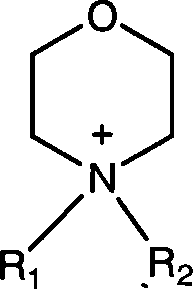
Morpholine quaternary ammonium salt ion liquid and preparation method thereof
A technology of ionic liquid and quaternary ammonium salt, applied in the direction of organic chemistry, can solve the problems of high cost, difficult industrial production and application, and achieve the effects of less equipment, easy promotion and use, and increased yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
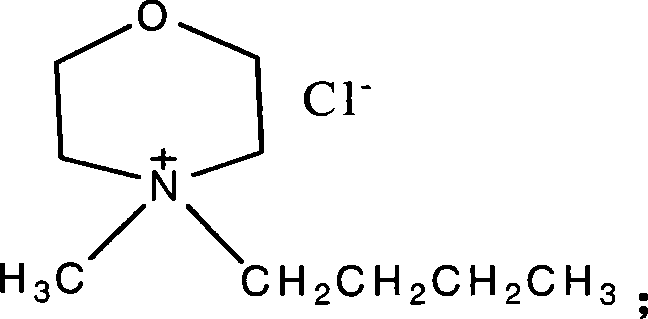
[0060] Preparation of N-methyl-N-butylmorpholine hydrochloride ionic liquid.
[0061] Weigh 10.1g (0.1mol) of N-methylmorpholine and 11.25g (0.12mol) of 1-chlorobutane, add 20ml of acetonitrile as solvent, mix them in a 100ml three-necked flask, heat for 20h under vigorous stirring, and control the temperature At 85°C, a small amount of white crystals were produced after 20 hours, and the solvent was evaporated with a rotary evaporator, and a large amount of crystals were produced after cooling. After suction filtration, washed with 20ml of acetone, and dried, 8.10g of white solids were obtained, namely N- Methyl-N-butylmorpholine hydrochloride ionic liquid.
[0062] The calculated yield was 42%. Adopt nuclear magnetic resonance instrument to detect, the parameter of gained product is: 1 H NMR (300MHz, CDCl 3 ), δ: 1.00ppm (t, 3H, CH 3 ), 1.47ppm (m, 2H, CH 2 ), 1.79ppm (m, 2H, CH 2 ), 3.53ppm (s, 3H, NCH 3 ), 3.62-4.12ppm (m, 10H).
Embodiment 2
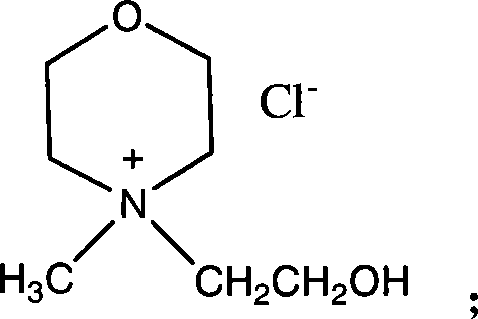
[0064] Preparation of N-methyl-N-hydroxyethylmorpholine hydrochloride ionic liquid.
[0065] Weigh 10.1g (0.1mol) of N-methylmorpholine and 9.66g (0.12mol) of 2-chloroethanol, add 20ml of acetonitrile as solvent, mix them in a 100ml three-necked flask, heat for 35h under vigorous stirring, and control the temperature at After 35 hours at 85°C, a colorless transparent liquid was obtained, and the solvent was evaporated by a rotary evaporator to obtain a white transparent solid. After drying, 11.80 g of the product was obtained, which was N-methyl-N-hydroxyethyl morpholine salt salt ionic liquid.
[0066] The calculated yield was 65.2%. Adopt nuclear magnetic resonance instrument to detect, the parameter of gained product is:
[0067] 1 H NMR (300MHz, CDCl 3 ), δ: 1.65ppm (s, 1H, OH), 2.02ppm (s, 3H, CH 3 ), 2.11(t, 2H, CH 2 ), 3.69ppm (t, 6H, CH 2 , CH 2 NCH 2 ), 4.90ppm (t, 4H, CH 2 OCH 2 ).
Embodiment 3
[0069] Preparation of N-methyl-N-allylmorpholine hydrochloride ionic liquid.
[0070] Weigh 20.2g (0.2mol) of N-methylmorpholine, 14.3g (0.2mol) of allyl chloride, and 40ml of acetonitrile as a solvent, mix them into a 100ml three-necked flask, control the temperature at 45°C and stir for about 0.5h. The mixture becomes turbid. A white solid appeared, and there were more and more solids. After 2 hours, the reaction was basically completed, and the solvent was evaporated with a rotary evaporator to obtain a large amount of white solid. Add a small amount of acetone (10ml) to wash off the solvent, filter with suction and dry to obtain 15.02g of the product , which is N-methyl-N-allyl morpholine hydrochloride ionic liquid.
[0071] After calculation, the yield is 85%. Adopt nuclear magnetic resonance instrument to detect, the parameter of gained product is:
[0072] 1 H NMR (300MHz, CDCl 3 ), δ: 3.53ppm (s, 3H, CH 3 ), 3.74ppm (s, 4H, CH 2 NCH 2 ); 4.06ppm (s, 4H, CH 2 OC...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com