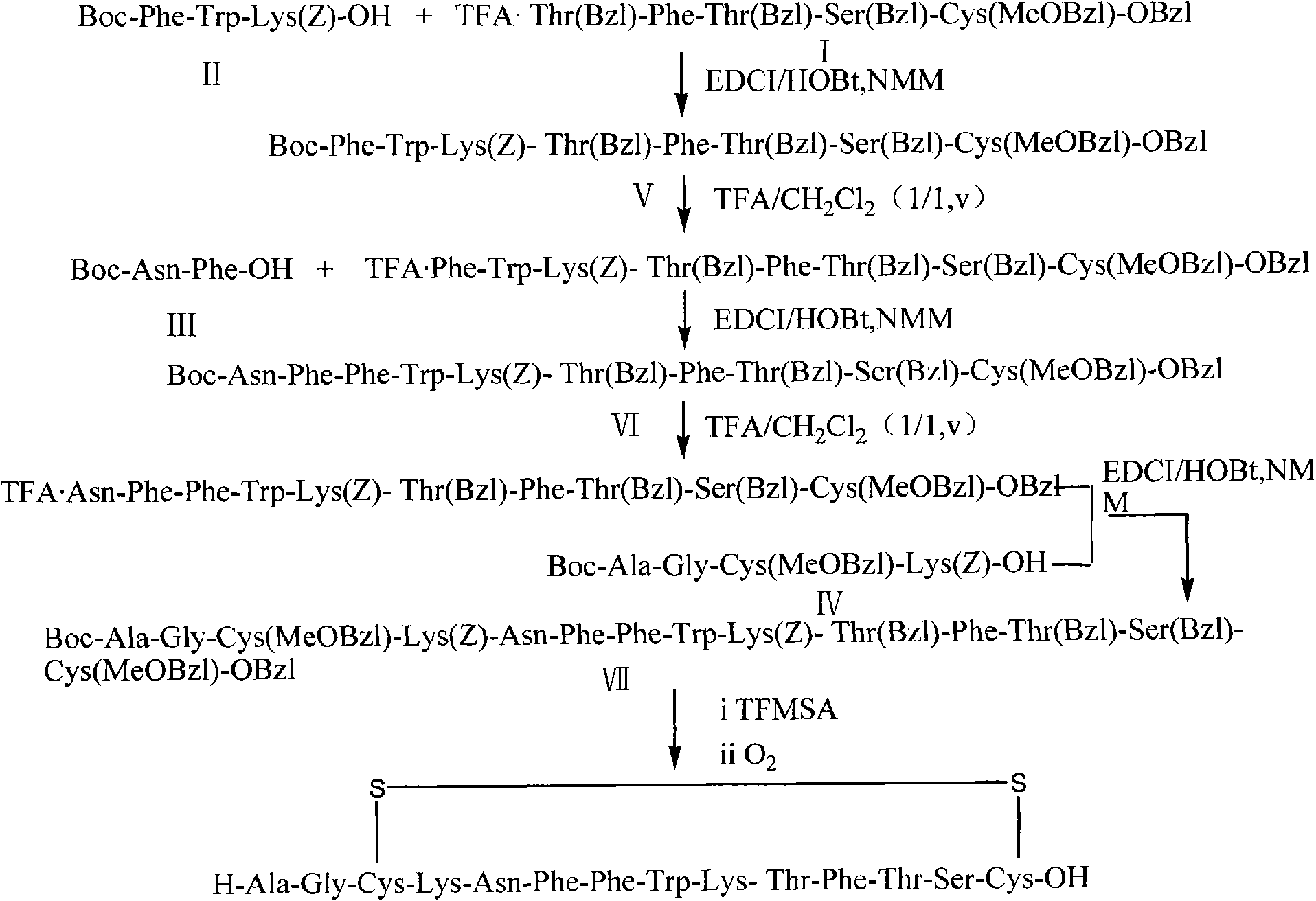
Synthetic method for somatostatin
A technology of somatostatin and synthesis method, which is applied in the field of liquid phase synthesis of somatostatin, can solve the problems of steric hindrance, reduction of product optical rotation, hindering amino acid synthesis and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0050] Step 1, Fragment I: Synthesis of TFA Thr(Bzl)-Phe-Thr(Bzl)-Ser(Bzl)-Cys(MeOBzl)-OBzl
[0051] Weigh 4.1g (13.9mmol) of Boc-Ser(Bzl)-OH and dissolve it in 50ml of tetrahydrofuran, add 1.53ml (13.9mmol) of N-methylmorpholine, slowly add 1.8 ml (13.9 mmol) of isobutyl chloroformate, after 2-4 minutes, add 20 ml of a tetrahydrofuran solution of 4.6 g (13.9 mmol) of Cys(MeOBzl)-OBzl pre-cooled to below 0°C in the refrigerator. After the reaction was maintained at the low temperature for 30 minutes, the ice-salt bath was removed, and the reaction was resumed at room temperature for 4-6 hours, and the reaction was detected by TLC. Filter to remove salt, concentrate the filtrate, dissolve the residue in ethyl acetate, wash with 5% NaOH, water, 5% HCl, water, and saturated brine successively, dry with anhydrous NaSO4, concentrate and remove the solvent under reduced pressure to obtain a white solid Boc-Ser(Bzl)-Cys(MeOBzl)-OBzl 8.04g, yield 95%.
[0052] Pour 8.04 g of the obt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com