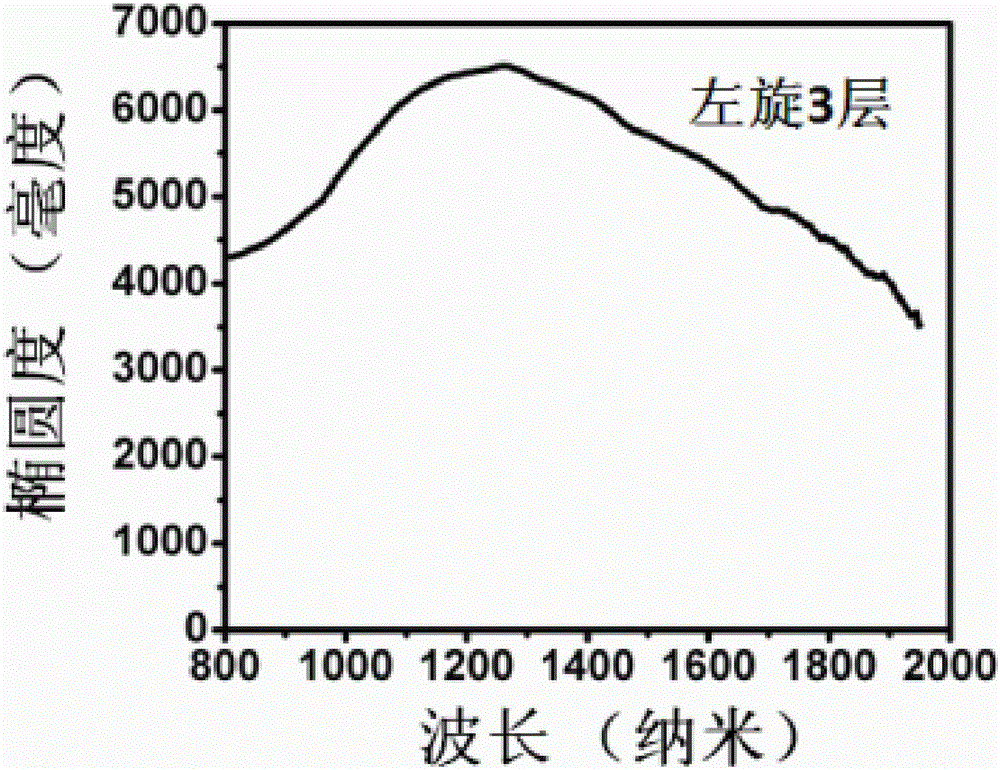
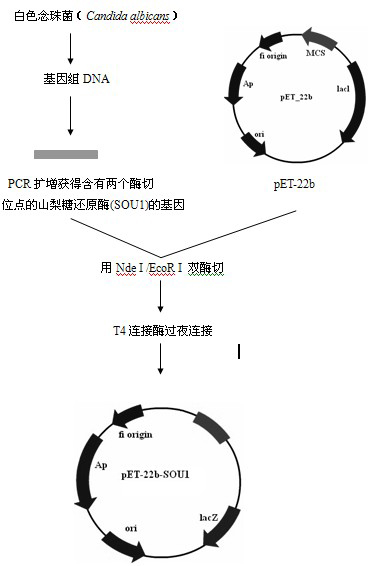
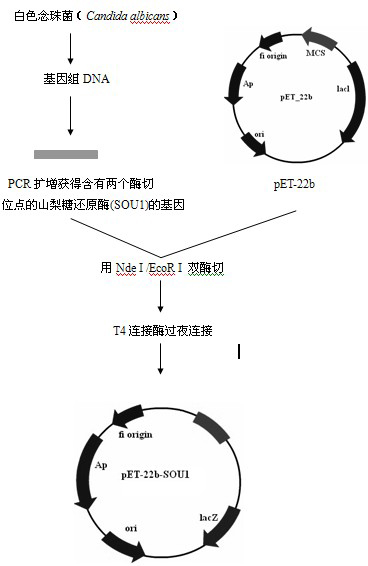
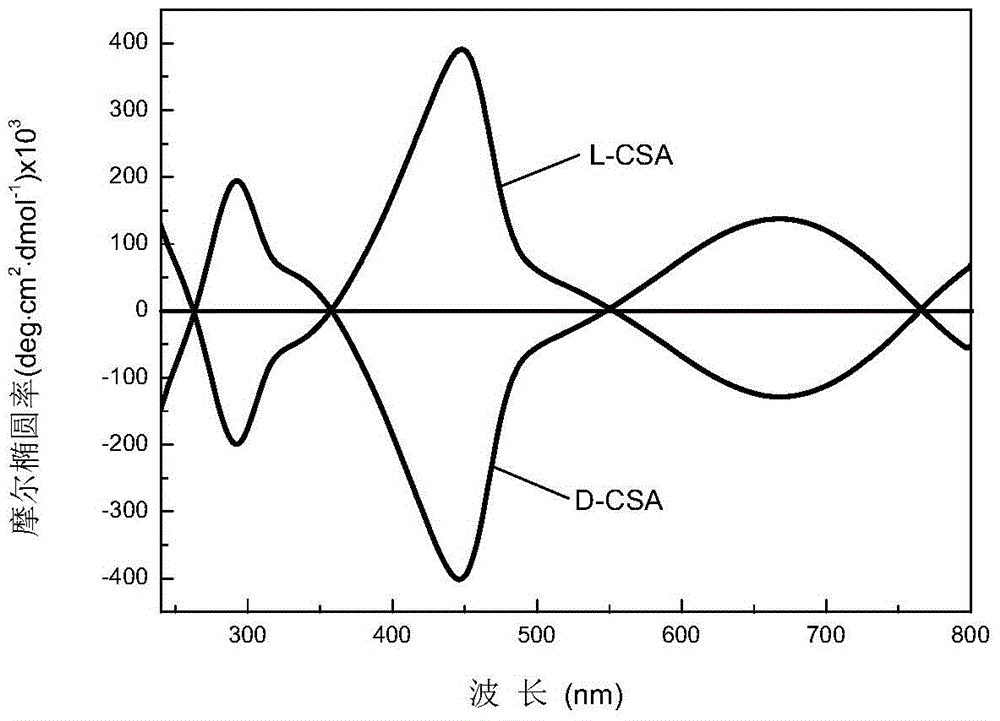
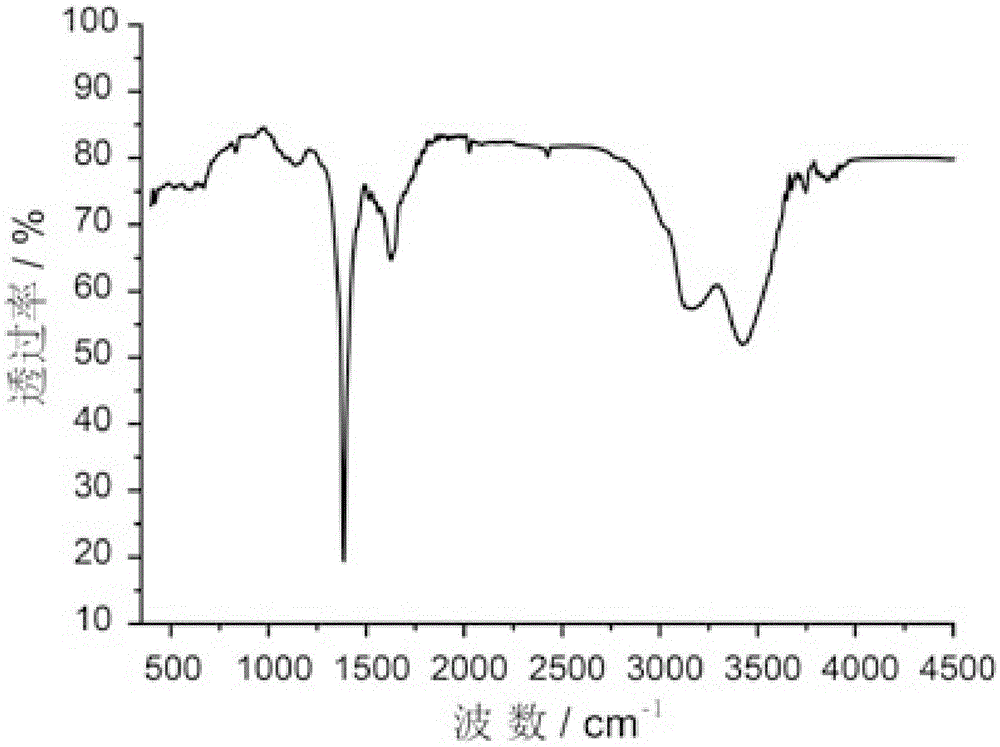
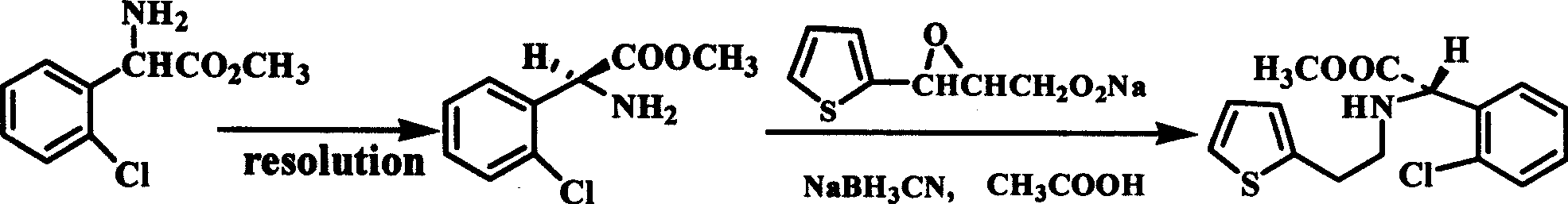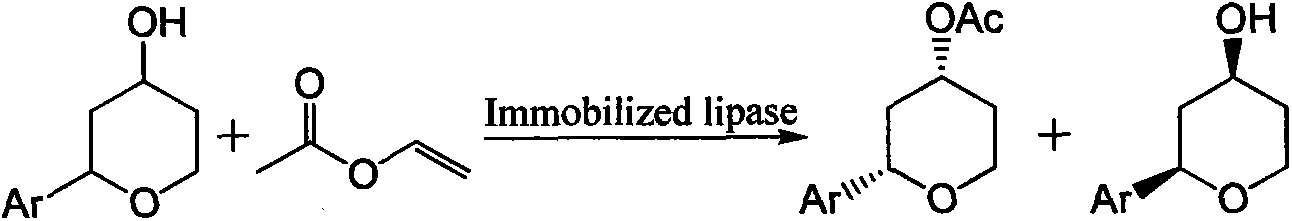Patents
Literature
147results about How to "High optical activity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
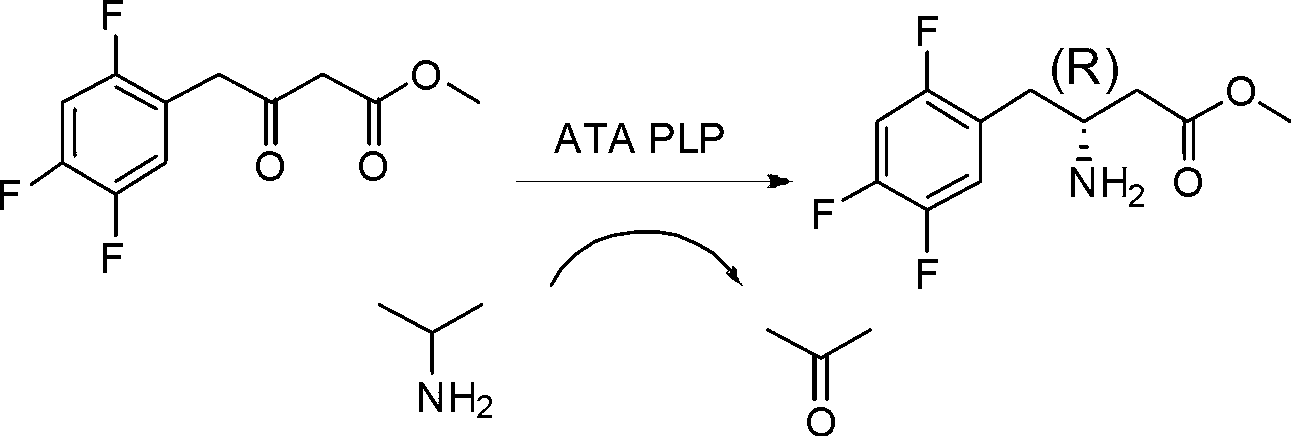
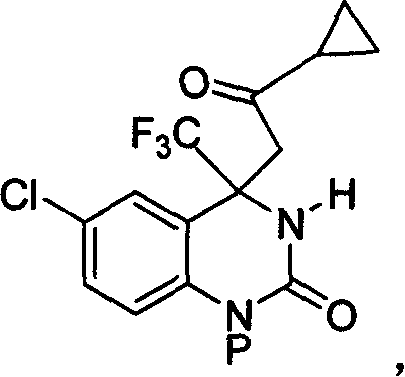
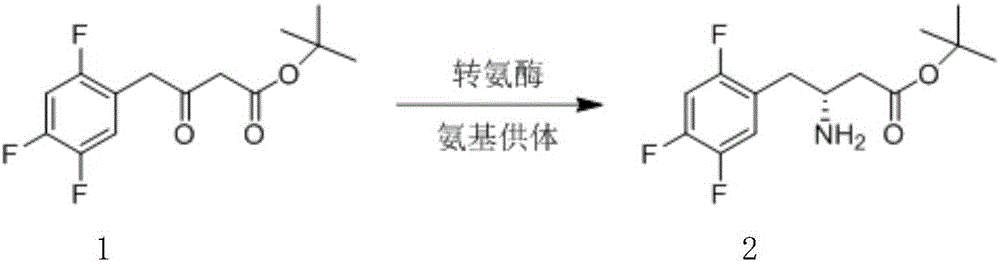
Biological preparation method of 3-amino-4-(2,4,5-trifluorophenyl)methyl butyrate
InactiveCN103014081AReduce energy consumptionEasy to operateTransferasesFermentationAminopheraseSolvent
The invention relates to a biological preparation method of 3-amino-4-(2,4,5-trifluorophenyl)methyl butyrate. According to the biological preparation method, 3-carbonyl-4-(2,4,5-trifluorophenyl)methyl butyrate is used as a substrate; under the condition that a biocatalyst, a cofactor, an amino donor and a cosolvent exist, the substrate performs the reaction to generate a target product 3-amino-4-(2,4,5-trifluorophenyl)methyl butyrate; the biocatalyst is recombination aminopherase; and the reaction is carried out in water-phase buffer solution with the pH of 6.0 to 9.0. The biological preparation method realizes that 3-carbonyl-4-(2,4,5-trifluorophenyl)methyl butyrate is converted into 3-amino-4-(2,4,5-trifluorophenyl)methyl butyrate by adopting the recombination aminopherase for the first time; compared with a conventional chemical method, the biological preparation method has low energy consumption, is simple and convenient to operate, generates a small number of by-products; and moreover, the product has high optical activity.
Owner:ENZYMEWORKS
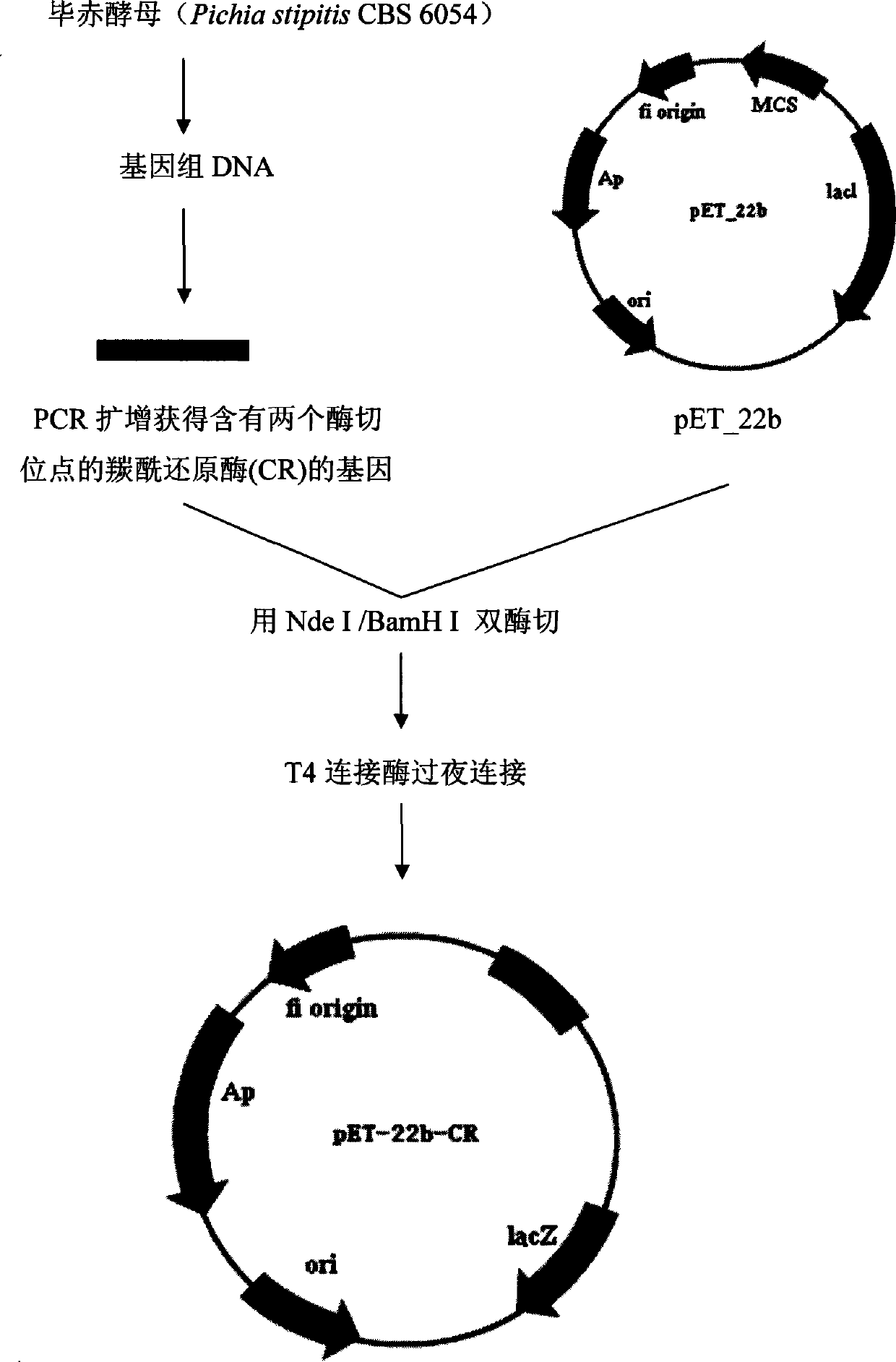
Use of carbonyl reductase in (S)-4-chloro-3 hydroxy butyric ether production
The invention discloses an application of carbonyl reductase with the amino acid sequence as shown in SEQ ID NO:2 to prepare (S)-4-chloro-3-hydroxybutanoic ethyl ester by asymmetric reduction of 4-chloracetyl ethyl acetate. The carbonyl reductase with the amino acid sequence as shown in SEQ ID NO:2 is taken as a catalyst, the ethyl 4-chloracetyl ethyl acetate is taken as a substrate, and NADPH is taken as a cofactor, and the (S)-4-chloro-3-hydroxybutanoic ethyl ester is prepared by the asymmetric reduction. The carbonyl reductase with the amino acid sequence as shown in SEQ ID NO:2 is applied to preparing the (S)-4-chloro-3-hydroxybutanoic ethyl ester by the asymmertric reduction of the 4-chloroacetyl ethyl acetate for the first time, which produces good effect; the yield of the substrate is up to 95%, and the enantiomer excess value of the product is 100%, the yield is high, and the production cost is greatly reduced.
Owner:NANJING UNIV OF TECH
Preparation method of chiral nanometer copper oxide with optical activity
InactiveCN103864134AHigh optical activityEnhance amphipathicMaterial nanotechnologyCopper oxides/halidesRoom temperatureCopper oxide
The invention discloses a preparation method of chiral nanometer copper oxide with optical activity. The preparation method comprises the following steps of: (1) dissolving an achiral anionic surface-active agent and a chiral small molecule into water at room temperature to obtain an achiral anionic surface-active agent and chiral small molecule water solution; (2) adding inorganic copper salt to the achiral anionic surface-active agent and chiral small molecule water solution, and stirring to react for 10-60 minutes; (3) adding alkali to reaction liquid obtained from the step (2), then increasing temperature to 100-200 DEG C, and reacting for 30 minutes-6 hours; (4) after the reaction is finished, centrifugalizing or filtering the reaction liquid obtained from the step (3), and washing and drying to obtain the chiral nanometer copper oxide with the optical activity. The prepared chiral nanometer copper oxide with the optical activity shows very uniform flower-shaped appearance and shows outstanding optical activity by having chirality.
Owner:SHANGHAI JIAO TONG UNIV
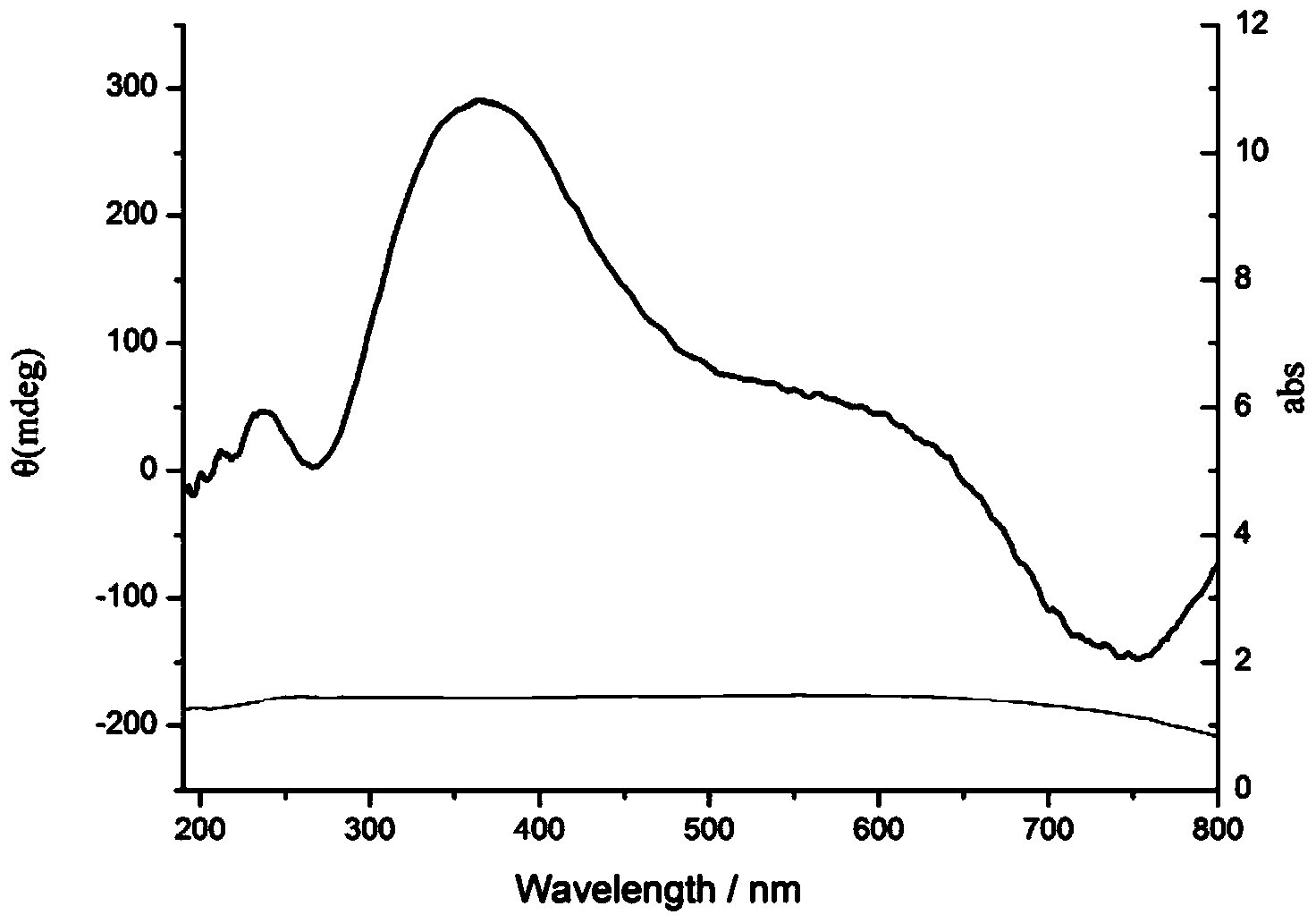
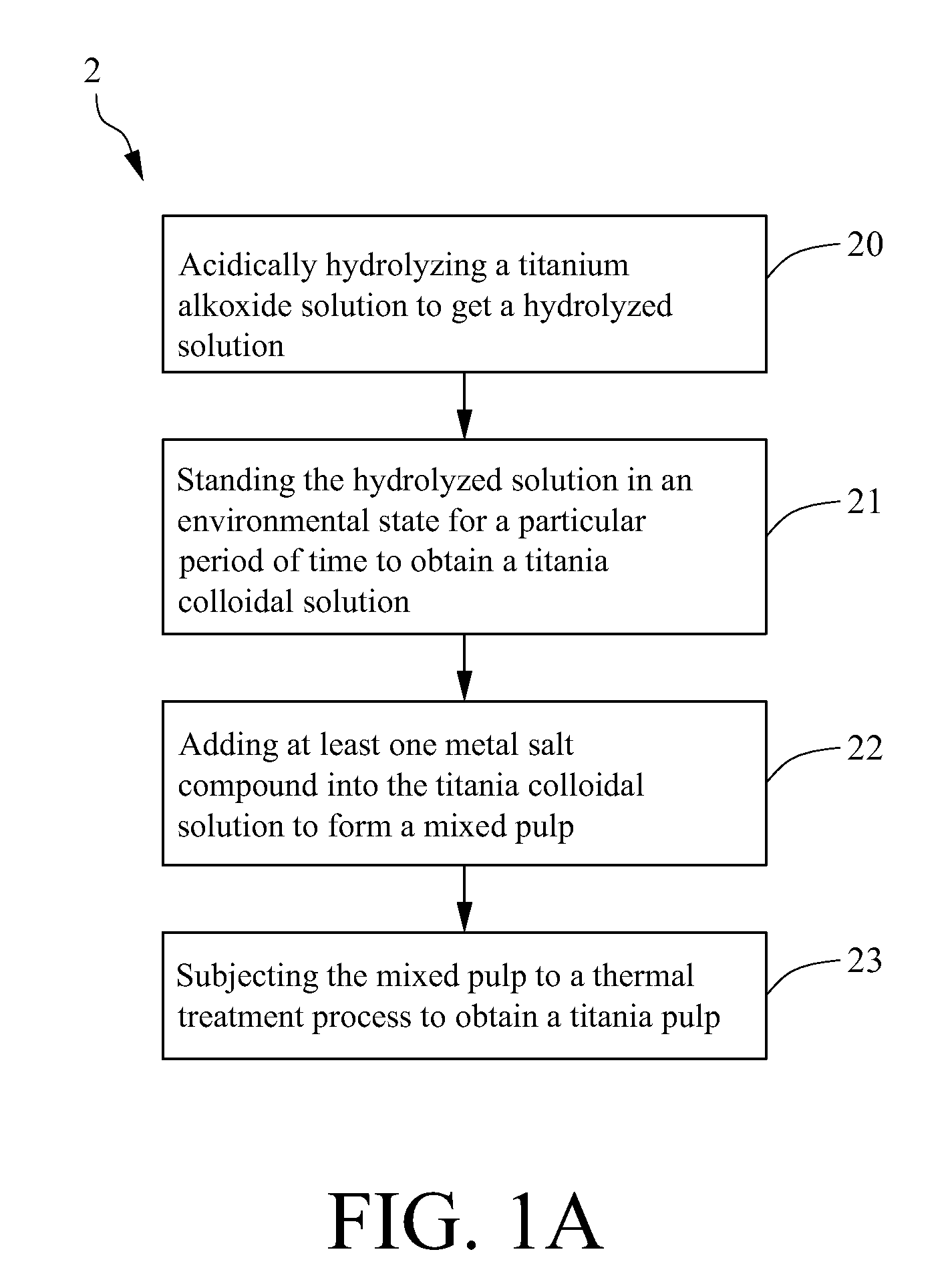
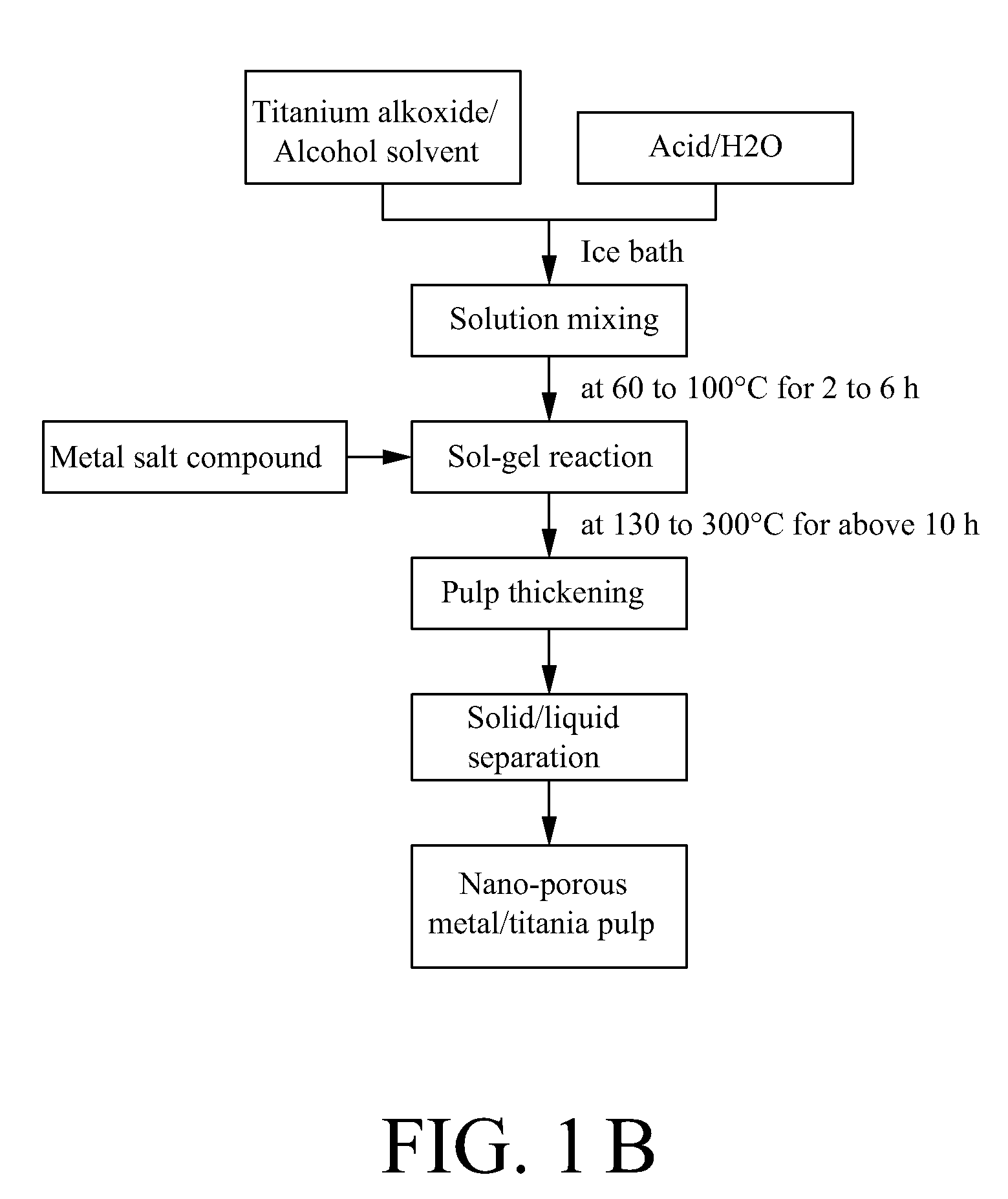
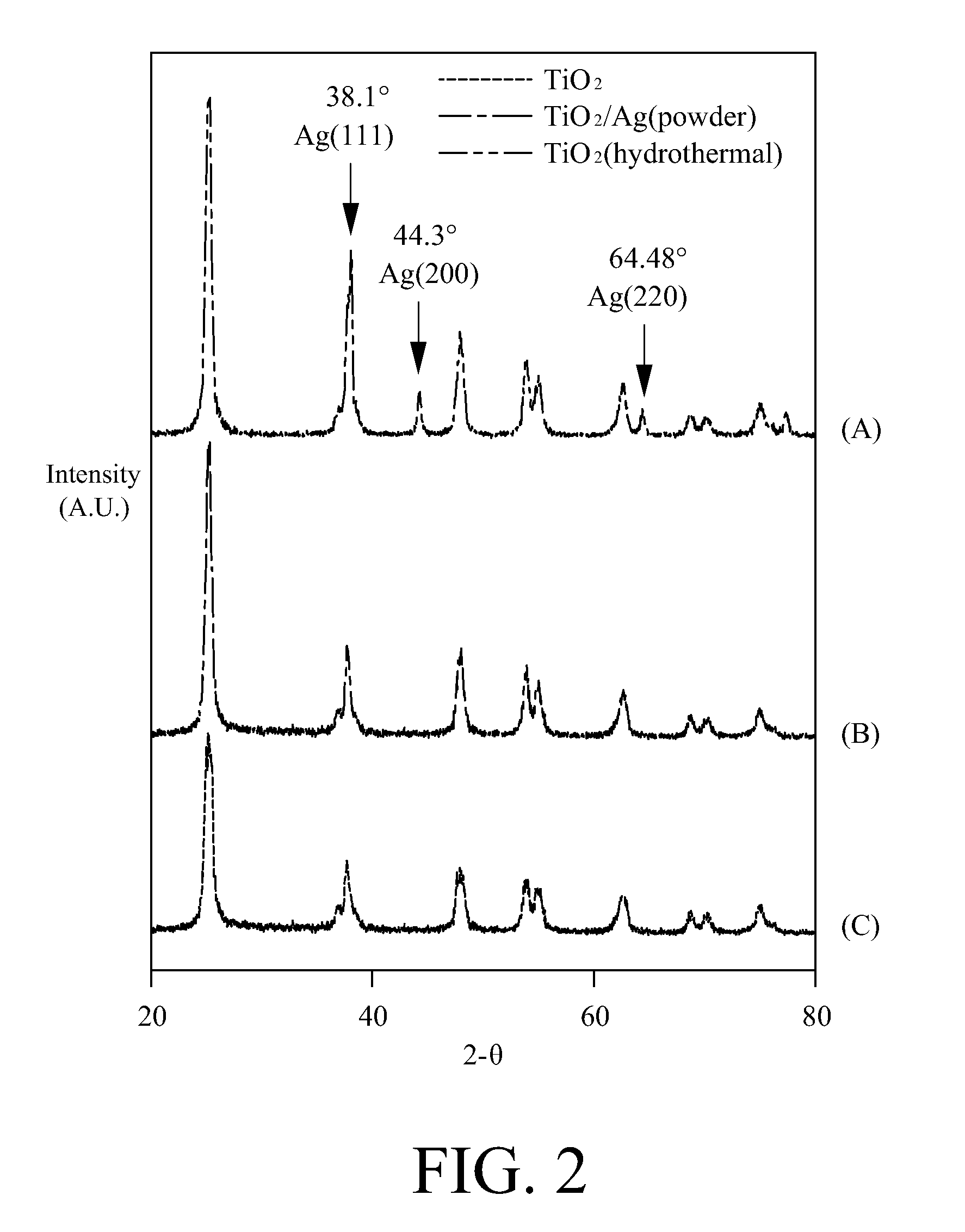
Method for making metal/titania pulp and photocatalyst
ActiveUS20100105549A1Convenient lightingIncrease production capacityUranium oxides/hydroxidesIncadescent envelopes/vesselsDecompositionSolvent
A method for making a metal-titania pulp and photocatalyst is provided, including firstly acidically hydrolyzing a titanium alkoxide solution in presence of an alcohol solvent to get a colloidal solution; then, adding at least one metal salt solution into the colloidal solution to produce a nano-porous metal / titania photocatalyst under appropriate conditions by appropriate reaction. The nano-porous metal / titania photocatalyst thus prepared has excellent optical activity and is applicable in research of water decomposition with light to improve production efficiency of hydrogen energy. In addition, the photocatalyst is further processed in the form of powder or film to facilitate industrial application in wastewater treatment.
Owner:INST NUCLEAR ENERGY RES ROCAEC
Graphene oxide antibacterial composite PVC material and preparation method thereof
The invention belongs to the field of high polymer materials and particularly relates to a graphene oxide antibacterial composite PVC material and a preparation method thereof. An antibacterial composite is prepared in the way that nano-silver and nanometer titania are loaded on graphene oxide, and zinc oxide capable of improving the optical activity of titania is added, so that the antibacterial performance is greatly improved. The mechanical property and structural characteristics of graphene oxide are given into full play, so that nano-silver and nanometer titania can be closely combined with graphene oxide and a PVC material, and the function is effective for a long time. The mechanical property of the material is enhanced while the antibacterial effect is improved, the preparation technological conditions are mild, and the graphene oxide antibacterial composite PVC material is suitable for industrial production.
Owner:江苏苏沃尚新材料科技有限公司
Application of carbonyl acyl reductase in preparing (S)-4-chlorine-3 hydroxyl ethyl butyrate
InactiveCN101962661AHigh yieldHigh optical activityFermentationDihydronicotinamide Adenine DinucleotideAcyl group
The invention discloses an application of a carbonyl acyl reductase in preparing (S)-4-chlorine-3 hydroxyl ethyl butyrate with an amino acid sequence as shown in SEQ ID NO: 2 by utilizing 4-chloracetyl acetidin asymmetrical reduction, which takes the carbonyl acyl reductase with the amino acid sequence as shown in SEQ ID NO: 2 as a catalyst, 4-chloracetyl acetidin as a substrate and NADH (Dihydronicotinamide Adenine Dinucleotide) as a cofactor to prepare the (S)-4-chlorine-3 hydroxyl ethyl butyrate by utilizing the asymmetrical reduction. The invention has the advantages that the carbonyl acyl reductase with the amino acid sequence as shown in SEQ ID NO: 2 is applied to the preparation of the (S)-4-chlorine-3 hydroxyl ethyl butyrate by utilizing the 4-chloracetyl acetidin asymmetrical reduction to obtain good effect; the enzyme activity thereof can reach 15U / mg; the yield of the substrate thereof reaches 91%; the enantiomer excessive value of products is 100%; the yield is high; and the production cost is greatly reduced.
Owner:NANJING TECH UNIV
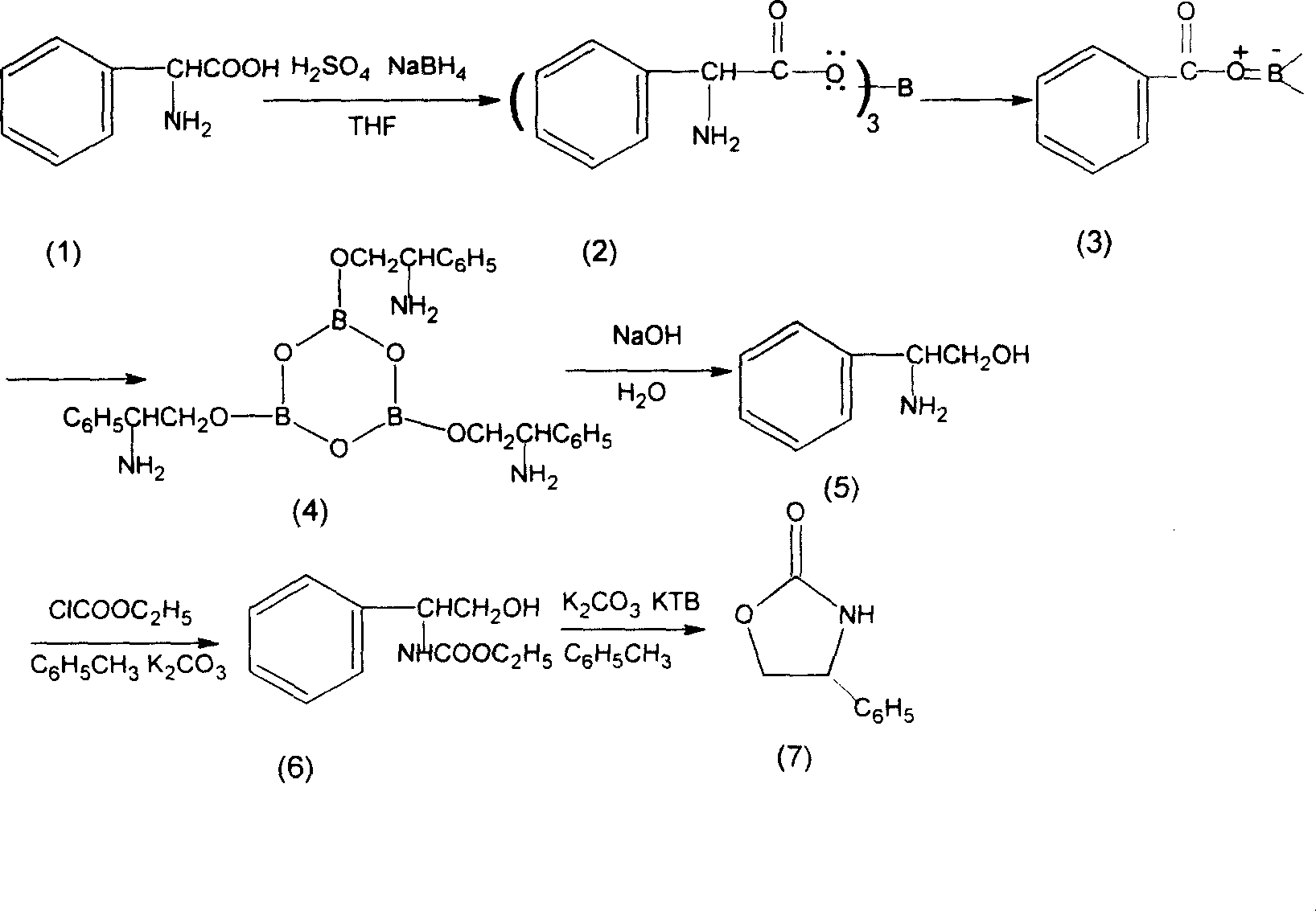
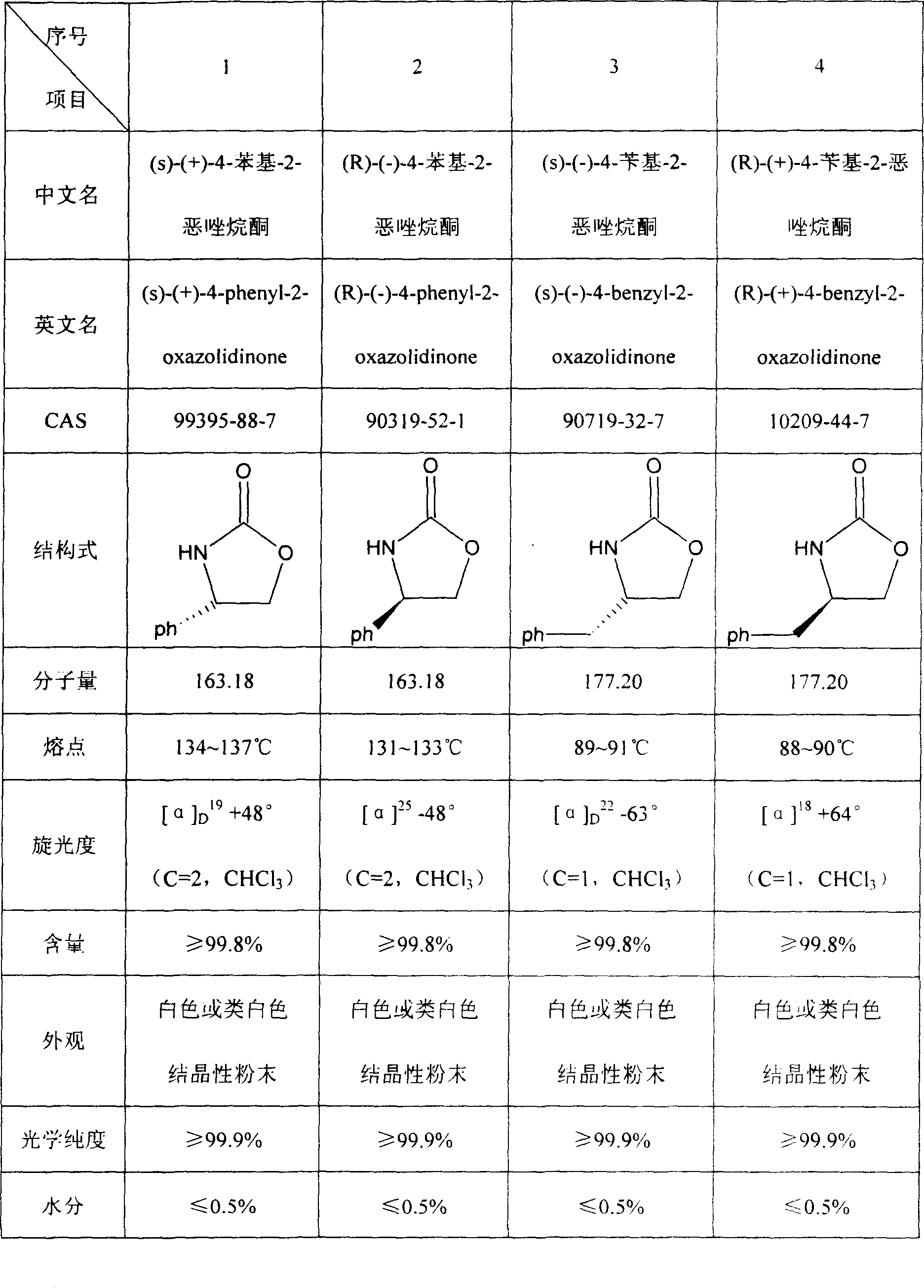
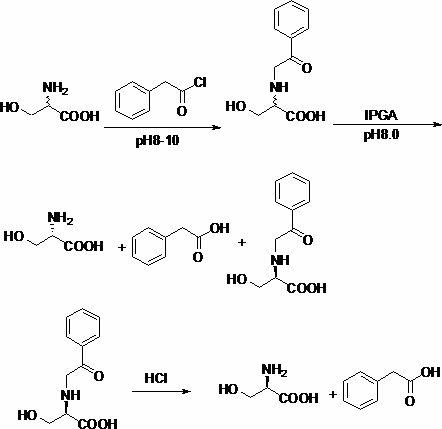
Prepn process of chiral 4-substituent-2-oxazolidone
InactiveCN1931847AMild reaction conditionsCommon reaction conditionsOrganic chemistryAlcoholMetallole
The present invention discloses the preparation process of one kind of chiral 4-substituent-2-oxazolidone. The preparation process includes the first reducing chiral amino acid into alcohol in the presence of Lewis acid and catalyst metal boron compound, the subsequent acylation with acyl chloride compound and no phosgene, and cyclization in buffering solution with strong alkali catalyst. The present invention has low cost, less reaction steps, less wastes produced, high product quality and high total yield, and is suitable for industrial production.
Owner:上海五洲药业股份有限公司 +1
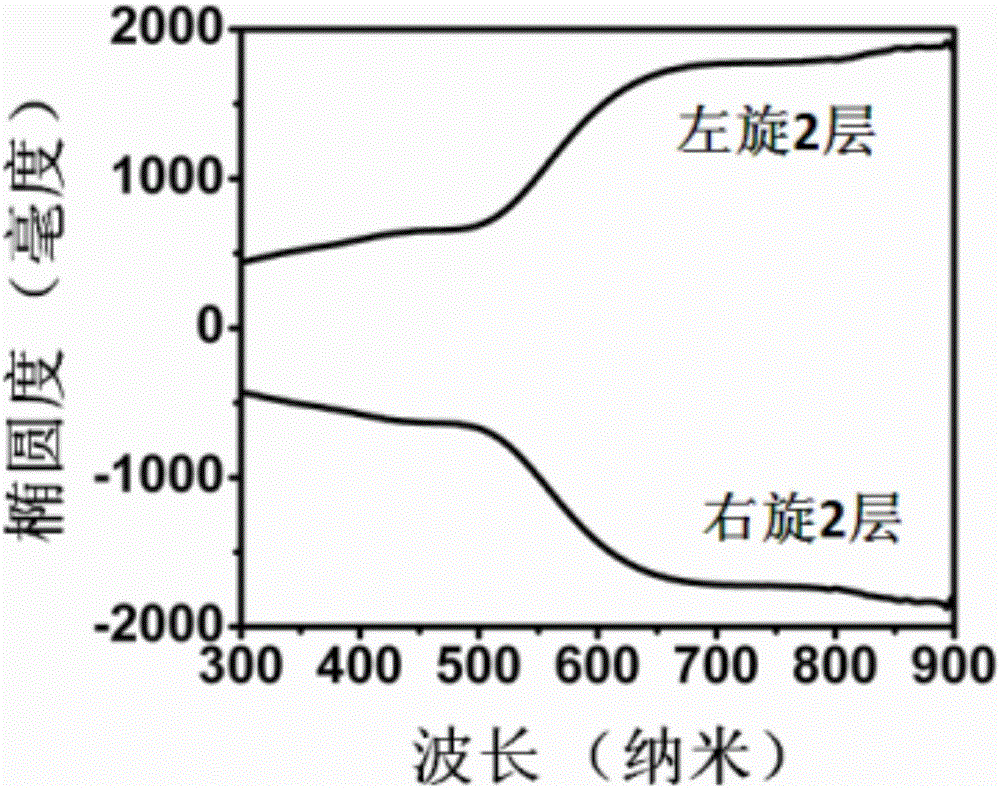
Chiral nano film and preparation method and application thereof
InactiveCN106829854ALong-range orderOptically activeSpecific nanostructure formationMaterial nanotechnologyColloidSolvent
The invention provides a chiral nano film and a preparation method and application thereof. The preparation method of the chiral nano film comprises the steps that a one-dimensional nanomaterial solution is spread on a lower phase in a Langmuir-Blodgett groove, a slide barrier located on the surface of the lower phase pushes a one-dimensional nanomaterial to move on the surface of the lower phase after the solvent is completely volatilized, the area of the one-dimensional nanomaterial is compressed to obtain a one-dimensional nanomaterial assembly film consistent in orientation; a Langmui-Schaeffer transfer method is adopted to transfer the assembly film to a substrate, and the chiral film provided with at least two layers of assembly films is obtained, wherein the assembly films are sequentially staggered clockwise or anticlockwise. The chiral film has ultra-high optical activity, an anisotropy factor is as high as 0.3, the optical activity is highest compared with chiral materials obtained by adopting an existing assembly means, and the preparation method is simple and has universality and wide application prospect.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA +1
Application of a kind of sorbose reductase in biological preparation of ethyl (s)-4-chloro-3-hydroxybutyrate
InactiveCN102286556AHigh yieldHigh optical activityMicroorganism based processesFermentationHydroxybutyric acidPtru catalyst
The invention discloses application of sorbose reductase in preparation of (S)-4-chloro-3-hydroxyethyl butyrate by a biological method. Sorbose reductase of which the amino acid sequence is disclosed as SEQ ID NO:2 is used as a catalyst, 4-chloracetylethyl acetate is used as a substrate, and NADPH (nicotinamide adenine dinucleotide phosphate) is used a cofactor to prepare the (S)-4-chloro-3-hydroxyethyl butyrate in an asymmetric reduction mode. In the invention, the sorbose reductase of which the amino acid sequence is disclosed as SEQ ID NO:2 is applied to preparation of (S)-4-chloro-3-hydroxyethyl butyrate in an asymmetric reduction mode for the first time, which has a good effect. The enzyme activity of the sorbose reductase is up to 5.6U / mg, the yield for substrate is up to 90%, and the enantiomeric excess of the product is 100%. Besides, the invention has the advantage of high yield, and greatly lowers the production cost.
Owner:NANJING UNIV OF TECH
Synthetic method of polyaniline nanofiber with helical structure
The invention provides a synthetic method of polyaniline nanofiber with a helical structure. The method comprises the following steps: firstly synthesizing N, N'-2(4-phenylene diamine)-1 and 4-phenylene diamine; secondly taking N, N'-2(4-phenylene diamine)-1 and 4-phenylene diamine as a polymerization reaction catalyst, chiral camphor sulfonic acid as an inducer and a doping agent, and ammonium persulfate as an oxidizing agent; finally synthesizing the polyaniline nanofiber with the helical structure through adopting the chemical oxidative polymerization. According to the synthetic method, the chiral camphor sulfonic acid is adopted as the doping agent to be interacted with the polyaniline main chain and to induce the polyaniline main chain to form the helical structure, and meanwhile, N, N'-2(4- phenylene diamine)-1 and 4-phenylene diamine with the oxidation potential lower than that of monomer aniline is further added to serve as a 'seed' and catalyze the polymerization reaction, so that the polymerization rate is greatly increased with the addition of phenylene diamine, and besides, heterogeneous nucleation is under restriction in the polymerization process, giving rise to the predominance of homogeneous nucleation, so that secondary growth is inhibited, the formation of the polyaniline helical structure is facilitated, and the optical activity of polyaniline is greatly improved.
Owner:HARBIN ENG UNIV
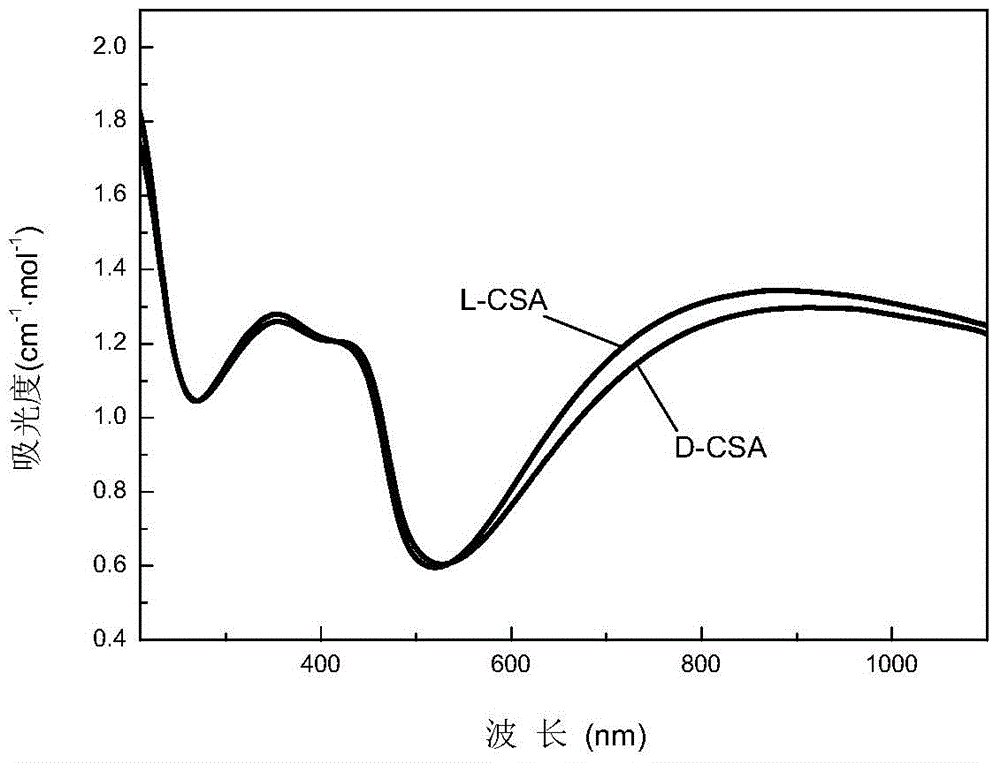
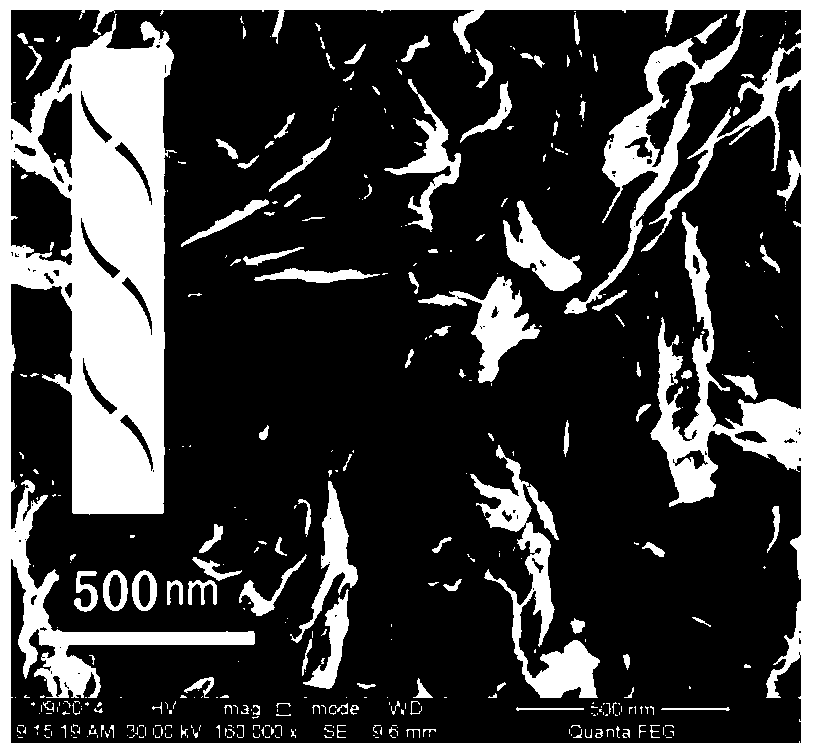
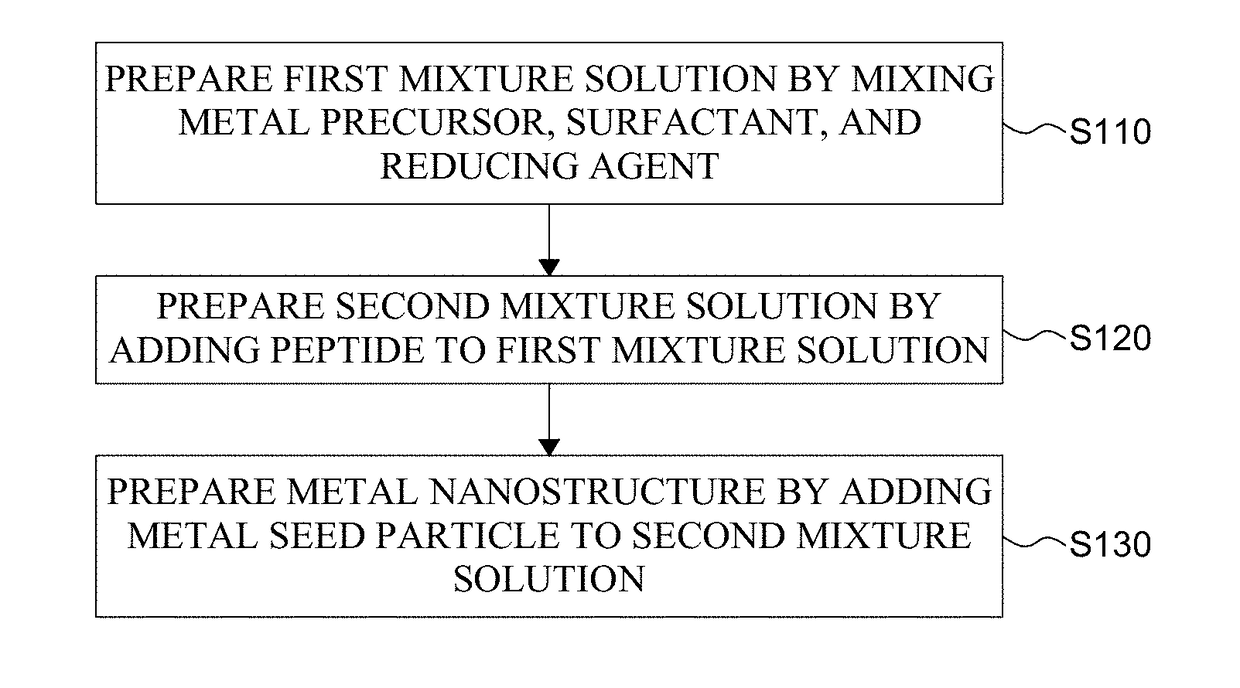
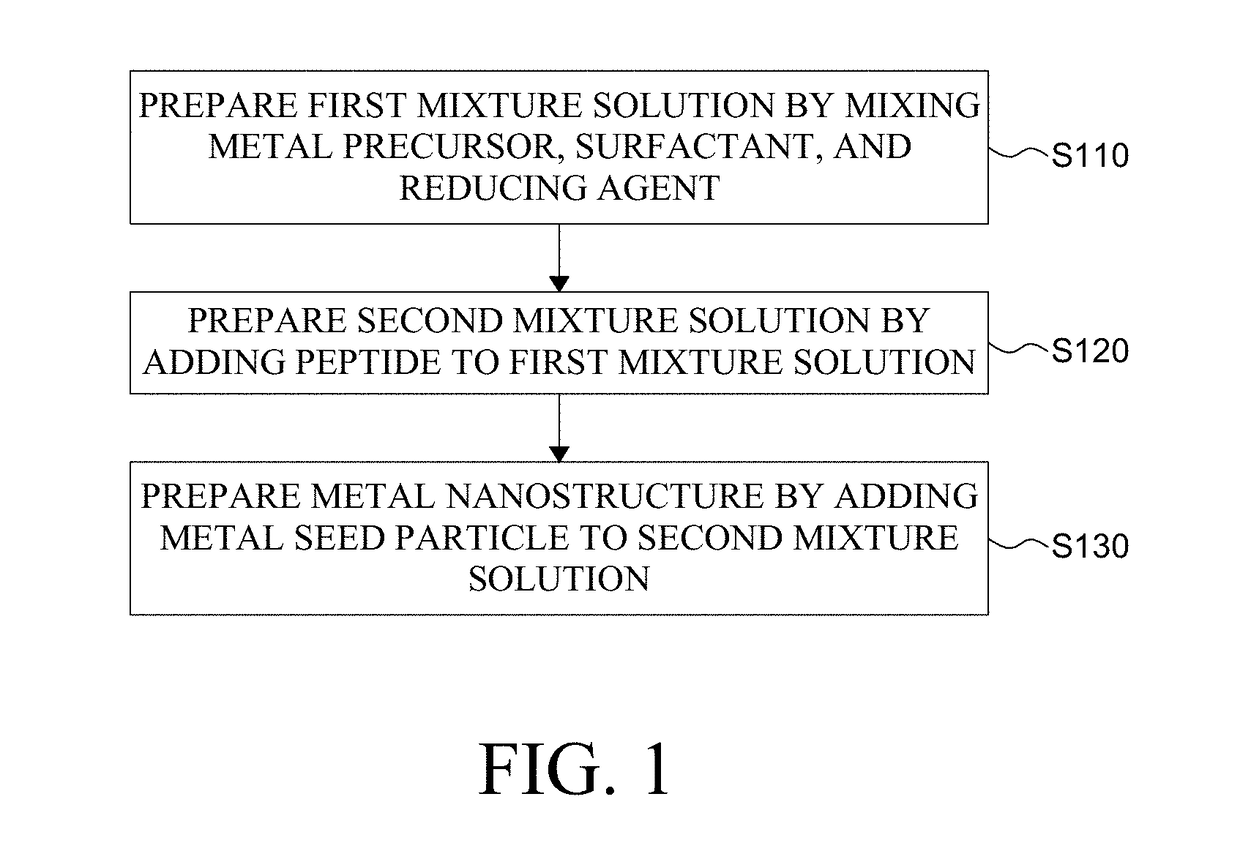
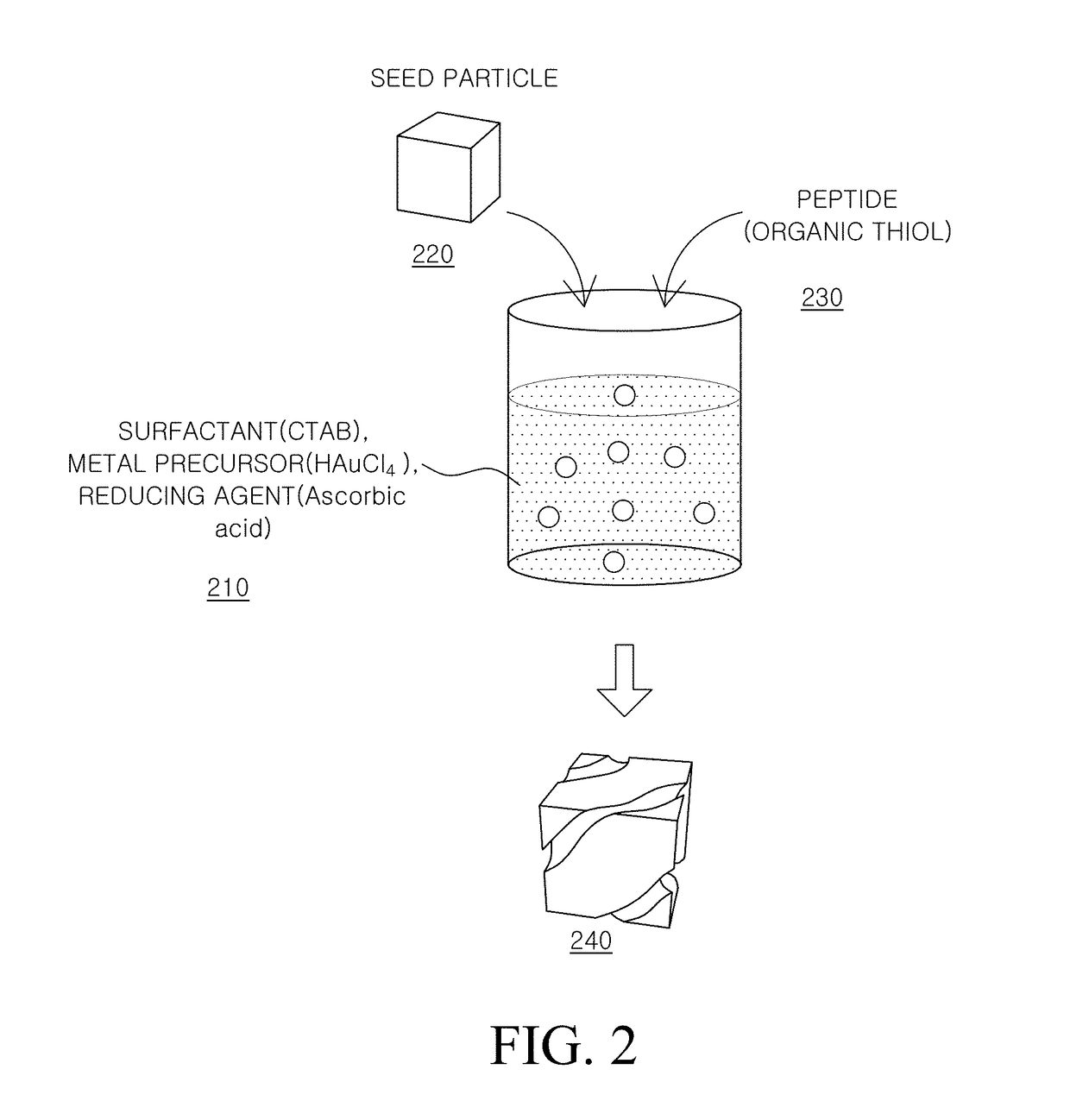
Metal Nanostructure and Method for Manufacturing Thereof
ActiveUS20180312636A1High optical activityGood optical performanceMaterial nanotechnologyFrom normal temperature solutionsPeptideMixed metal
The present disclosure relates to a method for manufacturing a metal nanostructure having a chiral structure. The method for manufacturing a metal nanostructure comprises: preparing a first mixture solution by mixing a metal precursor, a surfactant, and a reducing agent; preparing a second mixture solution by adding a peptide to the first mixture solution; and preparing a metal nanostructure having a chiral structure by adding a metal seed particle to the second mixture solution.
Owner:LG DISPLAY CO LTD +1
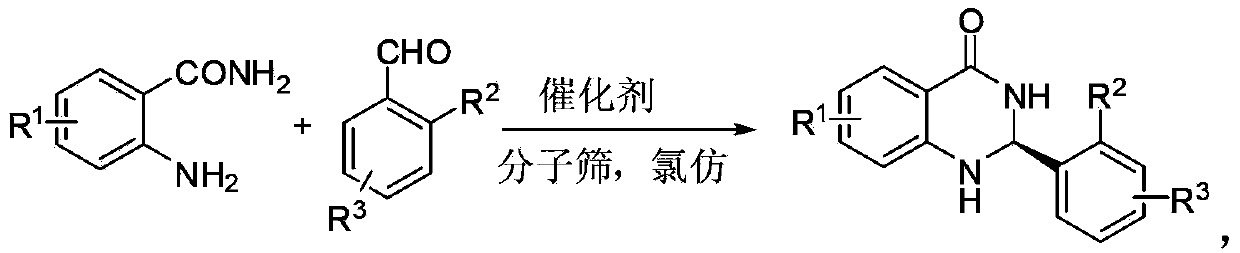
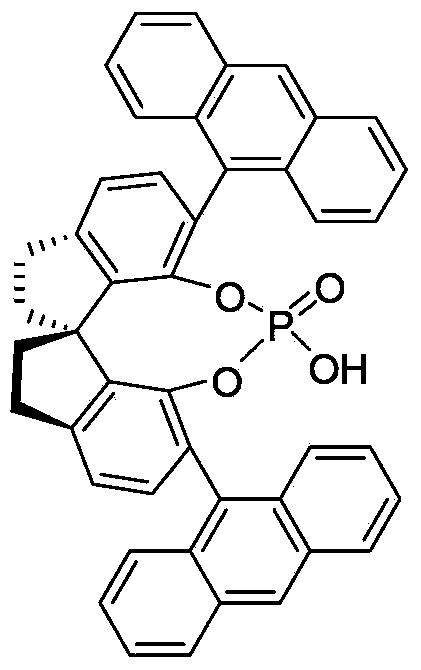
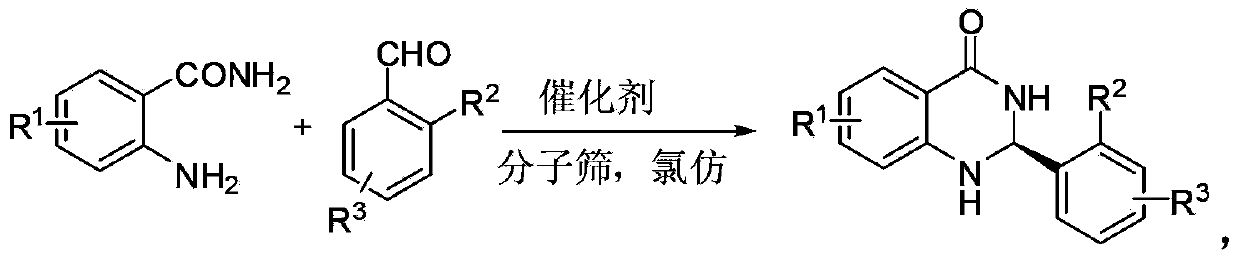
Method for synthesizing optically-active 2,3-dihydro quinazolinone derivative by using chiral spiro phosphoric acid as catalyst
InactiveCN103420921AMild reaction conditionsHigh optical activityOrganic chemistryMolecular sievePhosphoric acid
The invention discloses a method for synthesizing an optically-active 2,3-dihydro quinazolinone derivative by using chiral spiro phosphoric acid as a catalyst. The optically-active 2,3-dihydro quinazolinone derivative is synthesized by taking anthranilamide and aldehyde as raw materials, the chiral spiro phosphoric acid as the catalyst and chloroform as a reaction solvent, reacting for 24 hours at room temperature in the presence of molecular sieve powder, and purifying and separating by column chromatography. The method is mild in reaction condition, simple in process and convenient and rapid to operate; the synthesized optically-active 2, 3-dihydro quinazolinone derivative has potential good bioactivity and can be used as an intermediate in medicine synthesis.
Owner:ZHEJIANG UNIV
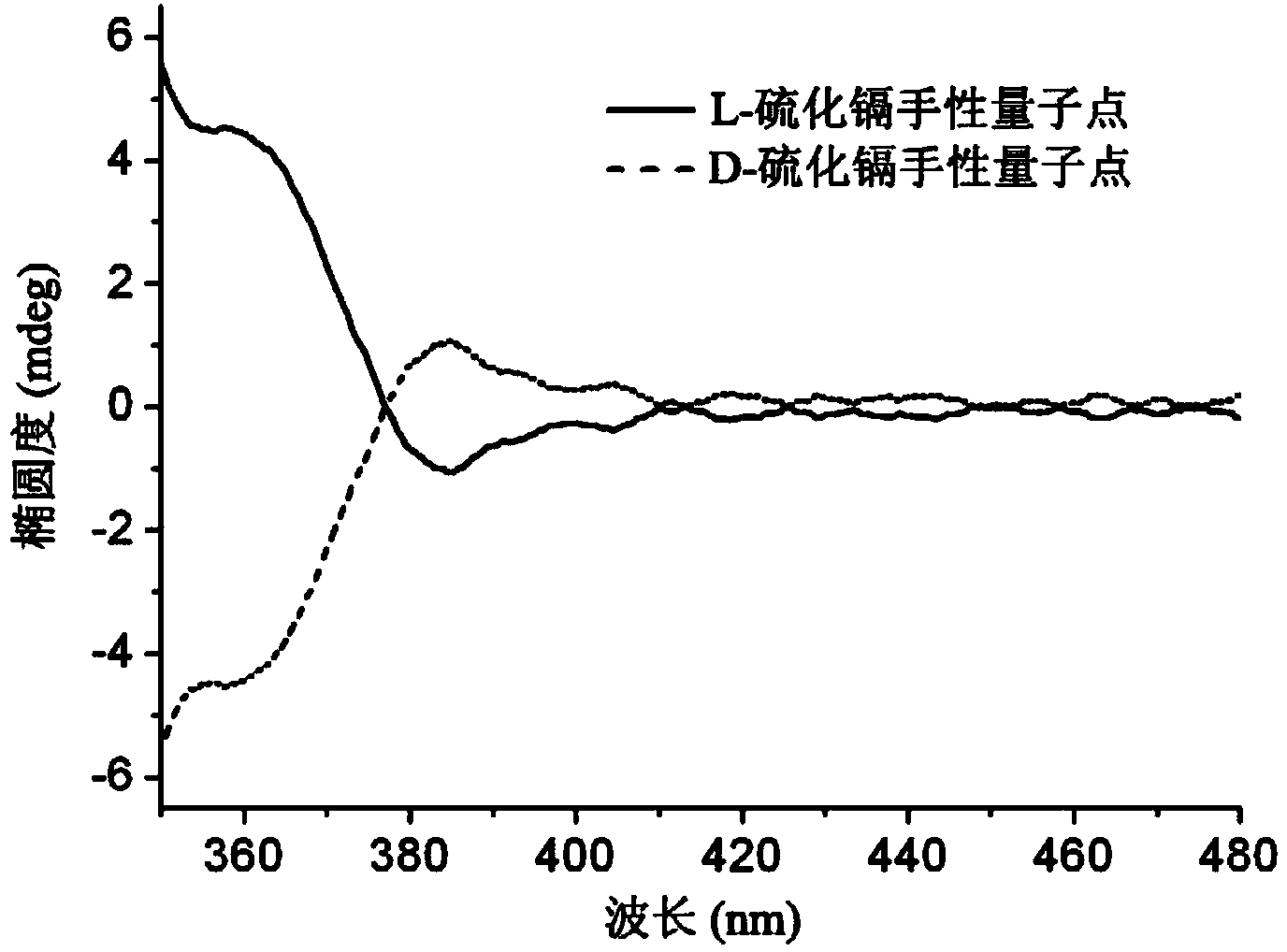
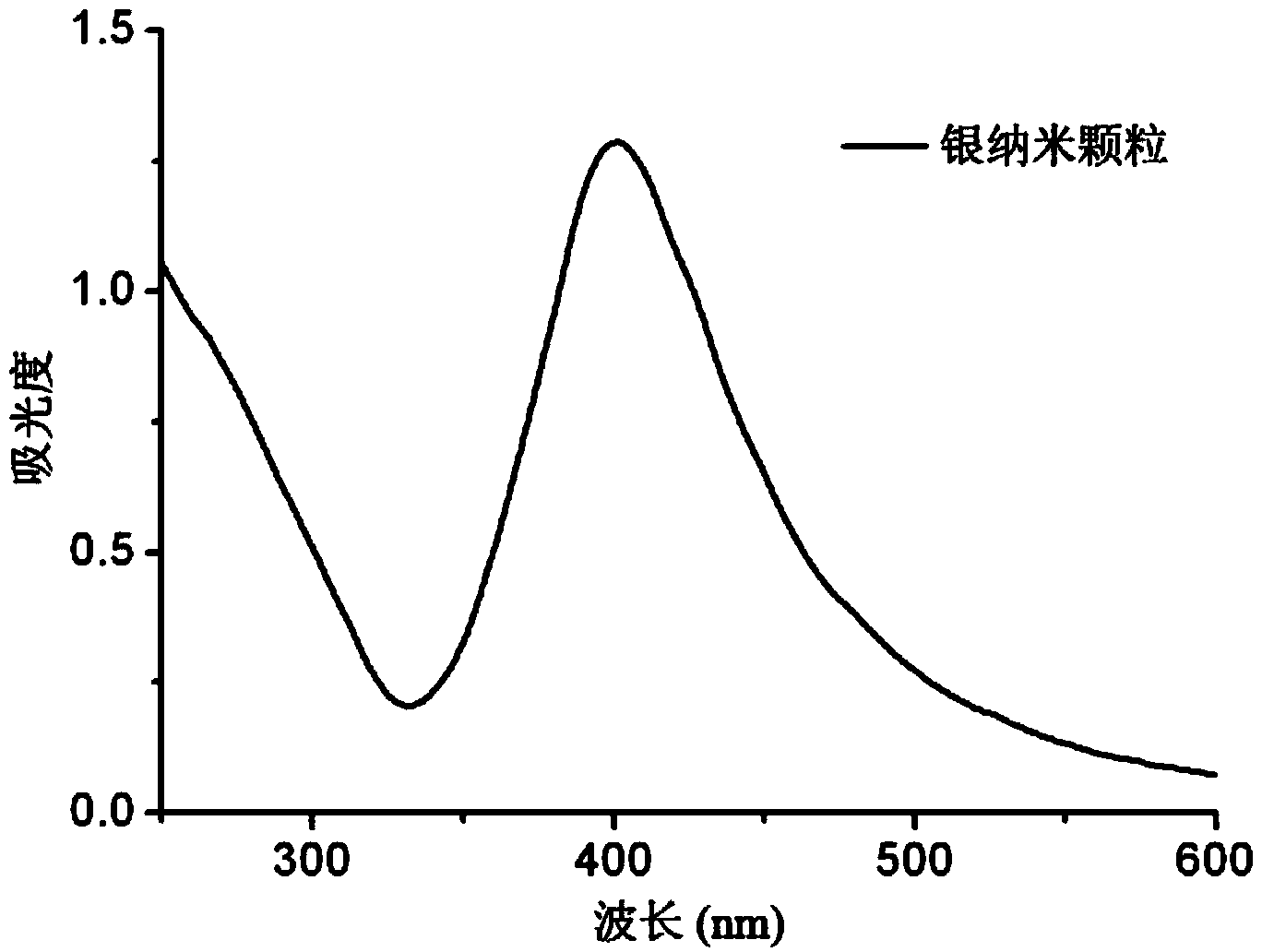
Silver composite chiral quantum dot nanomaterial and preparation method thereof
ActiveCN103923643AHigh optical activityStrong optical activityMaterial nanotechnologyLuminescent compositionsQuantum dotMaterials science
The invention relates to a silver composite chiral quantum dot nanomaterial. The silver composite chiral quantum dot nanomaterial is a silver nanoparticle coated with chiral quantum dots. The invention also discloses a preparation method. The method comprises the following steps: (1) preparing the chiral quantum dot nanoparticle with negative charges; (2) preparing a silver nanoparticle with positive charges; (3) self-assembling the chiral quantum dot nanoparticle with the negative charges obtained in the step (1) and the silver nanoparticle with the positive charges obtained in the step (2), so as to obtain the silver composite chiral quantum dot nanomaterial. The silver composite chiral quantum dot nanomaterial provided by the invention has strong optical activity, and the prepared silver composite chiral quantum dot nanomaterial is simple in preparation method, low in cost, diverse in component and property, wide in application range, and large in development space.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
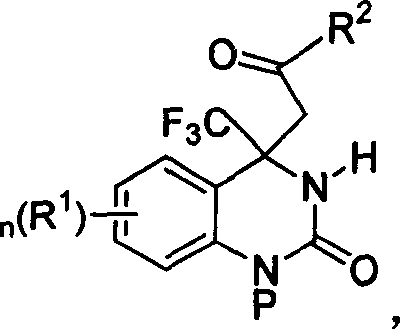
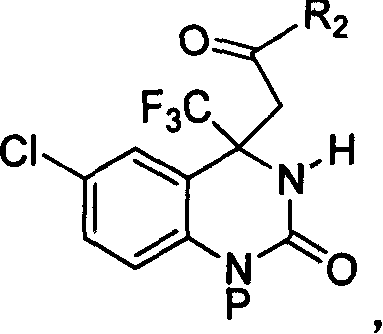
4,4-disubstituted-3,4-dihydro-2(1H)- quinolones and synthesis process and use thereof
InactiveCN1827605AHigh optical activityMild reaction conditionsOrganic active ingredientsOrganic chemistryQuinoloneFunction group
The invention relates to a 4, 4-disubstitutional-3,4-dihydro-2(one hydro)-quinaldinicketones compound and its method for synthesizing and its application. The said compound can be a compound with its constitutional formula on tha right, or its stereoisomer, its stereoisomeric mixture or a salt which can be accepted in the pharmacy. The method for synthesizing chiral pure isomer is carried out mainly by the asymmetric additive reaction between substrates of methyl ketone and trifluoromethylketone imine. Additionally, we can construct the most critical trifluoromethyl quaternary carbon chiral center of the target compound DPC083 easily and efficiently. Afterwards, the synthesis of the target compound DPC083 is realized by the transformation of the function group.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
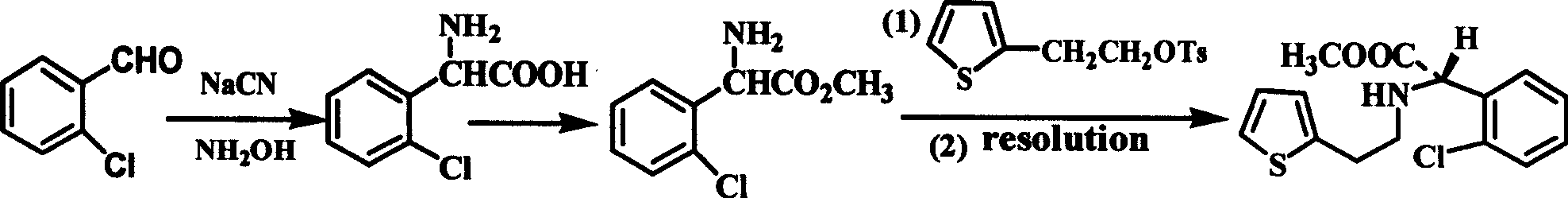
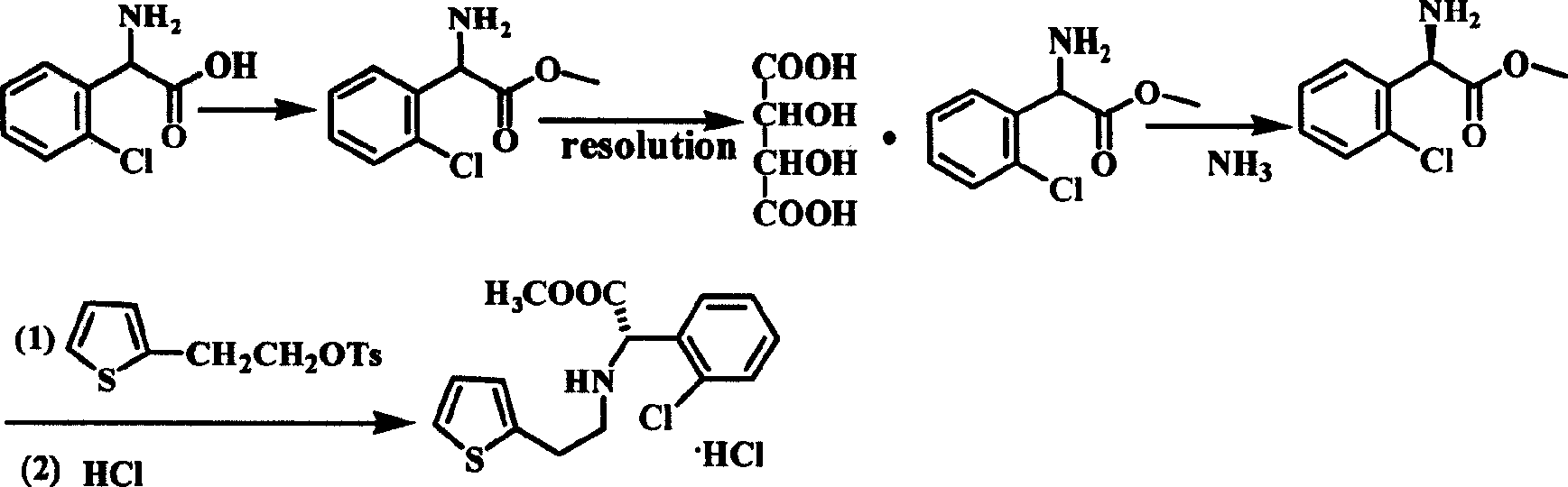
Intermediate (S)-2-(2-thiophene ethylamine)(2-chlorphenyl)methyl acetate of clopidogrel and method for preparing salts thereof
ActiveCN101519401ASimplified condensation reaction stepsThe reaction steps are simpleAsymmetric synthesesMethyl acetateEthyl ester
The invention relates to an intermediate (S)-2-(2-thiophene ethylamine)(2-chlorphenyl)methyl acetate of clopidogrel and the preparation of salts thereof. The invention improves the existing technologyThe invention relates to an intermediate (S)-2-(2-thiophene ethylamine)(2-chlorphenyl)methyl acetate of clopidogrel and the preparation of salts thereof. The invention improves the existing technology of preparing the intermediate and the salts thereof, and adopts (S)-(+)-chlorophenylglycine methyl ester L-tartrate as raw materials which directly react with p-substituted thiofuran benzenesulphonatof preparing the intermediate and the salts thereof, and adopts (S)-(+)-chlorophenylglycine methyl ester L-tartrate as raw materials which directly react with p-substituted thiofuran benzenesulphonate-2-ethyl esters compounds for preparing the intermediate. Compared with the prior art, the improved method has the advantages that the raw materials and reagents are cheap and easily available, the pe-2-ethyl esters compounds for preparing the intermediate. Compared with the prior art, the improved method has the advantages that the raw materials and reagents are cheap and easily available, the production yield is high, the optical purity of the product is high, the technological operation is simple, and the like, thus being a method for commercially producing the intermediate of clopidogrelroduction yield is high, the optical purity of the product is high, the technological operation is simple, and the like, thus being a method for commercially producing the intermediate of clopidogreland salts thereof.and salts thereof.
Owner:SHANGHAI ECUST BIOMEDICINE CO LTD +1
Biological preparation method of (R)-3-amino-4-(2,4,5-trifluorophenyl) tert-butyl butyrate
The invention discloses a biological preparation method of (R)-3-amino-4-(2,4,5-trifluorophenyl) tert-butyl butyrate. According to the method, a 3-carbonyl-4-(2,4,5-trifluorophenyl) tert-butyl butyrate is used as a substrate; under the existence of transaminase, amino donors, auxiliary factors and cosolvents, a reaction is taken to produce a target product of (R)-3-amino-4-(2,4,5-trifluorophenyl) tert-butyl butyrate. The reaction is performed in a water phase buffer solution with the pH being 6.0 to 10.0. The transaminase is used for converting the 3-carbonyl-4-(2,4,5-trifluorophenyl) tert-butyl butyrate into the (R)-3-amino-4-(2,4,5-trifluorophenyl) tert-butyl butyrate for the first time. Compared with the prior art, the process has the advantages that the substrate stability is good; the enzymatic conversion rate is high; the operation is simple and convenient; the subsequent product does not have protein residue; a better green and economical performance is realized; the optical activity of the product is high.
Owner:ENZYMEWORKS
Preparation method of photoresponseive cellulose nanocrystalline/fluorine-containing polyacrylate self-repairing material
The invention discloses a p photoresponseive cellulose nanocrystalline / fluorine-containing polyacrylate self-repairing material. A preparation method specifically comprises the following steps: step 1, preparing a Pickering emulsion; wherein uniformly mixing photoresponsive amphiphilic block copolymer graft-modified cellulose nanocrystalline, water and a mixed monomer according to a mass ratio of(0.01-0.03): (2-60): (1-3) to obtain the Pickering emulsion; and 2, preparing the photoresponsive cellulose nanocrystalline / fluorine-containing polyacrylate composite emulsion. The photoresponsive cellulose nanocrystalline / fluorine-containing polyacrylate self-repairing material disclosed by the invention has the characteristic of self-repairing.
Owner:SHAANXI UNIV OF SCI & TECH
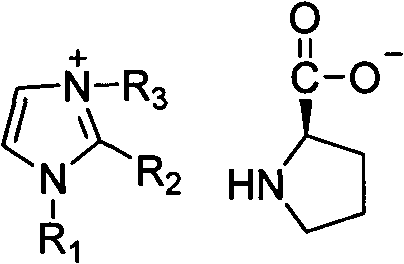
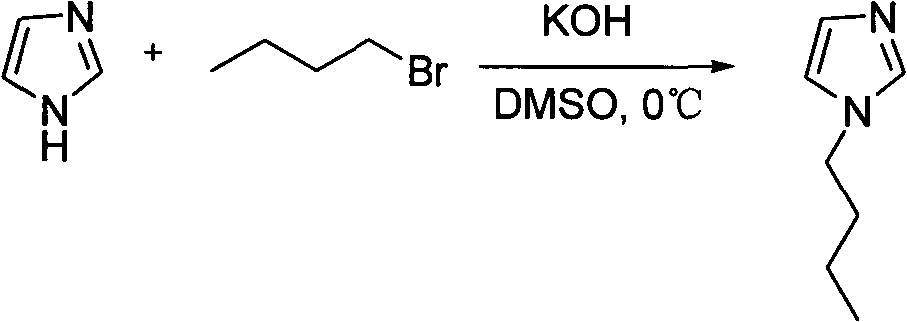
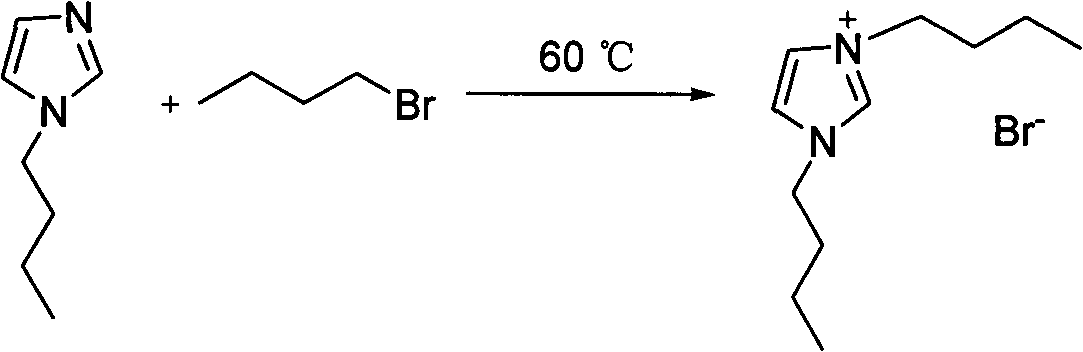
Alkyl imidazole-L-proline salt chiral ionic liquid and preparation method thereof
InactiveCN101891685ANo secondary pollutionHigh optical activityOrganic chemistryPotassium hydroxideStructural formula
The invention relates to an alkyl imidazole-L-proline salt chiral ionic liquid and a preparation method thereof. The structural formula of the alkyl imidazole-L-proline salt chiral ionic liquid is shown in formula (I), and the preparation method thereof comprises the following steps: enabling alkyl imnidazole bromide and potassium hydroxide to react in ethanol solution for 4h; removing generated salt; stirring with L- L-proline at the temperature of 0 DEG C and reacting for 12h; and carrying out vacuum drying on products for 24h. The optical rotation and optical purity OP%ee of the alkyl imidazole-L-proline salt chiral ionic liquid prepared by the invention are measured at the temperature of 25 DEG C; according to the method provided by the invention, the preparation process is green and environmental friendly, and no secondary pollution is generated; and the alkyl imidazole-L-proline salt chiral ionic liquid synthesized in the invention has favorable optical activity. The formula (I) is shown in the specification, wherein R1 is CH3 or (CH2)n-CH3, and n is from 1 to 5; R2 is H, CH3 or (CH2)n-CH3, and n is from 1 to 5; and R3 is (CH2)n-CH3, and n is from 1 to 5.
Owner:QIQIHAR UNIVERSITY
Graphene crystal composite polylactic acid antibacterial material and preparation method thereof
The invention belongs to the field of high molecular materials, and particularly relates to a modified graphene polylactic acid antibacterial composite material and a preparation method of the composite material. An antibacterial mixture used in the composite material comprises nano silver and nano titanium oxide, and nano zinc oxide capable of improving the optical activity of nano titanium oxide is added, so that an antibacterial property is greatly improved, a nature is more stable, and antibacterial action is more persistent. The composite material fully exerts antibacterial properties of nano silver and nano titanium oxide and a mechanical characteristic of a graphene material, so that a mechanical property of the material is improved under the condition that an antibacterial effect is maximized; and the preparation method is mild in technological condition, and is suitable for industrial production.
Owner:江苏苏沃尚新材料科技有限公司
Method for preparing 4-hydroxyl tetrahydropyran derivative with optical activity through enzymatic transesterification
InactiveCN103194520AHigh optical activityHigh reaction enantioselectivityFermentationChromatographic separationHigh activity
The invention discloses a method for a preparing 4-hydroxyl tetrahydropyran derivative with optical activity through enzymatic transesterification. The method comprises the following steps: adding a mixture of a racemic 4-hydroxyl tetrahydropyran derivative and an acyl donor with the molar ratio of 1:1 to 1:4 into an organic solvent; adding magnetic immobilized lipase; reacting under stirring at the temperature of 10 to 60 DEG C for 2 to 36 hours, wherein the relative using amount of the magnetic immobilized lipase and the racemic 4-hydroxyl tetrahydropyran derivative is controlled to be from 1mg:1ml to 100mg:1ml; separating out the immobilized enzyme from the magnetic field and stopping reaction; and performing column chromatographic separation to obtain the 4-hydroxyl tetrahydropyran derivative with optical activity and acetic acid ester of the derivative. The method is realized by utilizing high activity, high selectivity and high stability of the magnetic immobilized enzyme; the immobilized enzyme is separated quickly under the action of the magnetic field; mechanical loss is avoided; high-efficiency recycling is realized; the catalytic efficiency is high; the cost is low; and the process is simple and quick.
Owner:NINGXIA UNIVERSITY
Azobenzene diamine chromophore with photoelectric activity and its preparing method
InactiveCN1900170AHigh absorption wavelengthGood Photochromic PropertiesMonoazo dyesRecord information storageSynthesis methodsMonomer
The present invention belongs to the field of photoelectronic functional material technology, and is especially one kind of optical active organic chromophore molecule of azometaphenylene diamine and its synthesis process. The present invention features that the azometaphenylene diamine compound is synthesized with metaphenylene diamine and aromatic diazo salt and through coupling reaction, has absorption wavelength in the visible light area, and possesses two reaction active primary amine groups. The azometaphenylene diamine compound may be mixed with polymer to obtain non-linear optical performance and may be reacted with diisocyanate and other active monomer to introduce polymer chain, so as to obtain polymer with high chromophore content, excellent heat stability, etc. It may be used in preparing light memory medium with high storing density and high stability.
Owner:TONGJI UNIV
Chemical-enzymatic method for preparing D-serine
InactiveCN102321695AConvenient sourceEfficient separationOrganic compound preparationAmino-carboxyl compound preparationPenicillinPhenylacetic acid
The present invention provides a chemical-enzymatic method for preparing D-serine. According to the method, DL-serine is adopted as a raw material and is derived into DL-N-phenylacetyl serine by using an acylating agent; immobilized penicillin acylase is adopted as a biocatalyst, correspondingly and selectively catalyze the DL-N-phenylacetyl serine in an aqueous medium to obtain L-serine, phenylacetic acid and D-N-phenylacetyl serine; a metal complexation method and an isoelectric point crystallization method are adopted to carry out separation to obtain optically pure D-phenylacetyl serine; the D-N-phenylacetyl serine is subjected to acid hydrolysis, concentration and crystallization to obtain the D-serine, wherein the yield of the D-serine is 45%, ee of the D-serine is 99.6%. The method provided by the present invention has characteristics of high yield, high chemical purity, high optical purity, environment-friendly property, and is suitable for the large-scale production of the D-serine.
Owner:CHONGQING UNIV OF POSTS & TELECOMM
Method for extracting fluorescent carbon quantum dots from semi coke
InactiveCN105621391AStrong fluorescenceFixed carbon heightMaterial nanotechnologyLuminescent compositionsDistillationCentrifugation
The invention provides a method for extracting fluorescent carbon quantum dots from semi coke. The method comprises the following steps: firstly, adding semi coke powder into a 4M to 6M nitric acid solution, and carrying out stirred refluxing at the temperature of 80 DEG C to 120 DEG C; carrying out natural cooling, then, carrying out centrifugation for 30 minutes to 60 minutes at the rate of 10,000r / min to 15,000r / min, collecting supernatant, removing precipitates, and drying the obtained supernatant by distillation in a reduced-pressure distillation manner, so as to obtain black solids; dispersing the black solids in deionized water, carrying out neutralizing by ammonia water, then, carrying out centrifugation for 30 minutes to 60 minutes at the rate of 10,000r / min, and collecting supernatant; dialyzing the supernatant by dialysis bags, collecting solutions in the dialysis bags, and carrying out vacuum drying for 24 hours to 48 hours at the temperature of 60 DEG C to 80 DEG C, thereby obtaining the carbon quantum dots. According to the method, the semi coke which is low in cost and easy in raw material obtaining is adopted as a carbon source, and the carbon quantum dots can be obtained through simple chemical oxidation, evaporation, neutralization, centrifugation and dialysis treatment processes. The carbon quantum dots have the particle size of 3nm to 5nm and are uniform in dispersion, and surfaces of the carbon quantum dots have a large number of carboxyl groups and hydroxyl groups, so that a relatively good fluorescent property is shown.
Owner:XINJIANG UNIVERSITY
Production method of optically active N-benzyl-1-phenylethylamine
InactiveCN101525293AHigh optical activityRacemization does not occurOrganic compound preparationAmino compound preparationChemical industryN-benzyl-1-phenylethylamine
The invention relates to a production method of optically active N-benzyl-1-phenylethylamine, in particular to the production of optically active N-benzyl-1-phenylethylamine in the field of chemical industry. Optically active phenylethylamine and benzaldehyde as well as a palladium-charcoal as a catalyst are respectively put into a pressure kettle and replaced by N2 thrice, hydrogen gas is introduced into the pressure kettle when the reactants are stirred and heated until no hydrogen is absorbed in the reaction, the reaction is kept for half an hour under the conditions of heat preservation and pressure maintaining, the mixture is discharged after being cooled and the palladium-charcoal as a catalyst is filtered out; the reaction products with the catalyst being filtered out is dehydrated through atmospheric distillation, then a small amount of front cut fractions are removed through reduced pressure distillation and 169 DEG C to 173 DEG C / 15mmHg of fractions, namely the finished products are collected. Not only higher optical activity of the optically active N-benzyl-1-phenylethylamine produced by the method can be obtained, but also the yield can be stabilized at 90 percent to 93 percent and the chemical purity can reach more than 99.8 percent.
Owner:SHANDONG YUEXING CHEM CO LTD
Preparation process of antibacterial wool knitted fabric
InactiveCN109837739AImprove antibacterial propertiesImprove securityWarp knittingDyeing processWoolChemistry
The invention discloses a preparation process of antibacterial wool knitted fabric. The antibacterial wool knitted fabric comprises the following components in parts by weight: 25-35 parts of wool, 40-46 parts of polyester fibers, 45-56 parts of flax and 10-16 parts of a modified antibacterial agent. According to the preparation process of the antibacterial wool knitted fabric, an antibacterial treatment process is additionally arranged between a drying treatment process and a bristle treatment process; the antibacterial performance of the fabric can be greatly improved, the modified antibacterial agent is prepared, and under the illumination condition, titanium dioxide in the modified antibacterial agent can be in contact with water and oxygen in an environment to generate negative oxygenions and hydroxyl radicals. The fabric has high purification effect, is convenient for cleaning stains and has strong sterilization performance. The use safety of the fabric is ensured, and the surfaces of the woven fabric wool, the polyester fibers and the flax can be prevented from being stained with oil stain on the surface of a weaving machine in the woven fabric. Relative stability can be ensured, and transverse vibration of the weaving mechanism can be effectively reduced.
Owner:广州市雅娜琪服装有限公司
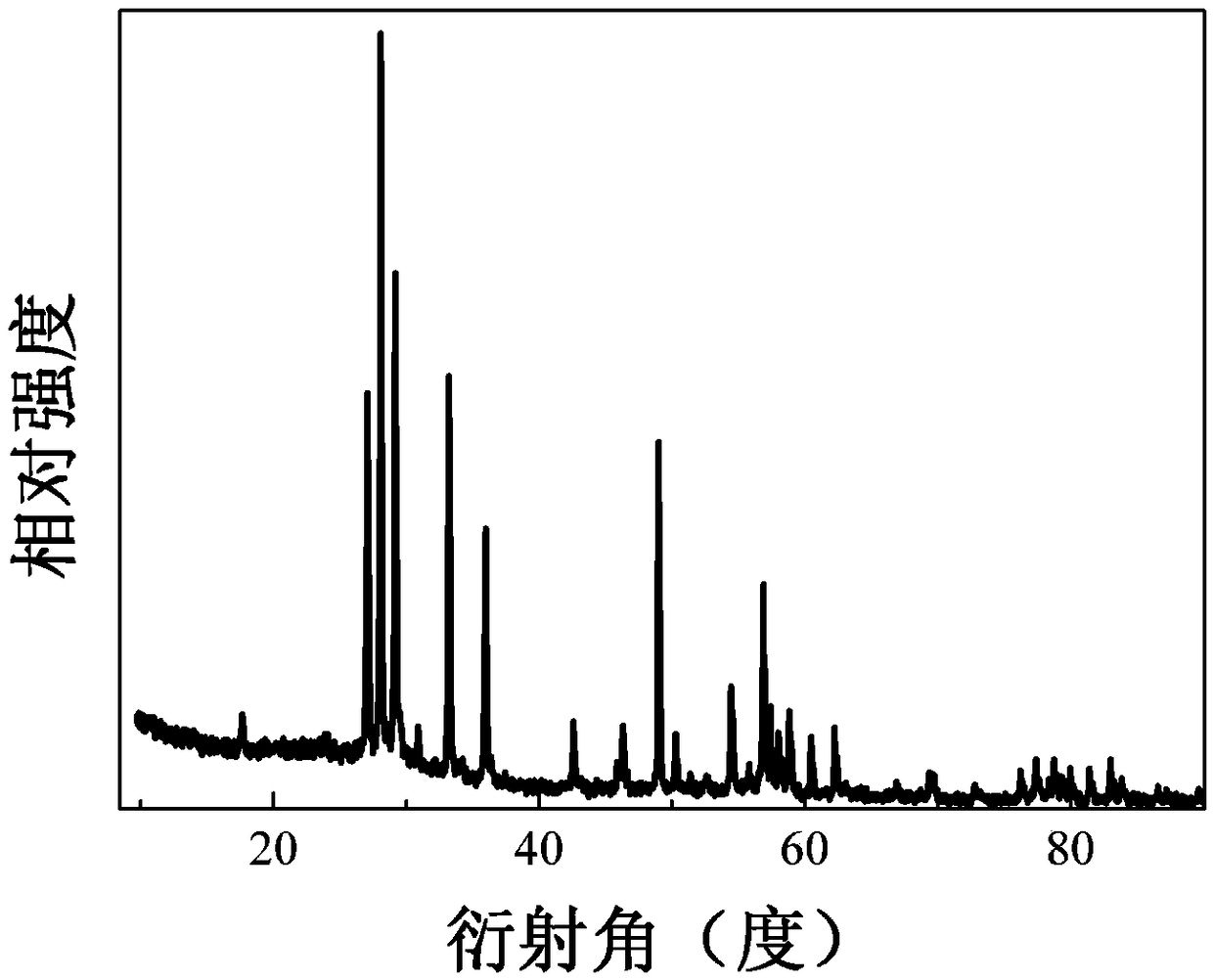
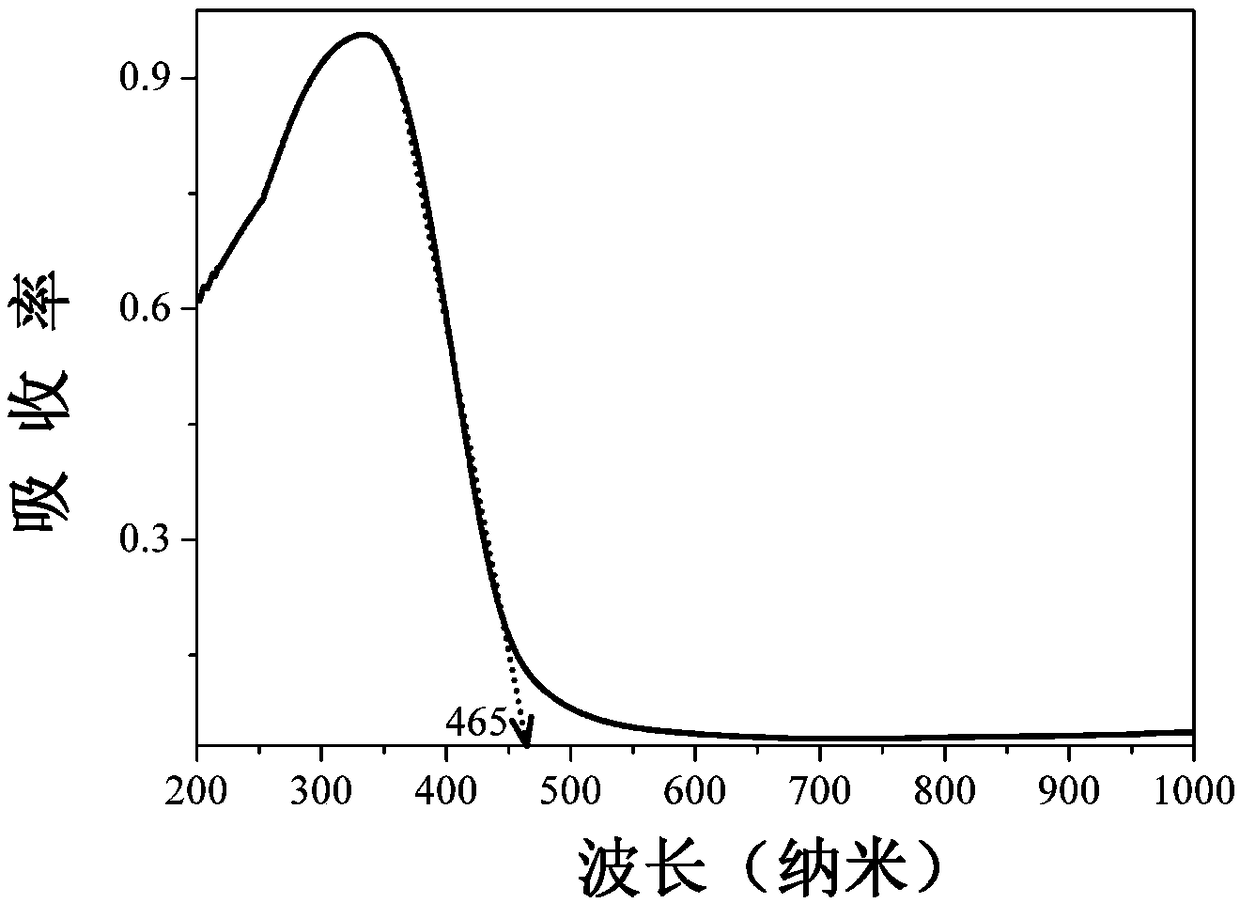
Cerium-titanium-doped bismuth tantalate niobate photocatalytic material and preparation method and application thereof
ActiveCN109395713AHigh crystallinityHas a porous structureWater/sewage treatment by irradiationWater treatment compoundsHigh absorptionCerium
The invention discloses a cerium-titanium-doped bismuth tantalate niobate photocatalytic material and a preparation method and application thereof. A chemical general formula of the photocatalytic material is Bi<8(1-x)>Ce<8x>Ta<8(1-y)>Ti<8y>Nb<10>O<57>, wherein bismuth tantalate niobate is used as a host material, x is a mole percent of a Ce<3+> and Bi<3+> doped site, y is a mole percent of a Ti<4+> and Ta<5+> doped site, x is greater than or equal to 0.005 and less than or equal to 0.07, y is greater than or equal to 0.005 and less than or equal to 0.07, and x equals y; the photocatalytic material is prepared through a high-temperature solid phase method, is pure in phase and good in crystallinity, is provided with a porous structure, has high absorption capacity within the range of 200-465 nanometers, has good optical activity and can efficiently degrade methylene blue in a photocatalytic mode. The tantalate light emitting material is easy to prepare and low in production cost.
Owner:XUZHOU NORMAL UNIVERSITY +1
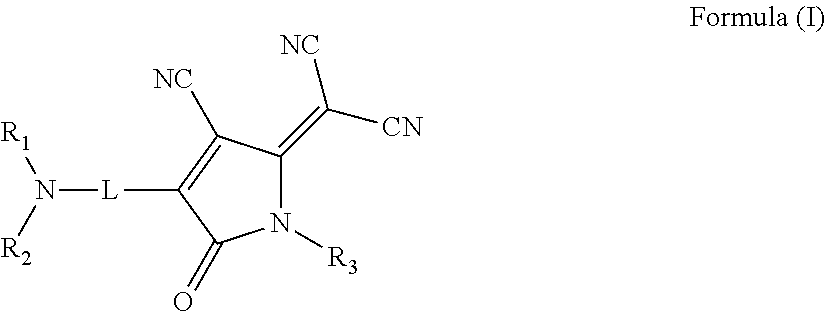
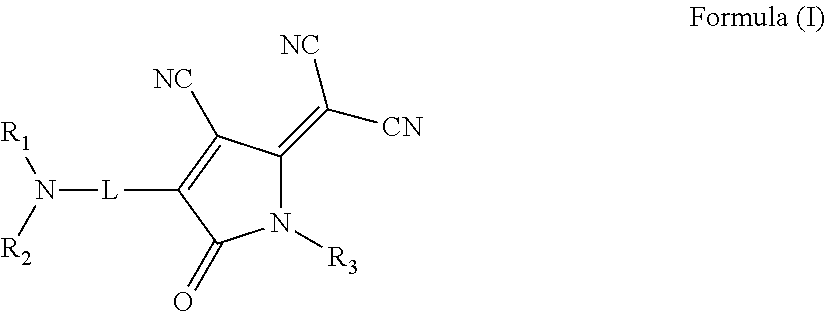
Nonlinear optical materials and nonlinear optical device using the same
InactiveUS20150014607A1Improve photostabilityGood optical performanceOrganic chemistryPolarising elementsArylPolymer science
There is provided an organic nonlinear optical material including a polymer binder and a compound represented by the following Formula (I):wherein, in Formula (I),R1 and R2 each independently represent a substituted or unsubstituted alkyl group or a substituted or unsubstituted aryl group;R3 represents a hydrogen atom, a substituted or unsubstituted alkyl group, or a substituted or unsubstituted aryl group; andL represents a divalent linking group connecting a nitrogen atom and an oxopyrroline ring having a dicyanomethylidene group in a π-conjugated system containing an azo group (—N═N—).
Owner:FUJIFILM CORP
Method for preparing Alpha-methyl-Alpha, Alpha-disubstituted-Alpha-aminophenol and derivatives thereof
InactiveCN101565390ASave raw materialsEasy to buyCarbamic acid derivatives preparationOrganic compound preparationSodium iodideMethylene Dichloride
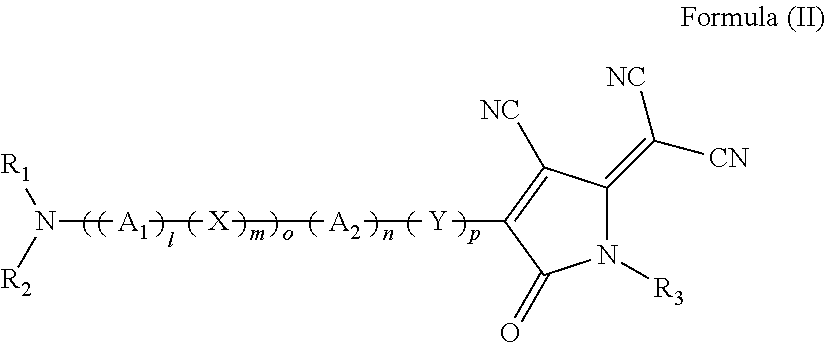
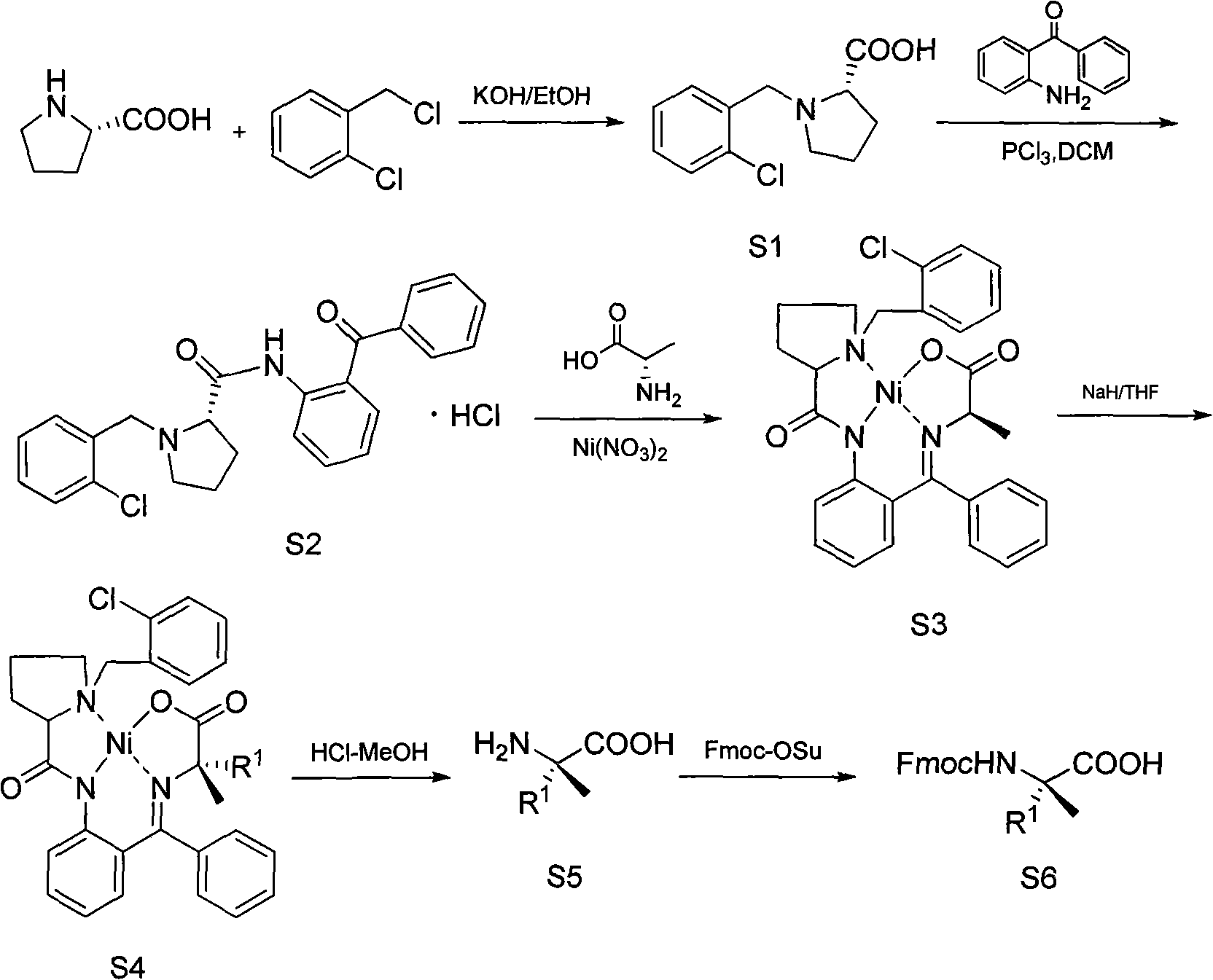
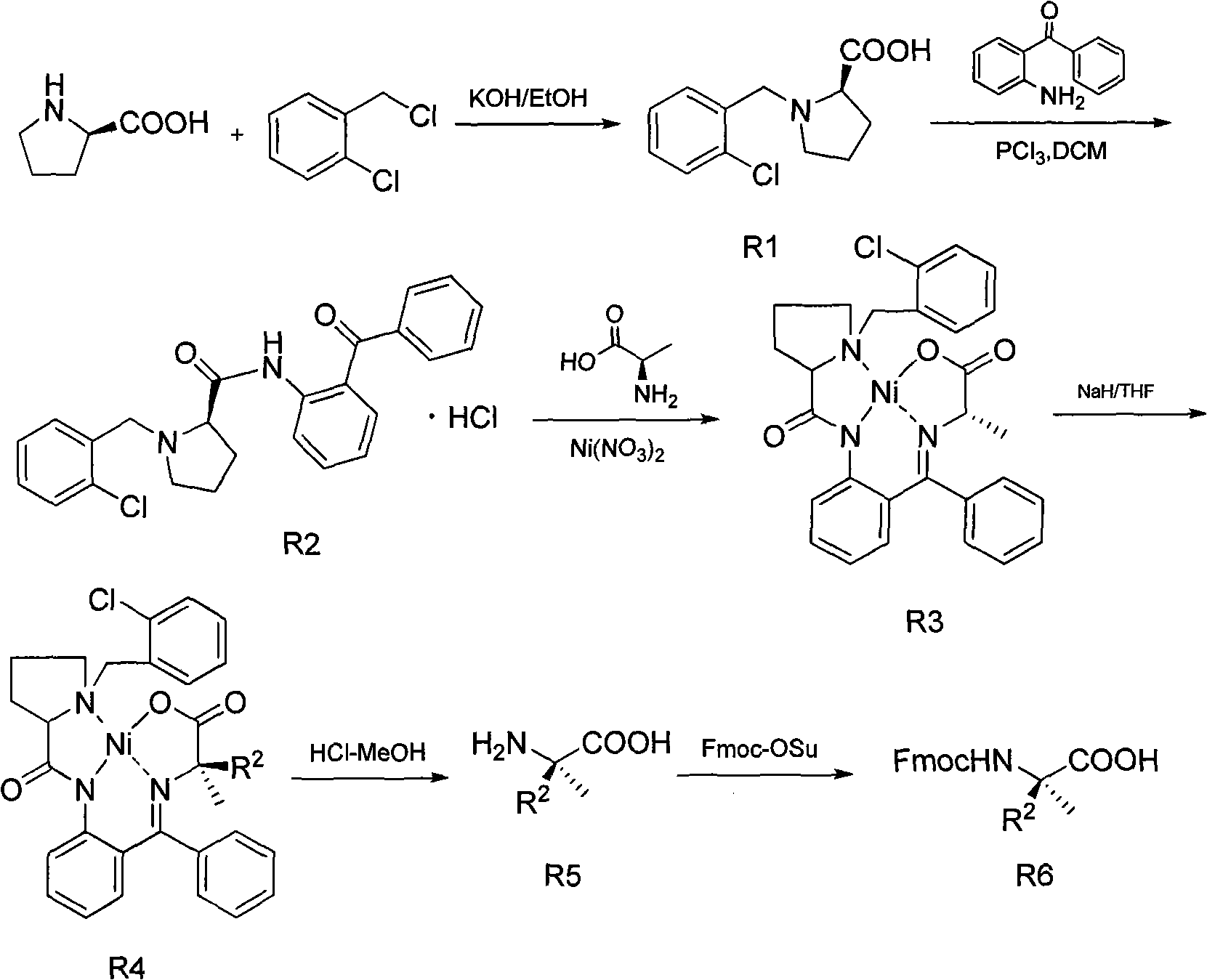
The invention discloses a method for preparing an Alpha-methyl-Alpha, Alpha-disubstituted-Alpha-aminophenol and derivatives thereof, comprising the following steps of: a) synthesizing S1 / R1: leading proline to react with o-chlorobenzyl chloride; b) synthesizing S2 / R2: leading methylene dichloride solution of S1 / R1 to react with phosphorus trichloride and 2-aminobenzophenone; c) synthesizing S3 / R3: leading S2 / R2 and alanine to react with nickelous nitrate hexahydrate; d) synthesizing S4 / R4: leading the redistilled tetrahydrofuran to react with sodium iodide, sodium hydride and RX / RX; e) synthesizing S5 / R5: leading the methanol solution of S4 / R4 to react with hydrochloric acid and concentrated ammonia liquor; and f) synthesizing S6 / R6: leading S5 / R5 to react with anhydrous sodium carbonate and Fmoc-Osu. The invention uses easily obtained raw materials with low price to obtain the Alpha-methyl-Alpha, Alpha-disubstituted-Alpha-aminophenol and derivatives thereof with extremely high optical activity by a normal six-step organic chemistry method. The method has moderate reaction condition and is easy for operation and amplification.
Owner:BEIJING OKEANOS TECH
Preparation method for (2S, 3aR, 7aS)-octahydro indole-2-benzyl formate
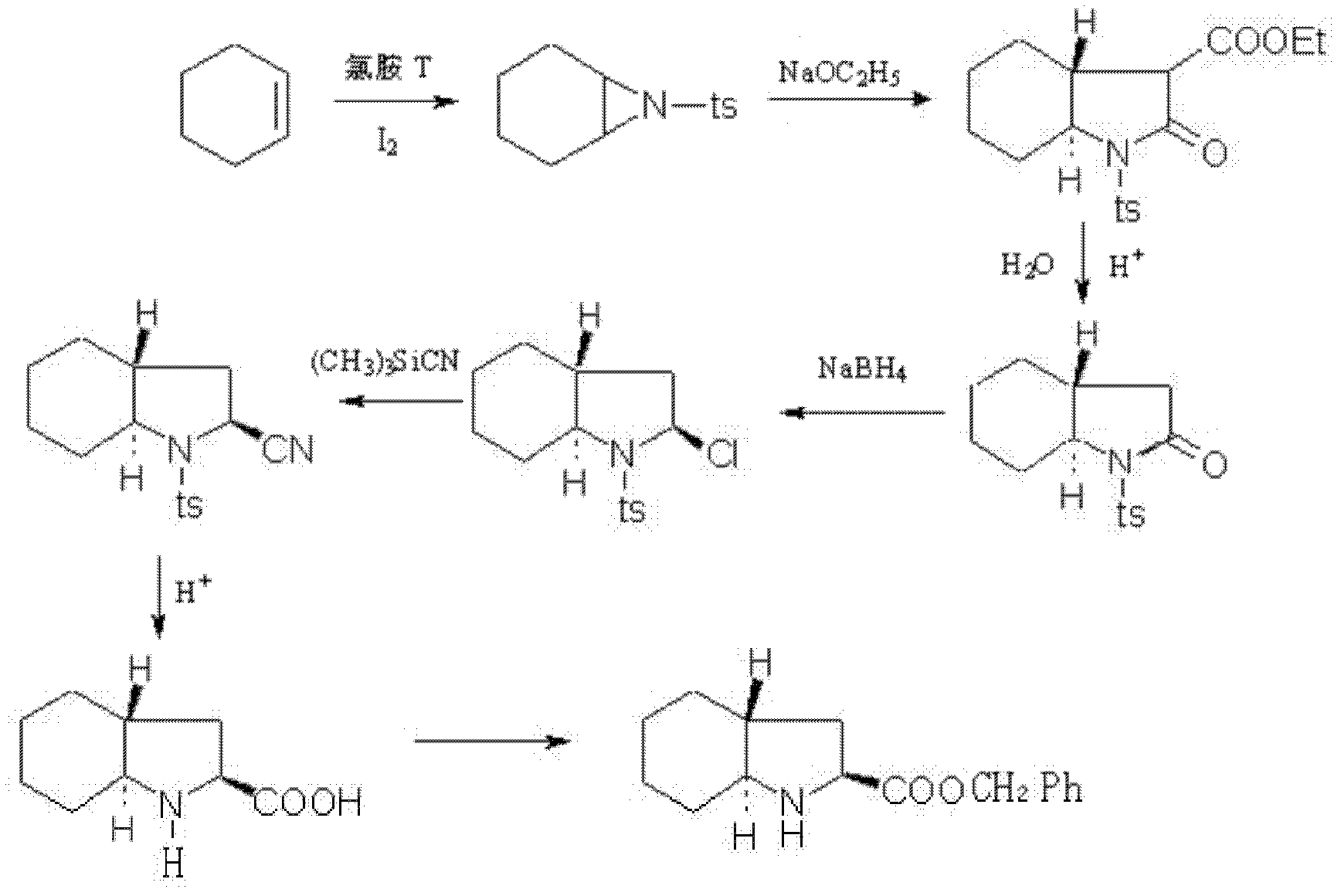
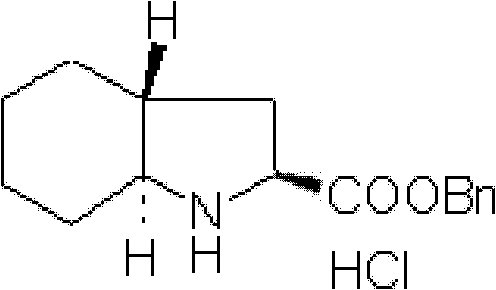
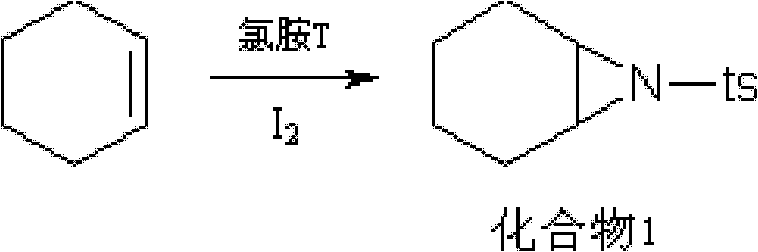
ActiveCN102321010AOvercome flammable and explosiveOvercome high pricesOrganic chemistryStrong acidsBENZYL ALCOHOL/WATER
The invention relates to a preparation method for a medicine intermediate and aims to provide a preparation method for (2S, 3aR, 7aS)-octahydro indole-2-benzyl formate. The method is high in yield and steps are reduced. The method comprises the following steps of: performing cis-form addition on cyclohexene and chloramines-T under the condition of adding a catalyst I2; heating and refluxing diethyl malonate and the product of the cis-form addition to perform ring-closure reaction; hydrolyzing the product of the ring-closure reaction; heating the hydrolysate to perform decarboxylation; performing sodium borohydride reducing chlorination on the decarboxylate; adding trimethyl cyano silane into the product of the reducing chlorination to perform substitution reaction; performing acidolysis on the substitution product by using concentrated hydrochloric acid and acetic acid as catalysts under the condition of heating and refluxing; performing refluxing water distribution on the product of the acidolysis and benzyl alcohol under the action of a strong acid catalyst to perform esterification; and further purifying the esterification product by using a resolving agent.
Owner:ANHUI TOPSUN PHARMA
Biodegradable rare-earth fluorescent film taking polyhydroxybutyrate as matrix as well as preparation method and application of biodegradable rare-earth fluorescent film
ActiveCN105038166AGood biocompatibilityHigh optical activityLuminescent compositionsPlant protective coveringsFluorescenceEuropium
The invention provides a biodegradable rare-earth fluorescent film taking polyhydroxybutyrate (PHB) as a matrix as well as a preparation method and an application of the biodegradable rare-earth fluorescent film. The fluorescent film takes PHB as the matrix, wherein a europium complex Eu(TTA)2TPY-OCH3 is doped in the matrix. The preparation method comprises the following steps: selecting the PHB which has excellent biocompatibility, biodegradability and optical activity as a polymer matrix, dissolving and blending the rare-earth europium complex Eu(TTA)2TPY-OCH3 with the matrix according to a certain proportion by using a doping method, and dropping liquid to form the film. The preparation method is relatively low in cost, simple in operation and low in risk, and a rare earth luminescent material is in biodegradable combination with the matrix, so that the innovative biodegradable fluorescent film with high luminescent property and high environment-friendliness is prepared. The film material provided by the invention has a very broad application prospect in the fields of agricultural film materials and medical materials.
Owner:CHONGQING UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com