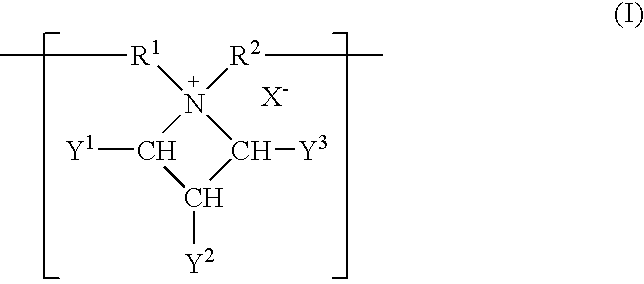
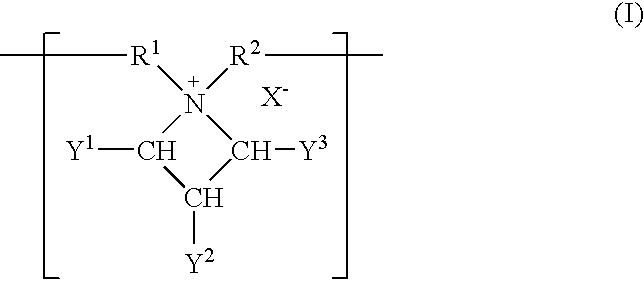
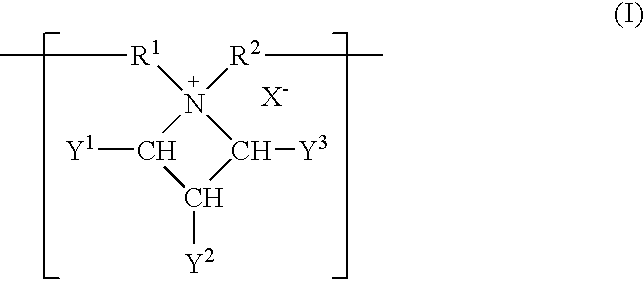
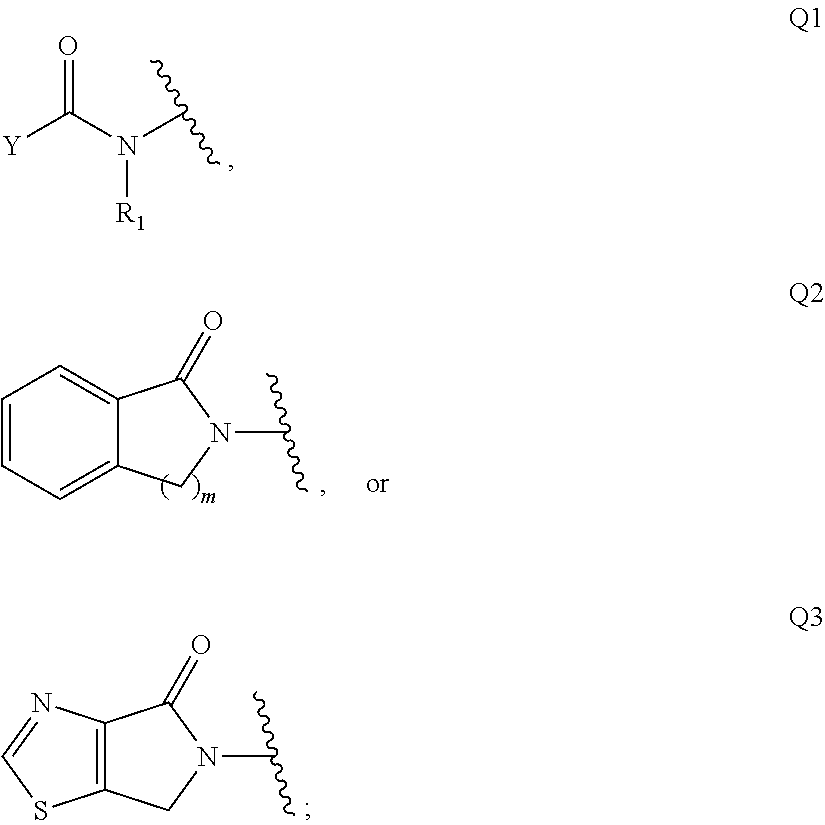
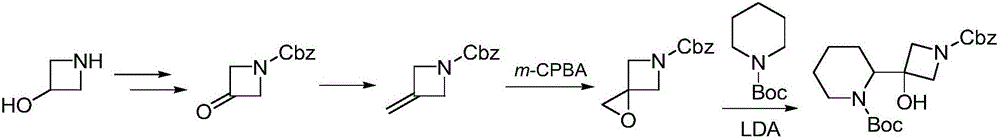Patents
Literature
153 results about "Azacyclobutane" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Ink-jet recording medium with an opaque or semi-opaque layer coated thereon, method for recording an image, and a recorded medium with at least one layer rendered clear or semi-opaque
InactiveUS20040109958A1Extended drying timeAvoid excessive adhesionLayered productsCoatingsAziridineCross linker
The present invention features a multi-layer ink-jet recording medium, suitable for recording images with dye and pigmented inks and thereby providing light-emitting, reflective, glossy, metallic-looking or holographic images, comprising a substrate coated with at least two layers comprising: (a) a first transparent ink-receptive layer comprising a polymeric binder and a cross-linker and optionally having a plasticizer and pigment particles such as alumina and silica coated over the substrate, wherein the cross-linker comprises and azetidinium polymer or a salt thereof, and / or a polyfunctinal aziridine or a salt thereof or a polyfunctional oxazoline or a salt thereof; and (b) a second ink-receptive layer comprising an opaque or semi-opaque coating composition, wherein the opaque or semi-opaque coating composition is capable of accepting a printed image and thereby becoming semi transparent or clearly transparent from application of ink-jet printing ink or similar inks, while presenting a light-emitting, reflective, glossy, metallic-looking or holographic image of high clarity and quality, wherein said first layer is located between said second layer and the substrate in said recording medium and the first and second layers are chemically coupled.
Owner:PIXTERRA
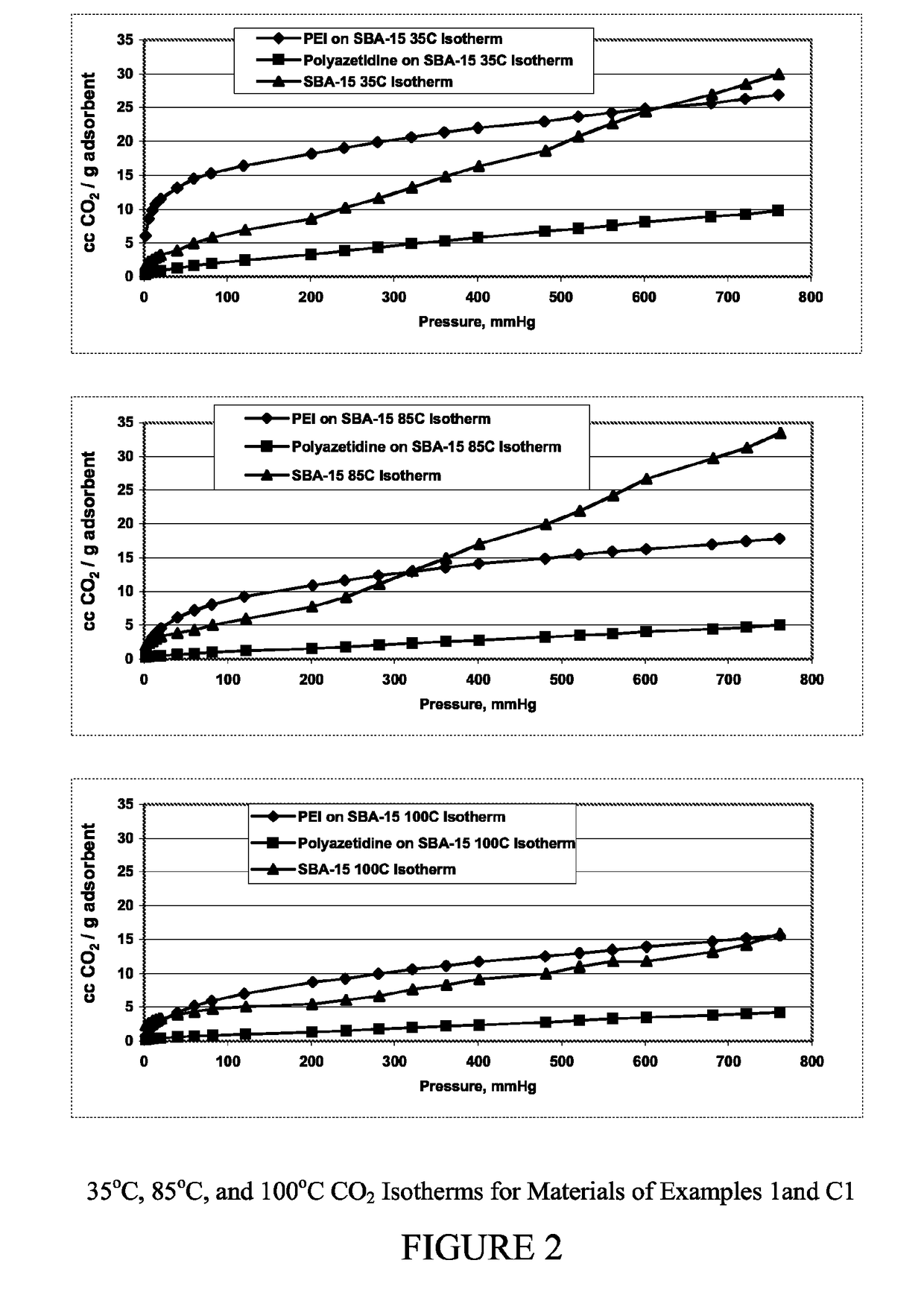
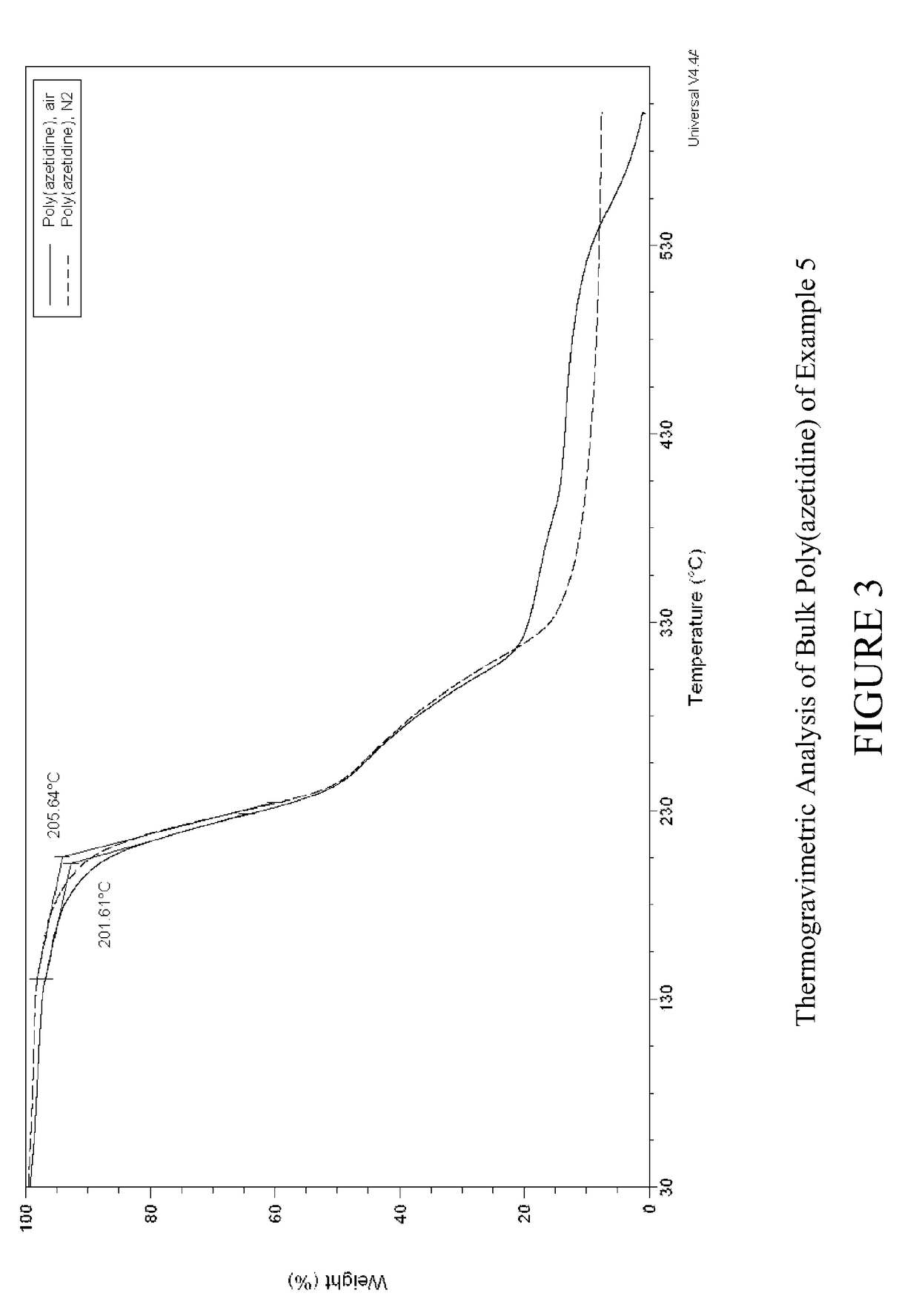
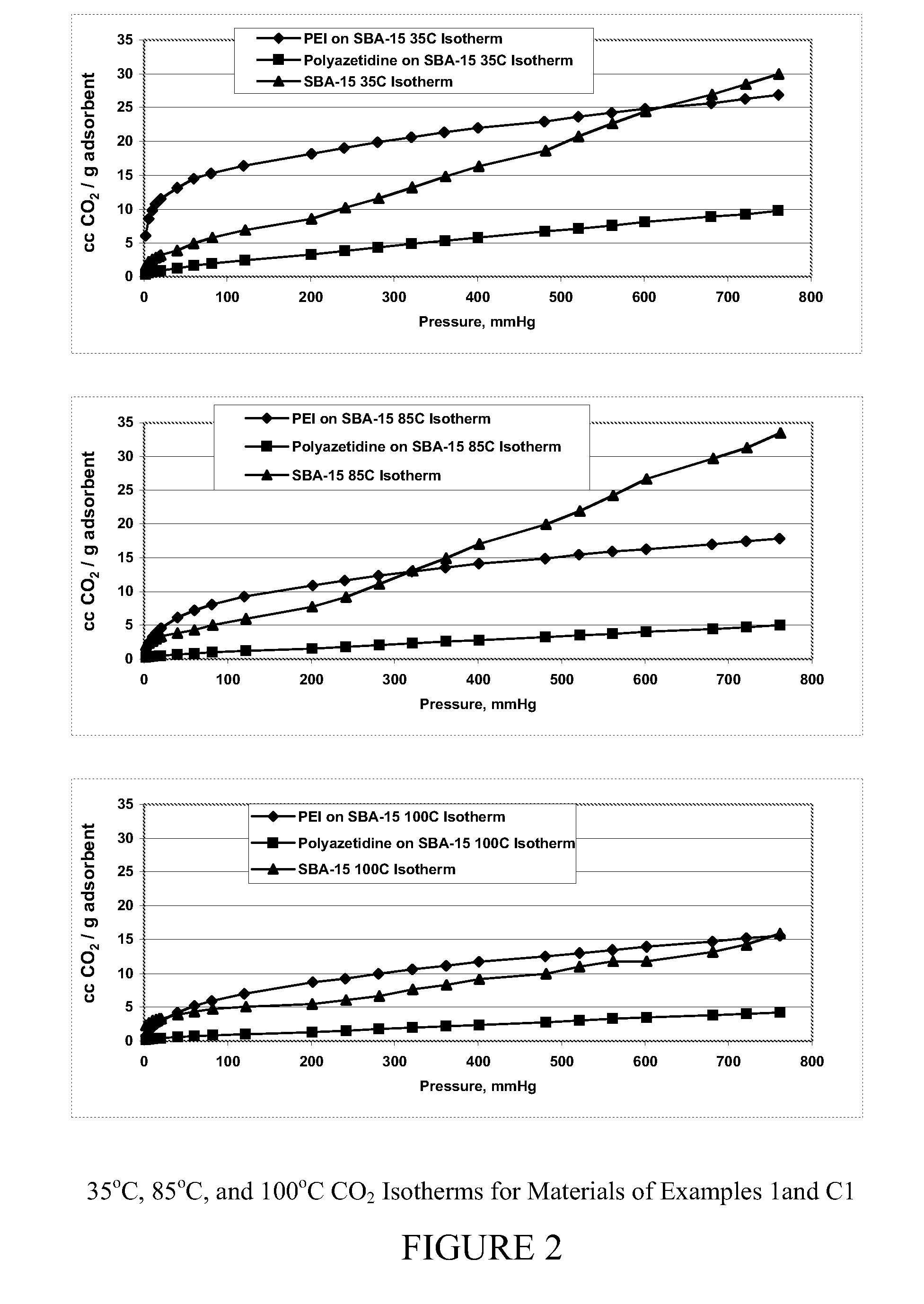
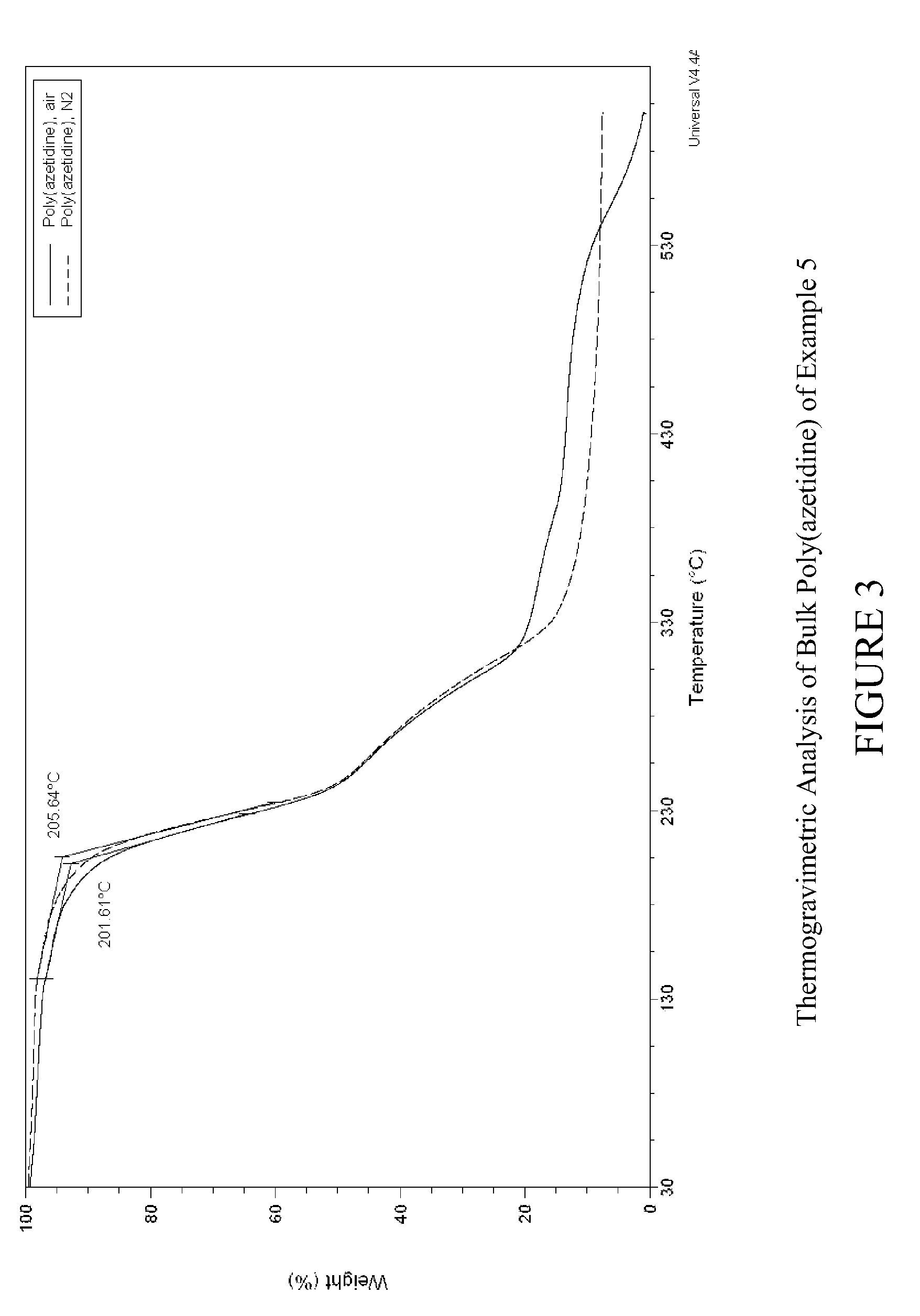
Carbon dioxide sorbents
Owner:EXXON RES & ENG CO
beta-Lactamyl vasopressin V1a Antagonists
Owner:EHJZEVAN FARMASJUTIKLZ INK
Azetidinium modified polymers and fabric treatment composition
InactiveUS7429558B2Organic detergent compounding agentsFibre treatmentCross-linkCrosslinked polymers
The invention relates to a fabric treatment composition which comprises a self-crosslinking polymer possessing pendant azetidinium groups. A first aspect of the invention comprises an azetidinium functionalized polymer containing primary or secondary amine groups. Cross-linking reactions between the azetidinium group and primary or secondary amine groups do not form quaternary groups and consequently do not result in a charged, cross-linked polymer. This lack of charge is believed to overcome the problems of stain fixing and dye adsorption. A second aspect of the present invention subsists in an azetidinium functionalized polymer of which the monomers comprise: an amino-acrylate and / or amino-alkacrylate monomer, and, optionally, further non-amino acrylate and / or alkacrylate monomer. A third aspect of the present invention provides a textile treatment composition which comprises an azetidinium functionalized polymer in accordance with the first or second aspect of the invention and a textile compatible carrier.
Owner:HENKEL IP & HOLDING GMBH
Ink-jet recording medium with an opaque or semi-opaque layer coated thereon, method for recording an image, and a recorded medium with at least one layer rendered clear or semi-opaque
InactiveUS6936316B2Avoid excessive adhesionExtended drying timeCoatingsThermographyAziridineCross linker
The present invention features a multi-layer ink-jet recording medium, suitable for recording images with dye and pigmented inks and thereby providing light-emitting, reflective, glossy, metallic-looking or holographic images, comprising a substrate coated with at least two layers comprising:(a) a first transparent ink-receptive layer comprising a polymeric binder and a cross-linker and optionally having a plasticizer and pigment particles such as alumina and silica coated over the substrate, wherein the cross-linker comprises and azetidinium polymer or a salt thereof, and / or a polyfunctinal aziridine or a salt thereof or a polyfunctional oxazoline or a salt thereof; and(b) a second ink-receptive layer comprising an opaque or semi-opaque coating composition, wherein the opaque or semi-opaque coating composition is capable of accepting a printed image and thereby becoming semi transparent or clearly transparent from application of ink-jet printing ink or similar inks, while presenting a light-emitting, reflective, glossy, metallic-looking or holographic image of high clarity and quality,wherein said first layer is located between said second layer and the substrate in said recording medium and the first and second layers are chemically coupled.
Owner:PIXTERRA
Carbon dioxide sorbents
Improved CO2 sorbents comprised of a mesoporous silica functionalized with a polyamine are obtained by the in-situ polymerization of azetidine. Also included herein are processes utilizing the improved CO2 sorbents wherein CO2 is chemisorbed onto the polyamine portion of the sorbent and the process is thermally reversible.
Owner:EXXON RES & ENG CO
1-((5-aryl-1,2,4-oxadiazol-3-yl) benzyl)azetidine-3-carboxylates and 1-((5-aryl-1,2,4-oxadiazol-3-yl)benzyl) pyrrolidine-3-carboxylates as edg receptor agonists
The present invention encompasses compounds of Formula I: as well as the pharmaceutically acceptable salts and hydrates thereof. The compounds are useful for treating immune mediated diseases and conditions, such as bone marrow, organ and tissue transplant rejection. Pharmaceutical compositions and methods of use are included.
Owner:MERCK & CO INC
Therapeutic compounds and their use in cancer
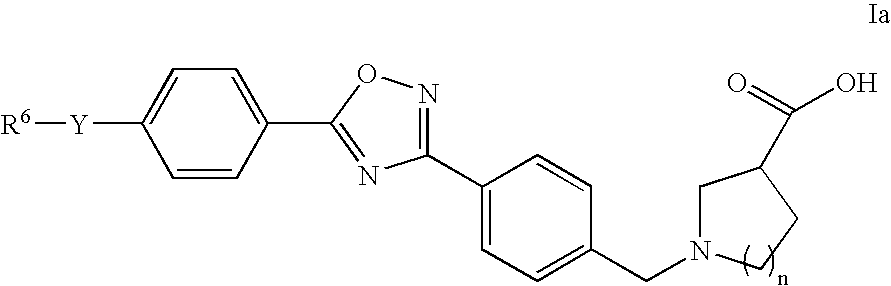
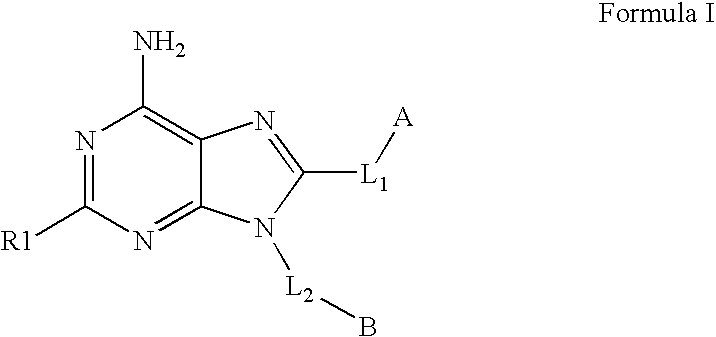
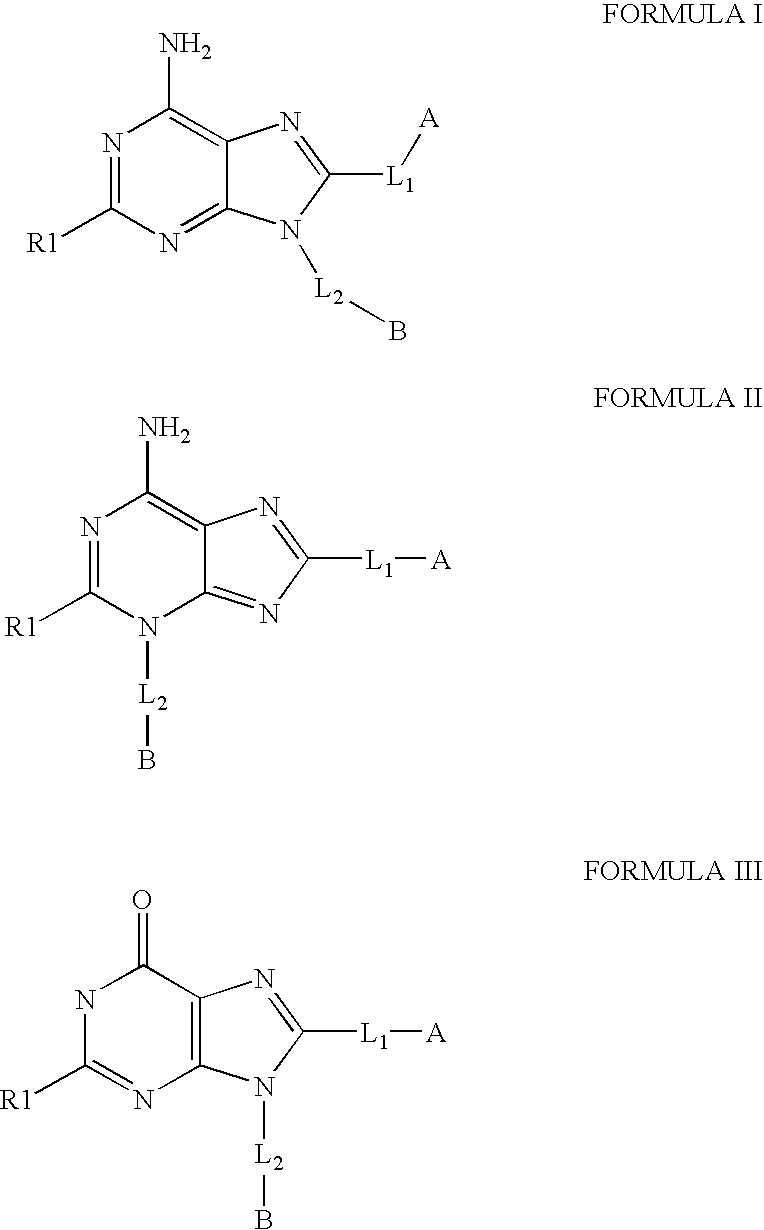
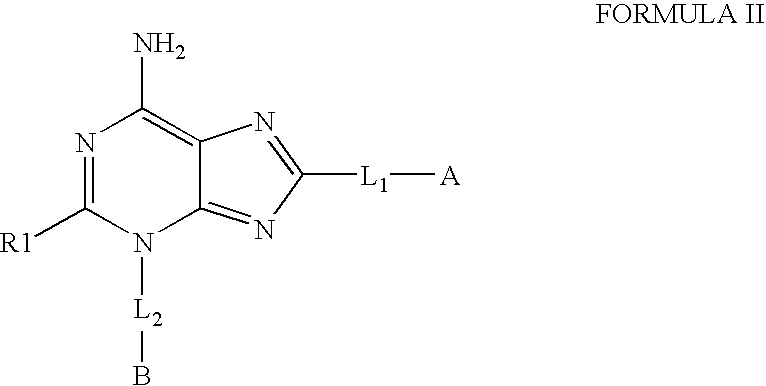
The invention relates to compounds of Formula Iand their therapeutic uses, wherein substituent A is chosen from a substituted or unsubstituted aryl, heteroaryl, heterocyclic, or carboxylic group, B is chosen from a substituted or unsubstituted piperidine, homopiperidine, piperazine, pyrrolidine or azetidine group, R1 is chosen from hydro, alkyl, aryl, heteroaryl amino and halo, and L1 and L2 are as defined in the specification.
Owner:SUNFLOWER RES
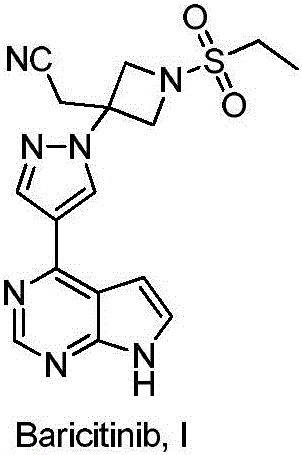
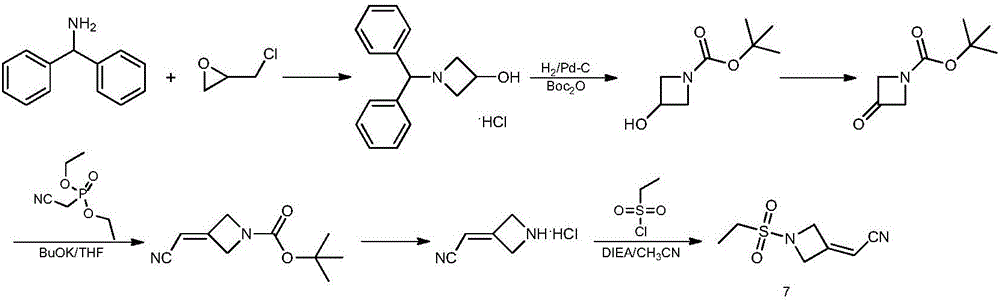
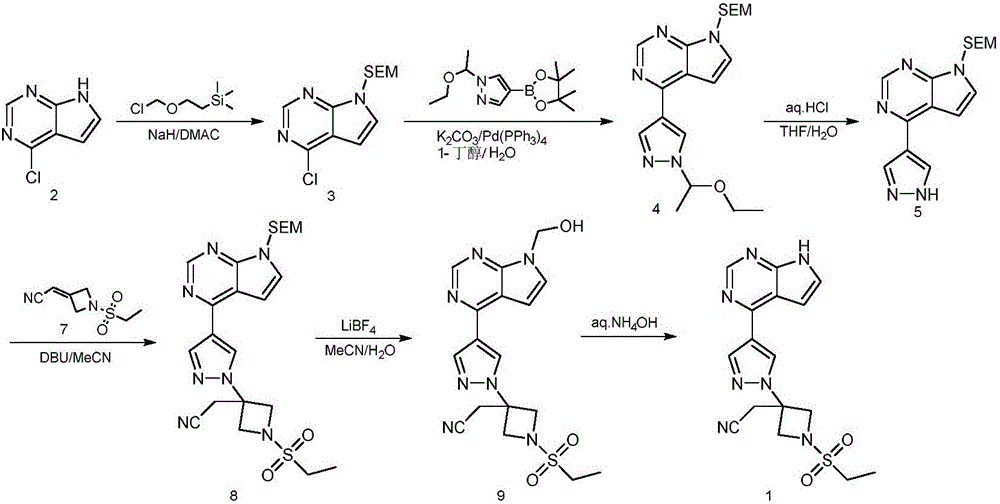
Method for preparing baricitinib
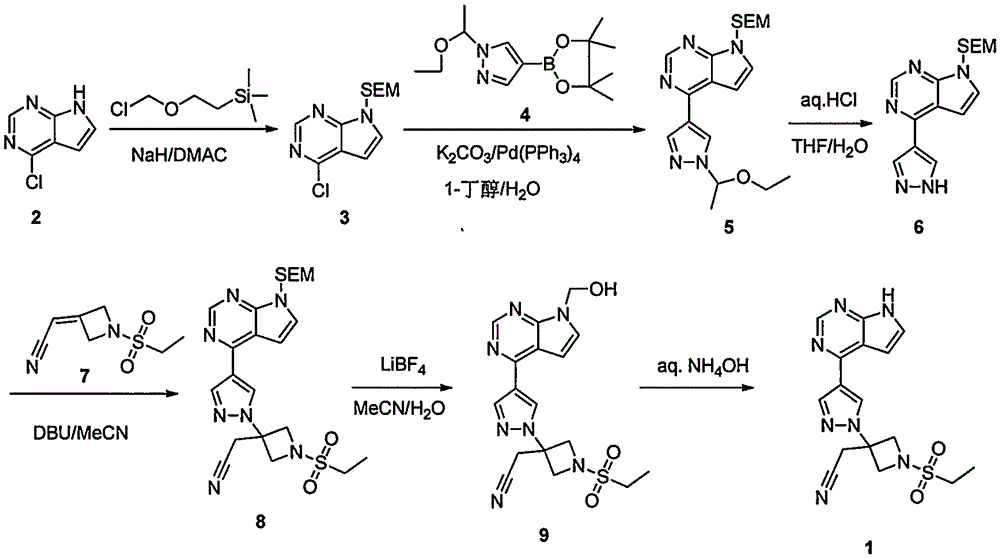
ActiveCN105294699ARaw materials are easy to getSimple processOrganic chemistryCyclobutaneCyanomethylidyne
The invention provides a method for preparing baricitinib. The method comprises the following steps: by taking 4-pyrazol boric acid pinacol ester (10) as an initial raw material, performing Michael addition reaction on the initial raw material and 3-(icyanomethylene) azacyclo-cyclobutane-1-tert-butyl formate (11) so as to prepare an intermediate 12, and performing catalytic coupling reaction on 12 and 13, thereby preparing an intermediate 14; removing two-molecule tert-butyl formate of the intermediate 14, thereby preparing an intermediate 15; performing sulfamide reaction on the intermediate 15 and ethanesulfonyl chloride in an organic solvent, thereby obtaining a final product baricitinib (1). The method for preparing baricitinib has the advantages that the raw materials are easy to obtain, the process is simple, the operation is convenient, the reaction yield is high when being compared with that of document records, the atom utilization rate is high, industrial production can be easily achieved, and the like. The reaction general formula is as shown in the specification.
Owner:SHANGHAI XUNHE PHARMA TECH CO LTD
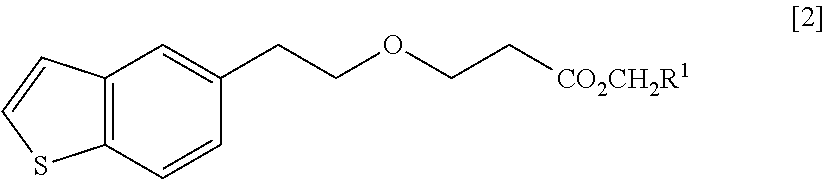
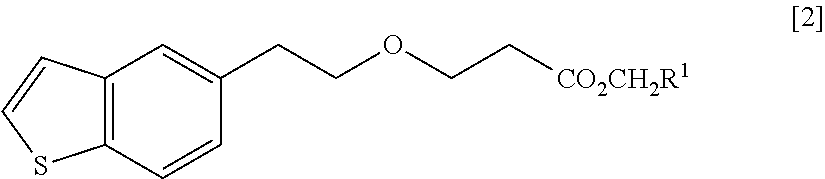
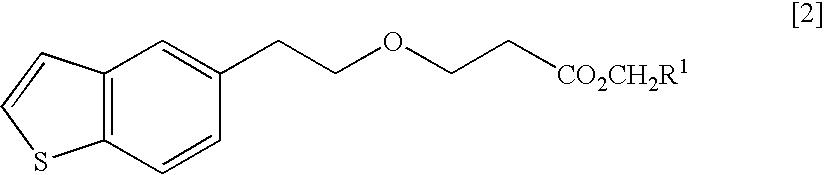
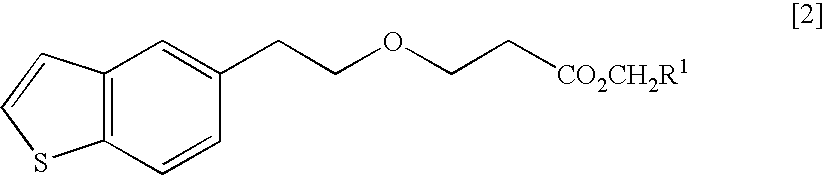
Process for production of 1-(3-(2-(1-benzothiophen-5-yl)- ethoxy)propyl)azetidin-3-ol or salts thereof
ActiveUS20110098484A1High yieldSmall amount of wasteNervous disorderOrganic chemistryAcetic acidDisease
Owner:TOYAMA CHEM CO LTD
Method for producing phenoxypyridine derivative
InactiveCN101454286AHas HGFR inhibitory effectHas anti-tumor effectOrganic active ingredientsOrganic chemistryHydrogen atomHydrogen
A process for preparing a compound represented by the formula (I): comprising reacting a compound represented by the formula (II) or salt thereof: with a compound represented by the formula (III): in the presence of a condensation reagent, wherein R 1 represents 1) optionally substituted azetidin-1-yl, 2) optionally substituted pyrrolidin-1-yl, 3) optionally substituted piperidin-1-yl, etc, R2, R3, R4 and R5 may be the same or different and each represents hydrogen or fluorine; and R6 represents hydrogen or fluorine.
Owner:EISIA R&D MANAGEMENT CO LTD
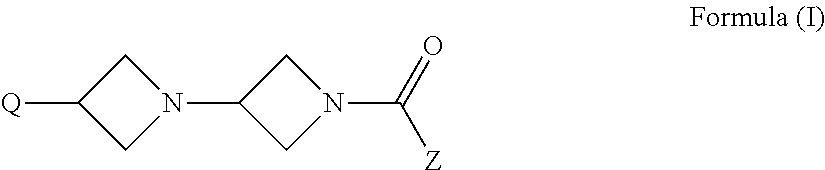
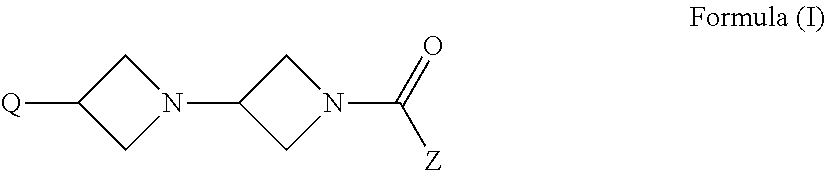
Azetidine derivatives, pharmaceutical compositions and uses thereof
The invention relates to new azetidine derivatives of the formula Ito their use as medicaments, to methods for their therapeutic use and to pharmaceutical compositions containing them.
Owner:BOEHRINGER INGELHEIM INT GMBH
Binders curable at room temperature with low blocking
ActiveUS7297231B2Increase viscosityEasy to useNatural cellulose pulp/paperPaper after-treatmentRoom temperaturePaper towel
Owner:KIMBERLY-CLARK WORLDWIDE INC
Beta-lactam cannabinoid receptor modulators
Owner:EHJZEVAN FARMASJUTIKLZ INK
Method for Making Waterborne Polyurethane with a Reactive Functional Group and a Nanocomposite Made of the Same
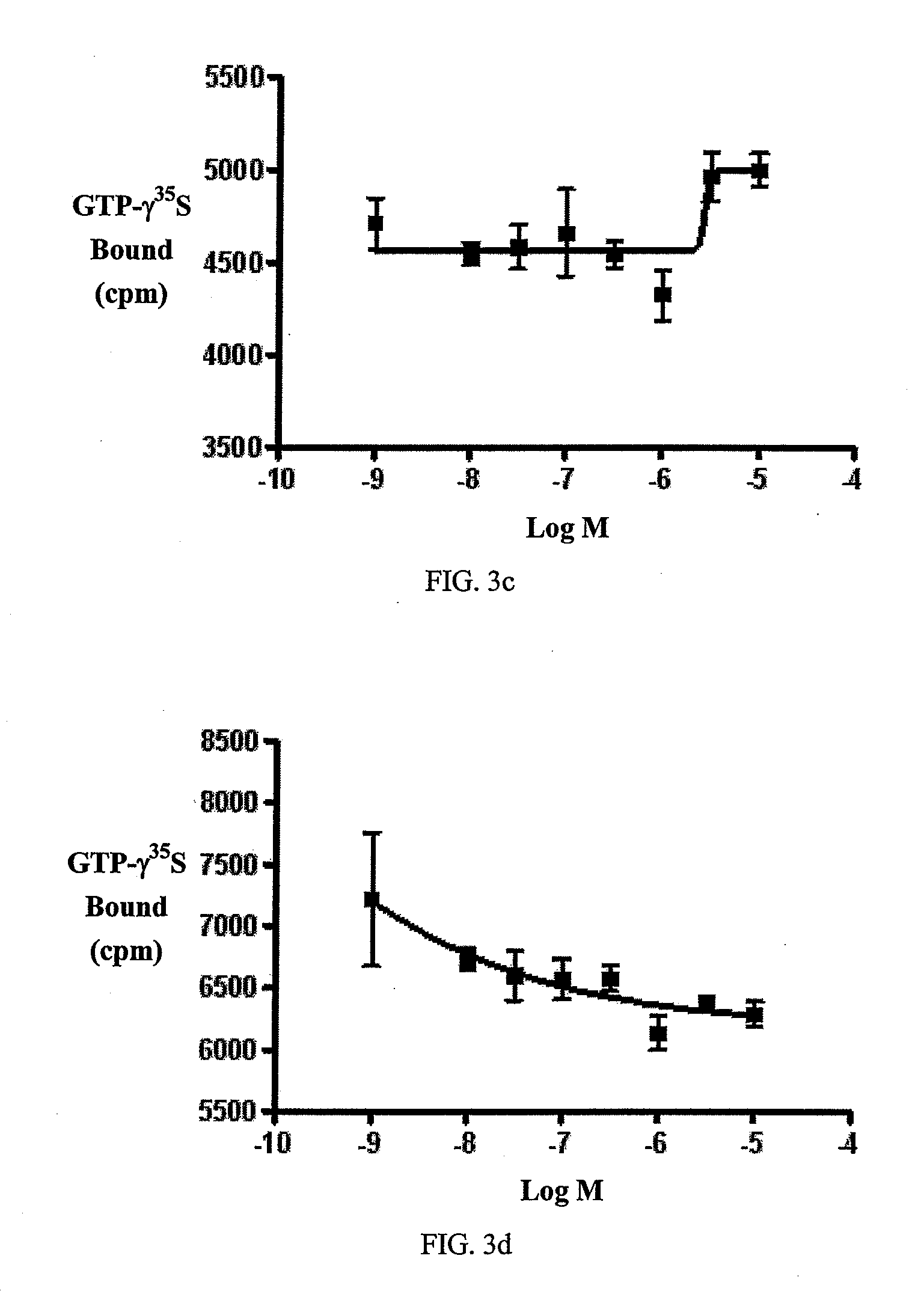
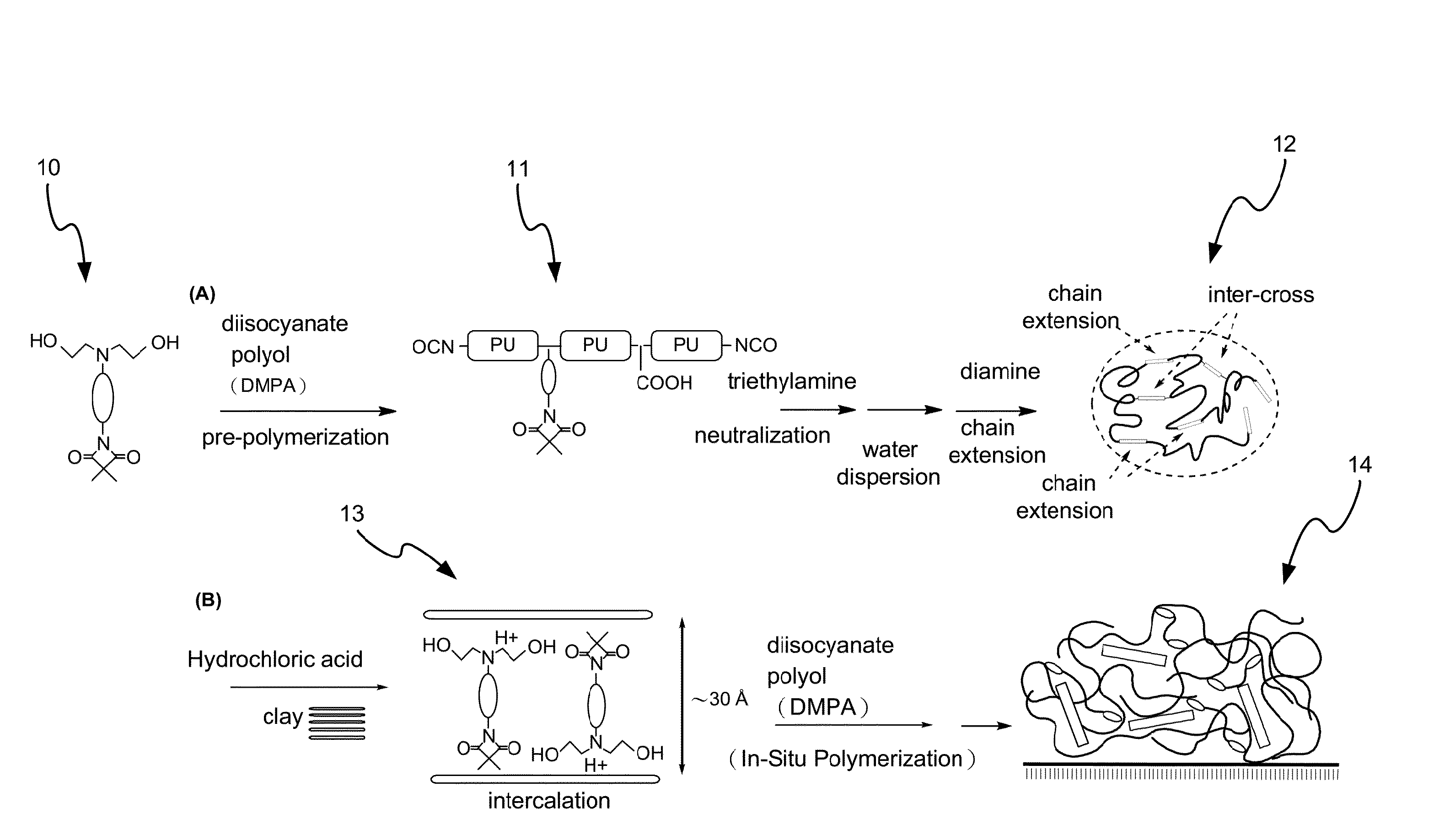
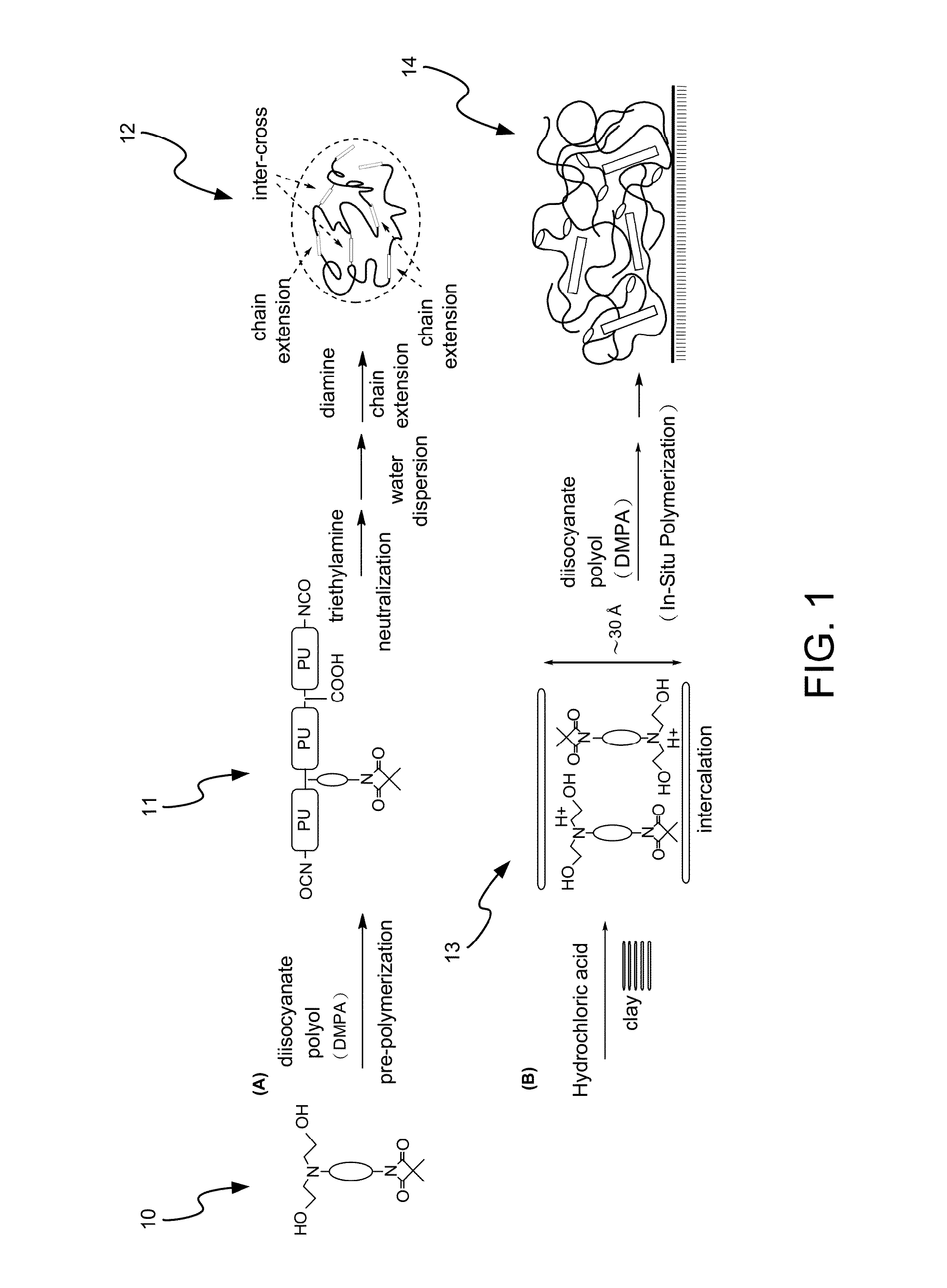
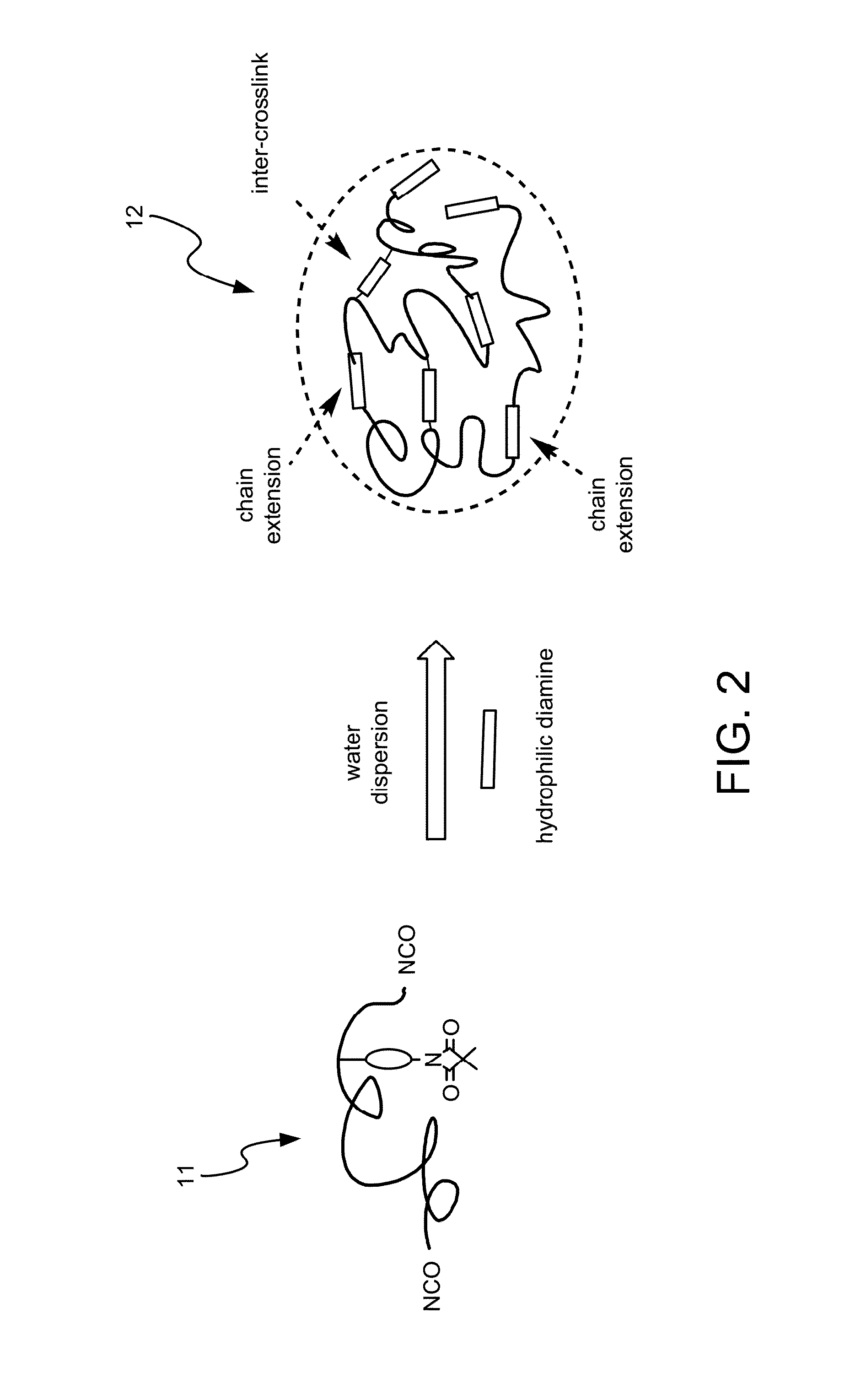
Disclosed is a method for making waterborne polyurethane with a reactive functional group. The method includes the step of introducing a short-chain diol monomer with a reactive functional group into waterborne polyurethane polymer by pre-polymerization to provide a polyurethane pre-polymer with the reactive functional group connected to a side chain and the step of reacting the pre-polymer with hydrophilic diamine for chain extension and inter-crosslink to provide waterborne polyurethane with different crosslink degree. The short-chain diol monomer is expressed by structural formula (I) and the polyurethane pre-polymer with the reactive functional group connected to the side chain is expressed by structural formula (II) as follows:A is azetidine-2,4-dione functional group or malonamide-linked alkyl group and B is nitrogen-linked di-hydroxy terminal group or tertiary amine-linked di-hydroxy terminal group R1 is diisocyanate, and R2 is a polyol backbone and wherein IG is a neutralized ionic group.
Owner:NAT CHUNG SHAN INST SCI & TECH
Preparation method of baricitinib
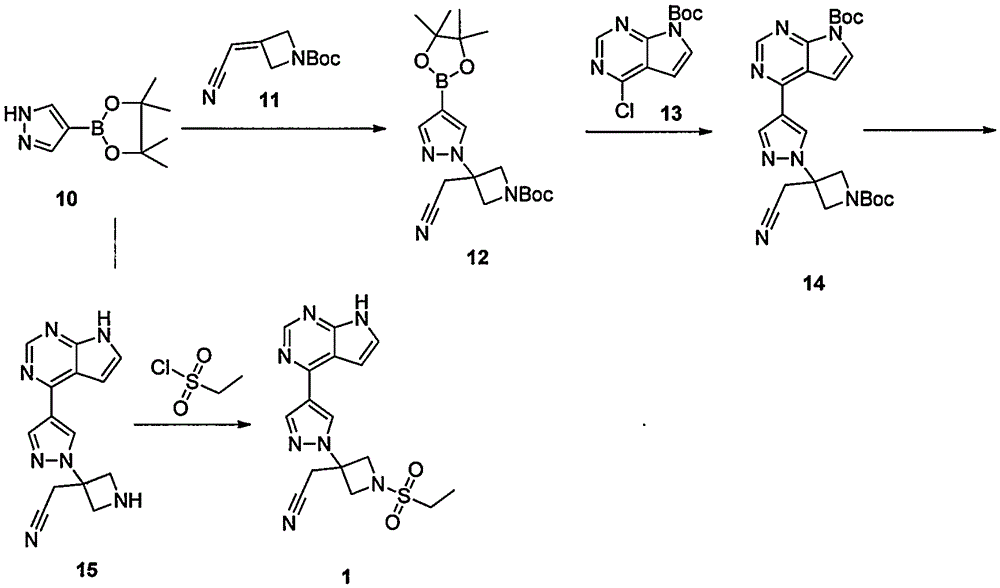
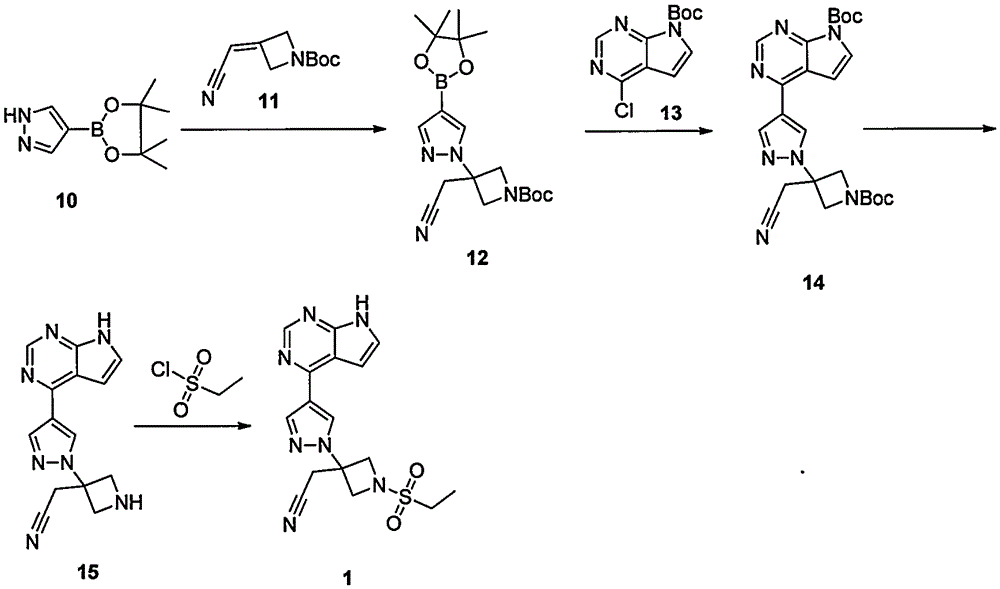
ActiveCN107176955ARaw materials are easy to getSimple processOrganic chemistrySulfonyl chlorideBoronic acid
The invention discloses a preparation method of baricitinib. The method comprises the following steps: performing a substitution reaction on 4-chloro-7H-pyrrolo[2,3-d]pyrimidine (II) serving as a raw material and benzene sulfonyl chloride in the presence of an alkali to obtain an intermediate III; then, performing a Suzuki coupling reaction on the intermediate III and 4-pyrazole-4-boronic acid pinacol ester in the presence of a palladium catalytic system and an alkali to obtain an intermediate V; then performing a Michael addition reaction on the intermediate V and 3-(cyanomethylene)azetidine-1-tert-butyl formate in the presence of a catalyst to obtain an intermediate VII; then removing Boc protection from the intermediate VII under the action of hydrochloric acid to obtain an intermediate VIII; then performing a sulfoamidate reaction on the intermediate VIII and ethyl sulfonyl chloride in an organic solvent in the presence of an alkali to obtain an intermediate IX; lastly, removing benzenesulfonyl protection from the intermediate IX under the action of tetramethylammonium fluoride or tetrabutylammonium fluoride or a trihydrate of the tetramethylammonium fluoride or the tetrabutylammonium fluoride to obtain baricitinib (I). Compared with the prior art, the method has the advantages of adoption of readily-available raw materials, low cost, high product yield and easiness for industrial production.
Owner:NANJING YOKO PHARMA +2
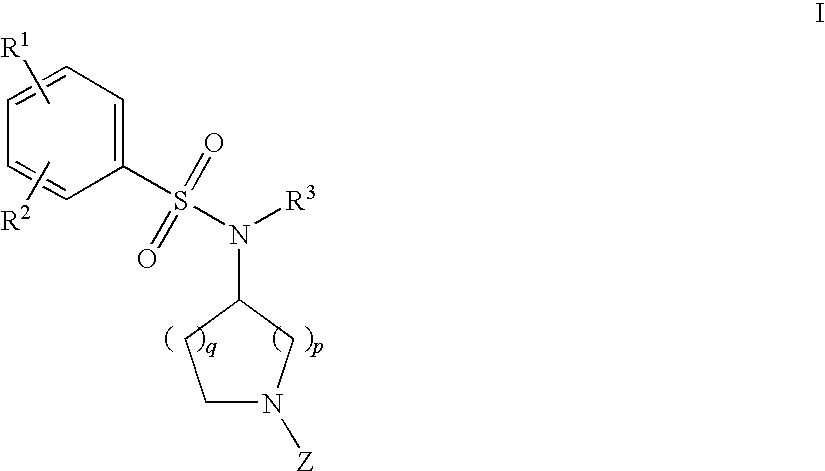
Benzenesulfonamide Compounds and the Use Thereof
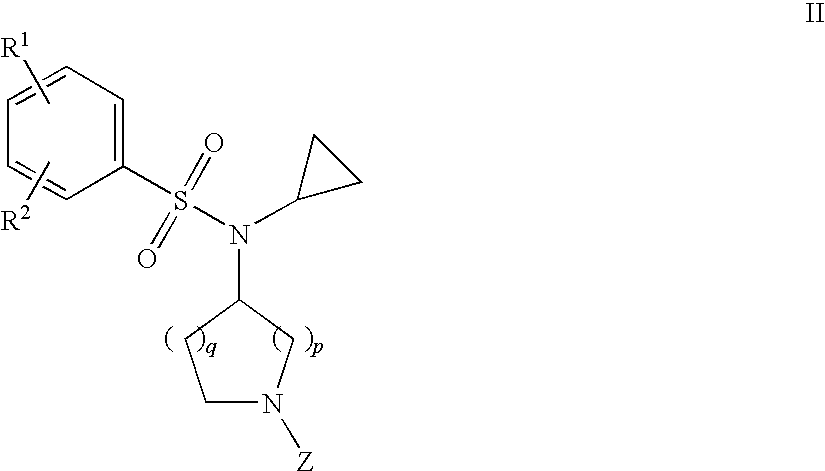
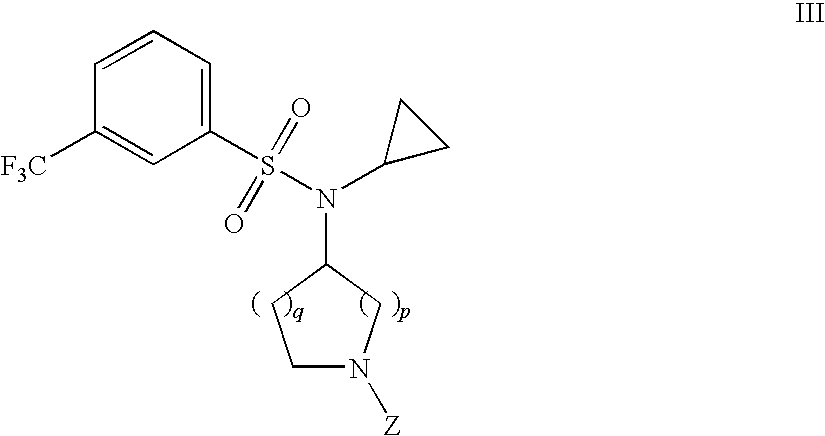
The invention relates to azetidinyl, pyrrolidinyl, piperidinyl, and hexahydroazepinyl compounds of Formula (I): and pharmaceutically acceptable salts, prodrugs, or solvates thereof, wherein R1, R2, R3, Z and q are defined as set forth in the specification. The invention is also directed to the use compounds of Formula I to treat a disorder responsive to the blockade of calcium channels, and particularly N-type calcium channels. Compounds of the present invention are especially useful for treating pain.
Owner:PURDUE PHARMA LP
Fabric pretreatment for digital printing
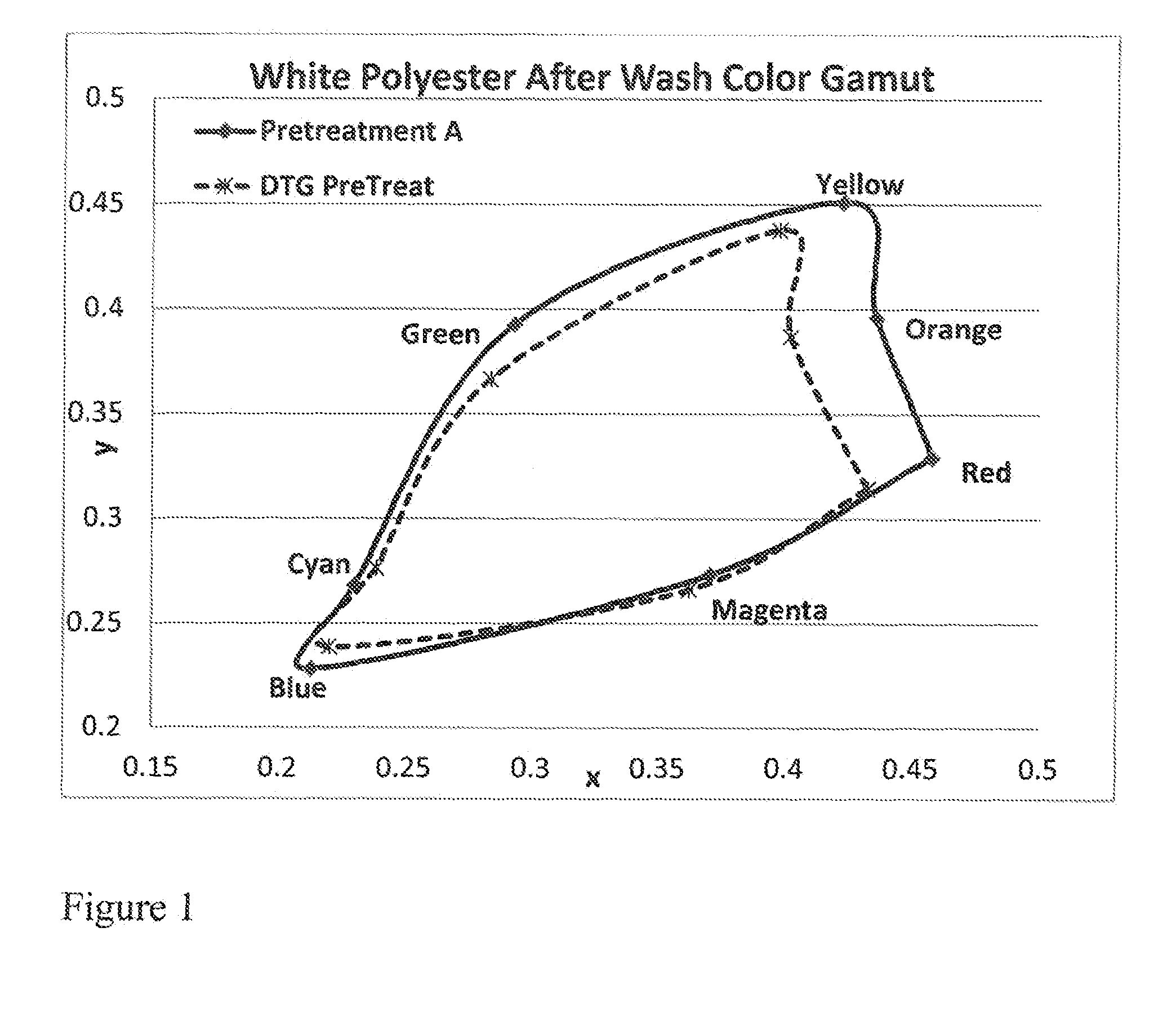
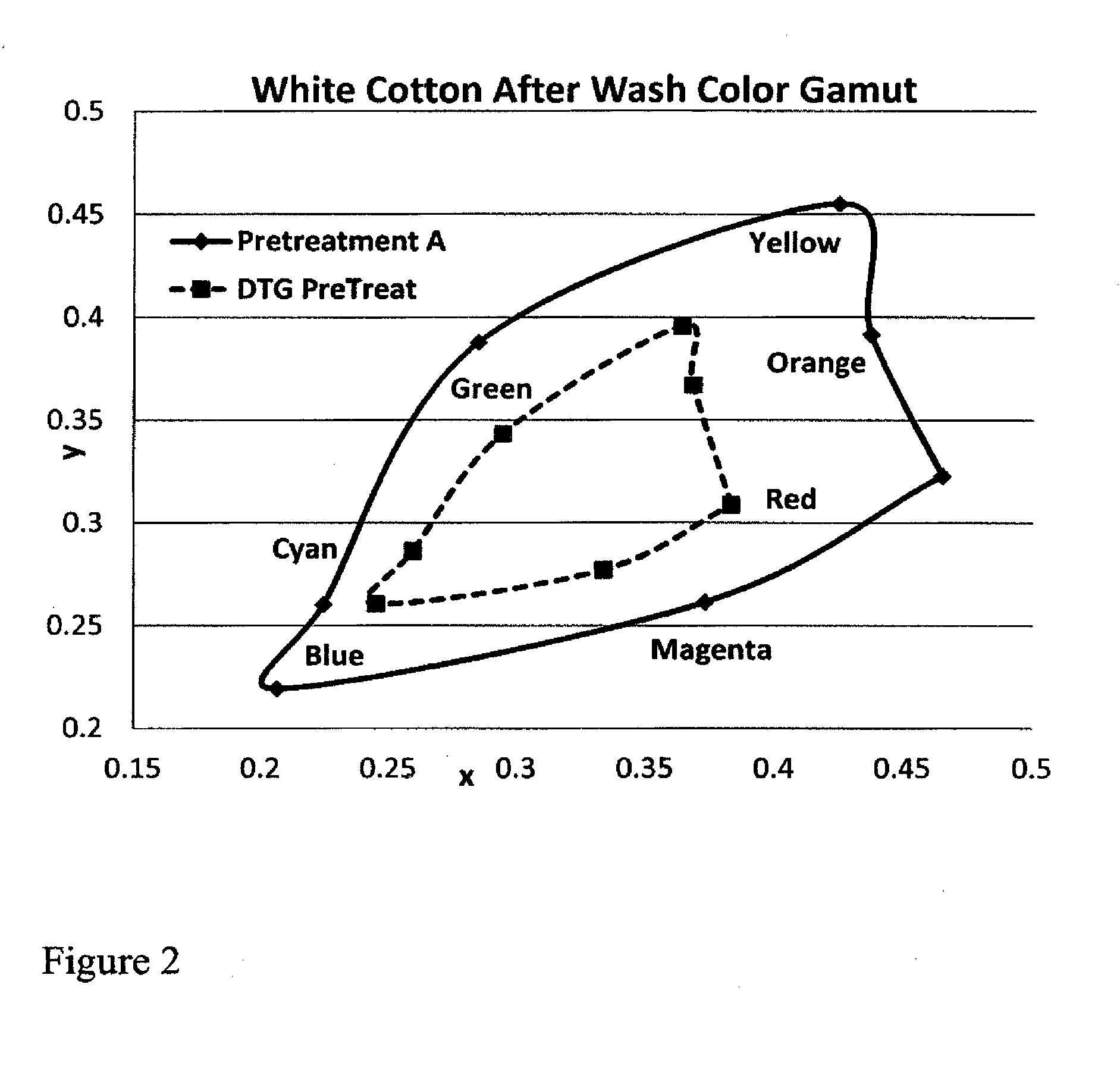
ActiveUS20160312404A1Keep the lookMaintain colorDuplicating/marking methodsDyeing processPrinting inkPolymer chemistry
An aqueous blend of an azetidinium functionalized polymer and a polymer having quaternary amine groups is disclosed for use as aqueous pretreatment for substrates such as textiles and garments that are going to be digitally printed. The pretreatment may further comprise wetting agents, surfactants, and preservatives. The pretreatment may be dry or wet immediately prior to digital printing and may be heat treated to bond the pretreatment to the substrate and / or the subsequent print ink.
Owner:LUBRIZOL ADVANCED MATERIALS INC
Solid pharmaceutical composition containing 1-(3-(2-(1-benzothiophen-5-yl)ethoxy)propyl)azetidin-3-ol or salt thereof
ActiveUS20150045345A1Excellent in dissolvabilityGood moldabilityOrganic active ingredientsBiocideCombinatorial chemistryAzacyclobutane
Owner:TOYAMA CHEM CO LTD
Anthranilamide insecticides
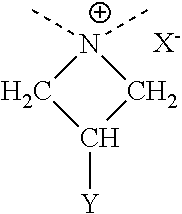
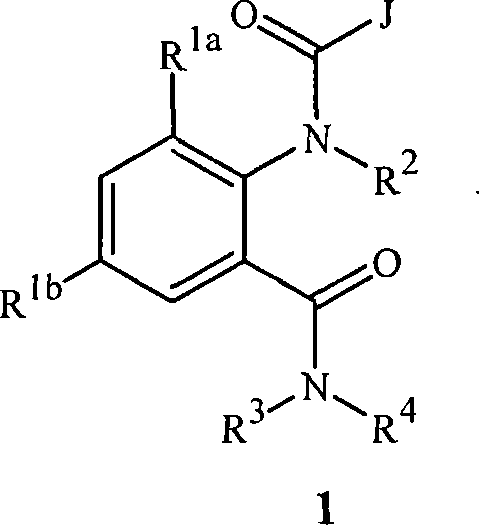
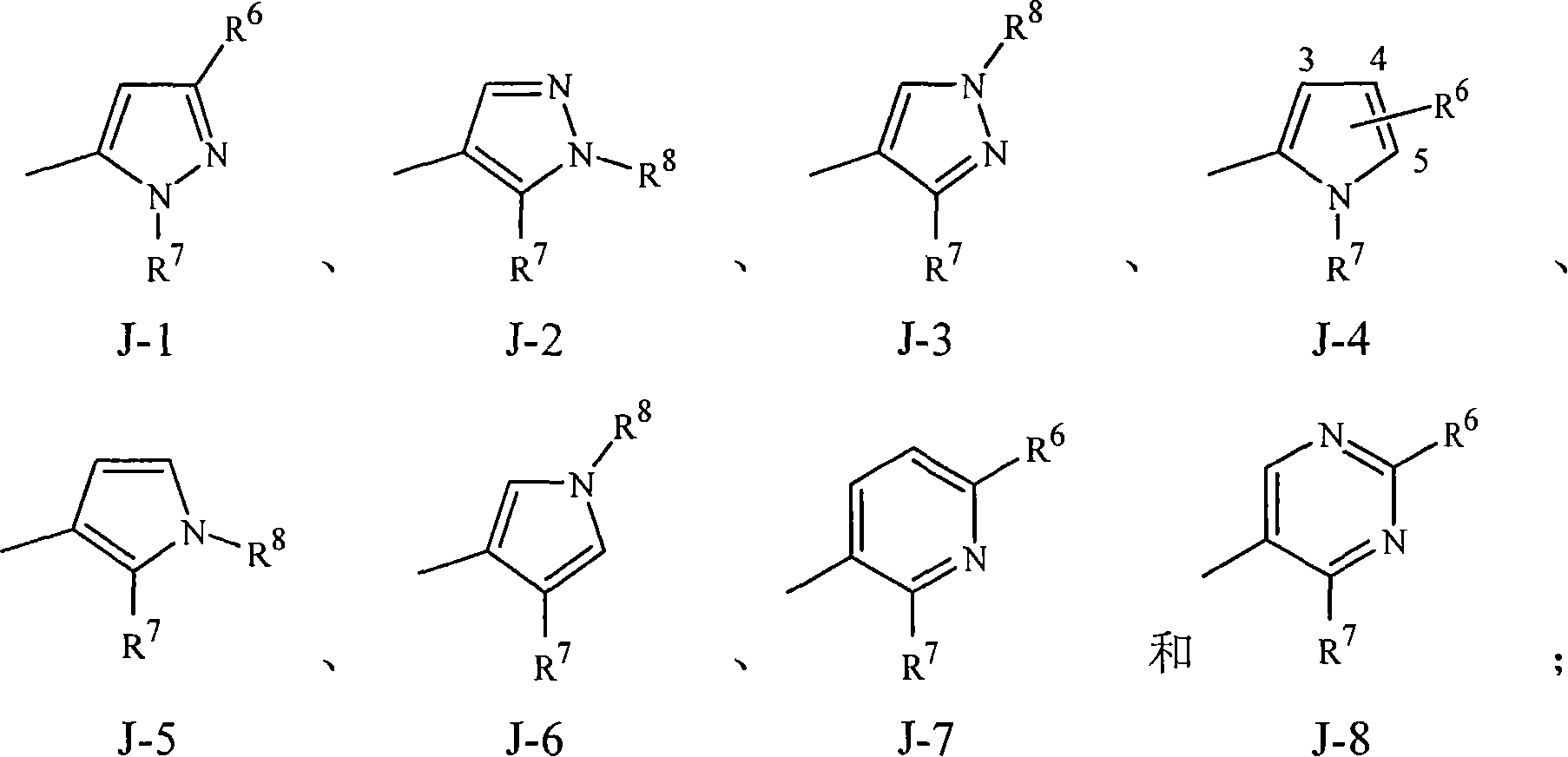
Disclosed are compounds of Formula 1, including all geometric and stereoisomers, N-oxides, and salts thereof, wherein J is a phenyl optionally substituted with one to four substituents independently selected from R5; or J is a heterocyclic ring selected from the group consisting of J-1 to J-8; R4 is C4-C12 alkylcycloalkyl, C5-C12 alkenylcycloalkyl, C5-C12 alkynylcycloalkyl, C4-C12 cycloalkylalkyl, C5-C12 cycloalkylalkenyl, C5-C12 cycloalkylalkynyl, C4-C12 cycloalkenylalkyl or C4-C12 alkylcycloalkenyl; each optionally substituted with one to six substituents selected from CH3 and halogen; or R4 is C3-C5 oxiranylalkyl, C3-C5 thiiranylalkyl, C4-C6 oxetanylalkyl, C4-C6 thietanylalkyl, 3-oxetanyl or 3-thietanyl, each optionally substituted with one to five substituents independently selected from C1-C3 alkyl, C1-C3 haloalkyl, halogen, CN, C2-C4 alkoxycarbonyl and C2-C4 haloalkoxycarbonyl; or R4 is C3-C5 aziridinylalkyl, C4-C6 azetidinylalkyl or 3-azetidinyl, each with R10 attached to the nitrogen atom, and optionally substituted on carbon atoms with one to five substituents independently selected from Cl-C3 alkyl, C1-C3 haloalkyl, halogen, CN, C2-C4 alkoxycarbonyl and C2-C4 haloalkoxycarbonyl; and Rla. R1b, R2, R3 and R5 are as defined in the disclosure. Also disclosed are compositions containing the compounds of Formula 1 and methods for controlling an invertebrate pest comprising contacting the invertebrate pest or its environment with a biologically effective amount of a compound or a composition of the invention.
Owner:FMC AGRO SINGAPORE PTE LTD +1
Salts
Owner:NOVARTIS AG
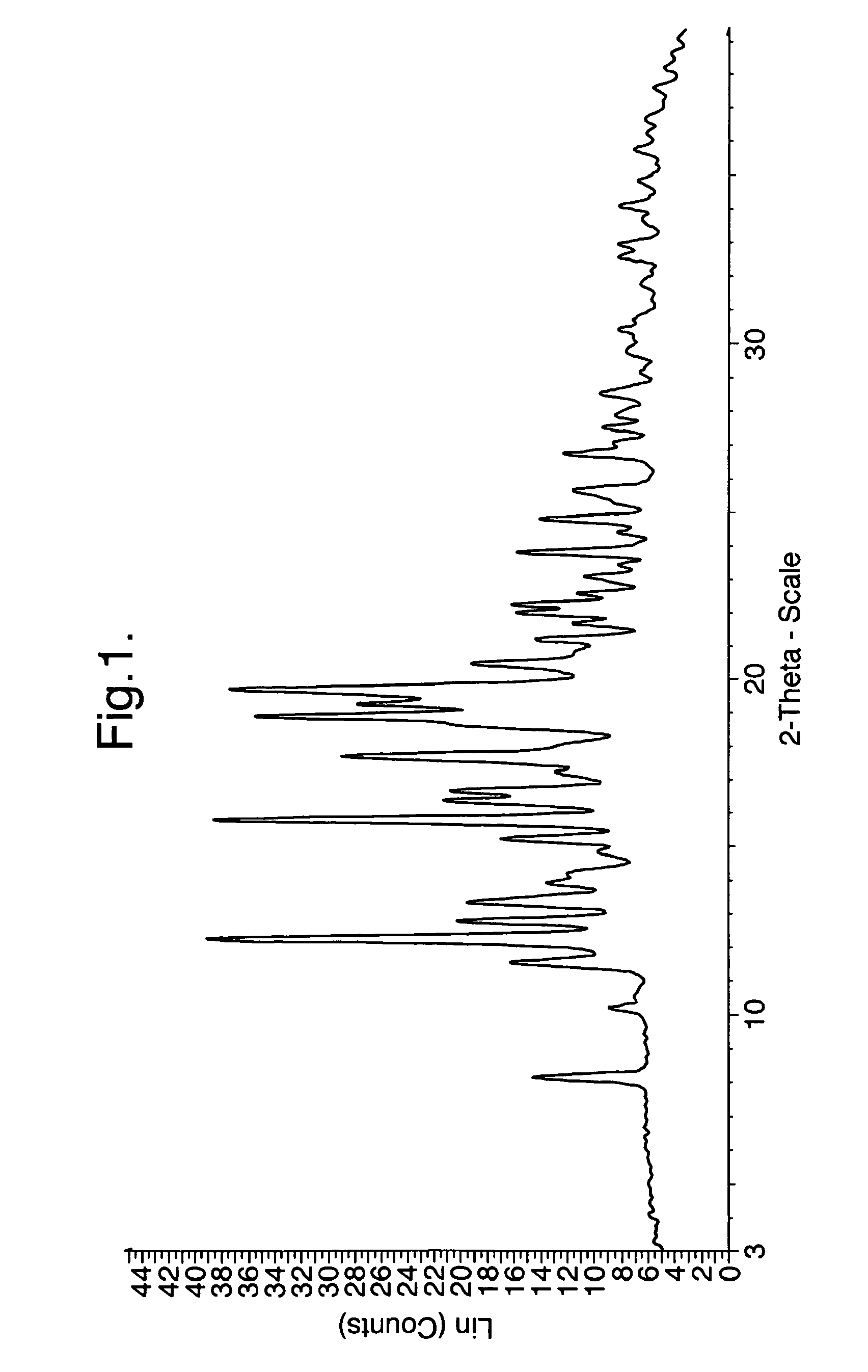
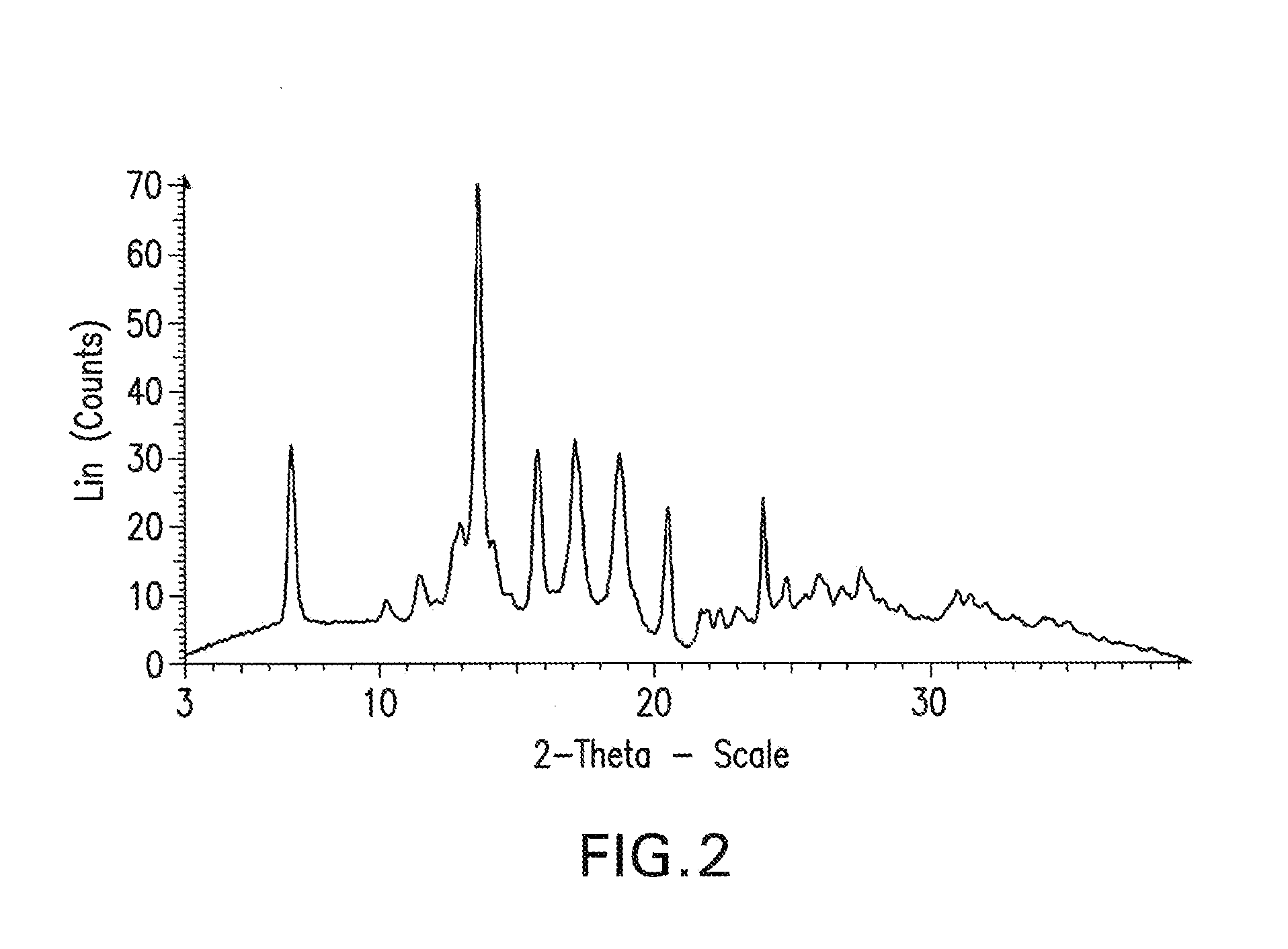
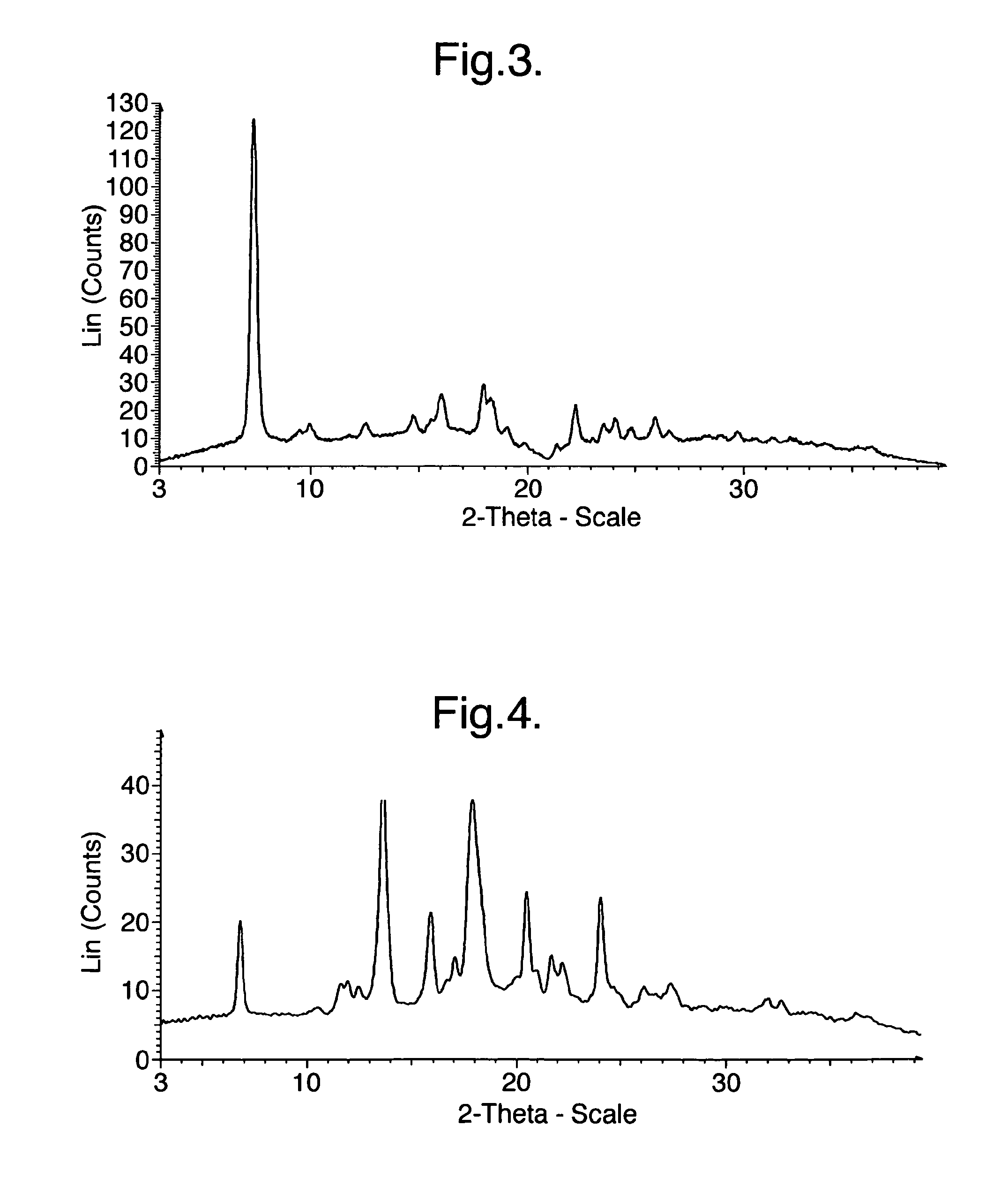
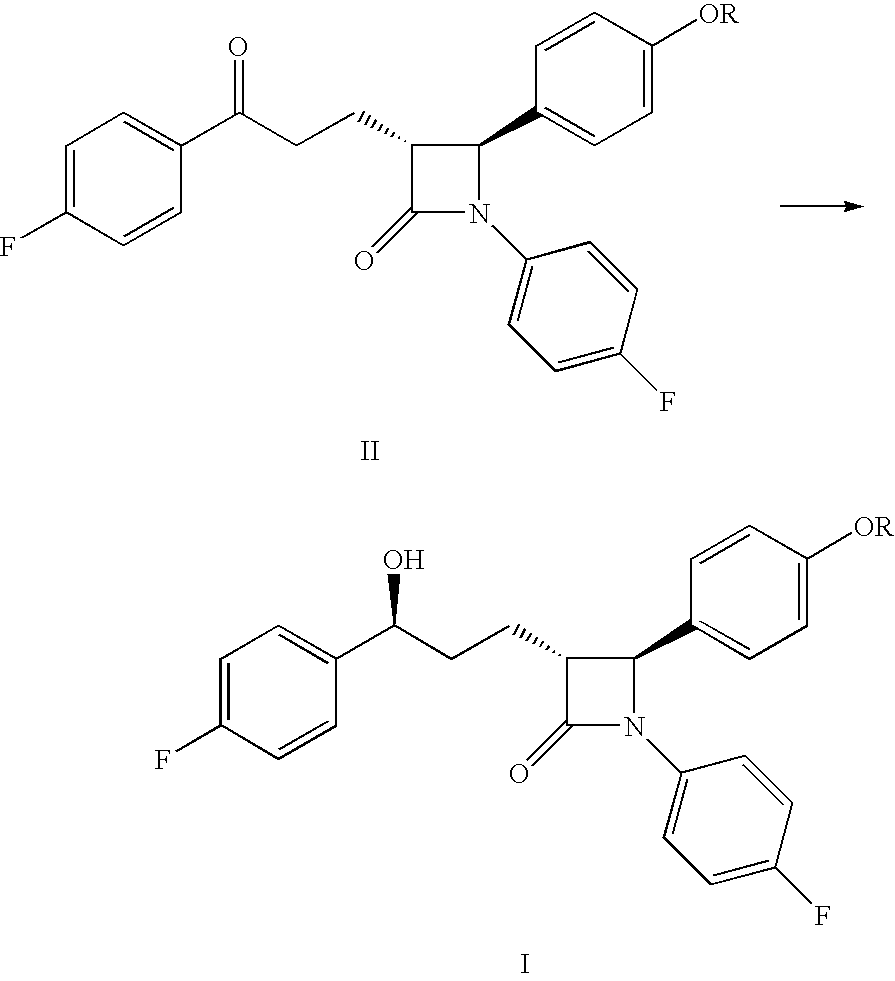
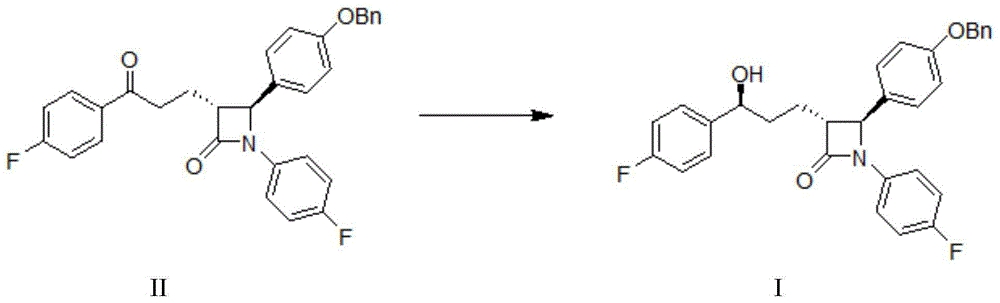
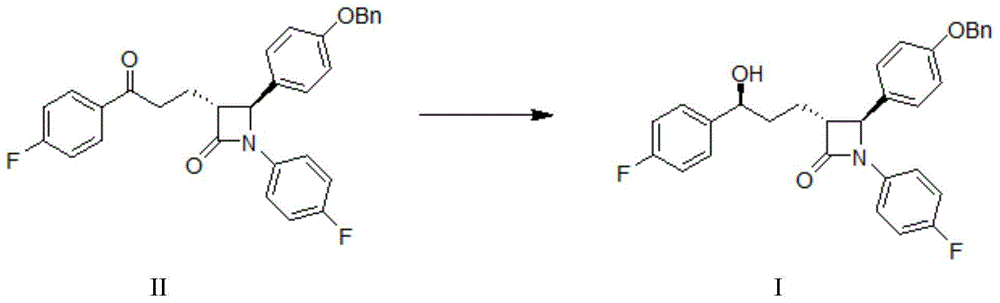
Processes for preparing intermediate compounds useful for the preparation of ezetimibe
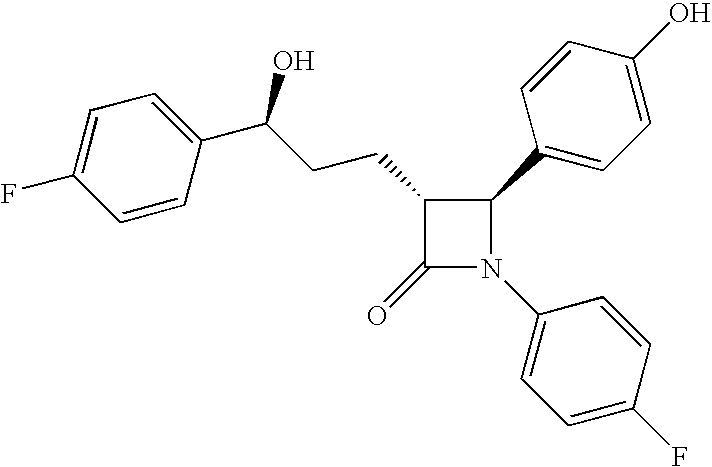
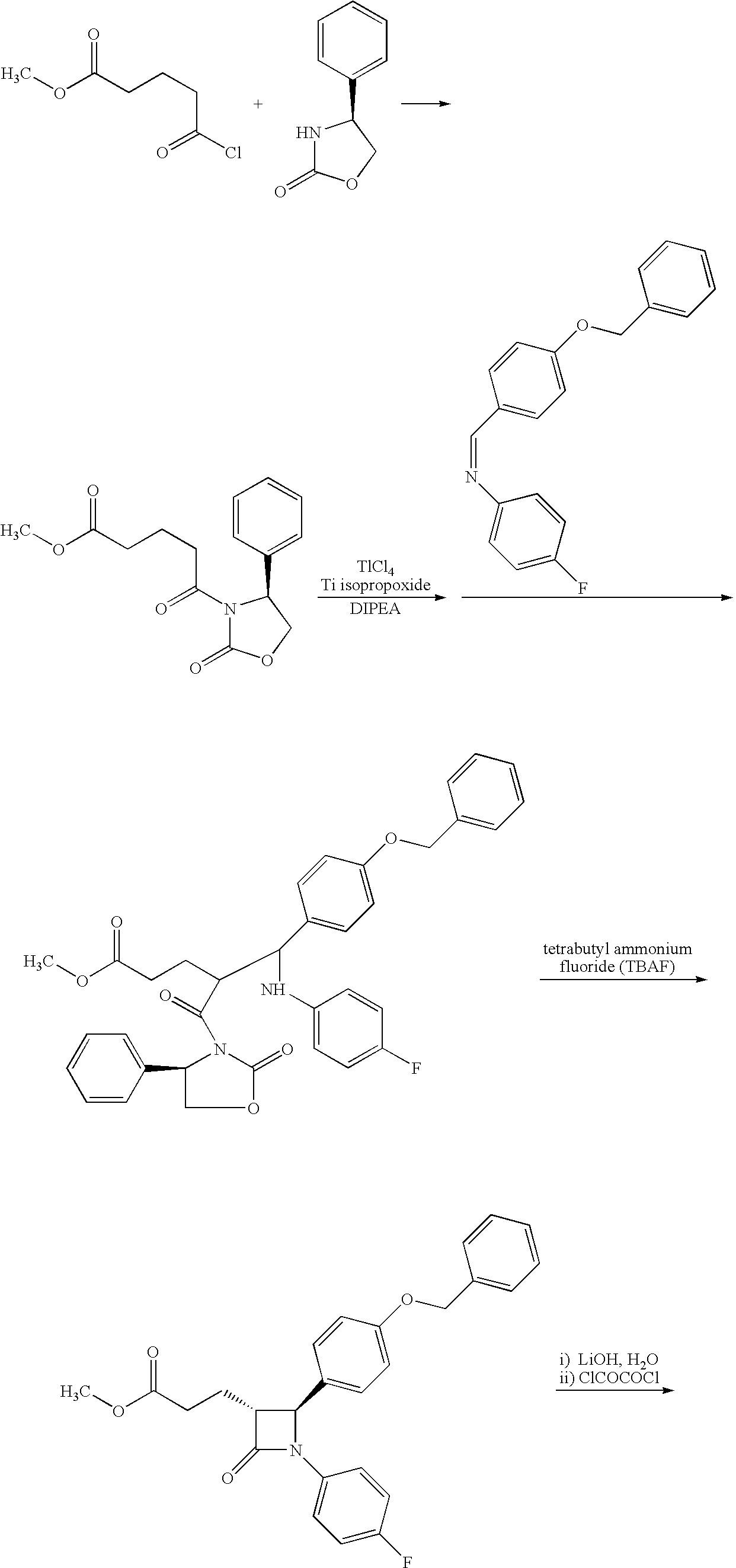
InactiveUS20090227786A1Easy to handleSilicon organic compoundsGroup 3/13 element organic compoundsEzetimibeKetone
The invention relates, in general, to an improved process for the preparation of the compounds (3R,4S)-4-(4-(benzyloxy)phenyl)-1-(4-fluorophenyl)-3-[3-(4-fluorophenyl)-3-oxopropyl]azetidin-2-one and (3R,4S)-1-(4-fluorophenyl)-3-[3-(4-fluorophenyl)-3-oxopropyl]-4-(4-hydroxyphenyl)-azetidin-2-one, which are key intermediates for the synthesis of ezetimibe, as well as the use of these intermediates for the preparation of ezetimibe.
Owner:MEDICHEM
Di-azetidinyl diamide as monoacylglycerol lipase inhibitors
Owner:JANSSEN PHARMA NV
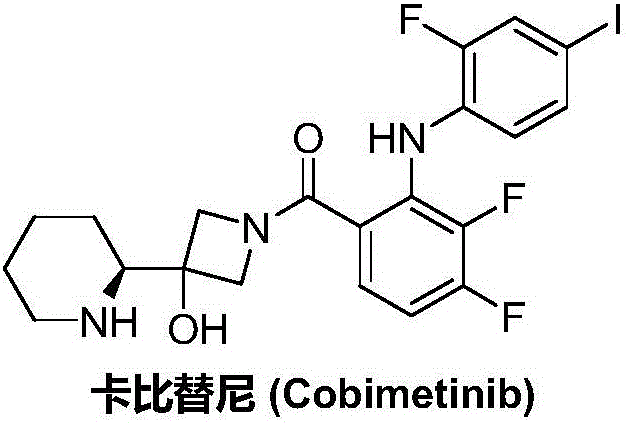
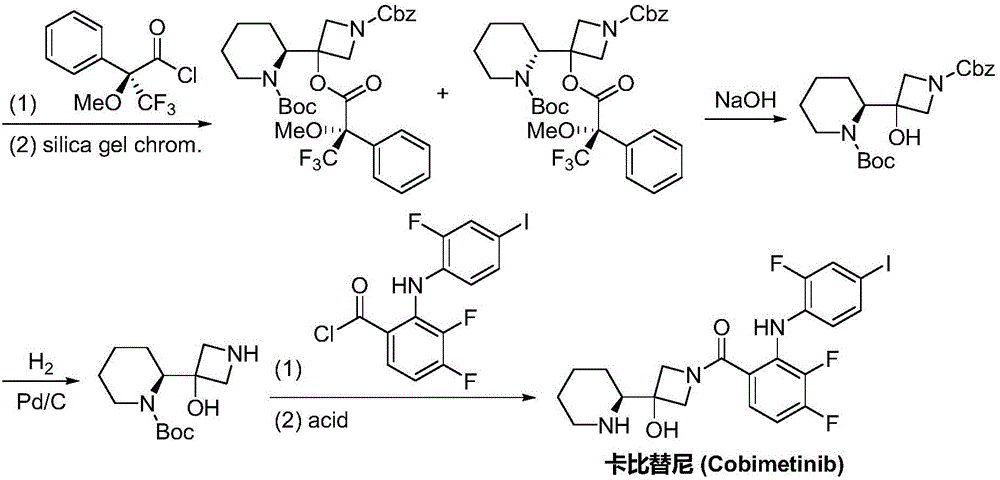
Synthesis method of cobimetinib
ActiveCN106045969AThe synthesis steps are simpleMeet the needs of useOrganic chemistryCobimetinibChloride
The invention discloses a synthesis method of cobimetinib. The method comprises the following steps: respectively carrying out salt forming reaction and bromination reaction on (R)-N-Boc-2-piperidinecarboxylic acid, silver nitrate (or mercuric oxide) and bromine water successively; reacting obtained (R)-N-Boc-2-bromopiperidine and a magnesium rod at first to generate a Grignard reagent, and then carrying out Grignard reaction on the (R)-N-Boc-2-bromopiperidine and 1-carbobenzoxy azetidine-3-one; carrying out deamination protection reaction on obtained 1-carbobenzoxy-3-hydroxy-3-[(2S)-N-Boc-2-piperidyl]-azetidine; carrying out amidation on obtained 3-hydroxy-3-[(2S)-N-Boc-2-piperidyl]-azetidine and 2,3,4-trifluorobenzoyl chloride; carrying out substitution reaction on obtained [2,3,4-trifluorophenyl] [3-hydroxy-3-(2S)-N-Boc-2-piperidyl-1-azetidinyl] ketone and 2-chloro-4-iodoaniline, and finally carrying out deamination protection reaction to obtain the cobimetinib. The method is reasonable and simple in process route, simple to operate and relatively low in cost, is an environmentally friendly method, and is suitable for industrial production.
Owner:湖南欧亚药业有限公司
Compounds having selective inhibiting effect at GSK3
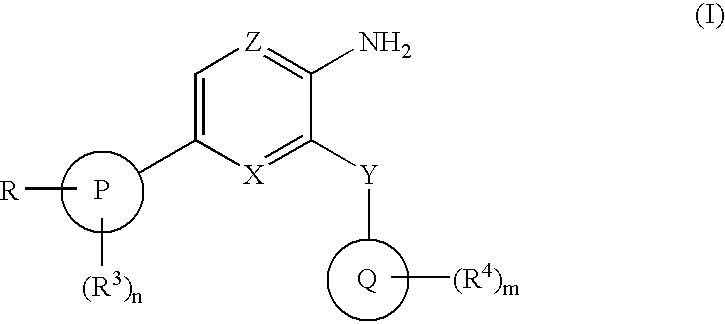
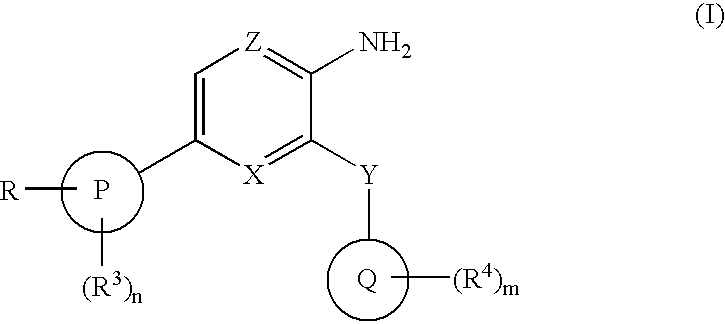
The present invention relates to new compounds of formula (I) wherein: Z is N and X is CH or N; Y is CONR5; P is phenyl or a 5 or 6 membered heteroaromatic ring containing one or more heteroatoms selected from N, O or S; Q is phenyl or a 5 or 6 membered aromatic heterocyclic ring containing one or more nitrogen atoms; R is C1-6alkylNR10R11 or C1-6alkylazetidine; R10 is hydrogen, C1-6alkyl, C2-6alkenyl, C2-6alkynyl, C0-6alkylC3-6cycloalkyl, C0-6alkylaryl, C0-61alkylheteroaryl or C1-6alkylNR8R9; R11 is C1-6alkylNR8R9, C0-6alkylC3-6cycloalkyl or C0-6alkylheterocycloalkyl; as a free base or a pharmaceutically acceptable salt, solvate or solvate of salt thereof, a process for their preparation and new intermediates used therein, pharmaceutical formulations containing said therapeutically active compounds and to the use of said active compounds in therapy.
Owner:ASTRAZENECA AB
Process for production of 1-(3-(2-(1-benzothiophen-5-yl)-ethoxy)propyl)azetidin-3-ol or salts thereof
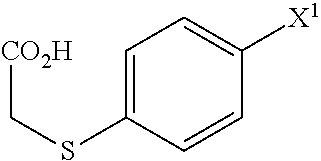
ActiveUS20090069576A1High yieldSmall amount of wasteNervous disorderOrganic chemistryDiseaseAcetic acid
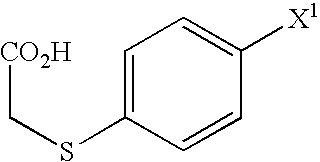
A Process for production of 1-(3-(2-(1-benzothiophen-5-yl)ethoxy)propyl)azetidin-3-ol or salts thereof which comprises using as a starting compound as a (phenylthio)acetic acid derivative or salts thereof represented by the general formula:wherein X1 represents a halogen atom, is useful as a safe process for mass production of 1-(3-(2-(1-benzothiophen-5-yl)ethoxy)propyl)azetidin-3-ol or salts thereof which is useful as a remedy for disease of central and peripheral nerve.
Owner:TOYAMA CHEM CO LTD
Combination of a CB1 receptor antagonist and of a product which activates dopaminergic neurotransmission in the brain, the pharmaceutical compositions comprising them and their use in the treatment of parkinson's disease
InactiveUS7217705B2Potentiate symptomatic effectEliminate side effectsBiocideNervous disorderDiseaseDopaminergic neurotransmission
The present invention relates to the combination of one or more CB1 antagonist azetidine derivatives and of one or more products which activate dopaminergic neurotransmission in the brain, to the pharmaceutical compositions comprising them and to their use in the treatment of Parkinson's disease.
Owner:AVENTIS PHARMA SA (US)
Strengthened composite products and methods for making and using same
The disclosure provides strengthened products, including strengthened fibrous composite products and methods for making and using same and strengthened particulates, such as particulate fertilizer products, and methods for making and using same. The fibrous composite product can include a plurality of fibers and an at least partially cured strengthening resin. The fertilizer composition can include a particulate core that can include a plant nutrient, at least one coating layer of the strengthening resin, and at least one coating layer of a water insoluble material. The strengthening resin can include one or more aldehyde-based resins and one or more crosslinked resins. The crosslinked resin can include one or more polyamines at least partially crosslinked by one or more symmetric crosslinks and can include one or more azetidinium functional groups.
Owner:GEORGIA PACIFIC CHEM LLC
Preparation method for Ezetimibe
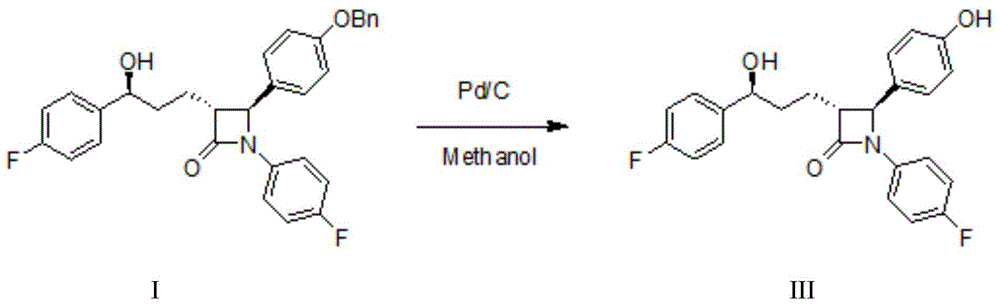
The invention discloses a preparation method for Ezetimibe. The preparation method comprises the following steps: adding (3R, 4S)-4-[4-(benzyloxy) phenyl]-1-(4-fluorophenyl)-3-[3-(4-fluorophenyl)-3-oxo propyl] azacyclobutane-2-one (RM1 for short) into a borane / dimethylsulfide reaction system, and then adding an oxidant to react to generate an Ezetimibe intermediate; and carrying out hydrogenation reduction on the Ezetimibe intermediate to obtain Ezetimibe with a structural formula III. According to the preparation method, in the preparation process of the Ezetimibe intermediate, RM1 is taken as a raw material, dichloromethane is taken as a raw material solvent and (R)-MeCBS / methylbenzene solution is taken as a reaction catalyst, so that the reaction can be accelerated; the yield of the Ezetimibe intermediate can be improved; in the preparation method of Ezetimibe, methanol is taken as a solvent, 10% Pd / C is taken as a reactant, so that low-temperature reaction is carried out; meanwhile, air oxidation reaction is prevented so as to further improve the yield of Ezetimibe.
Owner:CHENGDU SENKE PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com