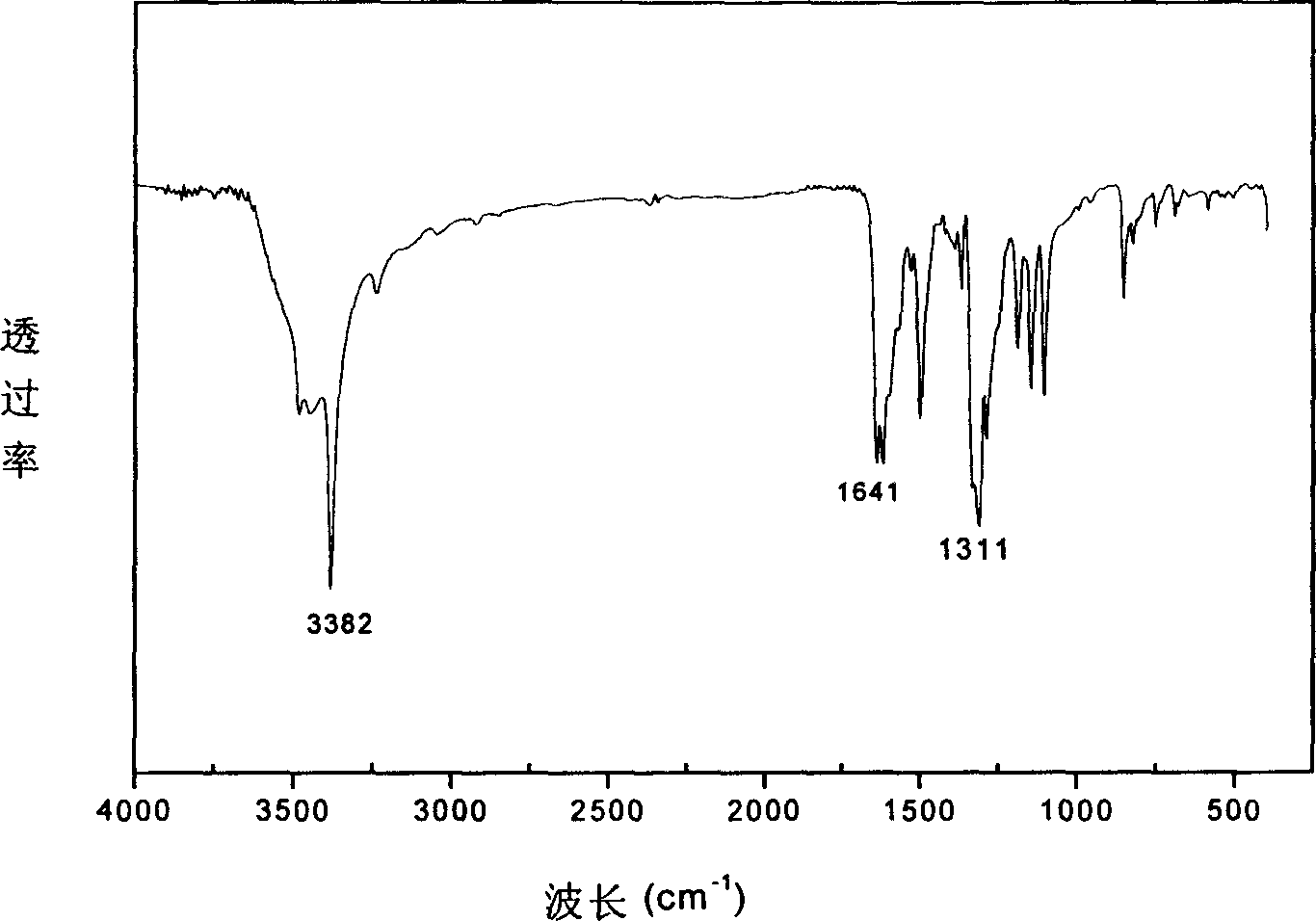
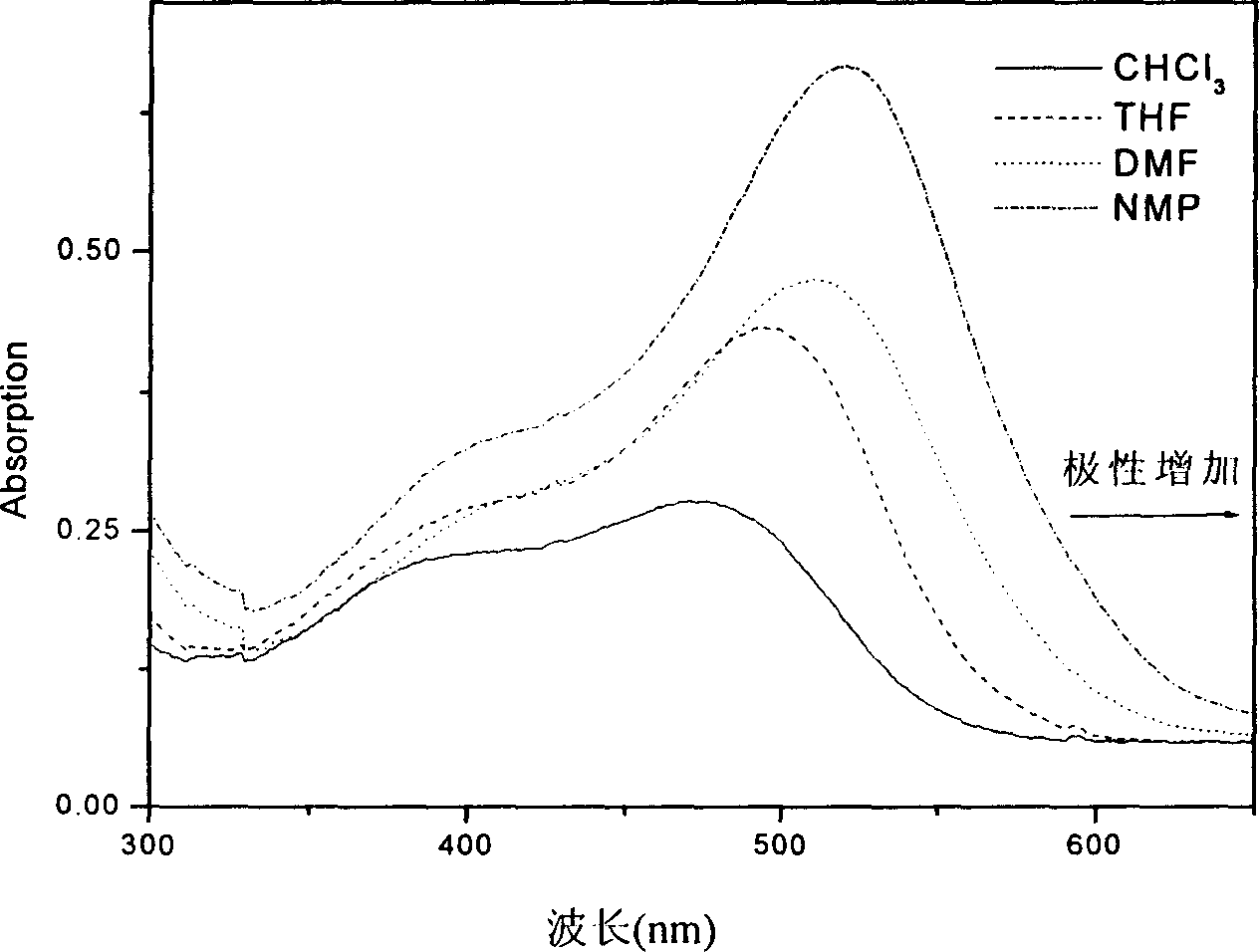
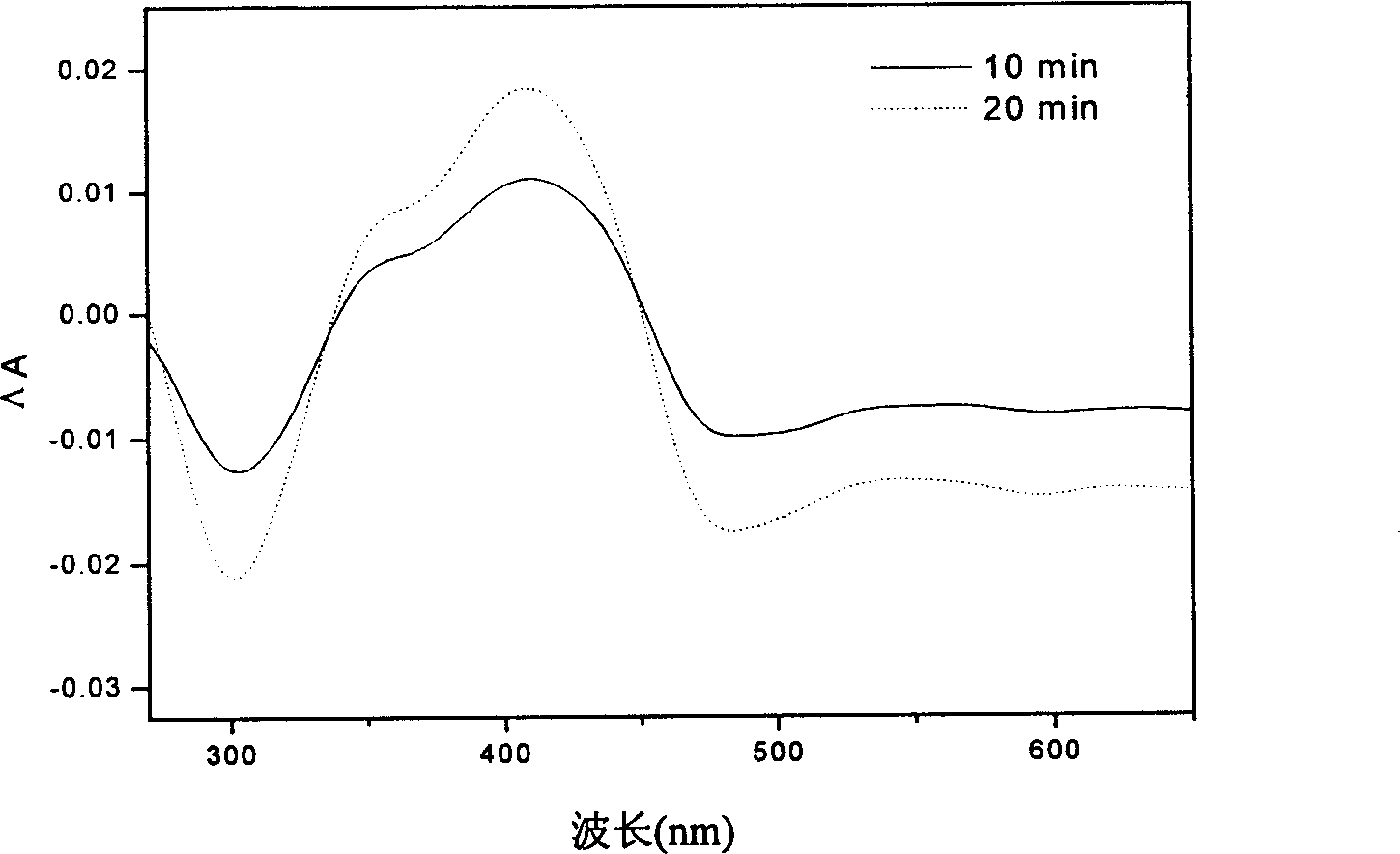
Azobenzene diamine chromophore with photoelectric activity and its preparing method
A technology of azophenylenediamine and chromophore, which is applied in the field of optoelectronic functional materials, can solve the problems of high price of laser light source, no contribution to polymer stability, and difficulty in accumulating chromophore, etc., and achieves excellent photochromic performance, Excellent thermal stability and high chromophore content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The raw materials used in the embodiment are as follows:
[0033] m-Phenylenediamine (analytical grade) was provided by China National Pharmaceutical (Group) Shanghai Chemical Reagent Company.
[0034] p-Nitroaniline (analytical grade) was provided by China National Pharmaceutical (Group) Shanghai Chemical Reagent Company.
[0035] Sodium nitrite (analytical grade) was provided by Shanghai Shisi Hewei Chemical Co., Ltd.
[0036] Hydrochloric acid (analytical grade) was provided by China Pharmaceutical Group Shanghai Chemical Reagent Co., Ltd.
[0037] Tetrahydrofuran (analytical grade) was provided by China National Pharmaceutical (Group) Shanghai Chemical Reagent Company.
[0038] The ratio of raw materials used is as follows:
[0039] 1 part m-phenylenediamine (moles)
[0040] 1 part of p-nitroaniline (number of moles)
[0041] 1 part of sodium nitrite (number of moles)
[0042] 5.2 parts of hydrochloric acid (the number of moles of HCl)
[0043] Synthesis of 2...
Embodiment 2
[0053] The raw materials used in the embodiment are as follows:
[0054] m-Phenylenediamine (analytical grade) was provided by China National Pharmaceutical (Group) Shanghai Chemical Reagent Company.
[0055] Aniline (analytical grade) was provided by China National Pharmaceutical (Group) Shanghai Chemical Reagent Company.
[0056] Sodium nitrite (analytical grade) was purchased from Shanghai Shisi Hewei Chemical Co., Ltd.
[0057] Hydrochloric acid (analytical pure), China Pharmaceutical Group Shanghai Chemical Reagent Co., Ltd.
[0058] The ratio of raw materials used is as follows:
[0059] 1 part m-phenylenediamine (moles)
[0060] 1 part of aniline (number of moles)
[0061] 1 part of sodium nitrite (number of moles)
[0062] 5.2 parts of hydrochloric acid (the number of moles of HCl)
[0063] Synthesis of 2,4-diaminoazobenzene:
[0064] Measure 6.0ml of concentrated hydrochloric acid and pour it into 60ml of water, add 3.6g of m-phenylenediamine to make m-phenylen...
Embodiment 3
[0067] The raw materials used in the embodiment are as follows:
[0068] m-Phenylenediamine (analytical grade) was provided by China National Pharmaceutical (Group) Shanghai Chemical Reagent Company.
[0069] p-methylaniline (analytical grade) was provided by China National Pharmaceutical (Group) Shanghai Chemical Reagent Company.
[0070] Sodium nitrite (analytical grade) was purchased from Shanghai Shisi Hewei Chemical Co., Ltd.
[0071] Hydrochloric acid (analytical pure), China Pharmaceutical Group Shanghai Chemical Reagent Co., Ltd.
[0072] The ratio of raw materials used is as follows:
[0073] 1 part m-phenylenediamine (moles)
[0074] 1 part of p-methylaniline (number of moles)
[0075] 1 part of sodium nitrite (number of moles)
[0076] 4 parts of hydrochloric acid (the number of moles of HCl)
[0077] Synthesis of 2,4-diamino-p-methylazobenzene:
[0078] Measure 6.0ml of concentrated hydrochloric acid and pour it into 60ml of water, add 3.6g of m-phenylenedia...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com