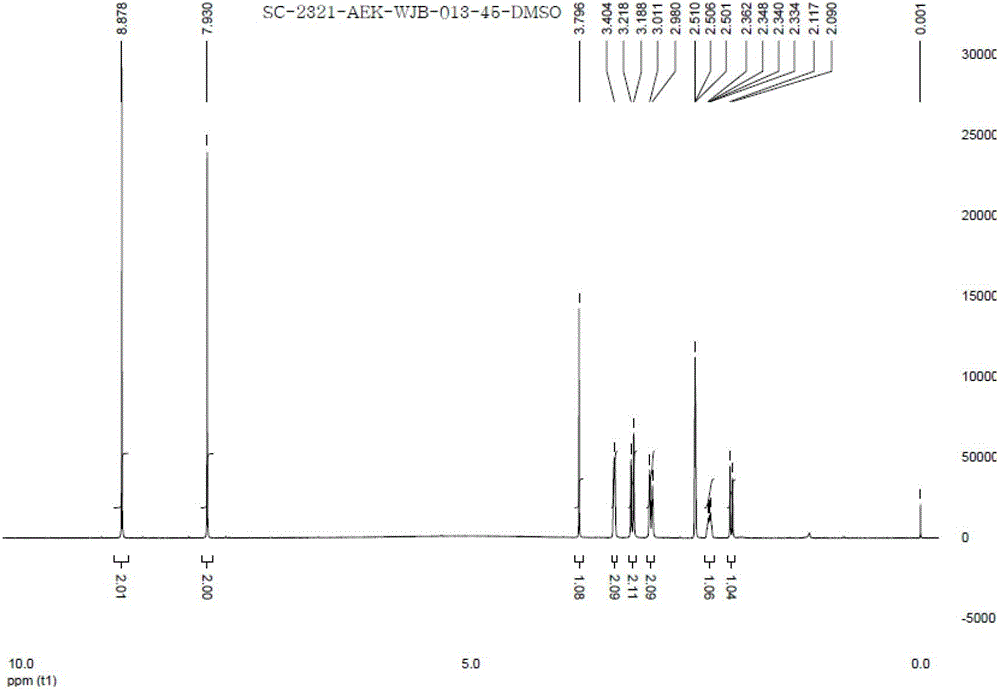
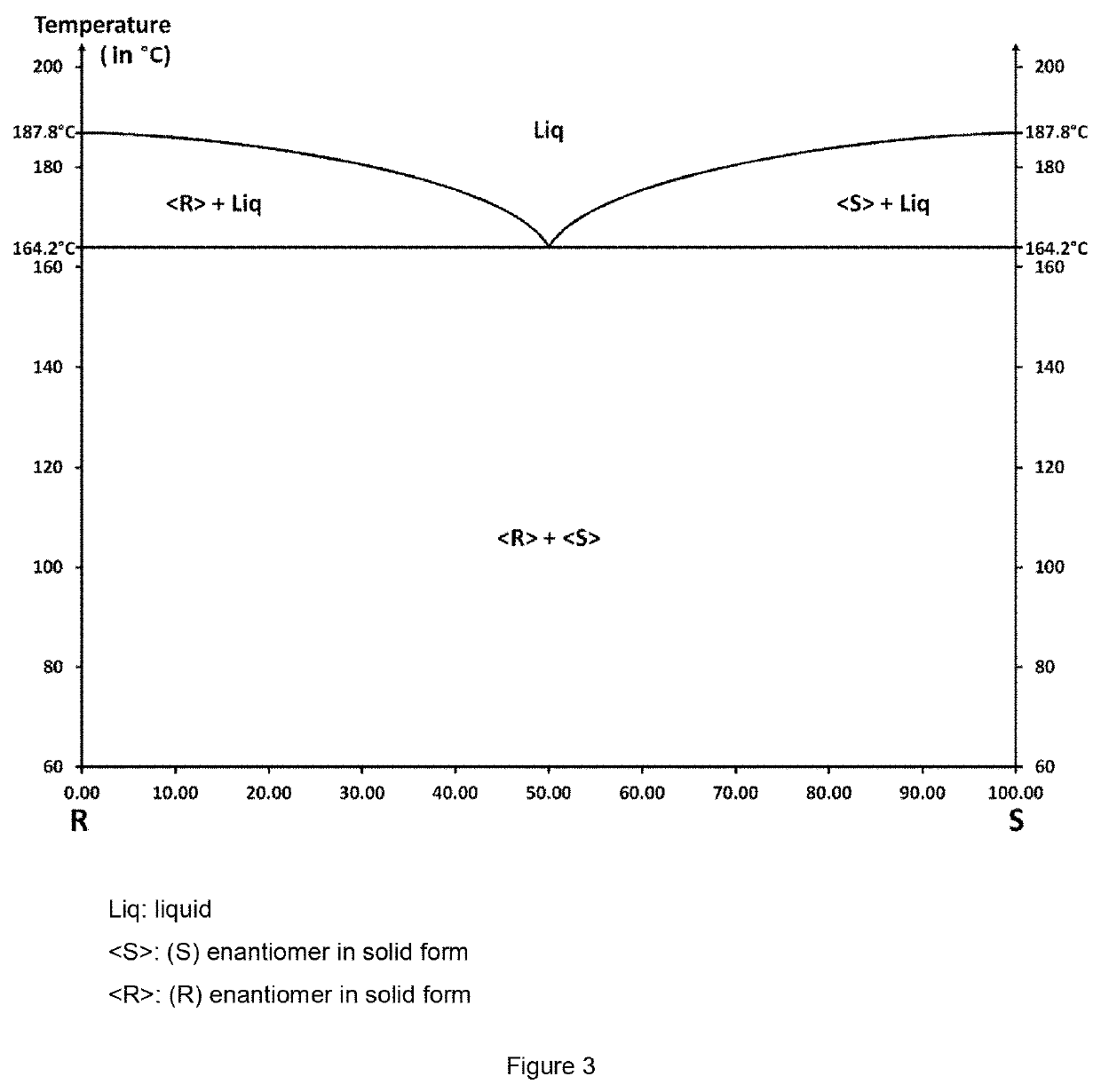
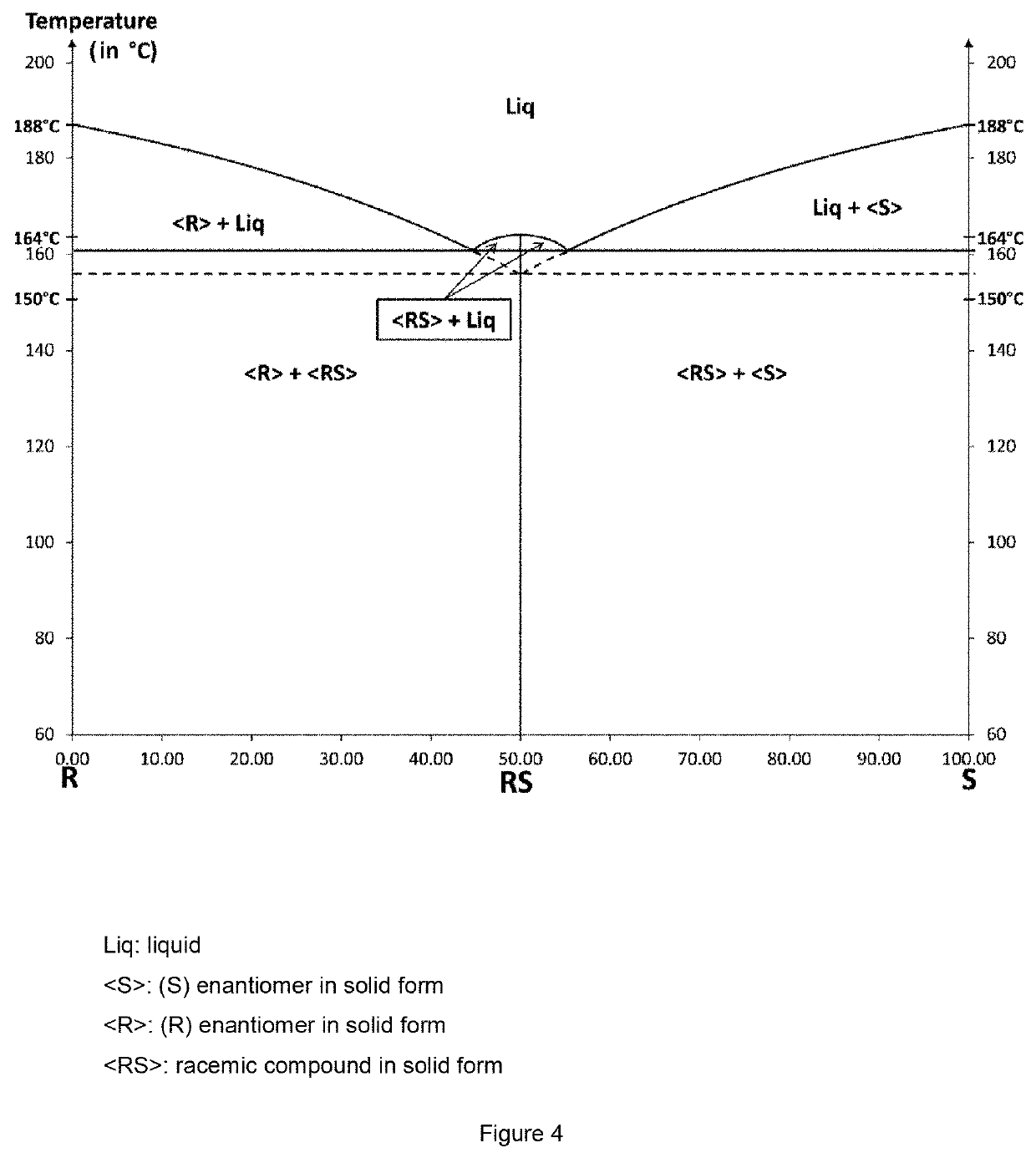
Patents
Literature
62results about How to "Solubility" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
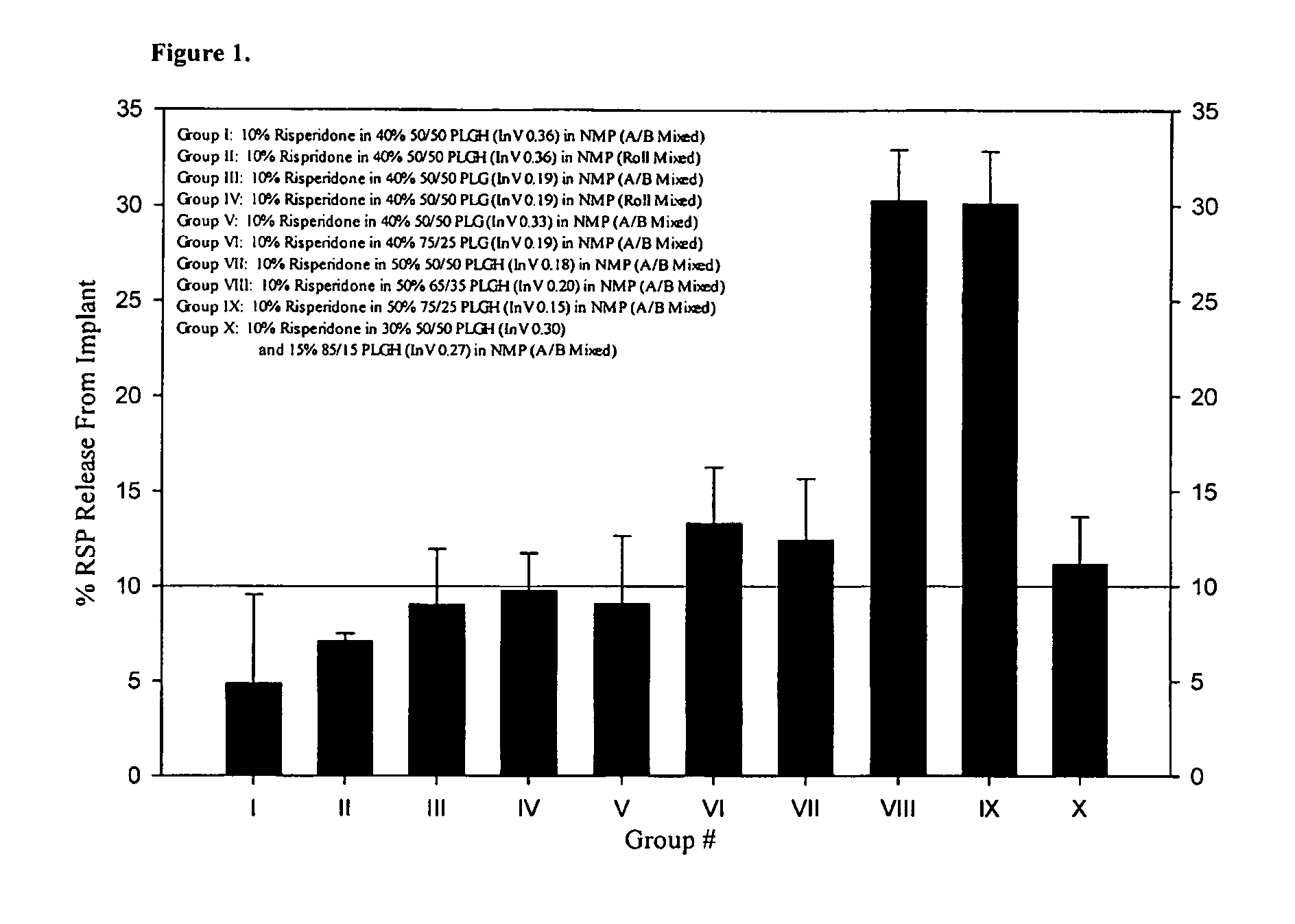
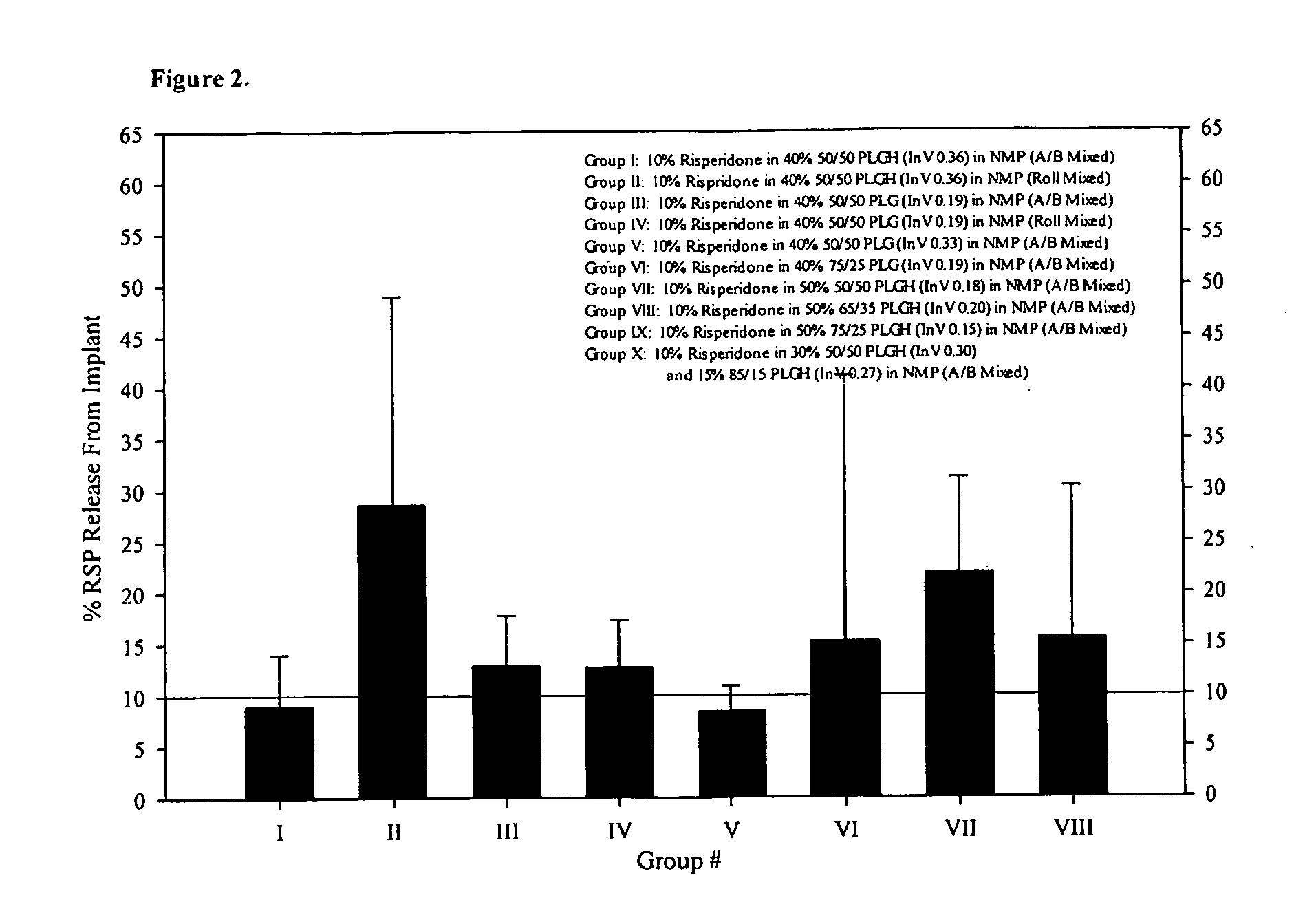
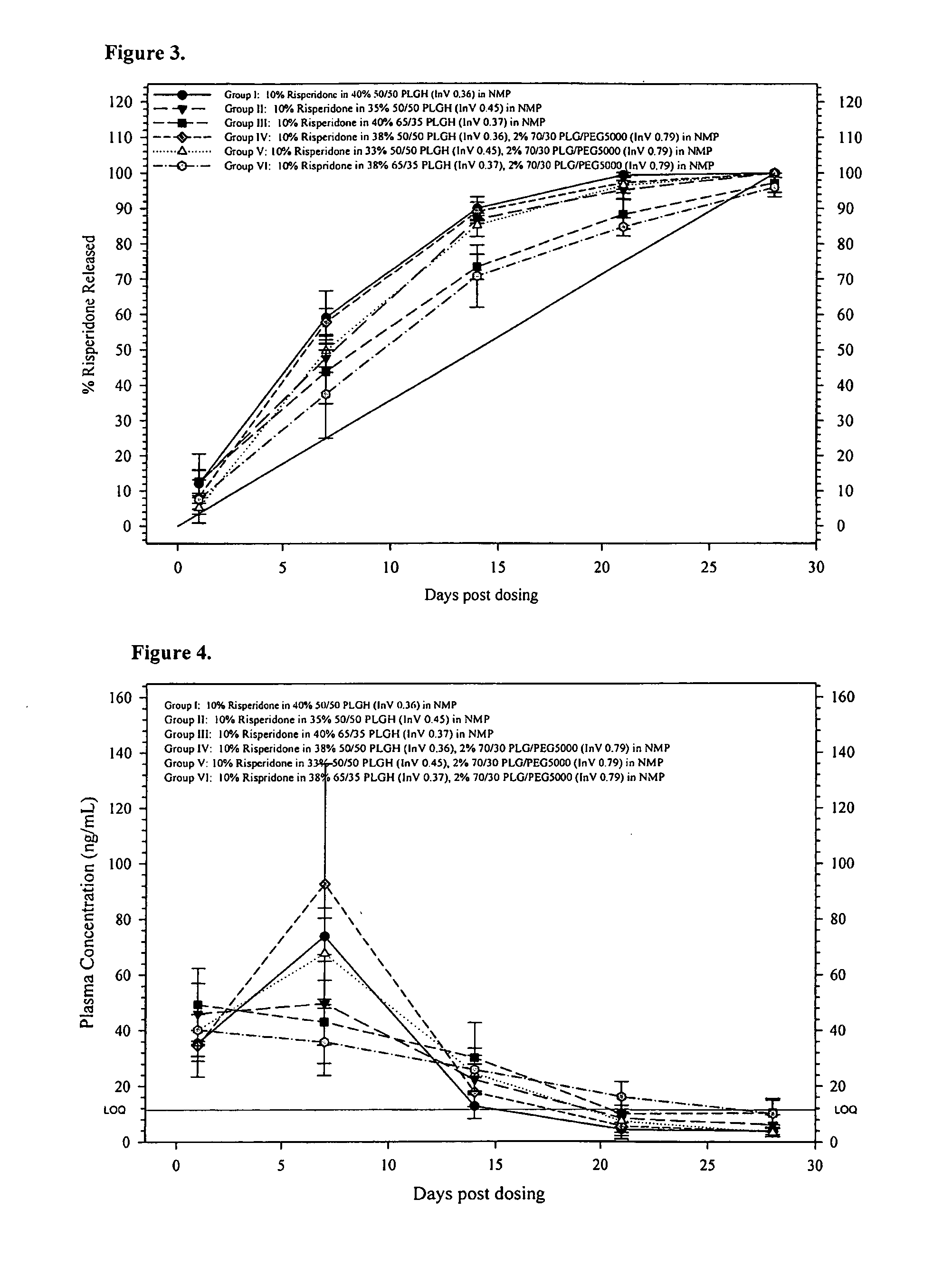
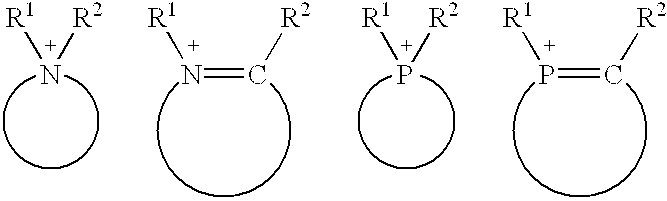
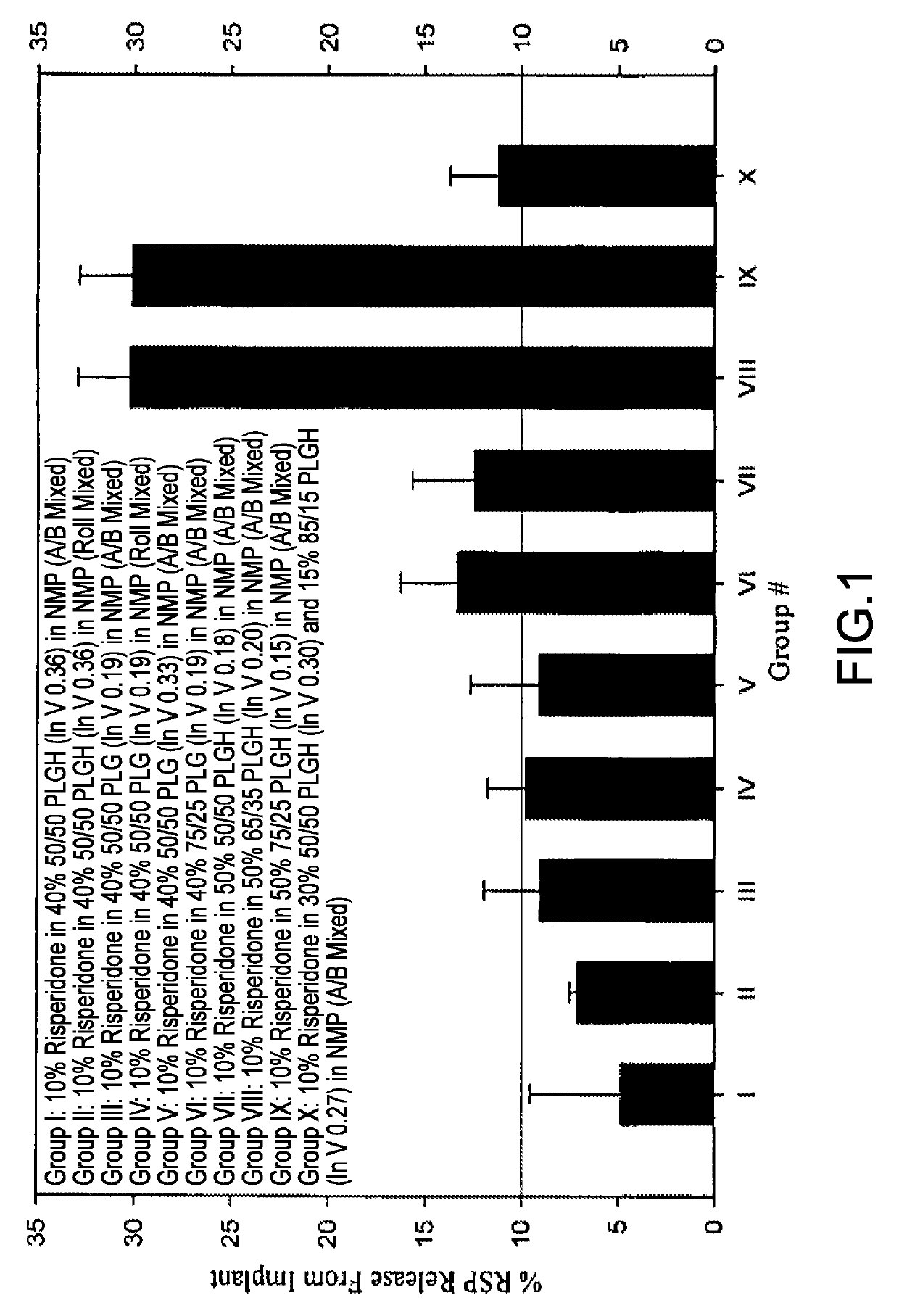
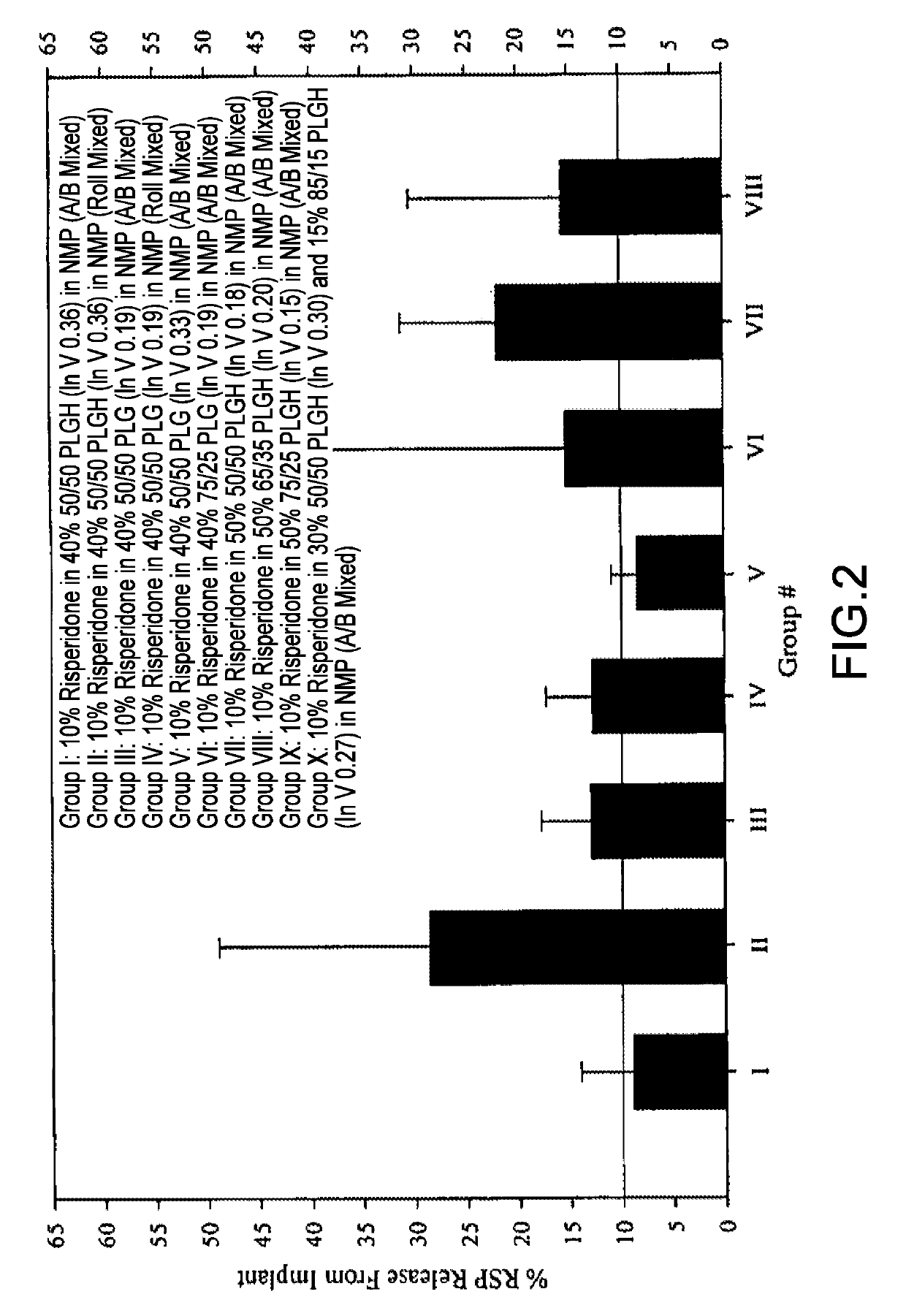
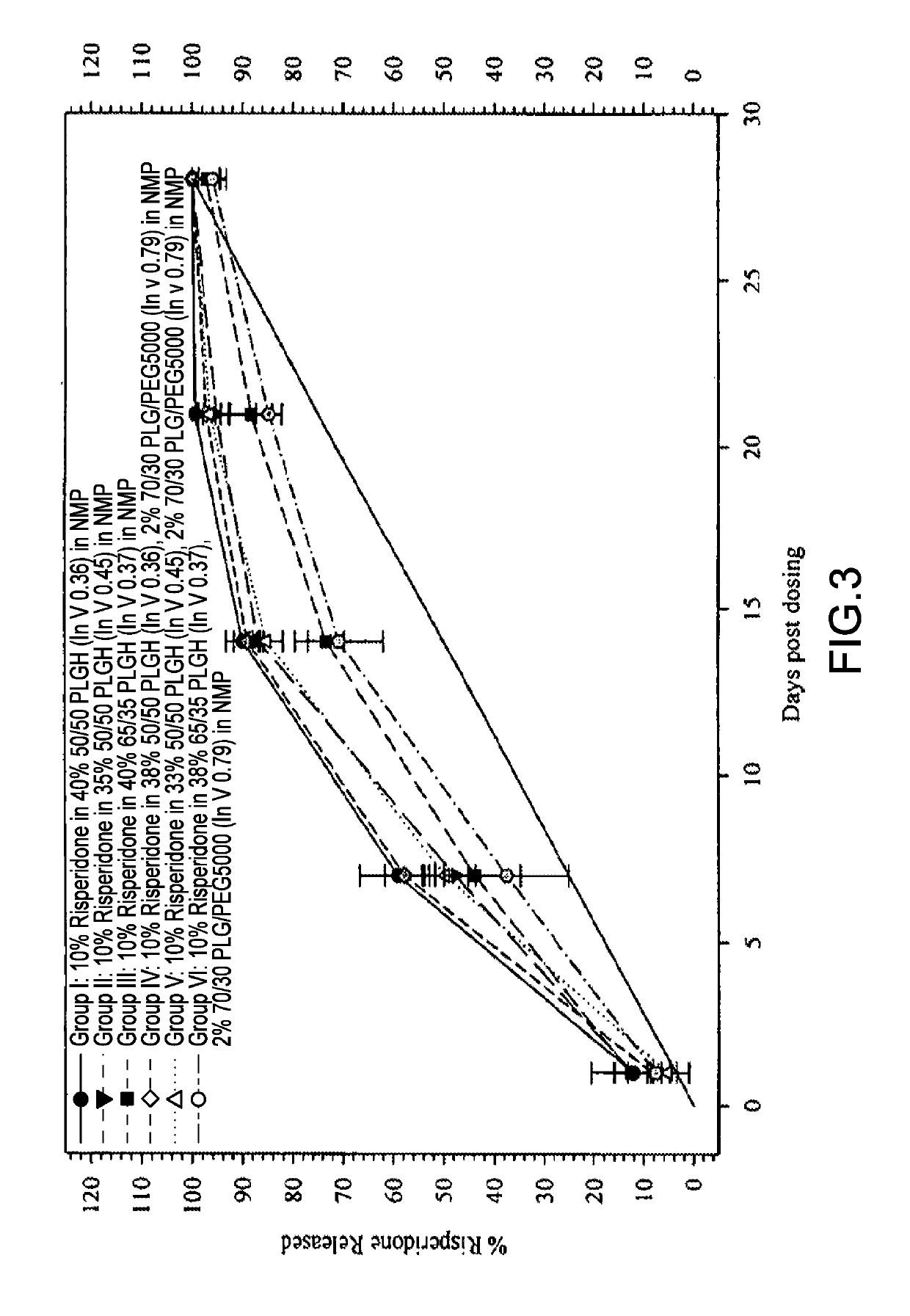
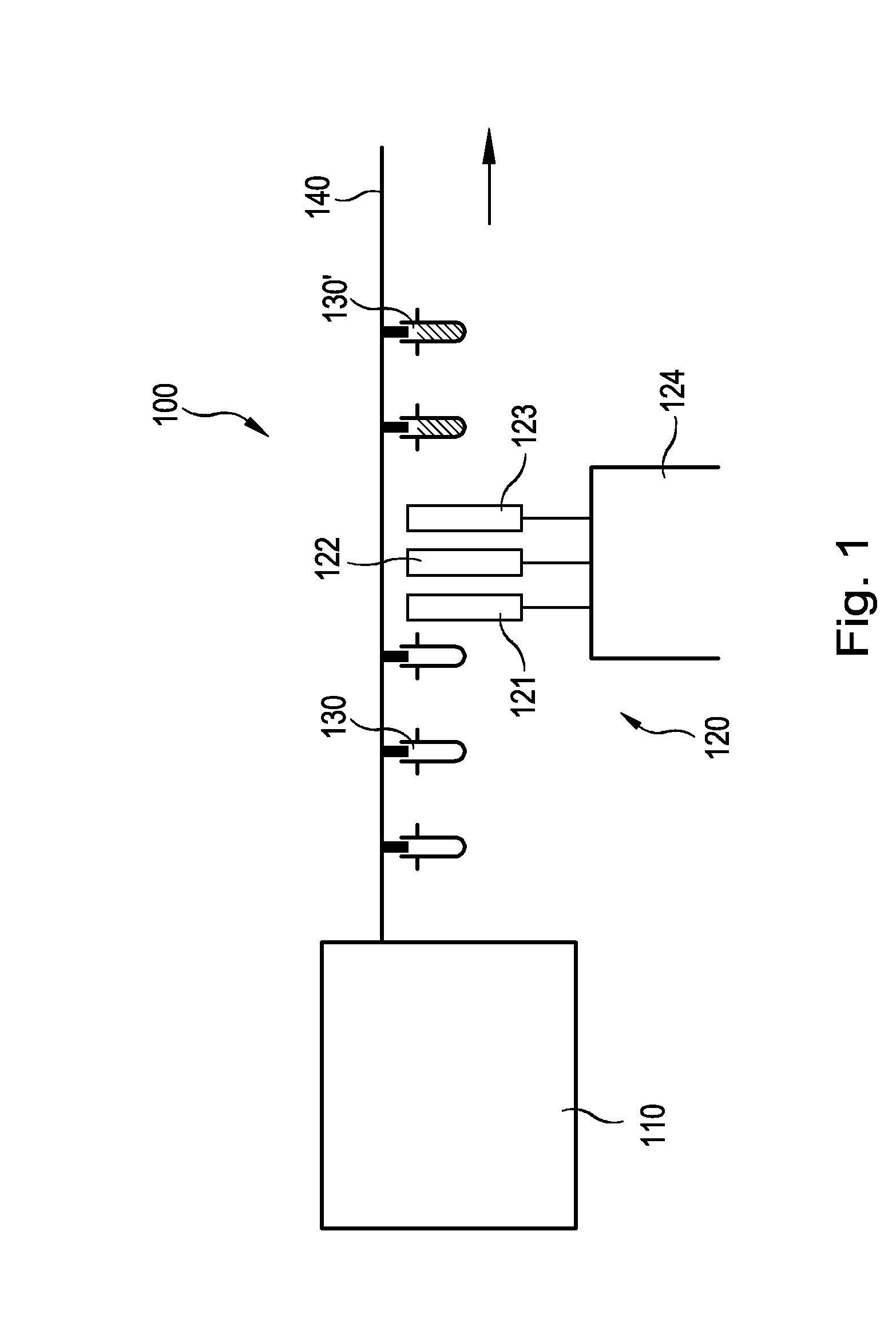
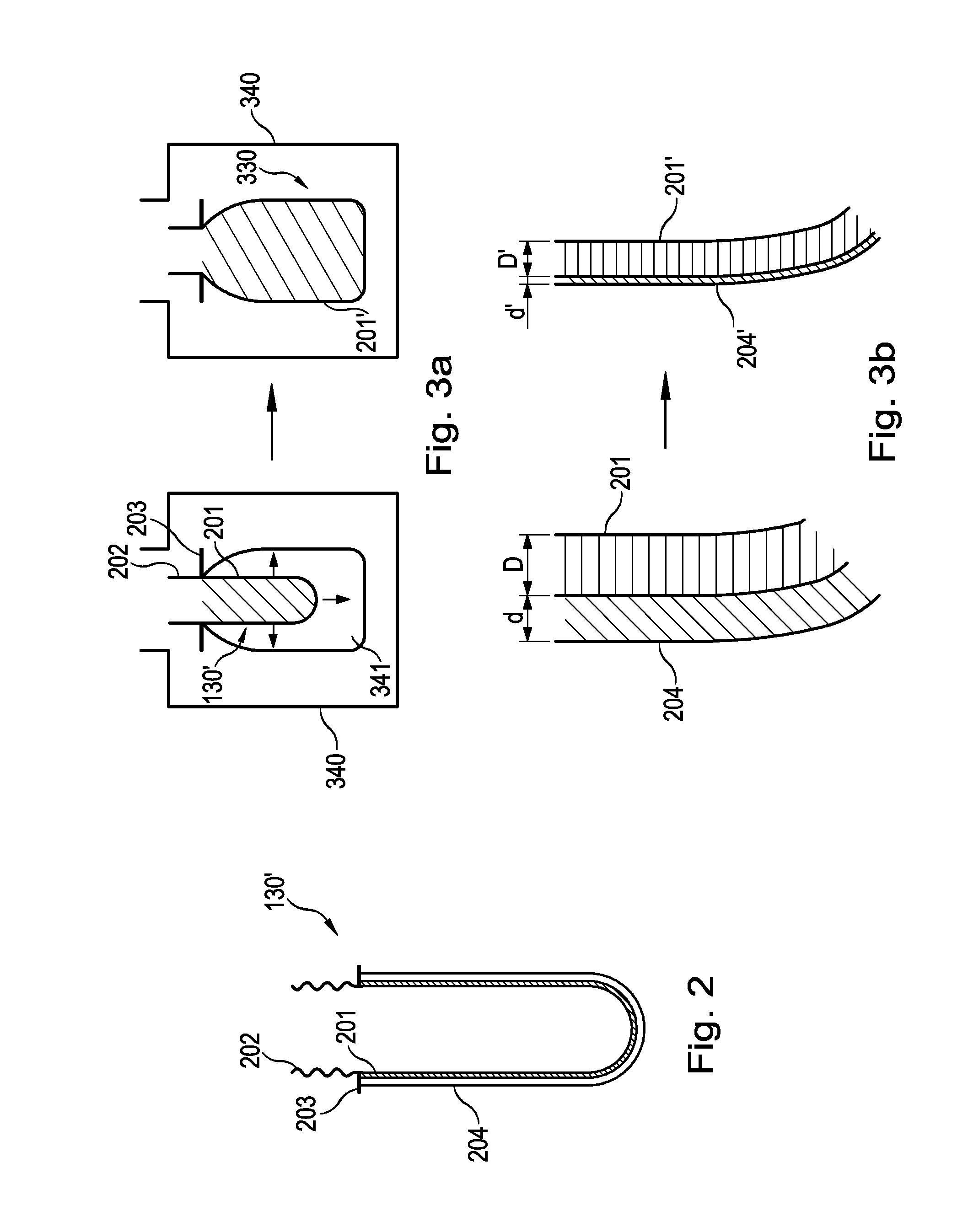
Sustained delivery formulations of risperidone compounds
ActiveUS20100266655A1Improve bioavailabilityLeast riskBiocideOrganic active ingredientsMetaboliteOrganic fluid
The present invention relates to a risperidone sustained release delivery system for treatment of medical conditions relating delusional psychosis, schizophrenia, bipolar disorder, psychotic depression, obsessive-compulsion disorder, Tourette syndrome, and autistic spectrum disorders. The sustained release delivery system includes a flowable composition containing risperidone, a metabolite, or a prodrug thereof and an implant containing risperidone, a metabolite, or a prodrug thereof. The flowable composition may be injected into tissue whereupon it coagulates to become the solid or gel, monolithic implant. The flowable composition includes a biodegradable, thermoplastic polymer, an organic liquid, and risperidone, a metabolite, or a prodrug thereof.
Owner:INDIVIOR UK
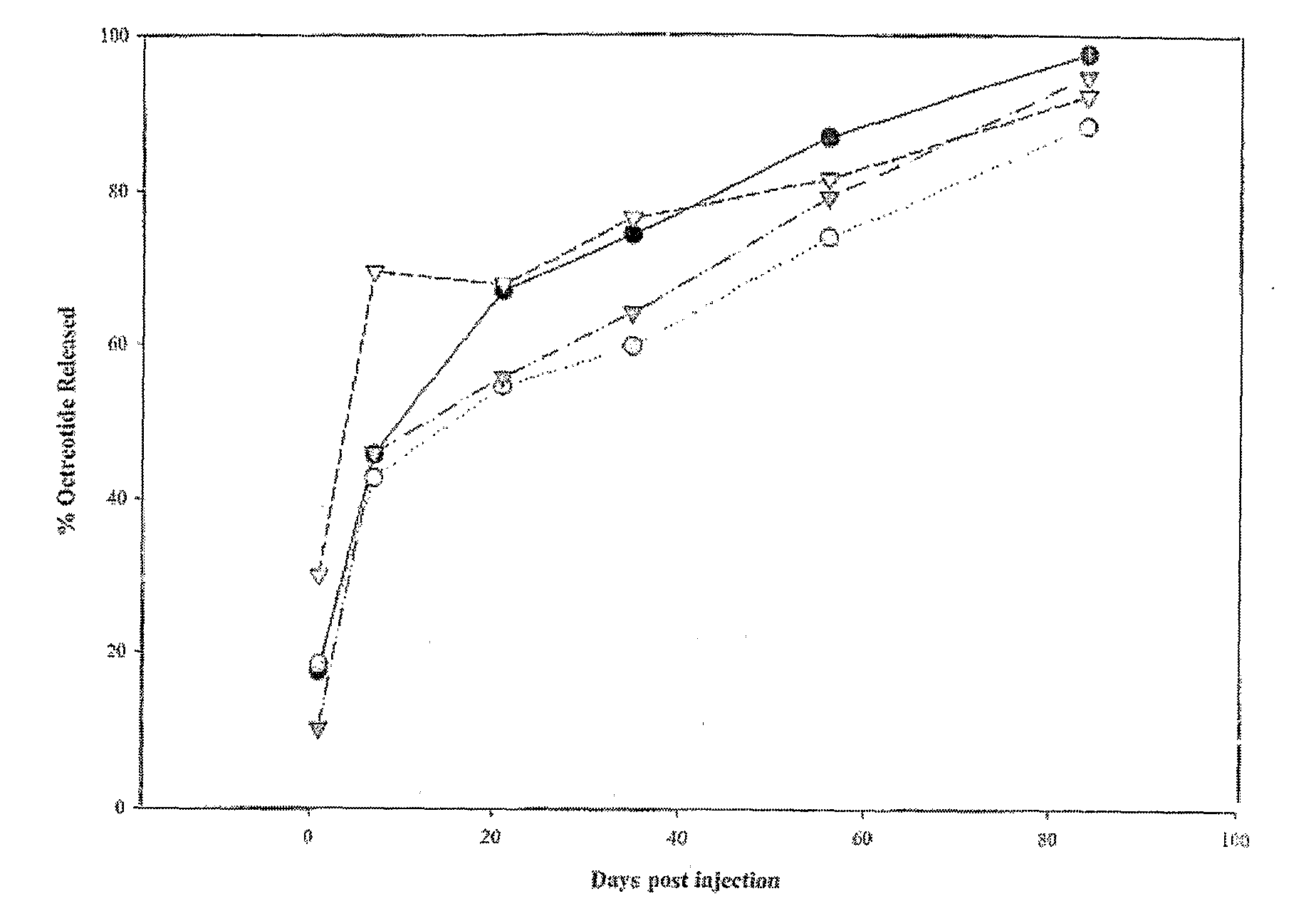
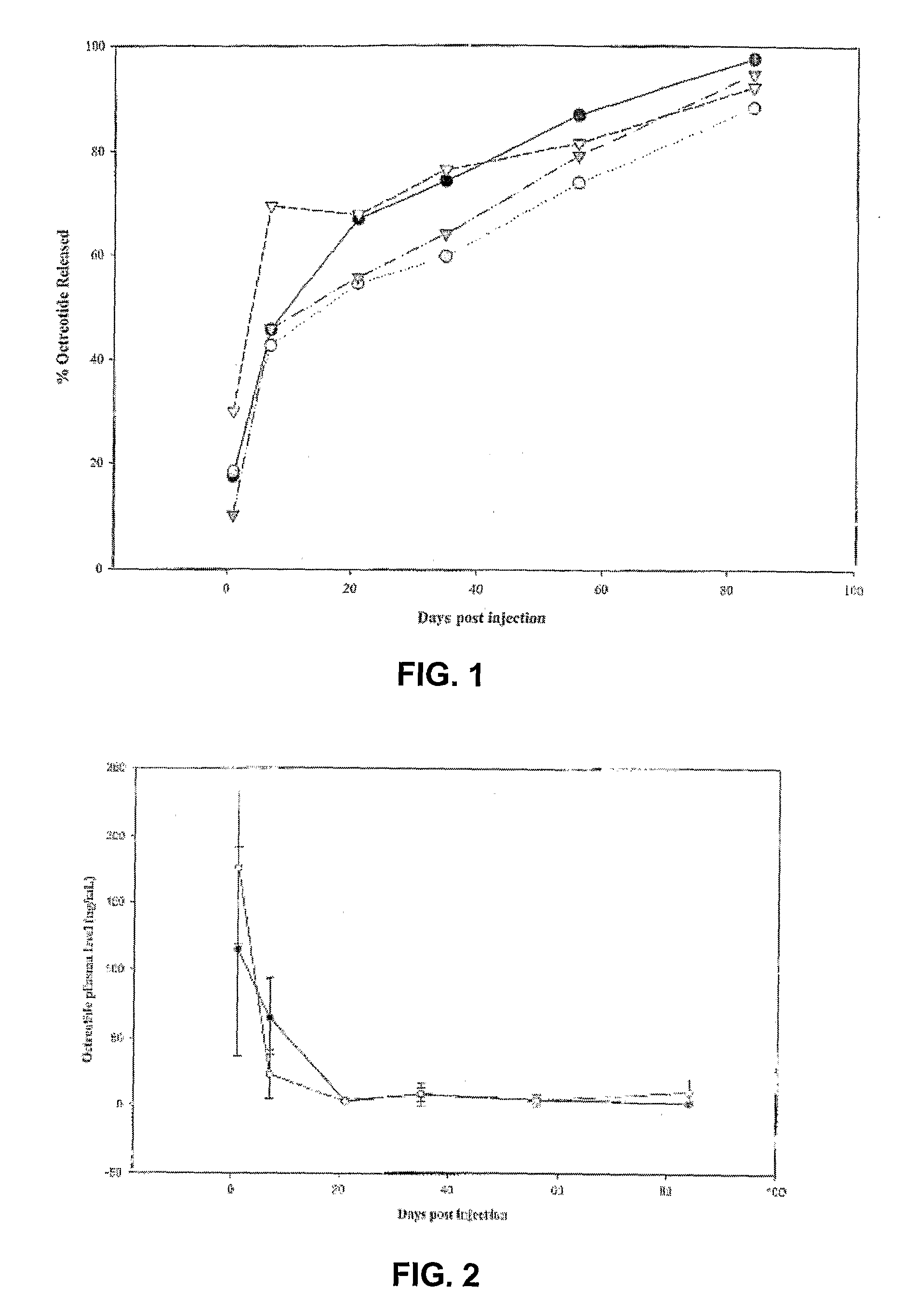
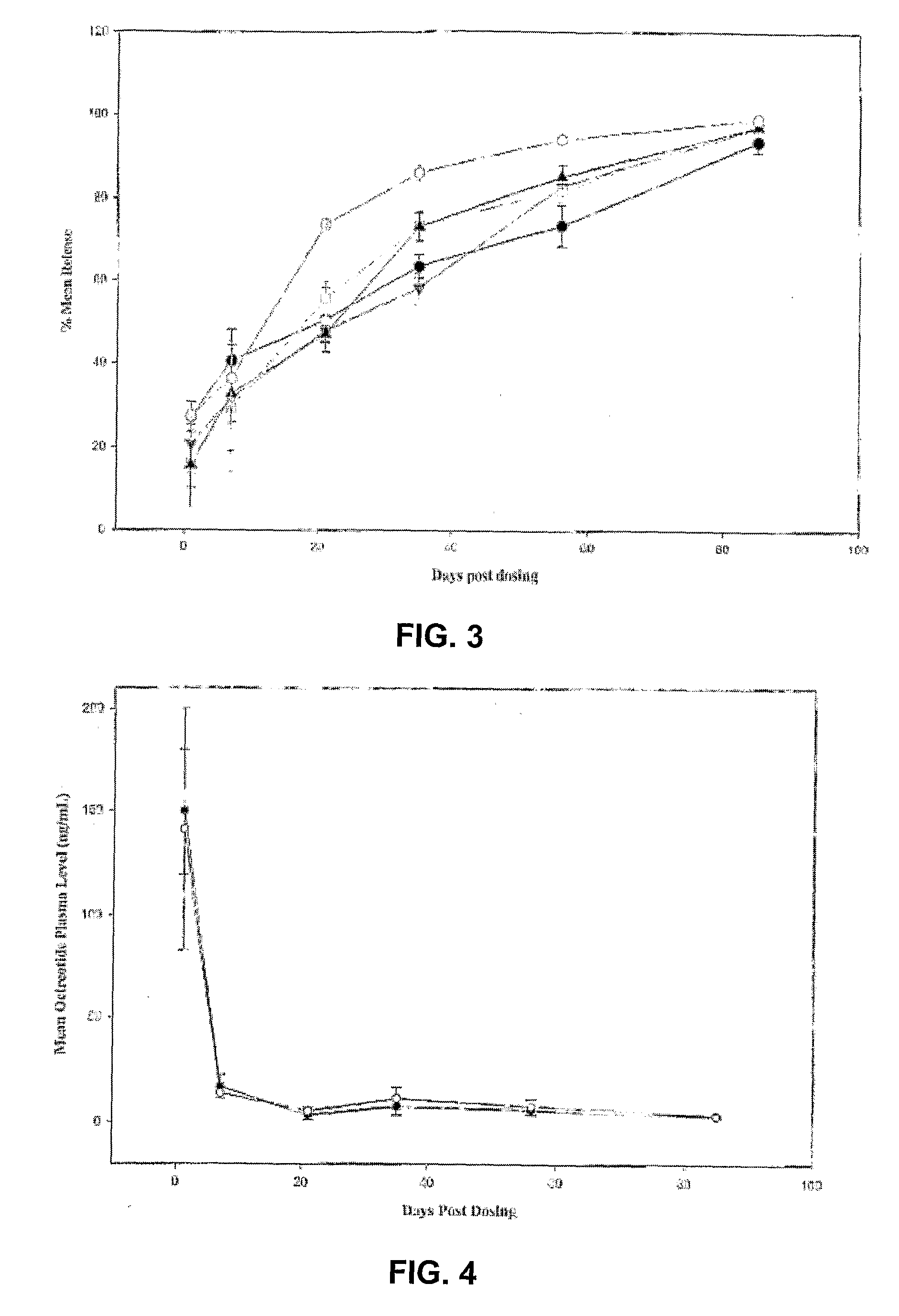
Sustained Delivery Formulations of Octreotide Compounds
InactiveUS20090092650A1Improve bioavailabilityLeast riskSenses disorderPeptide/protein ingredientsMedicineOrganic liquids
The present invention relates to an octreotide sustained release delivery system for treatment of diseases relating to somatotropin and / or somatostatin. The sustained release delivery system of the invention includes a flowable composition containing an octreotide compound, and an implant containing the octreotide compound. The flowable composition may be injected into tissue whereupon it coagulates to become the solid or gel, monolithic implant. The flowable composition includes a biodegradable, thermoplastic polymer, an organic liquid and an octreotide compound.
Owner:QLT USA INC
Composition of catalyst and solvent and catalysis processes using this composition
ActiveUS7256152B2High selectivityHigh activityHydrocarbon by isomerisationOrganic-compounds/hydrides/coordination-complexes catalystsAlkaneLiquid medium
A composition defined:either as comprising at least one Broensted acid, designated HB, dissolved in a liquid medium with an ionic nature of general formula Q+A−, in which Q+ represents an organic cation and A− represents an anion that is different from B,or as resulting from dissolving at least one Broensted acid, designated HB, in a non-aqueous liquid medium with an ionic nature of general formula Q+A−, in which Q+ represents an organic cation and A− represents an anion that is identical to the anion B, can be used as a catalyst and solvent in acid catalysis processes, in particular in the alkylation of aromatic hydrocarbons, the oligomerization of olefins, the dimerization of isobutene, the alkylation of olefins by isoparaffins, the isomerization of n-paraffins into isoparaffins, the isomerization of n-olefins into iso-olefins, the isomerization of the double bond of an olefin and the purification of an olefin mixture that contains branched alpha olefins as impurities.
Owner:INST FR DU PETROLE
Vehicle for topical delivery of anti-inflammatory compounds
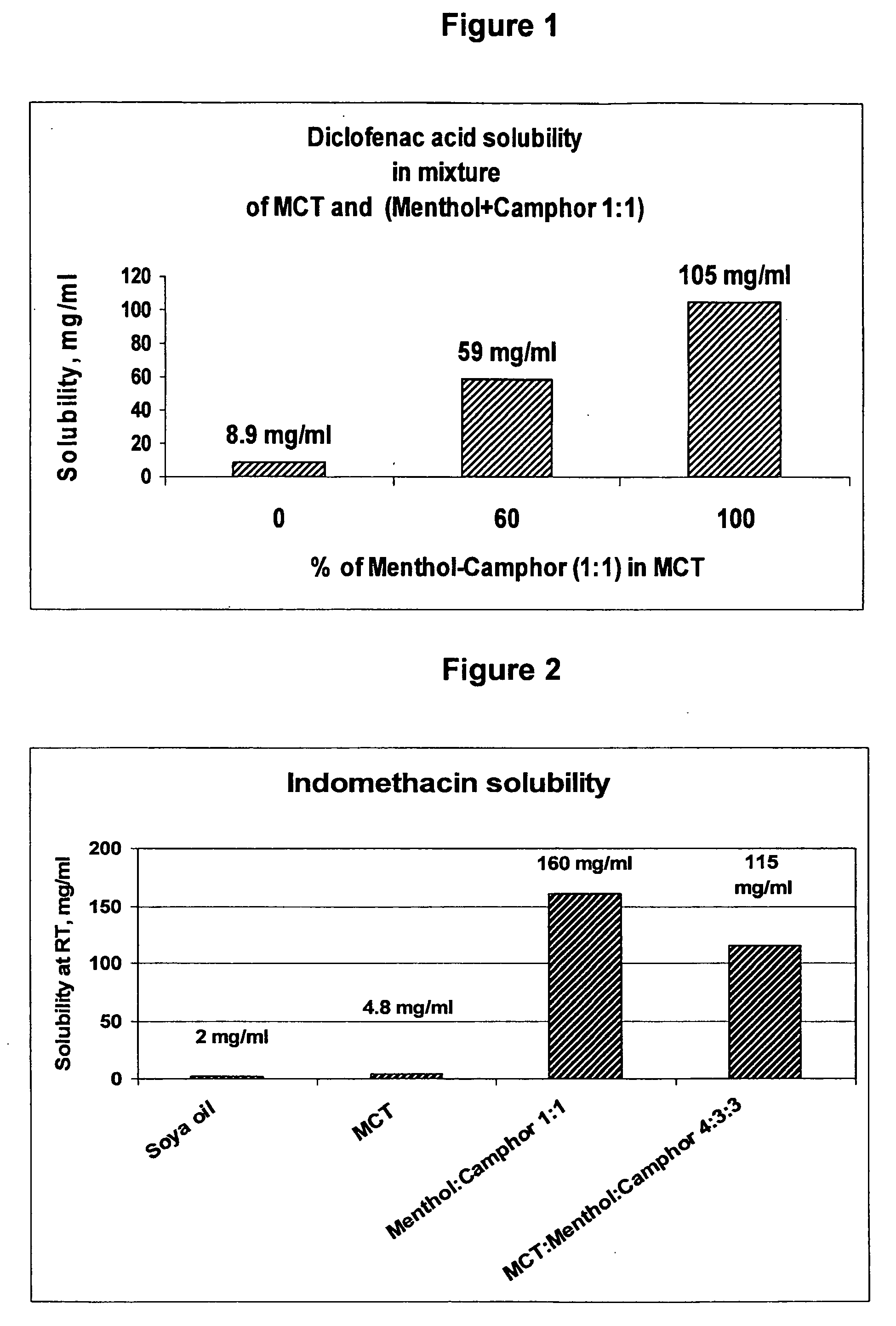
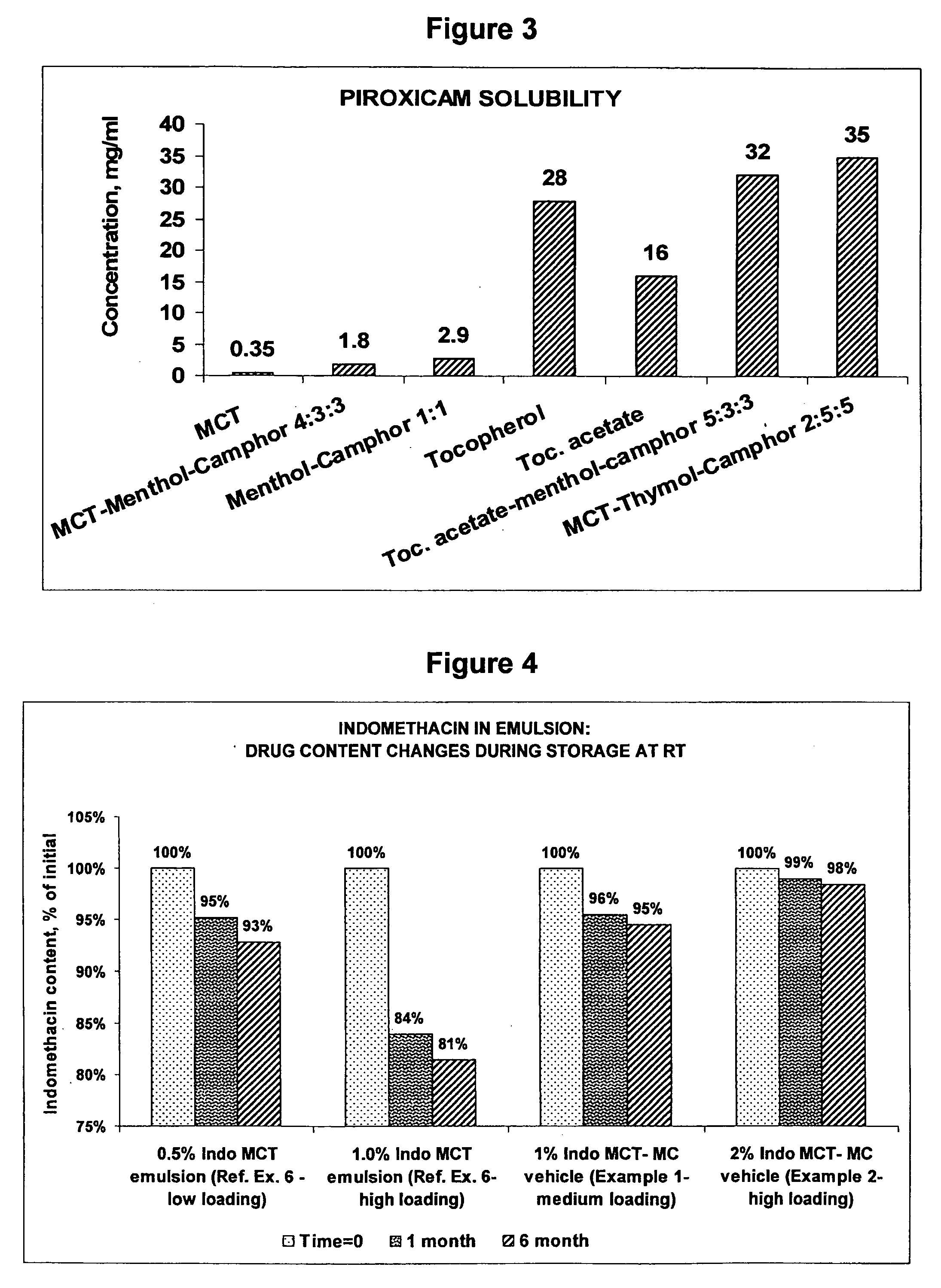
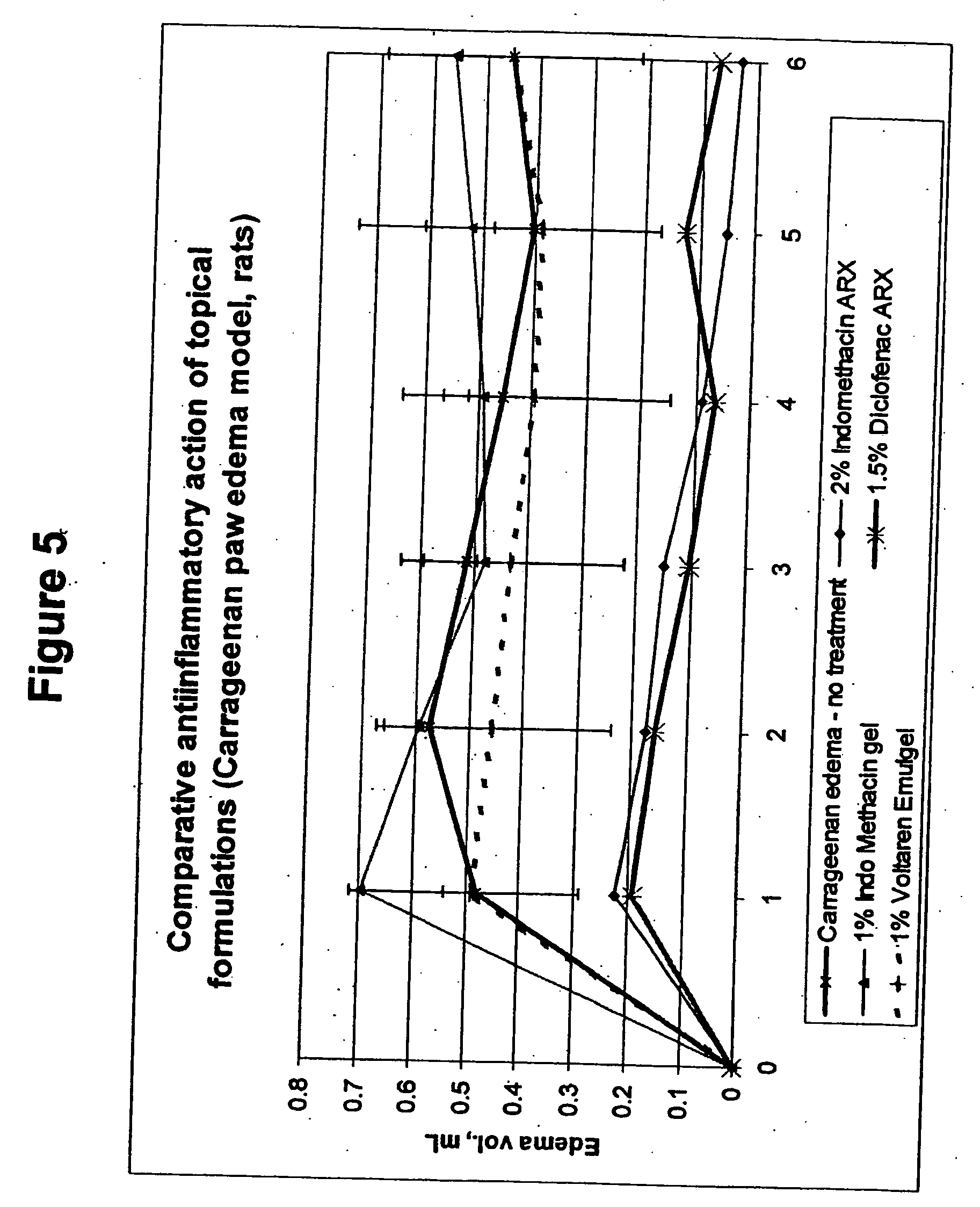
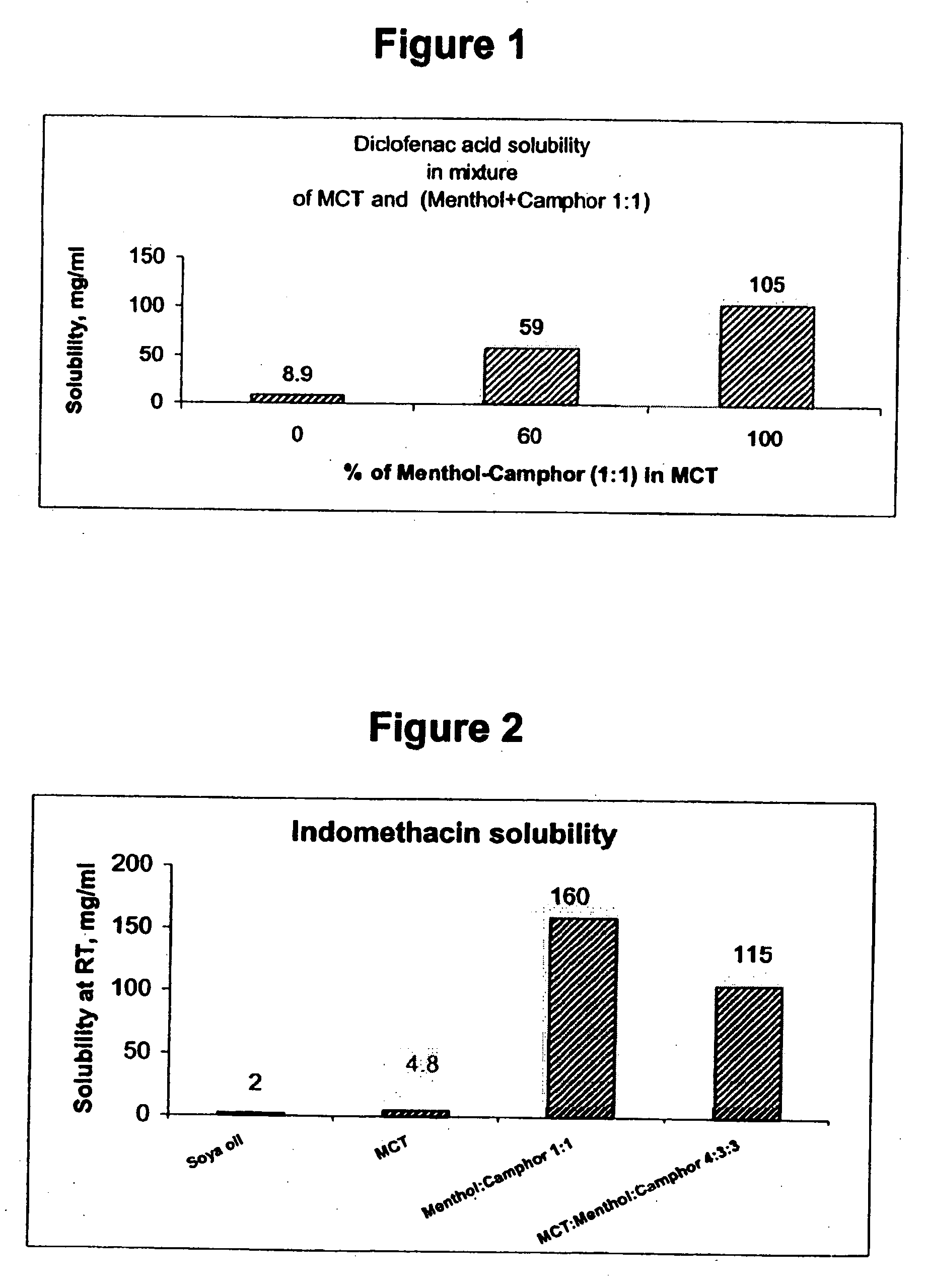
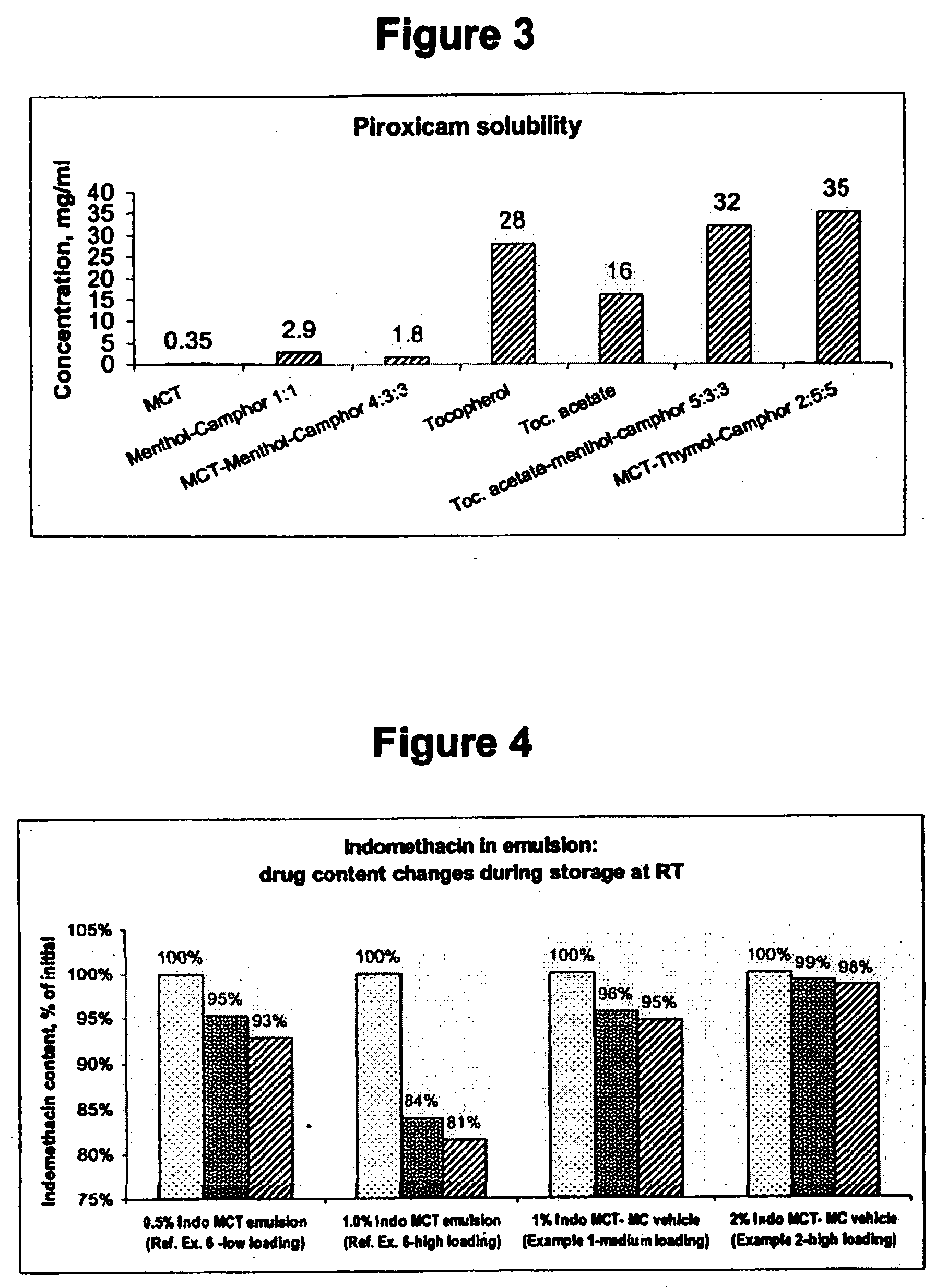
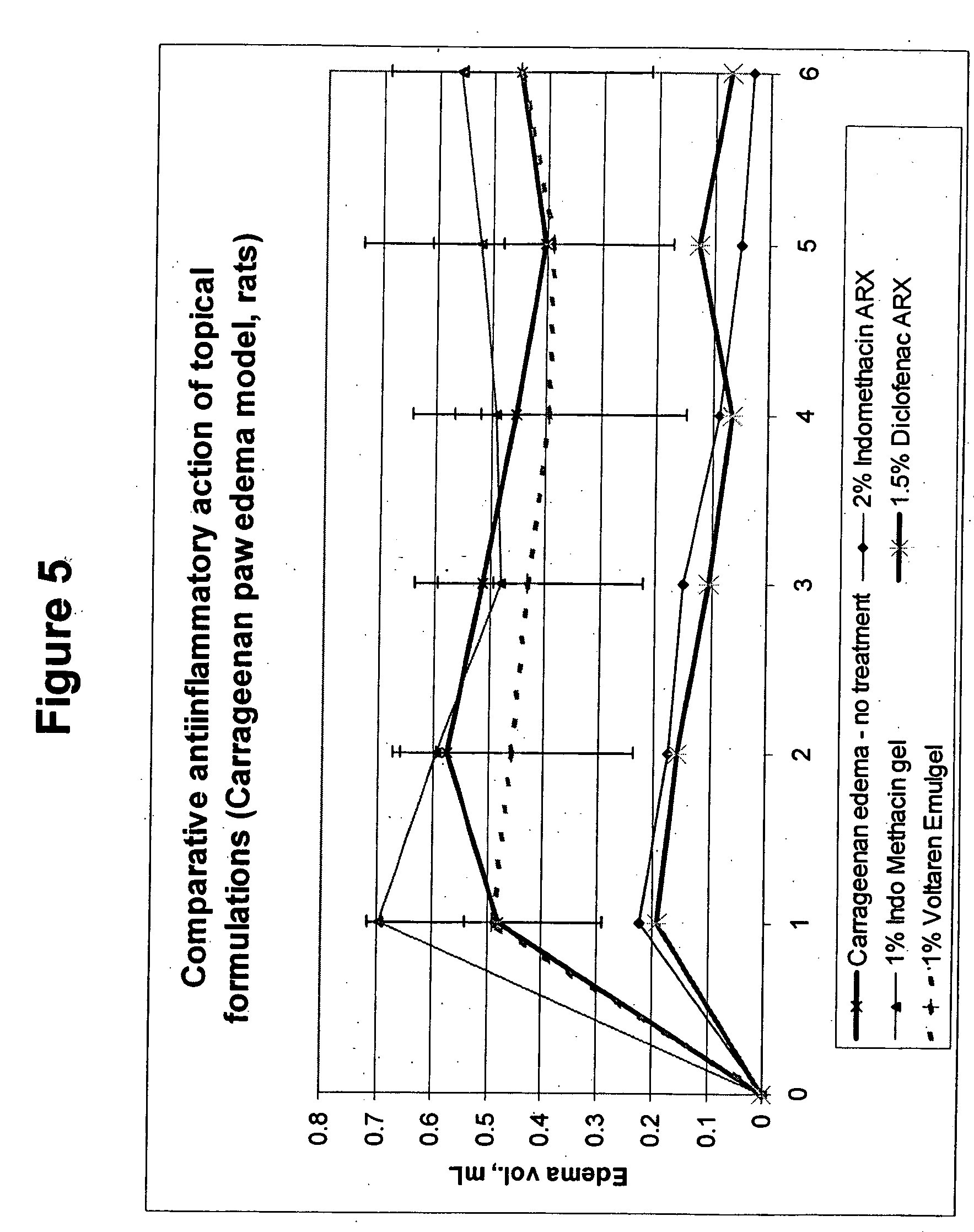
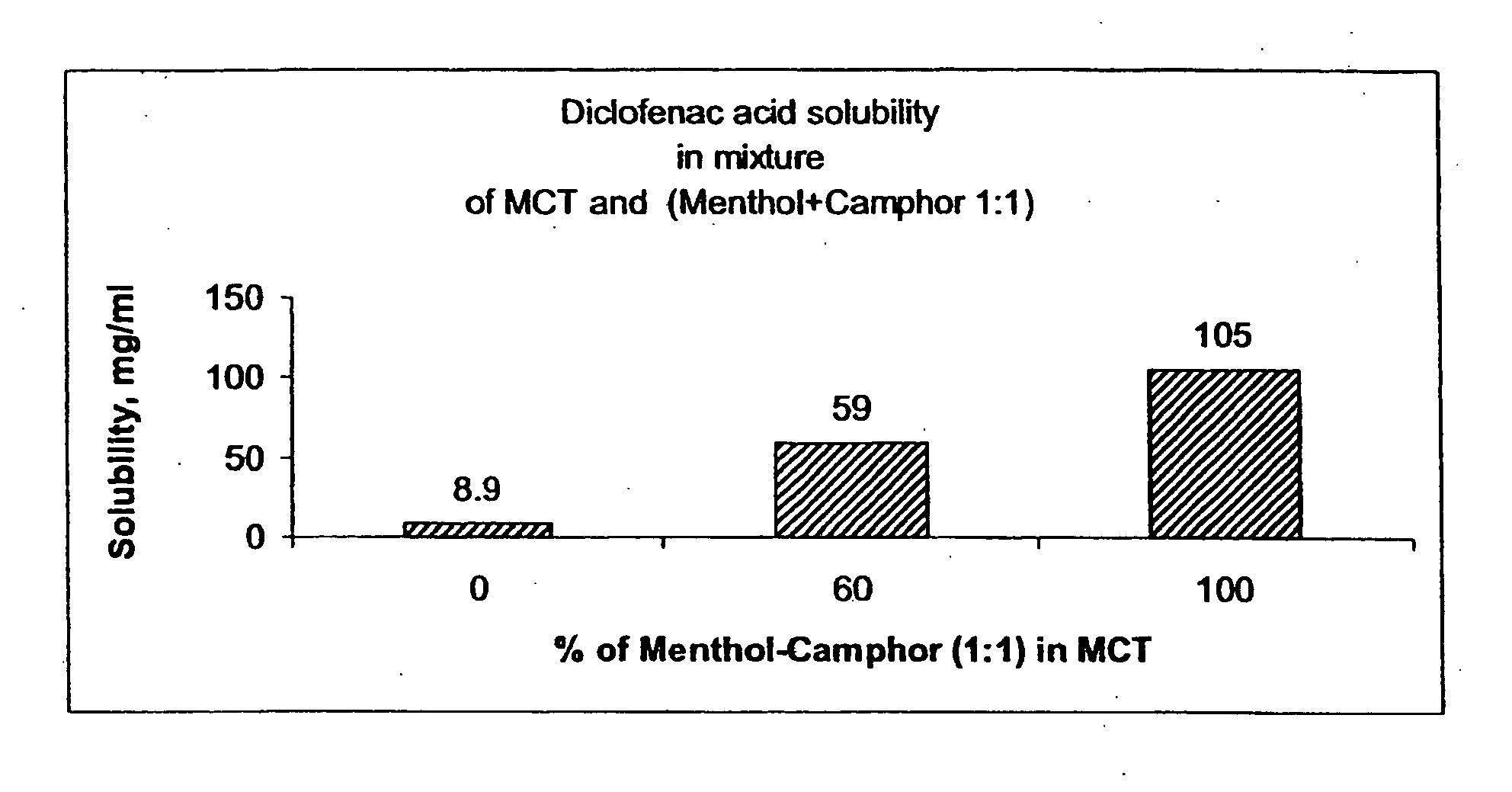
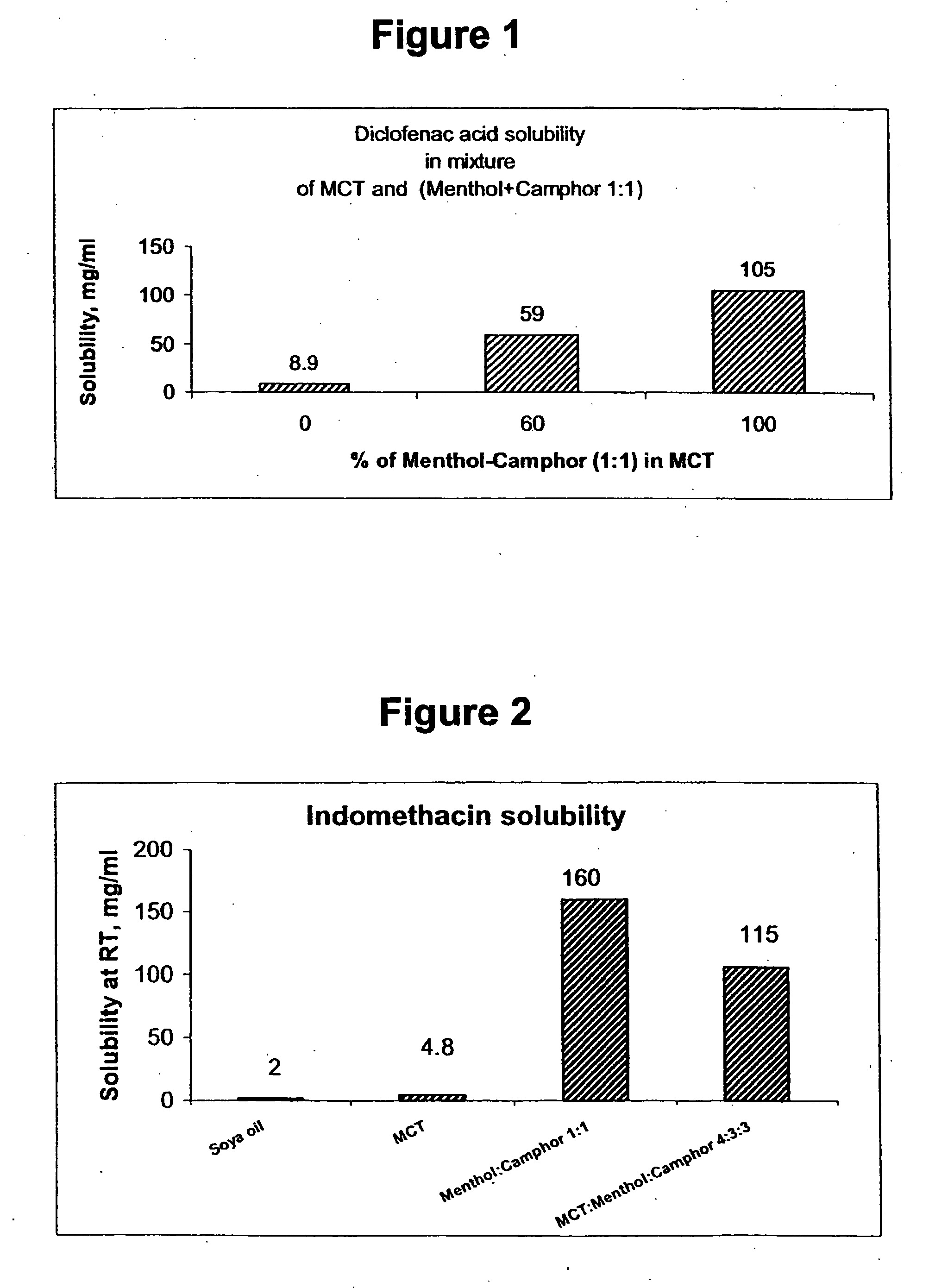
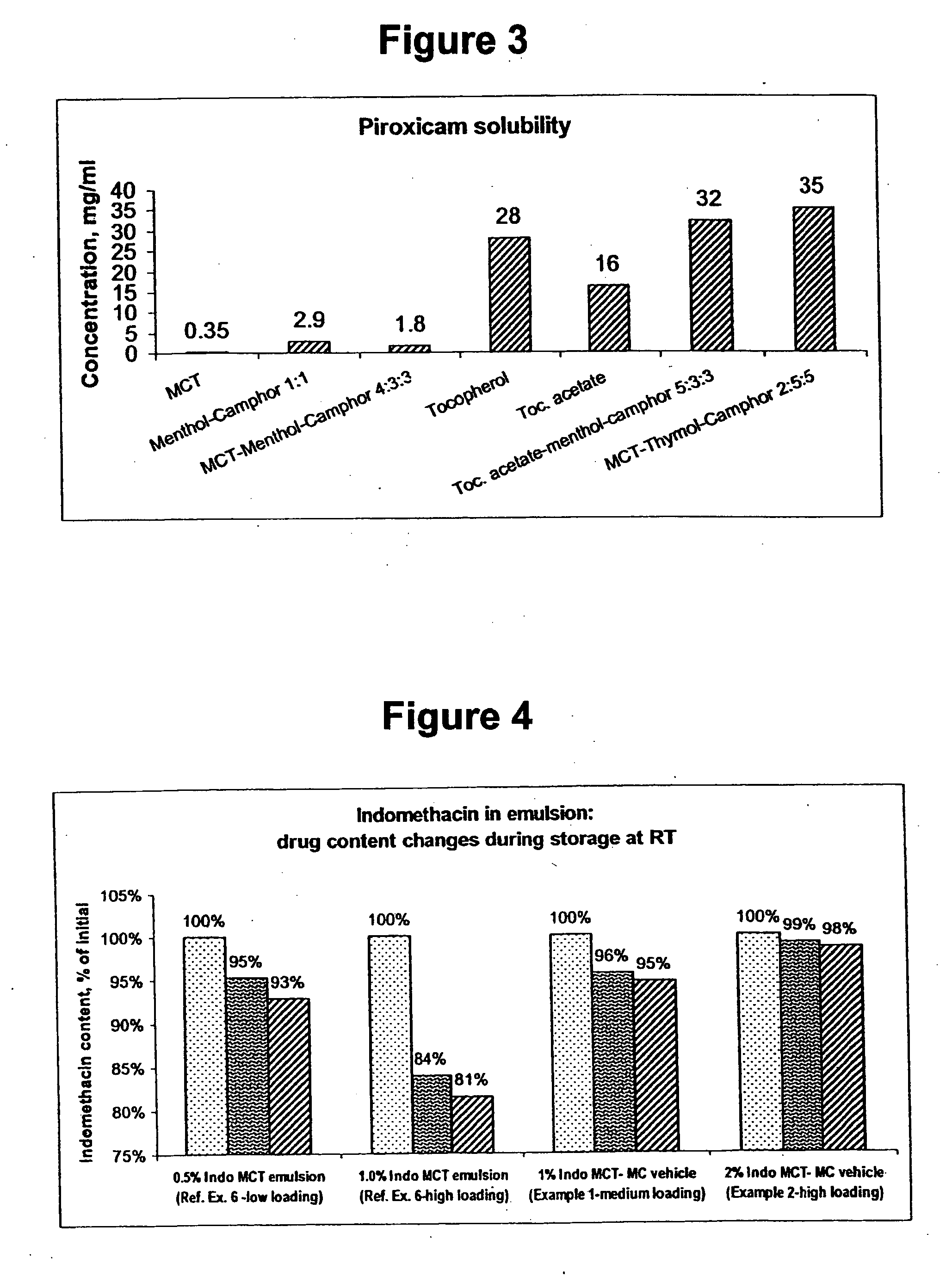
InactiveUS20060241175A1SolubilityPresent synergistic behavior in anti-inflammatory actionBiocideHydroxy compound active ingredientsCompound aMedicine
Owner:ALPHARX
Vehicle for topical delivery of anti-inflammatory compounds
InactiveUS20050158348A1SolubilityImprove delivery capabilitiesSalicyclic acid active ingredientsNervous disorderCompound aSolubility
A vehicle for topical application which contains a liquid eutectic mixture of hydrophobic compounds to improve solubility of pharmaceutically active component and enhance topical and transdermal delivery.
Owner:ALPHARX
Powerful cleaning agent
InactiveCN105273861AEasy to useSafe storageInorganic/elemental detergent compounding agentsOrganic detergent compounding agentsSide effectAlcohol
The invention relates to a powerful cleaning agent and belongs to the field of fine chemical automotive chemicals. The cleaning agent is prepared from surface active agents, alcohol additives, oiliness additives, auxiliary agents and water. The surface active agents and the oiliness additives play roles of solubilization, dissolving and washing, the alcohol additives jointly act with the two mentioned components for forming stable microemulsion and have certain permeation, moistening and dissolving functions, the three components jointly act so that the cleaning agent can achieve a good cleaning effect, and the auxiliary agents are functional additives. The cleaning agent has no side effect on cast iron, cast aluminum, leather, fabric, rubber, plastic and the like and can effectively clean an engine or the surface of the interior without generating phenomena of corrosion, color fading, bubbling, falling and the like.
Owner:蚌埠市神丰贸易有限公司
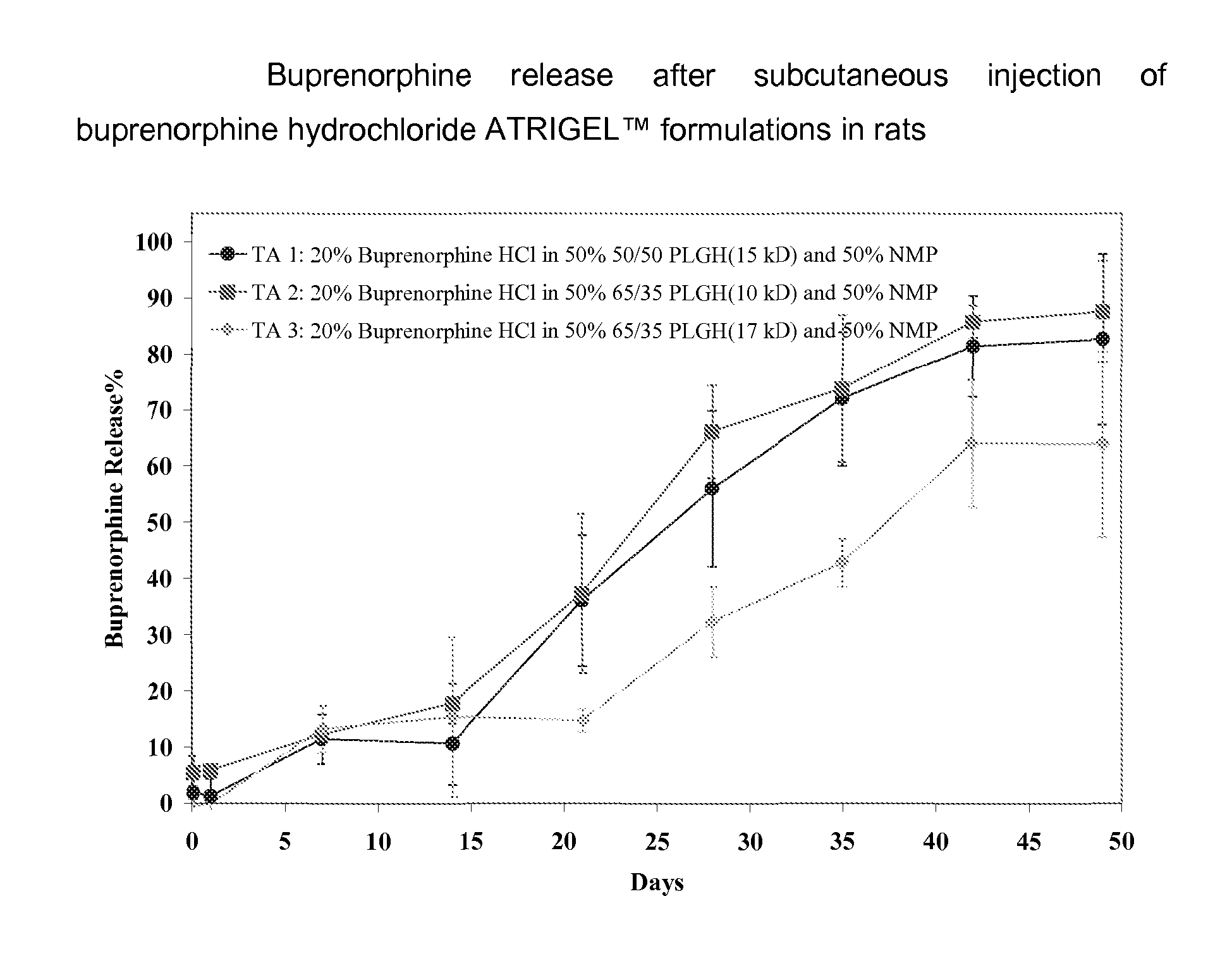
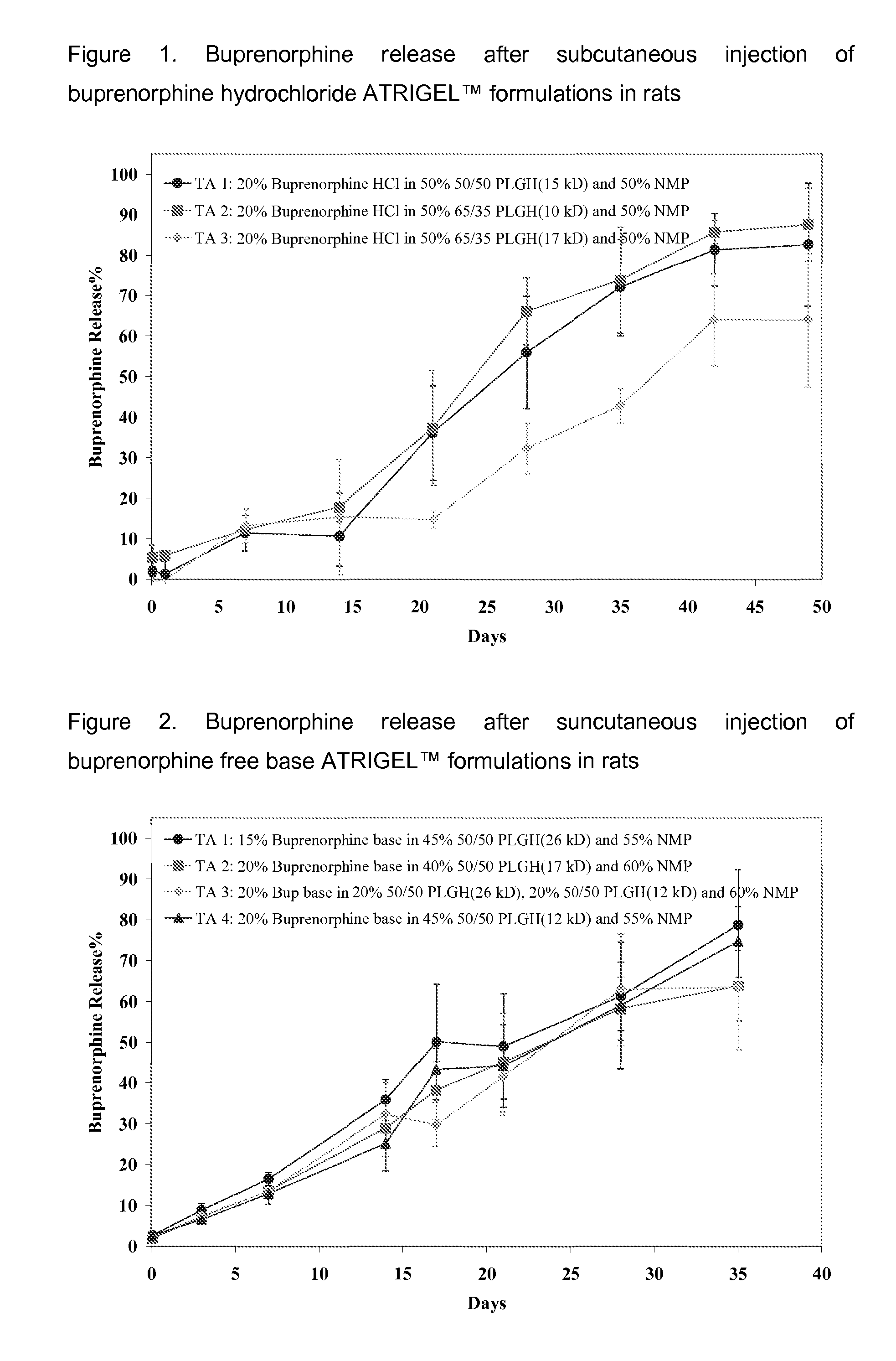
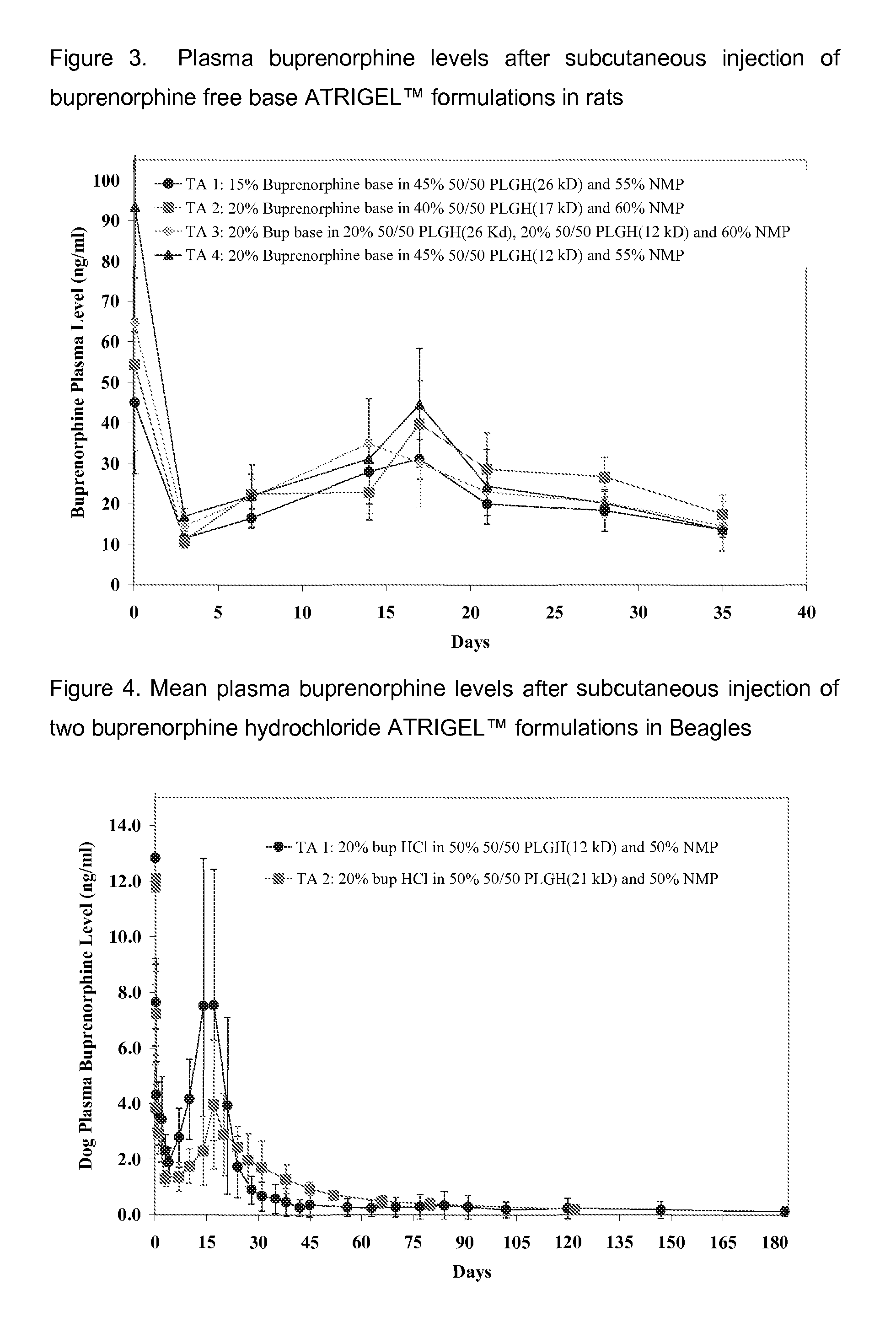
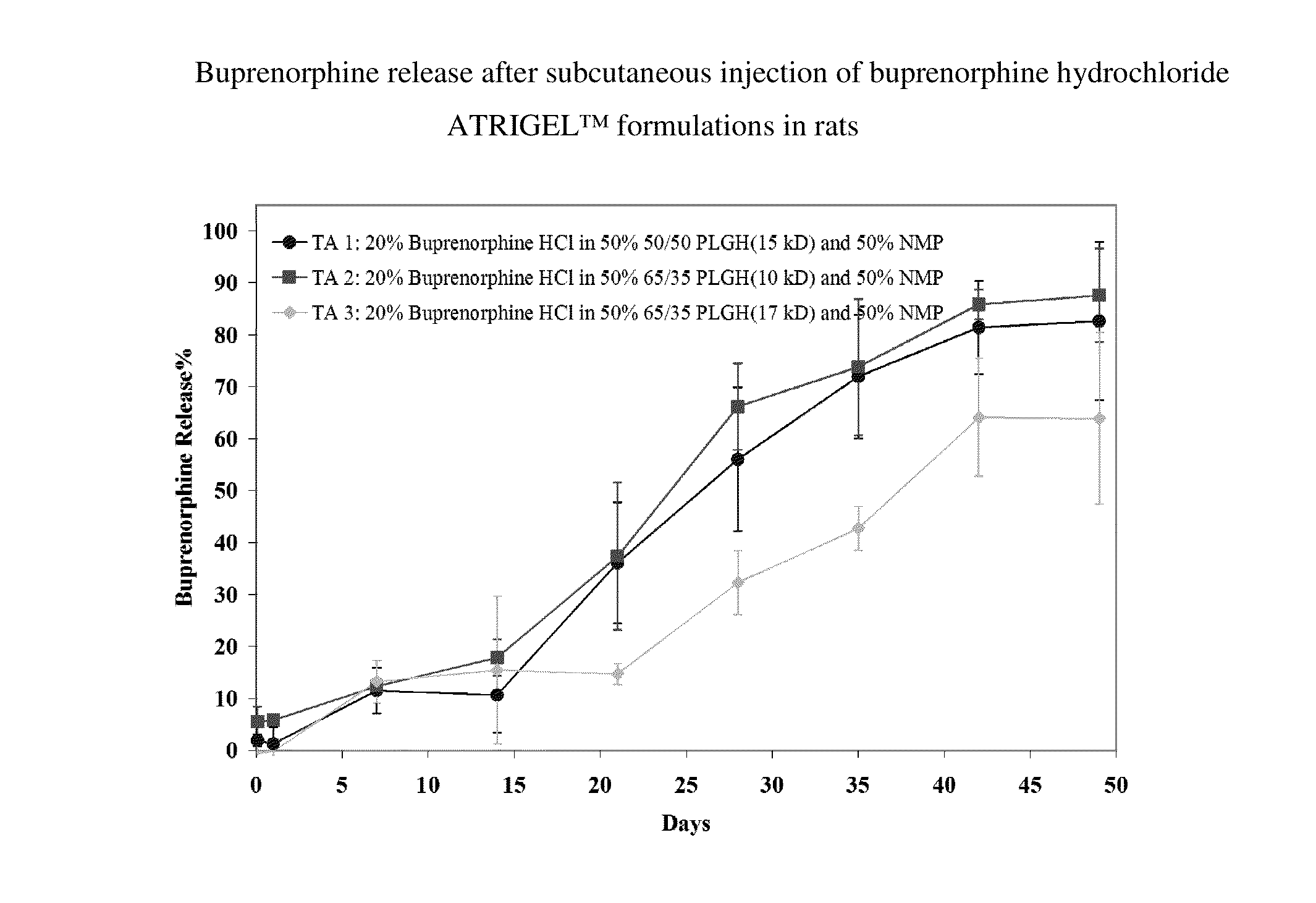
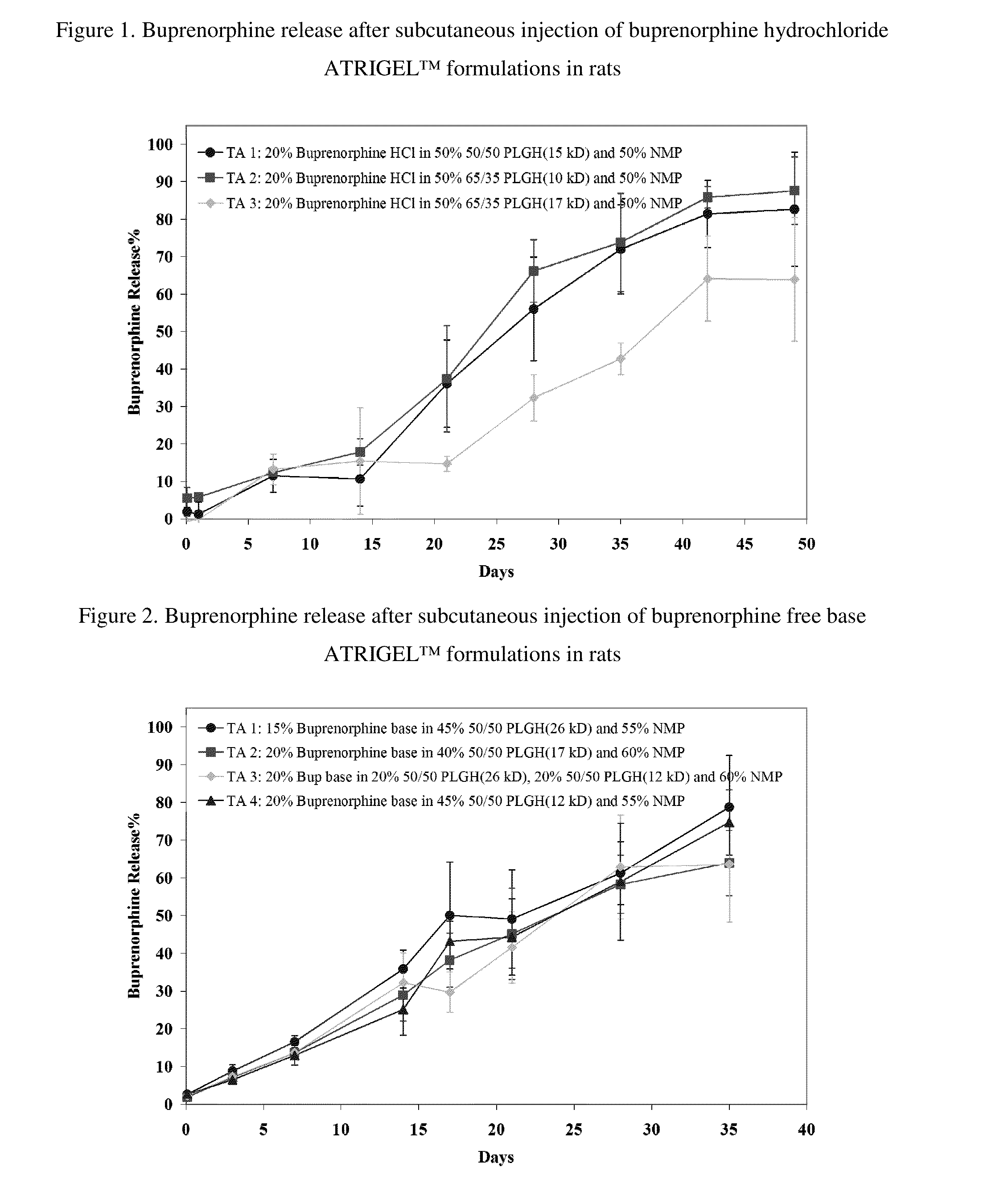
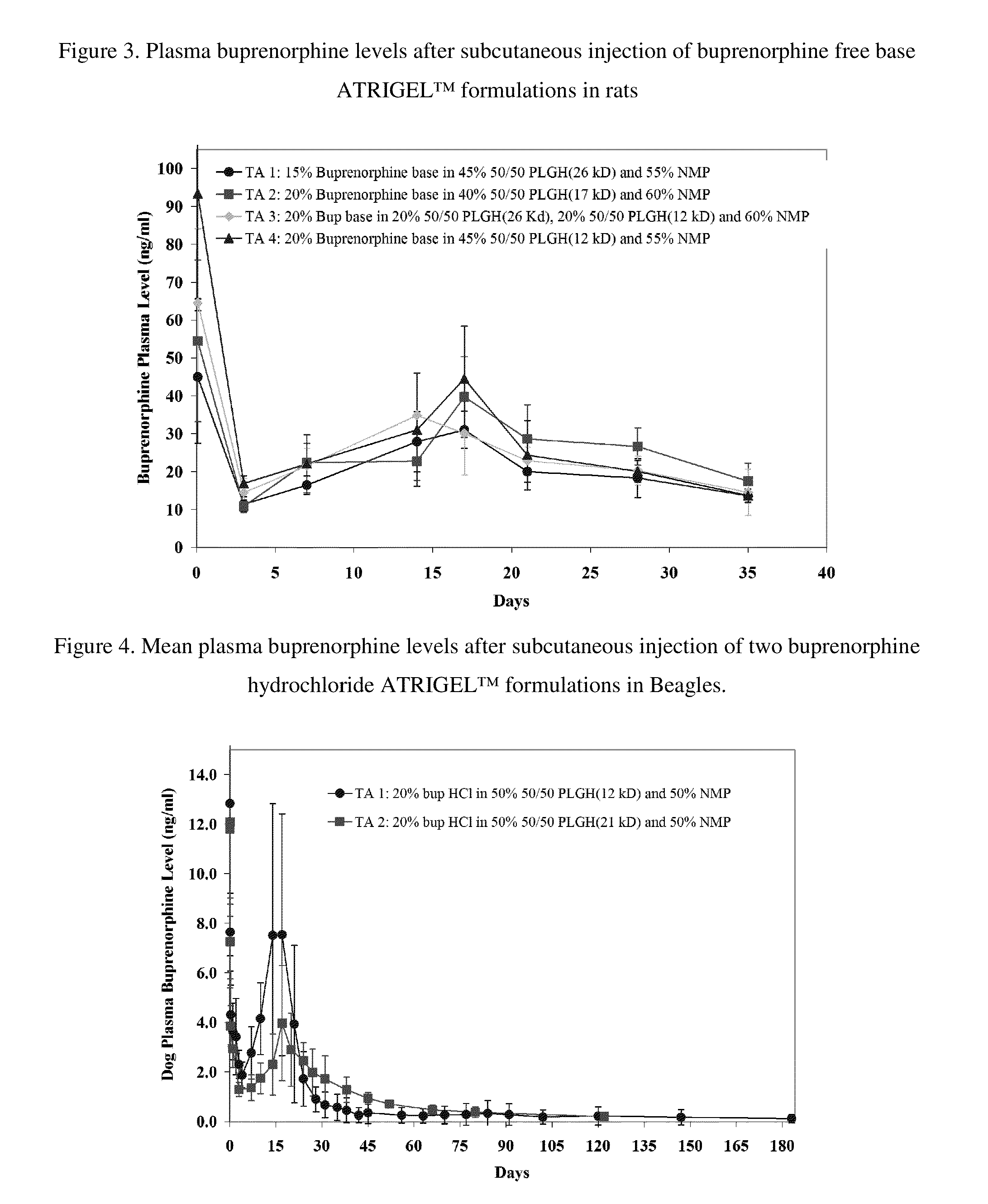
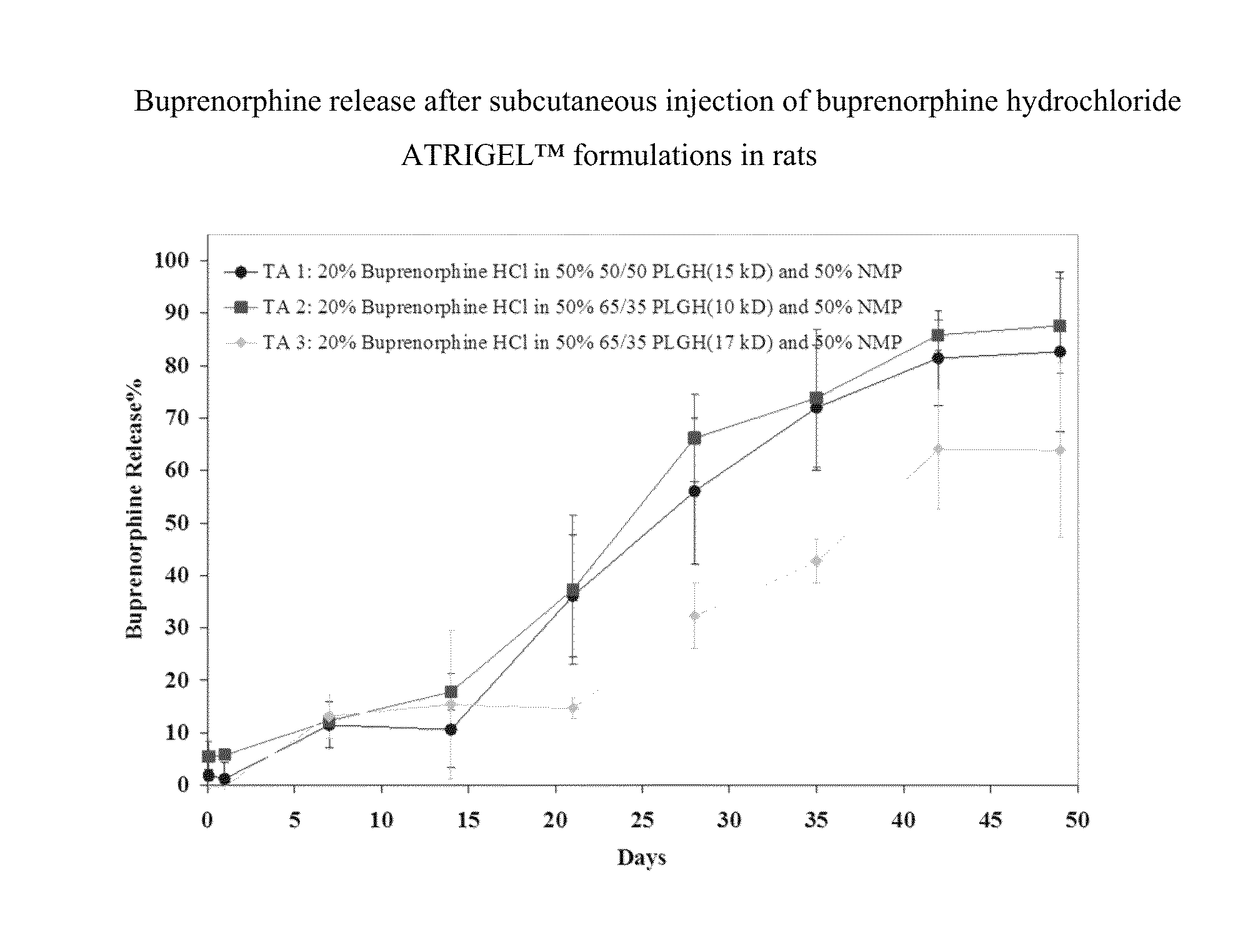
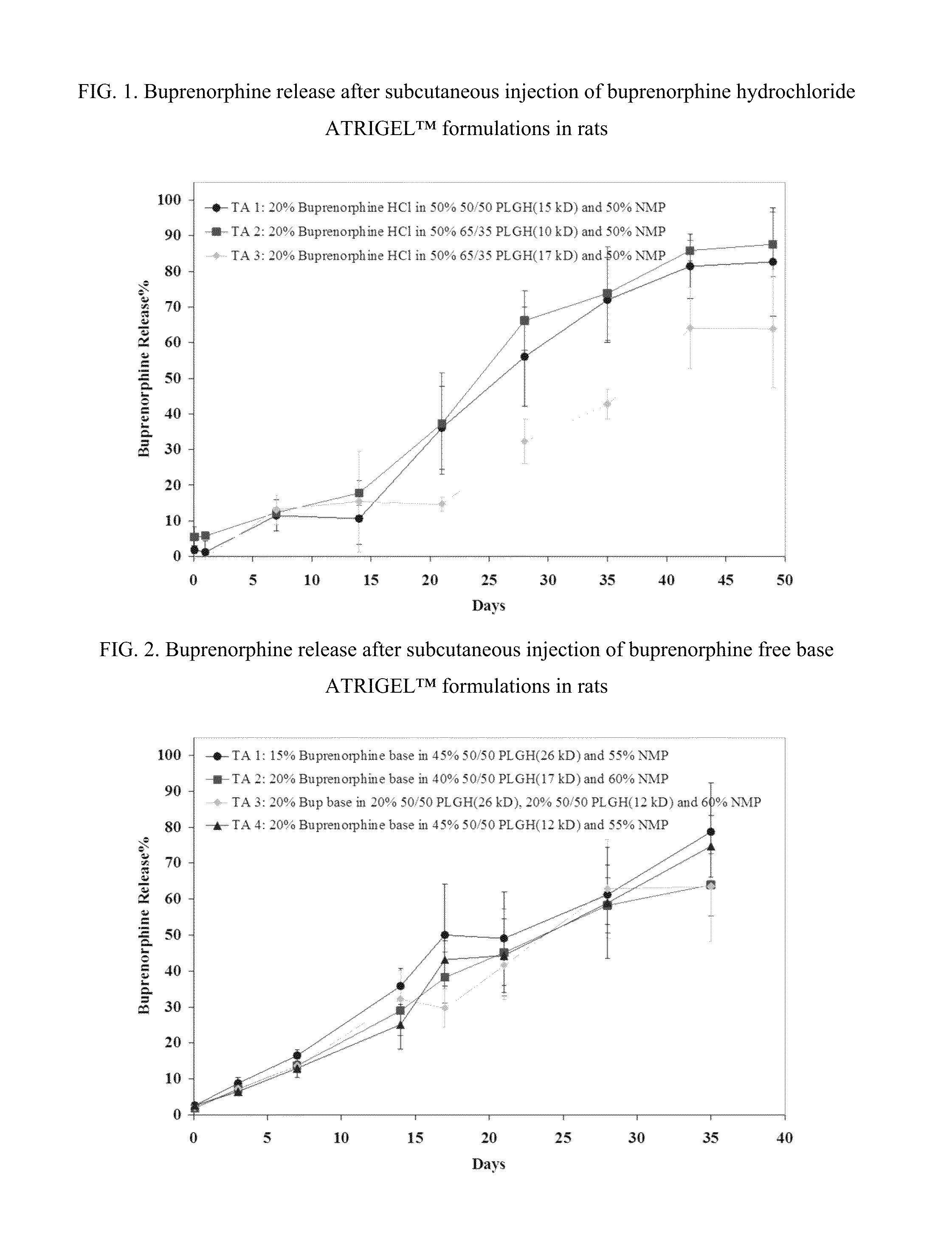
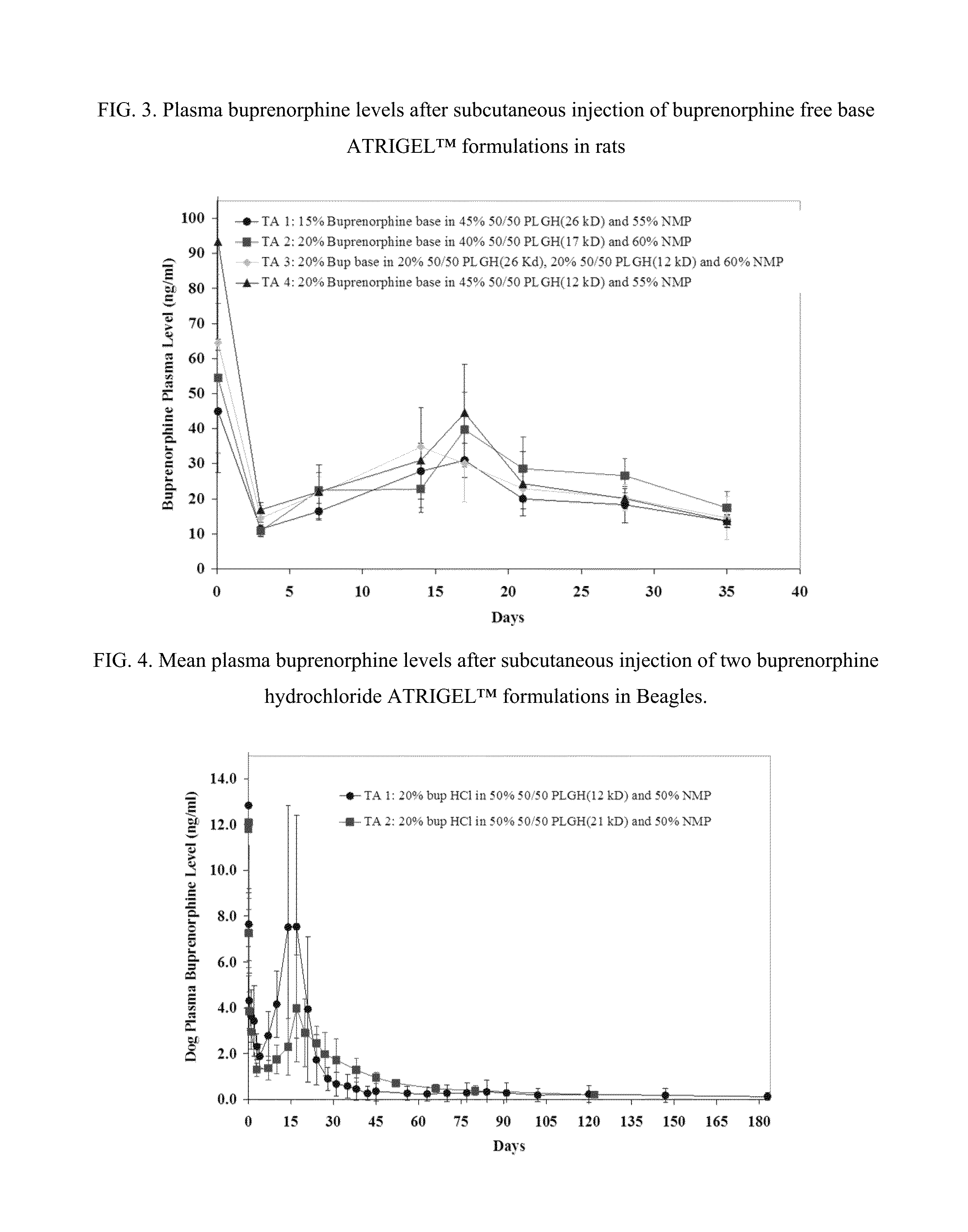
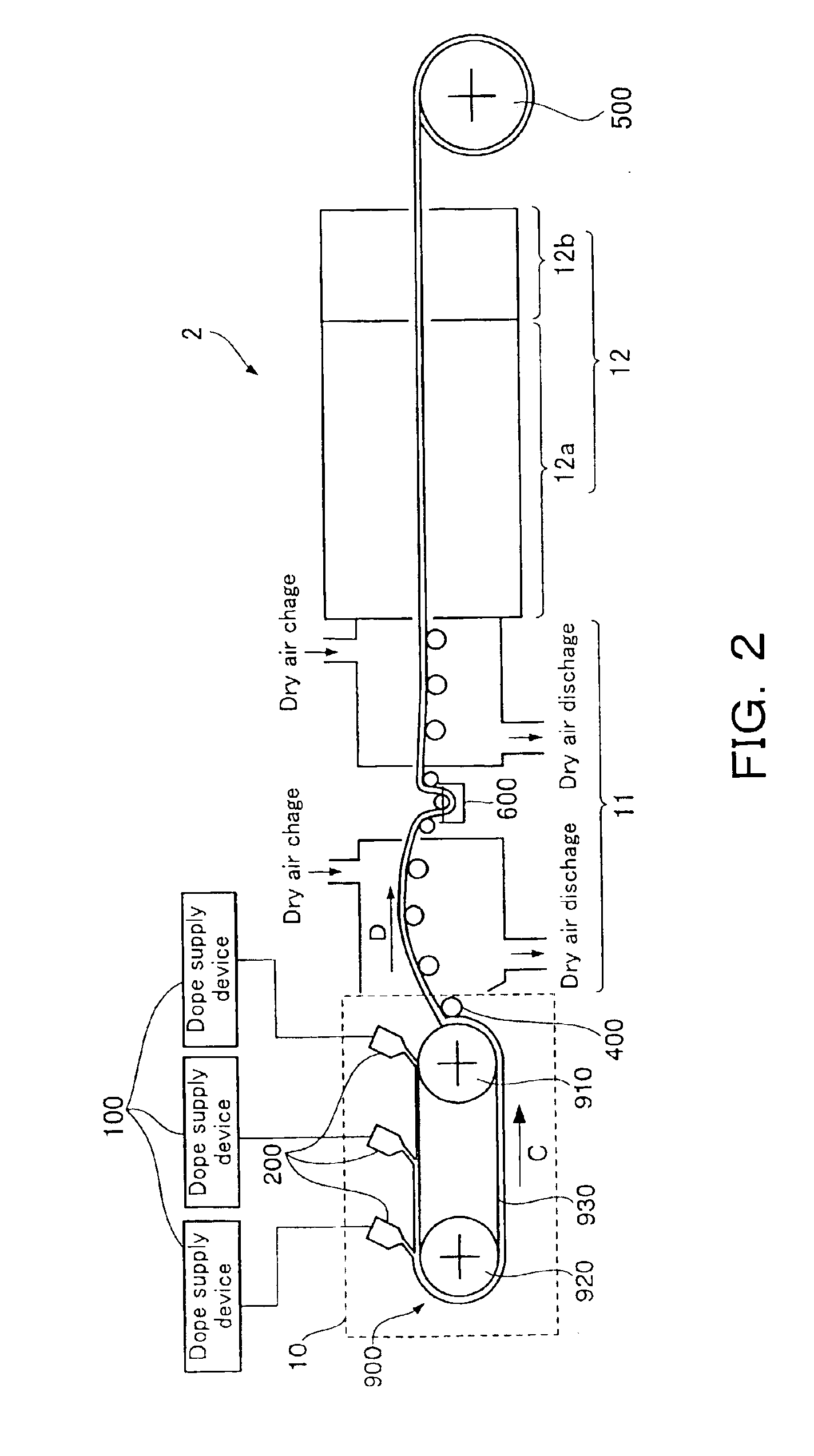
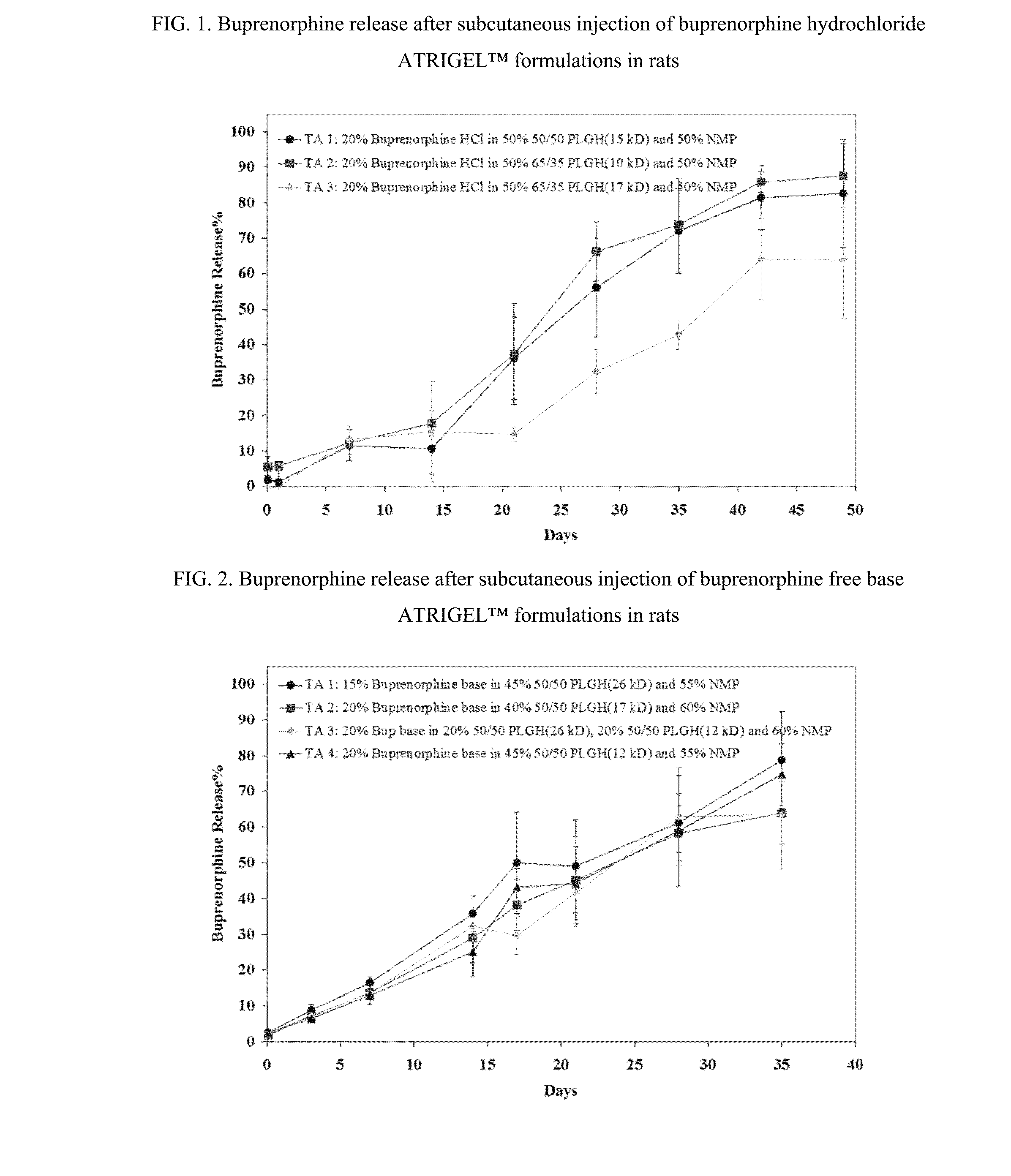
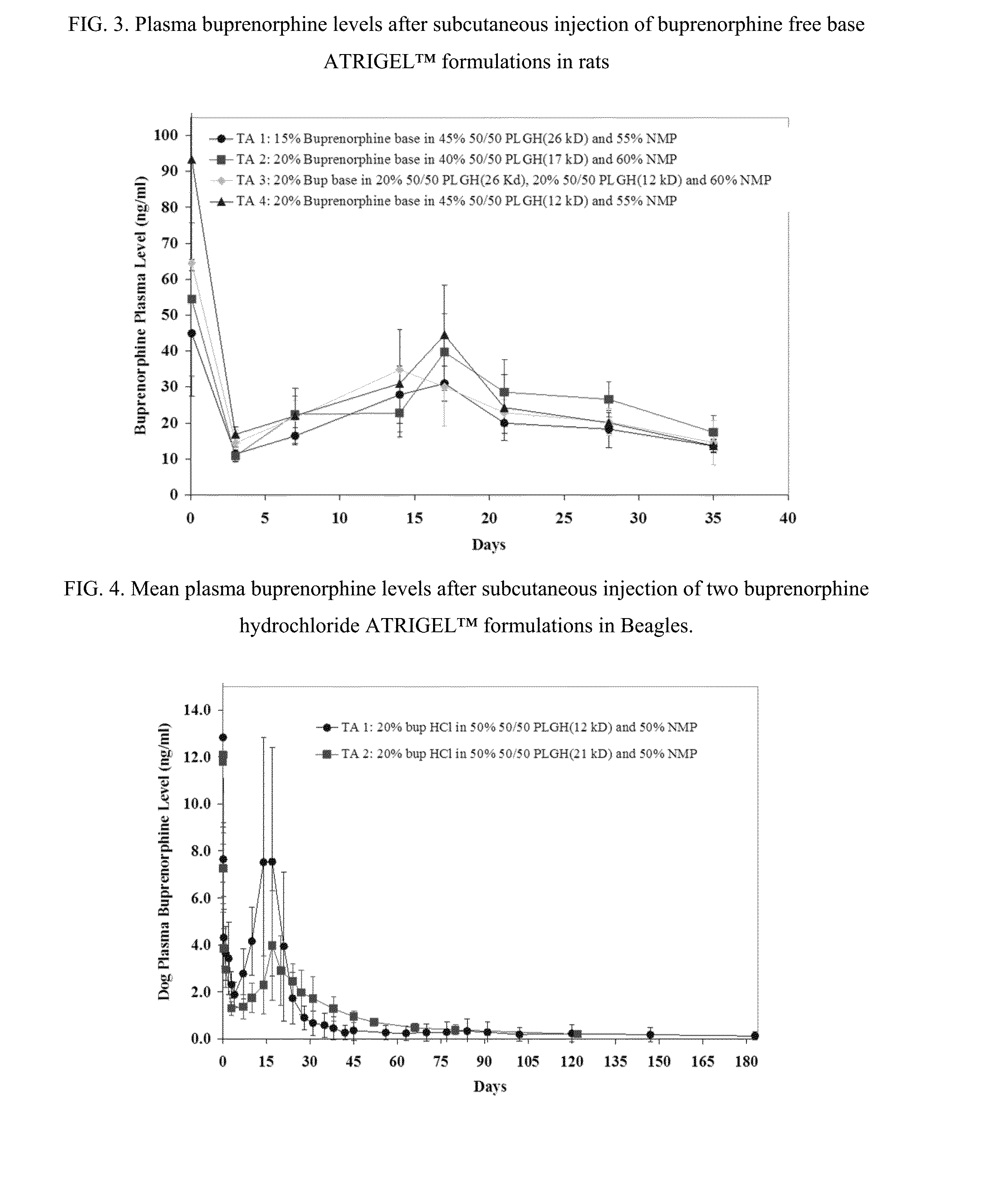
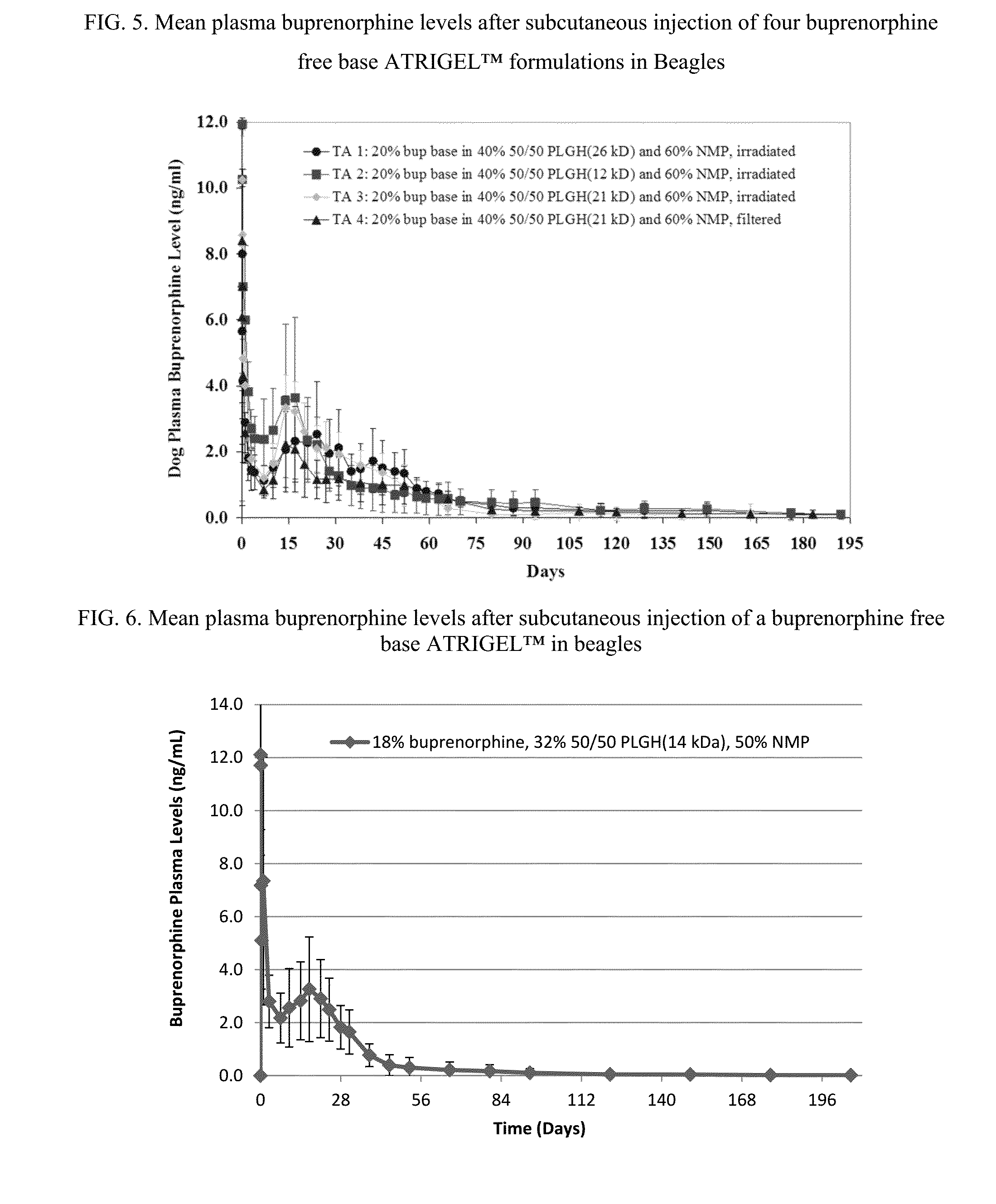
Injectable flowable composition comprising buprenorphine
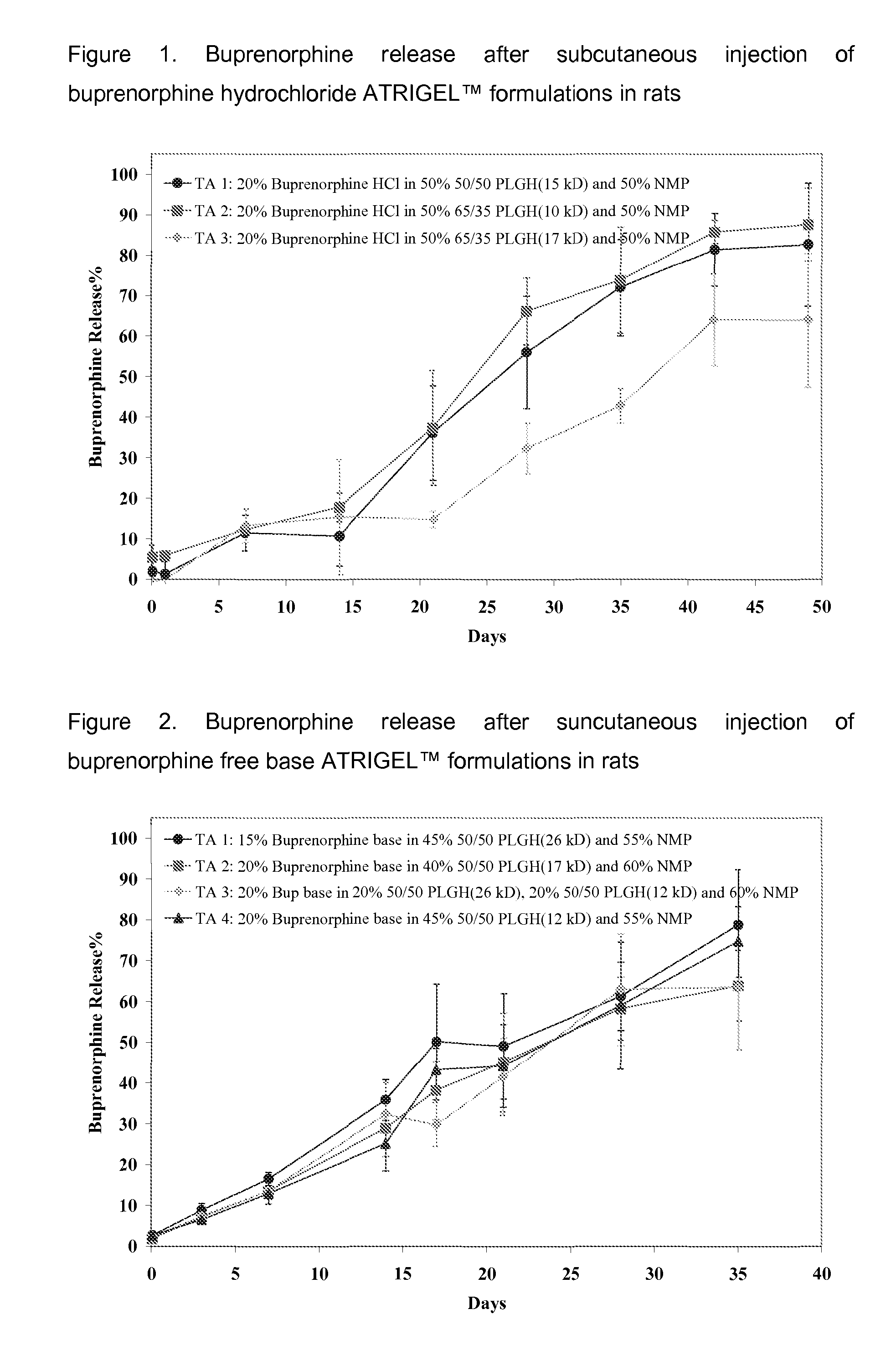
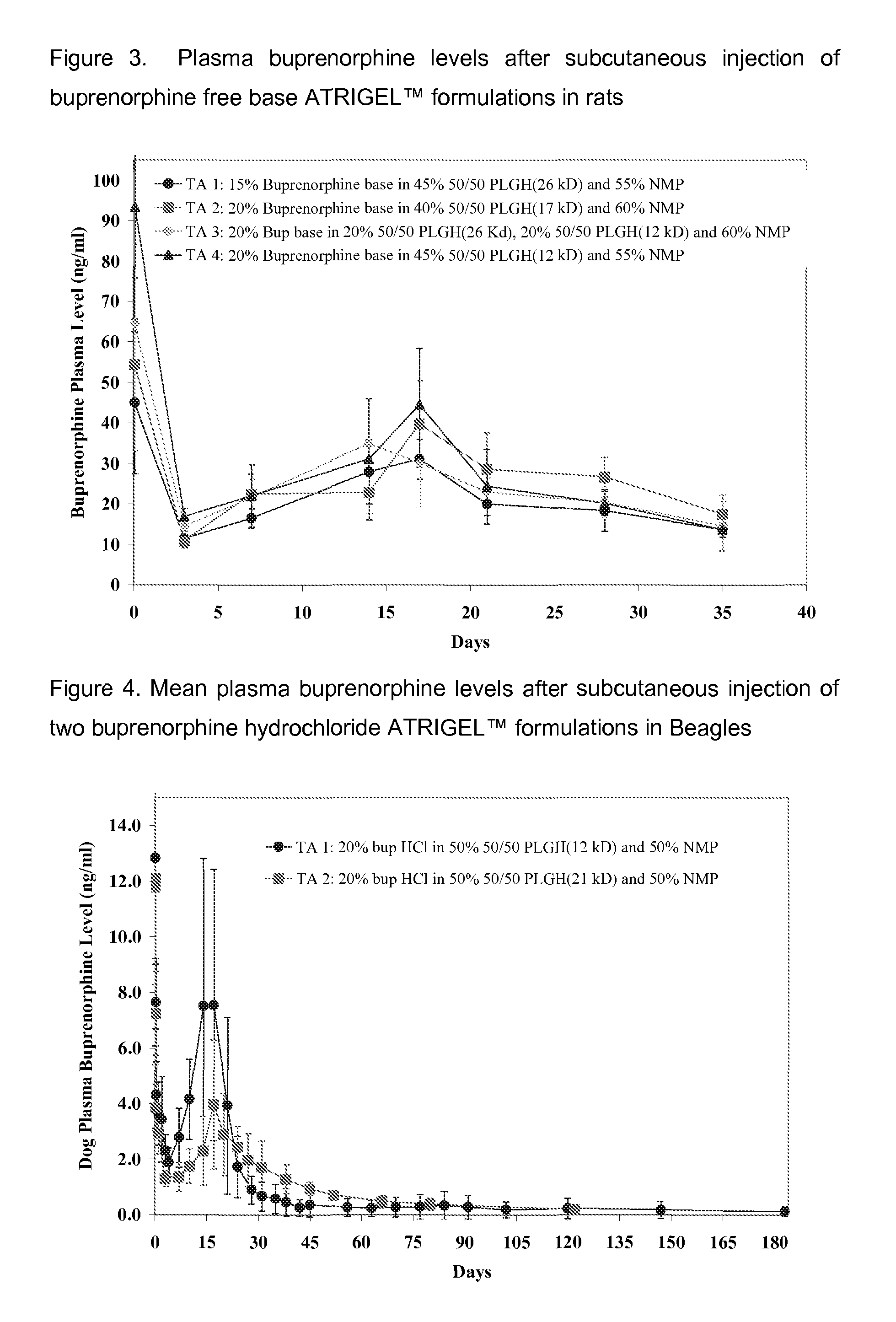
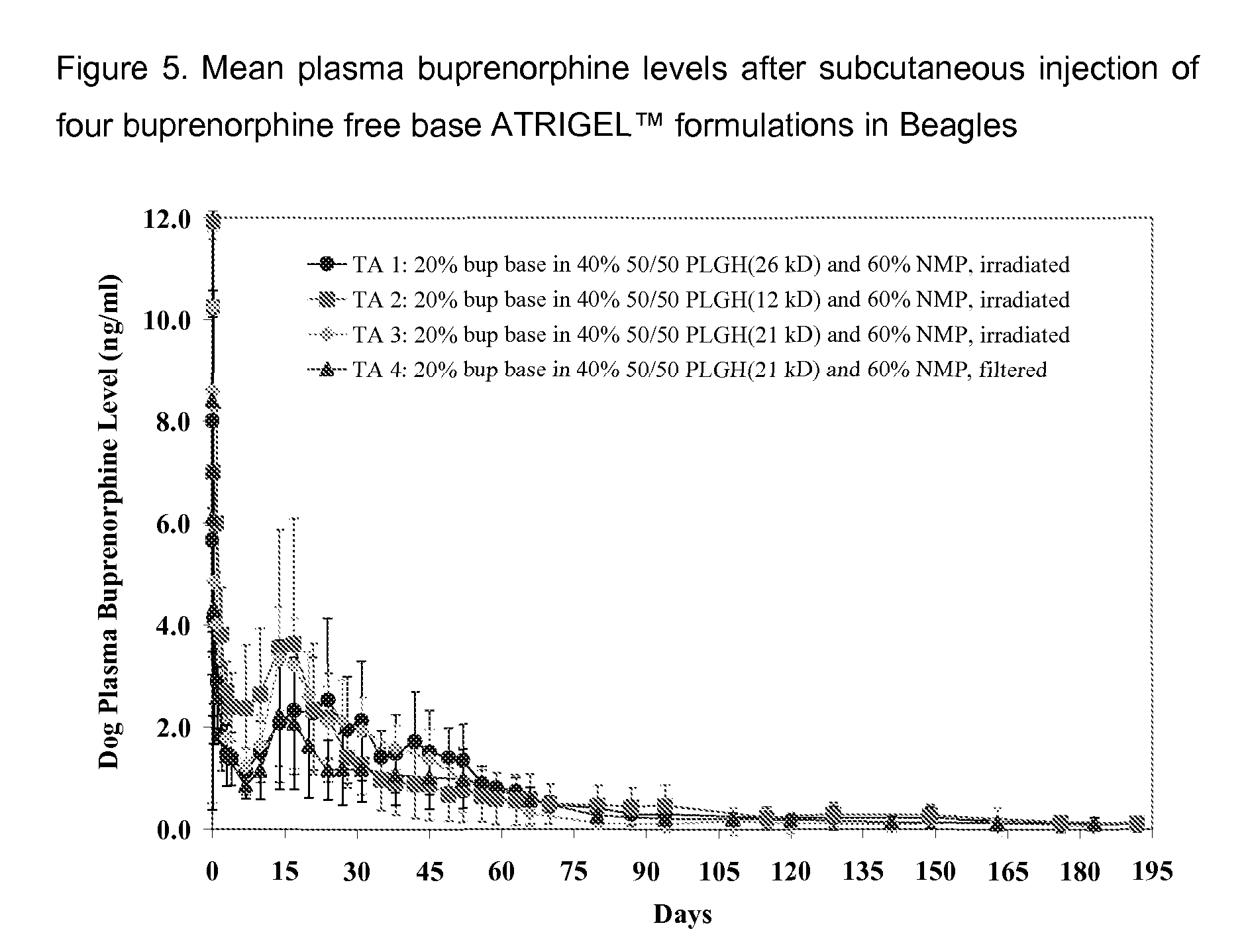
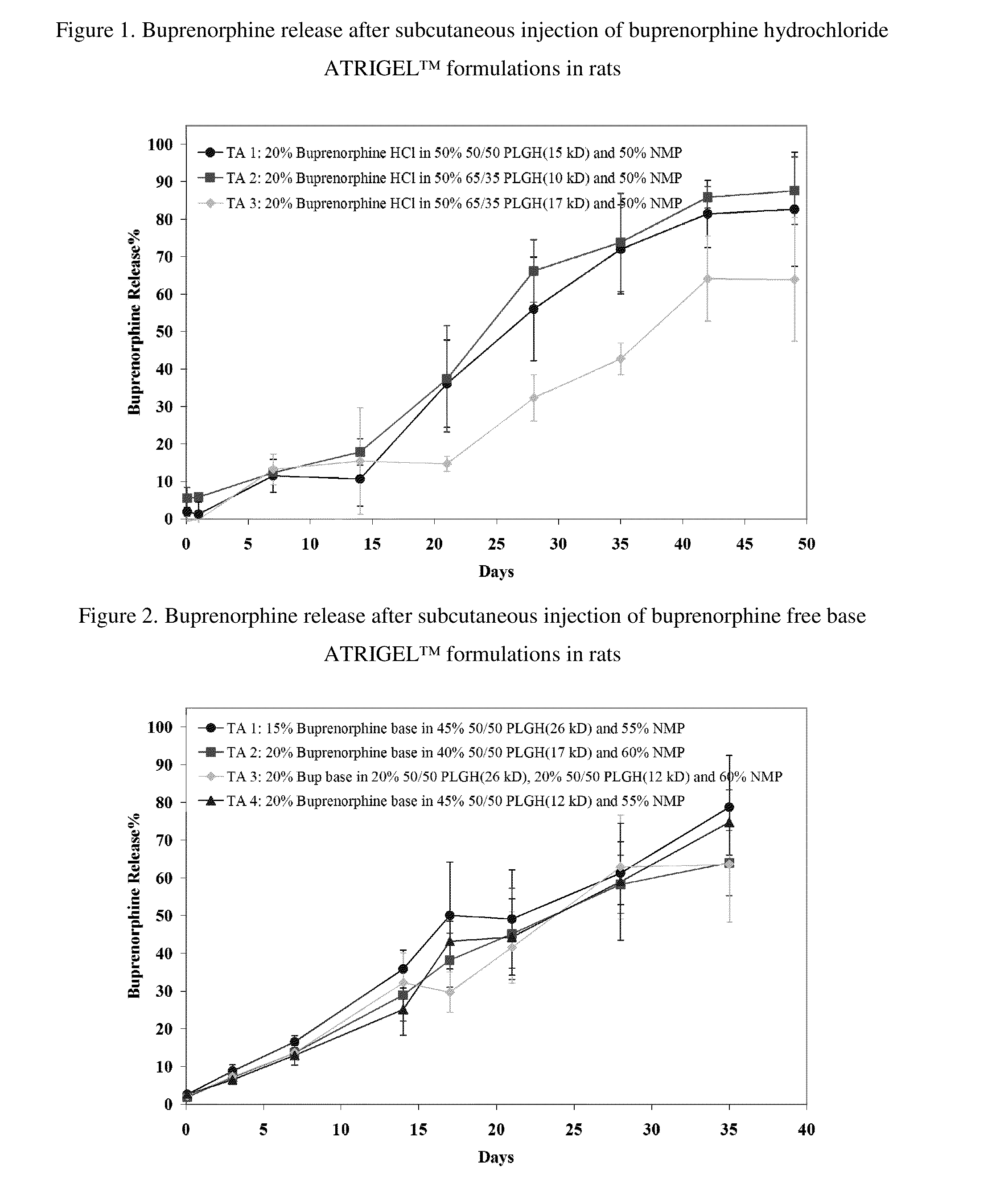
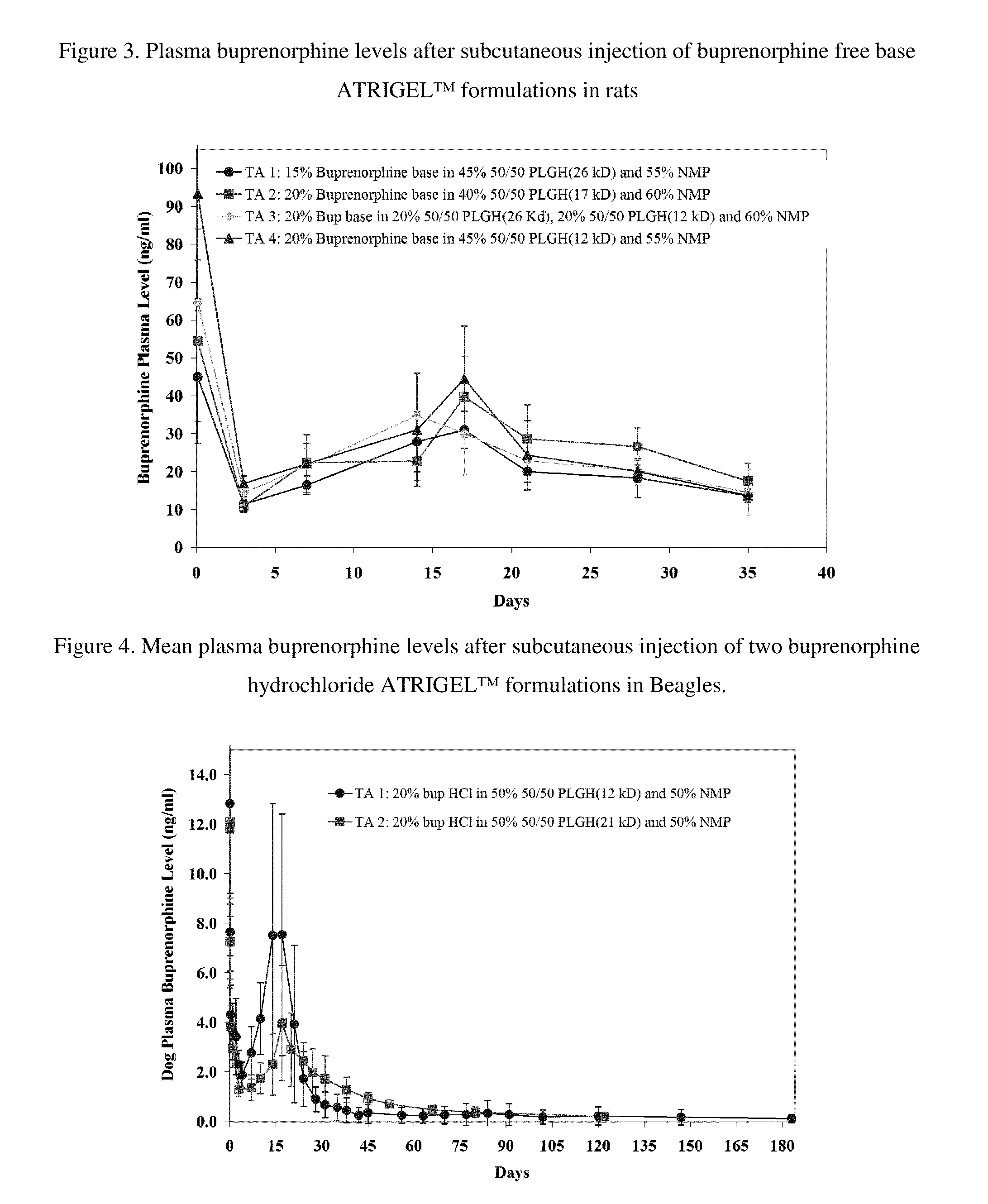
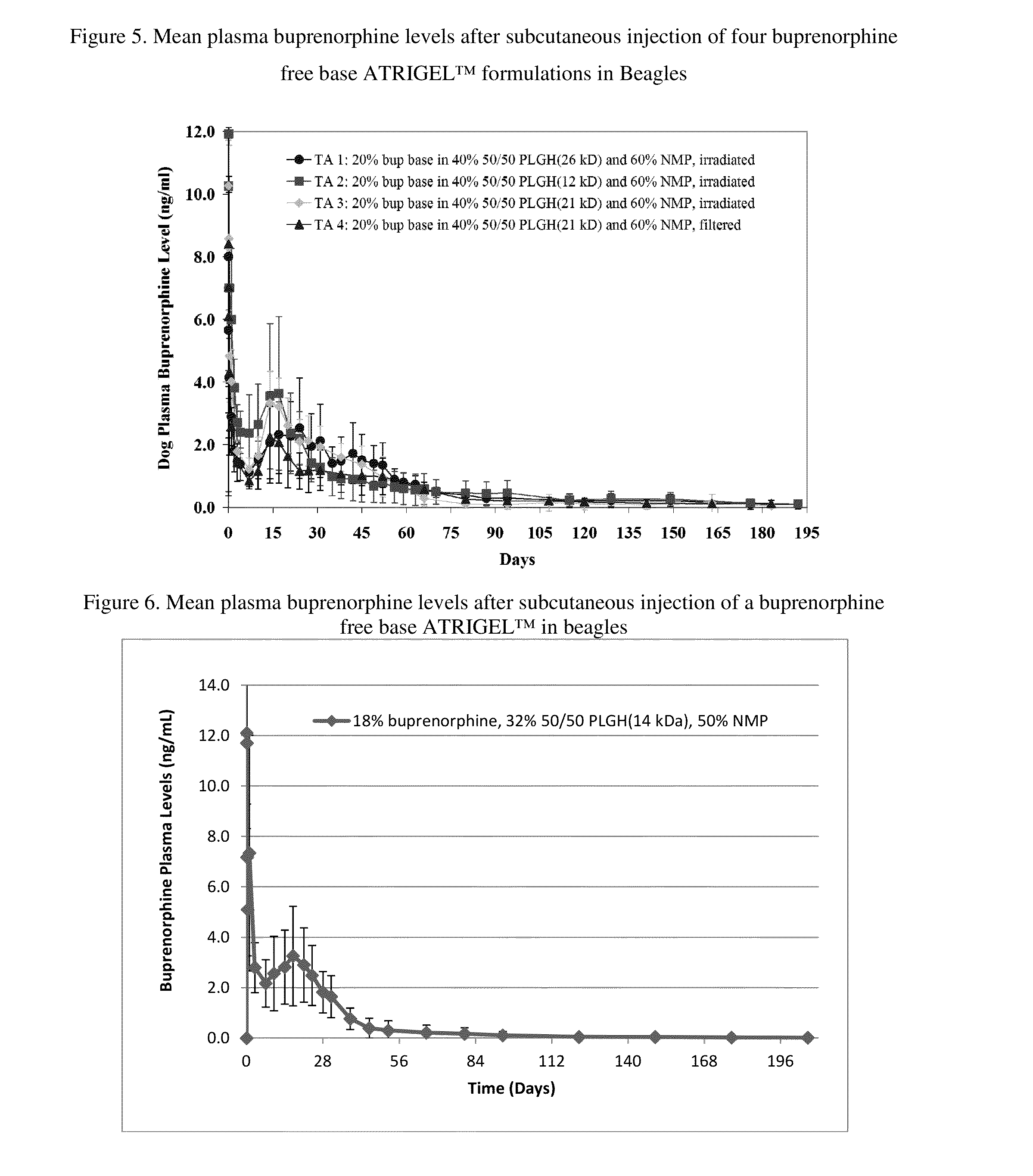
The present invention is directed to a buprenorphine sustained release delivery system capable of delivering buprenorphine, a metabolite, or a prodrug thereof for a duration of about 14 days to about 3 months. The buprenorphine sustained release delivery system includes a flowable composition and a solid implant for the sustained release of buprenorphine, a metabolite, or a prodrug thereof. The implant is produced from the flowable composition. The buprenorphine sustained release delivery system provides in situ 1-month and 3-month release profiles characterized by an exceptionally high bioavailability and minimal risk of permanent tissue damage and typically no risk of muscle necrosis.
Owner:INDIVIOR UK
Skin caring composition having function of removing acne marks, preparation and preparation method of skin caring composition
ActiveCN103622885AEffectively removeThe formula is scientific and rigorousCosmetic preparationsToilet preparationsCentella asiatica extractSide effect
The invention discloses a skin caring composition having a function of removing acne marks, a preparation and a preparation method of the skin caring composition. The skin caring composition consists of the following functional components in parts by weight: 0.01-2 parts of zinc carbonate hydroxide, 0.01-0.1 part of borax, 0.001-0.01 part of amber powder, 0.001-0.05 part of pearl powder, 0.05-0.5 part of allantoin, 1-5 parts of butyrospermum parkii, 0.1-0.5 part of angelica extract, 0.1-0.5 part of bighead atractylodes rhizome extract, 0.01-0.1 part of centella extract and 0.025-0.1 part of borneol. The skin caring composition is scientific and strict in formula, definite in effect and free from toxic and side effect, and is capable of effectively removing acne marks and other scar tissues; the skin caring composition has a function of removing acne marks, and is capable of obviously reducing skin roughness and content of skin melanin and the like; and the skin caring composition can guarantee a good skin status.
Owner:WUHAN MAYINGLONG MASSIVE HEALTH CO LTD
Methods for immobilizing molecules to a solid phase and uses thereof
InactiveUS20030096273A1Low costProduct qualityBioreactor/fermenter combinationsBiological substance pretreatmentsBiological activationSolid phases
Various methodologies for the immobilization of molecules such, as multistranded nucleic acid molecules, are described. The methodologies include activation of solid supports, as well as treatment of, e.g. termini of nucleic acid molecules to render them more receptive to immobilization on surfaces.
Owner:NEW YORK INSTITUTE OF TECHNOLOGY
Injectable flowable composition comprising buprenorphine
ActiveUS8975270B2Least riskImprove bioavailabilityBiocidePharmaceutical delivery mechanismMetaboliteMUSCLE NECROSIS
The present invention is directed to a buprenorphine sustained release delivery system capable of delivering buprenorphine, a metabolite, or a prodrug thereof for a duration of about 14 days to about 3 months. The buprenorphine sustained release delivery system includes a flowable composition and a solid implant for the sustained release of buprenorphine, a metabolite, or a prodrug thereof. The implant is produced from the flowable composition. The buprenorphine sustained release delivery system provides in situ 1-month and 3-month release profiles characterized by an exceptionally high bioavailability and minimal risk of permanent tissue damage and typically no risk of muscle necrosis.
Owner:INDIVIOR UK
Injectable flowable composition buprenorphine
ActiveUS9272044B2Least riskImprove bioavailabilityOrganic active ingredientsPharmaceutical delivery mechanismMetaboliteMUSCLE NECROSIS
The present invention is directed to a buprenorphine sustained release delivery system capable of delivering buprenorphine, a metabolite, or a prodrug thereof for a duration of about 14 days to about 3 months. The buprenorphine sustained release delivery system includes a flowable composition and a solid implant for the sustained release of buprenorphine, a metabolite, or a prodrug thereof. The implant is produced from the flowable composition. The buprenorphine sustained release delivery system provides in situ 1-month and 3-month release profiles characterized by an exceptionally high bioavailability and minimal risk of permanent tissue damage and typically no risk of muscle necrosis.
Owner:INDIVIOR UK
Method for producing nitride crystal
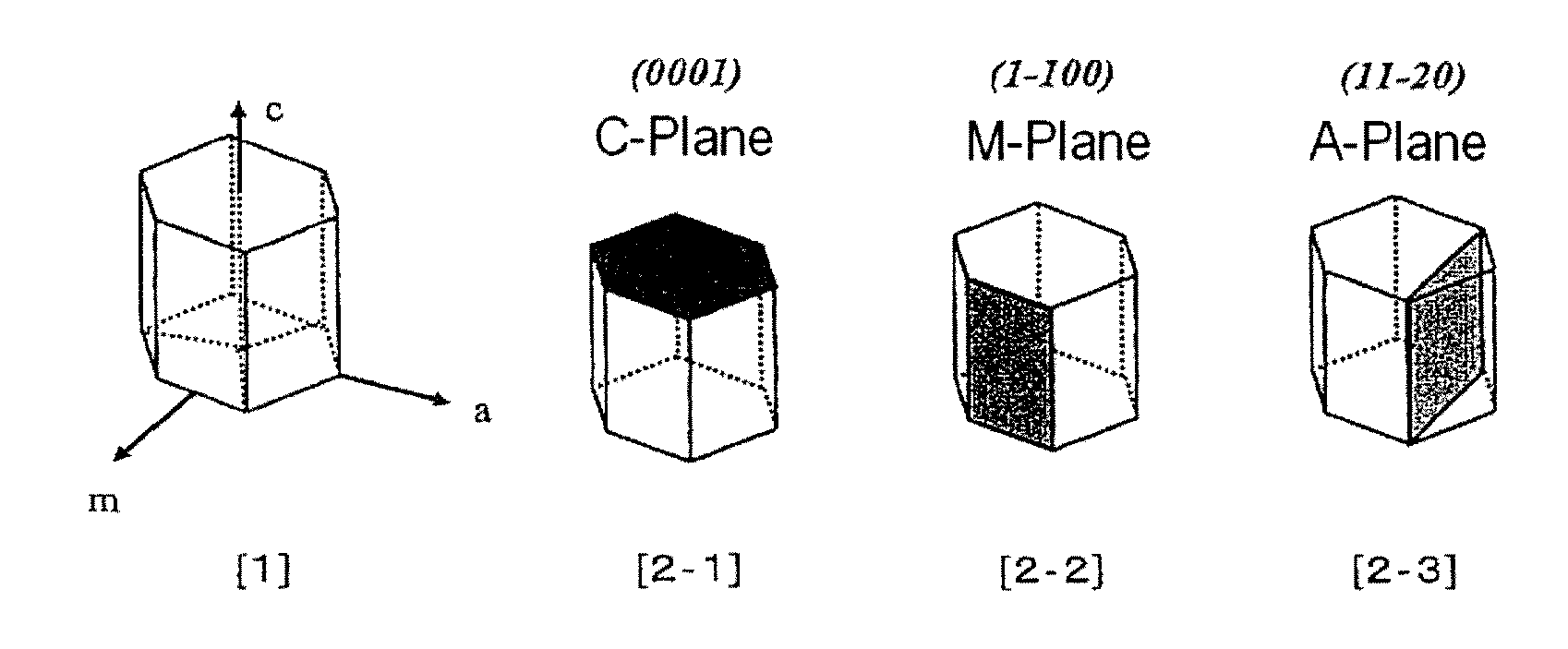
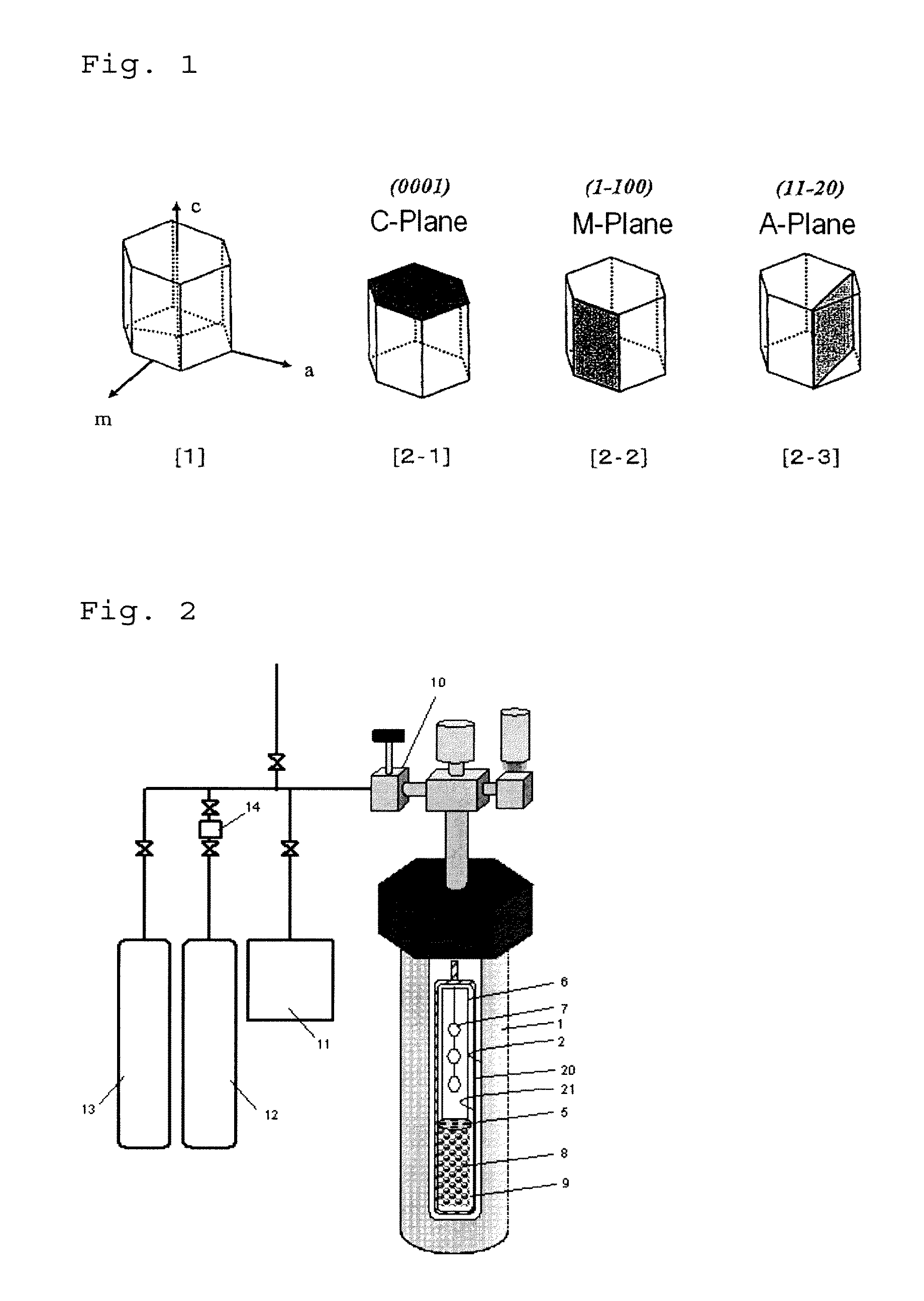
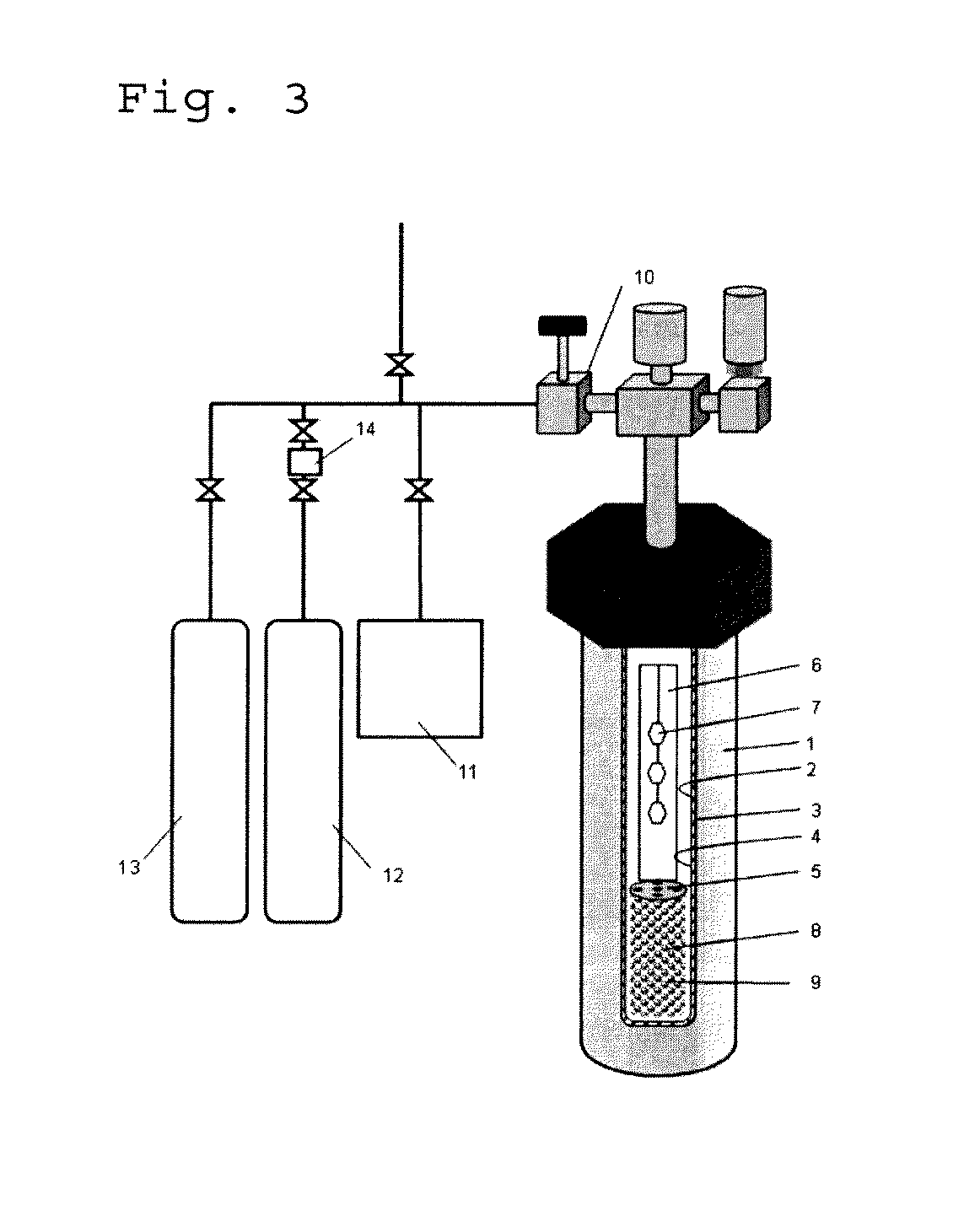
ActiveUS20120251431A1Efficient productionReadily and efficiently producedPolycrystalline material growthFrom normal temperature solutionsHalogenBromine
A method for producing a nitride crystal, comprising growing a nitride crystal on the surface of a seed crystal put in a reactor while the temperature and the pressure inside the reactor that contains, as put thereinto, a seed crystal having a hexagonal-system crystal structure, a nitrogen-containing solvent, a starting material, and a mineralizing agent containing fluorine and at least one halogen element selected from chlorine, bromine and iodine are so controlled that the solvent therein could be in a supercritical state and / or a subcritical state to thereby grow a nitride crystal on the surface of the seed crystal in the reactor.
Owner:MITSUBISHI CHEM CORP
Organic-inorganic composite particles
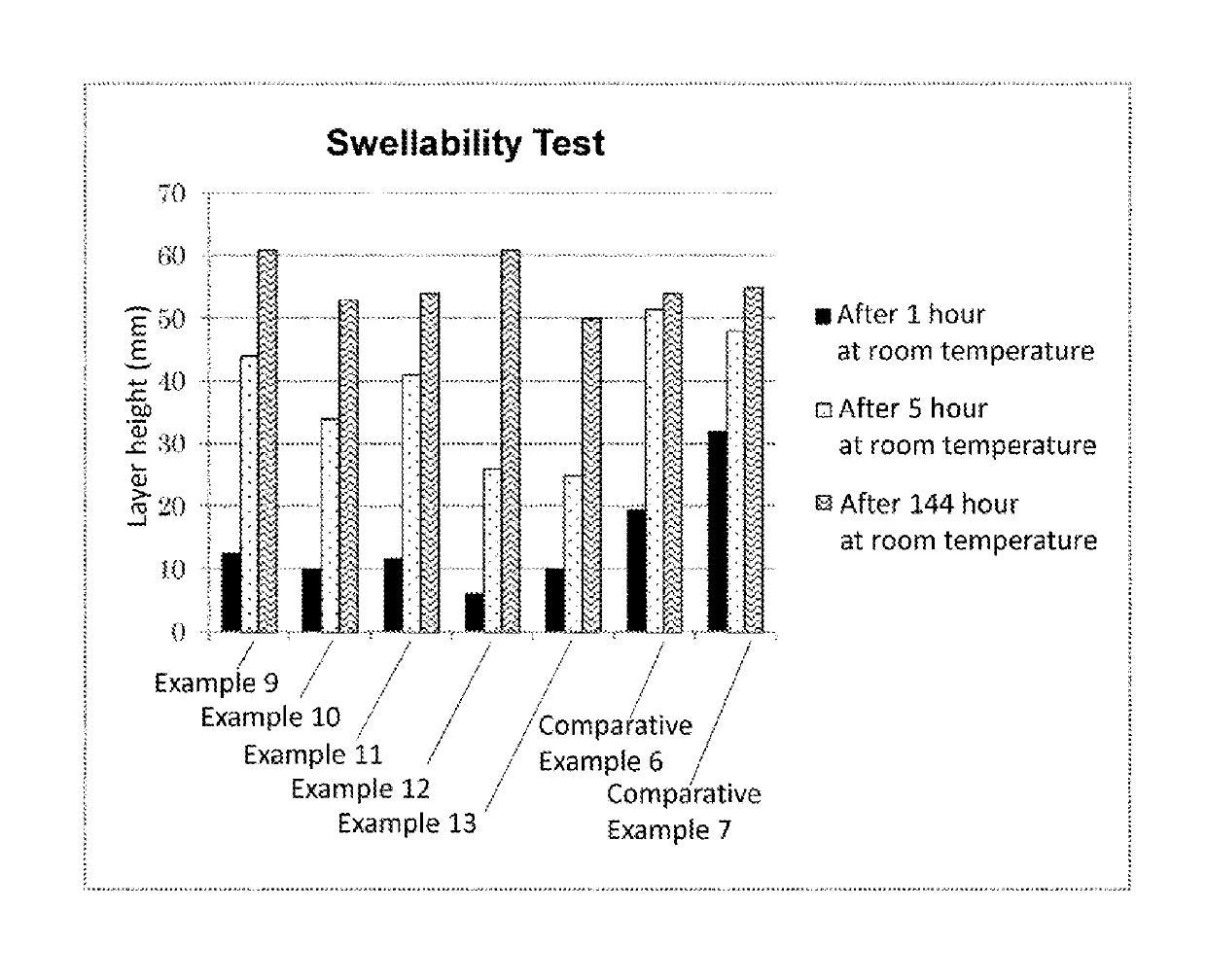
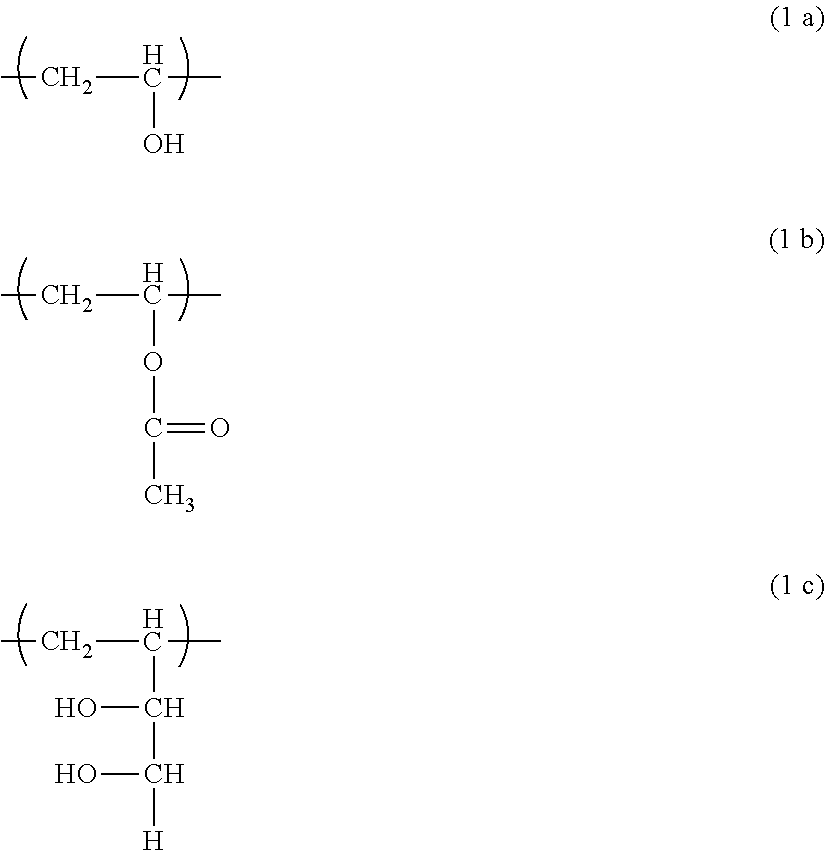
ActiveUS20190153290A1Reduce agglomerationReduced lump formationFluid removalDrilling compositionWater dispersiblePolyvinyl alcohol
An object of the present invention is to improve the water dispersibility of the PVA-based resin and also to suppress the swelling of the PVA-based resin.The object is achieved by organic-inorganic composite particles, which have an average particle size of preferably 1 μm to 3,000 μm, including a polyvinyl alcohol-based resin and a component having a three-dimensional siloxane crosslinked structure that is derived from a hydrolysis-polycondensation product of an alkoxysilane and / or a low condensate thereof, wherein the alkoxysilane includes a T unit and / or a Q unit as a structural unit (s), and a Si content with respect to a total weight of the composite particles is 0.1 wt % or more and 23 wt % or less in terms of SiO2.
Owner:MITSUBISHI CHEM CORP
Injectable Flowable Composition Comprising Buprenorphine
ActiveUS20130210853A1High bioavailabilityMinimal riskBiocideNervous disorderTissue damageBiomedical engineering
The present invention is directed to a buprenorphine sustained release delivery system capable of delivering buprenorphine, a metabolite, or a prodrug thereof for a duration of about 14 days to about 3 months. The buprenorphine sustained release delivery system includes a flowable composition and a solid implant for the sustained release of buprenorphine, a metabolite, or a prodrug thereof. The implant is produced from the flowable composition. The buprenorphine sustained release delivery system provides in situ 1-month and 3-month release profiles characterized by an exceptionally high bioavailability and minimal risk of permanent tissue damage and typically no risk of muscle necrosis.
Owner:INDIVIOR UK
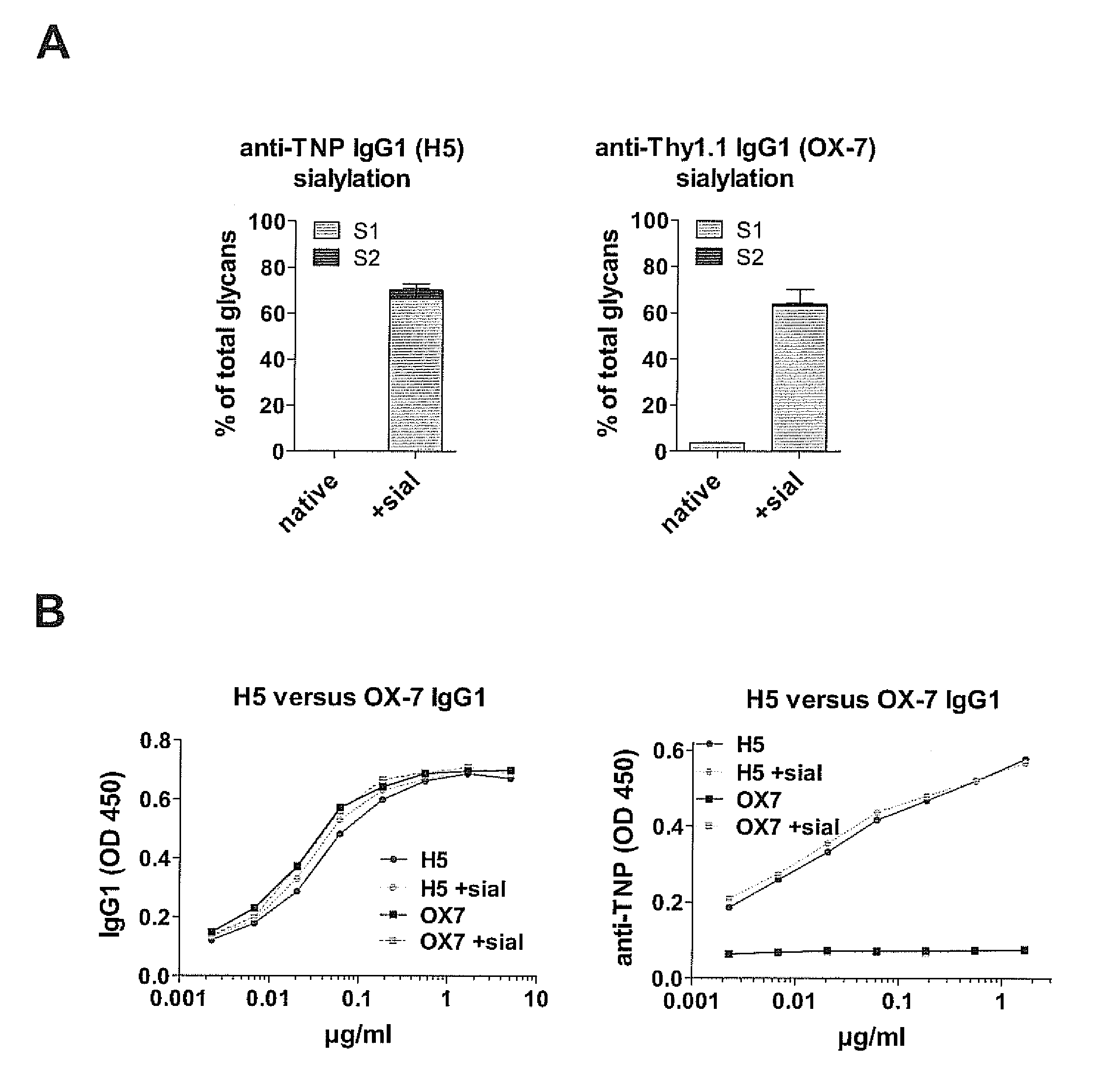
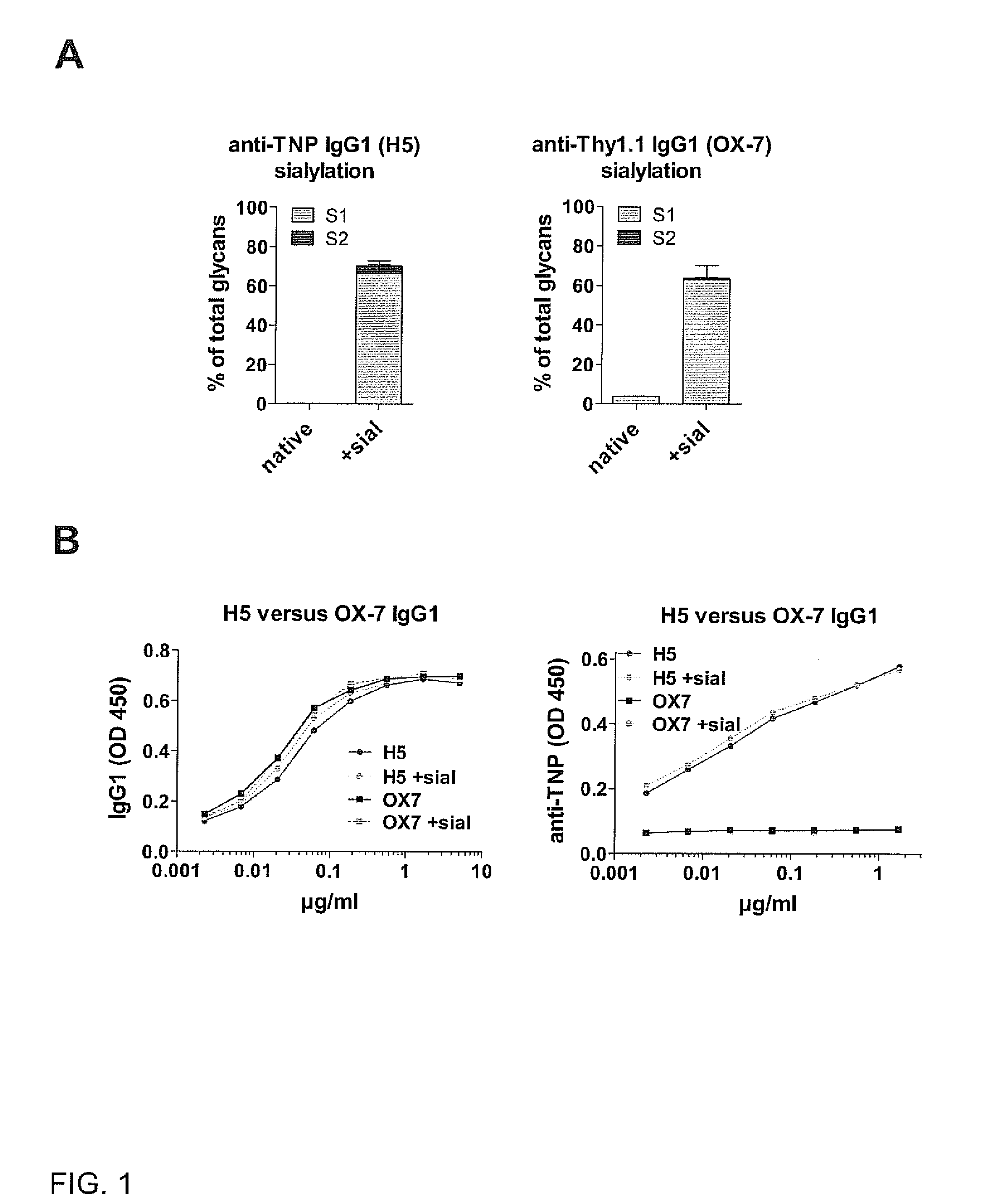
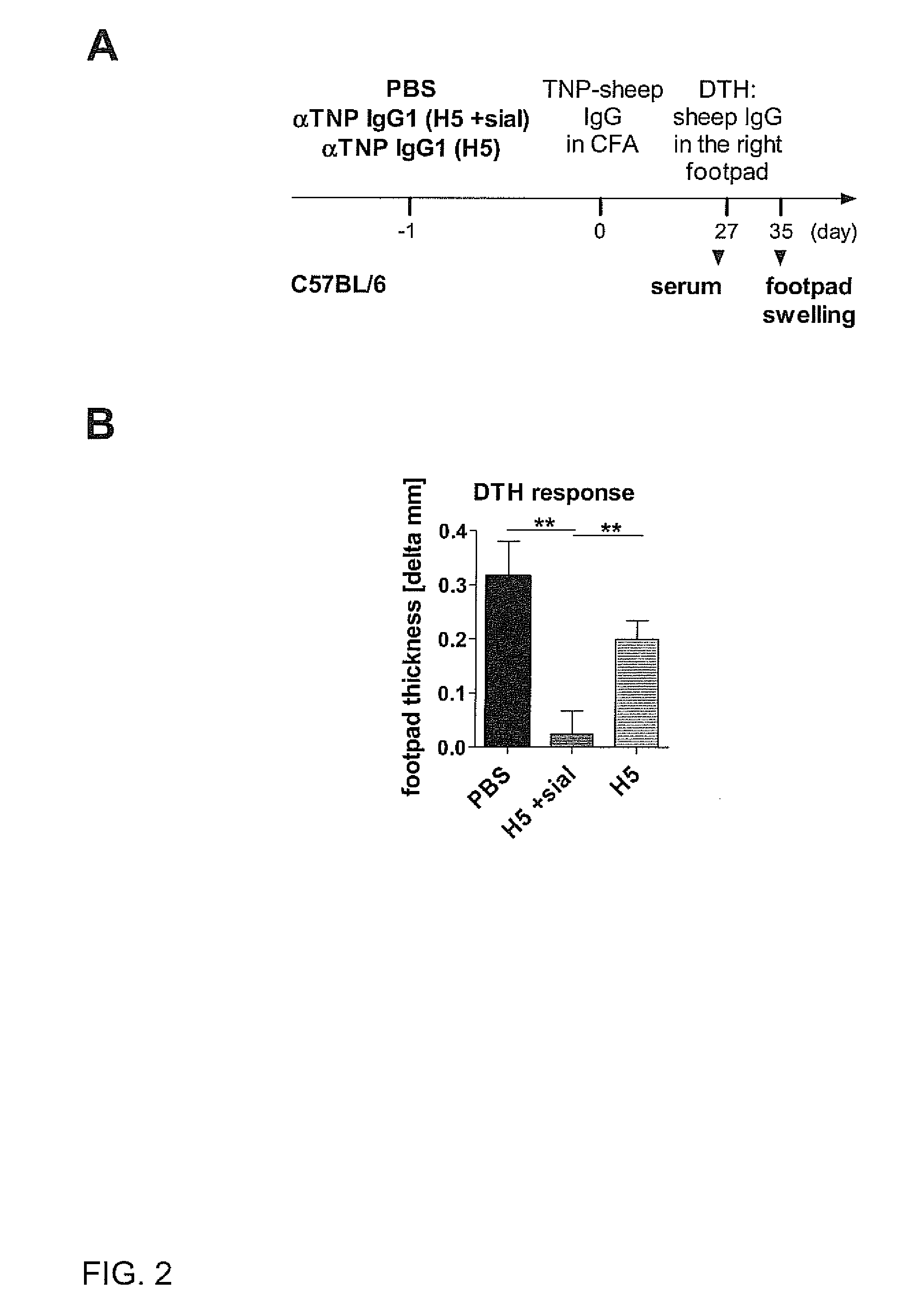
Sialylated antigen-specific antibodies for treatment or prophylaxis of unwanted inflammatory immune reactions and methods of producing them
InactiveUS20120058111A1SolubilityImprove solubilityImmunoglobulins against blood group antigensImmunoglobulins against cell receptors/antigens/surface-determinantsTransplant rejectionSialic acid
The present invention is directed to a sialylated isolated antibody specific for an antigen selected from autoimmune antigens, allergens, MHC molecules or Rhesus factor D antigen, comprising an Fc-portion of IgG type, and exhibiting a sialic acid residue at the Fc-portion for use in treatment and / or prophylaxis of autoimmune disease, allergy, transplant rejection or Rhesus factor D reactivity and to methods of producing such an antibody.
Owner:EHLERS MARC +3
Production method of cellulose film, cellulose film, protective film for polarizing plate, optical functional film, polarizing plate, and liquid crystal display
InactiveUS6887415B2Residue reductionReduce production efficiencySemi-permeable membranesMembranesCelluloseSolubility
A production method of cellulose film wherein cellulose film is produced by preparing a polymer solution through dissolving cellulose ester in a solvent containing a prescribed organic solvent as the main component, forming a filmy object from the prepared polymer solution, and evaporating the solvent in the filmy object; the residual amount of the organic solvent is reduced while the film quality is not degraded, and the production efficiency is degraded to a least possible extent; a poor solvent, highest in boiling point among the materials contained in the solvent, is added in the content ranging from 0.1 wt % to 1.0 wt %, taking the total amount of the solvent in the prepared polymer solution to be 100 wt %; and the solubility of cellulose ester in the poor solvent is inferior to the solubility of the cellulose ester in the organic solvent which is the main component of the solvent.
Owner:FUJIFILM HLDG CORP +1
Injectable flowable composition comprising buprenorphine
ActiveUS20130203796A1Least riskImprove bioavailabilityBiocidePharmaceutical delivery mechanismMetaboliteBioavailability
The present invention is directed to a buprenorphine sustained release delivery system capable of delivering buprenorphine, a metabolite, or a prodrug thereof for a duration of about 14 days to about 3 months. The buprenorphine sustained release delivery system includes a flowable composition and a solid implant for the sustained release of buprenorphine, a metabolite, or a prodrug thereof. The implant is produced from the flowable composition. The buprenorphine sustained release delivery system provides in situ 1-month and 3-month release profiles characterized by an exceptionally high bioavailability and minimal risk of permanent tissue damage and typically no risk of muscle necrosis.
Owner:INDIVIOR UK
Vehicle for topical delivery of anti-inflammatory compounds
InactiveUS20060211688A1Present synergistic behavior in anti-inflammatory actionSolubilityBiocideNervous disorderCompound aMedicine
Owner:ALPHARX
Sustained delivery formulations of risperidone compound
ActiveUS10376590B2Least riskImprove bioavailabilityNervous disorderPharmaceutical delivery mechanismBipolar mood disorderMetabolite
The present invention relates to a risperidone sustained release delivery system for treatment of medical conditions relating to delusional psychosis, schizophrenia, bipolar disorder, psychotic depression, obsessive-compulsion disorder, Tourette syndrome, and autistic spectrum disorders. The sustained release delivery system includes a flowable composition containing risperidone, a metabolite, or a prodrug thereof and an implant containing risperidone, a metabolite, or a prodrug thereof. The flowable composition may be injected into tissue whereupon it coagulates to become a solid or gel, monolithic implant. The flowable composition includes a biodegradable, thermoplastic polymer, an organic liquid, and risperidone, a metabolite or a prodrug thereof.
Owner:INDIVIOR UK
Method for immobilizing multistranded nucleic acid molecules by modifying more than one strand thereof, and binding each strand to a solid support
InactiveUS6936461B2SolubilityAvoid problemsBioreactor/fermenter combinationsBiological substance pretreatmentsBiological activationNucleic acid molecule
Various methodologies for the immobilization of molecules such, as multistranded nucleic acid molecules, are described. The methodologies include activation of solid supports, as well as treatment of, e.g. termini of nucleic acid molecules to render them more receptive to immobilization on surfaces.
Owner:NEW YORK INSTITUTE OF TECHNOLOGY
Paint remover for removing paint film on surface of tin-plated steel plate for food can and using method thereof
The invention relates to a paint remover for removing a paint film on the surface of a tin-plated steel plate for a food can and a using method thereof, wherein the paint remover is prepared from the following raw materials in percentage by weight: 25-35% of dibasic acid ester, 20-35% of 1, 2-propylene glycol carbonate, 8-15% of absolute ethyl alcohol, 16-25% of lactic acid and 2.5-6% of cetyl trimethyl ammonium bromide. The paint remover is prepared by a method comprising the following steps: firstly, burdening the raw materials of the paint remover according to the content thereof; adding the raw materials according to the sequence from solid to liquid respectively, then stirring for 5-15 minutes at room temperature, and sealing and reserving after mixing uniformly. The using method of the prepared paint remover for removing the paint film on the surface of the tin-plated steel plate for the food can comprises the following step of: immersing objects for removing the paint completely in the paint remover, wherein the using temperature of the paint remover is 25-80 DEG C, the best using temperature is 60-80 DEG C, and the immersing time is 1-15 minutes. The paint remover for removing the paint film on the surface of the tin-plated steel plate for the food can and the using method thereof provided by the invention have the characteristics of good environment-friendly performance, stable solvent components, paint removing efficiency and tiny metal corrosion performance.
Owner:WUHAN UNIV OF SCI & TECH
Calcium phosphate bioactive ceramic and preparation method thereof
InactiveCN104803672AGood mechanical stabilityPromote bone growthProsthesisCalcium EDTABiocompatibility Testing
The invention discloses a calcium phosphate bioactive ceramic and a preparation method thereof, and relates to the field of biomaterials. The calcium phosphate bioactive ceramic is prepared from the following ingredients in parts by weight: 50-75 parts of tetracalcium phosphate, 3-9 parts of titanium oxide, 3-8 parts of aluminum silicate fiber, 2-5 parts of zinc oxide, 10-22 parts of tertiary calcium phosphate and 6-10 parts of calcium oxide. The preparation method comprises the following steps: (1) weighing; (2) ball milling; (3) cold press molding; (4) high-temperature calcining; (5) cooling. The calcium phosphate bioactive ceramic has certain mechanical stability and better biocompatibility; meanwhile, the calcium phosphate bioactive ceramic can slowly dissolve and release the bone nutrition elements of calcium and phosphorus, promotes bone growth, and is an ideal bone renovating biomaterial.
Owner:SUZHOU VIVOTIDE BIOTECH
Plant-based biologically active substance having a polypharmacological effect
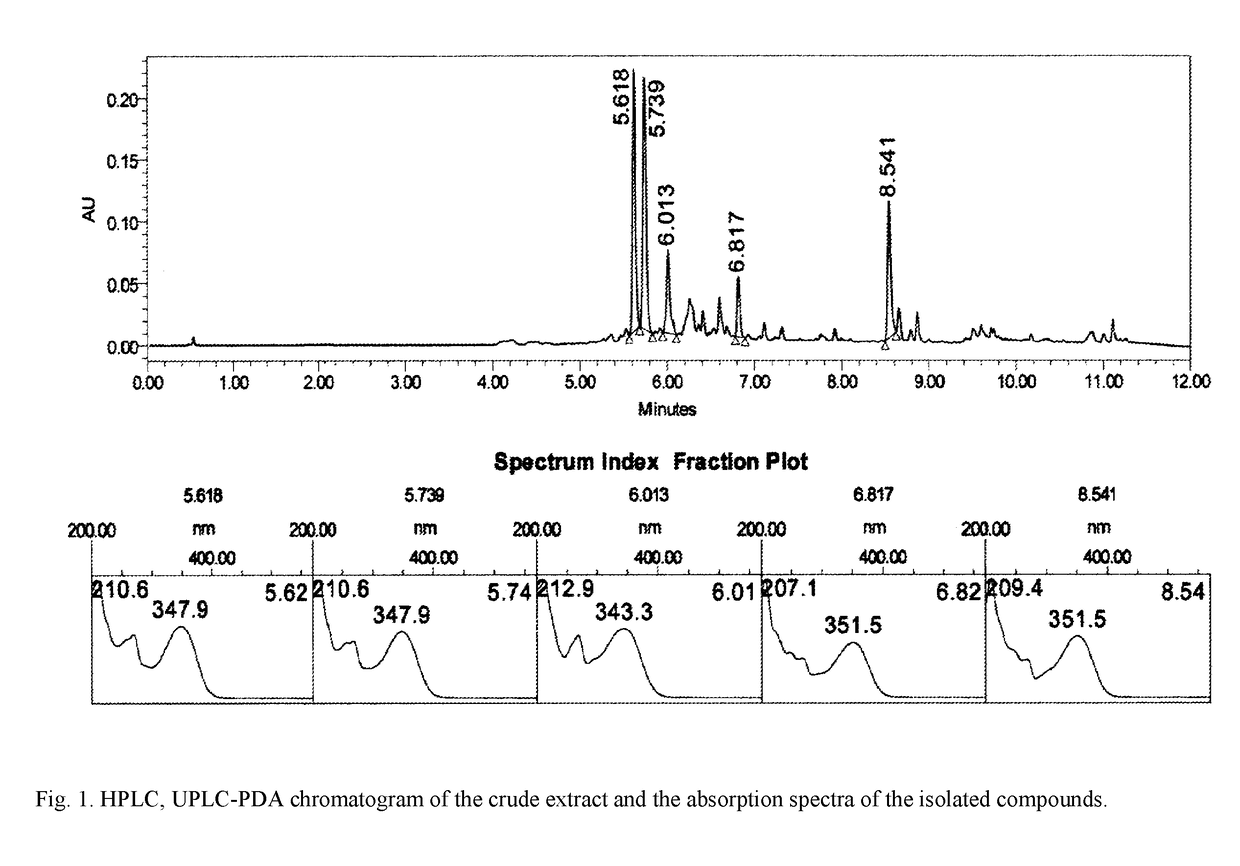
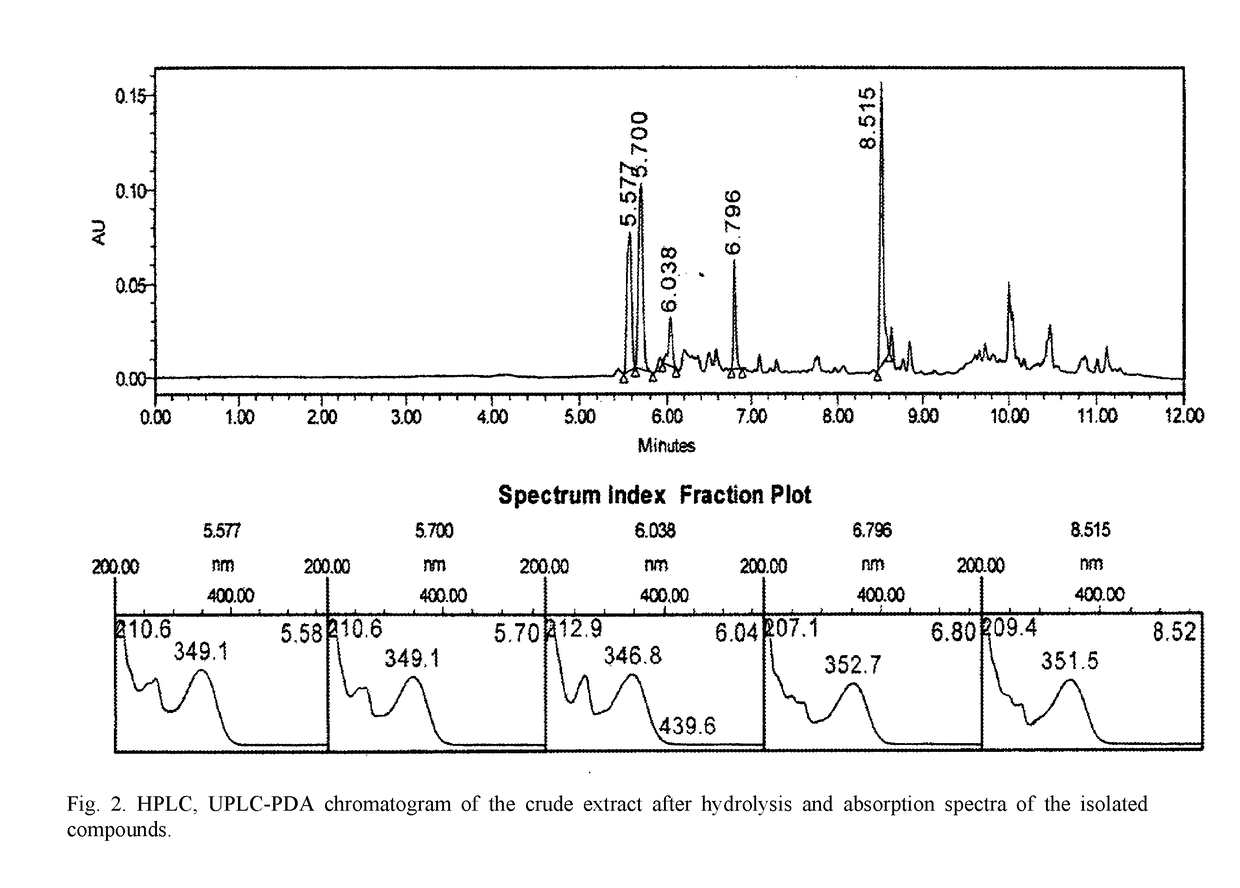
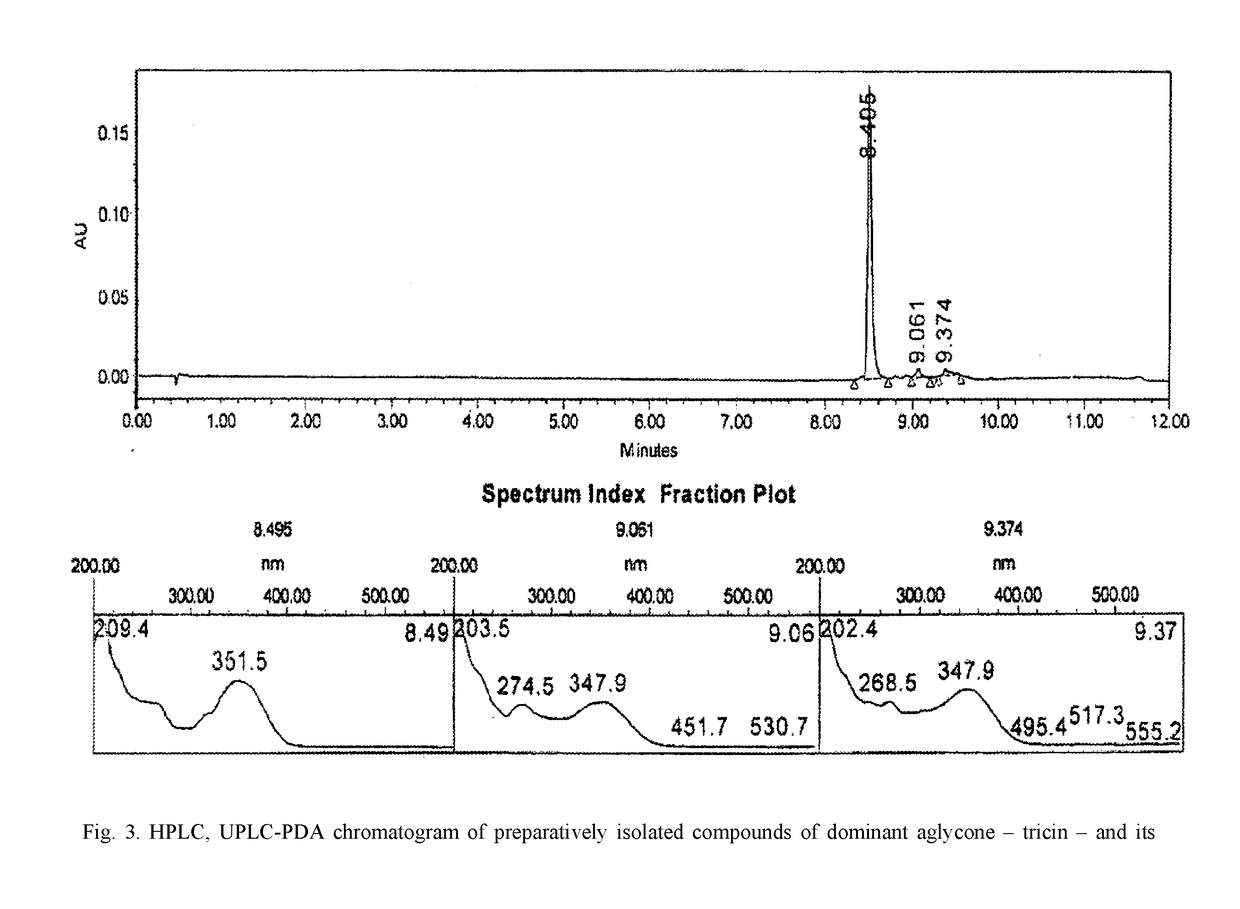
InactiveUS20180133278A1SolubilityImprove solubilityOrganic active ingredientsAntinoxious agentsBovine Viral Diarrhea VirusesApoptosis
The invention relates to pharmacology and may be used for producing medicinal agents for treating and preventing viral diseases caused by the herpes, influenza and hepatitis B and C viruses, and also virus-induced immunodeficiencies. A biologically active substance having a polypharmacological effect is made from the green parts and spikelets of cereals of the family Gramineae, genus Calamagrostis Adans and / or genus Deschampsia Beauv, and contains flavonoids, specifically aglycones of the flavonoids tricin, apigenin, luteolin, quercetin and rhamnazin, and / or flavonoid glycosides of tricin, apigenin, luteolin, quercetin, and rhamnazin, and excipients, and has the following composition by mass percent: tricin flavonoid aglycone and / or tricin flavonoid glycosides: 0.016-2.062%; apigenin flavonoid aglycone and / or apigenin flavonoid glycosides: 0.010-1.393%; luteolin flavonoid aglycone and / or luteolin flavonoid glycosides: 0.01-4.979%; quercetin flavonoid aglycone and / or quercetin flavonoid glycosides: 0.001-0.771%; rhamnazin flavonoid aglycone and / or rhamnazin flavonoid glycosides: 0.104-0.203%; excipients: 99.868-90.592%. In this way, the biologically active substance is perfected by determining the specific compositions of the active ingredients thereof, and the physical, chemical and biological characteristics thereof, resulting in the invention of an optimal composition (FIG. 1) for achieving an antiviral effect with regard to specific viruses, and dosages when creating medicinal forms. In addition, it has been determined that the biologically active substance is an inducer of a type-γ endogenous interferon, displays an apoptosis-modulating effect, has antioxidant properties and enhances cell resistance to free radical stress. The antiviral effect with regard to specific viruses has been established to be an antiviral effect with regard to the type 2 herpes simplex virus, the influenza virus, and the bovine viral diarrhea virus (hepatitis C virus).
Owner:ATAMANIUK VICTOR +1
Ginkgo leaf tea and preparation method thereof
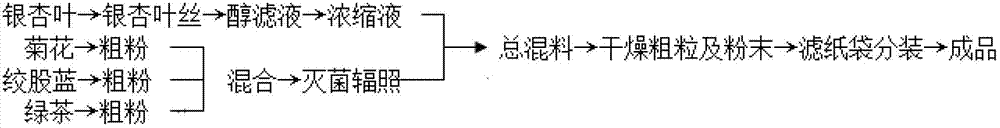
ActiveCN102920850AReduce contentElevated HDLMetabolism disorderTea substituesGynostemmaEpigallo-catechin gallate
The invention relates to ginkgo leaf tea and a preparation method thereof. The method comprises the following steps of: (1) cutting ginkgo leaves into ginkgo leaf threads, extracting ginkgo leaves with alcohol, preparing an alcohol filtrate, and concentrating; (2) grinding chrysanthemum, fiveleaf gynostemma herb and green tea into coarse powder, mixing coarse powder, and sterilizing; and (3) fully stirring and uniformly mixing a concentrated solution obtained in the step (1) and mixed powder, drying, sieving, and drying coarse particles and powder to obtain ginkgo leaf tea. In the tea components, fiveleaf gynostemma herb contains a plurality of gypenosides, and has the effects of lowering TC (Total Cholesterol) and TG (Triglyceride); ginkgo leaf contains flavone, and has the effect of lowering TC; fiveleaf gynostemma herb and ginkgo leaf co-act with each other, so that blood fat is lowered; chrysanthemum can be used for increasing high-density lipoprotein, lowering serum cholesterase and triglyceride acid, playing a certain role in adjusting blood fat, and adjusting flavor appropriately simultaneously; tea-catechin in green tea is used for lowering serum cholesterase level by increasing the excretion of lipoid substances; and the four types of raw materials act in a synergistic way, so that a very good effect of lowering blood fat is achieved.
Owner:TIANJIN KAIYONG PHARMA
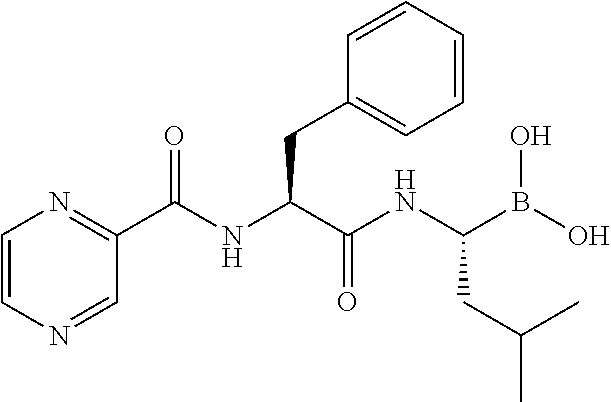
A kind of varenicline salt and preparation method thereof
The invention relates to varenicline hemitartrate and a preparing method thereof. The salt has a structure shown as the formula II. The varenicline hemitartrate is good in stability, low in moisture adsorption and high in safety. The preparing method is simple in operation and good in reproducibility.
Owner:SHANGHAI SYNCORES TECH INC
Method for Resolution of Baclofen Salts
ActiveUS20190345098A1Low compositionSubstantial discriminationOrganic compound preparationAmino-carboxyl compound preparationButyrateEnantiomer
The invention relates to the field of resolution of chiral compounds existing in the form of two optical antipodes (enantiomers), such as Baclofen. More particularly, the invention relates to the production of the pure enantiomer (R)(−) Baclofen, of chemical nomenclature (R)-4-amino-3-(4-chlorophenyl)-butanoic acid, and the hydrogen maleate salt thereof. More specifically, the invention relates to the resolution of hydrogen maleate salts of racemic Baclofen by preferential crystallisation and particularly by the AS3PC method (auto-seeded and programmed polythermal preferential crystallisation).
Owner:UNIV DE ROUEN (FR)
Injectable flowable composition buprenorphine
ActiveUS20150216988A1Improve bioavailabilityLeast riskBiocidePharmaceutical delivery mechanismMetaboliteBioavailability
The present invention is directed to a buprenorphine sustained release delivery system capable of delivering buprenorphine, a metabolite, or a prodrug thereof for a duration of about 14 days to about 3 months. The buprenorphine sustained release delivery system includes a flowable composition and a solid implant for the sustained release of buprenorphine, a metabolite, or a prodrug thereof. The implant is produced from the flowable composition. The buprenorphine sustained release delivery system provides in situ 1-month and 3-month release profiles characterized by an exceptionally high bioavailability and minimal risk of permanent tissue damage and typically no risk of muscle necrosis.
Owner:INDIVIOR UK
Method and apparatus for producing plastic preforms
InactiveUS20150158204A1Easy to disassembleSimple processConfectioneryPlastic recyclingAqueous solutionChemistry
Method for producing a plastic preform, the method comprising a first step of producing a plastic preform from raw material, and a second step of applying an outer layer to the plastic preform, wherein the outer layer forms a bond with the plastic preform, and is insoluble in aqueous solutions having a pH-value between 3 and 10, and well soluble in aqueous solutions having a pH-value in a range of less than 3 and / or more than 10.
Owner:KRONES AG
Peony seed oil cleansing oil and preparation method thereof
InactiveCN110538095AAntioxidantSolve problems that easily burden the skinCosmetic preparationsMake-upVegetable oilEthylhexyl palmitate
The invention discloses peony seed oil cleansing oil and a preparation method thereof. The peony seed oil cleansing oil is prepared from the following components in percentage by mass: 42-50% of peonyseed oil, 30-40% of olive oil, 8-10% of ethylhexyl palmitate, 8-10% of PEG-20 glyceryl triisostearate, and 1-1.5% of tocopheryl acetate. By deploying the type and content of vegetable oil, the problems that when the peony seed oil is independently used, the cost is high, and when the olive oil is independently used, burden is prone to being generated on the skin are solved; and through optimization of an emulsifier and other assistants, the prepared cleansing oil has the high dissolving capacity on cosmetics, when fresh water is added for massage and emulsification after cleansing, the particle size of an emulsion is small, flushing is easy, no oil film is left on the skin after flushing, pores can be deeply cleaned without excessive cleaning, and the skin is not tight after washing.
Owner:广州雍和生物科技有限公司
Composition comprising bortezomib
InactiveUS10314880B2Cost effectiveEasy to diluteDipeptide ingredientsPharmaceutical delivery mechanismSolubilitySolvent
The present invention relates to ready to dilute injectable formulations of bortezomib comprising bortezomib, non-aqueous solvents enhancing the solubility and pH modifying agent.
Owner:FTF PHARMA LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com