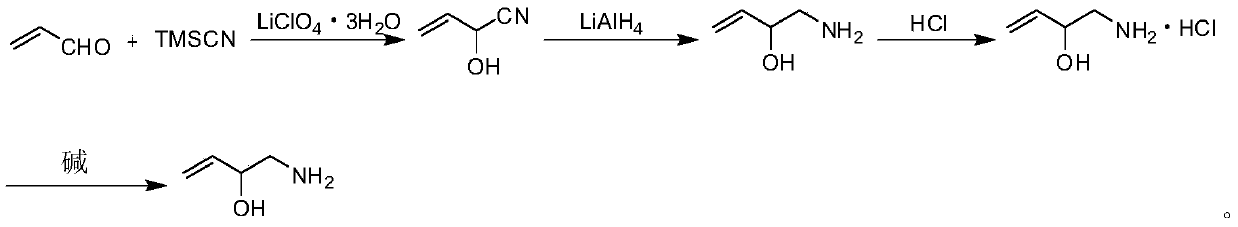
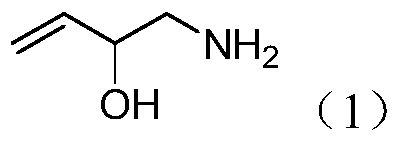
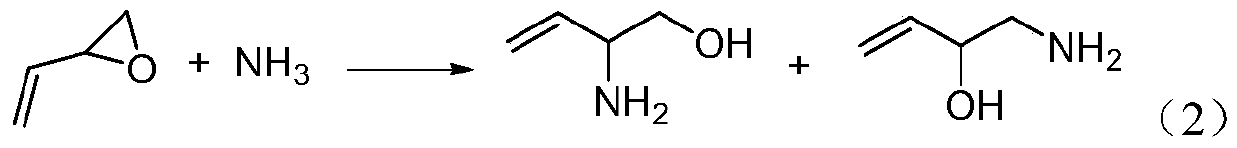
Preparation method of 2-hydroxyl-3-butene-1-amine
A technology of butene and hydroxyl, applied in the field of preparation of 2-hydroxy-3-butene-1-amine, can solve the problems of cumbersome route, long time-consuming, large workload and the like, and achieves simple operation, short time-consuming and cost-effective low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] A preparation method of 2-hydroxyl-3-butene-1-amine, specifically comprising the following steps:
[0046] (1) Add acrolein (1.12g, 0.02mol), trimethylsilyl cyanide (2.98g, 0.03mol) and lithium perchlorate trihydrate (3.21g, 0.02mol) successively to the reactor, and control the stirring speed to React at room temperature at 500r / min. During the reaction, the starting material acrolein used in the reaction was monitored by TLC. After the complete disappearance of the starting material, ethyl acetate was added, and the solid was removed by filtration. The obtained organic phase was washed three times with saturated brine, anhydrous Na 2 SO 4 After drying, 2-hydroxy-3-butene-1-carbonitrile was obtained by rotary evaporation.
[0047] In the above-mentioned TLC monitoring reaction process, the developing agent used is petroleum ether: ether=7:1;
[0048] The acrolein used in the above reaction: trimethylsilyl cyanide: lithium perchlorate trihydrate is 1mol: 1.5mol: 1mol; ...
Embodiment 2
[0062] A preparation method of 2-hydroxyl-3-butene-1-amine, specifically comprising the following steps:
[0063] (1) Add acrolein (1.12g, 0.02mol), trimethylsilyl cyanide (2.98g, 0.03mol) and lithium perchlorate trihydrate (6.42g, 0.04mol) successively to the reactor, and control the stirring speed to React at room temperature at 700r / min. During the reaction, the starting material acrolein used in the reaction was monitored by TLC. After the complete disappearance of the starting material, ethyl acetate was added, and the solid was removed by filtration. The obtained organic phase was washed three times with saturated brine, anhydrous Na 2 SO 4 After drying, 2-hydroxy-3-butene-1-carbonitrile was obtained by rotary evaporation.
[0064] In the above-mentioned TLC monitoring reaction process, the developing agent used is petroleum ether: ether=7:1;
[0065] The acrolein used in the above reaction: trimethylsilyl cyanide: lithium perchlorate trihydrate is 1mol: 1.5mol: 2mol; ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com