Antibacterial Compounds
a technology of compounds and compounds, applied in the field of antibacterial compounds, can solve the problems of unfavorable clinical treatment of single agents, lethal mdr-tb, high cost, and marginal effectiveness, and achieve the effect of uniform dosage and ease of administration
Inactive Publication Date: 2018-06-07
JANSSEN SCI IRELAND UC
View PDF3 Cites 1 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
[0130]It is especially advantageous to formulate the aforementioned pharmaceutical compositions in unit dosage form for ease of administration and uniformity of dosage. Unit dosage form as used herein refers to physically discrete units suitable as unitary dosages, each unit containing a predetermined quantity of active ingredient
Problems solved by technology
There exists no single agent that is effective in the clinical treatment of tuberculosis, nor any combination of agents that offers the possibility of therapy of less than six months' duration.
MDR-TB is lethal when untreated and cannot be adequately treated through the standard therapy, so treatment requires up to 2 years of “second-line” drugs.
These drugs are often toxic, expensive and marginally effective.
The reactivation of latent TB is a high risk factor for disease development and accounts for 32% deaths in HIV infected individuals.
The efficacy of the treatment regime is still not clear and furthermore the length of the treatments is an important constrain in resource-limited environments.
In addition to the management of the TB epidemic, there is the emerging problem of resistance to first-line antibiotic agents.
The consequences of resistance
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
 Login to View More
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Login to View More
Abstract
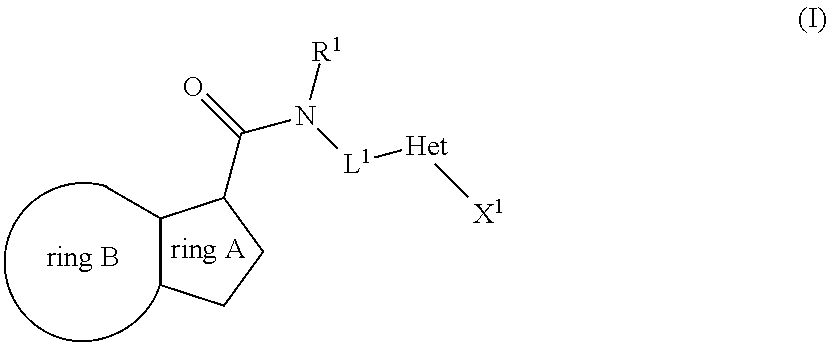
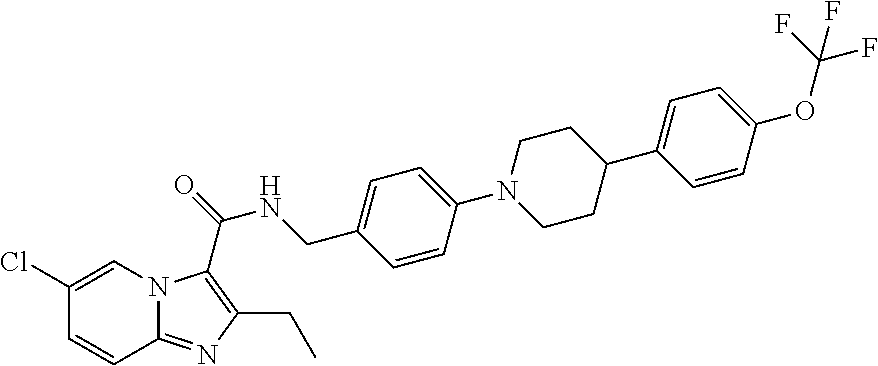
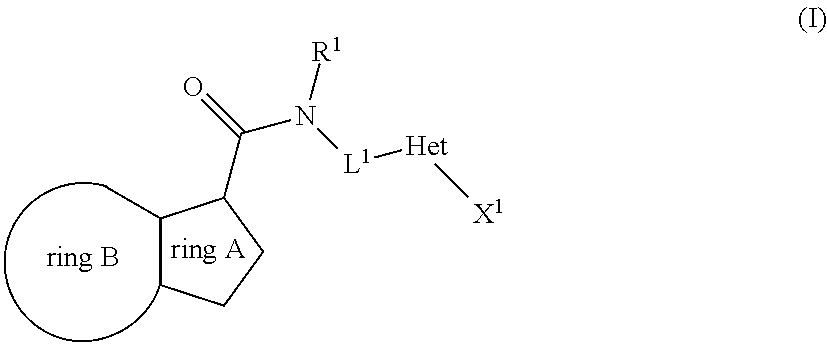
The present invention relates to the following compounds
wherein the integers are as defined in the description, and where the compounds may be useful as medicaments, for instance for use in the treatment of tuberculosis.
Description
[0001]The present invention relates to novel compounds. The invention also relates to such compounds for use as a pharmaceutical and further for the use in the treatment of bacterial diseases, including diseases caused by pathogenic mycobacteria such as Mycobacterium tuberculosis. Such compounds may work by interfering with ATP synthase in M. tuberculosis, with the inhibition of cytochrome bc1 activity as the primary mode of action. Hence, primarily, such compounds are antitubercular agents.BACKGROUND OF THE INVENTION[0002]Mycobacterium tuberculosis is the causative agent of tuberculosis (TB), a serious and potentially fatal infection with a world-wide distribution. Estimates from the World Health Organization indicate that more than 8 million people contract TB each year, and 2 million people die from tuberculosis yearly. In the last decade, TB cases have grown 20% worldwide with the highest burden in the most impoverished communities. If these trends continue, TB incidence will in...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07D471/04C07D487/04A61K31/519A61P31/04A61K45/06A61K31/4709C07D513/04A61K31/496A61K31/498
CPCC07D471/04C07D487/04A61K31/519A61P31/04A61K45/06A61K31/4709C07D513/04A61K31/496A61K31/498C07D519/00A61P31/06C07D403/12C07D401/14C07D487/10A61K31/404A61K31/429A61K31/437A61K31/438A61K31/444A61K31/4745A61K31/4985C07D401/12
Inventor GUILLEMONT, JEROME EMILE GEORGESMOTTE, MAGALI MADELEINE SIMONERABIOSSON, PIERRE JEAN-MARIE BERNARDTAHRI, ABDELLAH
Owner JANSSEN SCI IRELAND UC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com