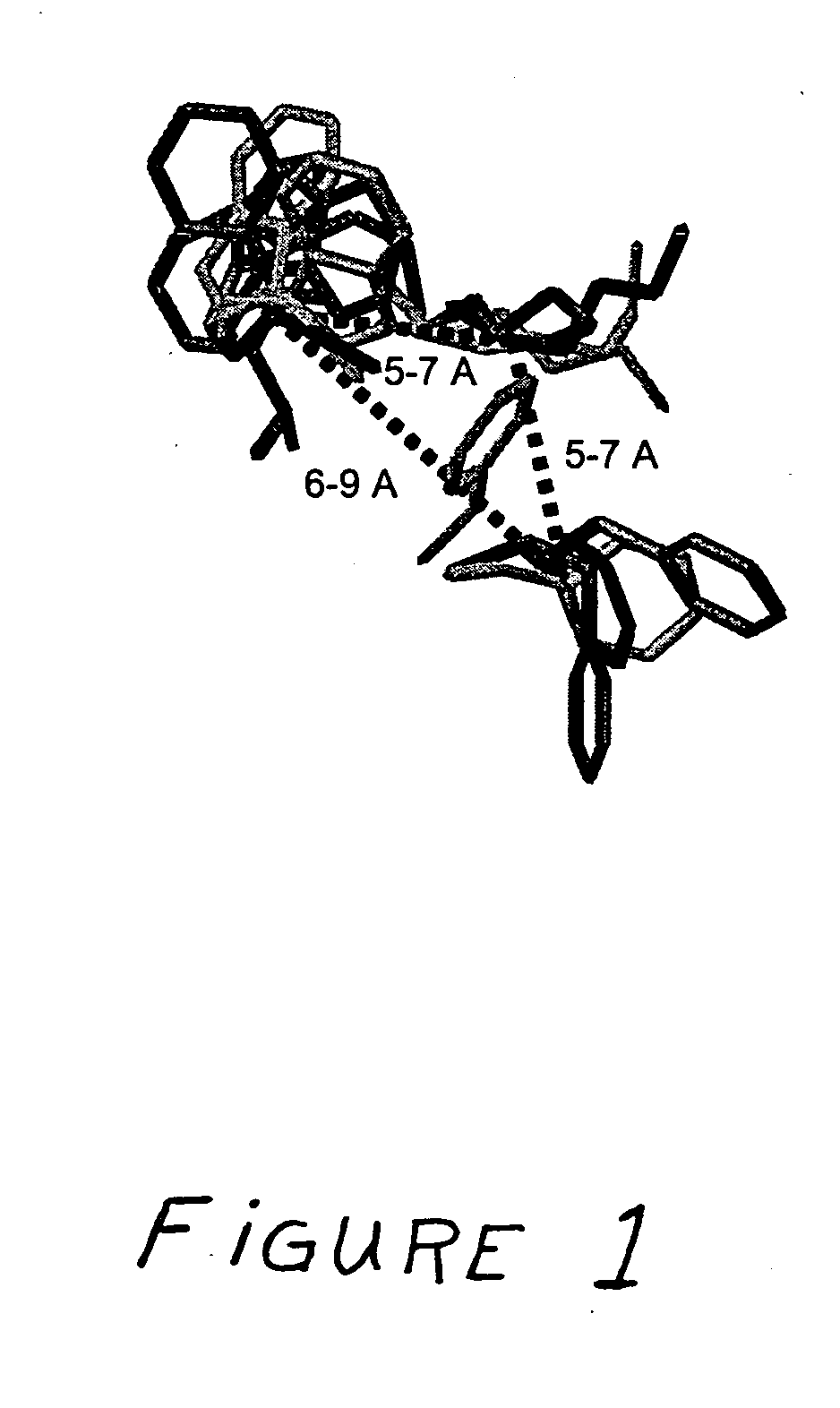
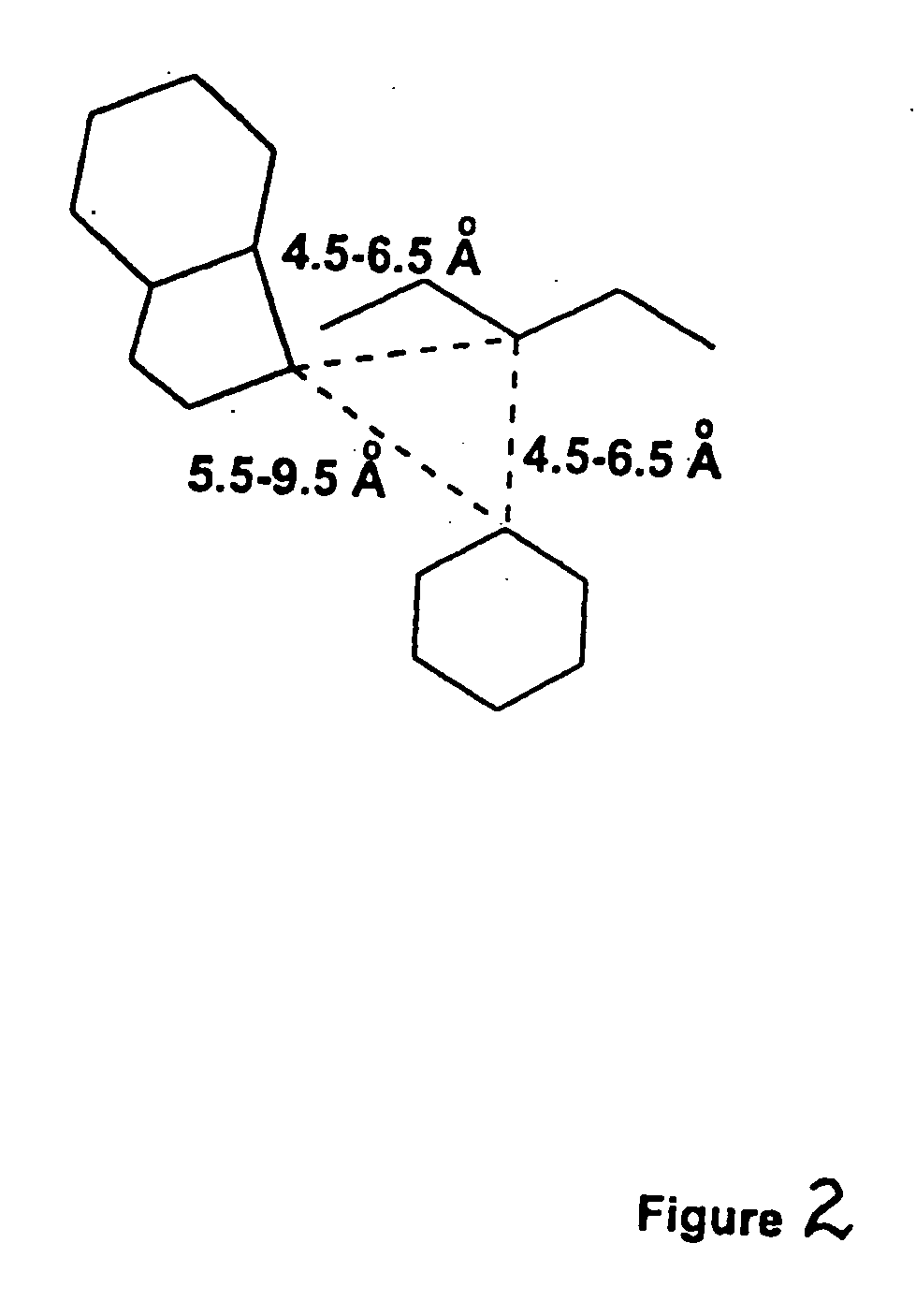
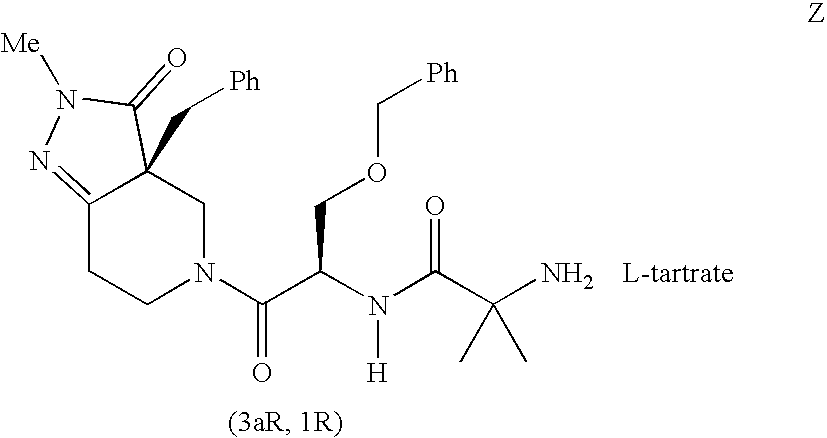
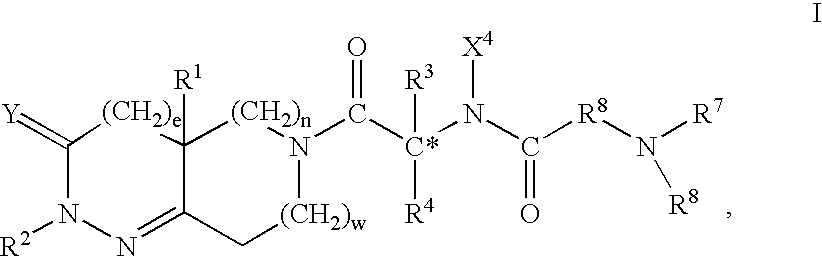
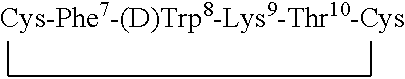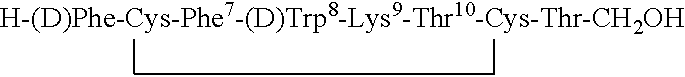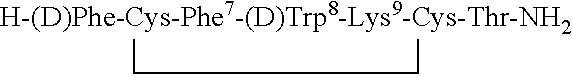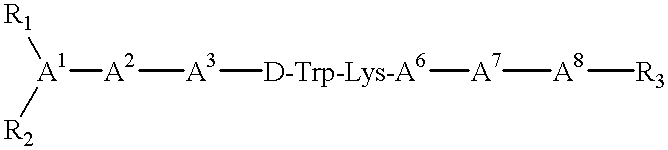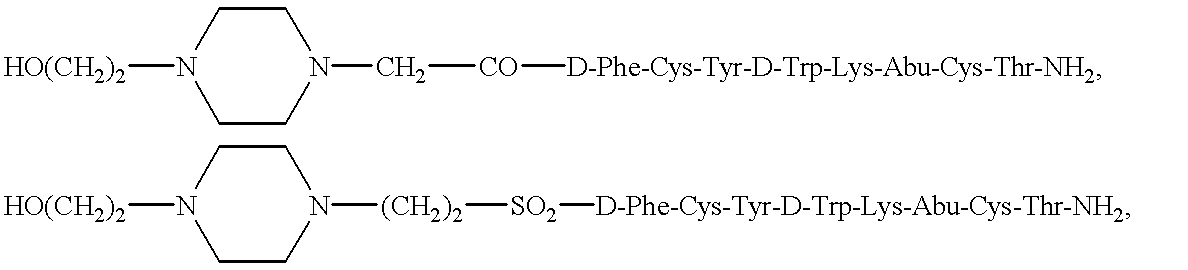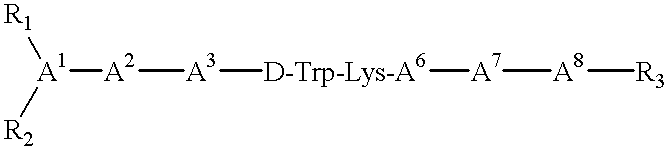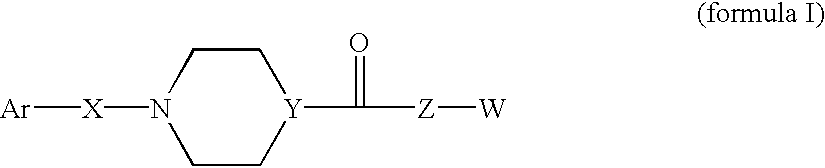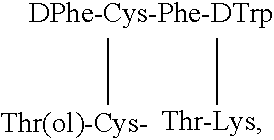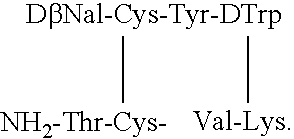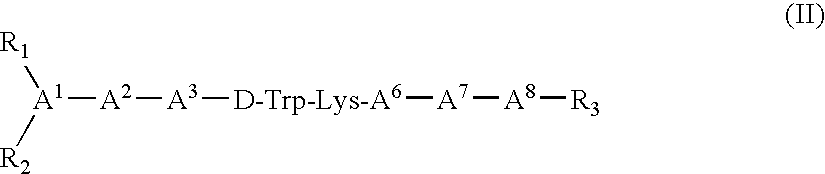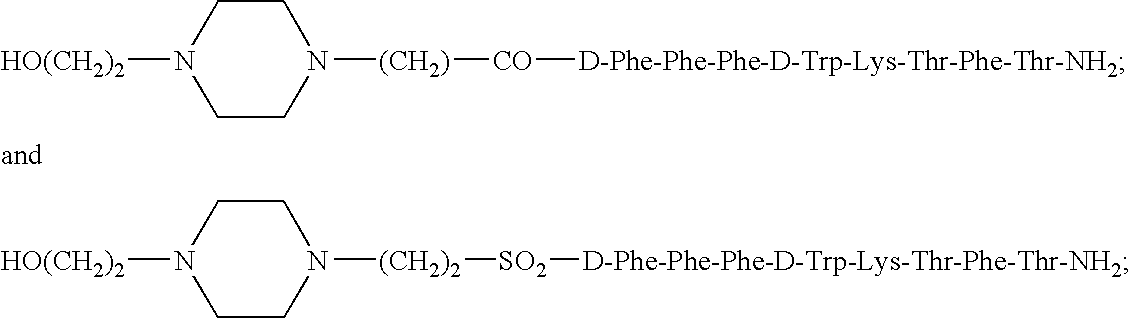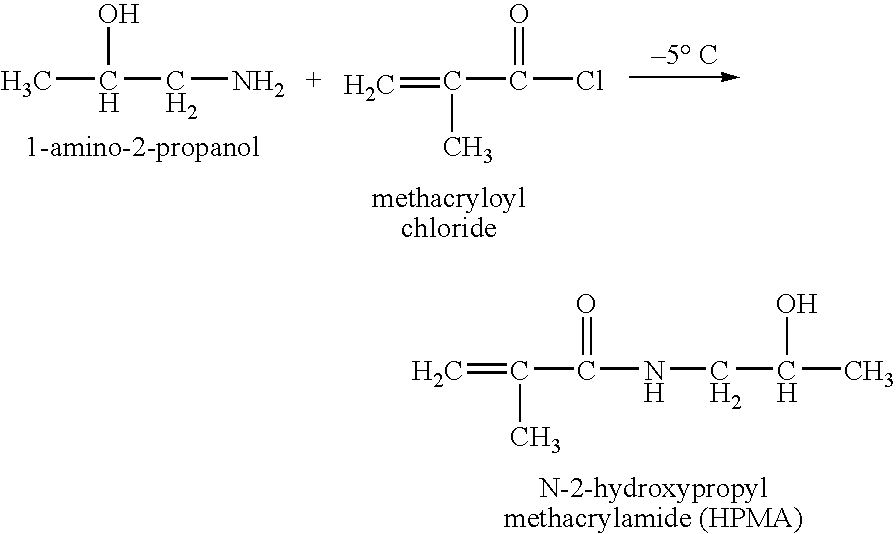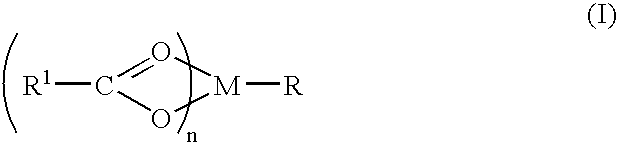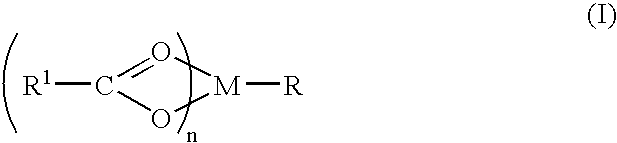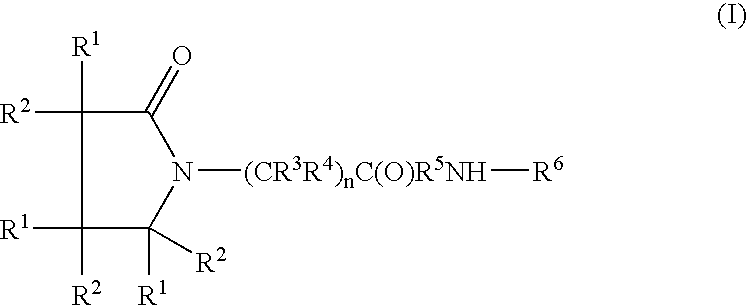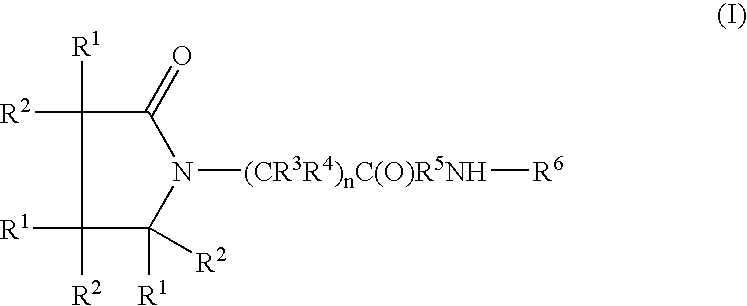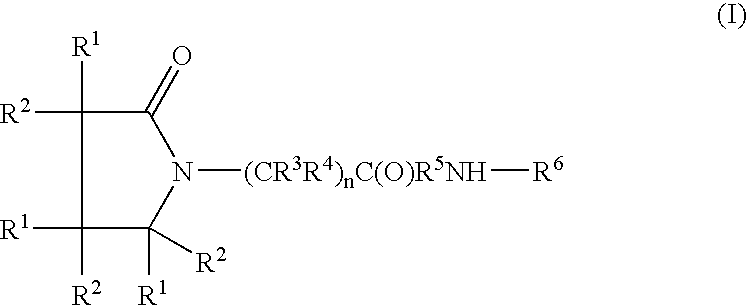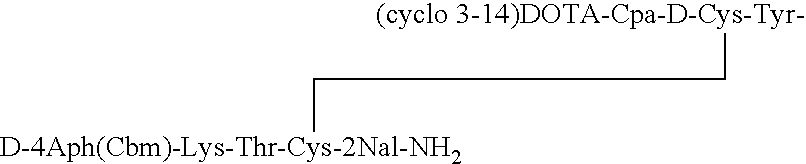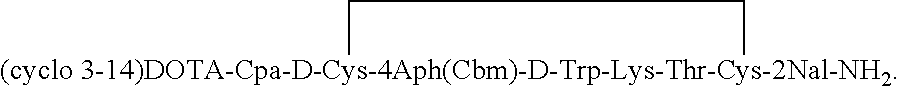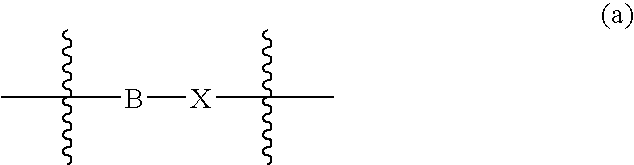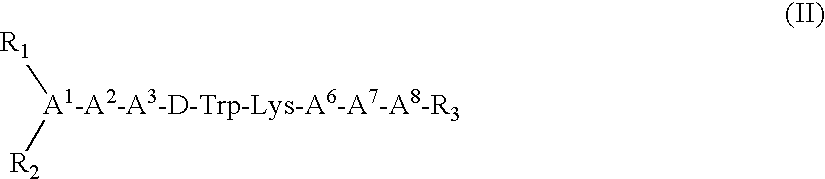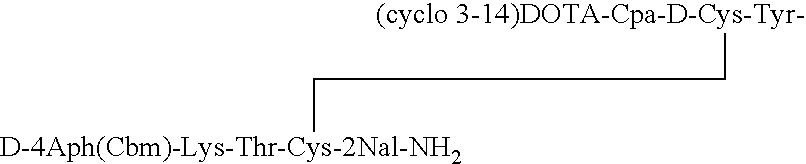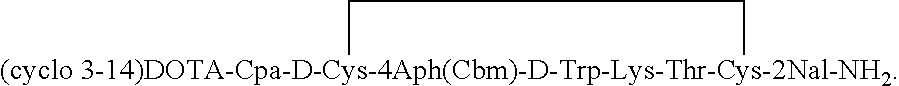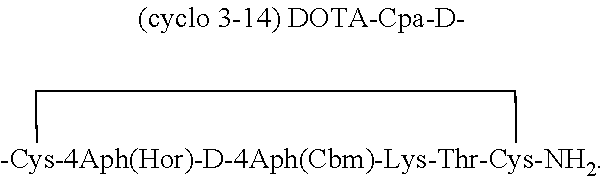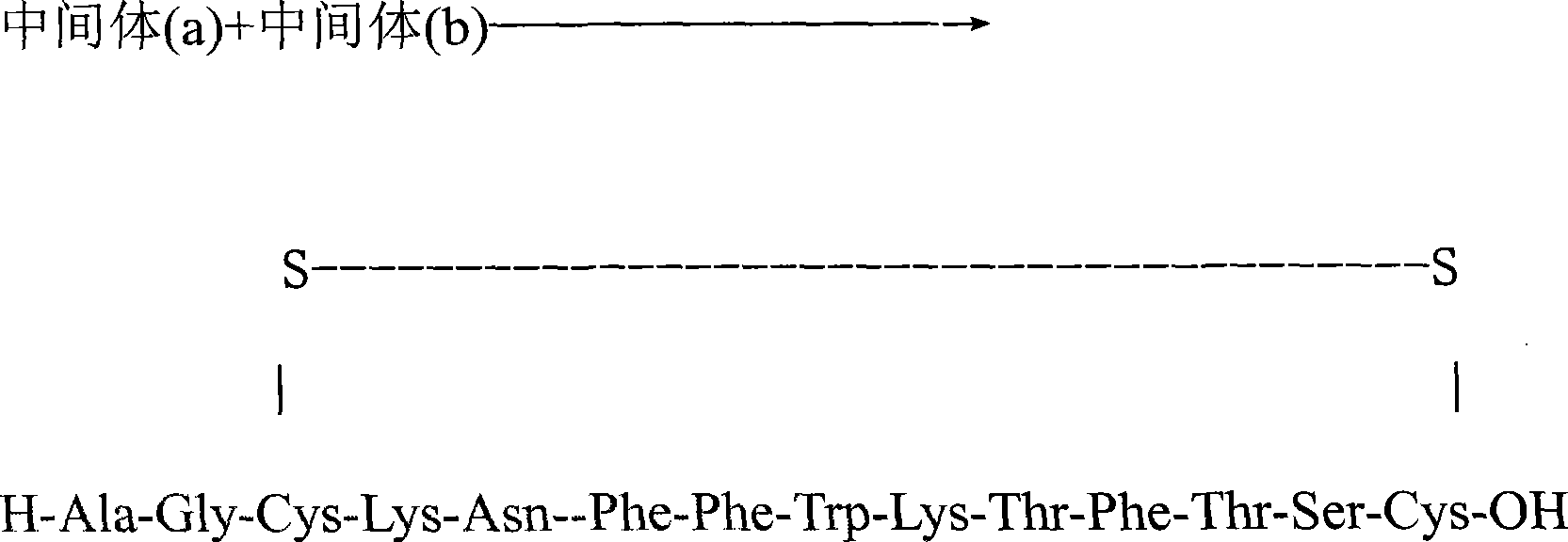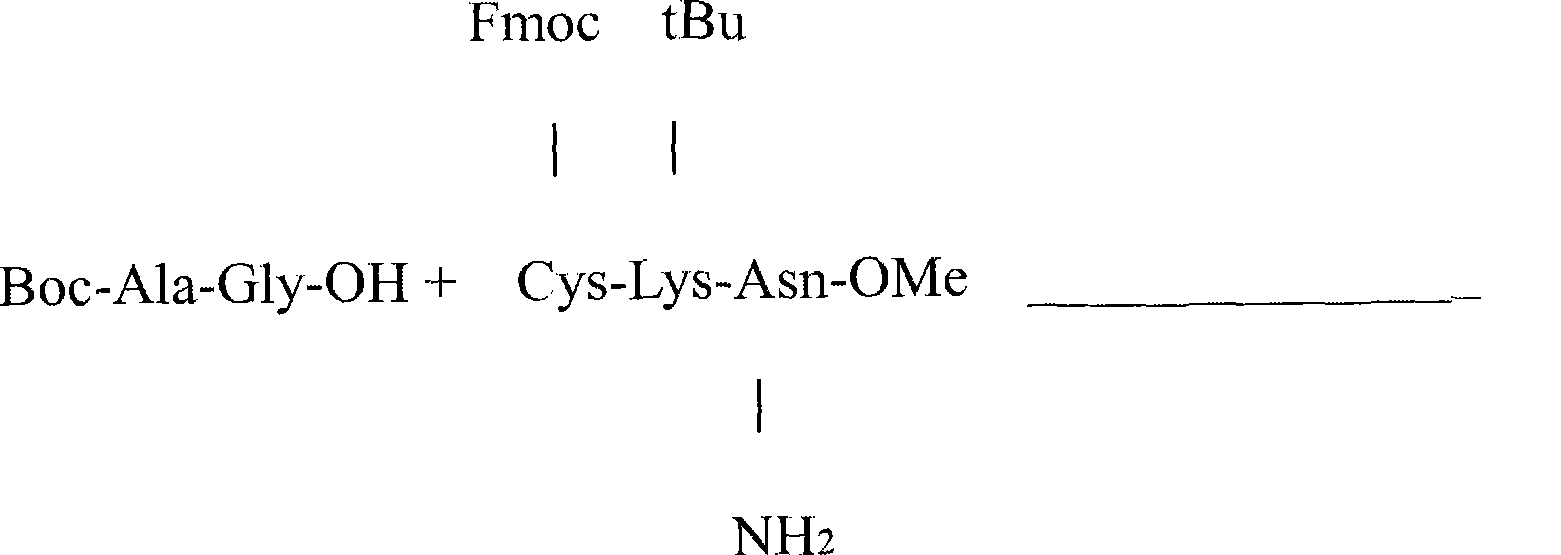Patents
Literature
222 results about "Somatostatin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Somatostatin, also known as growth hormone-inhibiting hormone (GHIH) or by several other names, is a peptide hormone that regulates the endocrine system and affects neurotransmission and cell proliferation via interaction with G protein-coupled somatostatin receptors and inhibition of the release of numerous secondary hormones. Somatostatin inhibits insulin and glucagon secretion.
Amine compounds, their production and use
InactiveUS6329389B1Enhance and inhibit production and secretionLow toxicityBiocideOrganic chemistryArylNitrogen
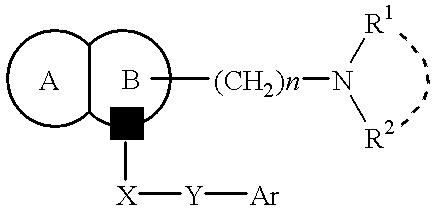
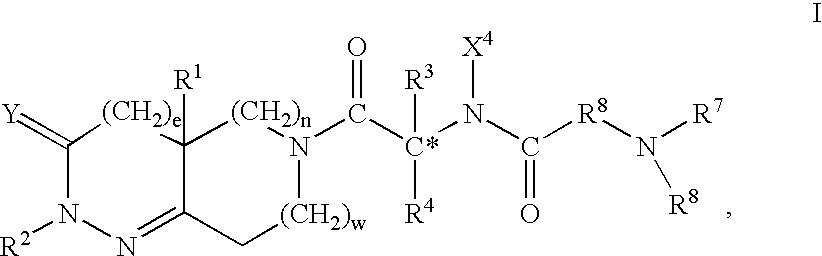
The present invention provides a compound of the formula:wherein Ar represents an aromatic group which may be substituted;X represents methylene, S, SO, SO2 or CO;Y represents a spacer having a main chain of 2 to 5 atoms;n represents an integer of 1 to 5;i) R1 and R2 each represents a hydrogen atom or a lower alkyl which may be substituted,ii) R1 and R2 form, taken together with the adjacent nitrogen atom, a nitrogen-containing heterocyclic ring which may be substituted, oriii) R1 or R2 together with -(CH2)n-N= form, bonded to a component atom of Ring B, a spiro-ring which may be substituted;Ring A represents an aromatic ring which may be substituted;Ring B represents a 4- to 7-membered nitrogen-containing non-aromatic ring which may be further substituted by alkyl or acyl,with a proviso that X represents S, SO, SO2 or CO when Ring A has as a substituent a group represented by the formula:where R11 represents alkyl, alkoxyalkyl, alkylthioalkyl, cycloalkyl, cycloalkylalkyl, aryl, arylalkyl or a group represented by the formula:where R12 represents alkyl, cycloalkyl, cycloalkylalkyl, aryl or arylalkyl, or a salt thereof; which has an excellent somatostatin receptor binding inhibition action.
Owner:TAKEDA PHARMACEUTICALS CO LTD
Conformationally constrained backbone cyclized peptide analogs
Novel backbone cyclized peptide analogs are formed by means of bridging groups attached via the alpha nitrogens of amino acid derivatives to provide novel non-peptidic linkages. Novel building units disclosed are Nα(ω-functionalized) amino acids constructed to include a spacer and a terminal functional group. One or more of these Nα(ω-functionalized) amino acids are incorporated into a peptide sequence, preferably during solid phase peptide synthesis. The reactive terminal functional groups are protected by specific protecting groups that can be selectively removed to effect either backbone-to-backbone or backbone-to-side chain cyclizations. The invention is specifically exemplified by backbone cyclized bradykinin antagonists having biological activity. Further embodiments of the invention are somatostatin analogs having one or two ring structures involving backbone cyclization.
Owner:DEVELOGEN ISRAEL
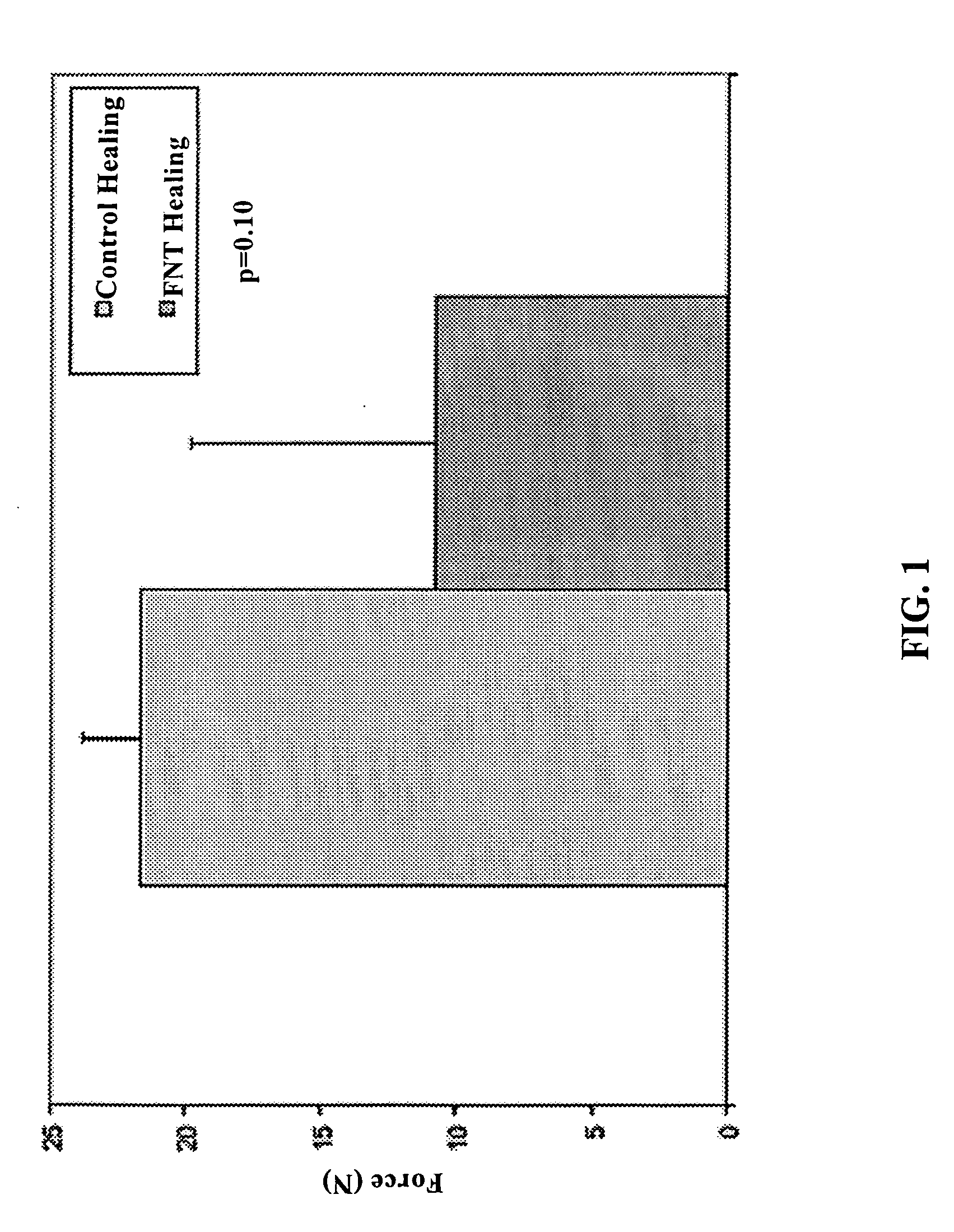
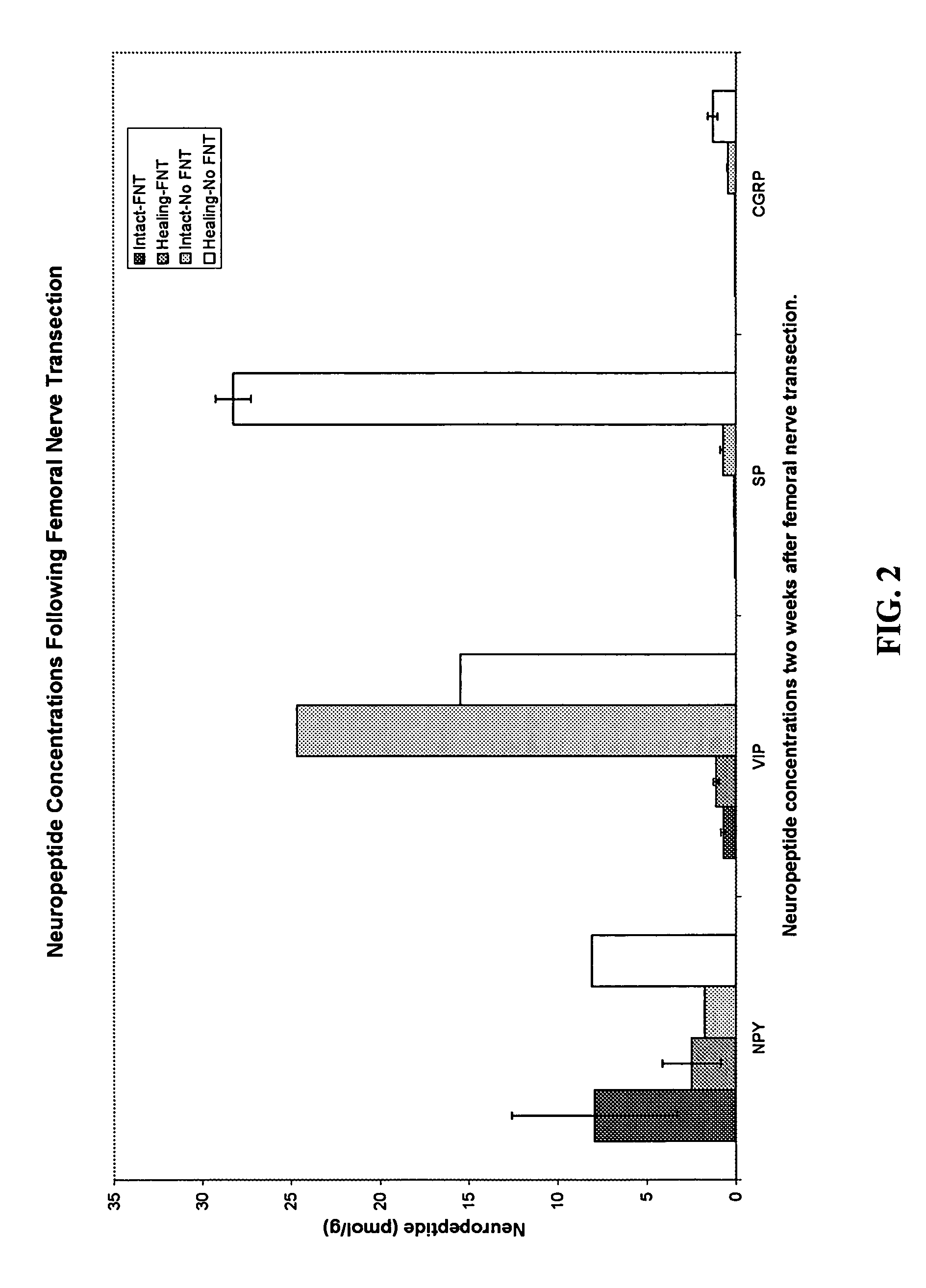
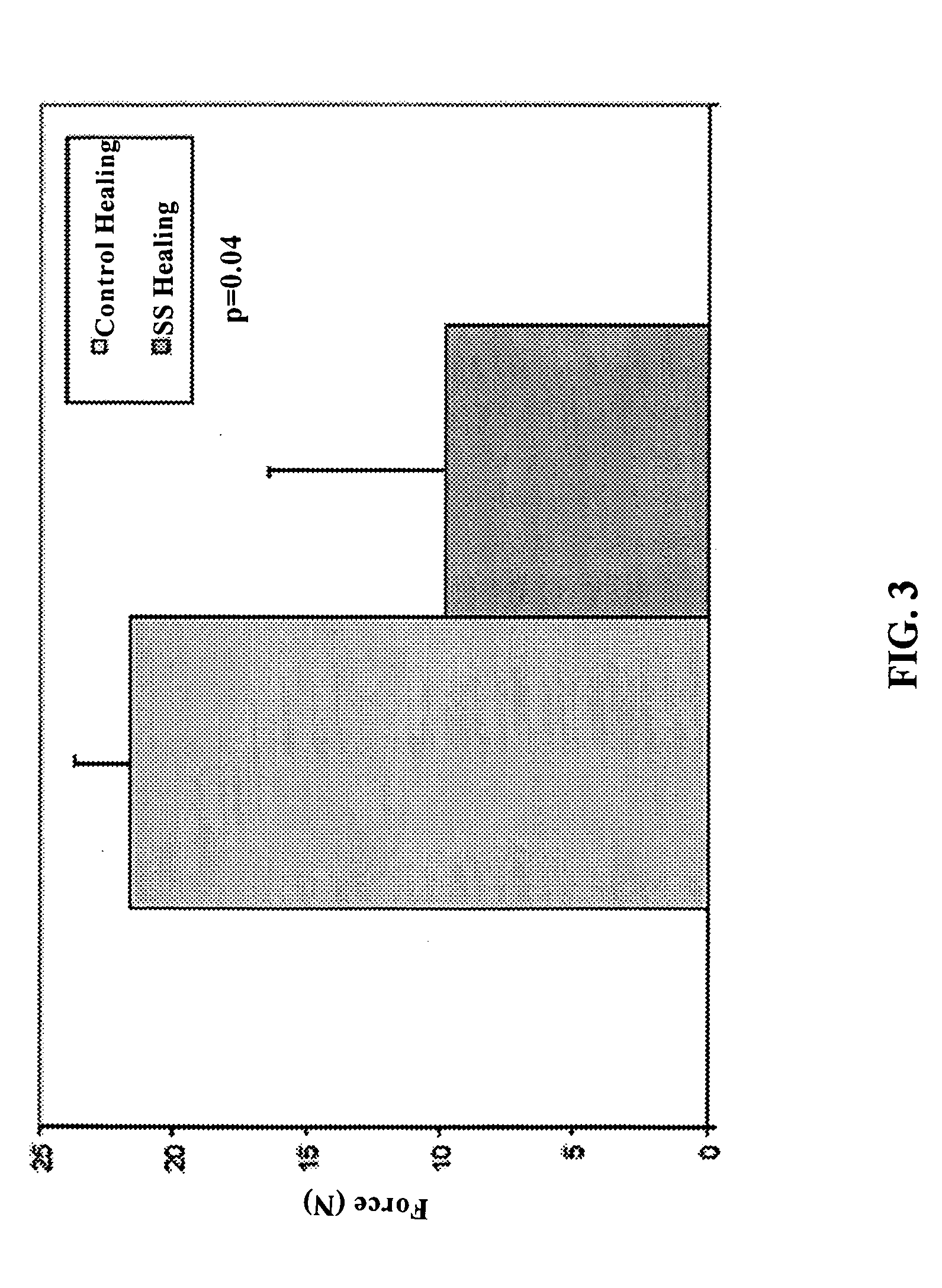
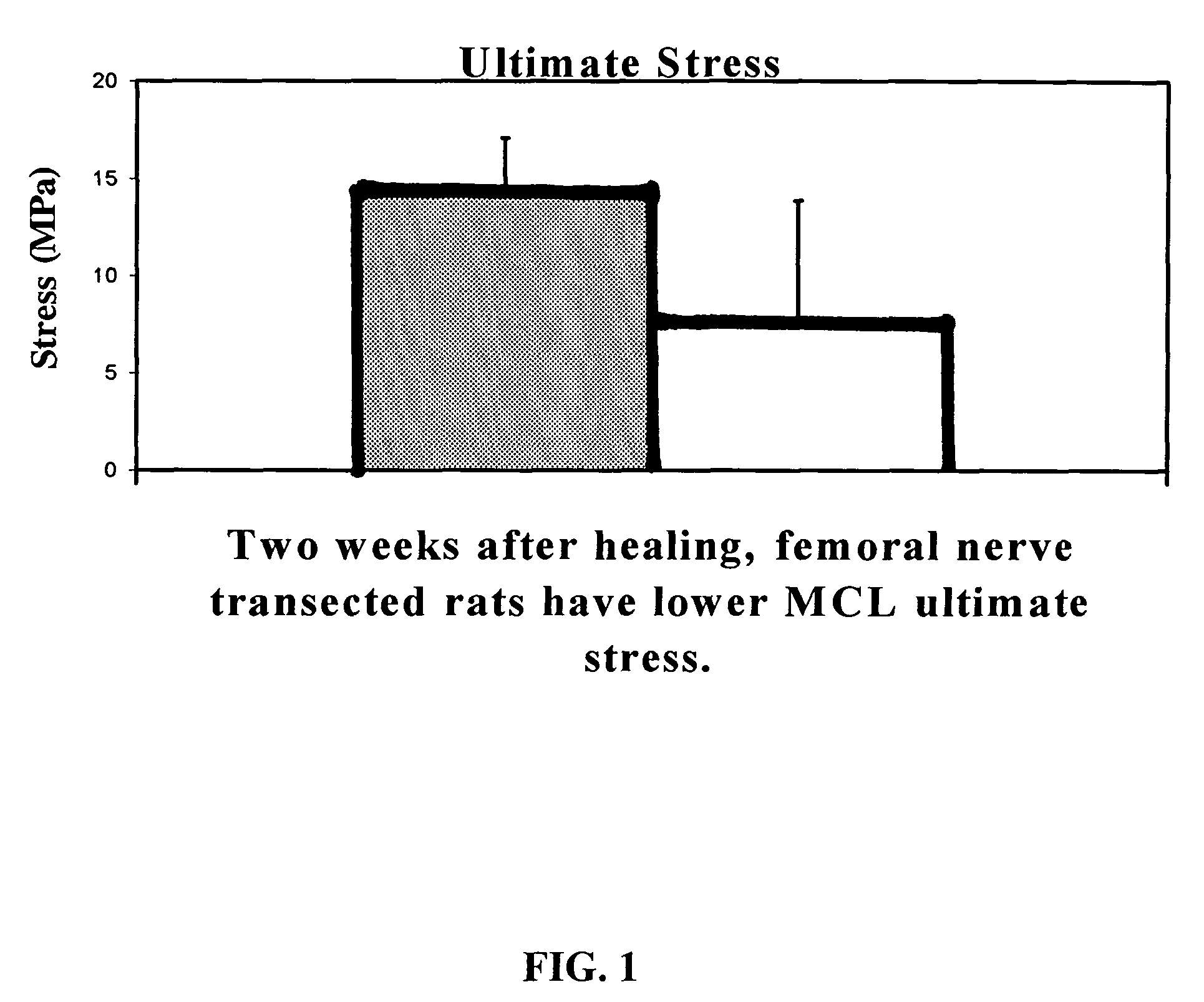
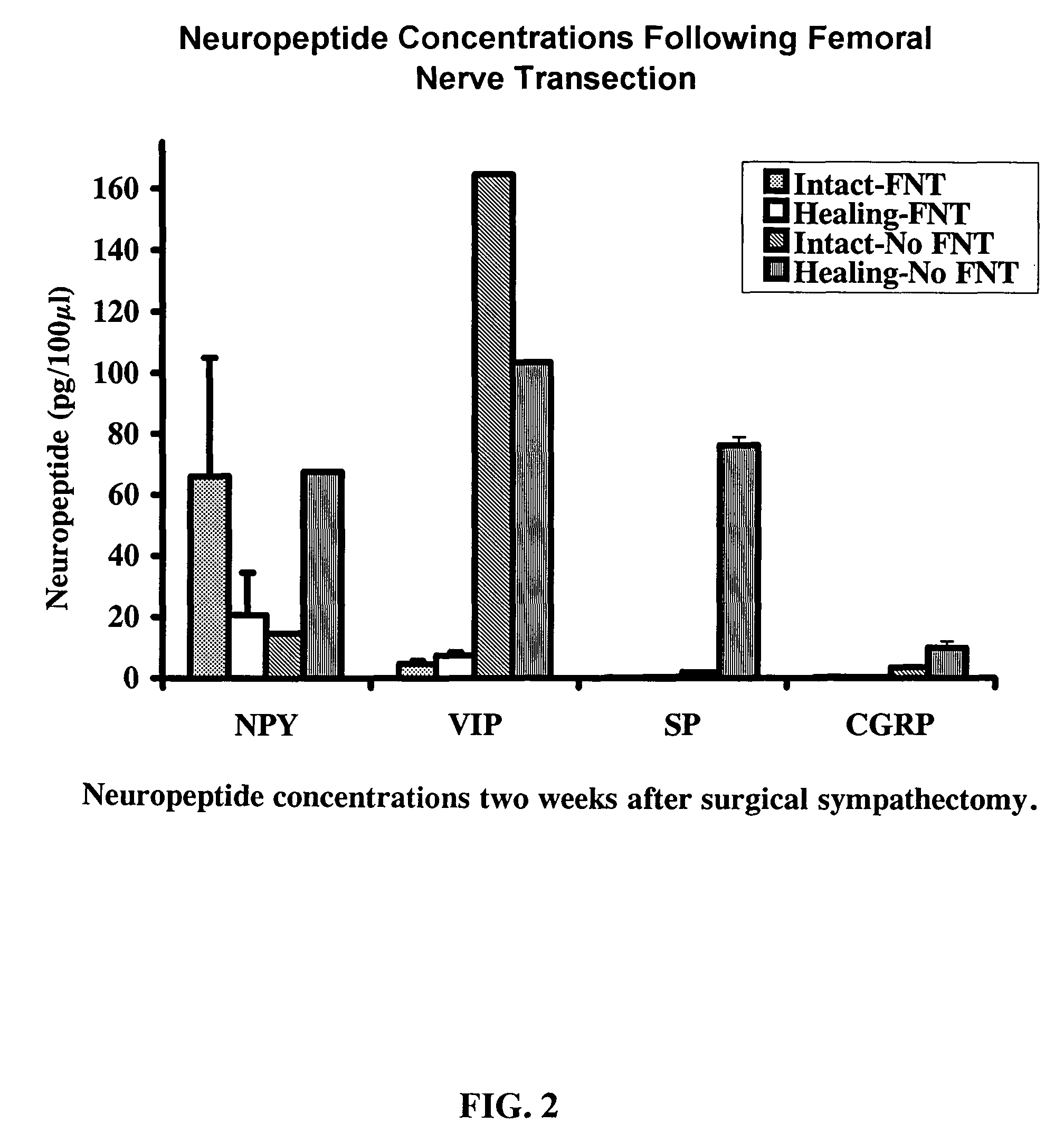
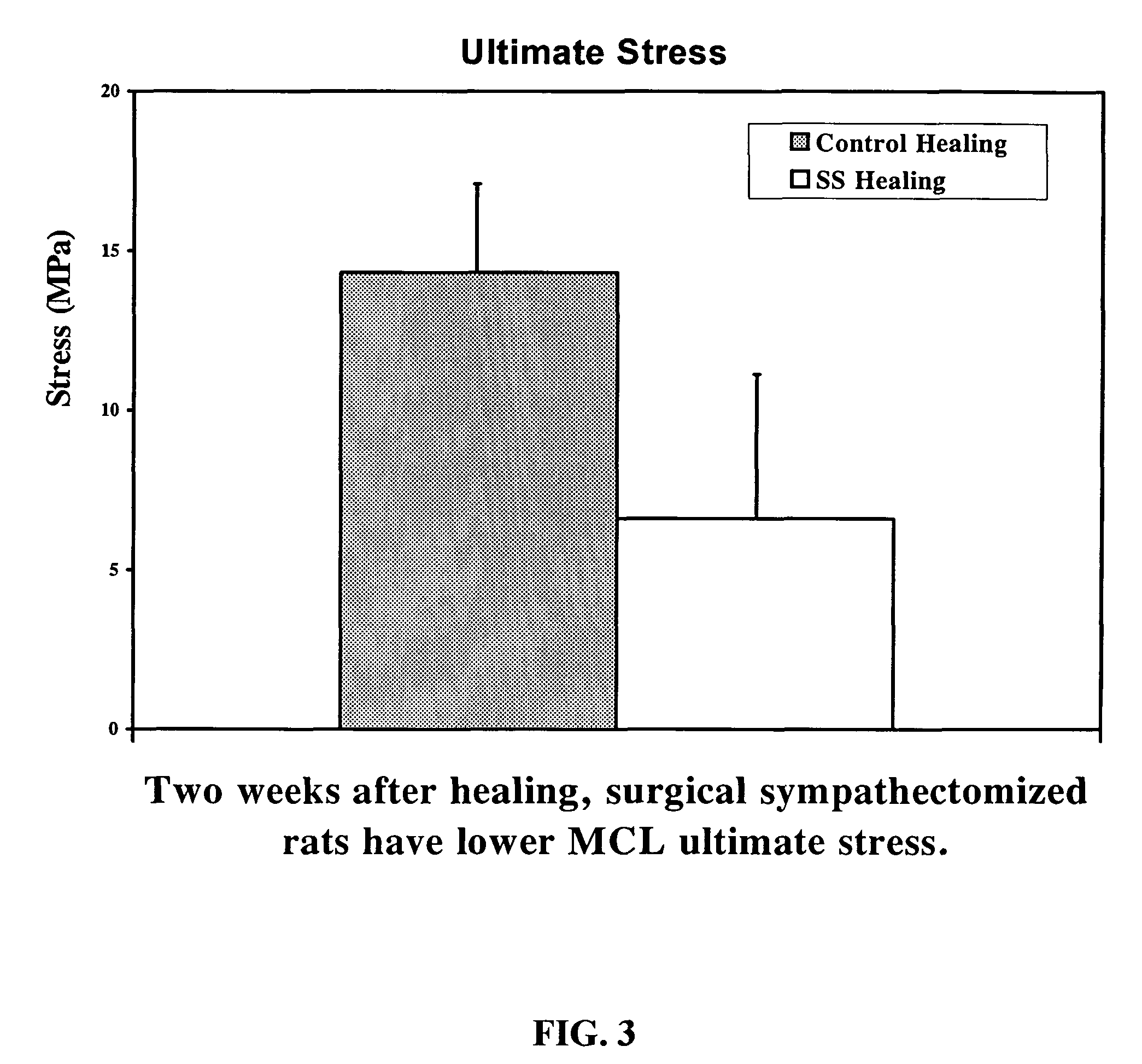
Use of neuropeptides for ligament, cartilage, and bone healing
Disclosed are a method and a corresponding pharmaceutical composition for treating damaged cartilage and subchondral bone. Neurogenic compounds in general and neuropeptides in particular have been found to be highly effective in stimulated repair of cartilage and bone damaged due to traumatic injury, ligament disease, and disuse. Preferred active ingredients for use in the method and corresponding pharmaceutical composition include calcitonin gene-related peptide (CGRP), cholecystokinin (CCK), dynorphin, enkephalin, galanin, neuropeptide Y (NPY), neurotensin, somatostatin, substance P (SP), thyrotropin-releasing hormone (TRH), vasoactive intestinal peptide (VIP).
Owner:WISCONSIN ALUMNI RES FOUND
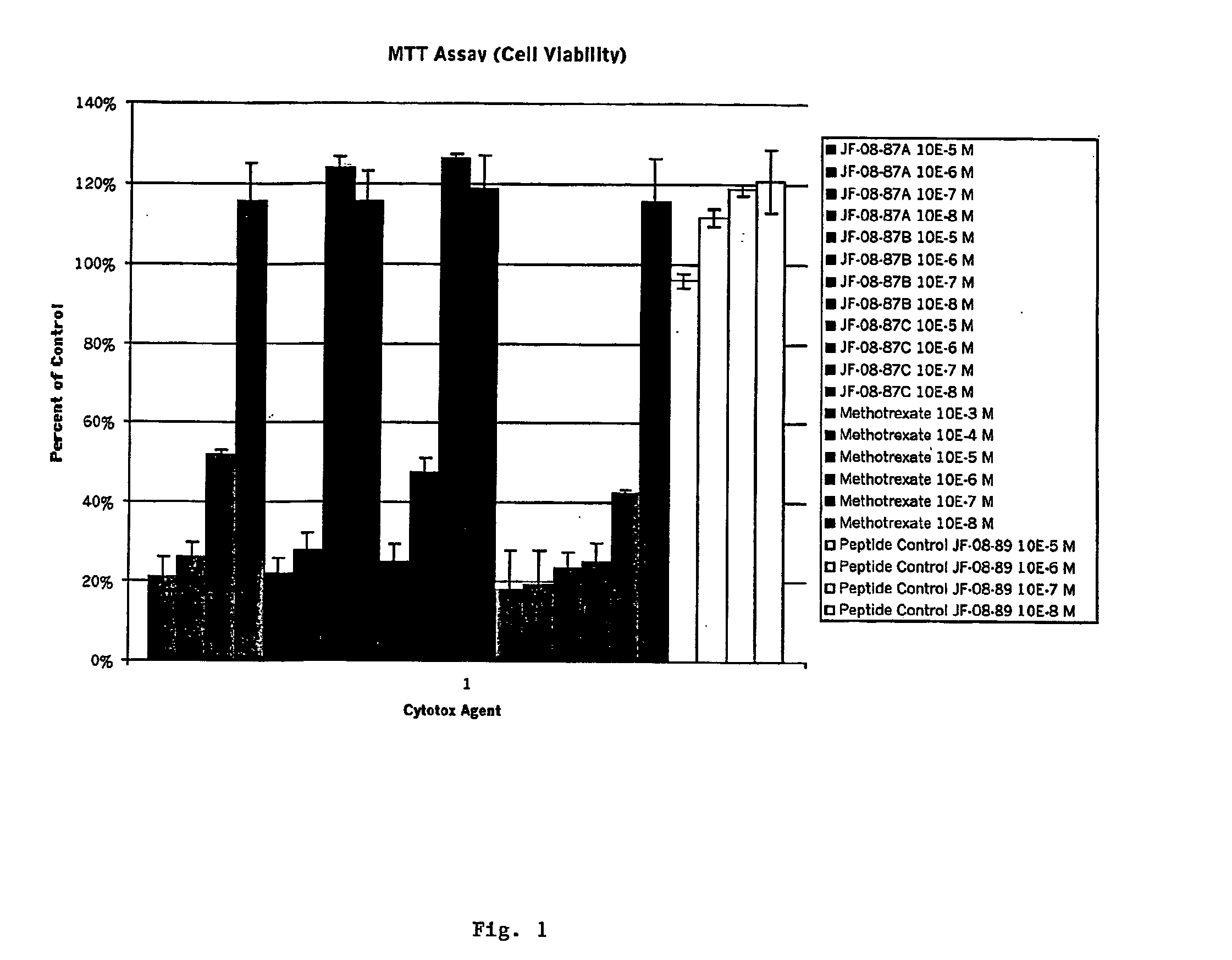
Diagnostic or therapeutic somatostatin or bombesin analog conjugates and uses thereof
InactiveUS20050070470A1Quick eliminationReduce drug toxicityOrganic active ingredientsSenses disorderDiseaseCytotoxicity
Disclosed are peptide agents and uses thereof that are analogs of biologically active peptides such as somatostatin and bombesin. The compounds of the invention have the general formula X-Y-Z-Q, where X is a cytotoxic agent, therapeutic agent, detectable label or chelating group, and Q is a biologically active peptide. In peptide agents of the invention Y is optionally a hydrophilic polymer or peptide, and Z is a linking peptide bonded to Q at the amino terminus of Q, having two, three, four, or five, amino acid residues selected to link X to Q, while retaining the biological activity of Q. Methods of using these peptide agents in the diagnosis and treatment of diseases are also disclosed.
Owner:TULANE EDUCATIONAL FUND
Method of inhibiting fibrosis with a somatostatin agonist
The present invention relates to a method of inhibiting fibrosis in a patient. The method comprises administering a therapeutically effective amount of a somatostatin, a somatostatin agonist or apharmaceutically acceptable salt thereof to said patient.
Owner:IPSEN PHARMA SAS
Somatostatin antagonists and agonists that act at the sst subtype 2 receptor
InactiveUS20020128206A1Ease of detectabilityEasy to prepareDipeptide ingredientsMetabolism disorderArylMammal
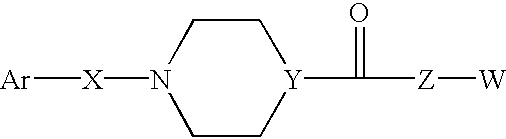
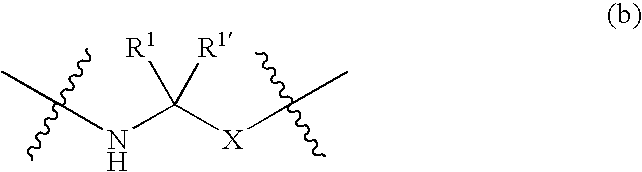
Compounds according to the formula: 1 and pharmaceutically acceptable salts, solvates or hydrates thereof, wherein group Ar is optionally substituted (C.sub.6-C.sub.10)aryl or (C.sub.1-C.sub.9)heteroaryl; X is a direct link, --CH.sub.2 --, --SO.sub.2 --, --CO--, --CHR.sup.1-- where R.sup.1 is(C.sub.1-C.sub.6) alkyl, or --CR.sup.1'R.sup.1"-where both R.sup.1' and R.sup.1" are, independently, (C.sub.1-C.sub.6)alkyl; Y is N or CH; and Z and W are as herein defined, and pharmaceutical compositions thereof, and methods useful to facilitate secretion of growth hormone(GH) in mammals.
Owner:HAY BRUCE A +2
Compounds and method for the prevention and treatment of diabetic retinopathy
InactiveUS6440933B1Reduce deliveryBlock deliverySenses disorderSomatostatinsDiabetic retinopathyDipeptide
The invention provides peptide derivatives designed to deliver peptides having growth factor inhibitory activity, especially somatostatin analogs, to the retina by sequential metabolism. The peptide derivatives, which comprise a dihydropyridine<CUSTOM-CHARACTER FILE="US06440933-20020827-P00900.TIF" ALT="custom character" HE="20" WI="20" ID="CUSTOM-CHARACTER-00001" / >pyridinium salt-type redox targetor moiety, a bulky lipophilic function and an amino acid / dipeptide / tripeptide spacer, are used in the prevention and treatment of diabetic retinopathy.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
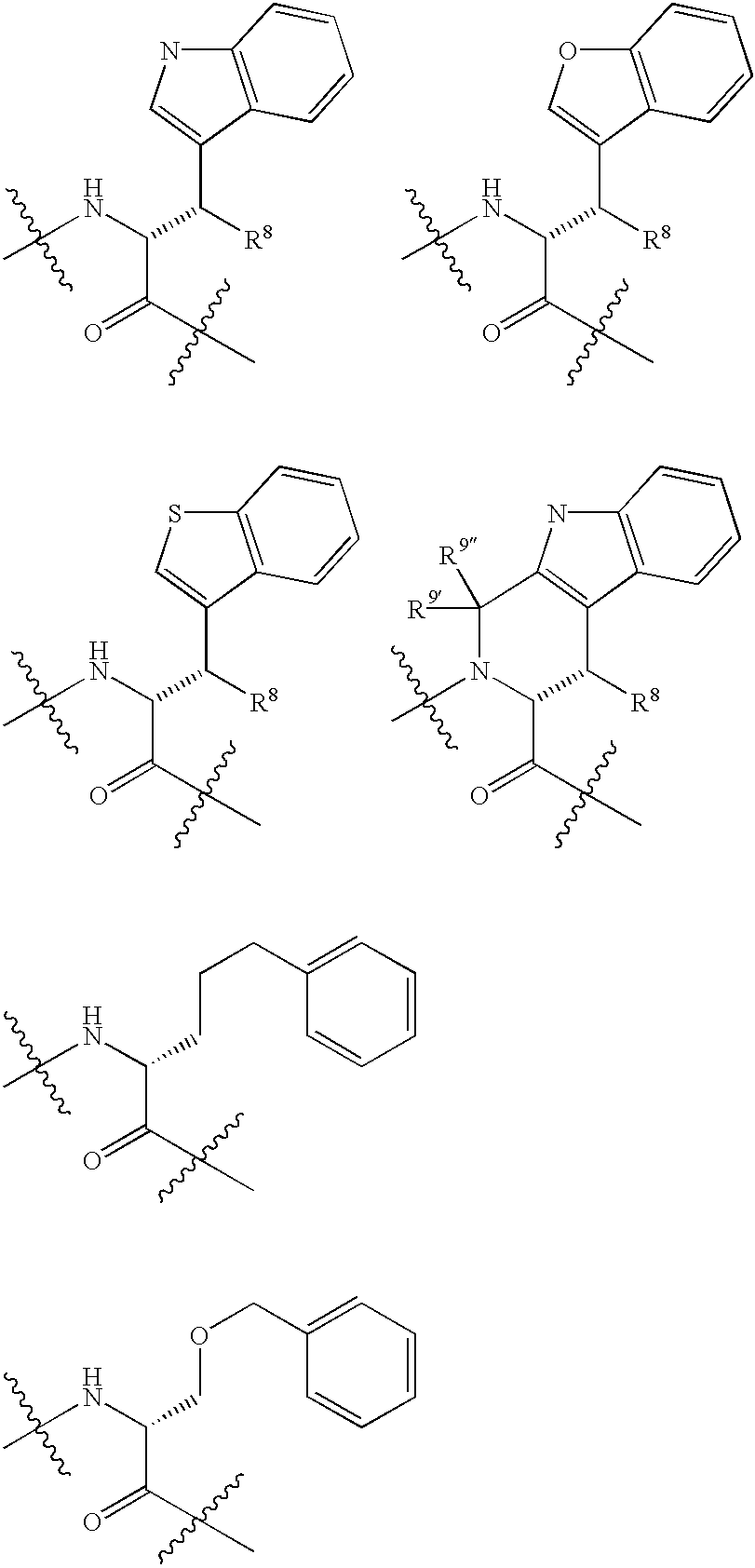
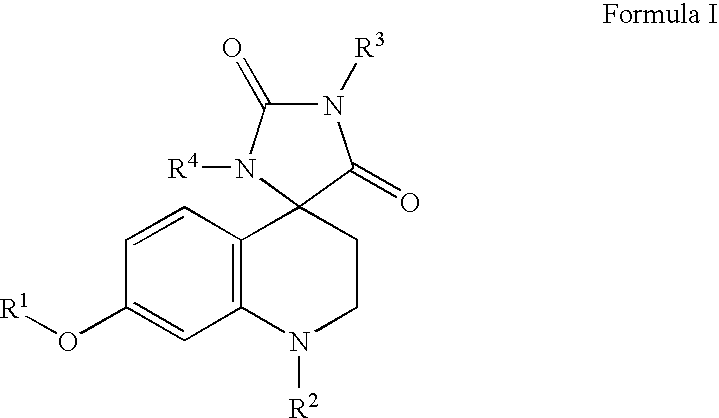
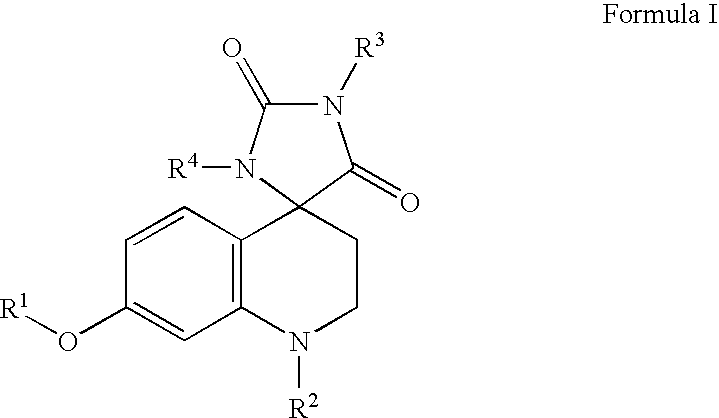
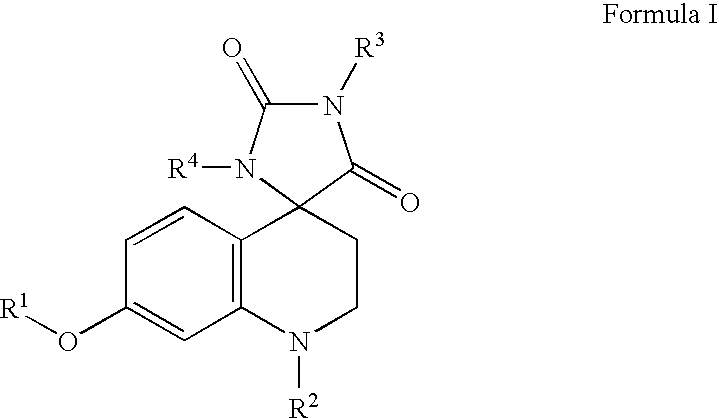
Somatostatin analogue compounds
Compounds having somatostatin activity of the following Formula I, wherein,R1 is aryl, substituted-aryl, and aryl-(lower-alkyl)-;R2 is lower alkyl, amino substituted lower alkyl, -carboxy-(lower-alkyl), -carbamic acid-(lower-alkyl) and -carboxy-(lower-alkyl)-aryl; andR3 and R4 are independently, lower-alkyl, aryl, substituted-aryl, (substituted-aryl)-(lower-alkyl)-, heteroaryl, (heteroaryl)-(lower-alkyl)-, substituted-heteroaryl, (substituted heteroaryl)-(lower-alkyl)-, heterocyclic, heterocyclic-(lower-alkyl)-, substituted-heterocyclic, (substituted-heterocylic)-(lower alkyl)-, -carboxy-(lower-alkyl), and -carboxy-(lower-alkyl)-aryl; or a pharmaceutically acceptable, ester, ether, or salt thereof; methods for their use; and preparation.
Owner:CHEMBRIDGE CORP
Receptor(SSTR4)-selective somatostatin analogs
Analogs of SRIF which are selective for SSTR4 in contrast to the other cloned SRIF receptors are useful in determining tissue and cellular expression of the receptor SSTR4 and its biological role in regulating tumor growth. SRIF analog peptides, such as des-AA1,2,4,5,12,13[Ala7]-SRIF; des-AA1,2,4,5,12,13[Aph7]-SRIF, des-AA1,2,4,5,12,13[Aph7]Cbm-SRIF; des-AA1,2,4,5,12,13[Tyr2,Ala7]-Cbm-SRIF, and des-AA1,2,4,5,12,13[Tyr7,CβMe-L-2Nal8]-SRIF, and counterparts incorporating D-Cys3 and / or D-Trp8 and / or Ala11, bind with high affinity to the cloned human receptor SSTR4 and activate the receptor, but they do not bind with significant affinity to human SSTR1, SSTR2, SSTR3 or SSTR5. By incorporating an iodinated tyrosine in position-2 in these SSTR4-selective SRIF analogs, a labeled compound useful in drug-screening methods is provided. Alternatively, for use in therapy, cytotoxins or highly radioactive elements can be N-terminally coupled or complexed thereto.
Owner:SALK INST FOR BIOLOGICAL STUDIES
Somatostatin agonists
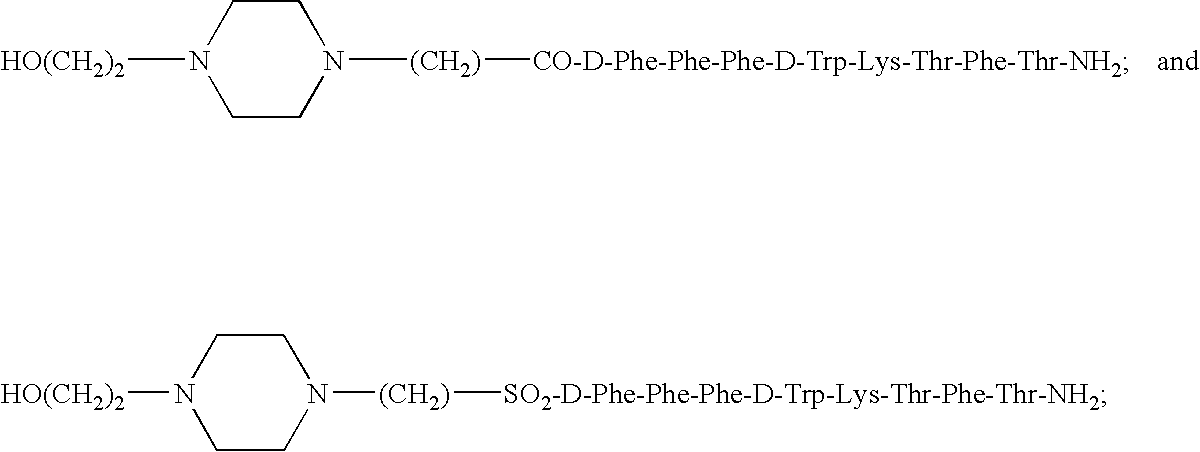
Claimed are a series of somatostatin agonists and uses thereof, according to formula (I),A1-cyclo[Cys-A2-D-Trp-A3-A4-Cys]-A5-Y1, (I)wherein A1, A2, A3, A4, A5 and Y1 are as defined in specification provided that the amine nitrogen of at least one peptide bond is substituted with a methyl group or pharmaceutically acceptable salts thereof.
Owner:TULANE EDUCATIONAL FUND THE ADMINISTRATORS +1
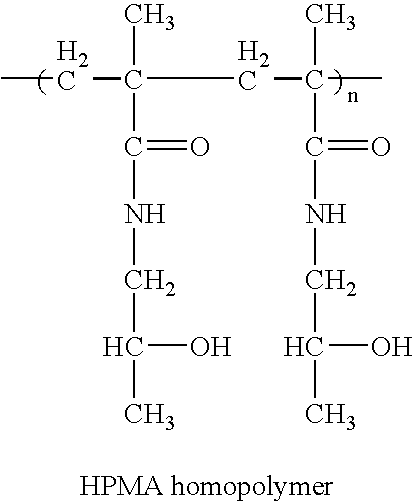
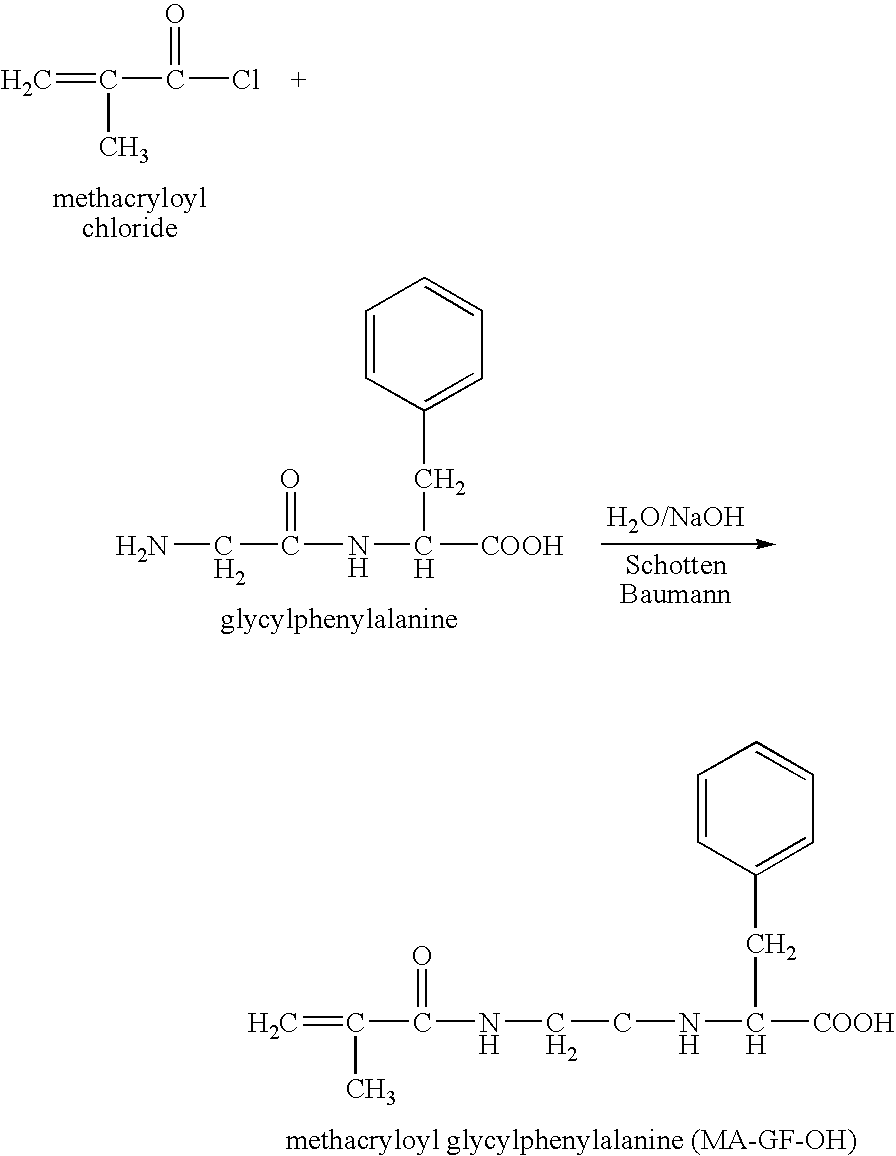
Hpma-polyamine conjugates and uses therefore
InactiveUS20060014695A1Prevent proliferationStimulate immune responsePeptide/protein ingredientsGenetic material ingredientsSomatostatin analogTreatment effect
The inventions provide compositions and methods for nucleic acid delivery comprising IIPMA conjugated to a polyamine. These compositions have the benefit of the steric hindrance of HPMA and the nucleic acid binding capability of a polyamine. Useful polyamines for this purpose include spermine, spermidine and their analogues, and DFMO. These polyamines have the ability not only to bind nucleic acids, but also have anti-cancer effects themselves. The compounds provided can also include ligand binding domains, such as vascular endothelial growth factors, somatostatin and somatostatin analogs, transferring, melanotropin, ApoE and ApoE peptides, von Willebrand's factor and von Willebrand's factor peptides, adenoviral fiber protein and adenoviral fiber protein peptides, PD 1 and PD 1 peptides, EGF and EGF peptides, RGD peptides, CCK peptides, antibody and antibody fragments, folate, pyridoxyl and sialyl-LewisX and chemical analogs. Methods for using these compositions to achieve a therapeutic effect, including for vaccination, are also provided.
Owner:UNIV OF MARYLAND
Compound and method of treating neurogenic conditions using non-steroidal anti-inflammatory drug complexes
InactiveUS7151084B2Nervous disorderPeptide/protein ingredientsMembrane TransportersPancreatic hormone
A complex is provided for the treatment of neurogenic conditions having the formula:where R1 isM is a metal ion Ca(II), Mg(II), Cu(II) or Ni(II); n is an integer 1 or 2; R is BBB peptide, transferrin, membrane transporter peptide, TAT peptide, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptidegluconate, L-lactate, L-leucine, L-tryptophan, and L-glutamate; and R is coupled to M through a carboxylate moiety. Magnesium(II) represents the preferred metal ion as magnesium is known to have neuroprotective effects. The metal ion is in part chelated by a non-steroidal anti-inflammatory drug that does not inhibit platelet activity and includes salicylate and ibuprofenate. The complex also includes a ligand operative in transport across the blood brain barrier. A process for making an inventive complex includes the stoichiometric addition of ligands containing carboxylate groups to a solution of the metal ion. In instances where the metal ion is magnesium(II), a stoichiometric ratio of 1:1:1 is found between the non-steroidal anti-inflammatory ligand:magnesium(II):transporter ligand.
Owner:MILLER LANDON C G
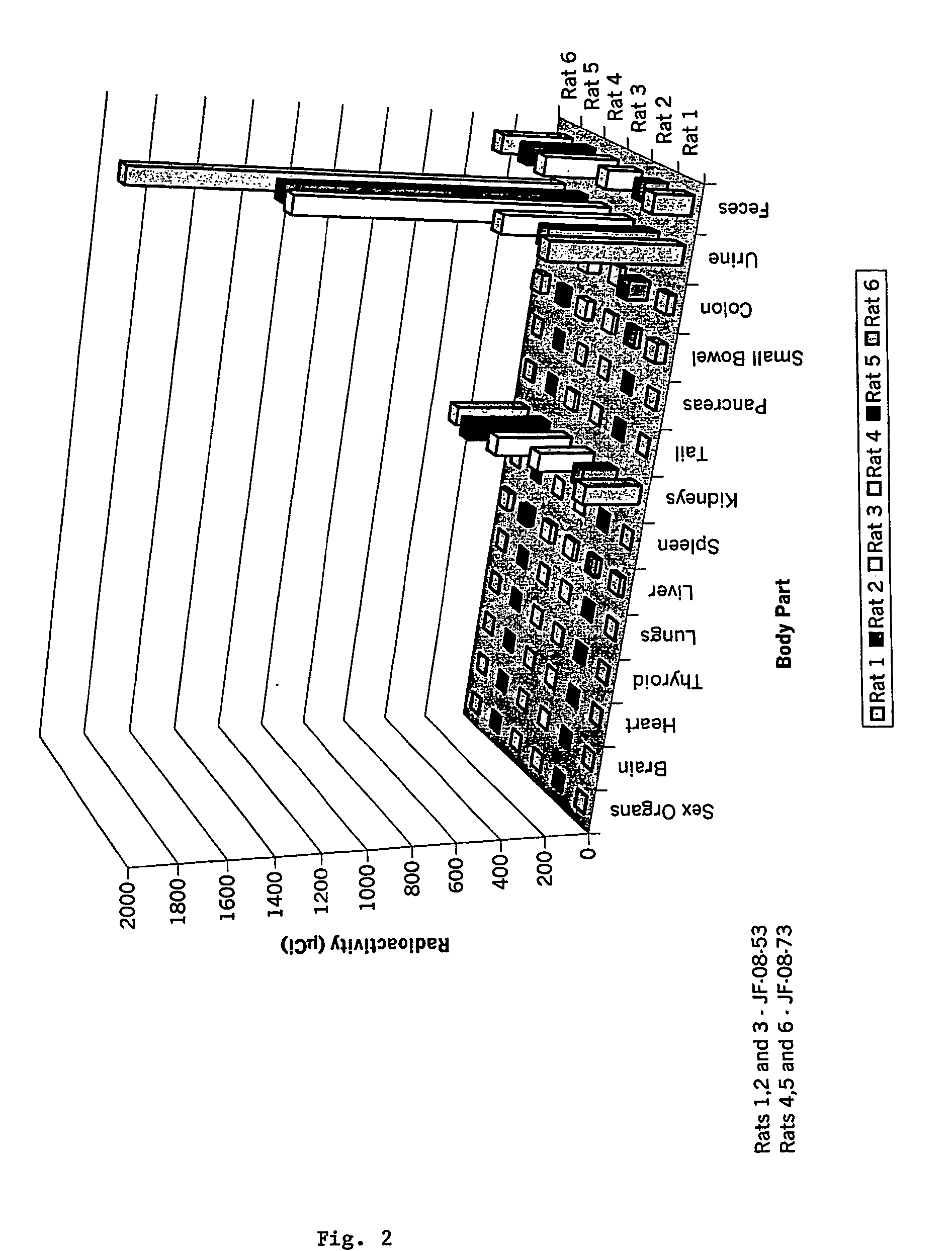
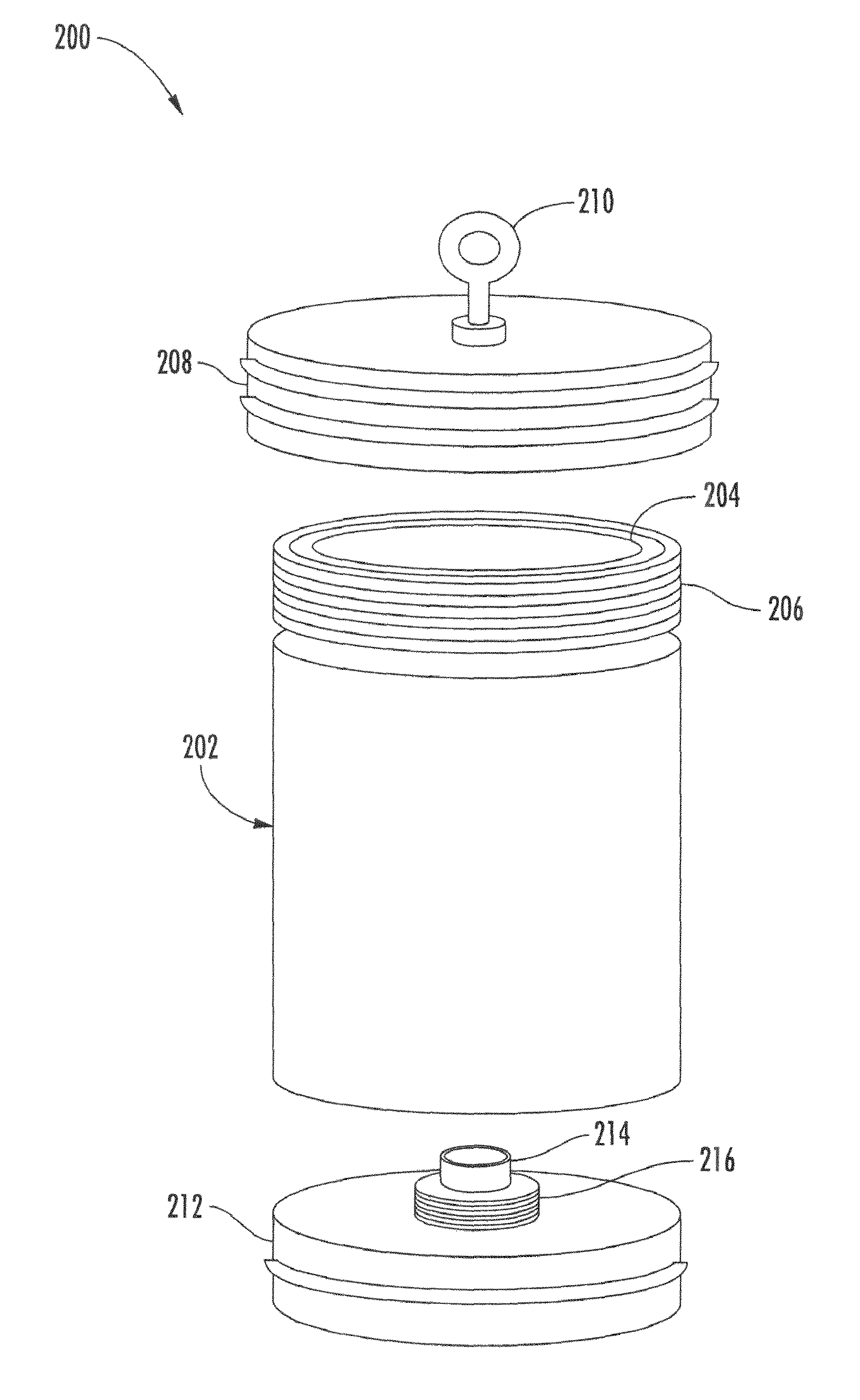
Radiolabeled treatment infusion system, apparatus, and methods of using the same
InactiveUS20110124948A1Useful applicationReduce radiation exposureIsotope delivery systemsRadiation applicationsRadioactive agentRadiation exposure
Described herein are methods and devices for infusion of a radioactive compound, such as yttrium-90 radiolabeled somatostatin peptide or analog. A radiation shield defining a shielded cavity suitable for storing a radioactive substance includes a first aperture providing external access to the shielded cavity and a second aperture suitable for transferring a dosage vial into and out of the shielded cavity. A removable shielded plug and panel are adapted to shield respective apertures of the radiation shield. At least one dose of a radiolabeled compound stored in a vial in the radiation shield is delivered through a fluid communication channel at a rate of about 500 mL / hour. The fluid communication channel is washed after delivery, such that the process substantially reduces radiation exposure during infusion of the radiolabeled compound into a patient.
Owner:MOLECULAR INSIGHT PHARMA
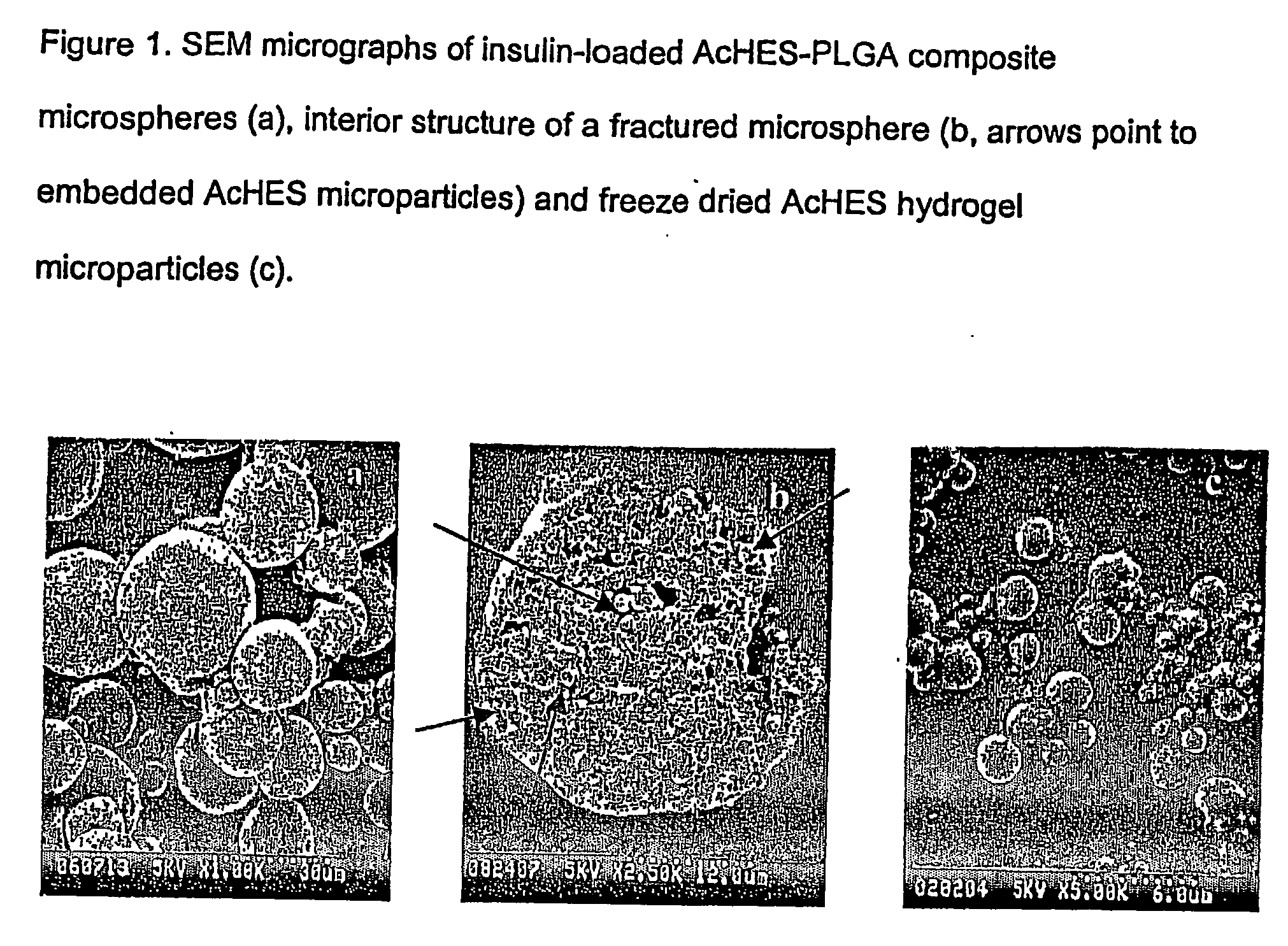
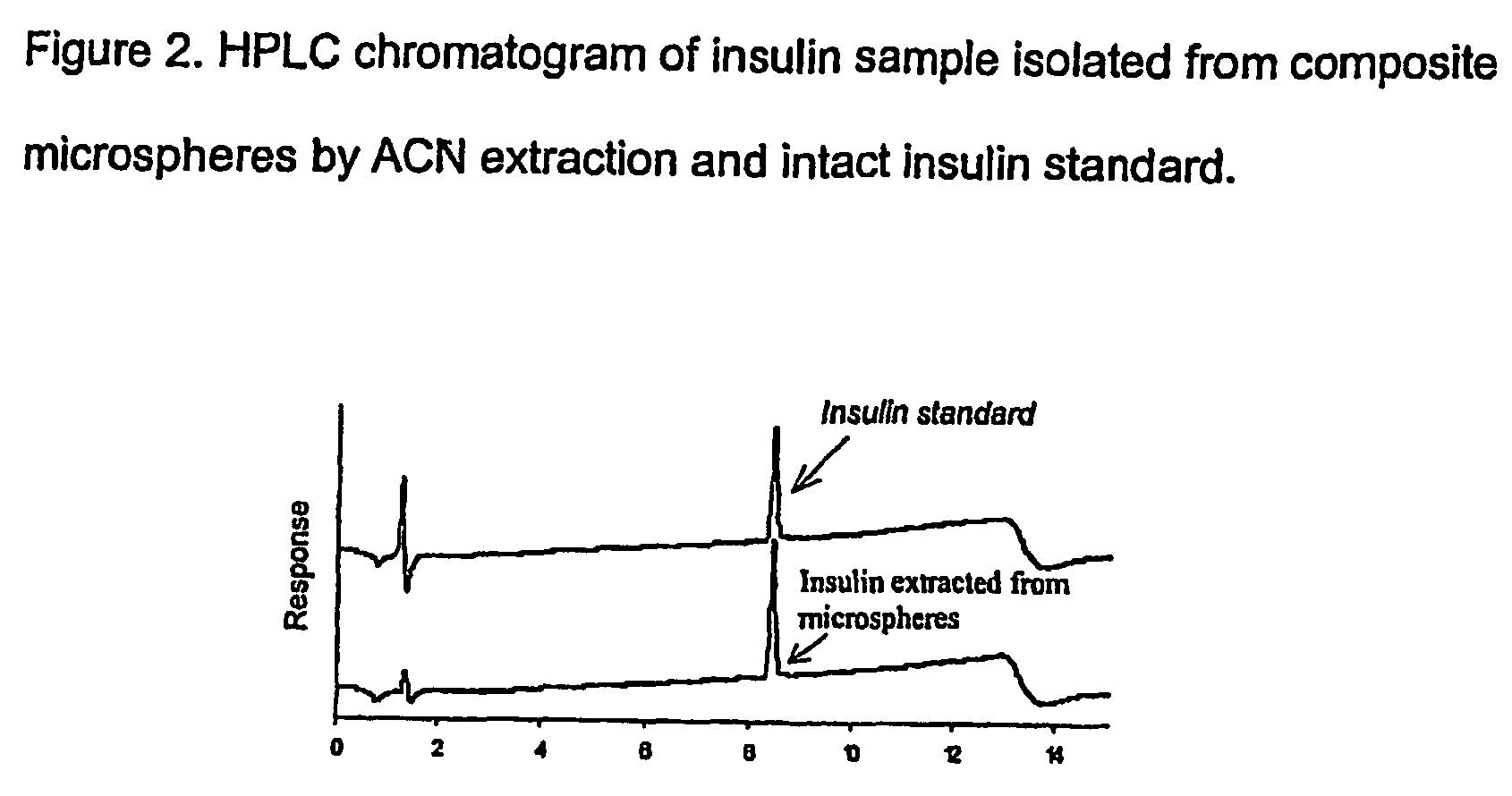
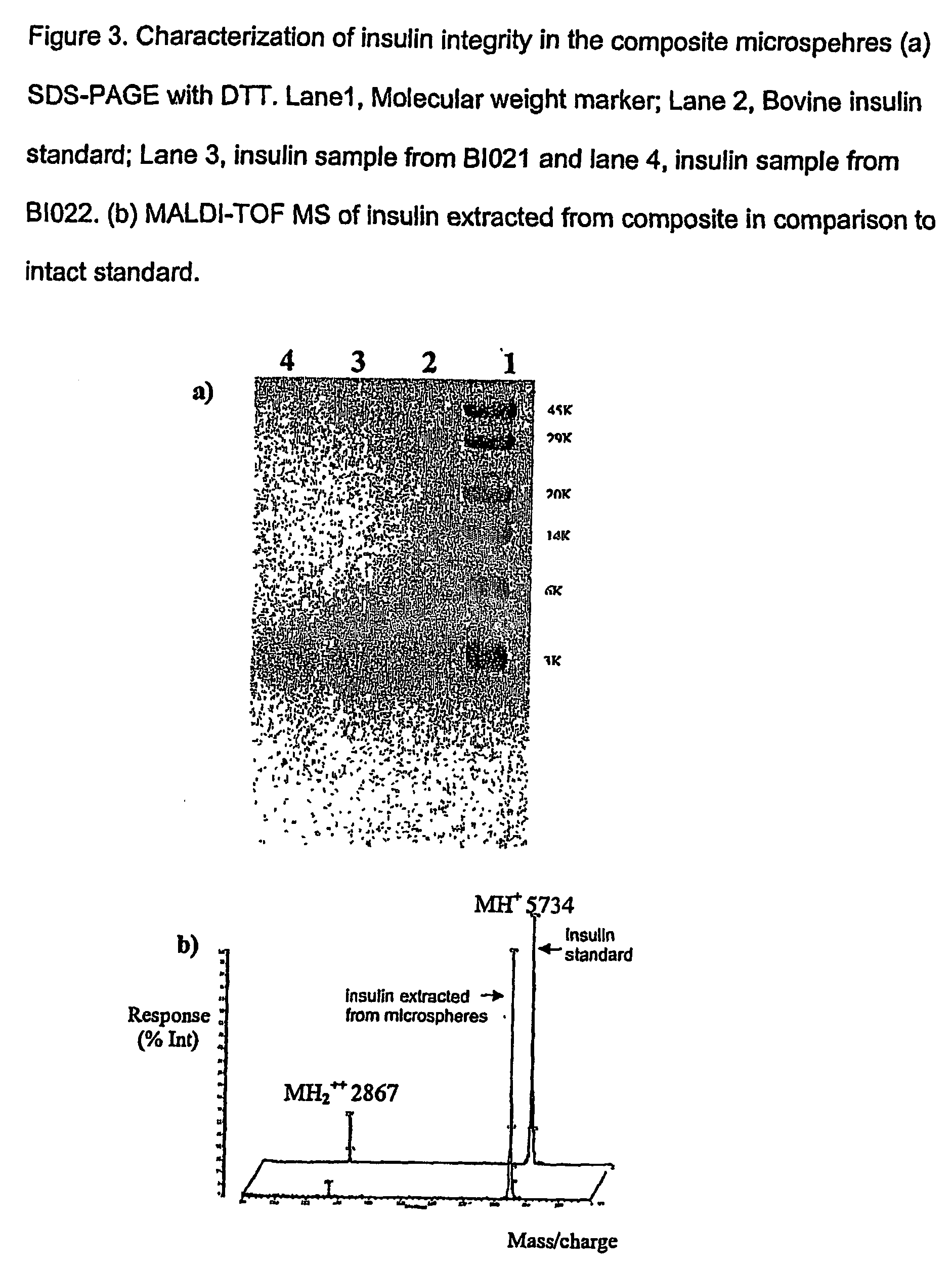
(Poly(acryloyl-hydroxyethyl starch)-plga composition microspheres
The present invention relates to a composite microsphere system comprising poly(D,L-lactide-co-glycolide) (PLGA), poly(acryloyl hydroxyethyl starch) (AcHES), and a pharmaceutically effective amount of a biologically active compound. The active compound may be, for example, an insulin, an interferon, a luteinizing hormone-releasing hormone (LHRH) analog, a somatostatin and / or derivatives thereof, a calicitonin, a parathyroid hormone (PTH), a bone morphogenic protein (BMP), an erythropoietin (EPO), an epidermal growth factor (EGF) or a growth hormone. This invention also relates to methods of using the composite microspheres, and methods of preparing same.
Owner:UNIV OF KENTUCKY RES FOUND
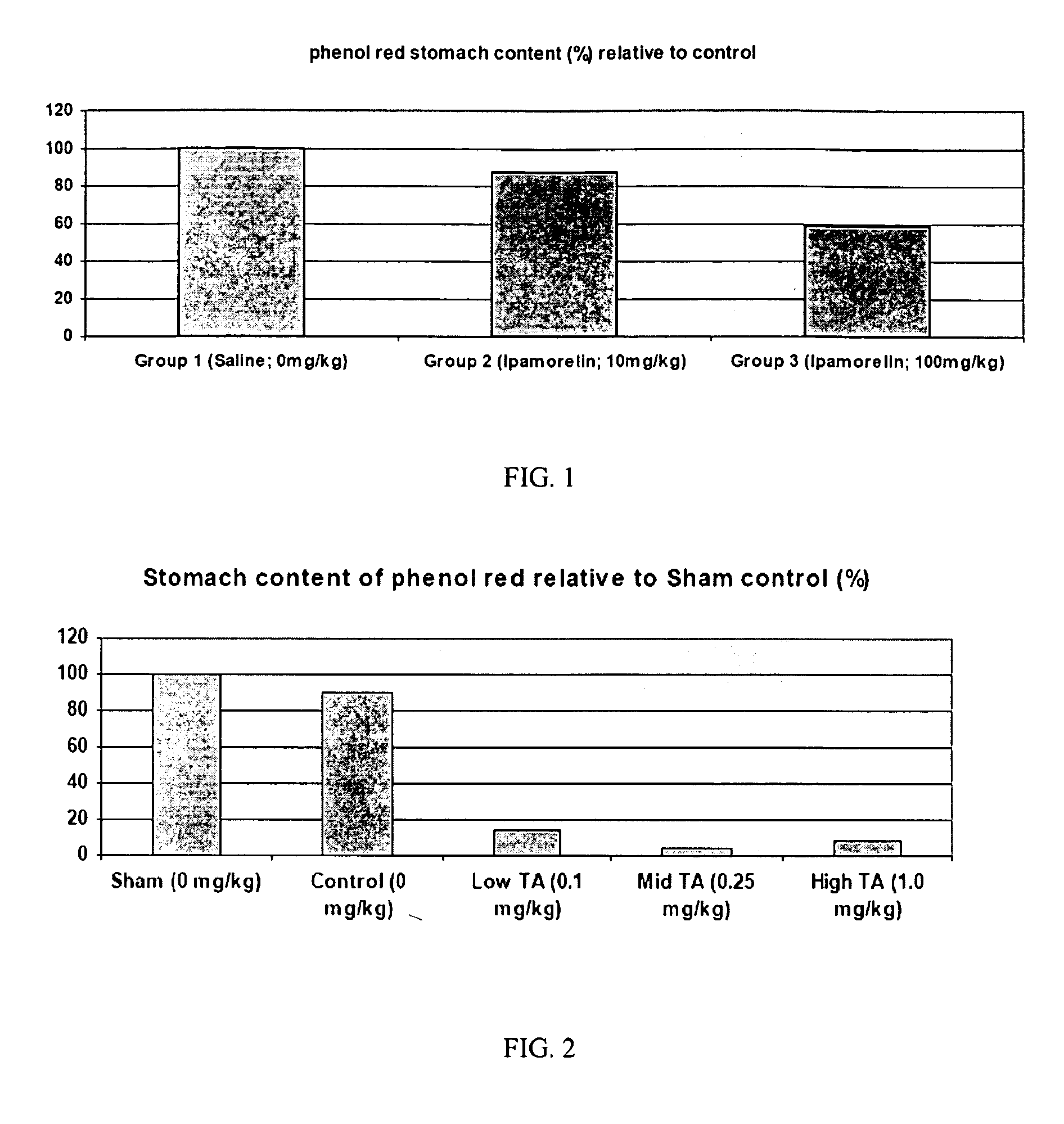
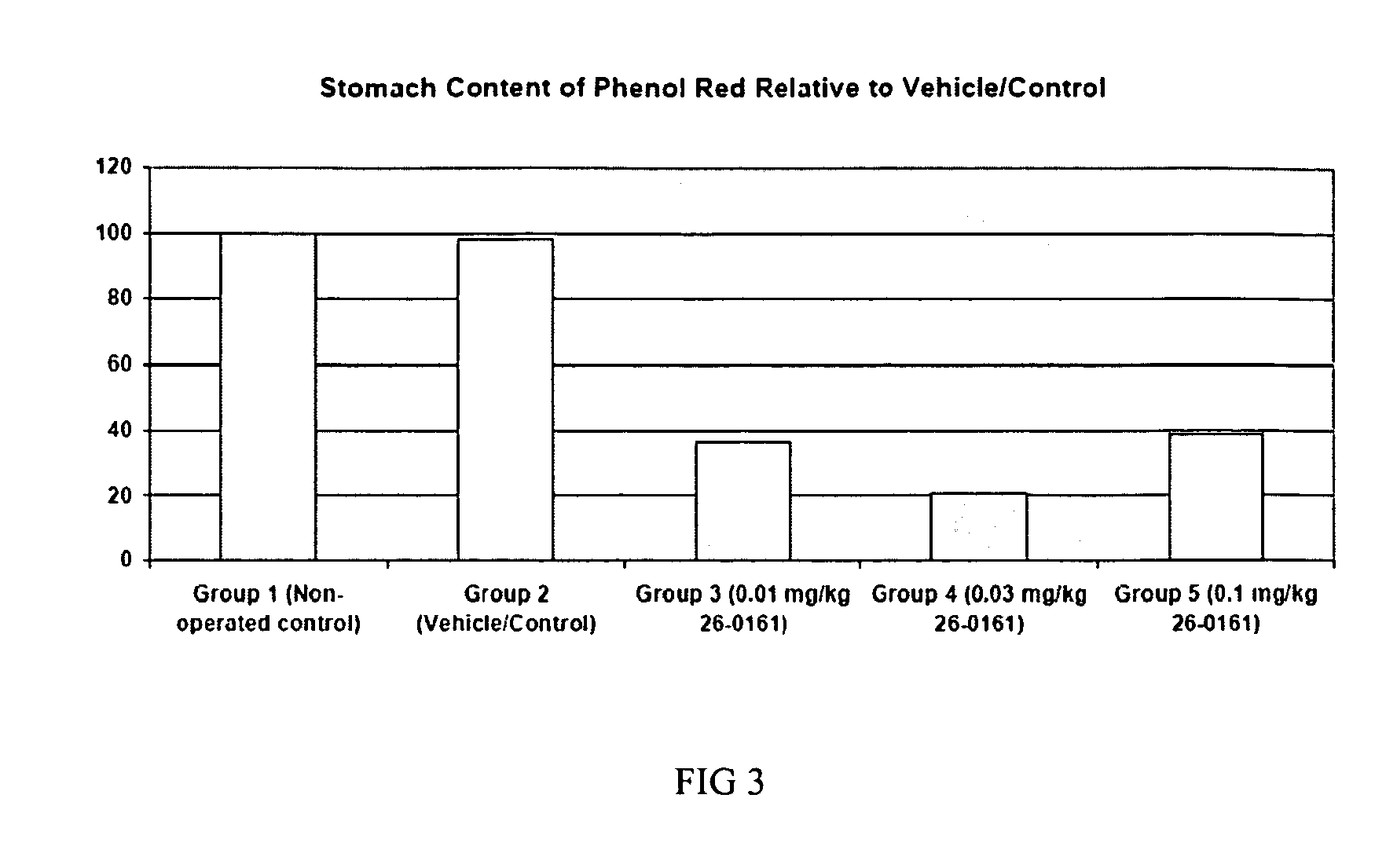
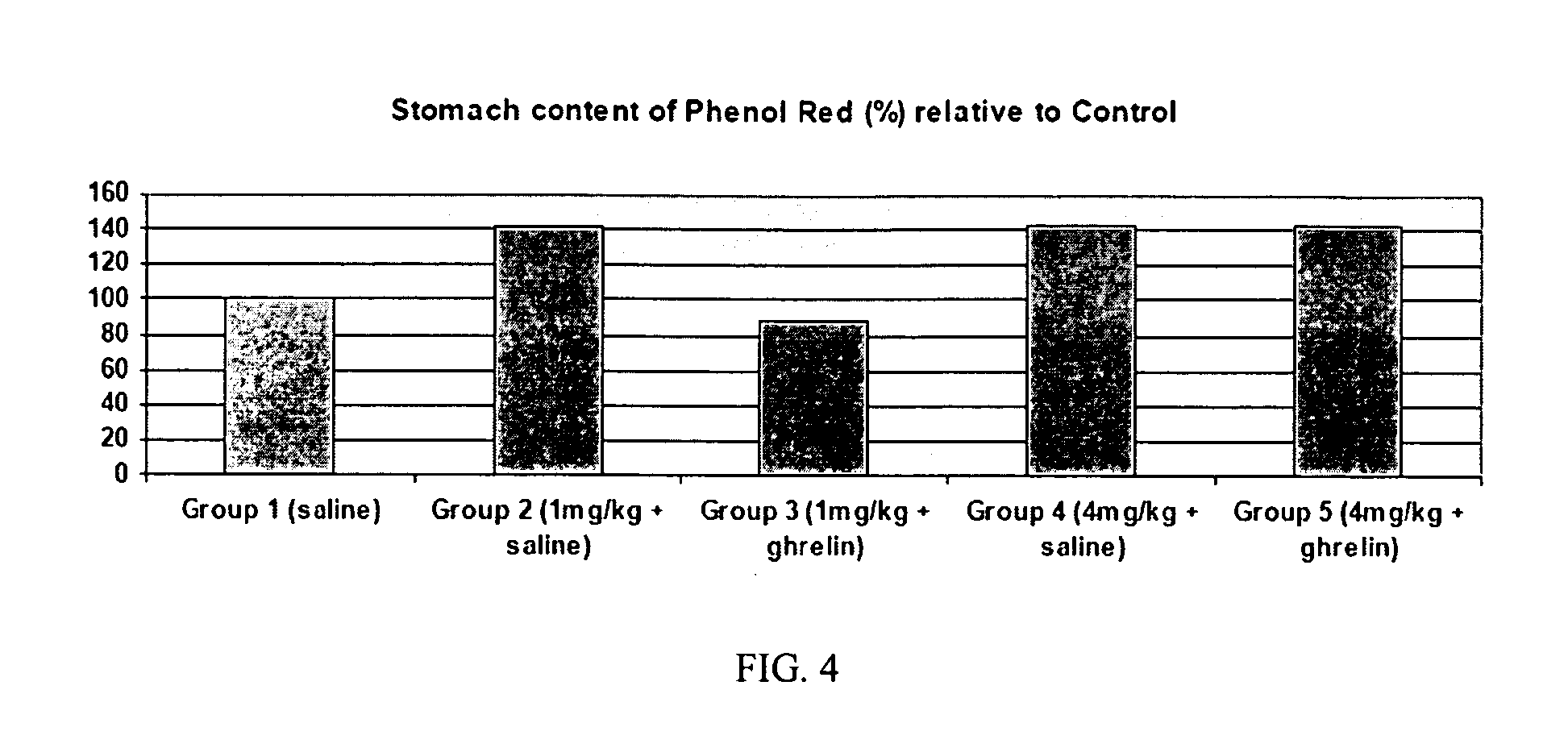
Method of stimulating the motility of the gastrointestinal system using ipamorelin
The present invention provides a method of stimulating the motility of the gastrointestinal system in a subject in need thereof, wherein the subject suffers from maladies (i.e., disorders, diseases, conditions, or drug- or surgery-induced dysfunction) of the gastrointestinal system, by administering to the subject a ghrelin mimetic, or pharmaceutically acceptable salt thereof. The invention also provides a method of treating a gastrointestinal malady by co-administering a ghrelin mimetic with a laxative, a H2 receptor antagonist, a serotonin receptor agonist, pure or mixed, an antacid, an opioid antagonist, a proton pump inhibitor, a motilin receptor agonist, dopamine antagonist, a cholinergic agonist, a cholinesterase inhibitor, somatostatin, octreotide, or any combination thereof.
Owner:HELSINN THERAPEUTICS (U S) INC
Use of neuropeptides for ligament healing
InactiveUS7776815B2High strengthDistinct utilityTachykinin ingredientsImmunoglobulinsDiseaseThyrotropin-releasing hormone
Disclosed are a method and a corresponding pharmaceutical composition for treating damaged ligaments. Neurogenic compounds in general and neuropeptides in particular have been found to be highly effective in stimulated repair of ligaments damaged due to traumatic injury, ligament disease, and disuse. Preferred active ingredients for use in the method and corresponding pharmaceutical composition include calcitonin gene-related peptide (CGRP), cholecystokinin (CCK), dynorphin, enkephalin, galanin, neuropeptide Y (NPY), neurotensin, somatostatin, substance P (SP), thyrotropin-releasing hormone (TRH), vasoactive intestinal peptide (VIP).
Owner:WISCONSIN ALUMNI RES FOUND
Piracetam and piracetam analog conjugate and a pharmaceutical composition for treatment of neuronal disorders
A compound of the formulawhere R1 is H, C1–C4 alkyl and OH; R2 in is H, C1–C4 alkyl and OH; R3 is H and C1–C4 alkyl; R4 is H and C1–C4 alkyl; n is an integer between 0 and 2 inclusive; R5 is a nullity, NHR7C(O)—, C6H4—, C6H4—O—; R7 is C2–C6 alkyl; and R6 is a moiety capable of crossing the blood brain barrier and is as a free compound serotonin, dopamine, blood brain barrier (BBB) peptide, membrane translocating peptide, TAT peptides, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptidegluconate, transferrin, glucosylamine, amino saccharin, saccharin ester, lactylamine, leucine, tryptophan, amino glutamate and amino cholines.
Owner:MILLER LANDON C G
Enhanced nasal composition of active peptide
InactiveUS20090035260A1Prevent oxidationImprove stabilityPeptide/protein ingredientsMetabolism disorderNasal cavityGoserelin
A pharmaceutical composition has a therapeutically effective amount of at least one of: a pharmaceutically active nasal peptide, its pharmaceutically acceptable salt and its peptidic fragment. The composition also contains an absorbefacient effective amount of THAM in a pharmaceutically acceptable, aqueous liquid diluent or carrier. The composition is provided in a convenient form for nasal administration. In one embodiment, the peptidic fragment may be selected physiologically active lymphokines and monokines, peptidic enzymes, proteic vaccines, peptidic toxoids and personalized proteins derived from genoma. In another embodiment, the peptidic fragment may be selected from the peptide hormones and hormone antagonists buserelin, desmopressin, vasopressin, angiotensin, felypressin, octreotide, somatropin, thyrotropin (TSH), somatostatin, gosereline, thryptorelin and insulin selected from the group consisting of cow and pig, synthetic and recombinant.
Owner:THERAPICON SRL
Somatostatin-dopamine chimeric analogs
The invention features somatostatin-dopamine chimeric analogs and methods relating to their therapeutic use for the treatment of neoplasia, acromegaly, and other conditions.
Owner:IPSEN PHARMA SAS
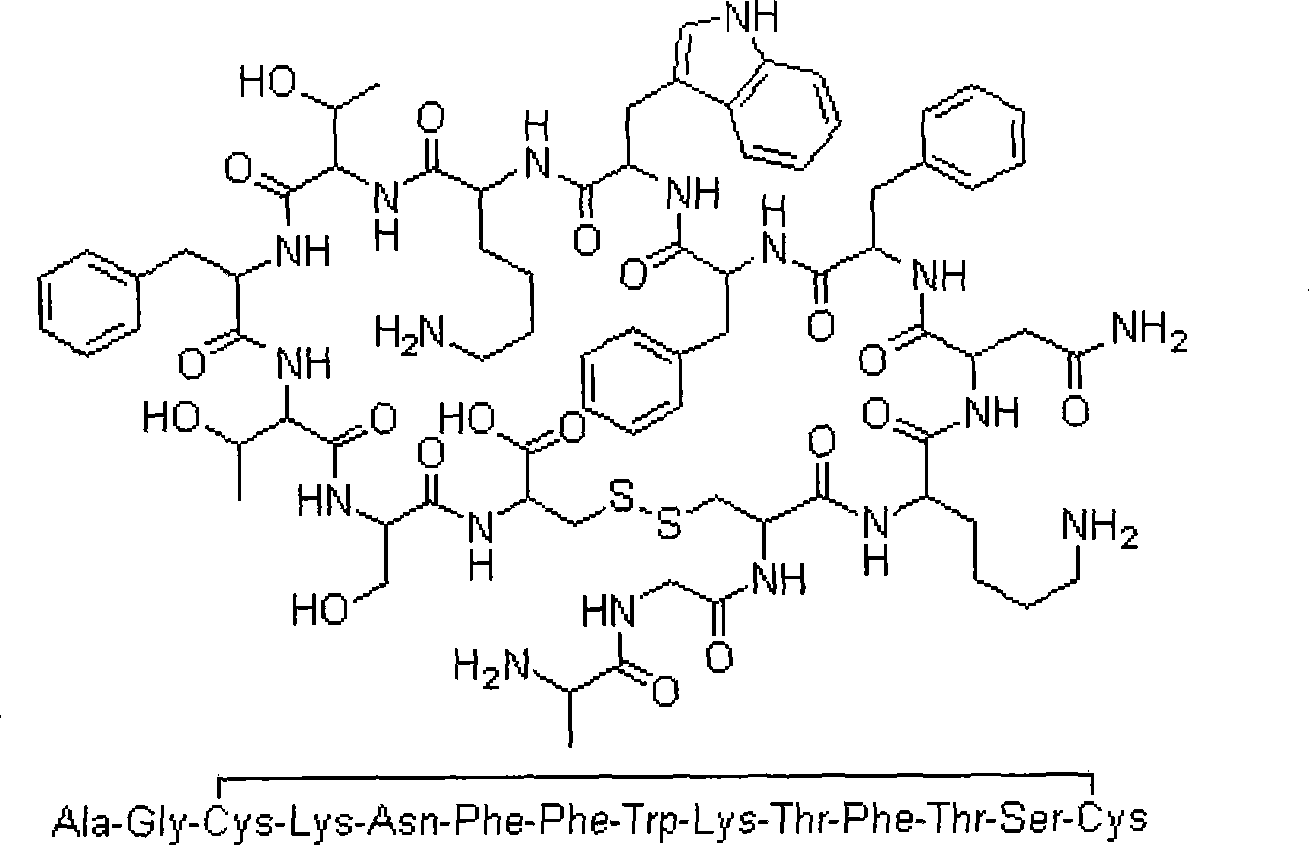
Synthetic method for somatostatin
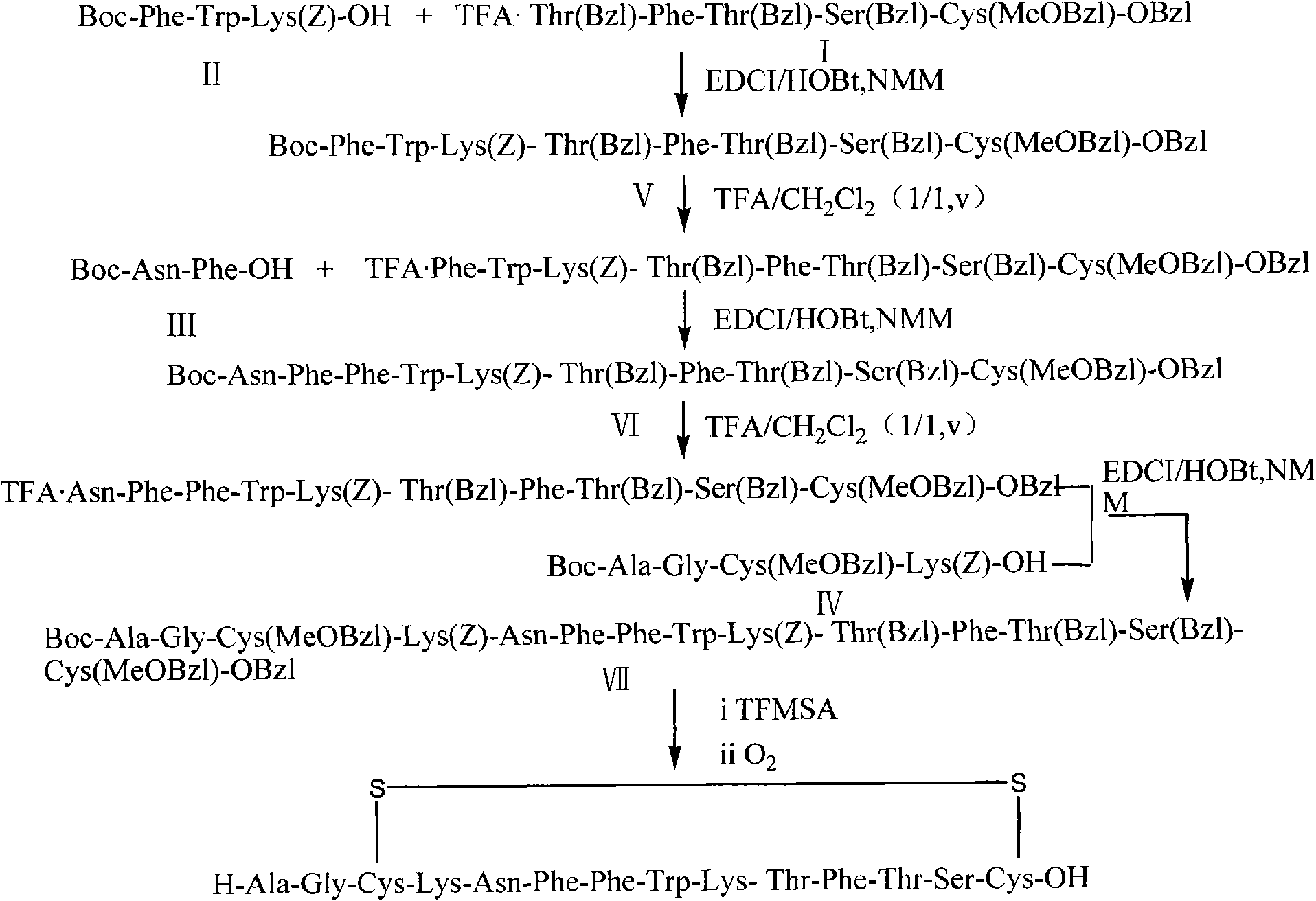
The invention relates to a liquid phase synthetic method for somatostatin, which is as follows: firstly, four segment peptides of Thr(Bzl)-Phe-Thr(Bzl)-Ser(Bzl)-Cys(MeOBzl)-Bzl, Boc- Phe-Trp-Lys(z)-OH, Boc-Asn-Phe-OH and Boc-Ala-Gly-Cys(MeOBzl)-Lys(z)-OH are respectively and total synthesized; and then EDCI / HOBt is used for fragment condensation to synthesize total protective reducibility tetradecapeptide; protecting groups possessed by trifluoromethanesulfonic acid and methyl-phenoxide system de-peptide chain are used for obtaining reducibility somatostatin which is oxidized to obtain somatostatin crude product; the somatostatin crude product is separated and purified by a C18 preparative column, is frozen and dried to obtain the somatostatin.
Owner:LANZHOU UNIVERSITY +1
Thiol-mediated drug attachment to targeting peptides
InactiveUS20050118099A1High affinityPeptide/protein ingredientsBiological material analysisThiolDiagnostic agent
Compositions and methods for thiol-specific attachment of therapeutic and diagnostic agents to somatostatin and other targeting peptides.
Owner:BIOGEN MA INC
Receptor(SSTR2)-selective somatostatin antagonists
ActiveUS20080260638A1Easy to markEasy to usePeptide/protein ingredientsMetabolism disorderSide effectHigh affinity binding
SRIF peptide antagonists, which are selective for SSTR2 in contrast to the other cloned SRIF receptors and which bind with high affinity to the cloned human receptor SSTR2 but do not activate the receptor, have many useful functions. Because they do not bind with significant affinity to SSTR1, SSTR3, SSTR4 or SSTR5, their administration avoids potential undesirable side effects. Because they block the receptor function, they can be used therapeutically to block certain physiological effects which SSTR2 mediates. By incorporating radioiodine or the like in these SSTR2-selective SRIF antagonists, a labeled compound useful in drug-screening methods is provided. Alternatively, for use in therapy, highly radioactive moieties can be N-terminally coupled, complexed or chelated thereto.
Owner:SALK INST FOR BIOLOGICAL STUDIES +2
Somatostatin antagonists and agonists that act at the sst subtype 2 receptor
InactiveUS20050054581A1Ease of preparation and detectabilityGood metabolic stabilityBiocidePeptide/protein ingredientsGrowth hormoneAgonist
Compounds according formula (I) A-G-Z-W and pharmaceutically acceptable salts, solvates or hydrates thereof; wherein, A is (C6-C10)aryl, (C6-C10)aryl-SO2, (C6-C10)aryl-CH2—, (C6-C10)arylcarbonyl, (C1-C9)heteroaryl, (C1-C9)heteroaryl-SO2—, (C1-C9)heteroaryl-CH2—; or (C1-C9)heteroarylcarbonyl; G is selected from the group consisting of: where B is (C6-C10)aryl or (C1-C9)heteroaryl, and X is CH2, SO2, or carbonyl; where X is CH2, SO2, or carbonyl; and R1 and R1′ are each independently selected from H, CN, (C1-C8)alkyl-, and phenyl(CH2)—, wherein said alkyl and phenyl groups are optionally substituted; and where Z and W are as defined in the present Specificiation; and pharmaceutical compositions and methods useful to increase secretion of growth hormone(GH) from the anterior pituitary of mammals, including on a sustained release basis.
Owner:HAY BRUCE A +2
Receptor(SSTR4)-selective somatostatin analogs
Owner:SALK INST FOR BIOLOGICAL STUDIES
Compositions and Methods for Enhanced Somatostatin Immunogenicity in the Treatment of Growth Hormone and Insulin-Like Growth Factor One Deficiency
ActiveUS20110195080A1Improving immunogenicityEffective and low cost materialMetabolism disorderAntibody mimetics/scaffoldsInsulinlike growth factorVaccine Immunogenicity
Compositions and methods are provided for treatment growth hormone and / or insulin-like growth factor 1 deficiency in a patient in need of such treatment. Compositions and methods include novel vaccines that provide immunogenicity for somatostatin and result in the increased release of endogenously produced growth hormone and / or insulin-like growth factor 1.
Owner:BRAASCH BIOTECH LLC
Pharmaceutical compositions which inhibit vascular proliferation and method of use thereof
The present invention relates to a method of treating vascular proliferation in a patient in need thereof. The method includes the step of administering a therapeutically effective amount of a type-1 somatostatin agonist to said patient.
Owner:IPSEN PHARMA SAS
Somatostatin agonists
Claimed are a series of somatostatin agonists typically characterized by alkylation of the amide nitrogen, and uses thereof. Examples of claimed compounds are those according to formula (I), A1-cyclo{Cys-A2-D-Trp-A3-A4-Cys}-A5-Y1, (I) wherein: A1 is an optionally substituted D- or L-aromatic α-amino acid or optionally substituted D- or L-cyclo(C3-6)alkylalanine; A2 is an optionally substituted aromatic α-amino acid or optionally substituted cyclo(C3-6)alkylalanine; A3 is Lys or Orn; A4 is β-Hydroxyvaline, Ser, hSer, or Thr; A5 is β-Hydroxyvaline, Ser, hSer, or Thr; and Y1 is OH, NH2 or NHR1, where R1 is (C1-6)alkyl; wherein each said optionally substituted aromatic α-amino acid and each said optionally substituted cyclo(C3-6)alkylalanine is optionally substituted with one or more substituents each independently selected from the group consisting of halogen, NO2, OH, CN, (C1-6)alkyl, (C2-6)alkenyl, (C2-6)alkynyl, (C1-6)alkoxy, Bzl, O-Bzl, and NR9R10, where R9 and R10 each is independently H or (C1-6) alkyl; and wherein the amine nitrogen of each peptide bond and the amino group of A1 of formula (I) is optionally substituted with a methyl group, provided that there is at least one said methyl group; and further provided that said compound is not D-Phe-cyclo{Cys-Phe-D-Trp-Lys-(N-Me-Thr)-Cys}-Thr-NH2; or a pharmaceutically acceptable salt thereof.
Owner:THE ADMINISTRATORS OF THE TULANE EDUCATIONAL FUND
Receptor(SSTR2)-selective somatostatin antagonists
ActiveUS7960342B2Easy to markEasy to useIn-vivo radioactive preparationsPeptide/protein ingredientsSide effectMedicine
SRIF peptide antagonists, which are selective for SSTR2 in contrast to the other cloned SRIF receptors and which bind with high affinity to the cloned human receptor SSTR2 but do not activate the receptor, have many useful functions. Because they do not bind with significant affinity to SSTR1, SSTR3, SSTR4 or SSTR5, their administration avoids potential undesirable side effects. Because they block the receptor function, they can be used therapeutically to block certain physiological effects which SSTR2 mediates. By incorporating radioiodine or the like in these SSTR2-selective SRIF antagonists, a labeled compound useful in drug-screening methods is provided. Alternatively, for use in therapy, highly radioactive moieties can be N-terminally coupled, complexed or chelated thereto.
Owner:SALK INST FOR BIOLOGICAL STUDIES +2
Treatment of insulin resistance
This invention is directed to methods of treating insulin resistance in a mammal which comprise administering an effective amount of a compound of formula I, where the variables are defined in the specification, or the stereoisomeric mixtures, diastereomerically enriched, diastereomerically pure, enantiomerically enriched or enantiomerically pure isomers, or the pharmaceutically acceptable salts and prodrugs thereof to said mammal. The compounds of formula I are growth hormone secretagogues and as such are useful for increasing the level of endogenous growth hormone. In another aspect this invention provides certain intermediates which are useful in the synthesis of the foregoing compounds and certain processes useful for the synthesis of said intermediates and th compounds of formula I. This invention is further directed to methods comprising administering to a human or other animal a combination of a functional somatostatin antagonist such as an alpha-2 adrenergic agonist and a compound of formula I.
Owner:PFIZER INC
Method of preparing tetradecapeptide somatostatin
InactiveCN101508724AHigh optical activityHigh chemical purityDigestive systemPeptidesKetoneTyrosine methyl ester
The invention discloses a preparation method of tetradecapeptide somatostatin. The method comprises the following steps: DCC and 3-(diethoxyphosphoryl)-1,2,3,-phentriazine-4-ketone are taken as condensing agents for preparing an intermediate a, the DCC is taken as a condensing agent for preparing an intermediate b, and the intermediate b and an intermediate c are condensed by taking diphenylphosphorylazide as a condensing agent to obtain an intermediate d; and the intermediate a and the intermediate b are condensed to obtain the tetradecapeptide somatostatin. The preparation method has the advantages of complete peptide linkage, mild operating condition, simple and practical purification, high yield, high optical activity and high chemical purity of the target product tetradecapeptide somatostatin. The yield of the target product tetradecapeptide somatostatin is 8-10% based on tyrosine methyl ester.
Owner:吴永平
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com