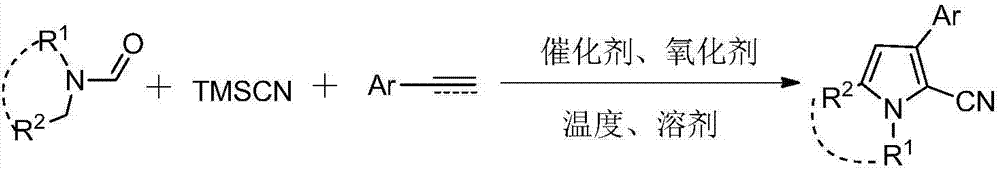
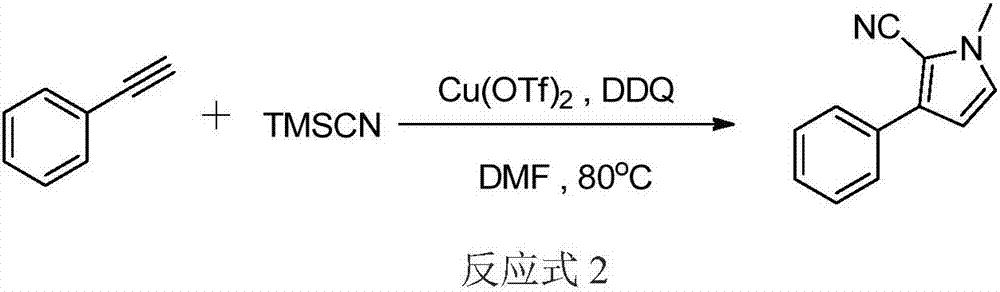
Three-component tandem synthesis method of multi-substituted pyrrole
A synthetic method and di-substitution technology, which is applied in the field of three-component tandem synthesis of 2-cyano-3-arylpyrrole compounds, can solve the problems of many by-products, cumbersome steps, and low product yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
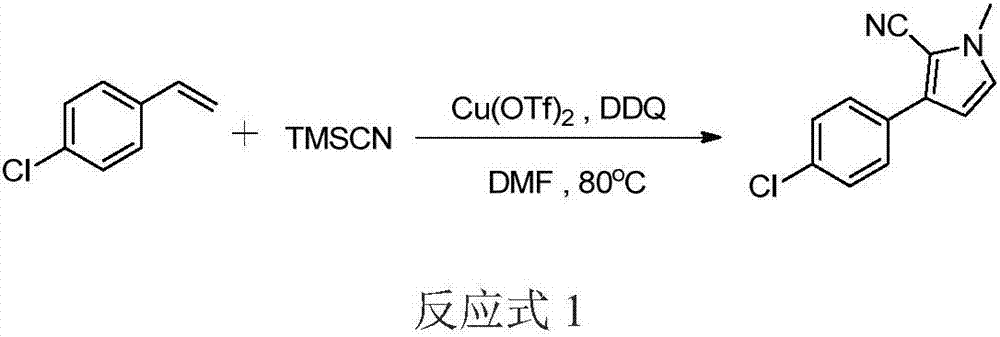
[0022] With N,N-dimethylformamide as both the reaction substrate and the reaction solvent, p-chlorostyrene is the aryl olefin substrate (reaction formula 1)
[0023]
[0024] Trimethylsilyl cyanide (240ul, 1.81mmol), copper trifluoromethanesulfonate (26mg, 0.072mmol), p-chlorostyrene (50mg, 0.36mmol) and DDQ (16mg, 0.072mmol) were sequentially added to the stirring In DMF (1mL), react at 80°C, add 16mg (0.072mmol) of DDQ every 2h for the first 8 hours of the reaction, and add 80mg (0.36mmol) of DDQ once at the 10th hour of reaction, and continue at 80°C Reaction 14h. After the reaction, the reaction system was extracted with ethyl acetate and washed with a saturated saline solution to obtain an organic phase, which was dried with anhydrous sodium sulfate for 0.5 h, and then the solvent was drained on a rotary evaporator. Finally, the spin-dried crude product was subjected to silica gel column chromatography. A white solid (67.3 mg, 86%) was obtained.
[0025] Product char...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com