method of a compound shown in a formula I. The preparation method comprises the following steps: carrying out a cyclization reaction on the compound 2 and glyoxal in the presence of a solvent and alkaline acid salt by using or not using inert gas protection to generate the compound as shown in the formula I, wherein R is an amino protecting group. The method can greatly shorten the reaction time of the cyclization reaction under the condition of ensuring the product yield and the product purity, obviously improve the production efficiency of the cyclization product, and greatly reduce the production cost and the time cost of the unit product; and meanwhile, the content of the impurity I in the varenicline intermediate product can be controlled within a relatively low level range, so that the product quality of the drug intermediate and the crude drug can be better guaranteed.
Owner:SHANGHAI VIWIT PHARMA CO LTD +1
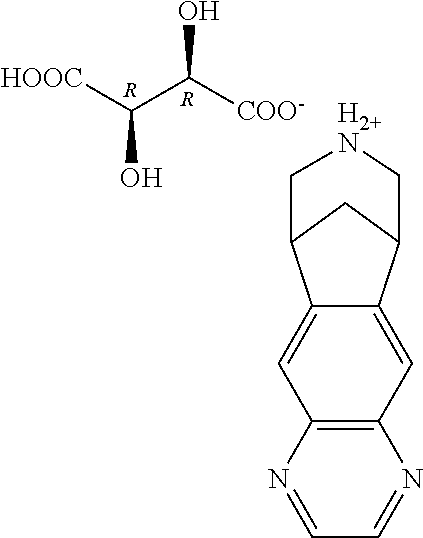
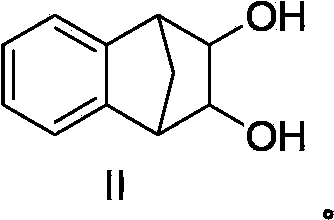
Varenicline salt and preparing method thereof
The invention relates to varenicline hemitartrate and a preparing method thereof. The salt has a structure shown as the formula II. The varenicline hemitartrate is good in stability, low in moisture adsorption and high in safety. The preparing method is simple in operation and good in reproducibility.
Owner:SHANGHAI SYNCORES TECH INC
Processes for the preparation of varenicline and intermediates thereof
InactiveUS20090318695A1Commercially viable and economical processSpeed up the processOrganic chemistryCombinatorial chemistryVarenicline
The invention provides an improved process for the preparation and purification of Varenicline and intermediates for the preparation of Varenicline.
Owner:TEVA PHARM USA INC
A kind of varenicline salt and preparation method thereof
The invention relates to varenicline hemitartrate and a preparing method thereof. The salt has a structure shown as the formula II. The varenicline hemitartrate is good in stability, low in moisture adsorption and high in safety. The preparing method is simple in operation and good in reproducibility.
Owner:SHANGHAI SYNCORES TECH INC
Process for Preparing Quinoxaline Derivatives
InactiveUS20120004239A1Increase productionSpeed up the processBiocideNervous disorderQuinoxalineTartrate
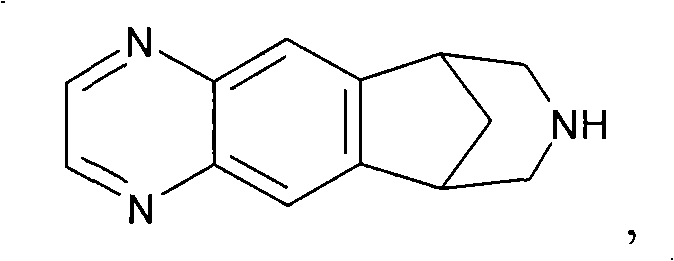
The present invention provides an improved process for preparing a compound of formula (IIIA), an intermediate of the synthesis of varenicline. Also, the present invention provides an improved process for preparing varenicline, or a pharmaceutically acceptable salt or solvate thereof. Furthermore, the present invention provides a process for decolorizing varenicline, or a salt or solvate thereof. Still further, the present invention provides a process of preparing varenicline L-tartrate with improved yield. Still further, the present invention relates to the use of compound of formula (V), or a salt or solvate thereof, as a reference marker and reference standard for assessing the purity of varenicline, or a salt or solvate thereof.
Owner:MEDICHEM
Method to predict the safety of the treatment with a nicotinic cholinergic receptor agonist
InactiveUS20150094220A1Easy to useNucleotide librariesMicrobiological testing/measurementAllelePharmacological treatment
The present invention generally relates to methods for predicting the safety of a nicotinic cholinergic receptor agonist drug-based pharmacological treatment, such as one based on varenicline, based on determining at least one allele of one or more of single-nucleotide polymorphisms (SNPs) rs9479757, rs7930792, rs12423809, rs4251417, rs7146, rs477292, rs495491, rs3778151, rs763132, rs4474069 and rs1183035, or of any SNP of the corresponding linkage groups thereof, in a biological sample of a subject.
Owner:GENETRACER BIOTECH +1
Depot formulation
ActiveUS10912734B2Promote absorptionReduce addictionPowder deliveryNervous disorderPharmaceutical drugPharmaceutical medicine
A pharmaceutical long acting depot composition is provided as an aid to smoking cessation treatment. The formulation comprises a therapeutically effective amount of varenicline or its pharmaceutically acceptable derivative and pharmaceutically acceptable excipients. The process of preparation of the formulation is also provided.
Owner:CIPLA LTD
Process for the preparation of varenicline
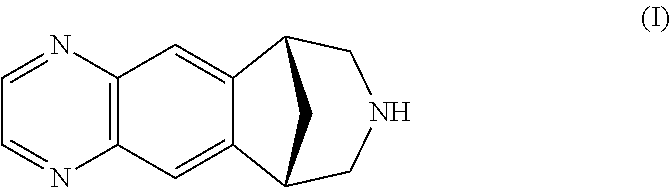
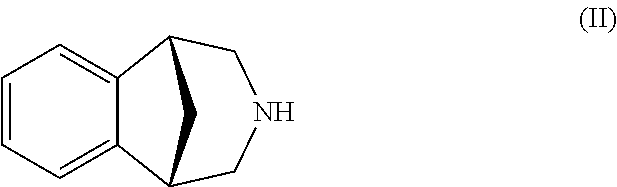
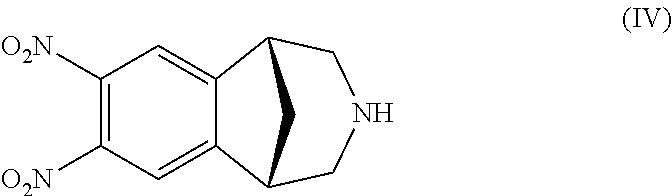
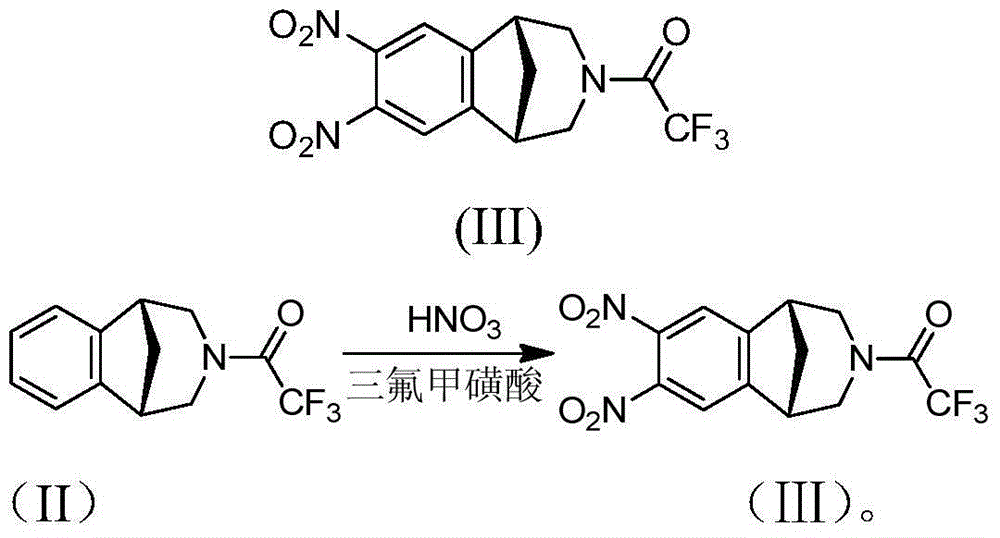
It is disclosed a process for the preparation of a compound of formula (I) or a salt thereof, comprising:nitrating a compound of formula (II) or a salt thereof,to obtain a compound of formula (IV) or a salt thereof,reducing it, to obtain a compound of formula (V) or a salt thereof, andsubsequently cyclizing it to obtain a compound of formula (I) or a salt thereof and, if desired, converting a compound of formula (I) to a salt thereof, or vice versa, characterized in that:the nitration of a compound of formula (II) or a salt thereof is carried out with concentrated nitric acid in the presence of a strong inorganic acid and that the amino group in the compound of formula (II) is not protected.
Owner:DIPHARMA FRANCIS
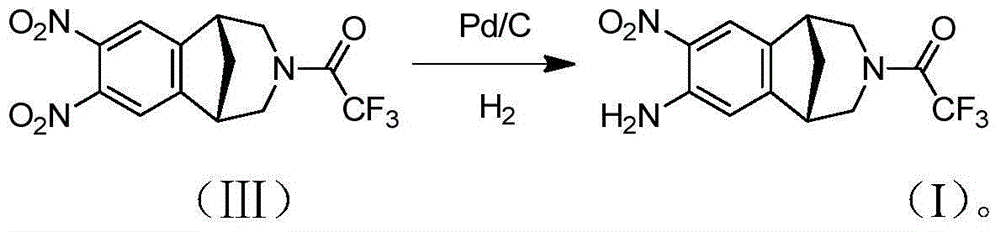
Preparation method of varenicline intermediate and nitroreduction impurity thereof
InactiveCN104478803ASelective reduction is goodReduce purification operationsOrganic chemistryBenzeneHydrogen
The invention discloses a preparation method of a varenicline intermediate and nitroreduction impurity thereof. The preparation method of the varenicline intermediate and nitroreduction impurity thereof comprises the following steps: nitrifying 2,3,4,5-tetrahydro-3-(trifluoroacetyl)-1,5-methano-1H-3-benazepine, hydrogenating Pd / C, and selectively reducing nitryl, thus obtaining a target compound shown in a formula (I). The preparation method of the varenicline intermediate and nitroreduction impurity thereof has the advantages that no pollution is produced to the environment, column chromatography operation is avoided, and yield and purity of a product are improved. The formula (I) is described in the specification.
Owner:连云港恒运药业有限公司
Preparation method for N-nitrosamine genotoxic impurity of varenicline tartrate
The invention provides a preparation method for an N-nitrosamine genotoxic impurity of varenicline tartrate. The preparation method comprises the following steps: with ammonium formate as a hydrogen donor, carrying out reduction reaction on dinitrate under the catalysis of palladium carbon to obtain a diamino compound, carrying out a cyclization reaction on the diamino compound and an aqueous glyoxal solution to obtain a cyclization product, carrying out a hydrolysis reaction on the cyclization product under the action of sodium hydroxide, removing trifluoroacetyl to obtain free alkali, and finally, subjecting the free alkali to reacting with sodium nitrite under the catalysis of protonic acid to obtain the N-nitrosamine impurity. According to the invention, the technical vacancy of preparation methods for the impurity at present is filled in, the prepared high-purity impurity can be applied as a control sample to the drug impurity research and production quality control process of varenicline tartrate, and a guarantee is provided for the genotoxicity research and comprehensive quality control of a varenicline tartrate bulk drug.
Owner:JIANGSU SINOBIOPHARMA
Transdermal system for varenicline
InactiveCN101232876AOrganic active ingredientsNervous disorderPharmaceutical medicineMedicinal chemistry
The present invention provides transdermal compositions comprising varenicline or a pharmaceutically acceptable salt or prodrug form thereof.
Owner:PFIZER PRODS ETAT DE CONNECTICUT
Varenicline pharmaceutical composition capable of reducing generation of nitrosamine impurities, and preparation and application of varenicline pharmaceutical composition
PendingCN113908131AInhibit and reduce generationImprove stabilityNervous disorderDigestive systemPharmaceutical drugPharmaceutical medicine
The invention discloses a varenicline pharmaceutical composition capable of reducing generation of nitrosamine impurities, and preparation and application of the varenicline pharmaceutical composition. According to the pharmaceutical composition disclosed by the invention, pharmaceutically acceptable acid is added into a secondary amine compound or the composition thereof, the generation of nitrosamine impurities can be effectively inhibited and reduced, the stability of the secondary amine compound or the composition thereof is improved, the content of nitrosamine genotoxic impurities is controlled at a relatively low level, and the safety requirement is met.
Owner:SHANDONG WEIZHI ZHONGKE PHARM CO LTD +2
Depot formulation
ActiveUS20190350844A1Promote absorptionReduce addictionPowder deliveryNervous disorderExcipientPharmaceutical preservatives
A pharmaceutical long acting depot composition is provided as an aid to smoking cessation treatment. The formulation comprises a therapeutically effective amount of varenicline or its pharmaceutically acceptable derivative and pharmaceutically acceptable excipients. The process of preparation of the formulation is also provided.
Owner:CIPLA LTD
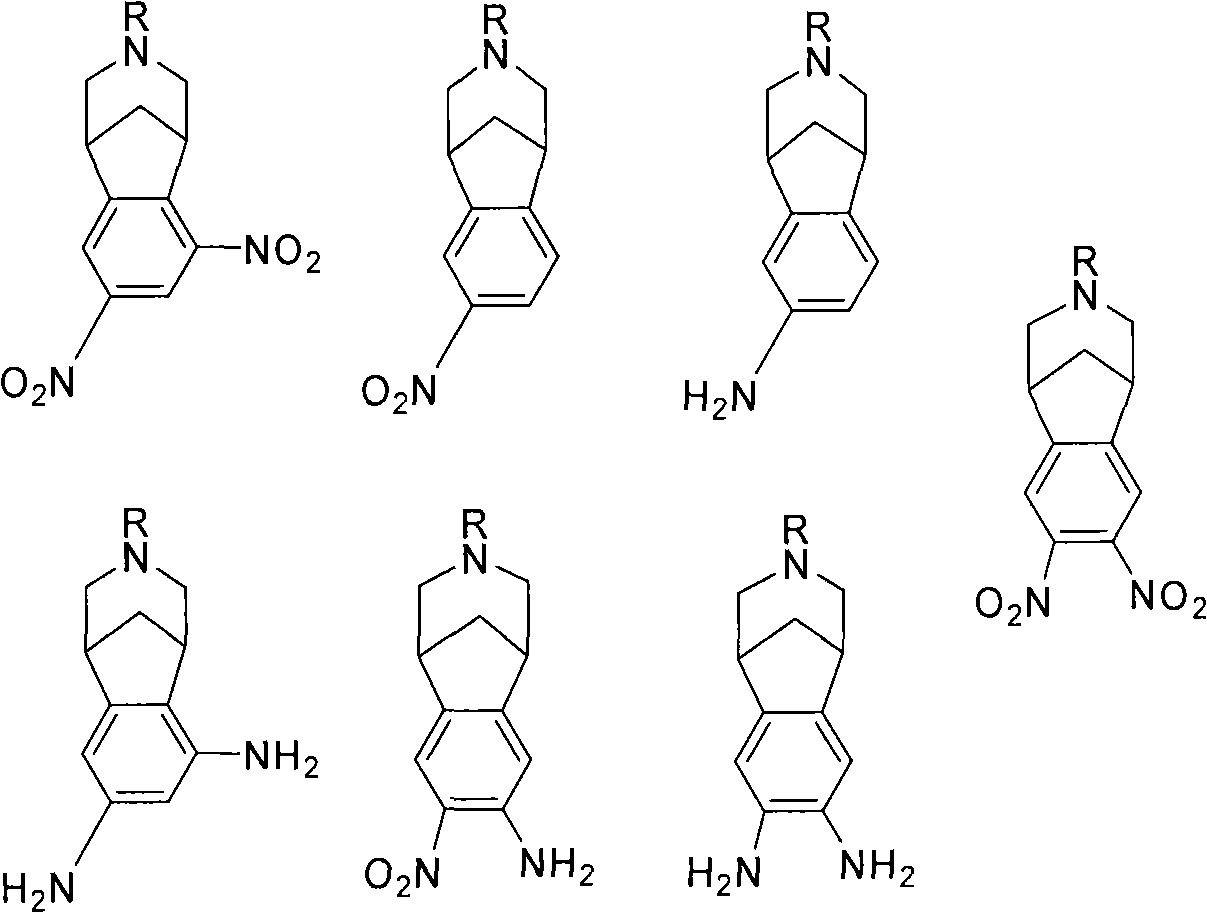
Varenicline standards and impurity controls
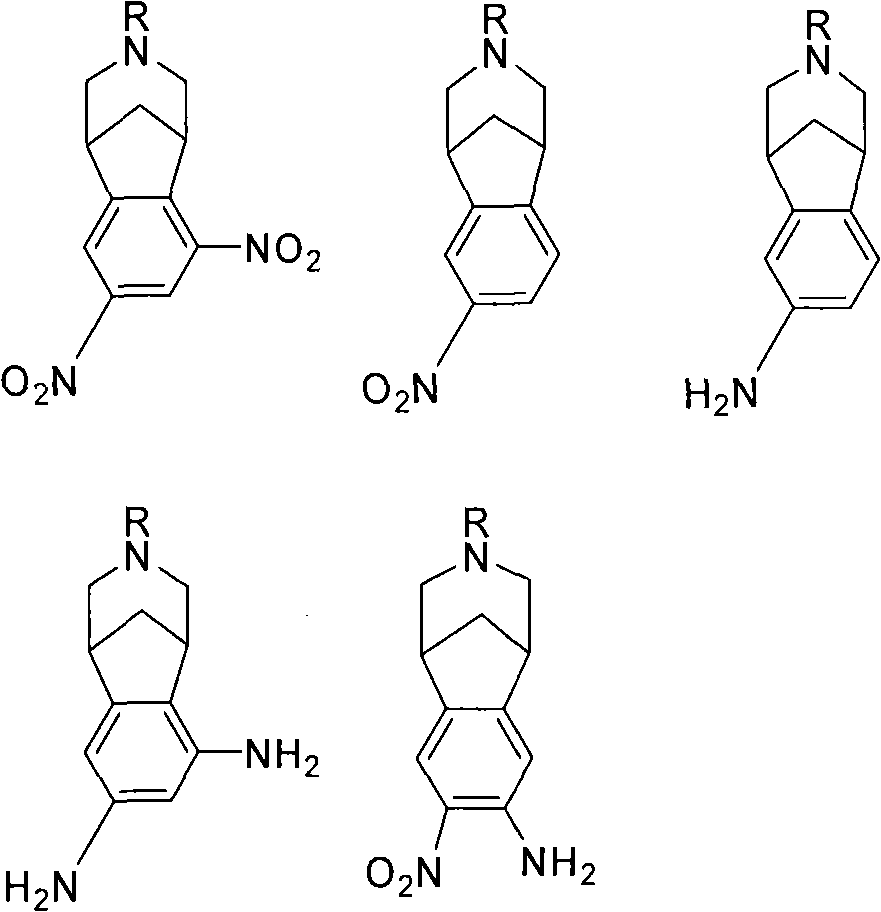
The subject invention provides a varenicline composition that comprises varenicline, or a pharmaceutically acceptable salt thereof, and an amount of a compound selected from one or more of several mononitro, monoamine mixed aminonitro, diamino or dinitro intermediates, and the concentration of said compound is greater than 0 ppm and not greater than about 500 ppm, not greater than about 100 ppm or not greater than about 10 ppm. Methods for synthesizing and using such varenicline compositions are also provided.
Owner:PFIZER PRODS ETAT DE CONNECTICUT
Varenicline tartrate intermediate impurity and preparation method thereof
PendingCN114685497AEnhanced authenticationEasy to separateOrganic chemistryPerfluoroacetic AcidNitration
The preparation method comprises the following steps: reacting an initial raw material with trifluoroacetic anhydride in dichloromethane under the catalysis of triethylamine to obtain an intermediate 1, carrying out nitration reaction on the intermediate 1 and fuming nitric acid under the catalysis of concentrated sulfuric acid, extracting and concentrating dichloromethane to obtain an intermediate 2, and purifying the intermediate 2 to obtain the varenicline tartrate intermediate impurity. Carrying out reduction reaction on the intermediate 2 under the catalysis of palladium carbon to obtain an intermediate 3; and carrying out cyclization reaction on the intermediate 3 and triethyl orthoformate under the catalysis of sulfamic acid to obtain the varenicline tartrate intermediate impurity. The preparation method of the impurity is provided for the first time and can be better applied to the existing preparation process of the varenicline tartrate key intermediate, the impurity identification and separation degree is improved, and the product quality is improved.
Owner:JIANGSU SINOBIOPHARMA
A kind of pharmaceutical composition of varenicline tartrate and preparation method thereof
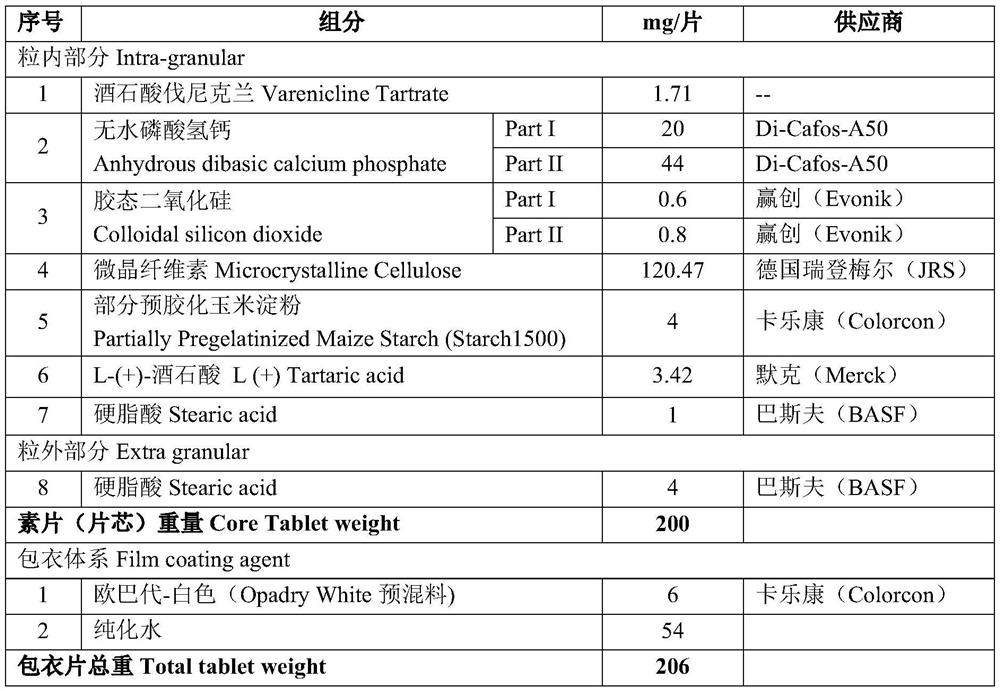
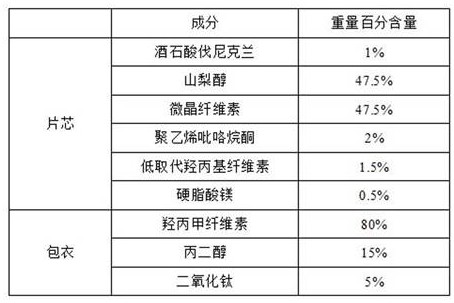
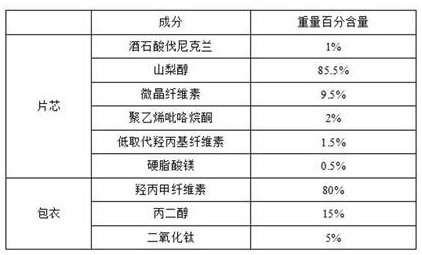
ActiveCN112057428BSimple prescriptionLow friabilityNervous disorderPill deliveryAdhesiveVarenicline Tartrate
The invention provides a varenicline tartrate composition and a preparation method thereof. In the composition, the weight percentage of sorbitol and microcrystalline cellulose is 5:1 to 10:1, and includes a disintegrant, a binder agents and lubricants. This product adopts the powder direct pressing process, the preparation process is simple, easy to operate, good reproducibility, and suitable for large-scale industrial production. Moreover, the varenicline tartrate composition prepared by the present invention not only does not need to control the particle size, but also has small friability, good fluidity, high dissolution rate, good stability and low probability of adverse reactions.
Owner:SHANGHAI HANSOH BIOMEDICAL +2
Varenicline synthesis method
The present invention relates to a process for the preparation of varenicline or a pharmaceutically acceptable salt thereof. The invention also relates to an intermediate compound useful in the process and the preparation of such intermediate compound.
Owner:唐山臻越科技有限公司
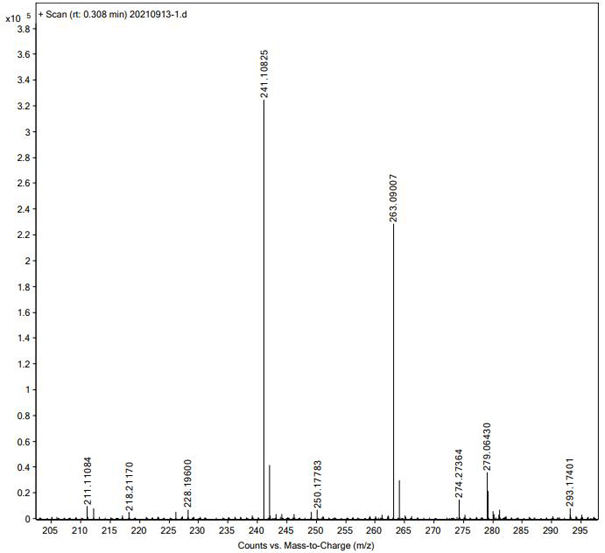
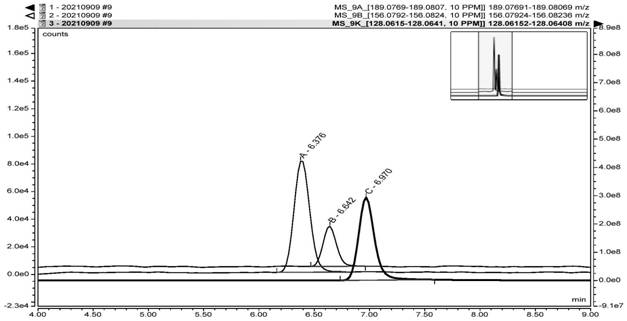
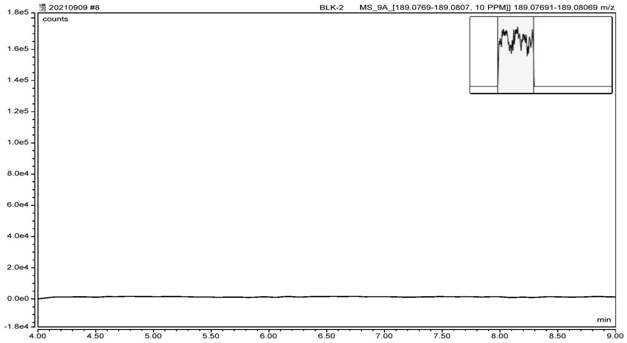
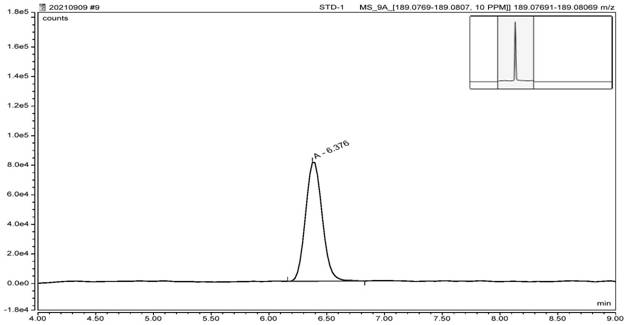
Method for detecting nitrosamine genotoxic impurities in varenicline intermediate
PendingCN114088843AHigh detection sensitivityQuality improvementComponent separationChemical structureResolution (mass spectrometry)
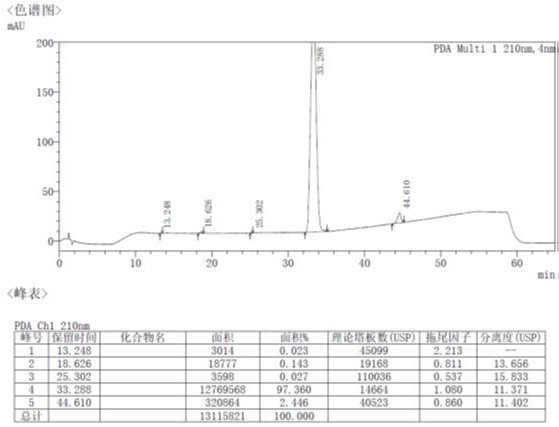
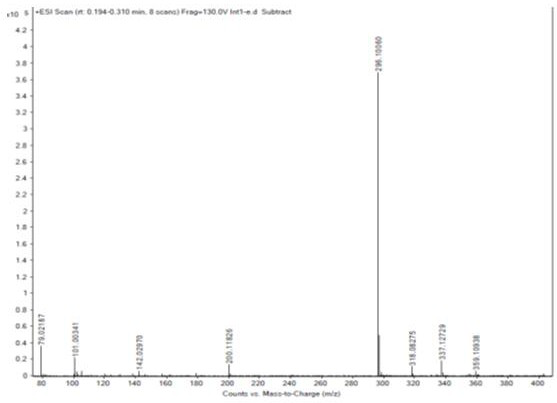
The invention provides a method for detecting nitrosamine genotoxic impurities in a varenicline intermediate. The method comprises the step of determining the content of the nitrosamine genotoxic impurities by adopting a high-resolution liquid chromatography and mass spectrometry technology, and the nitrosamine genotoxic impurities are compounds A, B and C with the chemical structural formulas shown in the specification, and the structure of the varenicline intermediate is shown in the specification. according to the method, the effective separation of the varenicline intermediate and the related genotoxic impurities can be achieved, and the quantitative detection of the micro-content can be achieved so as to effectively control the content of the genotoxic impurities in the bulk drug and ensure the medication safety; and the detection method provided by the invention adopts common reagents, and is simple and convenient to operate, good in separation degree, high in sensitivity and good in reproducibility.
Owner:上海皓鸿生物医药科技有限公司
Preparation method for varenicline intermediate
InactiveCN103570502AWide variety of sourcesReduce manufacturing costPreparation by oxidation reactionsOsmic AcidKetone solvents
The invention relates to a preparation method for a varenicline intermediate. The method includes: subjecting 1, 4-dihydro-1, 4-methanonaphthalene to an oxidation reaction under the action of an oxidizing agent so as to generate the varenicline intermediate. The oxidizing agent is an osmic acid salt or its hydrate, the oxidation reaction undergoes in a solvent in the presence of N-methyl-N-morpholine oxide, and the solvent is a mixed solvent of water and any one or more of the following organic solvents: C1-C6 alcohol solvents, ketone solvents, ether solvents and nitrile solvents. For the mixed solvent, water and the organic solvent are in a volume ratio of 1:0.1-9. The method provided by the invention has the advantages of low production cost, simple operation, mild reaction conditions and environmental friendliness, and can prepare the product that is easy to purify and has purity up to over 99%. With high yield, the method is suitable for both small-scale preparation in laboratories and large-scale industrialized production.
Owner:SHANGHAI SYNCORES TECH INC
The synthetic method of varenicline
ActiveCN113185513BOrganic chemistryBulk chemical productionCombinatorial chemistryPharmaceutical medicine
Owner:唐山臻越科技有限公司
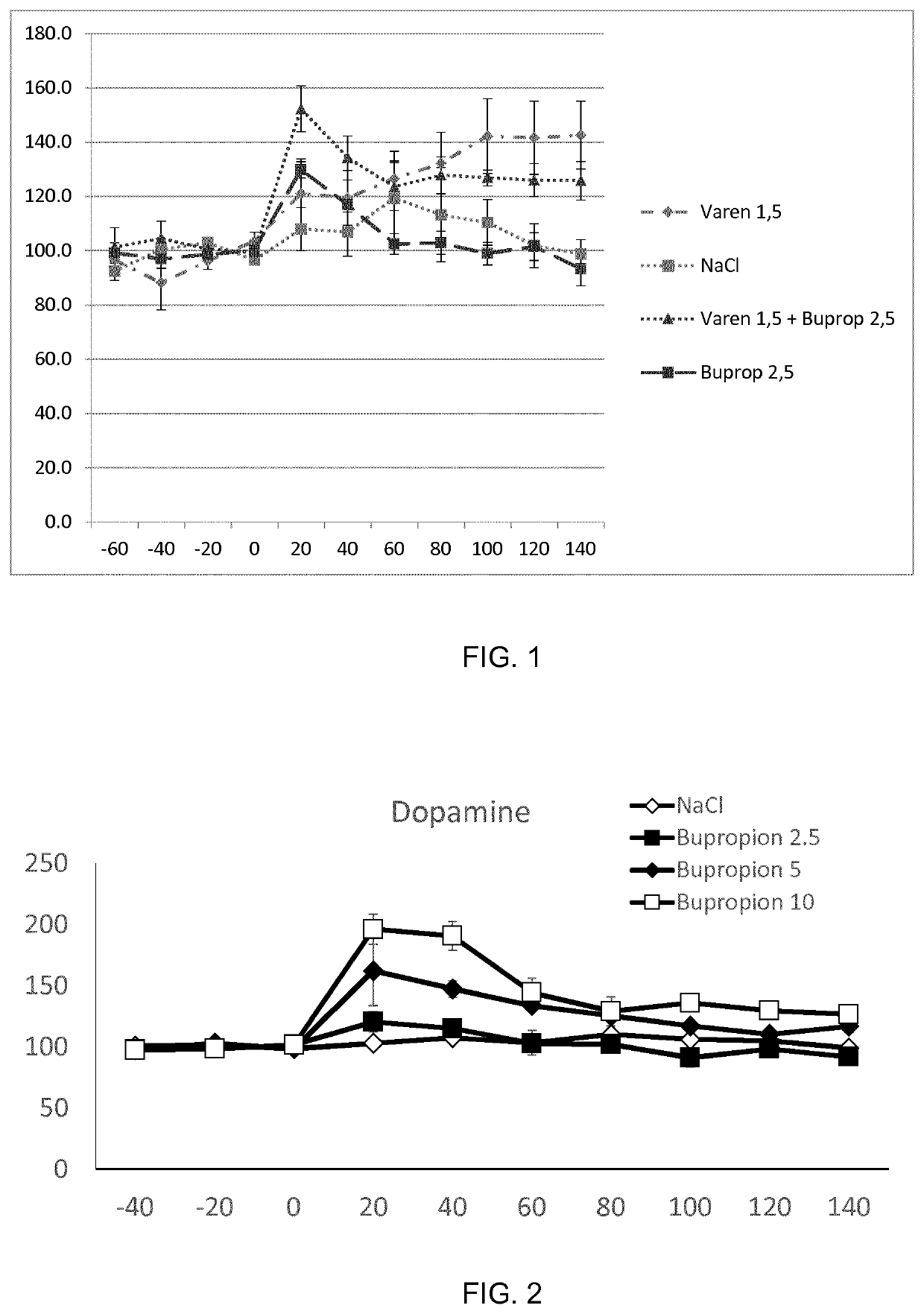
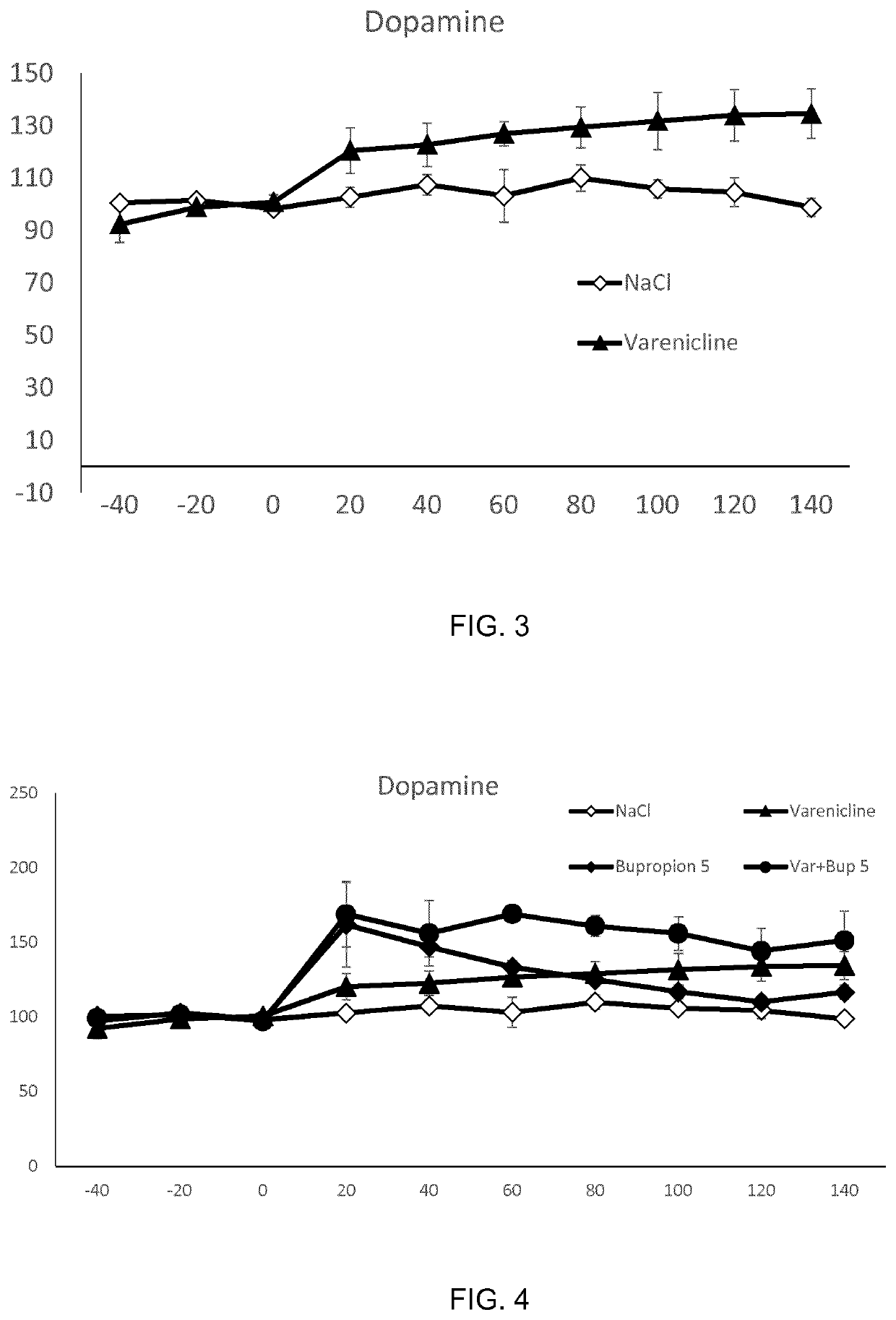
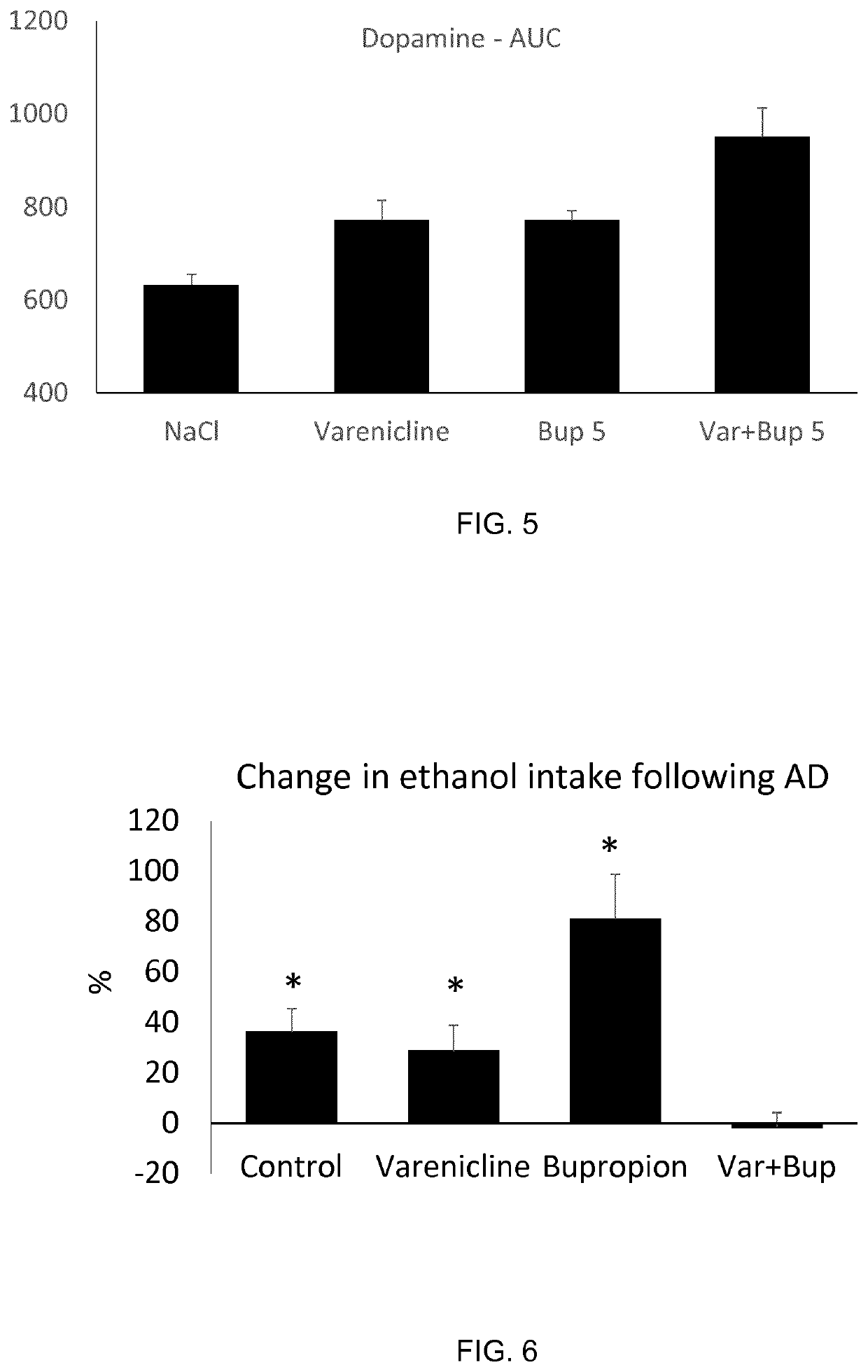
Treatment of alcohol use disorder
ActiveUS11318143B2Block nAChR activationFacilitated releaseNervous disorderHeterocyclic compound active ingredientsAlcohol abuse disorderPharmacology
The present invention is directed to a combination of varenicline and bupropion for use in treating alcohol use disorder (AUD) and / or treating alcohol risk consumption in a subject in need thereof. Corresponding compositions, uses and methods of treatment are also provided.
Owner:SODERPALM BO +1
A kind of varenicline tartrate tablet and preparation method thereof
ActiveCN112057431BSimple prescriptionLow friabilityNervous disorderPharmaceutical non-active ingredientsVarenicline TartrateMedicinal chemistry
Owner:JIANGSU HANSOH PHARMA CO LTD +1
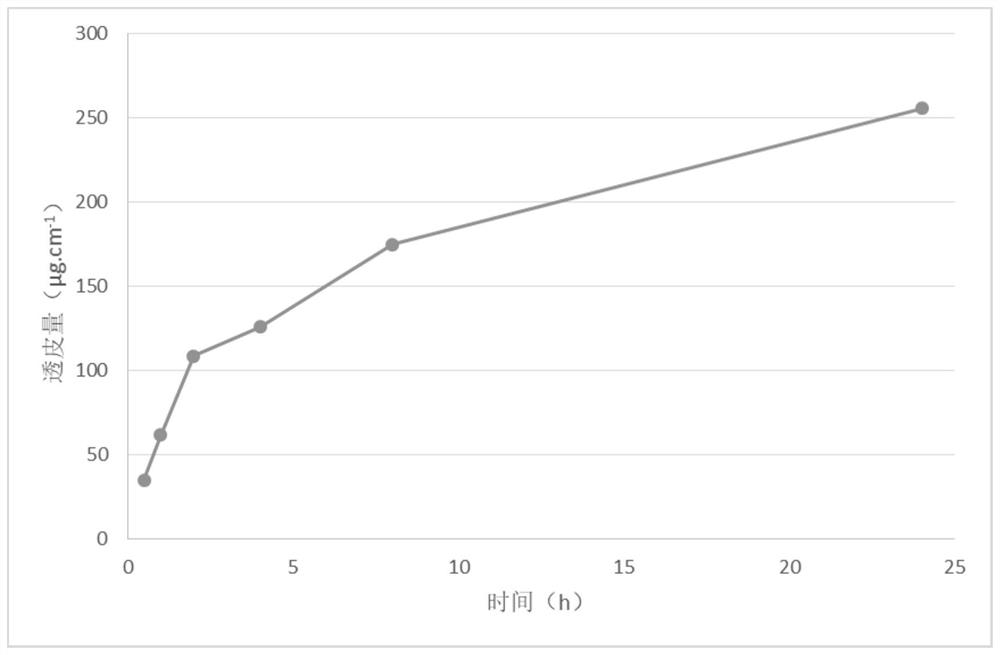
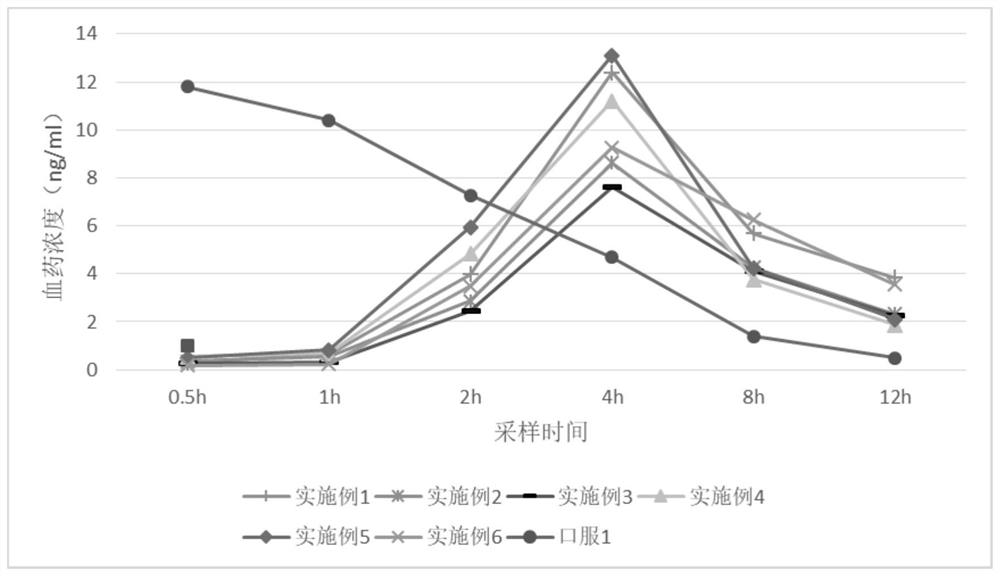
Varenicline transdermal solution as well as preparation method and application thereof
InactiveCN113116814AImprove solubilityImprove uniformityNervous disorderAntipyreticPatient complianceGlycerol
The present invention relates to a varenicline transdermal solution. The transdermal solution comprises varenicline or a pharmaceutically acceptable salt thereof and a pharmaceutically acceptable carrier, wherein the pharmaceutically acceptable carrier is selected from any one or a combination of a penetration enhancer, a humectant, a preservative and a solvent, and the solvent is selected from any one or a combination of glycerol, propylene glycol, ethanol and water. The solution is absorbed through skin, has the advantages of being long-acting, high in bioavailability, good in patient compliance, convenient to carry and use, flexible in medication position, capable of achieving postaural targeted administration, free of skin irritation and the like, and the effectiveness and safety of the medicine and the medication compliance of the patient are remarkably improved.
Owner:江苏山信药业有限公司
Orally disintegrating tablet containing varenicline solid dispersion, and preparation method of orally disintegrating tablet
PendingCN113425691AOrganic active ingredientsNervous disorderOral medicationOrally disintegrating tablet
The invention belongs to the field of medicine preparations, and particularly relates to a varenicline orally disintegrating tablet and a preparation method thereof. By aiming at the problems that varenicline has a bitter taste and has poor oral administration compliance, varenicline, a carrier and a solvent are prepared into a solid dispersion so as to cover the poor taste of a medicine; and the solid dispersion is adopted for preparing the orally disintegrating tablet, and the orally disintegrating tablet can be quickly disintegrated within 30 seconds, has a good taste, and remarkably improves the oral administration compliance.
Owner:BEIJING VENTUREPHARM BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com