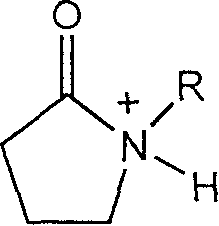
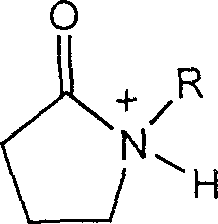
Method for catalyzing alcohol acid esterification using ion liquid
A technology for catalyzing alkyd esters and ionic liquids, applied in chemical instruments and methods, carboxylate preparation, physical/chemical process catalysts, etc., can solve the problem of colored products and corrosion equipment, difficult separation of products and catalysts, and waste acid pollution of the environment and other problems, to achieve the effect of short reaction time, high catalytic efficiency and low dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1: Weigh respectively n-butanol 9.25g (0.125mol), acetic acid 7.50g (0.125mol), ionic liquid N-methylpyrrolidone bisulfate 0.0025mol; ionic liquid, butanol, acetic acid are added successively with In the round-bottomed flask of stirrer, thermometer, reflux condenser, magnetically stirred, and reacted at reflux temperature for 2 hours; The obtained crude product was subjected to vacuum distillation, the conversion rate was 67.8%, and the selectivity was 100%. The ionic liquid is reused after simple dehydration.
Embodiment 2
[0019] Embodiment 2: Weigh respectively 5.75g (0.125mol) of ethanol, 7.50g (0.125mol) of acetic acid, and 0.0005mol of ionic liquid N-methylpyrrolidone sulfate; add the ionic liquid, ethanol, and acetic acid in sequence with a stirrer and a thermometer 1. In the round-bottomed flask of the reflux condenser, magnetically stirred, and reacted at reflux temperature for 0.5 hour; the obtained crude product was subjected to vacuum distillation, the conversion rate was 68.5%, and the selectivity was 100%. The ionic liquid is reused after simple dehydration.
Embodiment 3
[0020] Embodiment three: Weigh methanol 4.00g (0.125mol), acetic acid 7.50g (0.125mol), ionic liquid N-methylpyrrolidone bisulfate 0.000125mol; ionic liquid, methanol, acetic acid are added successively with agitator, In a round-bottomed flask with a thermometer and a reflux condenser, magnetically stir, and react at reflux temperature for 1 hour; the obtained crude product is subjected to vacuum distillation, the conversion rate is 69.2%, and the selectivity is 100%. The ionic liquid is reused after simple dehydration.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com