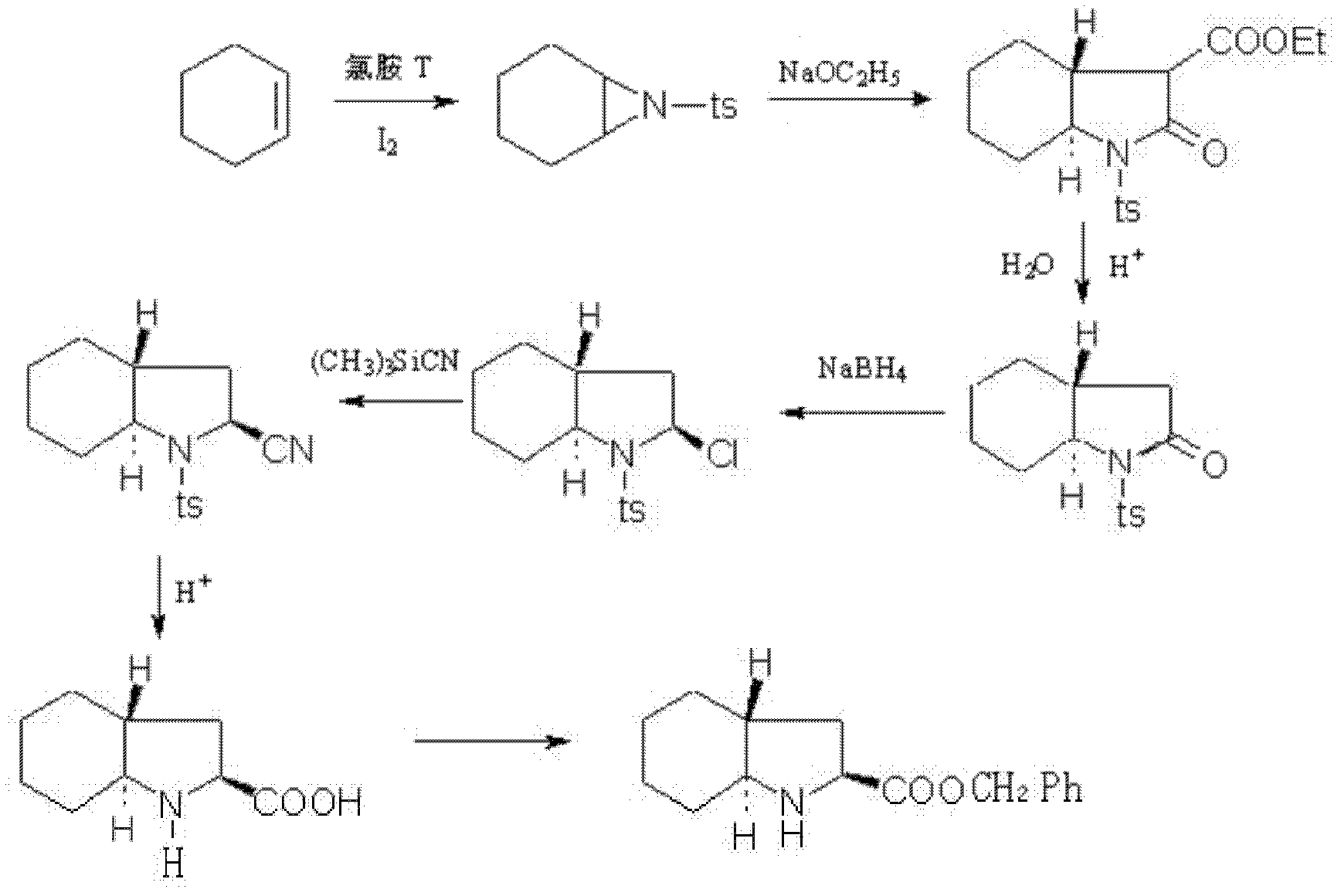
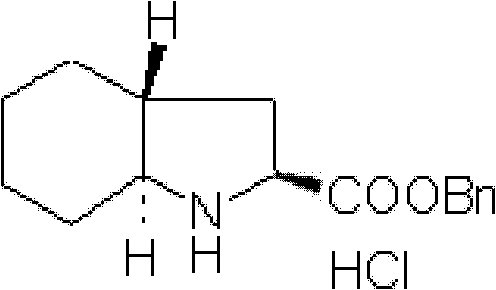
Preparation method for (2S, 3aR, 7aS)-octahydro indole-2-benzyl formate
A technology of benzyl formate and indoline, which is applied in the field of preparation of pharmaceutical intermediates, can solve the problems of carry-in, difficult separation of impurities, severe selective hydrogenation reaction, etc., and achieves high yield, good optical activity, and improved reaction. effect of speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
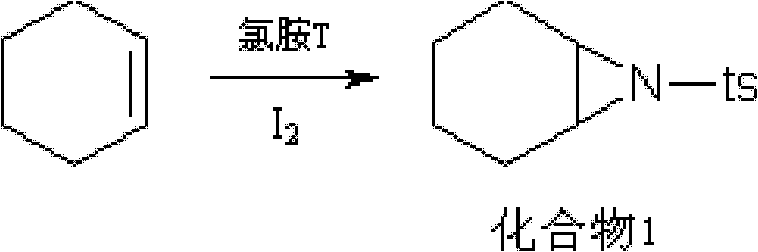
[0035] 1. Cis-addition reaction:
[0036] Chloramine-T (1.775mol), cyclohexene (4.4mol), I 2 (0.11mol) was added in 1491ml of acetonitrile, reacted at natural room temperature for 24 hours, and evaporated the solvent under reduced pressure to obtain 453.5g of the red oily product of this step, yield: 102%, 1 H NMR (CDCl 3 , 300MHz) δ(ppm): 1.16~1.26(m, 2H); 1.33~1.45(m, 2H); 2.42(s, 3H); 2.96(s, 2H); 7.46~7.57(m, 3H); 7.31 (d, 2H, J=7.78Hz); 7.80(d, 2H, J=6.7Hz);
[0037] 2. Closed-loop hydrolysis reaction:
[0038] Diethyl malonate (2.72mol) was added into a solution of tetrahydrofuran (1650ml) dissolved with sodium ethoxide (3.48mol), heated to reflux for 45 minutes, and slowly added dropwise to tetrahydrofuran (1767ml) of the product (453.5g) obtained in step (1). ) solution, reflux reaction for 15 hours, down to room temperature, and decompression to remove the solvent; add ethyl acetate to dissolve the resulting product, add 1256ml of water, stir and dissolve, adjust ...
Embodiment 2
[0052] 1. Cis-addition reaction:
[0053] Chloramine-T (1.775mol), cyclohexene (3.55mol), I 2 (0.10mol) was added in 1491ml of acetonitrile, reacted at natural room temperature for 24 hours, and evaporated the solvent under reduced pressure to obtain 444.6g of the red oily product of this step, yield: 100%, 1 H NMR (CDCl 3 , 300MHz) δ(ppm): 1.16~1.26(m, 2H); 1.33~1.45(m, 2H); 2.42(s, 3H); 2.96(s, 2H); 7.46~7.57(m, 3H); 7.31 (d, 2H, J=7.78Hz); 7.80(d, 2H, J=6.7Hz);
[0054] 2. Closed-loop hydrolysis reaction:
[0055] Diethyl malonate (2.7mol) was added into a solution of tetrahydrofuran (1345ml) dissolved with sodium ethoxide (3.3mol), heated to reflux for 30 minutes, and slowly added dropwise to tetrahydrofuran (1732ml) of the product (444.6g) obtained in step (1). ) solution, reflux reaction for 15 hours, down to room temperature, and evaporate the solvent under reduced pressure; add ethyl acetate to dissolve the resulting product, then add 1112ml of water, stir and diss...
Embodiment 3
[0069] 1. Cis-addition reaction:
[0070] Chloramine-T (1.775mol), cyclohexene (5.3mol), I 2 (0.14mol) was added in 1491ml of acetonitrile, and the natural room temperature was reacted for 24 hours under the condition of 25° C., and the solvent was evaporated under reduced pressure to obtain 462.4 g of the red oily product of step (1), yield: 104%, 1 HNMR (CDCl 3 , 300MHz) δ(ppm): 1.16~1.26(m, 2H); 1.33~1.45(m, 2H); 2.42(s, 3H); 2.96(s, 2H); 7.46~7.57(m, 3H); 7.31 (d, 2H, J=7.78Hz); 7.80(d, 2H, J=6.7Hz);
[0071] 2. Closed-loop hydrolysis reaction:
[0072] Diethyl malonate (3.2mol) was added into a solution of tetrahydrofuran (2000ml) dissolved with sodium ethoxide (4.8mol), heated to reflux for 2h, and slowly added dropwise to tetrahydrofuran (1800ml) of the product (462.4g) obtained in step (1) solution, reflux reaction for 18 hours, down to room temperature, and evaporate the solvent under reduced pressure; add 1800ml of ethyl acetate, 1380ml of water, stir and dissolv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com