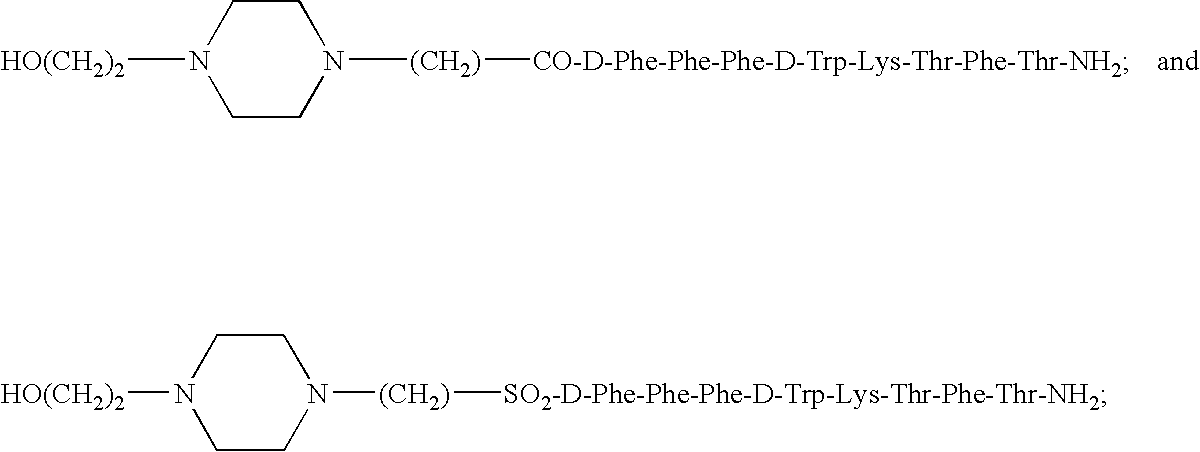
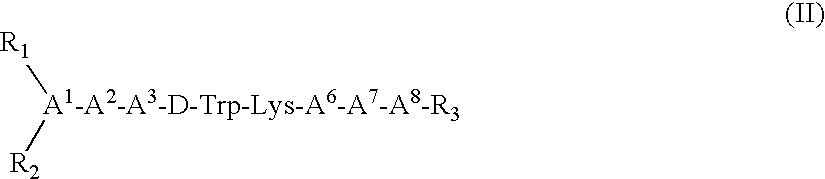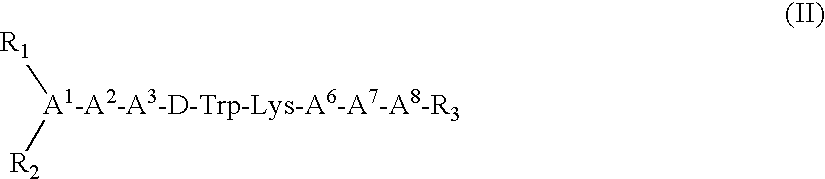Somatostatin agonists
a technology of somatostatin and agonist, which is applied in the field of somatostatin agonist, can solve the problems of loss of broad spectrum binding affinity, inability to detect the effects of all but one of the srif receptor subtypes in biological studies, and laborious synthesis of srif receptor subtypes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0247] One skilled in the art can, based on the description herein, utilize the present invention to its fullest extent. The following specific embodiments are, therefore, to be construed as merely illustrations of the invention and is not meant to be construed as limiting the full scope of the invention.
Synthesis
[0248] 4-Methylbenzhydrylamine hydrochloride resin (0.25 or 0.5 mequiv g−1) was obtained from Advanced ChemTech Inc., Louisville, Ky. Nα tert-Butyloxycarbonyl (Boc) protected amino acids were purchased from Bachem Inc., Torrance, Calif., Advanced ChemTech Inc., and Synthetech Inc., Albany, Oreg. The reactive side-chains of the amino acids were masked with one of the following groups: Cys, 4-methylbenzyloxycarbonyl; Lys, 2-chlorobenzyloxycarbonyl; Thr, O-benzyl; Tyr, O-2,6-dichlorobenzyl. All reagents and solvents were ACS grade or better and were used without further purification.
[0249] Compounds of the present invention, e.g., compounds of formula (I) can be and were s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| flow rate | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com