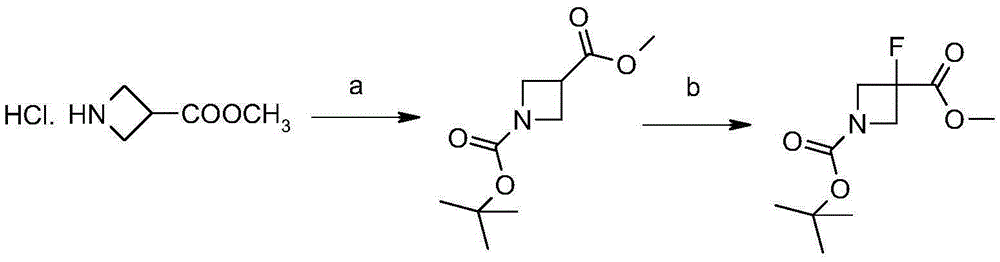
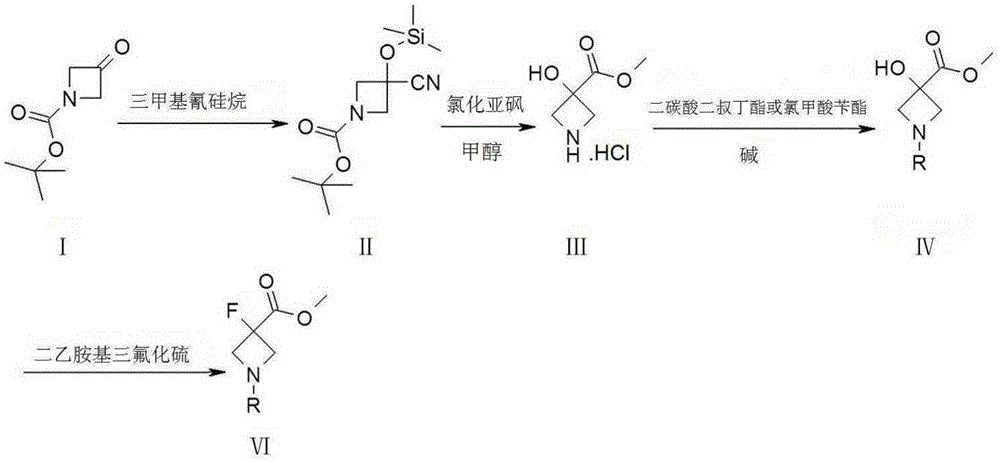
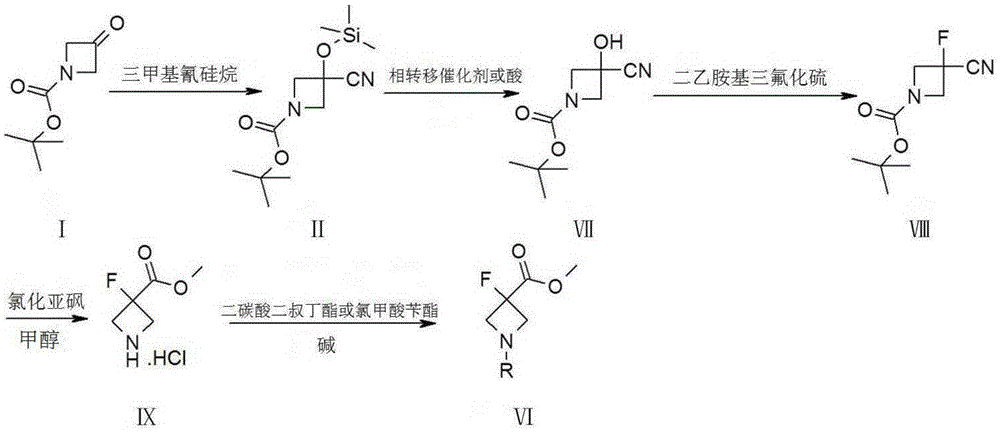
Synthetic method of 3-fluoro-azetidine derivative
A technology for the synthesis of azetidine, which is applied in the direction of organic chemistry, can solve the problems of high risk, low total yield, and high equipment requirements, and achieve the effects of high reaction yield, simple operation, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Synthesis of Compound II:
[0028]
[0029] Add raw material I (17.1g, 0.1mol, 1.0eq.), THF (100mL) and trimethylsilyl cyanide (10.4g, 0.105mol, 1.05eq.) into a 250mL four-neck flask, heat up to 65°C for 5h, TLC Shows complete reaction. Concentrate to obtain 27.0 g of off-white solid, theoretically 27.0 g, yield: 100%. 1 H-NMR (400MHz, CDCl3) δ (ppm) 4.41-4.33 (d, J = 9.8Hz, 2H), 4.10-3.99 (d, J = 9.8Hz, 2H), 1.52-1.39 (s, 9H), 0.32 -0.22(s,9H).
[0030] Synthesis of Compound III:
[0031]
[0032] Add intermediate II (27.0g, 0.1mol, 1.0eq.) and methanol (100mL) to a 250mL four-neck flask, add thionyl chloride (11.9g, 0.1mol, 1.0eq.) dropwise, reflux at 65°C for 3h, and LC - MS showed complete reaction. Concentrate to obtain 16.8 g of off-white solid, theoretically 16.76 g, yield: 100%. directly into the next reaction. 1 H-NMR (400MHz,D 2 O) δ (ppm) 4.55-4.41 (d, J = 12.5Hz, 2H), 4.21-4.05 (d, J = 12.5Hz, 2H), 3.90-3.78 (s, 3H).
[0033] Synthesis of Comp...
Embodiment 2
[0046] Synthesis of Compound II:
[0047]
[0048] Add raw material I (17.1g, 0.10mol, 1.0eq.), toluene (100mL) and trimethylsilyl cyanide (11.9g, 0.12mol, 1.2eq.) into a 250mL four-necked flask, reflux at 110°C for 3h, TLC shows the reaction completely. Concentrate to obtain 27.0 g of off-white solid, theoretically 27.0 g, yield: 100%. 1 H-NMR (400MHz, CDCl 3 )δ (ppm) 4.41-4.33 (d, J = 9.8Hz, 2H), 4.10-3.99 (d, J = 9.8Hz, 2H), 1.52-1.39 (s, 9H), 0.32-0.22 (s, 9H) .
[0049] Synthesis of compound VII:
[0050]
[0051] In a 500mL four-neck flask, add intermediate II (14.7g, 0.054mol, 1.0eq.), then add tetrahydrofuran (100mL) to dissolve, add tetraethylammonium fluoride trihydrate (12.2g, 0.060mol, 1.1eq. ), incubated at 40°C for 2h, and TLC detected that the reaction was complete. Add water (200mL), separate the layers, extract the aqueous layer with EA (100mL, 50mL) once respectively, combine the organic phases, wash with saturated aqueous sodium chloride solution...
Embodiment 3
[0065] Synthesis of Compound II:
[0066]
[0067] In a 2L four-neck flask, add intermediate I (200.0g, 1.168mol, 1.0eq.), dissolve it with EA (1.2L), add trimethylsilyl cyanide (173.8g, 1.752mol, 1.5eq.), 20 After reacting at ℃ for 4 hours, the reaction was complete as detected by TLC, and the solvent was removed by rotary evaporation to obtain 359.0 g of a yellow oil, the theoretical yield: 315.8 g, and the yield of the crude product was 100%. 1 H-NMR (400MHz, CDCl 3 )δ (ppm) 4.41-4.33 (d, J = 9.8Hz, 2H), 4.10-3.99 (d, J = 9.8Hz, 2H), 1.52-1.39 (s, 9H), 0.32-0.22 (s, 9H) .
[0068] Synthesis of Compound III:
[0069]
[0070] Add intermediate II (27.0g, 0.1mol, 1.0eq.) and MeOH (100mL) to a 250mL four-neck flask, add thionyl chloride (23.8g, 0.2mol, 2.0eq.) dropwise, react at 40°C for 4h, LC - MS showed complete reaction. Concentrate to obtain 16.8 g of off-white solid, theoretically 16.8 g, yield: 100%. directly into the next reaction. 1 H-NMR (400MHz,D 2 O) δ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com