Patents
Literature
93 results about "Trimethylsilyl cyanide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Trimethylsilyl cyanide is the chemical compound with the formula (CH₃)₃SiCN. This volatile liquid consists of a cyanide group, that is CN, attached to a trimethylsilyl group. The molecule is used in organic synthesis as the equivalent of hydrogen cyanide.
Three-component tandem synthesis method of multi-substituted pyrrole
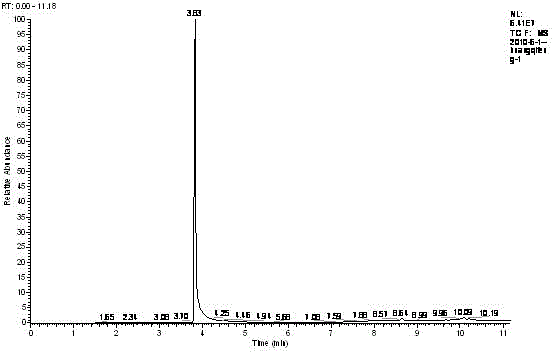
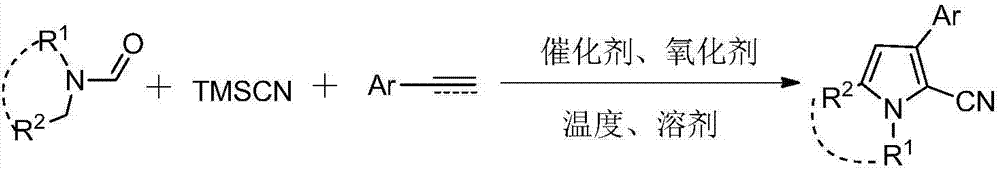
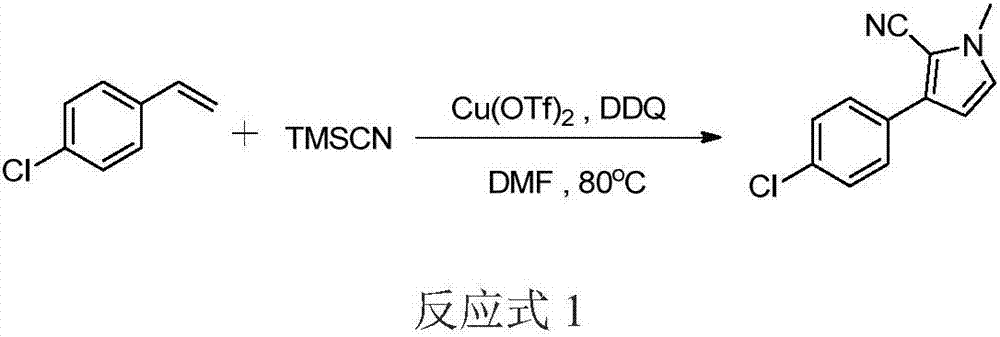
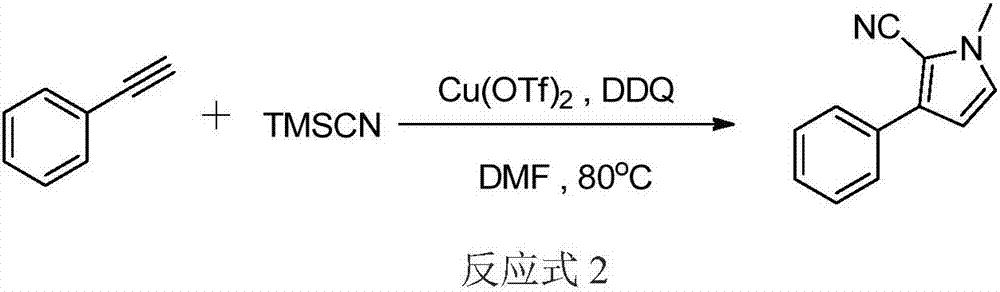
The invention relates to a three-component tandem synthesis method of a 2-cyano-3-arylpyrrole compound, wherein the reaction general formula is defined in the specification. According to the present invention, N,N-disubstituted formamide, trimethylsilyl cyanide and aryl olefin or aryl alkyne are subjected to one-pot synthesis under the actions of a Lewis acid catalyst and an oxidizing agent to prepare the 2-cyano-3-arylpyrrole compound; the used starting raw materials are simple; excluding the final product, the intermediates during the tandem conversion do not require separation and purification so as to reduce the coat input and the labor force input; and the simple and efficient preparation method is provided for the 2-cyano-3-arylpyrrole and the derivative thereof.
Owner:LANZHOU UNIVERSITY
Synthesis process of 2-thiopheneacetic acid
The invention discloses a synthesis process of 2-thiopheneacetic acid, which comprises the following steps: enabling thiophene as a raw material and formaldehyde react with hydrochloric acid gas to produce 2-chloromethyl thiophene in the presence of an organic solvent; enabling 2-chloromethyl thiophene to react with trimethylsilyl cyanide to generate 2-thiophene acetonitrile in a catalyst condition in the presence of an organic solvent; and generating the thiopheneacetic acid from 2-thiophene acetonitrile in an alkaline condition. The process disclosed by the invention has the positive effects that (1) the required raw materials are relatively simple, and the reaction residues thiophene, an organic solvent, ethanol and dichloromethane can be recycled, thereby greatly reducing the production cost; (2) 2-chloromethyl thiophene reacts in the organic solvent so that chloromethyl thiophene can be stored stably and the danger of explosion is avoided; (3) since trimethylsilyl cyanide is adopted as a cyaniding reagent, the use of highly-toxic substance sodium cyanide is avoided, the reaction yield is increased, the product quality and the safety performance are relatively high, and the industrial production is facilitated; and (4) the reaction conditions are mild, a small quantity of catalyst is used, and the process is simple; and compared with the existing synthesis process, the process disclosed by the invention has obvious economic benefits and environmental benefits.
Owner:SHANGQIU KOREATA CHEM
NiCo2O4/carbon nanotube composite electrode material and preparation method thereof
InactiveCN108376614ANovel structureHigh specific capacitanceHybrid capacitor electrodesHybrid/EDL manufactureCapacitanceChemical vapor deposition
The invention provides a NiCo2O4 / carbon nanotube composite electrode material and a preparation method thereof. To be specific, the invention relates to a NiCo2O4 / carbon nanotube composite electrode material having a core-shell structure using the carbon nanotube as a core and the NiCo2O4 nanotube as a shell and the overall shape of the material is a height-ordered nanotube array. The preparationmethod comprises: step one, selecting a double-pass aluminium oxide template with a pore diameter of 200 nm, carrying out magnetron sputtering on the back of the template to form a copper film with the thickness of 1 micron, and carrying out trimethylsilyl cyanide, ethyl alcohol, and distilled water ultrasonic cleaning successively, and then carrying out drying; step two, with processed porous aluminium oxide as a template, preparing a nickel-cobalt alloy nanotube array in an electrolytic tank by using a square-wave pulse electro-deposition method; step three, depositing carbon nanotubes in the nickel-cobalt alloy nanotubes by using a chemical vapor deposition method; and step four, removing the aluminium oxide template by using NaOH and carrying out calcination to obtain a NiCo2O4 / carbonnanotube composite material. The NiCo2O4 / carbon nanotube composite obtained by the method has a high specific capacitance value and good electrochemical performance stability when being applied to thesupercapacitor electrode material.
Owner:CHINA JILIANG UNIV
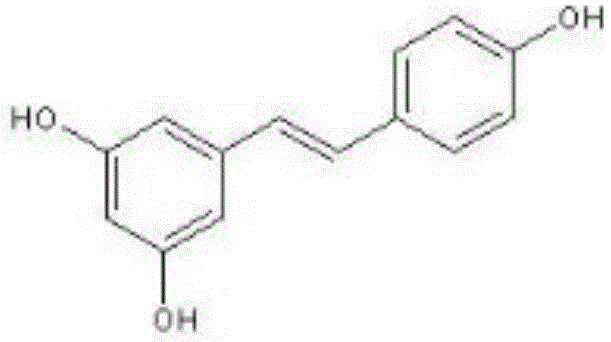
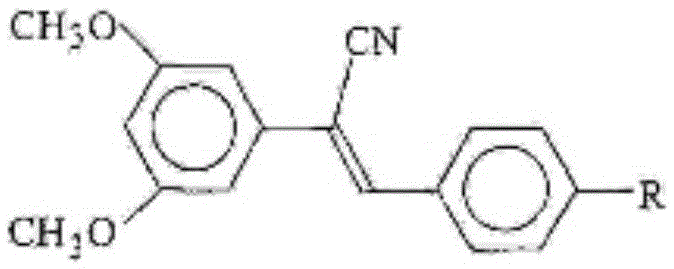
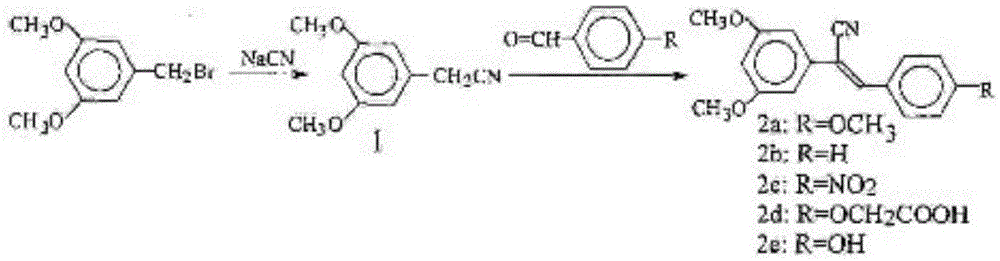
Cyano-containing resveratrol analogue and preparation method and purpose thereof
InactiveCN105503652AReduce harmAvoid harmCarboxylic acid nitrile preparationOrganic compound preparationBenzoic acidPotassium cyanide
The invention provides a cyano-containing resveratrol analogue and a preparation method and application thereof, and relates to the field of medical compounds. A structure of the cyano-containing resveratrol analogue is shown in the formula of the description; 3,5-dihydroxy-benzoic acid is mainly used as a raw material, and the cyano-containing resveratrol analogue is prepared by the following steps of methylation, reduction, brominating, Abozove rearrangement, Wittig-Hornor reaction and Knoevenegal reaction; in the preparation process of the cyano-containing resveratrol analogue, the chemical substances with lower toxicity, such as trimethylsilyl cyanide and tetrabutylammonium fluoride, are used for replacing chemicals with higher toxicity, such as sodium cyanide or potassium cyanide, to introduce cyano, so that the damage to the health of an operator is greatly decreased, and the serious harm to environments is avoided. The preparation method has the advantages that the yield is higher, the environment-friendly effect is good, and multiple types of cyano-containing resveratrol analogue with anti-cancer activity on cells can be obtained.
Owner:YANBIAN UNIV
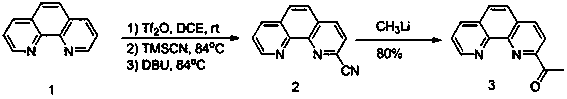
Method for synthesizing 2-acetyl-1, 10-phenanthroline (3)
ActiveCN110003203AHigh yieldReduce the impactOrganic chemistryTrifluoromethanesulfonic anhydrideLithium
The invention discloses a method for synthesizing 2-acetyl-1, 10-phenanthroline (3). The method comprises the following steps: using 1, 10-phenanthroline as a raw material to synthesize 2-cyano-1, 10-phenanthroline (2) by reacting with trimethylcyanosilane, trifluoromethanesulfonic anhydride, 1, 8-diazabicycloundec-7-ene; and then reacting 2-cyano-1, 10-phenanthroline (2) with methyl lithium to prepare 2-acetyl-1, 10-phenanthroline (3). The method provided by the invention prepares 2-acetyl-1, 10-phenanthroline (3) by adopting a two-step synthesis method, is beneficial to the increase of the yield, and purifies by quenching with a saturated sodium bicarbonate solution, extracting with dichloromethane and washing with a saturated sodium chloride solution after completing a first step, so that the influence of impurities generated in the first step on the reaction in a second step is minimized.
Owner:BEIJING YANLIAN CHEM TECH CO LTD
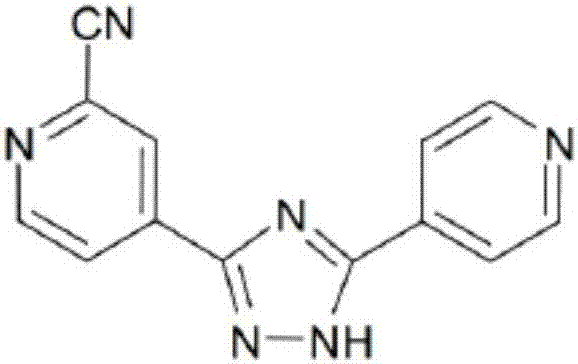
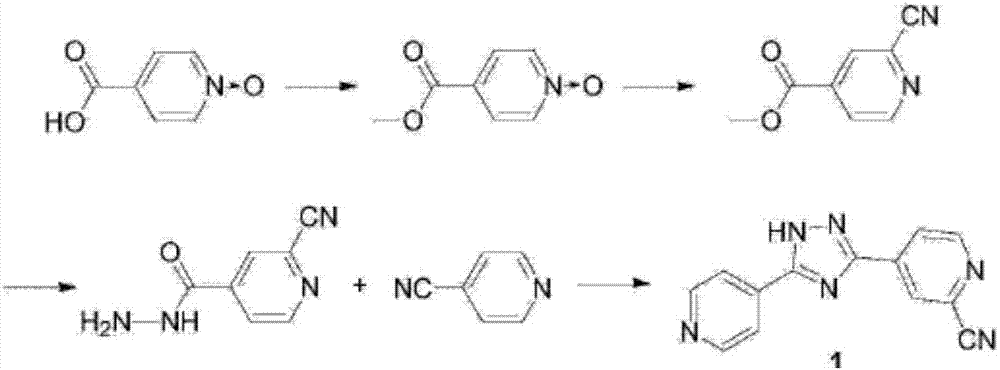
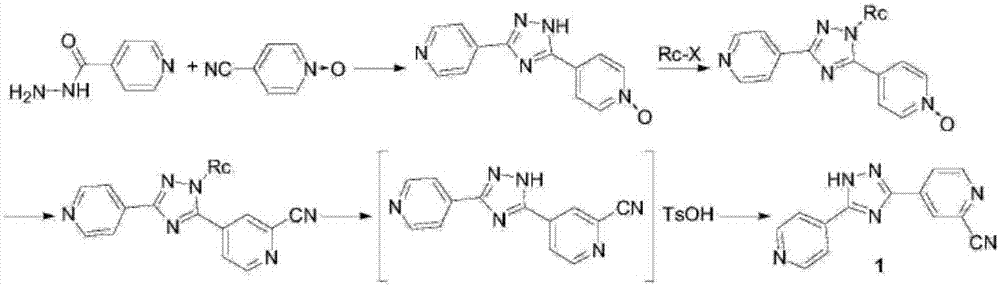
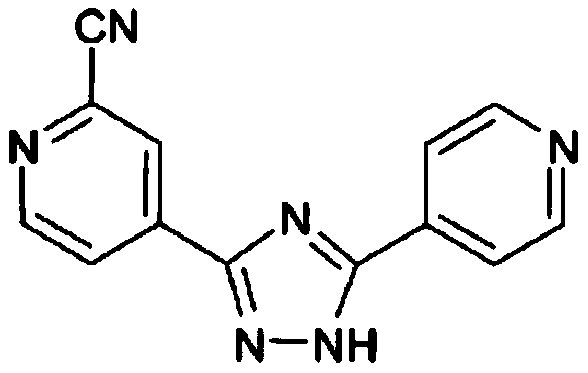
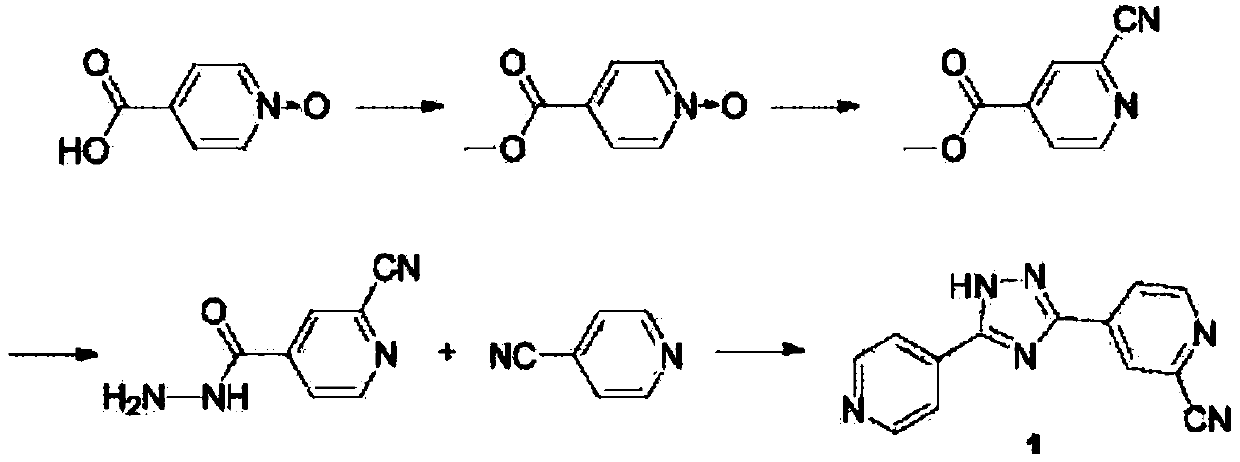
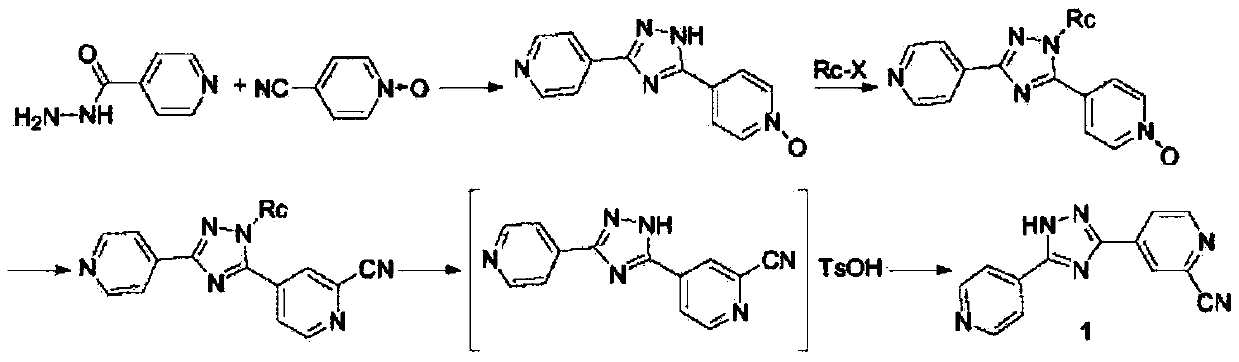
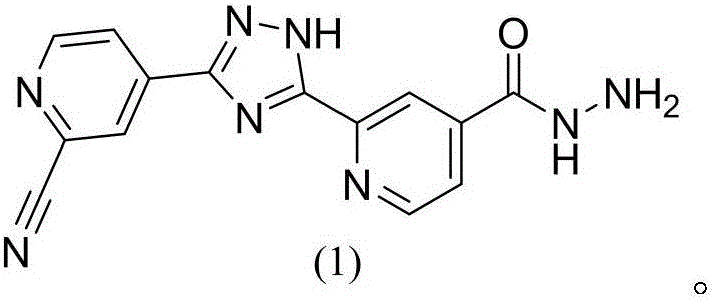
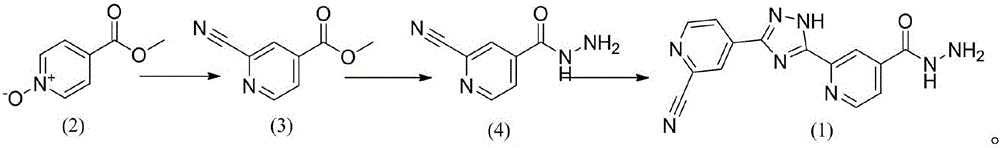
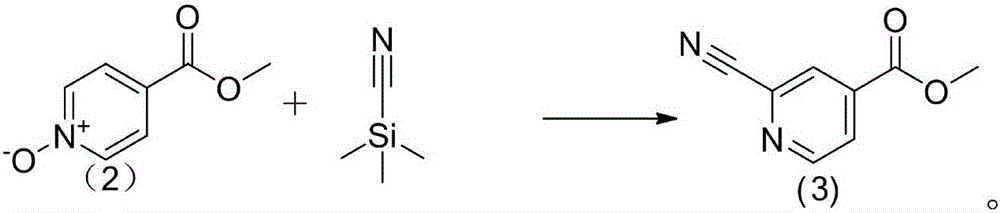
Topiroxostat impurity synthesis method
InactiveCN106008465APrecise positioningQualitative highOrganic chemistryIsoniazidTrimethylsilyl cyanide
The invention discloses a topiroxostat impurity synthesis method, and belongs to the chemical pharmaceutical technical field. The method comprises the steps: with methyl isonicotinic acid-N-oxide (2) as a starting material, generating 2-cyano-4-pyridine carboxylic acid methyl ester from methyl isonicotinic acid-N-oxide (2) and trimethylsilyl cyanide; carrying out hydrazinolysis of the compound (3) to generate 2-cyano-isoniazid (4); and carrying out ring self-formation of the compound (4) to generate a target 2-(3-(2-amino pyridine-4-yl)-1H-1,2,4-triazole-5-yl)isoniazide (1). The synthesized high-purity topiroxostat impurity can be used as an impurity standard in topiroxostat finished product detection analysis, so as to enhance accurate positioning and qualitation on the impurity in the topiroxostat finished product detection analysis, be conducive to strengthening of the control of the impurity, and improve the quality of the topiroxostat finished product; the method provided by the invention has the advantages of cheap and easily obtained raw materials, and simple operation; the yield of the obtained product is 85%+ / -5%, and the HPLC purity is not less than 98%.
Owner:JIANGSU YUEXING PHARMA
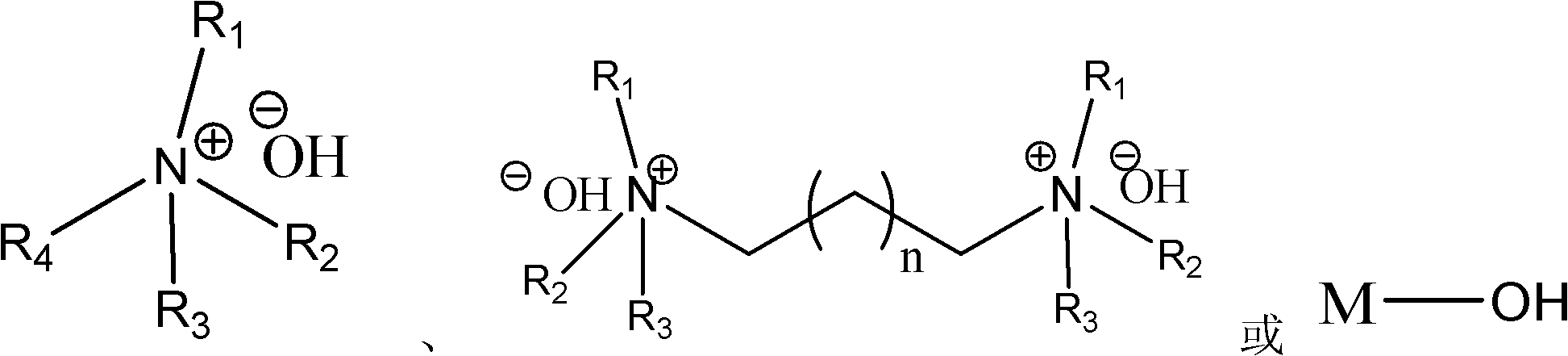
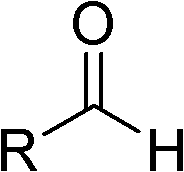
Highly active catalyst used for hydrosilation reaction of aldehyde and trimethylsilyl cyanide
InactiveCN102309985AReduce dosageMild reaction conditionsGroup 4/14 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsOrganic synthesisMetal catalyst
Belonging to the field of organic synthesis, the invention relates to a highly active catalyst used for a hydrosilation reaction of aldehyde and trimethylsilyl cyanide so as to synthesize a cyanohydrinsilylether compound. The catalyst is characterized by being a quaternary ammonium base or an alkali metal hydroxide both containing one or two hydroxyl anions. The catalyst can catalyze the hydrosilation reaction of aldehyde and trimethylsilyl cyanide efficiently in a mild condition and at a low catalyst concentration, and a racemic cyanohydrinsilylether compound can be obtained quantificationally, with the activity up to 3000000 turn over number / hour. Characterized by low price and easy availability, mild reaction condition, low catalyst dosage, short reaction time and wide application scope of substrate, the catalyst provided in the invention avoids the disadvantages of severe waterless and anaerobic conditions, high catalyst cost, environmental pollution and the like caused by employment of a metal catalyst, and has strong practicality.
Owner:DALIAN UNIV OF TECH
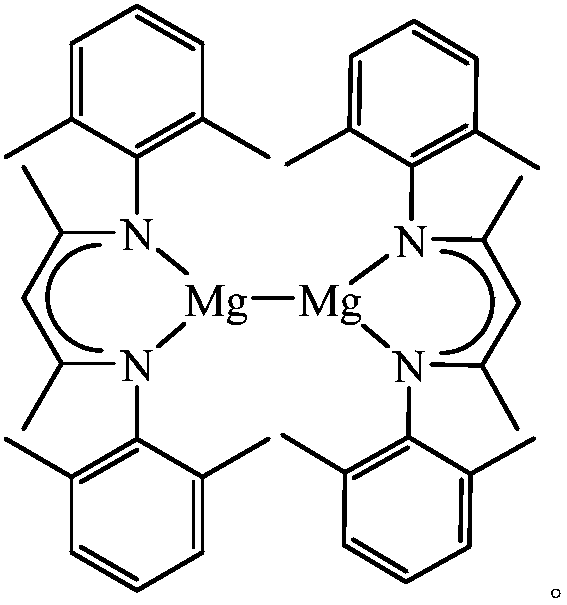
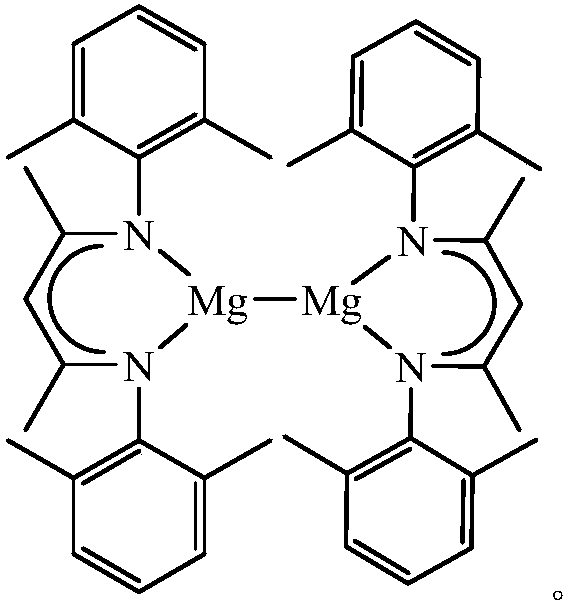
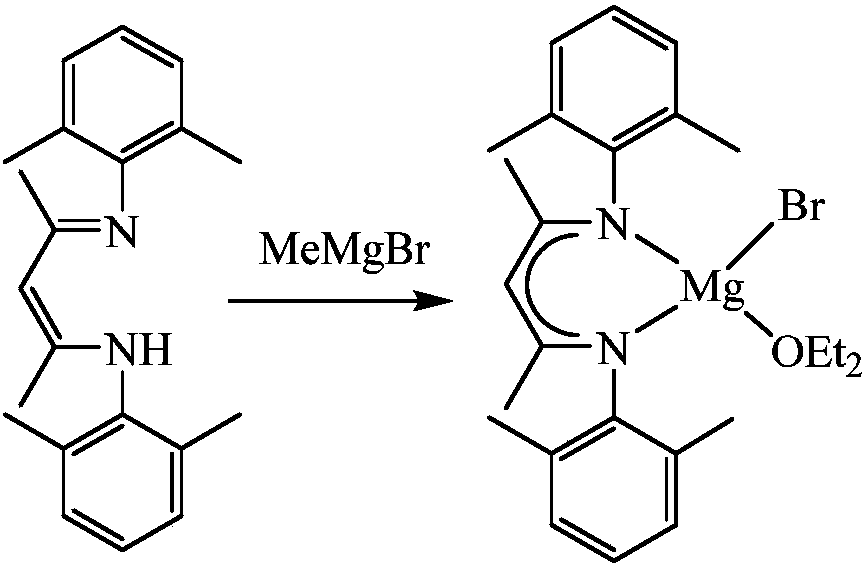
Beta-diimine univalence magnesium compound, preparation method and application thereof in aldosterone silicon cyanide
InactiveCN107556195AEasy to purifyLow toxicityCarboxylic acid nitrile preparationOrganic compound preparationGrignard reagentSilanes
The invention discloses a β-diimine monovalent magnesium compound and its preparation and application in silanization of aldehydes and ketones. The preparation method is as follows: under anhydrous and oxygen-free conditions, β-diimine ligand reacts with Grignard reagent to generate magnesium bromide, and then reduces it with sodium to obtain yellow crystal, which is β-diimine monovalent magnesium compound. The β-diimine monovalent magnesium compound of the present invention has simple synthesis, convenient separation and purification, clear structure, and high yield; it has high activity as a catalyst to catalyze the reaction of aldehydes or ketones with trimethylsilyl cyanide, and the substrate is universal wide sex.
Owner:NANJING FORESTRY UNIV +1
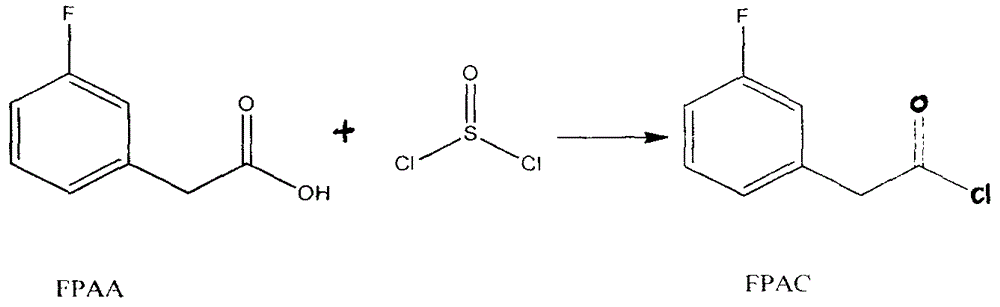
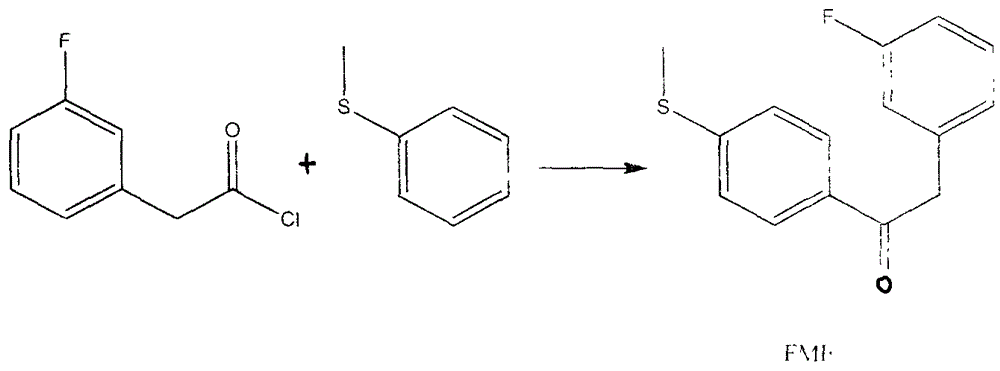
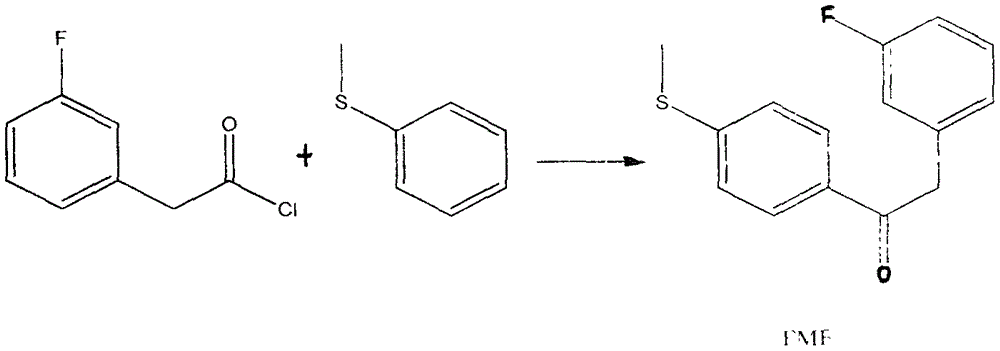
Preparation method for 4-(3-fluorine phenyl)-2,2-phenyl-5-(4-(methylmercapto-)phenyl) furan-3(2H)-ketone
The invention discloses a preparation method for 4-(3-fluorine phenyl)-2,2-phenyl-5-(4-(methylmercapto-)phenyl)furan-3(2H)-ketone. The preparation method comprises the following steps: m-fluorobenzene acetylchloride is generated under the existence of fluorobenzene acetic acid and thionyl chloride; 2-bromine-2-methyl propionyl-bromine is reacted with trimethyl cyanogen silane to generate 3-bromine-3-methyl-2-oxo butyl cyanide; m-fluorobenzene acetylchloride is reacted with thioanisole to generate 2-(3-fluorine phenyl)-1-(4-(methylmercapto-)phenyl) acetone under the existence of aluminium trichloride and dichloromethane; the 4-(3-fluorine phenyl)-2,2-phenyl-5-(4-(methylmercapto-)phenyl)furan-3(2H)-ketone is generated by the 2-(3-fluorine phenyl)-1-(4-( methylmercapto-)phenyl)acetone and the 3-bromine-3-methyl-2-oxo butyl cyanide; a best product is obtained through separation, crystallization, filtering and drying.
Owner:NANTONG HUAFENG CHEM
Method for preparing (1S, 5S)-6,6-dimethyl-3-azabicyclo [3.1.0] hexane-2-carboxylic acid alkyl ester
InactiveCN103864672AEasy to operateSimple post-processingOrganic chemistryTrimethylsilyl cyanideNitrogen
The invention discloses a method for preparing (1S, 5S)-6,6-dimethyl-3-azabicyclo [3.1.0] hexane-2-carboxylic acid alkyl ester. The method is characterized in that a compound shown in the formula II is subjected to cyanation reaction with trimethylsilyl cyanide by adopting a one-pot process to obtain a product, the product is subjected to hydrolysis reaction with acid-ROH solution to obtain the compound shown in the formula I, and the specific reaction formula is shown in the specification, wherein R is selected from C1-C5 alkyls. The compound shown in the formula I prepared by a one-pot reaction can be realized in the invention, and high-purity (1S, 5S)-6,6-dimethyl-3-azabicyclo [3.1.0] hexane-2-carboxylic acid alkyl ester can be prepared with high yield at low cost by the method disclosed by the invention. The method has advantages of simple process and no special requirement for equipment and can be put into large-scale production easily. The invention has an important significance and a practical value in industrial production of boceprevir.
Owner:SHANGHAI DESANO CHEM PHARMA
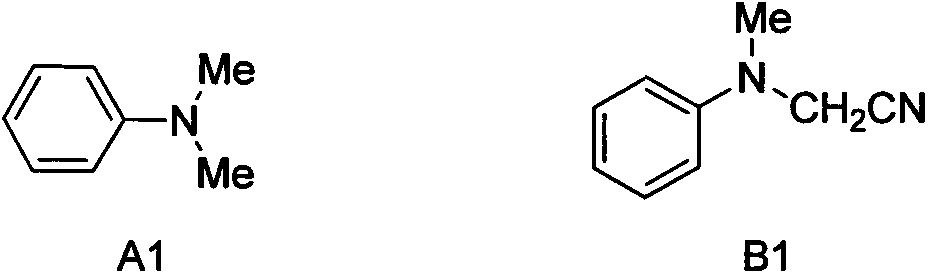
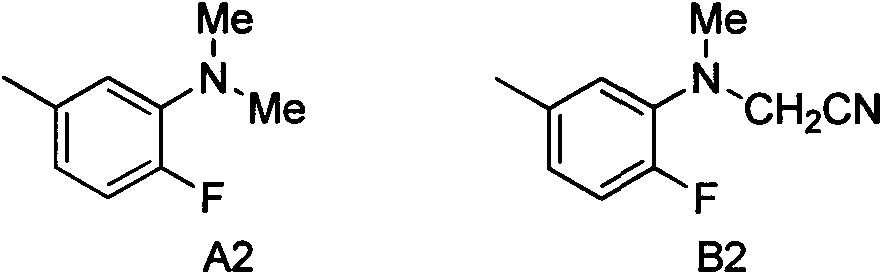
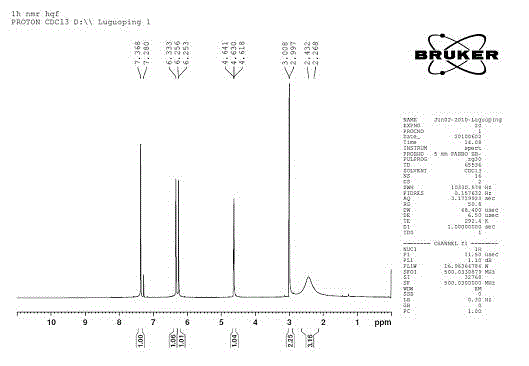
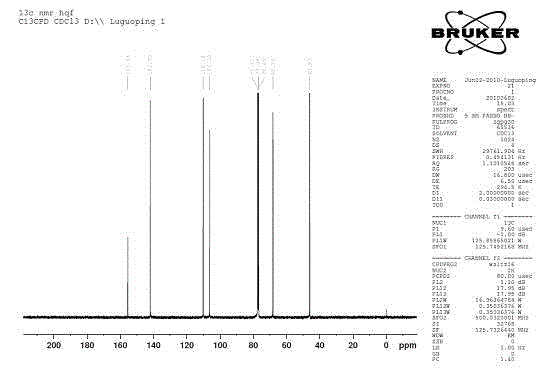
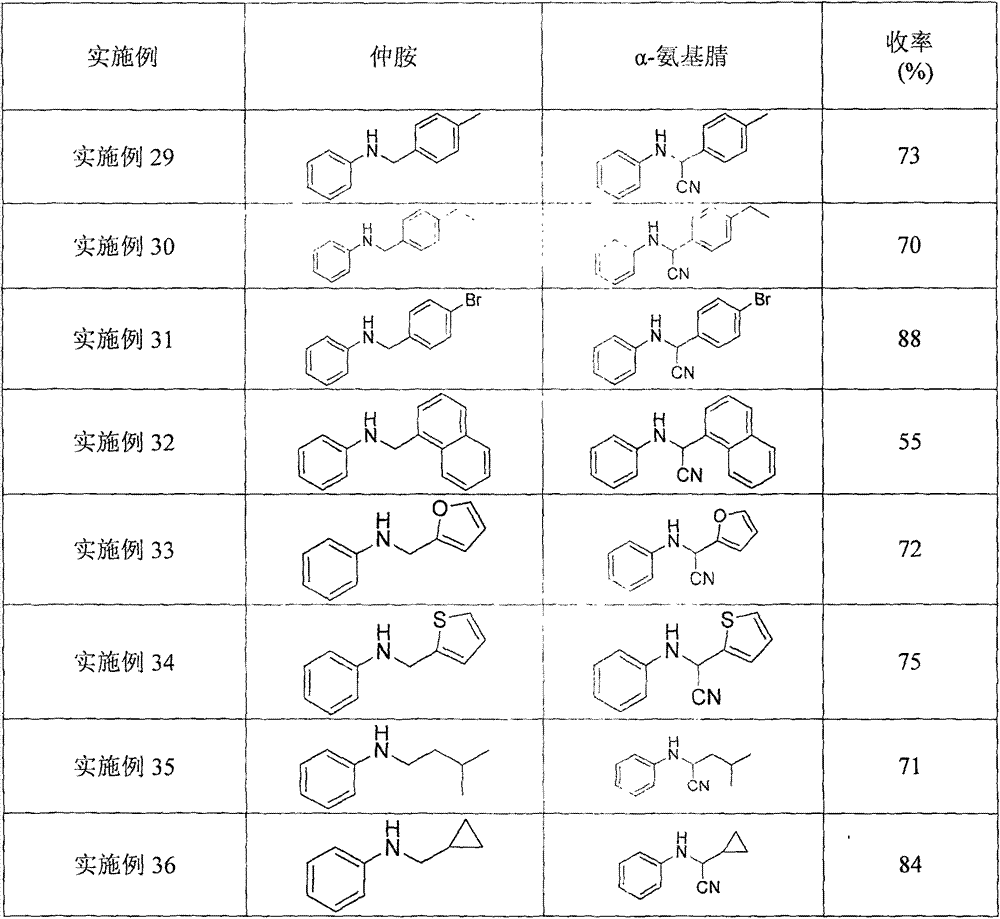
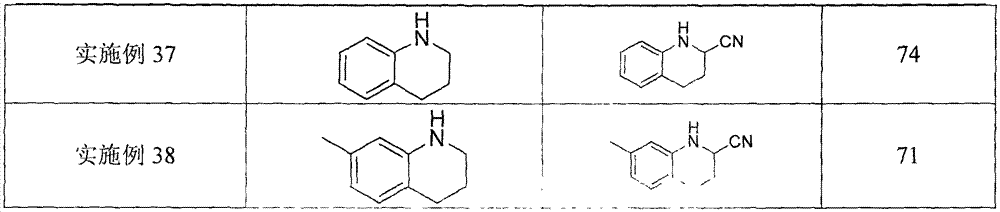
Alpha-cyanidation method of mono-alkyl substituted aniline
InactiveCN107129442AImprove responseMeet the needs of varietyCarboxylic acid nitrile preparationOrganic compound preparationSynthesis methodsCatalytic oxidation
The invention discloses an alpha-cyanidation method of mono-alkyl substituted aniline. According to the method, various aromatic, aliphatic, and heterocycle substituted secondary amines of anilines are taken as the raw materials, trimethylsilyl cyanide is taken as the cyanidation agent, divalent iron is taken as the catalyst, tert-butyl hydroperoxide is taken as the oxidizing agent, raw materials carry out reactions in a solvent, and the reaction product is processed by a simple concentrating and purifying technology to obtain the finished product. The invention aims to solve the shortages of a conventional catalytic oxidation cyanidation method of secondary amines and provides a novel synthesis method. Cheap, low toxin, and easily available divalent iron is taken as the catalyst, tert-butyl hydroperoxide is taken as the oxidizing agent, and the catalytic oxidation cyanidation reactions between a secondary amine substrate of various aromatic, aliphatic, and heterocycle substituted anilines and trimethylsilyl cyanide are realized efficiently. The provided method has the advantages of simple technology, safe production reactions, easy operation, short reaction time, and easiness for industrialization.
Owner:CHONGQING UNIV
Synthesis method of tert-butyl-8-oxo-2,6,9-triazaspiro[4.5]decane-2-formylate
InactiveCN109503605AReasonable reaction process designThe method route is simpleOrganic chemistrySynthesis methodsEthyl acetate
The invention relates to a synthesis method of tert-butyl-8-oxo-2,6,9-triazaspiro[4.5]decane-2-formylate. The technical problem that an appropriate industrial synthesis method is inexistent at presentis mainly solved. The synthesis method is divided into two steps: the first step, adding triethylamine in a methanol solution dissolved with a compound 1 and amino ethyl acetate hydrochloride, and then dropwise adding tetraisopropyl titanate in the reaction solution, after completing the dropwise adding, reacting the mixed system for 12 h at 25 DEG C; adding trimethylsilyl cyanide in the reactionsystem at 25 DEG C, and then reacting for 12 h at 25 DEG C, performing post-treatment to obtain a compound 2; and the second step, adding the compound 2, nickel and ammonia water in the methanol solution, and performing hydrogenation to obtain the final compound.
Owner:上海合全医药有限公司
Preparation method of topiroxostat
The invention provides a preparation method of topiroxostat. The method comprises the following steps: subjecting isonicotinic acid as a starting material to oxidation with hydrogen peroxide to obtainisonicotinic acid-nitric oxide (an intermediate 1); then esterifying with methanol to obtain methyl isonicotinate-nitric oxide (intermediate 2); then performing hydrazinolysis with hydrazine hydrateto obtain isoniazide-nitric oxide (intermediate 3); then reacting with 4-cyanopyridine to obtain an intermediate 4; then cyaniding with trimethylsilyl cyanide to obtain an intermediate 5; and finallyperforming dehydration and cyclization to generate topiroxostat. According to the method, initial raw materials and reagents are cheap and easily available; experimental operation is simple and controllable, extreme reaction conditions are avoided, and the method is suitable for laboratory development and even industrial production; the total yield is high, and the production cost is reduced; purity of the finished product can be ensured.
Owner:孙哲
2-cyanoindole substituted gem-difluoroolefin compound as well as preparation method and application thereof
The invention relates to the technical field of organic synthesis, in particular to a 2-cyanoindole substituted gem-difluoroolefin compound and a preparation method and application thereof. The preparation method of the compound comprises the steps: taking a trifluoromethylindole methanol compound and trimethylsilyl cyanide as raw materials, and under the action of a Lewis acid catalyst and inorganic alkali, carrying out a heating reaction in a solvent to obtain a 2-cyanoindole substituted gem-difluoroolefin compound. The invention provides the 2-cyanoindole substituted gem-difluoroolefin compound and the preparation method thereof. According to the method, the trifluoromethyl indole methanol compound and the trimethylsilyl cyanide which are simple and easy to obtain are used as raw materials, explosive diazo compounds do not need to be used, and the method has the advantages of being mild in reaction condition, easy to operate, high in yield, wide in reaction substrate range and the like.
Owner:NANJING FORESTRY UNIV
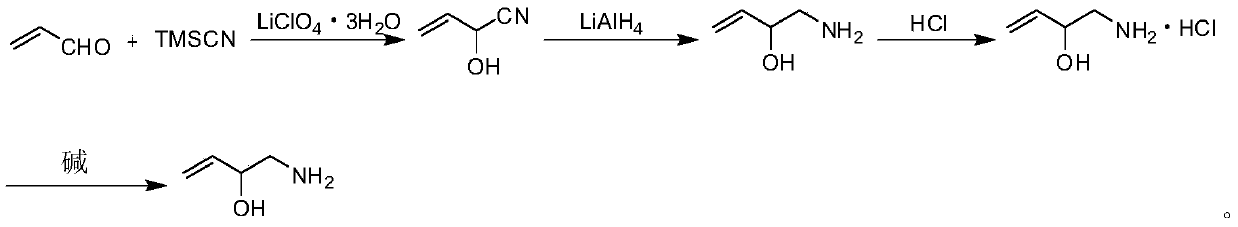
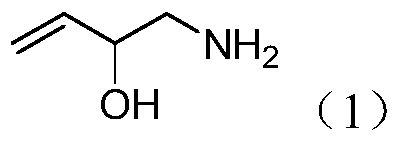
Preparation method of 2-hydroxyl-3-butene-1-amine
InactiveCN105503624AShort reaction pathEasy to operateCarboxylic acid nitrile preparationOrganic compound preparationDistillationDiethyl ether
The invention discloses a preparation method of 2-hydroxyl-3-butene-1-amine. The preparation method comprises the following specific steps: (1) taking acrolein as a start raw material to react with trimethylsilyl cyanide at room temperature in the presence of lithium perchlorate trihydrate to obtain 2-hydroxyl-3-butene-1-nitrile; (2) with diethyl ether or tetrahydrofuran as a solvent, reducing the 2-hydroxyl-3-butene-1-nitrile with lithium aluminum hydride to obtain a crude product of 2-hydroxyl-3-butene-1-amine; (3) making the crude product of 2-hydroxyl-3-butene-1-amine react with hydrochloric acid to obtain 2-hydroxyl-3-butene-1-amine hydrochloride; and (4) making the 2-hydroxyl-3-butene-1-amine hydrochloride react with alkali to finally obtain the 2-hydroxyl-3-butene-1-amine. According to the method disclosed by the invention, without a trimethylsilyl protection group removal process, the operation of reduced pressure distillation is avoided, the reaction path is shortened, and the operation is simple.
Owner:SHANGHAI INST OF TECH
Preparation method of topiroxostat
The invention relates to a preparation method of topiroxostat, which comprises the steps of taking isonicotinic acid as an initial raw material, taking acetic acid as a solvent, and reacting with hydrogen peroxide to generate an intermediate 1; reacting the intermediate 1 with methanol under the catalysis of concentrated sulfuric acid to generate an intermediate 2; carrying out reflux reaction on the intermediate 2 and hydrazine hydrate in methanol to generate an intermediate 3; carrying out reflux reaction on the intermediate 3 and 4-cyanopyridine to generate an intermediate 4; and reacting the intermediate 4 with trimethylsilyl cyanide to introduce a cyano group to generate an intermediate 5, and carrying out ring closing on the intermediate 5 under the action of inorganic acid by taking 2-butanol as a solvent to generate the target product topiroxostat. The raw materials and reagents are cheap and easy to obtain, the cost of the raw materials is low, the reaction conditions of each step are mild, the operation is simple, and the controllability is strong; and the large-scale industrial production and application can be realized. The yield is high, the purity is high, the total yield is 51.4%, the refining yield is 91.8%, the purity of the refined product reaches 99.82%, and the single impurity content is less than 0.1%.
Owner:SHANDONG UNIV
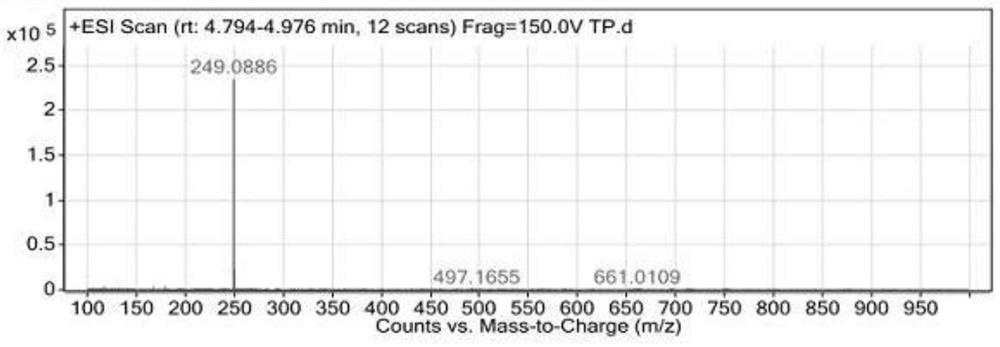
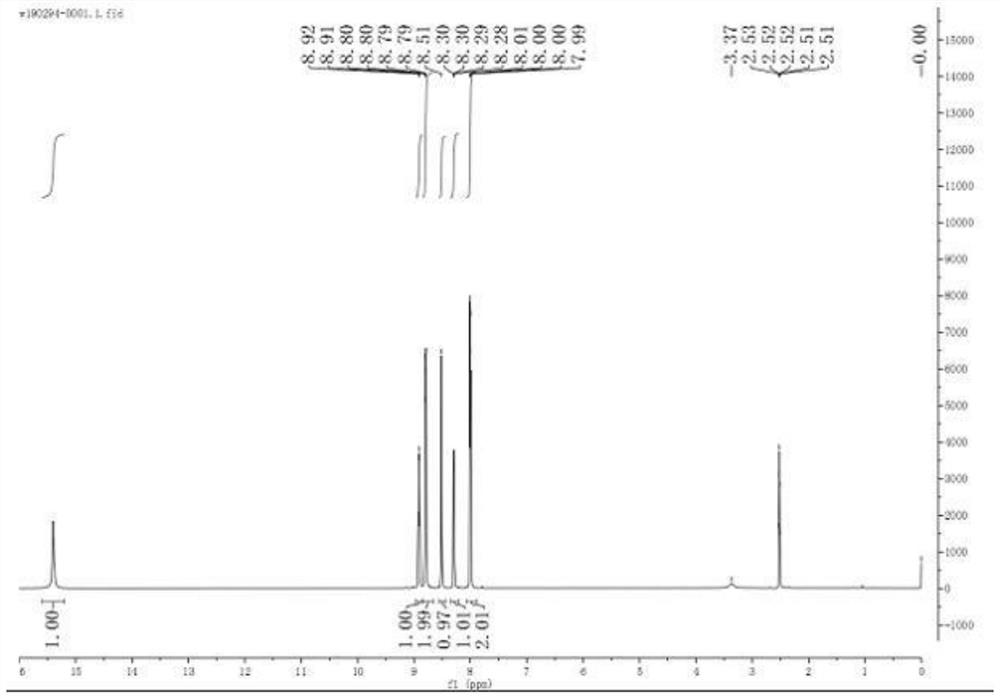
Synthesis method of 3-bromo-6-chloropyridyl-2-formic acid
InactiveCN103058921ALow costSuitable for large-scale industrial productionOrganic chemistrySynthesis methodsSodium cyanide
The invention provides a synthesis method of 3-bromo-6-chloropyridyl-2-formic acid, which comprises the following steps: by using 3-bromo-6-chloropyridine as an initial raw material, carrying out oxidation reaction with urea peroxide and trifluoroacetic anhydride to obtain 3-bromo-6-chloropyridine oxynitride; reacting with trimethylsilyl cyanide and triethylamine to obtain 3-bromo-6-chloropyridyl-2-cyanide; and finally, hydrolyzing in sulfuric acid to obtain the 3-bromo-6-chloropyridyl-2-formic acid. The synthesis method provided by the invention has the advantages of high operational safety, environment friendliness and the like; and especially, the synthesis method uses low-cost low-toxicity raw materials in stead of such expensive raw material as 2-fluoro-3-bromo-6-chloropyridine and virulent raw material sodium cyanide, and is suitable for large-scale industrial production.
Owner:SUZHOU ZIKANG PHARMA INC
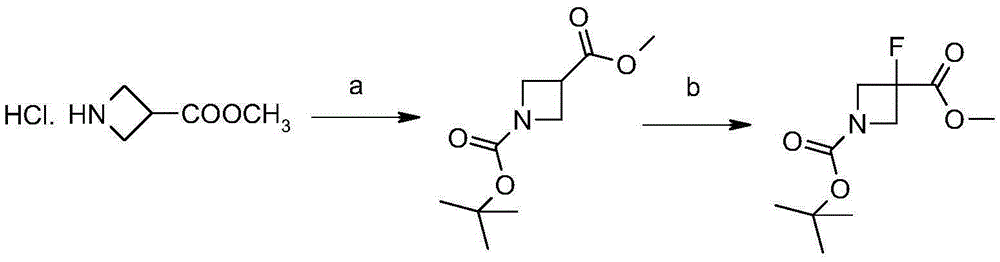
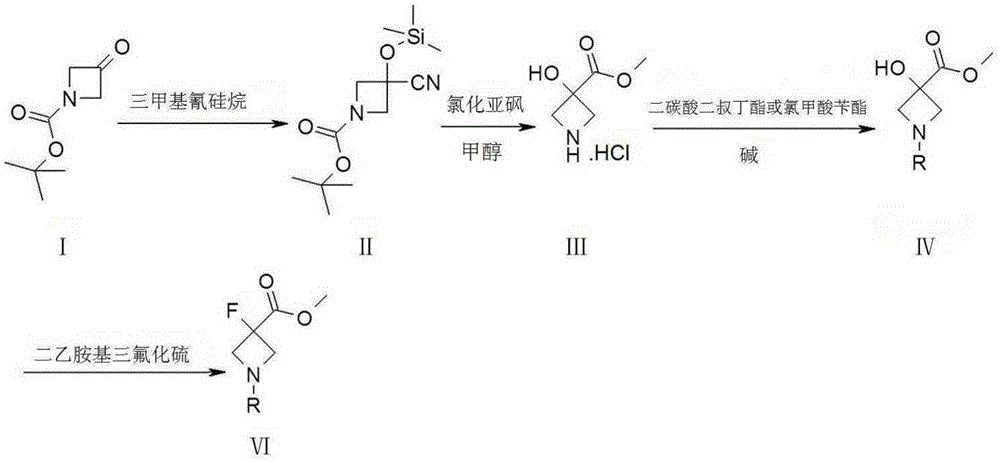
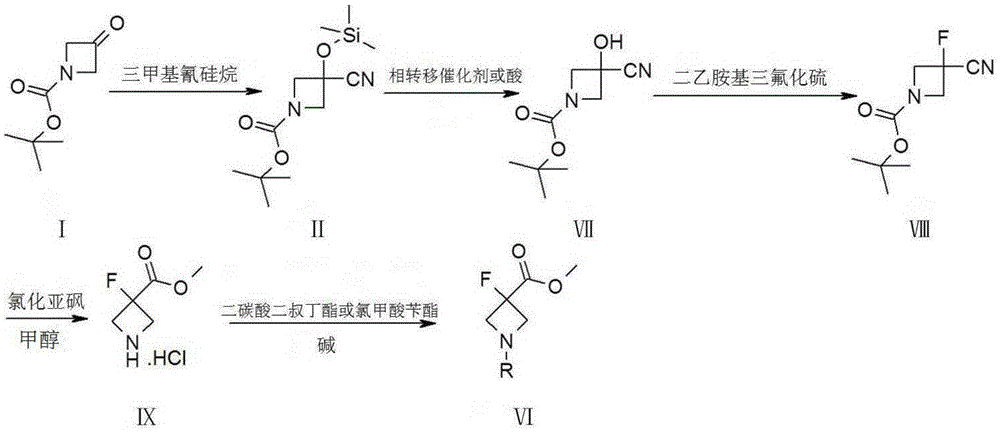
Synthetic method of 3-fluoro-azetidine derivative
ActiveCN105384673AMild reaction conditionsEasy to operateOrganic chemistryTrimethylsilyl cyanideTotal recovery
The invention provides a synthetic method of a 3-fluoro-azetidine derivative. The synthetic method takes a compound I as the raw material, the compound I is reacted with trimethylsilyl cyanide to obtain a compound II, and a compound VI is obtained through esterification, N-protection and fluoronation or through hydrolysis, fluoro, esterification and N-protection. The synthetic method is mild in reaction condition and easy to operate, the yield of a reaction of each step is high, and the total recovery can reach 85 percent.
Owner:NANJING FURUN KAIDE BIOLOGICAL PHARMA CO LTD
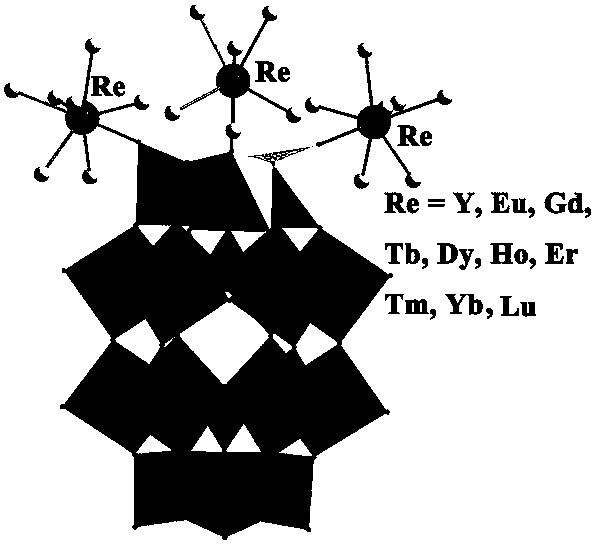
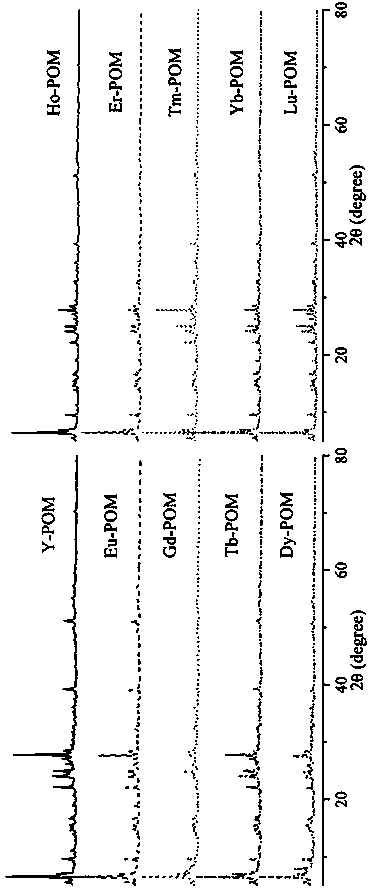
Method for preparing tantalum-based rare earth polyacid and nanocrystal of tantalum-based rare earth polyacid
InactiveCN107587195ALarge specific surface areaFacilitate catalytic reactionsMaterial nanotechnologyPolycrystalline material growthBenzaldehydeLewis acid catalysis
The invention discloses a method for preparing tantalum-based rare earth polyacid and a nanocrystal of tantalum-based rare earth polyacid, and belongs to the technical field of synthesis of polytantalate. The key points of the technical scheme are as follows: the tantalum-based rare earth polyacid is composed of three rare earth ions and one polyacid anion which are bonded through three RE-O-Ta bonds, the tantalum-based rare earth polyacid is overall an electrically neutral cluster and the molecular formula of the tantalum-based rare earth polyacid is [REH2O)7]3P2W15Ta3O62.nH2O. The inventionalso specifically discloses the method for preparing the nanocrystal of the tantalum-based rare earth polyacid. A large amount of coordinated water on the rare earth ions in the tantalum-based rare earth polyacid is easily lost under heating, and thus Lewis acid catalytic active sites of the rare earth ions are exposed, so that the tantalum-based rare earth polyacid can be used as a Lewis acid catalyst; the tantalum-based rare earth polyacid is insoluble in water and a common organic solvent, and therefore the tantalum-based rare earth polyacid can be used as a heterogeneous catalyst; besides,since the specific surface area of the tantalum-based rare earth polyacid is increased due to nanoparticles of the tantalum-based rare earth polyacid, a catalytic reaction is promoted, and when Y-POMis used for catalyzing a reaction of benzaldehyde with trimethylsilyl cyanide, the conversion rate within 4 hours reaches 99% or above.
Owner:HENAN NORMAL UNIV
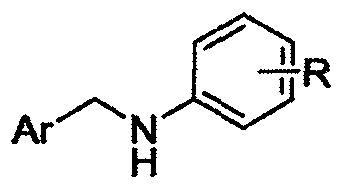
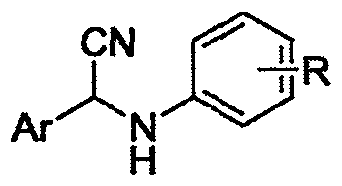
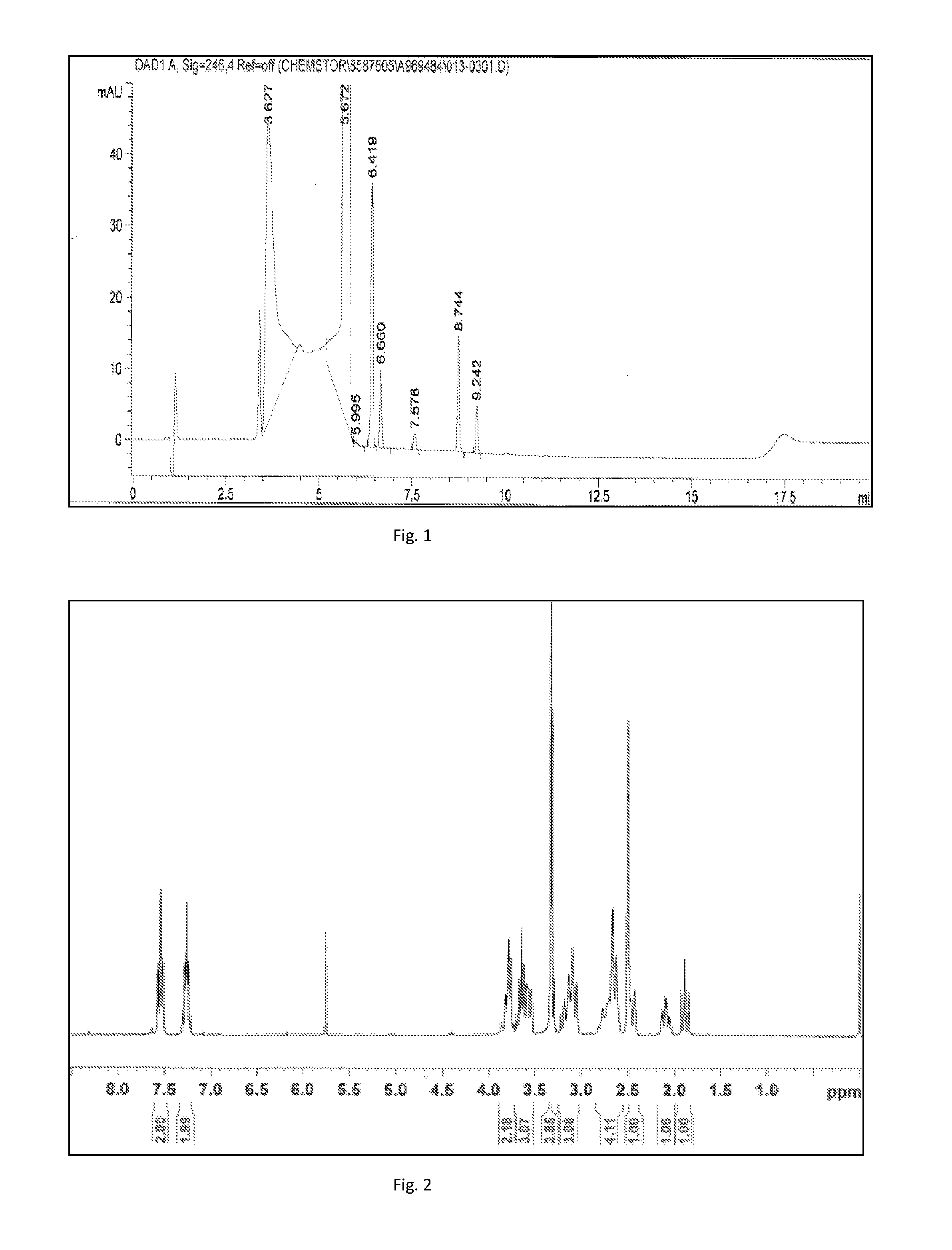
Alpha-cyaniding method of N-arylmethylaniline
InactiveCN109928893AImprove responseMeet the needs of varietyCarboxylic acid nitrile preparationOrganic compound preparationSynthesis methodsTrimethylsilyl cyanide
An alpha-cyaniding method of N-arylmethylaniline is disclosed. According to the method, a finished product is obtained through a simple process of concentrating and purifying after reaction of variousN-arylmethylaniline derivatives as raw materials, trimethylsilyl cyanide as a cyanating agent and 2,3-dichloro-5,6-dicyano-1,4-benzoquinone as an oxidant in a solvent. A new synthesis method is provided for solving the deficiencies of the existing oxidative cyanation process of N-arylmethylaniline. The method adopts the 2,3-dichloro-5,6-dicyano-1,4-benzoquinone which is low in price, low in toxicity and easily available as the oxidant, is free from metal ion catalysis, and efficiently realizes oxidative cyanation of various N-arylmethylaniline and the trimethylsilyl cyanide. The method disclosed by the invention is simple in process, safe in preparation reaction production, easy to operate, short in reaction time and beneficial to industrialization.
Owner:CHONGQING UNIV
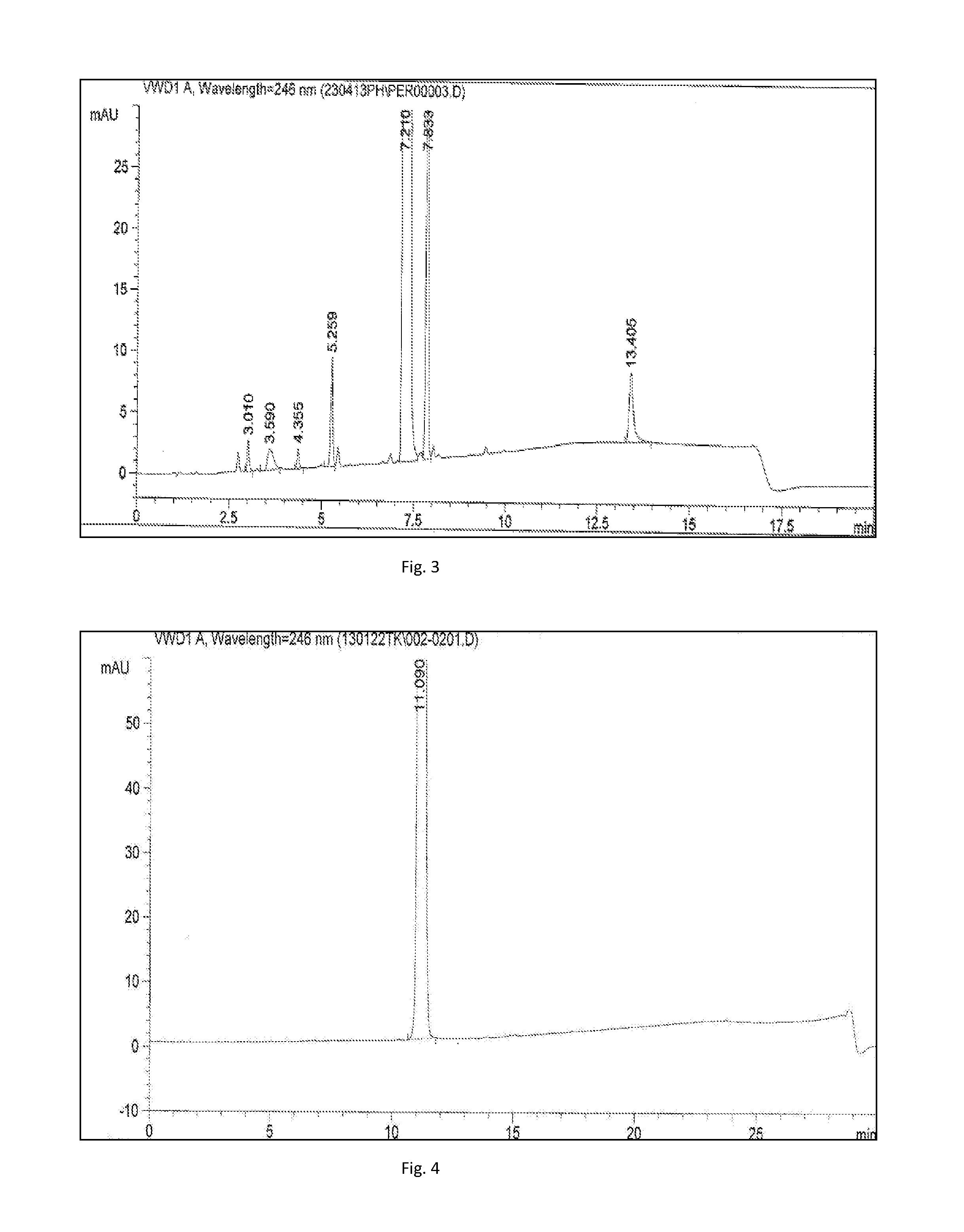
Process for the manufacture of spirocyclic substituted benzofuroquinolizines
ActiveUS20160168163A1Reduce usageOrganic chemistryRaney catalystsPolymer scienceTrimethylsilyl cyanide
The present invention relates to a process for the manufacture of N-(2-((2R,12bS)-2′-oxo-1,3,4,6,7,12b-hexahydrospiro[benzofuro[2,3-a]quinolizine-2,4′-imida-zolidine]-3′-yl)ethyl)-methanesulfonamide, where in the process trimethylsilyl cyanide is used.
Owner:VETCARE
N-[4-(alkoxyl)-benzenesulfonyl]-5-aryl-oxazole-2-thioketone neuraminidase inhibitor
ActiveCN107011282ANovel structureGood neuraminidase inhibitory activityOrganic chemistryDrug compositionsSulfonyl chlorideThioketone
The invention discloses an N-[4-(alkoxyl)-benzenesulfonyl]-5-aryl-oxazole-2-thioketone neuraminidase inhibitor. A preparation method specifically comprises the following steps: (1) performing a reaction on aromatic aldehyde ArCHO, lithium perchlorate and trimethylsilyl cyanide to obtain a cyan analogue; (2) reducing the cyan analogue II to obtain a hydroxyl-substituted primary ammonia compound; (3) performing a reaction on the hydroxyl-substituted primary ammonia compound and carbon disulfide to obtain a 1,3-oxazole-2-thioketone intermediate; (4) performing a reaction on sodium 4-hydroxybenzenesulfonate and an alkylation reagent to obtain an ether compound; (5) performing a reaction on the ether compound and a chlorination reagent to obtain a sulfonyl chloride compound; and (6) substituting active hydrogen of imino groups in the 1,3-oxazole-2-thioketone intermediate to obtain a target compound. The compound synthesized by the preparation method is novel in structure and has relatively good neuraminidase inhibiting activity.
Owner:SHANGHAI SCIENPHARM CO LTD
Preparation method of glufosinate-ammonium intermediate
InactiveCN109879910ALow toxicityEnhanced nucleophilicityGroup 5/15 element organic compoundsSynthesis methodsSodium cyanide
The invention relates to a preparation method of a glufosinate-ammonium intermediate. The preparation method comprises the following steps: with diethyl methylphosphite as a starting raw material, firstly carrying out a Michael reaction of diethyl methylphosphite and acrolein, and then carrying out a Streckker reaction on a product without treatment and trimethylsilyl cyanide and an ammonia watersolution of ammonium chloride to obtain the glufosinate-ammonium key intermediate, namely glufosinate-ammonium cyanamide. Compared with a traditional synthesis method, the method has the advantages that sodium cyanide is replaced by trimethylsilyl cyanide, the toxicity is greatly reduced, the operation safety is improved, meanwhile, the nucleophilic capacity is high, the reaction is facilitated, and the yield is high.
Owner:JIANGSU CHANGQING AGROCHEMICAL CO LTD
Synthesis method of topiroxostat
The invention belongs to the field of medicine and chemical industry and discloses a synthesis method of topiroxostat. The method comprises that 4-cyanopyridine as a starting raw material is oxidized by hydrogen peroxide to form an intermediate 1, the intermediate 1, sodium methoxide and ammonium chloride undergo a reaction to produce an intermediate 2, the intermediate 2 and 4-cyanopyridine undergo an annulation reaction under action of cuprous bromide and sodium carbonate to produce an intermediate 3, and the intermediate 3 and trimethylsilyl cyanide undergo a cyanation reaction to produce topiroxostat. The method utilizes low-price 4-cyanopyridine as a starting raw material, and in the first reaction step, methanol is used as a solvent and 4-cyanopyridine nitrogen oxide as the intermediate 1 is prepared. The method has a high yield and produces a high-purity product. The whole route is easy to operate and is conducive to industrial mass production.
Owner:KAIFENG PHARMA GRP +2
Method for visible light catalytic selective cyanation of piperazine compound
ActiveCN107226796AReduce dosageSimple and fast operationOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsAir atmosphereTrimethylsilyl cyanide
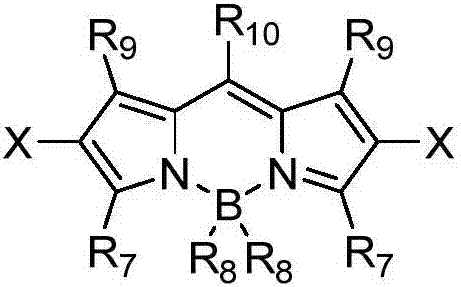
The invention discloses a method for visible light catalytic selective cyanation of a piperazine compound. The method comprises that a piperazine compound, trimethylsilyl cyanide and a multi-substituted BODIPY organic photocatalyst are stirred and undergo a reaction in the air atmosphere under visible light irradiation at 10 to 40 DEG C to produce a cyanopiperazine compound. Compared with the prior art, the method utilizes a small amount of a catalyst, is easy to operate, is environmentally friendly, needs mild reaction conditions, is fast and efficient, has a wide substrate application range, can selectively produce a cyanation product having a single structure and has a high conversion rate and selectivity. The catalyst system is suitable for selective cyanation of complex drug molecules.
Owner:SHAANXI NORMAL UNIV
A kind of synthetic method of topicastat
Owner:KAIFENG PHARMA GRP +2
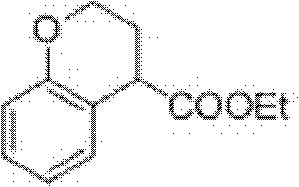
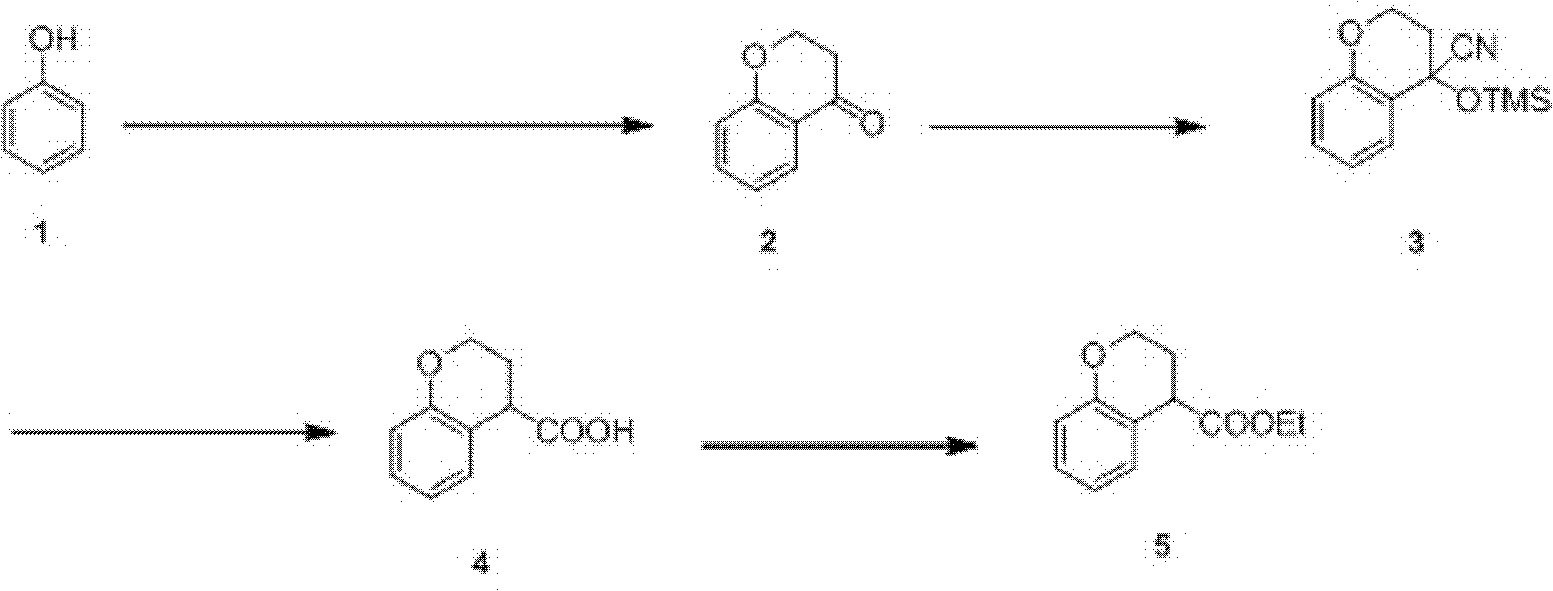
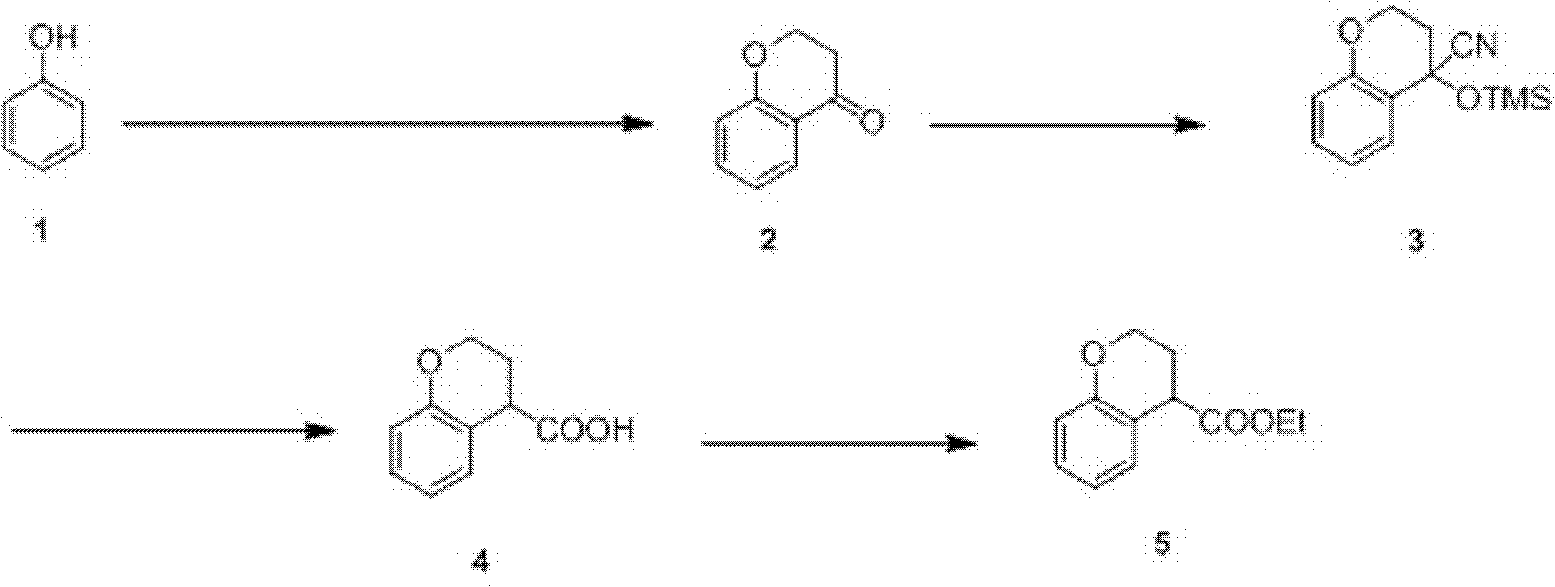
Preparation process of ethyl chromane-4-formate
The invention relates to a preparation process of ethyl chromane-4-formate. The preparation process is characterized by comprising the following steps of: carrying out Friedel-Crafts acylation reaction and cyclization, addition reaction, hydrolysis reaction and esterification reaction, wherein, in the Friedel-Crafts acylation reaction and cyclization, the Friedel-Crafts acylation reaction is carried out in the presence of anhydrous aluminium trichloride by using a compound of formula 1 and 3-chloropropionylchloride as initial raw materials, then carrying out cyclization on an obtained product and 10% NaOH to obtain a compound of formula 2; in the addition reaction, the addition reaction is carried out on the compound of formula 2 and trimethylsilyl cyanide in the presence of zinc iodide to obtain a compound of formula 3; in the hydrolysis reaction, the compound of formula 3 is hydrolyzed under the conditions of stannous chloride dihydrate, concentrated hydrochloric acid and acetic acid to obtain a compound of formula 4; and in the esterification reaction, the compound of formula 4 is esterified in the presence of concentrated sulfuric acid to obtain a compound of formula 5. According to the preparation process, expensive trifluoromethanesulfonic acid is replaced with cheap anhydrous aluminium trichloride in the Friedel-Crafts acylation step, and the yield is improved, so that the cost of preparing the compound is greatly reduced, and the high-quality product is obtained; and compared with the traditional method, the preparation process has obvious advantages.
Owner:苏州莱克施德药业有限公司
Preparation method of betamethasone intermediate
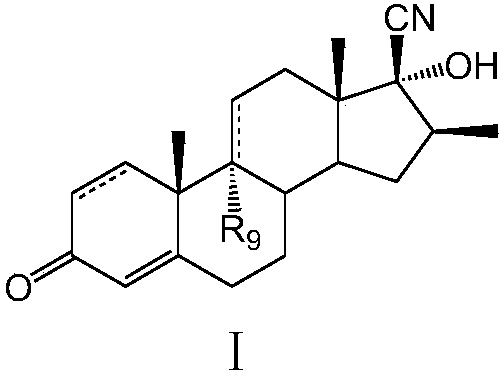
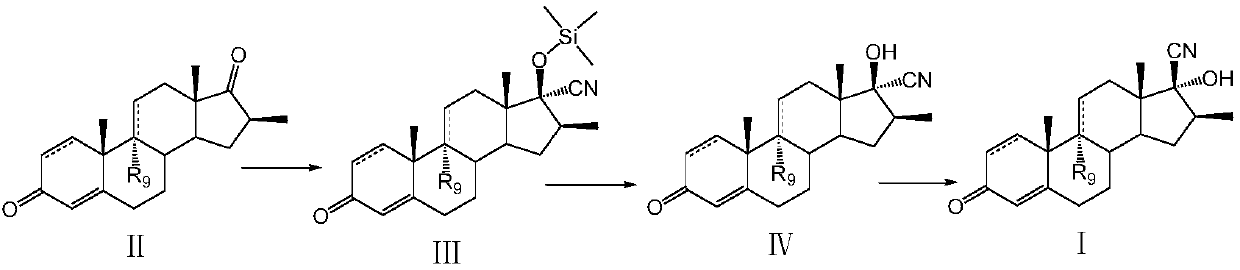
ActiveCN108047296AReaction steps increaseHigh product yieldSteroidsTrimethylsilyl cyanideBetamethasone
The invention discloses a preparation method of a betamethasone intermediate. The method includes the steps: taking a compound II as a substrate; performing silicon cyanide reaction to obtain a compound III; performing hydrolysis reaction to remove silicon alkyl groups to obtain a compound IV; performing transposition rearrangement reaction to prepare a compound I. According to the method, trimethylsilyl cyanide replaces sodium cyanide, the silicon cyanide reaction between the trimethylsilyl cyanide and the substrate is performed, the hydrolysis reaction and the transposition rearrangement reaction are performed to build 17 beta-CN, reaction steps are increased, reaction routes are simple, total reaction time is shortened, and the method is easy to operate and suitable for industrial production. More importantly, synthetic routes are achieved, side reaction is few, production yield and product quality are obviously increased, and production cost is greatly reduced.
Owner:江西赣亮医药原料有限公司
Method for N, N-dialkylaniline cyanation reaction
InactiveCN104672039AReduce pollutionReduce manufacturing costCarboxylic acid nitrile preparationOrganic compound preparationChemical synthesisKetone
The invention discloses a method for N, N-dialkylaniline cyanation reaction and belongs to the technical field of organic chemical synthesis. The method comprises the following steps: by taking benzene ring substituted N, N-dialkylaniline as a raw material, taking iodobenzene diacetate or di(trifluoroacetoxy) iodobenzene as an oxidant, taking trimethylsilyl cyanide as a cyaniding agent and taking anhydrous Na2SO4 as an additive, in the solvent, performing ice bath or performing one step reaction at room temperature, condensing and purifying to obtain the product. The method has the characteristics of simplicity, mild reaction condition, simplicity in operation, low production cost and convenience in popularization; the high-quality N, N-dialkylaniline cyanide product can be prepared by the method provided by the invention; the method can be widely used in the industrial production of the N, N-dialkylaniline cyanide; the product prepared by the method can be used for the synthesis of the bioactive compounds such as alpha-amino acid, 1, 2-diamine, tetrahydroquinoline, alpha-amino-aldehyde, ketone or beta-amino alcohol, and the wide demands of the market can be satisfied.
Owner:CHONGQING UNIV
Preparation method of furilazole
The invention discloses a preparation method of furilazole, which comprises the following steps: 1, carrying out a nucleophilic addition reaction on furfural and trimethylsilyl cyanide; 2, reducing a-(2-furan)-a-trimethylsiloxy acetonitrile; 3, carrying out an addition elimination reaction on a-aminomethyl-2-furancarbinol and acetone; and 4, amidating 5-(2-furan)-2,2-dimethyloxazolidine. The preparation method has the advantages of simple process operation, and pureness and high yield of the target product, and provides another approach for the synthesis of like herbicide safeners.
Owner:NANJING UNIV OF SCI & TECH +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
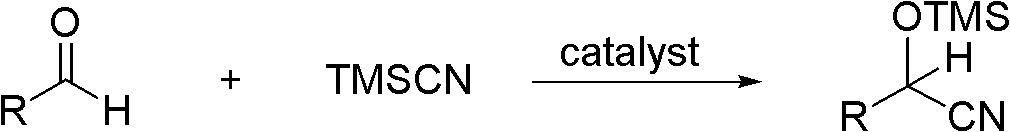
![Method for preparing (1S, 5S)-6,6-dimethyl-3-azabicyclo [3.1.0] hexane-2-carboxylic acid alkyl ester Method for preparing (1S, 5S)-6,6-dimethyl-3-azabicyclo [3.1.0] hexane-2-carboxylic acid alkyl ester](https://images-eureka.patsnap.com/patent_img/33006009-b8f9-492f-9cf2-4f4786465ca1/FDA00002610407400021.png)
![Method for preparing (1S, 5S)-6,6-dimethyl-3-azabicyclo [3.1.0] hexane-2-carboxylic acid alkyl ester Method for preparing (1S, 5S)-6,6-dimethyl-3-azabicyclo [3.1.0] hexane-2-carboxylic acid alkyl ester](https://images-eureka.patsnap.com/patent_img/33006009-b8f9-492f-9cf2-4f4786465ca1/BDA00002610407500012.png)
![Method for preparing (1S, 5S)-6,6-dimethyl-3-azabicyclo [3.1.0] hexane-2-carboxylic acid alkyl ester Method for preparing (1S, 5S)-6,6-dimethyl-3-azabicyclo [3.1.0] hexane-2-carboxylic acid alkyl ester](https://images-eureka.patsnap.com/patent_img/33006009-b8f9-492f-9cf2-4f4786465ca1/BDA00002610407500021.png)
![Synthesis method of tert-butyl-8-oxo-2,6,9-triazaspiro[4.5]decane-2-formylate Synthesis method of tert-butyl-8-oxo-2,6,9-triazaspiro[4.5]decane-2-formylate](https://images-eureka.patsnap.com/patent_img/6d130abe-9731-4832-84f2-27c828084aaa/858410DEST_PATH_IMAGE002.png)
![Synthesis method of tert-butyl-8-oxo-2,6,9-triazaspiro[4.5]decane-2-formylate Synthesis method of tert-butyl-8-oxo-2,6,9-triazaspiro[4.5]decane-2-formylate](https://images-eureka.patsnap.com/patent_img/6d130abe-9731-4832-84f2-27c828084aaa/DEST_PATH_IMAGE002.png)
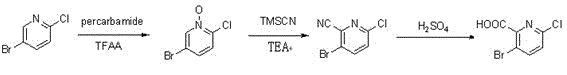
![N-[4-(alkoxyl)-benzenesulfonyl]-5-aryl-oxazole-2-thioketone neuraminidase inhibitor N-[4-(alkoxyl)-benzenesulfonyl]-5-aryl-oxazole-2-thioketone neuraminidase inhibitor](https://images-eureka.patsnap.com/patent_img/61704573-cca3-4a3f-88de-ccd5f332c5ba/BDA0001264684030000011.png)
![N-[4-(alkoxyl)-benzenesulfonyl]-5-aryl-oxazole-2-thioketone neuraminidase inhibitor N-[4-(alkoxyl)-benzenesulfonyl]-5-aryl-oxazole-2-thioketone neuraminidase inhibitor](https://images-eureka.patsnap.com/patent_img/61704573-cca3-4a3f-88de-ccd5f332c5ba/BDA0001264684030000012.png)
![N-[4-(alkoxyl)-benzenesulfonyl]-5-aryl-oxazole-2-thioketone neuraminidase inhibitor N-[4-(alkoxyl)-benzenesulfonyl]-5-aryl-oxazole-2-thioketone neuraminidase inhibitor](https://images-eureka.patsnap.com/patent_img/61704573-cca3-4a3f-88de-ccd5f332c5ba/BDA0001264684030000013.png)