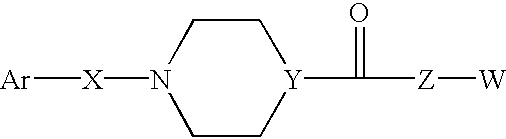
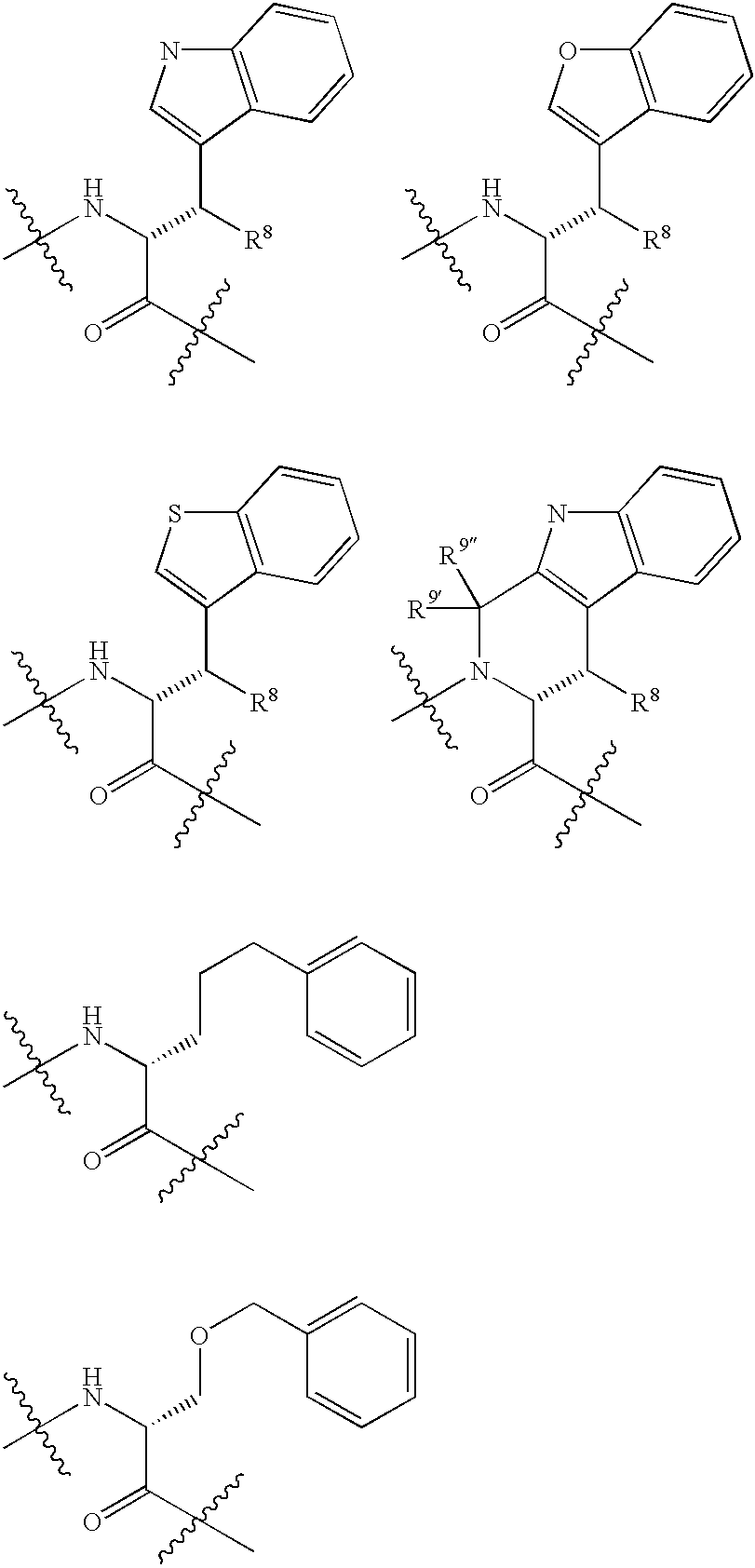
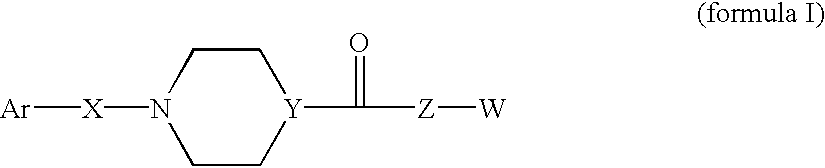Somatostatin antagonists and agonists that act at the sst subtype 2 receptor
a subtype 2 receptor and antagonist technology, applied in the field of pharmaceutical active compounds, can solve the problems of short half-life of growth hormone in the body, difficult in practice, and short permanent stature of growth hormone, and achieve the effects of increasing feed efficiency, and enhancing growth and performan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0180] Example 1 Synthesis of Certain Intermediates
[0181] CBZ-D-Trn-Lvs(OtBu)-OtBu
[0182] The following synthetic step is also evidenced in Scheme 2A., referring to reaction of compounds of types 11 and 10 therein, in order to form an intermediate 9. "CBZ" refers to phenyl-(CH.sub.2)--O--C(O)--, the carbobenzyloxy group.
[0183] To a solution of 406 mg of CBZ-D-Trp-OH (1.2 mmol), 302 mg of Lys(BOC)-OtBu HCl (1.0 mmol), 202 mg of hydroxybenzotriazole (1.5 mmol) and 366 mg of 4-dimethylaminopyridine (3 mmol) in 60 mL of methylene chloride was added 448 mg (1.5 mmol) of 1,3-dimethylaminopropyl-3-ethylca- rbodiimide hydrochloride (EDC). After stirring for 3 hours 100 mL more methylene chloride was added to the reaction, and it was washed four times with 25 mL portions of 0.1 N hydrochloric acid solution, twice with 25 mL of 50% saturated sodium bicarbonate solution, once with 50 mL of saturated brine, dried over anhydrous magnesium sulfate, filtered, and the solvent removed under reduced p...
example 2
[0185] Example 2 Synthesis of 6-Amino-2-(3-(1H-indol-3-yl)-2-{[4-(toluene4- -sulfonyl)-piperazine-1-carbony]1-amino}-propionyamino)-hexanoic acid tert-butyl ester
[0186] (a) BOC-D-Trp-Lys(CBZ)-OtBu
[0187] To a solution of 1.52 gm of BOC-D-Trp (5 mmol), 1.89 gm of Lys(Z)-OtBu HCI (5 mmol), 1.01 gm of hydroxybenzotriazole (7.5 mmol) and 1.83 gm of 4-dimethylaminopyridine (15 mmol) in 450 mL of methylene chloride was added 2.22 gm (11.6 mmol) of 1,3-dimethylaminopropyl-3-ethyl- carbodiimide hydrochloride. After stirring for 15 hours 300 mL more methylene chloride was added to the reaction, and it was washed four times with 100 mL portions of 50% saturated aqueous citric acid solution, once with 100 mL of 50% saturated sodium bicarbonate solution, once with 100 mL of saturated brine (NaCl), dried over anhydrous magnesium sulfate, filtered, and the solvent removed under reduced pressure to yield 2.95 gm (94%) of BOC-D-Trp-Lys(CBZ)-OtBu. (see Scheme IA, for reaction of reactants 5 and 4 .fw...
example 7
[0209] Example 7 bovine ("b")sst2 binding assay
[0210] The present example describes an assay for binding of pharmaceutically useful somatostatin agonists and antagonists at the bovine sst2 receptor.
[0211] Referring to the detailed protocols which follow, the methods for culturing Neuro2A cells and measuring competitive binding potency (IC.sub.50) were similar to those described by J. A. Koenig et al., "Somatostatin receptors in Neuro2A neuroblastoma cells:operational characteristics", British J. Pharmacol., 120, 45-51, 1997, with the following modifications.
[0212] Binding assays were conducted 72 hours after transiently transfecting the Neuro2A cells with a plasmid (PCI-bsst2) containing an insert coding for the bovine sst2 receptor, placed downstream of the cytomegalovirus promoter. In the transfection step, 6.5.times.106 Neuro2A cells were added in 35 ml of media to each tissue culture flask (162 cm.sup.2 surface area). The next day, transfection was conducted using Fugene 6 (Boeh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface area | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com